In Brief
This study provides, to the authors' knowledge, the highest-quality data to date on rod fracture (RF) rates following adult symptomatic lumbar scoliosis surgery. At a median 5.1-year follow-up, 38.8% of patients had at least one RF. The estimated RF rates at 2, 4, and 8 years were 11%, 24%, and 49%, respectively. Greater blood loss and postoperative pelvic tilt were significant risk factors for RF. These findings emphasize the importance of long-term follow-up to accurately estimate the true incidence of RF.
Keywords: adult scoliosis, spine deformity, spinal alignment, spinal instrumentation, rod fracture, spine surgery, lumbar, thoracic
ABBREVIATIONS : ASD = adult spinal deformity, ASLS = adult symptomatic lumbar scoliosis, ASLS-1 = Adult Symptomatic Lumbar Scoliosis–1, BDRF = bilateral displaced RF, BNRF = bilateral nondisplaced RF, EBL = estimated blood loss, HRQOL = health-related quality of life, LL = lumbar lordosis, MCS = mental component summary, NRS = numeric rating scale, ODI = Oswestry Disability Index, PCS = physical component summary, PI = pelvic incidence, PI-LL = PI minus LL, PLIF = posterior lumbar interbody fusion, PROM = patient-reported outcome measure, PSO = pedicle subtraction osteotomy, PT = pelvic tilt, RF = rod fracture, rhBMP-2 = recombinant human bone morphogenetic protein-2, SRS-22r = Scoliosis Research Society-22r, SVA = sagittal vertical axis, T1PA = T1 pelvic angle, TLIF = transforaminal lumbar interbody fusion, UDRF = unilateral displaced RF, UNRF = unilateral nondisplaced RF, VCR = vertebral column resection
Abstract
OBJECTIVE
Previous reports of rod fracture (RF) in adult spinal deformity are limited by heterogeneous cohorts, low follow-up rates, and relatively short follow-up durations. Since the majority of RFs present > 2 years after surgery, true occurrence and revision rates remain unclear. The objectives of this study were to better understand the risk factors for RF and assess its occurrence and revision rates following primary thoracolumbar fusions to the sacrum/pelvis for adult symptomatic lumbar scoliosis (ASLS) in a prospective series with long-term follow-up.
METHODS
Patient records were obtained from the Adult Symptomatic Lumbar Scoliosis–1 (ASLS-1) database, an NIH-sponsored multicenter, prospective study. Inclusion criteria were as follows: patients aged 40–80 years undergoing primary surgeries for ASLS (Cobb angle ≥ 30° and Oswestry Disability Index ≥ 20 or Scoliosis Research Society-22r ≤ 4.0 in pain, function, and/or self-image) with instrumented fusion of ≥ 7 levels that included the sacrum/pelvis. Patients with and without RF were compared to assess risk factors for RF and revision surgery.
RESULTS
Inclusion criteria were met by 160 patients (median age 62 years, IQR 55.7–67.9 years). At a median follow-up of 5.1 years (IQR 3.8–6.6 years), there were 92 RFs in 62 patients (38.8%). The median time to RF was 3.0 years (IQR 1.9–4.54 years), and 73% occurred > 2 years following surgery. Based on Kaplan-Meier analyses, estimated RF rates at 2, 4, 5, and 8 years after surgery were 11%, 24%, 35%, and 49%, respectively. Baseline radiographic, clinical, and demographic characteristics were similar between patients with and without RF. In Cox regression models, greater postoperative pelvic tilt (HR 1.895, 95% CI 1.196–3.002, p = 0.0065) and greater estimated blood loss (HR 1.02, 95% CI 1.005–1.036, p = 0.0088) were associated with increased risk of RF. Thirty-eight patients (61% of all RFs) underwent revision surgery. Bilateral RF was predictive of revision surgery (HR 3.52, 95% CI 1.8–6.9, p = 0.0002), while patients with unilateral nondisplaced RFs were less likely to require revision (HR 0.39, 95% CI 0.18–0.84, p = 0.016).
CONCLUSIONS
This study provides what is to the authors’ knowledge the highest-quality data to date on RF rates following ASLS surgery. At a median follow-up of 5.1 years, 38.8% of patients had at least one RF. Estimated RF rates at 2, 4, 5, and 8 years after surgery were 11%, 24%, 35%, and 49%, respectively. Greater estimated blood loss and postoperative pelvic tilt were significant risk factors for RF. These findings emphasize the importance of long-term follow-up to realize the true prevalence and cumulative incidence of RF.
Adult spinal deformity (ASD) affects nearly 15% of the US population and is particularly highly prevalent among the growing population of older adults.1 ASD is a heterogeneous disease that comprises a wide array of spinal disorders, including adult idiopathic and degenerative scoliosis, sagittal malalignment, and iatrogenic deformity.2–4 Adult scoliosis, the most common form of ASD, can have a significant negative impact on health-related quality of life (HRQOL).5,6
As surgical approaches and technology have rapidly evolved, older patients with more comorbidities and complex deformities have been considered possible candidates for surgical treatment of ASD.7–10 Moreover, despite substantial advances in instrumentation, surgical techniques, and critical care, complication rates greater than 70% and substantial risk for revision surgery have been reported.11–13
Implant-related complications including rod fractures (RFs) are the most frequent sources of postoperative morbidity and revision surgery after long-segment spinal fusion for ASD.14,15 These fractures can produce mechanical pain and progression of deformity, potentially leading to revision surgery.16,17 Despite the substantial impact of RFs, there are relatively few reports that define the occurrence of and risk factors for this instrumentation failure.
Previous RF studies have been limited by relatively short follow-up, limited follow-up rates, single-center or single-surgeon cohorts, heterogeneous populations, and lack of granular data collection. Since the majority of RFs present more than 2 years after surgery,18 the true occurrence and revision rates of RF remain poorly defined and longer outcome studies are needed. In the present study, using data from the multicenter, prospective Adult Symptomatic Lumbar Scoliosis–1 (ASLS-1) study,1,19 we sought to do the following: 1) define the rates of RF in a relatively homogenous patient population, based on longer-term follow-up than in most previous studies; 2) assess risk factors for the occurrence of RFs; and 3) determine rates of and risk factors for revision surgery for RF.
Methods
Study Design and Inclusion Criteria
Patient records were obtained from a prospective, multicenter, consecutive series of patients from the ASLS-1 database. ASLS-1 was an NIH-sponsored study to assess operative versus nonoperative treatment for adult patients with symptomatic lumbar scoliosis.1,19 Patients were enrolled through a protocol approved by the institutional review boards of the 9 participating sites. All patients were aged 40–80 years and had undergone primary surgery (no prior spinal fusion or multilevel decompressions) for adult symptomatic lumbar scoliosis (ASLS), which was defined as the presence of a thoracolumbar curve with a coronal Cobb angle ≥ 30° and an Oswestry Disability Index (ODI) ≥ 20 or Scoliosis Research Society-22r (SRS-22r) score ≤ 4.0 in the domains of pain, function, and/or self-image. In the present study, we focused only on the patients from the primary ASLS-1 study who were operatively treated with posterior instrumented fusion of at least 7 vertebral levels that included the sacrum/pelvis. Indications for surgery were predominantly combinations of back and leg pain, disability, motor weakness, and progressive deformity.
Data Collection
Standardized forms were used to collect patient demographic data, clinical characteristics, and surgical information. Patient-reported outcome measures (PROMs) included the SRS-22r, SF-12 physical component summary (PCS) and mental component summary (MCS) scores, ODI, and back and leg numeric rating scale (NRS) scores.
Radiographic review was performed independently of the operative teams by two neurosurgeons and was based on full-length standing posteroanterior and lateral 36-inch radiographs. Baseline and 3-month postoperative films were compared to assess parameter changes associated with surgery. All radiographic measurements were performed using Surgimap Software (Nemaris Inc.) and included coronal Cobb angles, thoracic kyphosis (T4–12), lumbar lordosis (LL), C7–S1 sagittal vertical axis (SVA), T1 pelvic angle (T1PA), pelvic incidence (PI), PI minus LL (PI-LL) mismatch, pelvic tilt (PT), and global coronal alignment.
Study Outcomes
The primary objectives of this study were to determine risk factors for and rates of RF, as well as rates of and risk factors for revision surgery for RF. RFs were identified based on annual imaging and categorized according to the RF classification of El Dafrawy et al.20 into four groups: unilateral nondisplaced RF (UNRF), unilateral displaced RF (UDRF), bilateral nondisplaced RF (BNRF), and bilateral displaced RF (BDRF). Among RF patients, subgroup analysis was performed between patients with UNRF and those with other RFs (UDRF, BNRF, and BDRF) based on the hypothesis that UNRFs may be less likely to have clinical impact or need for revision surgery. Isolated RFs located between S1 and iliac fixation were not included in the present study because in those patients the rods do not span a spinal motion segment for which the desired result was fusion (i.e., the sacroiliac joints).
Data and Statistical Analysis
Follow-up started on the date of surgical treatment and ended at the time of loss to follow-up, death, or 8 years after surgery. Kaplan-Meier curves were used to estimate the proportion of patients developing RF across the follow-up time. Demographic, clinical, surgical, and radiographic parameters were described for patients with no RF, and for patients with each type of RF (UNRF, UDRF, BNRF, or BDRF) based on their first RF. For initial statistical comparisons, patients were classified as those who did and those who did not develop RF and were compared using the Wilcoxon rank-sum test for continuous variables and the chi-square test or Fisher exact test for categorical data according to the sample size. Differences were considered statistically significant for p < 0.05.
Multivariate Cox models were used to determine a subset of risk factors independently associated with RF and with the need for revision surgery. Random effects for study sites were included in the models to account for the potential correlation of patient characteristics, surgical approaches, and outcomes within each site. Initial multivariate models included all variables with p < 0.10 from the univariate comparisons. Variables that did not retain a p < 0.10 in multivariate analysis were then removed, and additional variables thought to be potentially important confounders (e.g., age) based on surgical expertise were considered for inclusion before determining the final multivariate model. The number of events (RFs) was considered when determining the number of variables selected for the final model.21 Among patients who developed RF, we used Kaplan-Meier curves to estimate the proportion of patients with a revision across time after RF. Cox regression models were used to estimate the association of UNRF and bilateral fracture with risk of revision. RF type was treated as a time-varying variable, in which unilateral RFs that progressed to bilateral RFs were recategorized to bilateral RFs for the remaining follow-up time. Due to sample size limitations, we were not able to assess the combined effects of multiple rod constructs. Statistical analyses were performed using SAS software (SAS Institute).
Results
Clinical Characteristics of the Patient Population
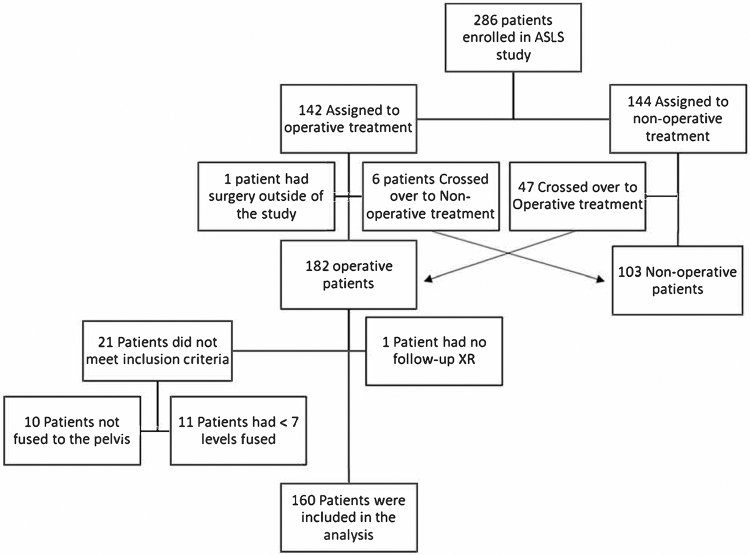
A total of 286 patients were enrolled in the ASLS-1 study between 2010 and 2014. Of the 182 patients who underwent surgery, 22 were excluded from our study (10 patients did not have instrumentation to the pelvis, 11 had < 7 levels fused, and 1 patient did not have radiographic follow-up) (Fig. 1). A total of 160 patients (141 women and 19 men) with a median age of 62 years (IQR 55.7–67.9 years) met inclusion criteria. Of these 160 patients, 122 (76%) had at least 5 years of follow-up. Additionally, 5 patients who crossed over to surgery after a period of nonoperative treatment in the study were not yet eligible for 5-year follow-up; therefore, we had 79% (122/155) 5-year follow-up among eligible patients.
FIG. 1.
Flowchart of patients in the study. XR = x-ray.
Baseline demographic and clinical characteristics were similar between patients with and without RF (Table 1). The overall median BMI was 26 (IQR 23.4–29.5), 92 patients had osteopenia (bone density T-score between −1 and −2.5), and 15 had osteoporosis (T-score ≤ −2.5). Baseline radiographic measures and PROMs were also similar between patients who did and those who did not develop RF (Table 1). On baseline radiographs, the overall median PI-LL mismatch was 19° (IQR 5°–32°), the median PT was 24° (IQR 20°–30°), the median C7–S1 SVA was 31.5 mm (IQR 13–66 mm), the median coronal Cobb angle was 51° (IQR 41.3°–67°), and the absolute median coronal alignment was 21 mm (IQR 9–38.6 mm). PROMs were reflective of moderate to severe disability and pain.
TABLE 1.
Baseline demographic, radiographic, and clinical characteristics of 160 ASLS patients stratified based on RF development
| Evidence of RF |
||||||
|---|---|---|---|---|---|---|
| No (n = 98) | Yes (n = 62) |
p Value: RF vs No RF | ||||
| UNRF (n = 28) | UDRF (n = 13) | BNRF (n = 6) | BDRF (n = 15) | |||
| Demographic parameter |
|
|
|
|
|
|
| Age, yrs |
62.7 (55.9–70.3) |
60 (51.8–62.6) |
60.3 (55.6–67.3) |
64.7 (57.8–68) |
63.1 (55.6–71.1) |
0.1633* |
| Female sex |
89 (90.8) |
25 (89.3) |
9 (69.2) |
5 (83.3) |
13 (86.7) |
0.1858† |
| Race |
|
|
|
|
|
>0.9999‡ |
| White |
94 (95.9) |
27 (96.4) |
12 (92.3) |
5 (83.3) |
15 (100.0) |
|
| Black |
3 (3.1) |
1 (3.6) |
1 (7.7) |
0 (0.0) |
0 (0.0) |
|
| Other |
1 (1.0) |
0 (0.0) |
0 (0.0) |
1 (16.7) |
0 (0.0) |
|
| Tobacco use |
|
|
|
|
|
0.8760 |
| Current |
1 (1.0) |
1 (3.6) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
| Former |
37 (37.8) |
8 (28.6) |
4 (30.8) |
3 (50.0) |
10 (66.7) |
|
| Never |
60 (61.2) |
19 (67.9) |
9 (69.2) |
3 (50.0) |
5 (33.3) |
|
| Clinical parameter |
|
|
|
|
|
|
| BMI |
25.6 (23.4–28.4) |
26.8 (23.6–31.1) |
28.4 (25.9–33.5) |
26.3 (24.9–30.6) |
24.6 (20.5–29.9) |
0.1347* |
| Osteopenia/osteoporosis T-score |
|
|
|
|
|
0.2694‡ |
| None/NA |
34 (34.7) |
10 (35.7) |
2 (15.4) |
2 (33.3) |
5 (33.3) |
|
| −1 to −1.5 |
28 (28.6) |
8 (28.6) |
3 (23.1) |
1 (16.7) |
6 (40.0) |
|
| −1.6 to −2.4 |
24 (24.5) |
9 (32.1) |
8 (61.5) |
3 (50.0) |
2 (13.3) |
|
| −2.5 or worse (or vertebral compression fracture) |
12 (12.2) |
1 (3.6) |
0 (0.0) |
0 (0.0) |
2 (13.3) |
|
| Diabetes |
|
|
|
|
|
0.0922‡ |
| No |
95 (96.9) |
26 (92.9) |
11 (84.6) |
4 (66.7) |
14 (93.3) |
|
| Yes, controlled w/ diet |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (16.7) |
0 (0.0) |
|
| Yes, controlled w/ oral medication |
2 (2.0) |
2 (7.1) |
2 (15.4) |
1 (16.7) |
0 (0.0) |
|
| Yes, insulin dependent |
1 (1.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (6.7) |
|
| Baseline radiographic measures |
|
|
|
|
|
|
| C7–S1 SVA, mm |
28 (11.8–73) |
24.5 (13–54.5) |
41 (33–59) |
42.5 (21–66) |
35 (20–64) |
0.7982 |
| T1PA, ° |
21.3 (13.8–30.3) |
19.3 (14.5–26.6) |
24.8 (14.7–27.5) |
26.1 (23.3–33) |
19.3 (13.2–23.9) |
0.7775 |
| TK (T4–12), ° |
30 (18.8–39) |
36.3 (21.2–43.6) |
28.1 (21.3–40.7) |
39.9 (21.9–43.1) |
36 (13.1–57) |
0.146 |
| LL (T12–sacrum), ° |
34 (22–46.6) |
37.9 (29.7–51) |
37 (18–58) |
38.5 (31.8–60) |
53 (26–58) |
0.0305 |
| PI, ° |
54.7 (47.4–64) |
52.8 (50–60.5) |
55 (51–62) |
50 (42–60) |
52 (45–60) |
0.7465 |
| PI-LL, ° |
23 (9–33) |
16.5 (7.5–25) |
23 (12–38) |
16.5 (8–27) |
9 (2–25) |
0.1273 |
| PT, ° |
23 (19.7–30) |
23.5 (19.5–26.3) |
23 (20–32) |
29.5 (27–31.3) |
24 (19–28.1) |
0.9413 |
| Lumbar Cobb angle, ° |
51 (43–67.6) |
53 (45.7–64.5) |
42 (38–69) |
36 (33–44.4) |
60 (46–70) |
0.9735 |
| Coronal alignment absolute value, mm |
23.5 (10–49) |
19.5 (8.5–31.5) |
23 (14–29) |
8.5 (3–22) |
18 (6–40) |
0.0516 |
| Baseline PROM scores* |
|
|
|
|
|
|
| ODI |
38 (26–50) |
34 (29–51) |
44 (34–46) |
53 (44–60) |
46 (36–60) |
0.0758 |
| SRS-22r subscore |
3.1 (2.7–3.5) |
3.1 (2.6–3.5) |
2.9 (2.7–3.4) |
2.6 (2.2–3.2) |
2.6 (2.2–3.4) |
0.0795 |
| SRS-22r pain |
2.8 (2.2–3.4) |
2.8 (2.4–3.2) |
2.4 (2.2–3) |
2.3 (1.8–2.8) |
2.3 (1.8–2.8) |
0.0590 |
| SRS-22r function |
3.2 (2.6–3.6) |
3.2 (2.7–3.7) |
2.8 (2.8–3.4) |
2.3 (2.2–3.4) |
2.6 (2–3.2) |
0.2167 |
| SRS-22r self-image |
2.8 (2.2–3.3) |
2.7 (2–3.1) |
2.7 (2.5–3) |
2.1 (2–3) |
2.5 (2–3.2) |
0.3057 |
| SRS-22r mental health |
3.8 (3.2–4.2) |
3.7 (2.8–4.1) |
3.6 (3.4–4) |
3.3 (2.6–4.6) |
3 (2.6–4.2) |
0.1196 |
| SRS-22r satisfaction |
3 (2–3.5) |
2.8 (2.3–3.5) |
2.5 (2–3.5) |
2.5 (2–3) |
2.5 (2–3) |
0.1306 |
| NRS back pain |
7 (5–8) |
7 (5–8) |
7 (7–8) |
8 (7–8) |
8 (7–8) |
0.1820 |
| NRS leg pain |
4 (1–6) |
5 (2.5–8) |
3 (2–7) |
6.5 (1–8) |
4 (1–8) |
0.1292 |
| MCS |
53.2 (43.7–60) |
46.4 (41.3–57) |
58.2 (48.9–59.8) |
41.2 (35.6–62.8) |
36.3 (32.1–53.9) |
0.0270
|
| PCS | 31.8 (25.8–39.4) | 35.9 (27.5–42.1) | 31.3 (26.1–35.7) | 30.1 (20.9–32.7) | 26.9 (24.4–40.2) | 0.5733 |
NA = not applicable; TK = thoracic kyphosis.
Values are presented as number (%) of patients or median (IQR) unless otherwise indicated. Boldface type indicates statistical significance; p values represent comparisons between patients without an RF and patients with any RF.
Wilcoxon rank-sum test.
Chi-square test.
Fisher exact test.
The overall median number of vertebral levels fused posteriorly was 11.5 (IQR 8–14), with 67 patients (41.9%) having an upper thoracic (T1–T4) uppermost instrumented vertebra (UIV), 20 patients (12.5%) having a UIV in the midthoracic spine (T5–T8), and 73 patients (45.6%) having a UIV in the lower thoracic spine (T9–T11) (Table 2). Only 18 patients had extra supporting rods.22,23 Of the primary rods used, 94.3% were 5.5 mm in diameter, 4.7% were 6 mm, and 1% were 7 mm in diameter. The most common rod material was cobalt-chromium (84.4%), followed by titanium alloy (10%) and stainless steel (4.4%). Grafting material was most commonly a combination of local bone and allograft, and recombinant human bone morphogenetic protein-2 (rhBMP-2) was used in 90.6% of patients. Posterior column osteotomies were used in the majority of patients, and three-column osteotomies (pedicle subtraction osteotomy [PSO] or vertebral column resection [VCR]) were used in 9 patients. Posterior interbody fusion (posterior lumbar interbody fusion [PLIF] or transforaminal lumbar interbody fusion [TLIF]) was performed in 65% of patients (Table 2). Postoperative radiographic measures, changes in radiographic measures from baseline, and PROMs obtained at last follow-up are summarized in Table 3.
TABLE 2.
Index operative parameters for 160 ASLS patients stratified based on RF development
| Evidence of RF |
||||||
|---|---|---|---|---|---|---|
| No (n = 98) | Yes (n = 62) |
p Value: RF vs No RF | ||||
| UNRF (n = 28) | UDRF (n = 13) | BNRF (n = 6) | BDRF (n = 15) | |||
| Op parameter |
|
|
|
|
|
|
| EBL, ml |
1500 (700–2500) |
1850 (850–2500) |
3000 (1800–3300) |
3250 (2000–3600) |
2400 (1400–4000) |
0.0045
*
|
| Op duration, hrs |
6.5 (5.5–7.3) |
6.5 (6–7.1) |
8 (6.5–8) |
5.4 (5–5.8) |
7 (6–8.5) |
0.2613* |
| Op approach |
|
|
|
|
|
0.2877‡ |
| Posterior only |
86 (87.8) |
26 (92.9) |
13 (100.0) |
6 (100.0) |
13 (86.7) |
|
| Anterior-posterior combined |
12 (12.2) |
2 (7.1) |
0 (0.0) |
0 (0.0) |
2 (13.3) |
|
| Posterior approach details |
|
|
|
|
|
|
| Grafting material |
|
|
|
|
|
|
| Local bone |
94 (95.9) |
26 (92.9) |
12 (92.3) |
5 (83.3) |
13 (86.7) |
0.1876‡ |
| Vol, ml |
55 (40–75) |
60 (45–72.5) |
70 (60–90) |
30 (30–30) |
60 (40–60) |
0.2538* |
| Allograft bone |
92 (93.9) |
26 (92.9) |
10 (76.9) |
5 (83.3) |
15 (100.0) |
0.4056† |
| Vol, ml |
60 (50–90) |
75 (50–100) |
60 (60–90) |
60 (60–60) |
60 (60–90) |
0.7056* |
| rhBMP-2 |
93 (94.9) |
25 (89.3) |
9 (69.2) |
5 (83.3) |
13 (86.7) |
0.0197† |
| Dose, mg |
48 (30–116) |
52 (24–150) |
54 (42–96) |
30 (24–36) |
36 (24–48) |
0.5750* |
| Other |
14 (14.3) |
1 (3.6) |
3 (23.1) |
1 (16.7) |
0 (0.0) |
0.2360† |
| Rod material/diameter |
|
|
|
|
|
|
| Material |
|
|
|
|
|
0.6327‡ |
| Titanium |
9 (9.2) |
4 (14.3) |
1 (7.7) |
1 (16.7) |
1 (6.7) |
|
| Stainless |
3 (3.1) |
2 (7.1) |
1 (7.7) |
0 (0.0) |
1 (6.7) |
|
| Cobalt-chrome |
85 (86.7) |
22 (78.6) |
11 (84.6) |
5 (83.3) |
12 (80.0) |
|
| Other |
1 (1.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (6.7) |
|
| Diameter, mm |
|
|
|
|
|
|
| Rod 1 |
|
|
|
|
|
0.4860‡ |
| 5.5 |
92 (93.9) |
27 (96.4) |
13 (100.0) |
5 (83.3) |
15 (100.0) |
|
| 6.0 |
6 (6.1) |
1 (3.6) |
0 (0.0) |
1 (16.7) |
0 (0.0) |
|
| Rod 2 |
|
|
|
|
|
0.1902‡ |
| 5.5 |
89 (90.8) |
28 (100.0) |
13 (100.0) |
5 (83.3) |
15 (100.0) |
|
| 6.0 |
6 (6.1) |
0 (0.0) |
0 (0.0) |
1 (16.7) |
0 (0.0) |
|
| 7.0 |
3 (3.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
| Rod 3 |
|
|
|
|
|
>0.9999‡ |
| 5.5 |
11 (91.7) |
1 (100.0) |
3 (100.0) |
1 (100.0) |
1 (100.0) |
|
| 6.0 |
1 (8.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
| Accessory rods |
|
|
|
|
|
|
| 0 |
88 (89.8) |
28 (100.0) |
12 (92.3) |
6 (100.0) |
14 (93.3) |
0.1301‡ |
| 1 |
10 (10.2) |
0 (0.0) |
1 (7.7) |
0 (0.0) |
1 (6.7) |
|
| Satellite rods |
|
|
|
|
|
|
| 0 |
94 (96.9) |
27 (96.4) |
11 (84.6) |
6 (100.0) |
15 (100.0) |
0.8287‡ |
| 1 |
2 (2.1) |
0 (0.0) |
2 (15.4) |
0 (0.0) |
0 (0.0) |
|
| 2 |
1 (1.0) |
1 (3.6) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
| No. of levels fused |
10 (8–14) |
13 (8–15) |
14 (8–16) |
8 (8–13) |
13 (9–15) |
0.1113* |
| Uppermost instrumented vertebra |
|
|
|
|
|
|
| Upper T-spine (T1–4) |
38 (38.8) |
13 (46.4) |
9 (69.2) |
0 (0.0) |
7 (46.7) |
0.3177† |
| Mid–T-spine (T5–8) |
12 (12.2) |
4 (14.3) |
0 (0.0) |
2 (33.3) |
2 (13.3) |
0.9024† |
| Lower T-spine (T9–12) |
48 (49.0) |
11 (39.3) |
4 (30.8) |
4 (66.7) |
6 (40.0) |
0.2841† |
| Pelvic fixation |
|
|
|
|
|
0.9822† |
| Iliac screws |
90 (91.8) |
27 (96.4) |
12 (92.3) |
5 (83.3) |
13 (86.7) |
|
| S2AI screws |
8 (8.2) |
1 (3.6) |
1 (7.7) |
1 (16.7) |
2 (13.3) |
|
| Osteotomies |
|
|
|
|
|
|
| Posterior column osteotomy |
62 (63.3) |
15 (53.6) |
9 (69.2) |
5 (83.3) |
10 (66.7) |
0.9631† |
| 3-column osteotomy |
5 (5.1) |
1 (3.6) |
2 (15.4) |
0 (0.0) |
1 (6.7) |
0.7357‡ |
| PSO |
3 (3.1) |
1 (3.6) |
2 (15.4) |
0 (0.0) |
0 (0.0) |
0.6777‡ |
| VCR |
2 (2.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (6.7) |
>0.9999‡ |
| PLIF/TLIF |
61 (62.2) |
16 (57.1) |
12 (92.3) |
5 (83.3) |
10 (66.7) |
0.3583† |
| Total no. of levels |
1 (0–2) |
1 (0–2) |
1 (1–2) |
1.5 (1–2) |
1 (0–2) |
0.7090* |
| Cage material |
|
|
|
|
|
0.0245
†
|
| PEEK |
23 (44.2) |
4 (30.8) |
1 (12.5) |
0 (0.0) |
2 (22.2) |
|
| Titanium |
29 (55.8) |
9 (69.2) |
7 (87.5) |
4 (100.0) |
7 (77.8) |
|
| Anterior approach details |
|
|
|
|
|
|
| ALIF, no. of levels |
|
|
|
|
|
0.4615‡ |
| 1 |
7 (58.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (50.0) |
|
| 2 |
4 (33.3) |
2 (100.0) |
0 (0.0) |
0 (0.0) |
1 (50.0) |
|
| 3 |
1 (8.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
| Cage material |
|
|
0 (0.0) |
0 (0.0) |
|
>0.9999‡ |
| PEEK |
5 (71.4) |
2 (100.0) |
0 (0.0) |
0 (0.0) |
1 (100.0) |
|
| Titanium |
1 (14.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
| Allograft | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
ALIF = anterior lumbar interbody fusion; S2AI = S2 alar iliac; T-spine = thoracic spine.
Values are presented as number (%) of patients or median (IQR) unless otherwise indicated. Boldface type indicates statistical significance; p values represent comparisons between patients without an RF and patients with any RF.
Wilcoxon rank-sum test.
Chi-square test.
Fisher exact test.
TABLE 3.
Postoperative radiographic outcome measures and PROMs for 160 ASLS patients stratified based on RF development
| Evidence of RF |
||||||
|---|---|---|---|---|---|---|
| No (n = 98) | Yes (n = 62) |
p Value: RF vs No RF | ||||
| UNRF (n = 28) | UDRF (n = 13) | BNRF (n = 6) | BDRF (n = 15) | |||
| Postop (3-mo radiographs) |
|
|
|
|
|
|
| C7–S1 SVA, mm |
23.4 (12–40.4) |
25.2 (12–35.5) |
29.4 (9–42.5) |
24.6 (18–38.1) |
27.3 (16.4–39) |
0.9664 |
| T1PA, ° |
15 (8.7–22.5) |
14 (7.8–22.5) |
22 (19.3–25) |
29.5 (16–31.3) |
17 (8–28.9) |
0.1822 |
| TK (T4–12), ° |
41 (30–47) |
44.7 (37.5–51.6) |
39 (29–42) |
53.1 (36.2–57) |
43 (32.7–49.8) |
0.1474 |
| LL (T12–sacrum), ° |
50.5 (43–61) |
57 (44.9–64) |
48 (43–52) |
46.5 (38–51) |
53.3 (47–65) |
0.51 |
| PI, ° |
53.5 (45–63) |
53 (49–60) |
56 (53–60) |
61.5 (47–67.5) |
54 (47–58) |
0.4063 |
| PI-LL, ° |
6 (3–11) |
9.5 (4–14.5) |
12 (7–25) |
13.3 (9–23.5) |
7 (5–10) |
0.0168 |
| PT, ° |
20.9 (15–24.5) |
21 (13.5–24.5) |
30 (23–32) |
33.5 (23–38.7) |
22 (16–31) |
0.0382 |
| Lumbar Cobb angle, ° |
21 (12.5–30.2) |
19.9 (13.1–27) |
13.6 (6–23) |
16.6 (14–20) |
15.5 (4–19.4) |
0.108 |
| Coronal alignment absolute value, mm |
15 (8–25) |
19 (11.5–30.1) |
17.8 (7.6–24.9) |
24.5 (14–33.2) |
11 (3.2–20.1) |
0.5686 |
| Change (postop – baseline) |
|
|
|
|
|
|
| C7–S1 SVA, mm |
40 (15–71.3) |
40.5 (20–57.3) |
63 (30.9–86) |
47.1 (39–63.1) |
35.6 (9.6–64) |
0.6108 |
| T1PA, ° |
8.1 (4.4–15) |
7.6 (3.2–10) |
9.7 (5.8–12.1) |
9.8 (7.2–12) |
7.1 (3.8–12.7) |
0.738 |
| TK (T4–12), ° |
12 (7.4–20.4) |
13.9 (9.6–23) |
13.5 (2.5–21) |
14.3 (13.2–15.7) |
17.6 (8–20.9) |
0.5334 |
| LL (T12–sacrum), ° |
18 (8–30.9) |
19 (9.1–23.8) |
23 (12–30) |
8 (5–10.2) |
13 (6–23) |
0.3651 |
| PI, ° |
3 (1.6–4) |
3 (1–4.5) |
3 (2–4) |
4.7 (4–25.5) |
2 (1–4) |
0.7529 |
| PI-LL, ° |
20 (10–30.3) |
16.5 (11–26.5) |
23 (12–32) |
3.5 (2–10.4) |
11 (8–24) |
0.2029 |
| PT, ° |
5.7 (3–10.8) |
7.5 (3.5–12.5) |
7 (3.2–13) |
8.2 (2–11.5) |
9.9 (3–15) |
0.1267 |
| Lumbar Cobb angle, ° |
33 (24.9–41.8) |
34.7 (24.5–41.2) |
36 (19.4–40) |
18 (16–25) |
39.7 (27.1–55) |
0.5041 |
| Coronal alignment absolute value, mm |
23.9 (10–49) |
32.6 (12.4–43.4) |
23.3 (7.6–43.6) |
30.2 (18.3–55.2) |
23.2 (7.8–46) |
0.9208 |
| PROM scores at last FU |
|
|
|
|
|
|
| ODI |
20 (8–36) |
20 (7–35) |
26 (12–42) |
25 (12–42) |
42 (28–62) |
0.0861* |
| SRS subscore |
4 (3.4–4.4) |
4.1 (3.4–4.4) |
3.7 (3.4–4.1) |
3.6 (3.2–4.2) |
2.8 (2.3–3.6) |
0.0416* |
| SRS pain |
4 (3.2–4.6) |
3.9 (3.2–4.5) |
3.8 (2.8–4.2) |
3.8 (2.2–4) |
2.8 (1.8–3.4) |
0.0179* |
| SRS function |
3.8 (3.2–4.2) |
3.9 (3–4.2) |
3.6 (3.2–4.2) |
3.1 (2.8–3.8) |
2.8 (2.2–3.4) |
0.1056* |
| SRS self-image |
4 (3.5–4.5) |
4.2 (3.5–4.5) |
3.8 (3.5–4.2) |
4.1 (2.8–4.7) |
3.5 (2.8–4) |
0.2150* |
| SRS mental health |
4.2 (3.6–4.6) |
4.2 (3.5–4.5) |
4.2 (3.8–4.2) |
4.1 (3.6–4.2) |
3.6 (3–4.4) |
0.1010* |
| SRS satisfaction |
4.5 (4–5) |
4.5 (4–5) |
4.5 (3.5–5) |
4.8 (4.5–5) |
5 (2.5–5) |
0.9749* |
| NRS back pain |
2 (0–4) |
3 (1–4.5) |
3 (2–6) |
3.5 (1–6) |
5.5 (2–8) |
0.0008 |
| NRS leg pain |
1 (0–4) |
2 (0–5) |
2 (0–3) |
3 (1–4) |
3.5 (1–5) |
0.2079 |
| MCS |
51.4 (41.7–59) |
54.2 (39.6–58.8) |
56.4 (40.4–59) |
55.5 (40.3–59.5) |
47.1 (33.8–56.8) |
0.7606 |
| PCS | 43.6 (32.2–52.1) | 47.7 (38.3–55.8) | 35.7 (31.9–45.9) | 31.1 (27.9–32) | 42.5 (24.4–53.3) | 0.4557 |
FU = follow-up.
The p values represent comparisons (Wilcoxon rank-sum test) between patients without an RF and patients with any RF.
The p values for comparison between no RF and BDRF: ODI score (p = 0.0010), SRS subscore (p = 0.0005), SRS pain (p = 0.0003), SRS function (p = 0.0010), SRS self-image (p = 0.0179), SRS mental health (p = 0.0184), and SRS satisfaction (p = 0.9892).
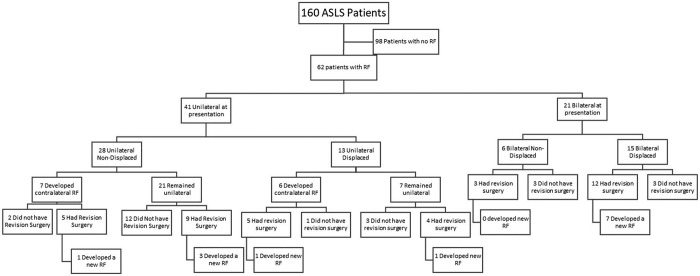
Assessment of Risk Factors for RF
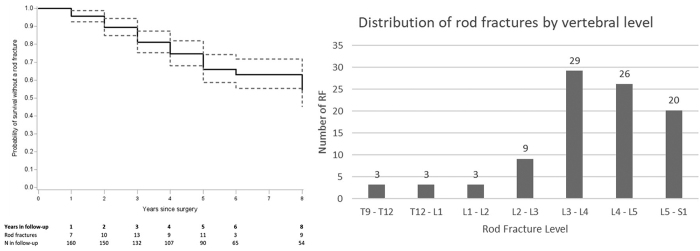
At a median follow-up of 5.1 years (IQR 3.8–6.6 years) that ranged from 0.1 to 9.2 years, a total of 93 RFs were identified in 62 patients (38.8%), including 41 that were unilateral and 21 that were bilateral at presentation. Thirteen of the patients who initially presented with unilateral RF later developed a contralateral fracture at a median of 0.88 years (IQR 0.51–1.3 years) following the initial RF (Fig. 2). The median time to the initial RF was 2.96 years (IQR 1.89–4.54 years), with 73% occurring more than 2 years following surgery. Based on Kaplan-Meier analyses, estimated RF rates at 2, 4, 5, and 8 years after surgery were 11%, 24%, 35%, and 49%, respectively (Fig. 3A). The most common levels for RF were L3–L4 (31.2%), L4–L5 (28%), and L5–S1 (21.5%) (Fig. 3B).
FIG. 2.
Flowchart of RF occurrence, whether managed operatively, and recurrent RF.
FIG. 3.
RF survival curve (left) and graph of vertebral level of RFs (right).
On univariate analysis, baseline demographic and radiographic parameters were not significantly associated with occurrence of RF, except for baseline LL, which was significantly greater among patients who developed RF (39.5° vs 34.0°, p = 0.0305), and baseline SF-12 MCS score, which was significantly worse among patients who developed RF (53.1 vs 47.3, p = 0.027) (Table 1). With regard to operative parameters, higher rates of RF were associated with greater operative estimated blood loss (EBL; 2250 vs 1500 ml, p = 0.0045) and use of titanium PLIF or TLIF cages (p = 0.0245) (Table 2). Although a greater percentage of patients in the non-RF group had rhBMP-2 used in their surgery (93% vs 83.8%, p = 0.0197), the overall dosage was similar between the groups (Table 2). Compared with patients who did not develop RF, the median postoperative PI-LL (absolute value of PI-LL: 6° vs 9°, p = 0.0168) and PT (20.9° vs 23°, p = 0.0382) were significantly higher in the RF group (Table 3). At last follow-up, patients with RF presented with significantly worse PROMs in terms of SRS-22r subscore, SRS-22r pain, and VAS (visual analog scale) back pain (all p < 0.05) (Table 3). The negative impact on PROMs was greatest for patients with BDRFs (Table 3).
In Cox regression models, greater 3-month postoperative PT (HR 1.895, 95% CI 1.196–3.002, p = 0.0065) and greater EBL (HR 1.02, 95% CI 1.005–1.036, p = 0.0088) were associated with an increased risk of developing RF (Table 4).
TABLE 4.
Multivariate analysis of factors associated with development of RF after accounting for clustering by study site
| Parameter | Adjusted HR (95% CI) | p Value |
|---|---|---|
| 3-mo postop PT |
1.895 (1.196–3.002) |
0.0065
*
|
| EBL |
1.02 (1.005–1.036) |
0.0088
†
|
| 3-mo postop PI-LL |
0.728 (0.529–1.002) |
0.0515* |
| Diabetes, yes vs no |
2.656 (0.935–7.542) |
0.0667 |
| rhBMP-2 used, yes vs no |
0.476 (0.205–1.102) |
0.0515 |
| Age |
0.76 (0.533–1.083) |
0.1284‡ |
| Preop global coronal alignment | 0.921 (0.813–1.044) | 0.2005*¶ |
Boldface type indicates statistical significance.
Modeled per 10° increase.
Modeled per 100-ml increase.
Modeled per decade increase.
Absolute value.
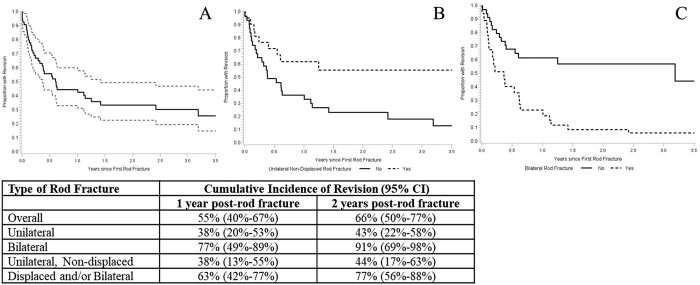
Assessment of Risk Factors for RF Revision Surgery
As of last follow-up, 38 patients (61.3% of patients with RF) had undergone revision surgery at a median of 97 days (IQR 38–224.25 days), ranging from 0 to 1163 days following discovery of RF. Of the patients who underwent revision surgery, 13 (34.2%) later developed another RF, with 4 RFs at the same level of the previous one and 11 RFs at a different level (Fig. 2). In Kaplan-Meier analyses among patients with an RF, an estimated 55% and 66% of patients had a revision surgery within 1 year and 2 years of RF, respectively (Fig. 4). The presence of bilateral RF was predictive of revision surgery (HR 3.52, 95% CI 1.8–6.9, p = 0.0002), while patients with UNRF were less likely to require revision surgery (HR 0.39, 95% CI 0.18–0.84, p = 0.0164). Associations between RF types and likelihood of undergoing revision surgery persisted even after adjusting for PROMs.
FIG. 4.
Survival curves for time to revision after the initial RF. A: All RFs. B: Only UNRFs. C: Only bilateral RFs. Grid below provides cumulative incidence of revision surgery based on RF type at 1 year and 2 years following occurrence of RF.
Discussion
Operative treatment of ASD has been shown to positively impact HRQOL, but there are high complication rates associated with these procedures.4,7,19,24 Instrumentation-related complications including RF are among the most frequent adverse events after ASD surgery. While previous reports have assessed RF, evidence is still limited, and this complication remains a significant challenge. To our knowledge, this study provides the longest follow-up of a multicenter primary ASLS cohort to date, and our aim was to define the rate of RF and assess risk factors for the occurrence of RF and revision surgery.
Our results demonstrate an overall RF rate of 38.8%, which is substantially higher than rates reported in previous studies, in which rates ranged from 6.8% to 24%.4,7,17,25,26 This marked difference in results is likely due to the considerably longer follow-up in the present study, which extended up to 9.2 years, with 76% (122/160) of patients followed for at least 5 years. Notably, of all the RFs encountered, 73% occurred more than 2 years following surgery. In a study that included 526 operatively treated patients with ASD, Lertudomphonwanit et al.4 reported an 18.4% RF rate at a mean 4.5-year follow-up. The mean time to RF was 39.6 months, with half of the fractures occurring within the first 3 years after surgery. The markedly higher rate of RF in the present study compared with that of the study by Lertudomphonwanit et al. likely resulted at least in part from the longer overall follow-up and range of follow-up, since nearly one-third of RFs in the present series occurred more than 4 years postoperatively and nearly one-fifth occurred more than 5 years after surgery.
In contrast to previous reports in which BMI, osteoporosis, and tobacco use have been associated with an increased risk of RF,12,27–30 in the present study no definite modifiable risk factors were identified. Multivariate analysis revealed that one of the strongest preoperative clinical variables predictive of RF was diabetes; however, this association did not reach statistical significance. Notably, the relatively small numbers of patients in the present study with diabetes, osteoporosis, or active smoking may have been insufficient to detect significant associations of these variables with RF.
The only baseline spinopelvic parameter associated with RF on univariate analysis in the present study was LL, which was significantly higher among patients who developed RF. This finding could be explained by the fact that rod contouring tends to cause notches that reduce fatigue life, or time before complete failure; hence, the more bending needed, the greater the risk of fracture.31–34 Notably, the severity of deformity present at baseline in the coronal or sagittal planes was not significantly associated with occurrence of RF. This result is in contrast to those in previously reported studies in which greater baseline sagittal deformity was identified as a significant risk factor for RF.4,12 These previous studies, however, included patient populations with heterogeneous deformity, with an overall marked degree of sagittal malalignment, whereas the patients in the present study primarily had coronal deformities with relatively limited sagittal malalignment.
On univariate assessment, the magnitude of deformity correction was not associated with development of RF. Smith and colleagues assessed RF rates in a series of 287 ASD patients and identified greater correction of SVA and PI-LL mismatch and less correction of coronal Cobb angle as risk factors for RF.12 However, the deformities in their series were very heterogeneous, including both thoracic and lumbar scoliosis, significant sagittal deformities (25% had a PSO), and primary and revision cases. Although the magnitude of change in spinal alignment was not associated with RF in our series, both greater postoperative PT and PI-LL mismatch were associated with increased rates of RF. Both increased PT and PI-LL mismatch may reflect residual malalignment that could add stress to the spinal instrumentation and reduce the fatigue life of the rods. This result is consistent with that reported by Smith et al.17 for a study in which residual postoperative sagittal malalignment was associated with a greater risk of RF in operatively treated ASD patients.
Numerous studies have demonstrated a higher RF rate at the level of or adjacent to a PSO.12,17,35–37 For one of the largest studies involving implant complications, Smith et al.12 reported RF rates of 22% and 4.7% among patients whose surgery did or did not include a PSO, respectively. Furthermore, in a study including more than 400 patients, the RF rate was more than twofold higher for cases including a PSO.17 This finding is thought to be a result of the greater biomechanical stress inherent to the destabilizing nature of the three-column osteotomy, combined with the need for additional rod contouring that could potentially weaken the rod.12,17,33,34 Our results did not show a statistically significant association between osteotomies and implant failure. However, in contrast with the aforementioned studies in which most patients had significant sagittal malalignment, thus requiring greater corrections through the use of PSO and VCR, our cohort consisted mainly of coronal deformities, and all were primary cases that markedly reduced the need for three-column osteotomies.
Univariate analysis suggested an association between the use of titanium interbody cages and RF. However, when controlling for potential confounders and study site clustering, no association between titanium interbody cages and RF was identified, consistent with previous reports in which fusion rates with PEEK and titanium cages are similar. A meta-analysis including 6 studies with 410 patients found no significant difference in fusion rates when comparing these two materials.38 In another systematic review and meta-analysis by Tan et al.39 that included 11 articles with 743 patients, fusion rates between titanium and PEEK cages also did not differ.
Substantial evidence in the literature supports higher fusion rates associated with the use of rhBMP-2.40,41 In the present study, use of rhBMP-2 was associated with reduced rates of RF on univariate analysis but not on multivariate analysis. Importantly, not all RFs are a result of nonunion, with many likely resulting at least in part from biomechanical compromise of the rods introduced by contouring.
The only intraoperative parameter significantly associated with RF was EBL, although the HR of 1.02 suggests that greater EBL did not dramatically change the risk of RF. The explanation for this association is not readily clear, but greater EBL may be a surrogate for greater overall case complexity and invasiveness. In contrast to previous studies that have suggested differences in RF rates based on rod material or size, the present study showed no similar associations; however, the vast majority of rods (84%) used in the present study were cobalt-chromium, and 95% were 5.5 mm in diameter, limiting the ability for comparisons. There are several reports supporting the use of accessory/satellite rods to provide additional strength and avoid instrumentation failure, especially in areas of greater stress and instability.22,23,37 Since there were only 18 patients with multirod constructs in our study population, there were insufficient numbers to draw conclusions regarding their effectiveness in preventing RF as opposed to standard two-rod constructs.
Reports of revision rates due to RF after deformity surgery are extremely variable in the literature and range from 41.2% to 87%.4,12,17,42,43 Most of the available studies have limitations that affect the proportion of patients requiring reoperation; thus, benchmark revision rates remain unknown. Some studies are limited by including only symptomatic patients, which can magnify revision rates, since many patients without symptoms may never need revision.17 Other studies do not focus solely on revision rates associated with RF, have a limited number of patients, or have low follow-up rates.12,37,43 We found that the cumulative incidence rates of revision surgery at 1 year and 2 years after RF were 55% and 66%, respectively, with 61.3% of all patients with RF eventually undergoing revision. The decision to revise is somewhat subjective but was usually directed by evidence of loss of correction or the patient reporting loss of function or increased pain. Considering that this study was prospectively performed with multiple surgeons from 9 participating sites, that the length and rate of follow-up exceeded those of previously published reports on ASD, and that the population was a very homogenous cohort, the present study likely represents one of the most complete assessments of revision rates for RF in ASLS to date.
There is growing evidence suggesting a relationship between the type of RF and the risk of revision surgery as well as patient-reported outcomes. In a study by Lertudomphonwanit et al.4 that included 526 ASD patients with an overall RF revision rate of 41.2%, 75% of bilateral RFs were revised, while only 21% of unilateral RFs required revision (p < 0.0001). In further analysis of RF characteristics, the same authors subclassified fractures into four groups: UNRF, UDRF, BNRF, and BDRF.18 Their results showed a statistically significant difference in revision rates between UNRF and bilateral RF (p < 0.0001), with 9.5% of UNRF and 53.6% of UDRF patients requiring revision, while 84.6% of patients with bilateral RF underwent revision surgery. We observed a similar trend, and we found that for unilateral RF the cumulative incidence rates of revision at 1 year and 2 years post-RF were 38% and 43%, respectively, compared with 77% and 91% at 1 year and 2 years after bilateral RF (Fig. 4). Furthermore, unadjusted models revealed that bilateral RF was predictive of revision surgery (HR 3.52, 95% CI 1.8–6.9, p = 0.0002), while patients with UNRF were less likely to require revisions (HR 0.39, 95% CI 0.18–0.84, p = 0.016).
Prior studies have reported sagittal decompensation, loss of correction, and more severe pain scores in patients with bilateral RF as a result of pseudarthrosis.18 Although we found no statistically significant difference between UNRF and all other fractures in terms of postoperative spinopelvic measurements, it is important to note that these values reflect measurements from the first postoperative standing radiographs and not immediately preceding revision for RF, since standing radiographs were not routinely collected by study sites at this time point.
The results of the present study suggest that the type of RF may have implications for risk of revision and may be important to consider for management decisions and patient counseling. Bilateral RFs were significantly more likely than unilateral RFs to necessitate revision surgery. Thus, a more conservative expectant approach might be a suitable option for a unilateral RF if asymptomatic, since many patients may never need revision. However, follow-up may still be warranted since 35% of unilateral fractures in our study subsequently became bilateral, and 57.5% of patients with unilateral RFs eventually underwent revision surgery. Collectively, patients who experienced an RF had worse PROMs at last follow-up, including SRS-22r subscore, SRS-22r pain domain, and NRS back pain score, suggesting the potential impact of this complication whether or not revision surgery is required.
A major strength of the present study is the homogeneous patient population. All patients were at least 40 years of age, had at least 7 levels of spinal fusion (including the sacrum), and had sacropelvic fixation. It is also important to recognize the limitations of this study. This was a post hoc study that was exploratory in nature. The cohort homogeneity potentially helped to reduce many confounders; however, this feature also created a gap of information due to the absence of other variables that have been associated with implant failure. The relatively balanced sagittal spinopelvic values in our cohorts required less-invasive procedures (e.g., three-column osteotomies) to obtain proper postoperative goals, thus limiting the ability to assess the impact of sagittal realignment on risk of RF. Due to the limitation in sample size, we were not able to assess the potential protective effects of multirod constructs on RF occurrence. In addition, realignment goals were not standardized across enrolling surgeons. Instead, a "usual care" approach was employed to make results more generalizable, as advocated by Dawson et al.44 Finally, since this study involved multiple surgeons from several institutions, there was the potential for selection, indication, and performance bias that could have influenced the results and the decision for revision surgery.
Conclusions
This study provides the highest-quality data to date, to our knowledge, on rates of RF following primary ASLS surgery. At a median 5.1-year follow-up, 38.8% of patients had at least one RF. The median time to RF was 2.96 years (IQR 1.89–4.54 years, range 0.8–8.7 years), 73% of RFs occurred > 2 years following surgery, and the estimated RF rates at 2, 4, 5, and 8 years after surgery were 11%, 24%, 35%, and 49%, respectively. On multivariate analysis, greater operative EBL and higher postoperative PT were significant risk factors for RF. A total of 38 patients (61% of all RFs) underwent revision surgery. The presence of bilateral RF was predictive of revision surgery, whereas patients with UNRF were less likely to require revision surgery. Collectively, these findings emphasize the importance of long-term follow-up to accurately assess the true prevalence and cumulative incidence of RF.
Acknowledgments
This trial was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases division of the NIH (5RO1-ARO55176) from 2010 to 2017, and since 2017 it has received funding through the Scoliosis Research Society.
Disclosures
Dr. Smith reports receiving consultancy fees from Zimmer Biomet, NuVasive, DePuy Synthes, SeaSpine, Stryker, Carlsmed, and Cerapedics; receiving royalties from Zimmer Biomet, NuVasive, and Thieme; holding stock options in Alphatec and NuVasive; receiving research funding to his institution from DePuy Synthes, the International Spine Study Group Foundation (ISSGF), AO Spine, NuVasive, NIH, and the Scoliosis Research Society (SRS); receiving fellowship grant funding to his institution from AO Spine; serving on the editorial boards of Journal of Neurosurgery: Spine, Neurosurgery, Operative Neurosurgery, and Spine Deformity; and serving on the Executive Committee of ISSGF and on the Board of Directors of SRS. Dr. Kelly reports receiving grants from NIH and SRS while conducting the present study and grants outside the submitted work from the Setting Scoliosis Straight Foundation, ISSGF, and AO Spine; receiving compensation for travel and being a member of the Board of Directors for SRS; and receiving honoraria from Spine. Dr. Gupta reports receiving consultancy fees from DePuy Synthes, Medtronic, Globus, and Alphatec Spine (began and ended in 2019); receiving royalties from DePuy Synthes, Medtronic, Alphatec Spine, Innomed, and Globus; receiving fellowship grant funding from OmeGA and AO Spine; serving on an advisory board/panel for DePuy Synthes and Medtronic; holding stock in Johnson & Johnson; receiving travel funding/honoraria from Globus, AO Spine, Louisiana State University, SRS, Wright State, and the Malaysia Spine Society; being on the SRS Board of Directors; receiving faculty travel reimbursement for chairing courses from DePuy, Globus, Medtronic, Zimmer, and AO Spine; and volunteering for the National Spine Health Foundation. Dr. Ames reports being an employee for University of California, San Francisco; receiving royalties from Stryker, Biomet, Zimmer Spine, DePuy Synthes, NuVasive, Next Orthosurgical, K2M, and Medicrea; being a consultant for DePuy Synthes, Medtronic, Medicrea, K2M, Agada Medical, and Carlsmed; participating in research for Titan Spine, DePuy Synthes, and International Spine Study Group (ISSG); being an editorial board member for Operative Neurosurgery and Neurospine; receiving grant funding from SRS; and serving on the Executive Committee of ISSG, as the director of Global Spinal Analytics, and as the Safety and Value Committee Chair for SRS. Dr. Yanik reports receiving grants from SRS during the conduct of the study. Dr. Yen reports receiving consultancy fees from NuVasive. Dr. Lafage reports receiving grants from NIH during the conduct of the study; receiving personal fees outside the submitted work from Globus Medical, NuVasive, Nemaris Inc., DePuy Synthes Spine, Implanet, the Permanente Medical Group, and K2M Medical; receiving honoraria from Stryker, Implanet, and DePuy Synthes Spine; being a consultant for Alphatec and Globus Medical; receiving royalties from NuVasive; and ownership of VFT Solutions, LLC. Dr. Bess reports receiving grants from DePuy Synthes, Globus, Medtronic, SI Bone, and Mirus; receiving grants and other support from K2, Stryker, and NuVasive; receiving other support from Carlsmed and Progenerative Medicine; receiving grants outside the submitted work from ISSGF; being a consultant for Mirus, Stryker, and ATEC Spine; being a patent holder for Stryker and NuVasive; receiving clinical or research support for study described (includes equipment or material) from ISSGF; receiving support of non–study-related clinical or research effort overseen by author from DePuy Synthes, Globus, Medtronic, Stryker, SeaSpine, Carlsmed, SI Bone, and ISSGF; serving on the speakers bureau for Stryker and ATEC Spine, and receiving royalties from Stryker and NuVasive. Dr. Schwab reports receiving personal fees outside the submitted work from Globus Medical Inc., K2 Medical LLC, Medicrea USA Corp., Medtronic Sofamor Danek USA Inc., and Zimmer Biomet; personal fees from Synthes GmbH and NuVasive Inc.; direct stock ownership in VFT Solutions and SeaSpine; being a consultant for Zimmer Biomet, Medtronic, and Mainstay Medical; receiving royalties from Zimmer Biomet, Medtronic, and Medicrea; receiving support of non–study-related clinical or research effort overseen by author from DePuy, K2M, NuVasive, Medtronic, Globus, AlloSource, Orthofix, and SI Bone—paid through ISSGF; and being on the Executive Committee of ISSG. Dr. Shaffrey reports receiving grants during the conduct of the study from NIH; receiving personal fees outside the submitted work from NuVasive, Medtronic, Zimmer Biomet, and SI Bone; being a consultant for NuVasive, Medtronic, SI Bone, and Proprio; having direct stock ownership in NuVasive; being a patent holder in NuVasive and Medtronic; and receiving royalties from NuVasive, Medtronic, and SI Bone. Dr. Bridwell reports receiving grants during the conduct of the study from SRS.
Author Contributions
Conception and design: Smith, Sardi, Lazaro, Kelly, Dial, Hills, Yanik, Gupta, Schwab, Shaffrey, Bridwell. Acquisition of data: Smith, Kelly, Baldus, Lafage, Schwab, Shaffrey, Bridwell. Analysis and interpretation of data: Smith, Sardi, Lazaro, Yanik, Baldus, Yen, Lafage, Ames, Bess, Bridwell. Drafting the article: Smith, Sardi, Lazaro. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Smith. Statistical analysis: Yanik. Administrative/technical/material support: Bess. Study supervision: Bess, Shaffrey, Bridwell.
Supplemental Information
Previous Presentations
This study was presented at the International Meeting for Advanced Spine Techniques (IMAST), April 6–9, 2022, Miami, FL.
References
- 1. Kelly MP, Lurie JD, Yanik EL, et al. Operative versus nonoperative treatment for adult symptomatic lumbar scoliosis. J Bone Joint Surg Am. 2019;101(4):338–352. doi: 10.2106/JBJS.18.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Youssef JA, Orndorff DO, Patty CA, et al. Current status of adult spinal deformity. Global Spine J. 2013;3(1):51–62. doi: 10.1055/s-0032-1326950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Birknes JK, White AP, Albert TJ, Shaffrey CI, Harrop JS. Adult degenerative scoliosis: a review. Neurosurgery. 2008;63(3 suppl):94–103. doi: 10.1227/01.NEU.0000325485.49323.B2. [DOI] [PubMed] [Google Scholar]
- 4. Lertudomphonwanit T, Kelly MP, Bridwell KH, et al. Rod fracture in adult spinal deformity surgery fused to the sacrum: prevalence, risk factors, and impact on health-related quality of life in 526 patients. Spine J. 2018;18(9):1612–1624. doi: 10.1016/j.spinee.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 5. Ames CP, Scheer JK, Lafage V, et al. Adult spinal deformity: epidemiology, health impact, evaluation, and management. Spine Deform. 2016;4(4):310–322. doi: 10.1016/j.jspd.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 6. Bess S, Line B, Fu KM, et al. The health impact of symptomatic adult spinal deformity: comparison of deformity types to United States population norms and chronic diseases. Spine (Phila Pa 1976) 2016;41(3):224–233. doi: 10.1097/BRS.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yadla S, Maltenfort MG, Ratliff JK, Harrop JS. Adult scoliosis surgery outcomes: a systematic review. Neurosurg Focus. 2010;28(3):E3. doi: 10.3171/2009.12.FOCUS09254. [DOI] [PubMed] [Google Scholar]
- 8. Smith JS, Shaffrey CI, Glassman SD, et al. Risk-benefit assessment of surgery for adult scoliosis: an analysis based on patient age. Spine (Phila Pa 1976) 2011;36(10):817–824. doi: 10.1097/BRS.0b013e3181e21783. [DOI] [PubMed] [Google Scholar]
- 9. Smith JS, Shaffrey CI, Ames CP, Lenke LG. Treatment of adult thoracolumbar spinal deformity: past, present, and future. J Neurosurg Spine. 2019;30(5):551–567. doi: 10.3171/2019.1.SPINE181494. [DOI] [PubMed] [Google Scholar]
- 10. Smith JS, Shaffrey CI, Bess S, et al. Recent and emerging advances in spinal deformity. Neurosurgery. 2017;80(3S):S70–S85. doi: 10.1093/neuros/nyw048. [DOI] [PubMed] [Google Scholar]
- 11. Smith JS, Lafage V, Shaffrey CI, et al. Outcomes of operative and nonoperative treatment for adult spinal deformity: a prospective, multicenter, propensity-matched cohort assessment with minimum 2-year follow-up. Neurosurgery. 2016;78(6):851–861. doi: 10.1227/NEU.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 12. Smith JS, Shaffrey E, Klineberg E, et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J Neurosurg Spine. 2014;21(6):994–1003. doi: 10.3171/2014.9.SPINE131176. [DOI] [PubMed] [Google Scholar]
- 13. Kelly MP, Lenke LG, Godzik J, et al. Retrospective analysis underestimates neurological deficits in complex spinal deformity surgery: a Scoli-RISK-1 Study. J Neurosurg Spine. 2017;27(1):68–73. doi: 10.3171/2016.12.SPINE161068. [DOI] [PubMed] [Google Scholar]
- 14. Soroceanu A, Diebo BG, Burton D, et al. Radiographical and implant-related complications in adult spinal deformity surgery: incidence, patient risk factors, and impact on health-related quality of life. Spine (Phila Pa 1976) 2015;40(18):1414–1421. doi: 10.1097/BRS.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 15. Smith JS, Klineberg E, Lafage V, et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J Neurosurg Spine. 2016;25(1):1–14. doi: 10.3171/2015.11.SPINE151036. [DOI] [PubMed] [Google Scholar]
- 16. Glassman SD, Bazzi J, Puno RM, Dimar JR. The durability of small-diameter rods in lumbar spinal fusion. J Spinal Disord. 2000;13(2):165–167. doi: 10.1097/00002517-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 17. Smith JS, Shaffrey CI, Ames CP, et al. Assessment of symptomatic rod fracture after posterior instrumented fusion for adult spinal deformity. Neurosurgery. 2012;71(4):862–867. doi: 10.1227/NEU.0b013e3182672aab. [DOI] [PubMed] [Google Scholar]
- 18. Lertudomphonwanit T, Bridwell KH, Kelly MP, et al. Relationship of the character of rod fractures on outcomes following long thoracolumbar fusion to the sacrum for adult spinal deformity. Spine J. 2020;20(9):1452–1463. doi: 10.1016/j.spinee.2020.05.553. [DOI] [PubMed] [Google Scholar]
- 19. Smith JS, Kelly MP, Yanik EL, et al. Operative versus nonoperative treatment for adult symptomatic lumbar scoliosis at 5-year follow-up: durability of outcomes and impact of treatment-related serious adverse events. J Neurosurg Spine. 2021;35(1):67–79. doi: 10.3171/2020.9.SPINE201472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El Dafrawy M, Bridwell K, Adogwa O, et al. Rod fractures and nonunions after long fusion to the sacrum for primary presentation adult spinal deformity: a comparison with and without interbody fusion in the distal lumbar spine. Spine Deform. 2021;9(1):231–237. doi: 10.1007/s43390-020-00174-6. [DOI] [PubMed] [Google Scholar]
- 21. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 22. Rabinovich EP, Buell TJ, Wang TR, Shaffrey CI, Smith JS. Reduced occurrence of primary rod fracture after adult spinal deformity surgery with accessory supplemental rods: retrospective analysis of 114 patients with minimum 2-year follow-up. J Neurosurg Spine. 2021;35(4):504–515. doi: 10.3171/2020.12.SPINE201527. [DOI] [PubMed] [Google Scholar]
- 23. Gupta S, Eksi MS, Ames CP, et al. A novel 4-rod technique offers potential to reduce rod breakage and pseudarthrosis in pedicle subtraction osteotomies for adult spinal deformity correction. Oper Neurosurg (Hagerstown) 2018;14(4):449–456. doi: 10.1093/ons/opx151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zuckerman SL, Cerpa M, Lenke LG, et al. Patient-reported outcomes after complex adult spinal deformity surgery: 5-year results of the Scoli-Risk-1 Study. Global Spine J. doi: 10.1177/2192568220988276. Published online February 9, 2021. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976) 2006;31(20):2329–2336. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 26. DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine (Phila Pa 1976) 2006;31(19 suppl):S144–S151. doi: 10.1097/01.brs.0000236893.65878.39. [DOI] [PubMed] [Google Scholar]
- 27. Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine (Phila Pa 1976) 2000;25(20):2608–2615. doi: 10.1097/00007632-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 28. Govindarajan V, Diaz A, Perez-Roman RJ, Burks SS, Wang MY, Levi AD. Osteoporosis treatment in patients undergoing spinal fusion: a systematic review and meta-analysis. Neurosurg Focus. 2021;50(6):E9. doi: 10.3171/2021.3.FOCUS2175. [DOI] [PubMed] [Google Scholar]
- 29. Jackson KL, II, Devine JG. The effects of smoking and smoking cessation on spine surgery: a systematic review of the literature. Global Spine J. 2016;6(7):695–701. doi: 10.1055/s-0036-1571285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khalid SI, Nunna RS, Maasarani S, et al. Association of osteopenia and osteoporosis with higher rates of pseudarthrosis and revision surgery in adult patients undergoing single-level lumbar fusion. Neurosurg Focus. 2020;49(2):E6. doi: 10.3171/2020.5.FOCUS20289. [DOI] [PubMed] [Google Scholar]
- 31. Moal B, Schwab F, Ames CP, et al. Radiographic outcomes of adult spinal deformity correction: a critical analysis of variability and failures across deformity patterns. Spine Deform. 2014;2(3):219–225. doi: 10.1016/j.jspd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 32. Lindsey C, Deviren V, Xu Z, Yeh RF, Puttlitz CM. The effects of rod contouring on spinal construct fatigue strength. Spine (Phila Pa 1976) 2006;31(15):1680–1687. doi: 10.1097/01.brs.0000224177.97846.00. [DOI] [PubMed] [Google Scholar]
- 33. Tang JA, Leasure JM, Smith JS, Buckley JM, Kondrashov D, Ames CP. Effect of severity of rod contour on posterior rod failure in the setting of lumbar pedicle subtraction osteotomy (PSO): a biomechanical study. Neurosurgery. 2013;72(2):276–283. doi: 10.1227/NEU.0b013e31827ba066. [DOI] [PubMed] [Google Scholar]
- 34. Sardi JP, Ames CP, Coffey S, et al. Accuracy of rod contouring to desired angles with and without a template: implications for achieving desired spinal alignment and outcomes. Global Spine J. doi: 10.1177/2192568221998371. Published online February 25, 2021. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang BP, Ondra SL, Chen LA, Jung HS, Koski TR, Salehi SA. Clinical and radiographic outcomes of thoracic and lumbar pedicle subtraction osteotomy for fixed sagittal imbalance. J Neurosurg Spine. 2006;5(1):9–17. doi: 10.3171/spi.2006.5.1.9. [DOI] [PubMed] [Google Scholar]
- 36. Bridwell KH, Lewis SJ, Edwards C, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine (Phila Pa 1976) 2003;28(18):2093–2101. doi: 10.1097/01.BRS.0000090891.60232.70. [DOI] [PubMed] [Google Scholar]
- 37. Lee KY, Lee JH, Kang KC, Im SK, Lim HS, Choi SW. Strategies for prevention of rod fracture in adult spinal deformity: cobalt chrome rod, accessory rod technique, and lateral lumbar interbody fusion. J Neurosurg Spine. 2021;34(5):706–715. doi: 10.3171/2020.8.SPINE201037. [DOI] [PubMed] [Google Scholar]
- 38. Seaman S, Kerezoudis P, Bydon M, Torner JC, Hitchon PW. Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J Clin Neurosci. 2017;44:23–29. doi: 10.1016/j.jocn.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 39. Tan JH, Cheong CK, Hey HWD. Titanium (Ti) cages may be superior to polyetheretherketone (PEEK) cages in lumbar interbody fusion: a systematic review and meta-analysis of clinical and radiological outcomes of spinal interbody fusions using Ti versus PEEK cages. Eur Spine J. 2021;30(5):1285–1295. doi: 10.1007/s00586-021-06748-w. [DOI] [PubMed] [Google Scholar]
- 40. Kim HJ, Buchowski JM, Zebala LP, Dickson DD, Koester L, Bridwell KH. RhBMP-2 is superior to iliac crest bone graft for long fusions to the sacrum in adult spinal deformity: 4- to 14-year follow-up. Spine (Phila Pa 1976) 2013;38(14):1209–1215. doi: 10.1097/BRS.0b013e31828b656d. [DOI] [PubMed] [Google Scholar]
- 41. Hamilton DK, Smith JS, Reames DL, Williams BJ, Chernavvsky DR, Shaffrey CI. Safety, efficacy, and dosing of recombinant human bone morphogenetic protein-2 for posterior cervical and cervicothoracic instrumented fusion with a minimum 2-year follow-up. Neurosurgery. 2011;69(1):103–111. doi: 10.1227/NEU.0b013e318214a9b1. [DOI] [PubMed] [Google Scholar]
- 42. Adogwa O, Buchowski JM, Lenke LG, et al. Comparison of rod fracture rates in long spinal deformity constructs after transforaminal versus anterior lumbar interbody fusions: a single-institution analysis. J Neurosurg Spine. 2020;32(1):42–49. doi: 10.3171/2019.7.SPINE19630. [DOI] [PubMed] [Google Scholar]
- 43. Puvanesarajah V, Shen FH, Cancienne JM, et al. Risk factors for revision surgery following primary adult spinal deformity surgery in patients 65 years and older. J Neurosurg Spine. 2016;25(4):486–493. doi: 10.3171/2016.2.SPINE151345. [DOI] [PubMed] [Google Scholar]
- 44. Dawson L, Zarin DA, Emanuel EJ, Friedman LM, Chaudhari B, Goodman SN. Considering usual medical care in clinical trial design. PLoS Med. 2009;6(9):e1000111. doi: 10.1371/journal.pmed.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]