Abstract
Previously, we reported that rdxB, encoding a likely membrane-bound two [4Fe-4S]-containing center, is involved in the aerobic regulation of photosystem gene expression in Rhodobacter sphaeroides 2.4.1. To further investigate the role of rdxB as well as other genes of the rdxBHIS operon on photosystem gene expression, we constructed a series of nonpolar, in-frame deletion mutations in each of the rdx genes. Using both puc and puf operon lacZ fusions to monitor photosystem gene expression, under aerobic conditions, in each of the mutant strains revealed significant increased photosynthesis gene expression. In the case of mutations in either rdxH, rdxI, or rdxS, the aerobic induction of photosystem gene expression is believed to be indirect by virtue of a posttranscriptional effect on cbb3 cytochrome oxidase structure and integrity. For RdxB, we suggest that this redox protein has a more direct effect on photosystem gene expression by virtue of its interaction with the cbb3 oxidase. An associated phenotype, involving the enhanced conversion of the carotenoid spheroidene to spheroidenone, is also observed in the RdxB, -H, -I, and -S mutant strains. This phenotype is also suggested to be the result of the role of the rdxBHIS locus in cbb3 oxidase activity and/or structure. RdxI is suggested to be a new class of metal transporter of the CPx-type ATPases.
The purple nonsulfur bacterium Rhodobacter sphaeroides 2.4.1 is able to grow aerobically as a chemoheterotroph, anaerobically using respiration or fermentation, and photosynthetically in either an autotrophic or heterotrophic mode. When grown under photosynthetic or anaerobic (semiaerobic) conditions, the photosynthetic apparatus including light-harvesting system I (B875), light-harvesting system II (B800-850), bacteriochlorophyll a (Bchl), and carotenoids (Crts) are synthesized and localized to the intracytoplasmic membrane (16).
Our previous results have shown that the cbb3 cytochrome oxidase (29, 31, 40) and the apparently membrane-bound RdxB (29) as well as known regulatory elements such as FnrL (39, 41), the PrrBA two-component activation system (6, 7, 8, 32), the AppA/PpsR antirepressor/repressor system (11, 12), and the outer membrane-localized TspO protein (37) are involved in the regulation of photosystem formation. It has been suggested that a redox signal originating from the cbb3 cytochrome oxidase normally acts to inhibit photosystem gene expression under aerobic conditions, most likely acting through the two-component activation system, PrrBA (30, 31).
This same redox flow through the cbb3 cytochrome oxidase also plays a role in controlling the relative accumulation of Crt, mainly spheroidene (SE) and spheroidenone (SO), under photosynthetic conditions (29, 30, 31, 38, 40). These studies also implicated the rdxB gene as being involved in the regulation of photosynthesis gene expression and Crt accumulation (29, 31). The rdxBHIS operon of R. sphaeroides 2.4.1 was initially identified through the discovery of rdxB as a homologue of the rdxA gene (25), which encodes a membrane-localized redox protein apparently containing two [4Fe-4S] clusters.
The rdxBHIS operon maps immediately downstream of the ccoNOQP operon, which encodes the cbb3 cytochrome oxidase. This oxidase consists of four subunits: CcoN, to which heme b and copper cofactors are bound; CcoO (monoheme cytochrome c); CcoQ (signal transponder protein); and CcoP (diheme cytochrome c) (10, 22, 24, 31). Microorganisms which contain a ccoNOQP/fixNOQP operon encoding a cbb3 cytochrome oxidase are also reported to possess the rdxBHIS/fixGHIS operon at the 3′ end of the ccoNOQP operon (5, 14, 21, 22, 29, 33).
Because of the apparent relationship of the rdxB locus to the O2-sensing signal transduction pathway involving the cbb3 cytochrome oxidase, as well as the relative abundance of the Crts SE and SO, we have undertaken a genetic and phenotypic study of each gene of the rdxBHIS locus. To facilitate this analysis, we have constructed nonpolar, in-frame deletion mutations in each of the rdx genes in order to ascertain the role(s) of each of the subunits.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The R. sphaeroides and Escherichia coli strains and plasmids used in this study are described in Table 1. E. coli strains were grown at 37°C on Luria-Bertani medium supplemented, when required, with tetracycline (20 μg/ml) and ampicillin, kanamycin, streptomycin, and spectinomycin (each at 50 μg/ml). R. sphaeroides strains were grown at 30°C on Sistrom's minimal medium A (SIS) containing succinate as a carbon source (3). Final concentrations of antibiotics were 1 μg/ml for tetracycline and 50 μg/ml for kanamycin, trimethoprim, spectinomycin, and streptomycin. Aerobic cultures were grown on a rotary shaker or sparged with 30% O2–69% N2–1% CO2. Photosynthetic cultures were grown at a light intensity of 50 W/m2 and sparged with 95% N2–5% CO2. Semiaerobic cultures were sparged with 2% O2–1% CO2–97% N2. Strains grown anaerobically in the dark were cultured in SIS supplemented with 0.1% yeast extract in the presence of 60 mM dimethyl sulfoxide (DMSO).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| E. coli | ||
| DH5αphe | DH5αphe::Tn10dCm | 6 |
| S17-1 | C600::RP4-2(Tc::Mu)(Km::Tn7) thi pro hsdR recA Tra+ | 35 |
| R. sphaeroides | ||
| 2.4.1 | Wild type | W. Sistrom |
| RDXBΔ | 2.4.1 derivative, in-frame deletion in rdxB | 31 |
| CCONΔ | 2.4.1 derivative, in-frame deletion in ccoN | 31 |
| JR112 | 2.4.1 derivative, in-frame deletion in rdxH | This study |
| JR114 | 2.4.1 derivative, in-frame deletion in rdxS | This study |
| JR117 | 2.4.1 derivative, in-frame deletion in rdxI | This study |
| Plasmid | ||
| pBluescript SK+ | AprlacPOZ′ | Stratagene |
| pRK415 | Tcr Mob+lacZα IncP | 15 |
| pBBR1MCS2 | Kmr | 18 |
| pLO1 | KmrsacB RP4-oriT ColE1-oriV | 20 |
| pUI8180 | Cosmid containing rdxBHIS operon | 39 |
| pCF200Km | Spr Str Kmr IncQ puc::lacZYA′ | 19 |
| pUI1663 | Spr Str Kmr IncQ puf::lacZYA | 8 |
| pJR101 | pBluescript SK+ :: 4.9-kb PstI fragment containing rdxBHIS operon | This study |
| pJR104 | pLO1::1.8-kb FspI + HincII fragment containing in-frame deleted rdxI | This study |
| pJR106 | pLO1::1.1-kb SspI + HincII fragment containing in-frame deleted rdxH | This study |
| pJR107 | pLO1::1.7-kb NruI fragment containing in-frame deleted rdxS | This study |
| pJR115 | pBBR1MCS2::0.6-kb SacII fragment containing rdxS gene | This study |
| pJR116 | pBBR1MCS2::1.1-kb XbaI + SacI fragment containing rdxH gene | This study |
| pJR120 | pBBR1MCS2::2.7-kb KpnI + HindIII fragment containing rdxI gene | This study |
| pJR121 | pBBR1MCS2::2.1-kb HindIII + SacII fragment containing rdxB gene | This study |
DNA manipulation and analysis.
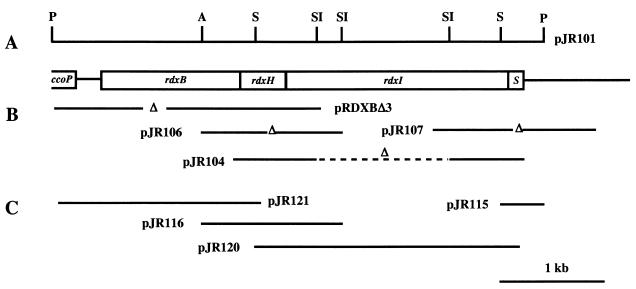
Cosmid pUI8180 was digested with PstI, which contains a recognition site upstream of the ccoP gene but no recognition sequence within the rdxBH genes. The digest was analyzed by Southern hybridization using the PCR-amplified rdxH gene as a probe. A specific 4.8-kb DNA fragment encompassing the entire rdxBHIS operon was observed following exposure of the probed gel to X-ray film (data not shown). The PstI fragment corresponding to this band was subcloned into the same site of pBluescript II KS+ and designated pJR101 (Fig. 1). The plasmid, pJR101, which was used for sequencing template DNA contained a portion of the ccoP gene, the entire rdxBHIS operon, and a downstream 148-bp flanking region.
FIG. 1.
Physical map of the R. sphaeroides 2.4.1 rdxBHIS operon. (A) Important restriction sites used in this study (P, PstI; A, AatII; S, SacII; SI, SacI) are shown. (B) Plasmids used in construction of the in-frame deletion mutants. Deleted regions are represented by Δ. (C) Chromosomal fragments used to complement the rdxB, rdxH, rdxI, and rdxS mutants. All DNA fragments were introduced in plasmid pBBR1MCS2 (18). Each gene of the rdxBHIS operon was expressed from the kanamycin resistance gene promoter on the plasmid.
Conjugation techniques.
Plasmids were mobilized by biparental or triparental mating from E. coli strains into R. sphaeroides strains as described elsewhere (4).
Construction of rdxBHIS in-frame deletion mutations.
The method and mutant selection for the rdxB in-frame deletion mutation were previously reported (31). Below we describe the methods for constructing the rdxHIS in-frame deletion mutations.
(i) rdxH mutation.
A 1.05-kb AatII-SacI fragment containing the rdxH gene from pJR101 (Fig. 1) was subcloned into the same site of pUC19 and designated pH1. Plasmid pH1 was used as template DNA for PCR to construct the rdxH in-frame deletion mutation. The PCR was performed with primers 18Reverse (5′-AGC GGA TAA CAA TTT CAC ACA GGA-3′) and MURDXH (5′-CCC CGC GGA GCC TGA GTT CGT GCT G-3′) to generate a DNA fragment with a 57-bp in-frame deletion (amino acid residues 89 to 107). The PCR-amplified DNA fragment was digested with SacII-SphI and inserted at the same site of pH1, yielding plasmid pH2. DNA sequencing of the PCR-amplified region of plasmid pH2 was performed to confirm the in-frame deletion as well as the absence of any additional mutations. Plasmid pH2 was blunt ended with SspI-HincII and then cloned into the PmeI site of the suicide vector, pLO1 (20). The lengths of the flanking DNA regions for homologous recombination were approximately 460 and 580 bp. The resulting plasmid, pJR106, was transferred to R. sphaeroides 2.4.1 by conjugation, and screening for single and double recombinants was performed as described previously (31). The rdxH in-frame deletion mutation was verified by DNA sequencing of the PCR-amplified DNA fragment containing the mutated region.
(ii) rdxI mutation.
Plasmid pJR101 has four SacI sites; one is derived from pUC19, and the remaining three (at 322, 904, and 1792 bp) are located within the rdxI gene (Fig. 1). Plasmid pJR101 was partially digested with 1 U of SacI for 10 min at 37°C and separated by agarose gel electrophoresis. The partially digested, larger DNA fragment was eluted, self-ligated, and transformed to E. coli DH5αphe. We found one plasmid having the deletion of two SacI fragments (corresponding to amino acids 108 to 597). This plasmid was blunt ended with FspI-HincII and cloned into the PmeI site derived from pLO1 (20). Mutant screening for the rdxI mutation was performed as described previously (31), and the in-frame deletion was confirmed by sequencing of the PCR-amplified DNA fragment of the RdxI mutant.
(iii) rdxS mutation.
The 1.6-kb AccI fragment containing rdxS of pJR101 was isolated and cloned into the same site of pBluescript II KS+; the resulting plasmid was designated pAC1. The 3′ flanking region of the rdxS gene within plasmid pJR101 was 148 bp, which is not sufficient for homologous recombination. We therefore amplified the 3′-terminal flanking region of the rdxS gene contained within cosmid pUI8180. The PCR was performed with primers MURDXS (5′-CCG GAT CCG CGC CAG TAG CAC GCG CTT T-3′) and pLA1 (5′-GGC GCA GGG GAT CA-3′), using cosmid pUI8180 as the template. The PCR product contained the 24-bp deletion (corresponding to amino acids 43 to 50) of the rdxS gene. The amplified DNA (about 1,350 bp) was digested with BamHI-NotI and exchanged into the same restriction sites of pAC1. A 580-bp BamHI fragment that was deleted by the above-described BamHI treatment used to subclone the PCR product was reintroduced into this plasmid. Finally, an NruI fragment containing the rdxS mutation was ligated into PmeI-digested pLO1 (20). Mutant screening and confirmation were performed as described above.
Spectral analysis.
A crude cell extract was prepared by passage through a French press (Aminco, Urbana, Ill.), followed by two rounds of centrifugation at 13,000 rpm to remove unbroken cells and cell debris. The membrane fraction was prepared by centrifugation at 100,000 × g for 1 h. The dithionite-reduced-minus-oxidized spectrum was recorded with 200 μg of membrane protein dissolved in Tris buffer (pH 7.5) containing 0.5% dodecyl maltoside. The sample was reduced for 2 min after adding 30 μl of 15% (wt/vol) sodium dithionite. The amounts of b- and c-type hemes were estimated by using the extinction coefficients ɛ561-575 of 22 mM−1 cm−1 and ɛ551-540 of 19 mM−1 cm−1, respectively. The amount of B800-850 and B875 light-harvesting complexes was determined as described elsewhere (23). Photopigments were extracted from cell pellets with acetone-methanol (7:2, vol/vol) mixture and quantitated as described previously (3). The extracted mixture was concentrated for high-pressure liquid chromatography (HPLC) analysis on a Shimadzu system equipped with an SPD-M10AV diode array detector as previously described (38). All analyses were performed in duplicate at least twice, and the data presented are the averages of the values obtained.
Enzyme assay and protein determination.
Assays of β-galactosidase and cbb3 cytochrome oxidase activity were performed on crude cell extracts of R. sphaeroides as previously described (31) at least twice, with standard deviations not exceeding 15%. Protein concentrations were determined with the Pierce (Rockford, Ill.) bicinchoninic acid protein assay reagent with bovine serum albumin as a standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of protein present in the membrane fraction was performed in a Tris-glycine-sodium dodecyl sulfate buffer system using a mini-slab gel apparatus. Western analysis was carried out as described previously (37), using rabbit anti-CcoO and anti-CcoP antibodies at a 1:5,000 dilution. Blots were then incubated with alkaline phosphatase-coupled goat anti-rabbit immunoglobulin G (1:10,000 dilution) and developed using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate. The specific band which appeared on the membrane was scanned and quantitated using the NIH Image 1.61 software.
Nucleotide sequence accession number.
The nucleotide sequence of the rdxBHIS operon was submitted to GenBank (accession no. AF202779).
RESULTS
DNA sequence of the rdxBHIS operon.
The rdxBHIS operon was partially sequenced in a previous study (29). In this study, we sequenced the remaining rdxI, rdxS, and flanking regions, which were contained within cosmid pUI8180, in addition to the previously reported rdxBHI′ region (29). Derived proteins homologous to rdxBHIS are reported for the phylogenetically related species Rhodobacter capsulatus (17), Bradyrhizobium japonicum (33), Rhizobium leguminosarum (GenBank accession no. AJ001522), and Rhizobium meliloti (14) (Table 2).
TABLE 2.
Amino acid sequence identity of RdxI and RdxS homologous proteins
| Proteina | % Identity
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
R. capsulatus
|
B. japonicum
|
R. leguminosarum
|
R. meliloti
|
|||||
| CcoI | CcoS | FixI | FixS | FixI | FixS | FixI | FixS | |
| RdxI | 74.1 | 45.4 | 45.5 | 45.0 | ||||
| RdxS | 70.6 | 57.8 | 57.1 | 57.1 | ||||
All Rdx components are apparently membrane proteins containing at least one membrane-spanning region (25, 29). Comparative amino acid sequence alignments of rdxI show that all of the putative functional regions of recently suggested CPx-type ATPases, such as a metal binding domain (C53-X-X-C56), a portion of a phosphatase domain (T308-G-E310), an ion transduction domain (C413-P-C415), E1-E2 ATPase phosphorylation sites (D457-K-T-G-T461), an ATP binding region (V634-G-D-G637), and eight putative transmembrane regions, are present (2, 14, 27, 33, 34, 36). RdxS, consisting of 52 amino acids, possesses no recognizable protein motifs.
Construction of in-frame deletion mutations.
The plasmids used in construction of the in-frame deletion mutations for each of the genes of rdxHIS are depicted in Fig. 1B. We also show construction of the rdxB mutation as described elsewhere (31).
All mutations of the rdxBHIS locus gave rise to colonies with increased red pigmentation on SIS plates in the presence of O2 compared to the wild type, 2.4.1, as previously reported for rdxB (29, 30, 31). This suggested the induction of photosynthesis genes in the presence of O2. In particular, in-frame mutations of rdxI and rdxS formed very dark red, very small colonies compared with the wild type. The colony sizes of rdxB and rdxH in-frame mutations were between those of the wild type, 2.4.1, and the RdxI and RdxS mutants on SIS plates. However, colony pigmentation of the rdxB in-frame mutation was substantially darker red than that of the rdxH mutation.
Complementation of Rdx mutants.
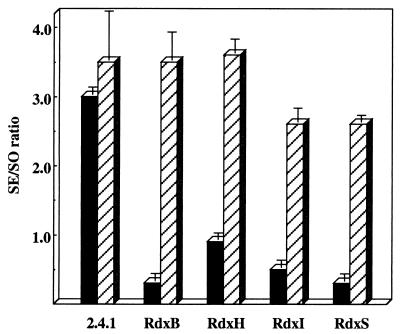
Complementation experiments were performed to ensure that each of the Rdx mutants possessed a specific in-frame deletion and that no additional extragenic mutations were present. The plasmids used for complementation are depicted in Fig. 1C. Each of the gene products was expressed from the kanamycin resistance gene promoter on plasmid pBBR1MCS2 (18). Complementation was determined by measurements of the Crt composition under phototrophic conditions, growth characteristics, and colony pigmentation. When complemented mutants were cultured photosynthetically, all mutant strains were reverted to the wild-type, green-brown coloration from the red pigmentation due to an excess of SO over SE (Fig. 2). The SE/SO ratio was also similar to that obtained with the wild type 2.4.1 (Fig. 2). When the complemented mutant strains were cultured under semiaerobic conditions, growth was restored to that of the wild type (data not shown). This complementation analysis also demonstrated that none of the in-frame deletions were polar on the downstream gene(s) of the rdx operon.
FIG. 2.
Complementation of rdxBHIS in-frame deletion mutants. Carotenoid composition (SE/SO ratio) of the wild-type strain, 2.4.1, and mutant strains was analyzed by HPLC as described in Materials and Methods. Strains were grown photosynthetically at a light intensity of 50 W/m2. Data are the mean values of two independent cultures. Dark blocks represent 2.4.1 and each of the rdxBHIS mutants; hatched blocks represent the complemented strain. Plasmid pBBR1MCS2 (18) was used to complement 2.4.1.
Therefore, these results revealed that each Rdx mutant contained a mutation in the designated gene only.
Growth of Rdx mutants.
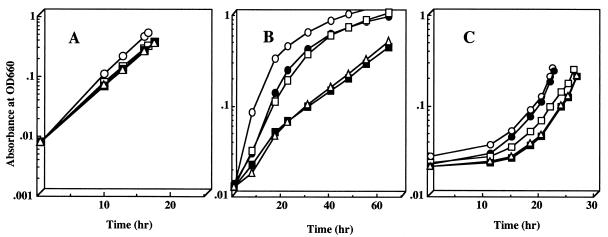
We next examined the growth characteristics and quantitated the levels of spectral complex formation under highly aerobic, semiaerobic, and photoheterotrophic conditions in order to more fully characterize each of the mutant strains. Although highly aerobic growth patterns of the mutants (Fig. 3A) were similar to one another and to the wild-type patterns (doubling time of approximately 3 h), growth under semiaerobic conditions revealed a different response (Fig. 3B). In-frame mutations of either rdxI and rdxS showed approximately 50% of the growth rate (doubling time of 9 to 10 h) of RdxB, RdxH, and wild-type strains (3 to 5 h) under semiaerobic conditions. Thus, we assumed that RdxI and RdxS might have a more significant physiological role under conditions of low O2 tensions.
FIG. 3.
Relative growth rates of mutant strains under aerobic conditions (A; sparged with 30% O2–1% CO2–69% N2), semiaerobic conditions (B; sparged with 2% O2–1% CO2–97% N2), and phototrophic conditions (C; sparged with 5% CO2–95% N2). ○, wild type; ●, rdxB; □, rdxH; ■, rdxI; ▵, rdxS; ▴, ccoN. OD660, optical density at 660 nm.
Spectral complex analysis under aerobic conditions.
It has been shown that spectral complex formation of R. sphaeroides 2.4.1 under aerobic conditions can be controlled by the cellular redox poise through the cbb3 terminal oxidase (29, 30, 31, 38, 40). These earlier studies also revealed that the rdxB gene was somehow involved in this response (29, 31). To begin to understand the role, if any, of each of the other rdx genes, we analyzed the spectral complex content of each mutant strain grown under highly aerobic conditions.
The mutant strains exhibited 3- to 7-fold and 7- to 13-fold increased levels of the B800-850 and B875 complexes, respectively (Table 3). The levels of total Crt (two- to fourfold) and Bchl (four- to ninefold) were also higher in all mutant strains than in the wild-type strain, 2.4.1. All Rdx mutants possessed SO as the major Crt under phototrophic conditions, with the level of SE being substantially lower than that in the wild type. The higher amounts of the B875 complex compared to the B800-850 complex are the result of low Bchl availability under aerobic conditions and its preferential use for B875 assembly relative to B800-850 assembly, as reported earlier (13, 26, 31, 38).
TABLE 3.
Spectral complex formation of R. sphaeroides strains grown under aerobic conditions
| Mutationa | Spectral complex formationb
|
|||
|---|---|---|---|---|
| B800-850 | B875 | Crt | Bchl | |
| None (2.4.1) | <0.1 | 0.3 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.2 |
| rdxB | 0.3 ± 0.1 | 2.0 ± 0.1 | 1.1 ± 0.1 | 2.4 ± 0.2 |
| rdxH | 0.5 ± 0.1 | 3.2 ± 0.1 | 1.6 ± 0.1 | 4.1 ± 0.3 |
| rdxI | 0.5 ± 0.1 | 3.3 ± 0.3 | 2.0 ± 0.1 | 5.2 ± 0.1 |
| rdxS | 0.7 ± 0.1 | 3.9 ± 0.2 | 2.1 ± 0.2 | 5.4 ± 0.4 |
Strains were grown by sparging with 69% N2–30% O2–1% CO2 to an optical density at 600 nm of 0.3.
B800-850 and B875 spectral complexes are expressed as nanomoles per milligram of protein. Bchl and Crt are expressed as micrograms per 100 ml of culture.
Analysis of puc and puf operon expression in Rdx mutants.
The relationship between the formation of spectral complexes in the presence of O2 and the actual expression of the puc and puf genes in the rdxB polar insertion mutation was reported previously (29). In these earlier experiments, transcription levels of the genes encoding the B800-850 and B875 apoproteins were approximately 13.5- and 4.3-fold, respectively, higher than in the wild type, 2.4.1.
To extend these analyses to each of the rdx genes described here, promoter activities of the puc and puf genes under aerobic conditions were determined. As shown in Table 4, the level of puc operon expression was increased approximately 4.1- to 6.5-fold in the different Rdx mutants compared with the wild type. The values for puf operon expression also increased approximately 2.8- to 5.0-fold in the mutant strains. Thus, all of the rdx in-frame deletion mutations when present individually gave rise to the derepression of both the puc and puf operons under aerobic conditions.
TABLE 4.
β-Galactosidase activities from cell extracts of R. sphaeroides strains containing puc::lacZ (pCF200Km) or puf::lacZ (pUI1663) fusion plasmid
| Mutationa | β-Galactosidase activity (nmol/min/mg of protein)b
|
|
|---|---|---|
| puc::lacZ | puf::lacZ | |
| None (2.4.1) | 221 ± 24 | 279 ± 15 |
| rdxB | 980 ± 12 | 769 ± 30 |
| rdxH | 1,427 ± 1 | 1,291 ± 99 |
| rdxI | 1,187 ± 28 | 1,381 ± 38 |
| rdxS | 900 ± 12 | 927 ± 43 |
Strains were grown aerobically with sparging of 69% N2–30% O2–1% CO2 to an A600 of 0.2.
Average of two independent experiments.
Spectral complex analysis under anaerobic conditions.
When wild type 2.4.1 is grown under dark, dimethyl sulfoxide (DMSO) conditions, pigmentation is the typical green-brown color resulting from the highly expressed Crt, SE, in the presence of Bchl. Under these conditions, the SE-to-SO ratio of 2.4.1 is 5.1 ± 0.8. However, differences in pigmentation were found when the individual RdxB, -I, and -S mutants (1.8 ± 0.1, 1.8 ± 0.1, 1.9 ± 0.1, respectively) were grown under dark, DMSO conditions, in which they appeared red. Only the RdxH mutant showed the green-brown pigmentation similar to that of the wild type, 2.4.1, with an SE/SO ratio of 4.2 ± 0.5. The result observed for the RdxH mutant is similar to that observed for the CcoQ mutant of the cbb3 cytochrome oxidase (31) and the PrrC mutant (9), both of which are defective in being able to transduce the inhibitory signal from cbb3 cytochrome oxidase to the membrane-bound histidine kinase, PrrB.
cbb3 cytochrome oxidase activity in the Rdx mutants.
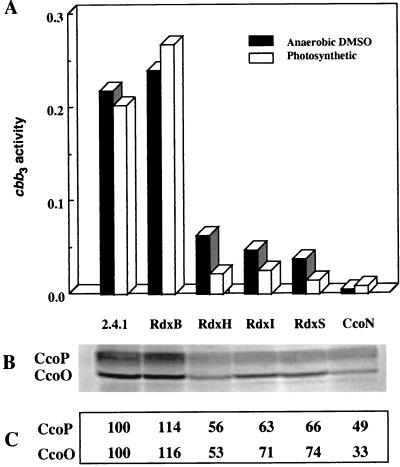
The rdxBHIS operon is located immediately 3′ to the ccoNOQP operon, which encodes the cbb3 cytochrome oxidase (29, 39). Because the RdxI and RdxS mutants showed slow growth under semiaerobic conditions, in which the cbb3 cytochrome oxidase is the major terminal oxidase (10, 21), we investigated both cbb3 cytochrome oxidase activity and the levels of the CcoO and CcoP proteins by Western blot analysis in each of the Rdx mutants. Previously, it was shown that with a ccoN in-frame deletion mutation grown under anaerobic conditions with DMSO, cbb3 oxidase activity was undetectable (31). All of the individual RdxB, -H, -I, and -S mutants together with the wild type 2.4.1 and the CcoN mutant as a control were grown under anaerobic dark, DMSO conditions, and cbb3 cytochrome oxidase activity was measured. This is the only terminal oxidase which is measurable under these conditions (31). As shown in Fig. 4A, the RdxB mutant had at least the same level of cbb3 oxidase activity as 2.4.1, while levels for mutants RdxH, RdxI, and RdxS were substantially lower. When the individual Rdx mutants were grown under photosynthetic conditions in SIS medium, cbb3 cytochrome oxidase activity was reduced even further to near background levels except for that of the RdxB mutant, which was at or greater than wild-type levels. In no case was the reduction in cbb3 oxidase activity quite as pronounced as in the CcoN mutant (31).
FIG. 4.
Activities and Western blot analysis of cbb3 cytochrome oxidase of R. sphaeroides 2.4.1 and mutant strains. (A) Strains were cultured under anaerobic dark, DMSO conditions (■) and under photosynthetic conditions (□). cbb3 oxidase activity was expressed as micromoles per minute per milligram of protein. (B) Western blot analysis was performed with 30 μg of membrane fraction from cells grown under anaerobic dark, DMSO conditions using R. sphaeroides CcoO/P-specific antibody. (C) Quantitation of protein levels by Western blotting. Quantitation was performed by subtracting the background levels for each lane from the specific band. The CcoP and CcoO levels of 2.4.1 were considered 100%, and others are given relative to that value.
To ascertain if the observed cbb3 activity reflected the amounts of cbb3 protein in the cell, we performed Western blot analysis using a CcoO/P-specific antiserum made against the R. sphaeroides 2.4.1 polypeptides (Fig. 4B). While the CcoO/P-specific signal is abundant in the wild type 2.4.1 and the RdxB mutant to approximately the same extent, reduced levels of these polypeptides were present in the membrane fractions isolated from the RdxH, RdxI, and RdxS mutant strains. However, the loss of cbb3-specific oxidase activity is clearly in excess of the residual levels of P and O polypeptides in the membrane.
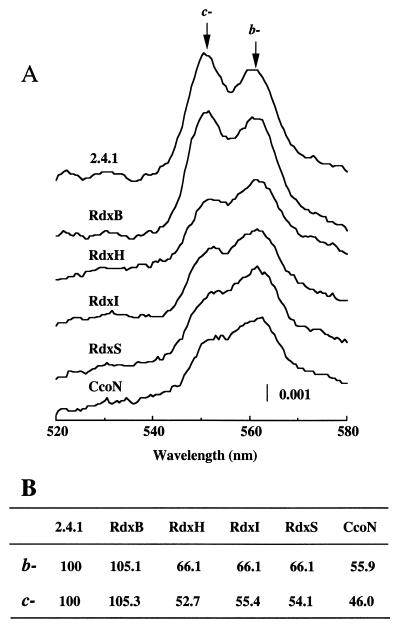
The dithionite-reduced-minus-air-oxidized spectra of the membrane fractions of each of the Rdx mutants showed both the b- and c-type cytochromes (Fig. 5). Amounts of the b (ɛ561-575) and c (ɛ551-540) cytochromes were similar when 2.4.1 and the RdxB mutant were compared. When the levels of the cytochromes in the RdxH, -I, and -S mutants were quantitated, the b type was reduced 33.9% in all of the mutants and the c type was reduced 47.3, 44.6, and 45.9%, respectively. These values are in relatively good agreement with the Western blot data provided earlier. Therefore, it is safe to conclude that the cbb3 oxidase activity is more severely affected than the physical presence of the component polypeptides within the membrane in these mutant strains.
FIG. 5.
Reduced-minus-oxidized spectra of R. sphaeroides strains (A) and the b- and c-type cytochrome content in the membrane fraction (B). The strains were grown under semiaerobic conditions (sparged with 2% O2–1% CO2–97% N2); 200 μg of total membrane protein was used. The b-type (0.39 μM−1 cm−1) and c-type (0.27 μM−1 cm−1) cytochrome content of 2.4.1 was considered 100%, and the contents of Rdx mutants are given relative to that value.
Finally, to determine whether the effect observed for each of the Rdx mutants on cbb3 activity was at the transcriptional or posttranscriptional level, the promoter activity of the cco operon was determined using a lacZ transcriptional fusion (24) under semiaerobic conditions. We observed that similar β-galactosidase activities were found in the wild type, 2.4.1, and all of the Rdx mutant strains, implying that the lower levels of cbb3 activity in the mutants was not the result of a transcriptional effect on the levels of cco operon expression (data not shown).
DISCUSSION
Photosystem gene expression in R. sphaeroides 2.4.1 is dependent on a variety of environmental conditions, such as O2 tension and light intensity (16). Studies from our laboratory have shown that R. sphaeroides 2.4.1 possesses at least four regulatory systems which mediate O2- and/or light-dependent control of photosystem gene expression (6, 7, 8, 9, 11, 12, 37, 39, 41). In addition to these regulatory systems, mutations in the ccoNOQP operon encoding the cbb3 cytochrome oxidase (29, 31, 40) and in the rdxB gene (29, 31) result in the O2-insensitive formation of the photosystem as well as altered Crt accumulation. It was proposed that these membrane-associated complexes are involved in generating and/or transmitting a signal to the membrane-localized PrrB histidine kinase, preventing its activation of the PrrA response regulator, which is required for photosystem gene expression (8, 30, 31). Unlike the well-studied cbb3 cytochrome oxidase gene, the rdxBHIS genes have not been characterized.
Previously, we reported that a polar insertion into rdxB (29) as well as an in-frame deletion mutation within rdxB (31) activates photosystem gene expression in the presence of high O2. To further understand the role of all genes of the rdxBHIS operon in this signal transduction pathway, we have constructed a series of in-frame deletion mutations which are nonpolar for each of the rdx genes.
On the basis of the data presented here and in consideration of our earlier results for RdxB mutant strains in our studies of the cbb3 oxidase, mutants of RdxB more closely resemble the wild type 2.4.1 than do any of the other Rdx mutants in terms of growth rate, cbb3 cytochrome oxidase activity, levels of the CcoO and CcoP proteins, and reduced-minus-oxidized spectra. Thus, the effects of nonpolar mutations in rdxB are limited to the activation of photosynthesis gene expression in the presence of O2 and an alteration in the ratio of SE to SO.
Conversely, the RdxH, -I, and -S mutant strains are appreciably different from the wild type, 2.4.1, and the RdxB mutant in that they show reduced growth rates, reduced levels of cbb3 oxidase activity, and reduced levels of the CcoO and CcoP polypeptides and b- and c-type cytochromes. On the other hand, like the RdxB mutant, they show increased photosynthesis gene expression in the presence of O2, and with one notable exception (see below), they show altered ratios of SE to SO.
Even within this latter grouping, the RdxH mutant does not appear to be as severely affected as the RdxI and -S mutant strains. For example, the SE-to-SO ratio of the RdxH mutant grown under dark, DMSO conditions is virtually normal, in contrast to the very abnormal ratio when this same mutant is grown photosynthetically. Thus, the RdxH mutant appears to possess the most complex phenotype.
Because of the relatively severe diminution of cbb3 oxidase activity but otherwise modest loss of CcoO and CcoP polypeptide levels in the RdxH, -I, and -S mutants, we can speculate that the rdx gene products play some role in developing and/or maintaining the integrity of the cbb3 oxidase. Further supportive of this hypothesis is the observation that RdxI possesses features highly similar to those of the metal-transporting CPx-type ATPases by virtue of amino acid sequence homologies (2, 27, 33, 36). The reported features of CPx-type ATPases are well conserved in the RdxI subunit, although we have not determined the number and topology of the transmembrane regions experimentally. Despite these similarities, the operon structures for P-type ATPases from Enterococcus hirae (27), Helicobacter pylori (2), and Helicobacter felis (2) are completely different from the R. sphaeroides 2.4.1 rdxBHIS operon structure (29). The operon copYZAB of E. hirae encoding two P-type ATPases (CopAB), a repressor (CopY), and an activator (CopZ) is a well-studied operon of this enzyme class which functions to maintain copper homeostasis (27, 28). The RdxI/FixI type of CPx-type ATPases might represent a new subfamily of these ATPases (14, 17, 21, 29, 33) that probably functions to maintain the activity of the cbb3 cytochrome oxidase through the maintenance of intracellular copper homeostasis (Fig. 4) (1, 33, 42). Therefore, we suggest that RdxI of R. sphaeroides 2.4.1 functions as a specific Cu transporter to and for the cbb3 cytochrome oxidase (Fig. 4).
RdxS is a small protein of approximately 6 kDa that is thought to be a membrane protein by virtue of its N-terminal region. Our results revealed that the phenotype of the RdxS mutant was most similar to that of the RdxI mutant. As discussed above, the RdxH mutant is the most complex, but because it more closely resembles the RdxI and -S mutant strains, we believe that it is also likely to be involved in making copper available to the cbb3 oxidase. However, because residual cbb3 oxidase activity as well as polypeptides are present in the RdxH, -I, and -S mutant strains compared to a CcoN mutant, other Cu transporters and a chaperone system must be available to satisfy the needs of the cbb3 oxidase, at least in part. Because the physical presence of the CcoO and CcoP polypeptides and the b- and c-type cytochromes is far in excess of the residual cbb3 activity, the data suggest that copper availability is not essential for the targeting of these subunits to the membrane but is required for activity.
Finally, because the RdxB mutant is defective only in the activation of photosynthesis gene expression in the presence of O2 and in the maintenance of the hypothesized 2-oxo donor to SE, in the reduced state (31, 38), we speculate that the RdxB protein can receive reducing equivalents from the cbb3 cytochrome oxidase, and thus mutants of RdxB can alter the flow of reductant through cbb3 oxidase in the presence of O2, resulting in photosynthesis gene expression. In the absence of O2, absence of RdxB would result in the excessive production of the 2-oxo donor, causing an accumulation of SO.
ACKNOWLEDGMENT
This work was supported by grant GM15590 to S.K.
REFERENCES
- 1.Batut J, Boistard P. Oxygen control in Rhizobium. Antonie Leeuwenhoek. 1994;66:129–150. doi: 10.1007/BF00871636. [DOI] [PubMed] [Google Scholar]
- 2.Bayle D, Wangler S, Weitzenegger T, Steinhilber W, Volz J, Przybylski M, Schafer K P, Sachs G, Melchers K. Properties of the P-type ATPases encoded by the copAP operons of Helicobacter pylori and Helicobacter felis. J Bacteriol. 1998;180:317–329. doi: 10.1128/jb.180.2.317-329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen-Bazire G, Sistrom W R, Stanier R Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Comp Physiol. 1957;49:25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- 4.Davis J, Donohue T J, Kaplan S. Construction, characterization, and complementation of a Puf− mutant of Rhodobacter sphaeroides. J Bacteriol. 1988;170:320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Gier J W, Schepper M, Reijnders W N, van Dyck S J, Slotboom D J, Warne A, Saraste M, Krab K, Finel M, Stouthamer A H, van Spanning R J, van der Oost J. Structural and functional analysis of aa3-type and cbb3-type cytochrome c oxidases of Paracoccus denitrificans reveals significant differences in proton-pump design. Mol Microbiol. 1996;20:1247–1260. doi: 10.1111/j.1365-2958.1996.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 6.Eraso J M, Kaplan S. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eraso J M, Kaplan S. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J Bacteriol. 1995;177:2695–2706. doi: 10.1128/jb.177.10.2695-2706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eraso J M, Kaplan S. Complex regulatory activities associated with the histidine kinase PrrB in expression of photosynthesis genes in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1996;178:7037–7046. doi: 10.1128/jb.178.24.7037-7046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eraso J M, Kaplan S. From redox flow to gene regulation: the role of the PrrC protein of Rhodobacter sphaeroides 2.4.1. Biochemistry. 2000;39:2052–2062. doi: 10.1021/bi9923858. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Horsman J A, Berry E, Shapleigh J P, Alben J O, Gennis R B. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry. 1994;33:3113–3119. doi: 10.1021/bi00176a046. [DOI] [PubMed] [Google Scholar]
- 11.Gomelsky M, Kaplan S. appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1995;177:4609–4618. doi: 10.1128/jb.177.16.4609-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomelsky M, Kaplan S. Molecular genetic analysis suggesting interactions between AppA and PpsR in regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1997;179:128–134. doi: 10.1128/jb.179.1.128-134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong L, Lee J K, Kaplan S. The Q gene of Rhodobacter sphaeroides: its role in puf operon expression and spectral complex assembly. J Bacteriol. 1994;176:2946–2961. doi: 10.1128/jb.176.10.2946-2961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn D, David M, Domergue O, Daveran M L, Ghai J, Hirsch P R, Batut J. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J Bacteriol. 1989;171:929–939. doi: 10.1128/jb.171.2.929-939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 16.Kiley P J, Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988;52:50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch H G, Hwang O, Daldal F. Isolation and characterization of Rhodobacter capsulatus mutants affected in cytochrome cbb3 oxidase activity. J Bacteriol. 1998;180:969–978. doi: 10.1128/jb.180.4.969-978.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovach M E, Phillips R W, Elzer P H, Roop R M, 2nd, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 19.Lee J K, Kaplan S. Isolation and characterization of trans-acting mutations involved in oxygen regulation of puc operon transcription in Rhodobacter sphaeroides. J Bacteriol. 1992;174:1158–1171. doi: 10.1128/jb.174.4.1158-1171.1992. . (Erratum, 174:2418.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenz O, Schwartz E, Dernedde J, Eitinger M, Friedrich B. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J Bacteriol. 1994;176:4385–4393. doi: 10.1128/jb.176.14.4385-4393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandon K, Kaminski P A, Mougel C, Desnoues N, Dreyfus B, Elmerich C. Role of the fixGHI region of Azorhizobium caulinodans in free-living and symbiotic nitrogen fixation. FEMS Microbiol Lett. 1993;114:185–189. doi: 10.1111/j.1574-6968.1993.tb06571.x. [DOI] [PubMed] [Google Scholar]
- 22.Mandon K, Kaminski P A, Elmerich C. Functional analysis of the fixNOQP region of Azorhizobium caulinodans. J Bacteriol. 1994;176:2560–2568. doi: 10.1128/jb.176.9.2560-2568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meinhardt S W, Kiley P J, Kaplan S, Crofts A R, Harayama S. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of energy transfer from light-harvesting complexes to the reaction center. Arch Biochem Biophys. 1985;236:130–139. doi: 10.1016/0003-9861(85)90612-5. [DOI] [PubMed] [Google Scholar]
- 24.Mouncey N J, Kaplan S. Oxygen regulation of the ccoN gene encoding a component of the cbb3 oxidase in Rhodobacter sphaeroides 2.4.1T: involvement of the FnrL protein. J Bacteriol. 1998;180:2228–2231. doi: 10.1128/jb.180.8.2228-2231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neidle E L, Kaplan S. Rhodobacter sphaeroides rdxA, a homolog of Rhizobium meliloti fixG, encodes a membrane protein which may bind cytoplasmic [4Fe-4S] clusters. J Bacteriol. 1992;174:6444–6454. doi: 10.1128/jb.174.20.6444-6454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neidle E L, Kaplan S. 5-Aminolevulinic acid availability and control of spectral complex formation in hemA and hemT mutants of Rhodobacter sphaeroides. J Bacteriol. 1993;175:2304–2313. doi: 10.1128/jb.175.8.2304-2313.1993. . (Erratum, 175:7123.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odermatt A, Suter H, Krapf R, Solioz M. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J Biol Chem. 1993;268:12775–12779. [PubMed] [Google Scholar]
- 28.Odermatt A, Solioz M. Two trans-acting metalloregulatory proteins controlling expression of the copper-ATPases of Enterococcus hirae. J Biol Chem. 1995;270:4349–4354. doi: 10.1074/jbc.270.9.4349. [DOI] [PubMed] [Google Scholar]
- 29.O'Gara J P, Kaplan S. Evidence for the role of redox carriers in photosynthesis gene expression and carotenoid biosynthesis in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1997;179:1951–1961. doi: 10.1128/jb.179.6.1951-1961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Gara J P, Eraso J M, Kaplan S. A redox-responsive pathway for aerobic regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1998;180:4044–4050. doi: 10.1128/jb.180.16.4044-4050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh J I, Kaplan S. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry. 1999;38:2688–2696. doi: 10.1021/bi9825100. [DOI] [PubMed] [Google Scholar]
- 32.Ouchane S, Kaplan S. Topological analysis of the membrane-localized redox-responsive sensor kinase PrrB from Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1999;274:17290–17296. doi: 10.1074/jbc.274.24.17290. [DOI] [PubMed] [Google Scholar]
- 33.Preisig O, Zufferey R, Hennecke H. The Bradyrhizobium japonicum fixGHIS genes are required for the formation of the high-affinity cbb3-type cytochrome oxidase. Arch Microbiol. 1996;165:297–305. doi: 10.1007/s002030050330. [DOI] [PubMed] [Google Scholar]
- 34.Rensing C, Ghosh M, Rosen B P. Families of soft-metal-ion-transporting ATPases. J Bacteriol. 1999;181:5891–5897. doi: 10.1128/jb.181.19.5891-5897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:37–45. [Google Scholar]
- 36.Solioz M, Vulpe C. CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem Sci. 1996;21:237–241. [PubMed] [Google Scholar]
- 37.Yeliseev A A, Kaplan S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1995;270:21167–21175. doi: 10.1074/jbc.270.36.21167. [DOI] [PubMed] [Google Scholar]
- 38.Yeliseev A A, Eraso J M, Kaplan S. Differential carotenoid composition of the B875 and B800-850 photosynthetic antenna complexes in Rhodobacter sphaeroides 2.4.1: involvement of spheroidene and spheroidenone in adaptation to changes in light intensity and oxygen availability. J Bacteriol. 1996;178:5877–5883. doi: 10.1128/jb.178.20.5877-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeilstra-Ryalls J H, Kaplan S. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: the role of the fnrL gene. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeilstra-Ryalls J H, Kaplan S. Control of hemA expression in Rhodobacter sphaeroides 2.4.1: regulation through alterations in the cellular redox state. J Bacteriol. 1996;178:985–993. doi: 10.1128/jb.178.4.985-993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeilstra-Ryalls J H, Kaplan S. Role of the fnrL gene in photosystem gene expression and photosynthetic growth of Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1998;180:1496–1503. doi: 10.1128/jb.180.6.1496-1503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zufferey R, Arslan E, Thony-Meyer L, Hennecke H. How replacements of the 12 conserved histidines of subunit I affect assembly, cofactor binding, and enzymatic activity of the Bradyrhizobium japonicum cbb3-type oxidase. J Biol Chem. 1998;273:6452–6459. doi: 10.1074/jbc.273.11.6452. [DOI] [PubMed] [Google Scholar]