In Brief
The primary assessment of school-age outcomes in the Management of Myelomeningocele clinical trial did not show better adaptive behavior and cognitive skills in the prenatal group. In this secondary analysis, the need for a cerebrospinal fluid shunt masked stronger adaptive behavior and cognitive skills in the unshunted groups (both prenatal and postnatal). Disease severity and dynamic changes in hydrocephalus status seem to be the primary factors in the need for shunting and a major determinant of adaptive behavior and cognitive outcomes after prenatal surgery.
Keywords: spina bifida, hydrocephalus, myelomeningocele, MOMS trial, neurodevelopmental outcomes, adaptive behavior, congenital
ABBREVIATIONS : ADHD = attention-deficit/hyperactivity disorder; BRIEF = Behavior Rating Inventory of Executive Function; CMS = Children’s Memory Scale; CNS = central nervous system; CSF = cerebrospinal fluid; CVLT-C = California Verbal Learning Test for Children; FRESNO = Functional Rehabilitation Evaluation of Sensori-Neurological Outcomes; KBIT-2 = Kaufman Brief Intelligence Test, Second Edition; MOMS = Management of Myelomeningocele Study; MOMS2 = follow-up of MOMS; SBM = spina bifida myelomeningocele; SNAP-IV = Swanson, Nolan and Pelham–IV; WJ-III = Woodcock-Johnson Tests of Achievement, Third Edition
Abstract
OBJECTIVE
The purpose of this secondary analysis was to assess the role of hydrocephalus on neurodevelopmental outcomes in a cohort of school-age children enrolled in the Management of Myelomeningocele Study (MOMS) clinical trial.
METHODS
The sample analyzed in this report consisted of 150 of 183 children aged 5–10 years (mean ± SD 7 years 8 months ± 1.2) who were randomly assigned between 20 and 26 weeks of gestational age to undergo either prenatal or postnatal surgery and were enrolled in the school-age follow-up study of MOMS. These 150 children (76 prenatal and 74 postnatal) were placed into three groups: no hydrocephalus (n = 22), unshunted hydrocephalus (n = 31), and shunted hydrocephalus (n = 97). Comparisons were made on the basis of measures of adaptive behavior, intelligence, reading and math skills, verbal and nonverbal memory, fine motor dexterity, and sensorimotor skills. Parent ratings of executive functions, inattention, and hyperactivity-impulsivity were also compared.
RESULTS
There were no statistically significant differences in neurodevelopmental outcomes between the groups with no hydrocephalus and unshunted hydrocephalus, or between the prenatal and postnatal groups with shunted hydrocephalus, so these groups were combined (no/unshunted vs shunted hydrocephalus). The no/unshunted group showed significantly better performance (p < 0.05) than the shunted group in terms of adaptive behavior, intelligence, verbal and nonverbal memory, reading skills (but not math), fine motor dexterity, sensorimotor skills (but not visual-motor integration), and inattention (but not hyperactivity-impulsivity or executive function ratings). An assessment of the prenatal surgery group showed that the combined no/unshunted group performed better than the shunted group in terms of adaptive behavior and verbal memory skills. Both the prenatal and postnatal surgery subgroups with unshunted hydrocephalus performed as well as the group with no hydrocephalus despite significantly enlarged ventricles.
CONCLUSIONS
Although the primary assessment of school-age outcomes in the MOMS clinical trial did not show better adaptive behavior and cognitive skills in the prenatal group, hydrocephalus and shunting were associated with poorer neurodevelopmental outcomes (both prenatal and postnatal groups). Disease severity and dynamic changes in hydrocephalus status may be the primary factors in the need for shunting and a major determinant of adaptive behavior and cognitive outcomes after prenatal surgery.
Spina bifida myelomeningocele (SBM) is the most common congenital birth defect affecting the central nervous system (CNS), with a prevalence rate of roughly 1 in 1500 births in the United States.1 SBM results from a failure of primary neurulation that leads to a typical spinal abnormality, as well as secondary anomalies that can affect brain development, especially in the cerebellum and corpus callosum.1,2 Additional injury results from exposure of the developing CNS to a toxic intrauterine environment and hydrocephalus.3 Virtually all children with myelomeningocele develop the Chiari II malformation, representing a spectrum of brain malformations primarily affecting the cerebellum and medulla as well as the midbrain. Many children with myelomeningocele develop hydrocephalus, which often requires cerebrospinal fluid (CSF) diversion. With the advent of prenatal diagnosis, fetal surgery has been developed in an effort to prevent secondary injury to the brain and hydrocephalus.1,3–5
The Management of Myelomeningocele Study (MOMS) was a prospective randomized controlled trial that compared the effects of prenatal repair of myelomeningocele between 20 and 26 weeks gestational age with traditional postnatal repair. The trial was initiated in 2003 and stopped in 2011 after 183 maternal/fetal dyads had been randomly assigned to treatment and a statistically significant reduction in the need for shunting and improved motor function were identified in the prenatal group at 12 months of age.4,6 At 30 months of age, 91 children in the prenatal surgery group were compared with 92 children the postnatal surgery group.7 Although there was continued evidence for improved motor functioning, there was no difference in the cognitive skills between the two groups.
The most recent follow-up of the MOMS participants (MOMS2) occurred when the children reached 5–10 years of age.8 This report replicated the earlier findings of fewer CSF diversionary procedures, as well as better ambulation, sensorimotor, and fine motor skills, in the prenatal group. The prenatal and postnatal surgery groups did not differ in terms of the primary outcome, a measure of adaptive behavior administered with a semi-structured interview of the primary caregiver that assessed everyday habitual performance of activities in the communication, daily living, and socialization domains. However, caregivers in the prenatal group reported less overall family burden and higher quality of life for the child and family, which was related to improved motor functioning and reduction in the need for neurosurgical procedures. There was no strong evidence of improved cognitive function. The prenatal group generally had higher levels of performance than the postnatal group, but only comparisons of reading skills and nonverbal memory achieved statistical significance. The prenatal group scored reliably and significantly higher on a measure of fine motor dexterity.
We hypothesized that heterogeneity in hydrocephalus status among the prenatal group, i.e., variations in hydrocephalus severity and the subsequent need for a CSF diversionary procedure, was responsible for the largely null results in terms of cognitive outcomes at school age. In addition, there is a unique opportunity to evaluate children with myelomeningocele who did not develop hydrocephalus, who were uncommon prior to prenatal surgery but are representative of a group that is becoming larger as more centers adopt prenatal surgical interventions. In a study before the advent of prenatal surgery,9 only 4/24 children with various types of spina bifida and without hydrocephalus had myelomeningocele, with this group largely representing closed lesions such as meningocele and lipoma; a group with ventricular dilation but no shunt consisted almost entirely of children with myelomeningocele (15/18). In the MOMS study, there was also a group of children with myelomeningocele who did not receive a shunt because ventricular dilation did not meet the pre-established criteria or because they met the established criteria but the treating neurosurgeon and family opted not to proceed with a shunt. This group may mimic the current neurosurgical practices at some centers that monitor hydrocephalus before shunt implantation.10
In the MOMS study, specific criteria were used to determine whether children met the threshold to receive a CSF shunt (Table 1). At 12 months of age, most children in the postnatal surgery group (98%) met the criteria for shunting, compared with 73% of the children in the prenatal group.6 However, the actual rates of shunting were 84% and 44% in the postnatal and prenatal groups, respectively. Thus, about 20% of the study cohort met the criteria for shunting but did not receive a CSF shunt. In additional analyses based on the 12-month MRI findings, 25% of the prenatal group had evidence of ventricular dilation and met the predetermined criteria, with another 27% not meeting the criteria for hydrocephalus.6
TABLE 1.
MOMS criteria for the presence of hydrocephalus indicating need for shunt
|
Criterion 1 At least 2 of the following: |
An increase in the greatest occipital-frontal circumference adjusted for gestational age defined as crossing percentiles (if a plateau was reached this did not qualify) |
| Bulging fontanelle or split sutures or sunsetting sign | |
| Increasing hydrocephalus on consecutive imaging studies determined by increase in ratio of biventricular diameter to biparietal diameter according to the method of O’Hayon et al.34
| |
| Head circumference > 95 percentile for gestational age; or
| |
|
Criterion 2
|
Presence of marked syringomyelia with ventriculomegaly; or
|
|
Criterion 3
|
Ventriculomegaly with symptoms of Chiari malformation (stridor, swallowing difficulties, apnea, bradycardia); or
|
| Criterion 4 | Persistent CSF leakage from the myelomeningocele wound or bulging at the repair site |
From The New England Journal of Medicine, Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele, Volume 364, pp 993-1004. © 2011 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Using data from 30-month visits, Houtrow et al.11 divided the 171 children from the MOMS trial who survived and were not lost to follow-up into three groups on the basis of the MOMS criteria for hydrocephalus (Table 1) and their actual shunt status at 12 months of age: no hydrocephalus, unshunted hydrocephalus, and shunted hydrocephalus. There were no significant differences among the three groups in terms of cognitive and language measures. However, additional analyses of the children who had unshunted or shunted hydrocephalus revealed that children with more severe hydrocephalus, defined by the number of hydrocephalus criteria met, performed more poorly on language and motor measures.11
The present analysis followed the procedures used by Houtrow et al.11 and grouped school-age children on the basis of the same criteria (Table 1), with the primary analysis comparing the three groups (no hydrocephalus, unshunted hydrocephalus, and shunted hydrocephalus) regardless of initial randomization. A secondary analysis compared outcomes according to hydrocephalus status in only the prenatal repair group to clarify the influence of hydrocephalus status on the overall 5–10 year follow-up results of the MOMS2 study. All comparisons used the neurodevelopmental measures from the school-age follow-up8 that are sensitive to the long-term effects of SBM and hydrocephalus status.9,12,13
Methods
The MOMS2 follow-up was conducted at the three centers that participated in the original MOMS trial (Children’s Hospital of Philadelphia, Vanderbilt University, and the University of California, San Francisco), along with the independent data-coordinating center at the George Washington University Biostatistics Center and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Institutional Review Board approval was obtained at each clinical site and the data-coordinating center. Caregivers gave written informed consent and children gave assent per local regulations.
Study Procedures
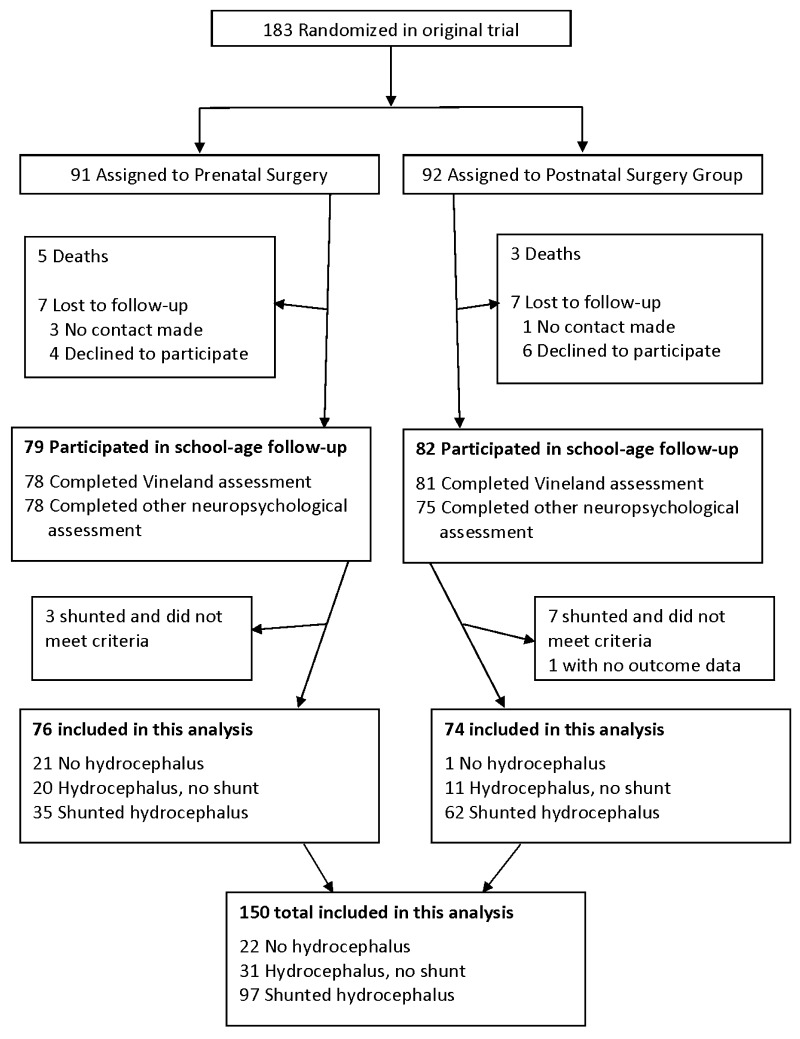
Families who participated in the original MOMS clinical trial were offered the opportunity to participate in the MOMS2 school-age follow-up study, with data available for 161 of the 183 original MOMS participants (Fig. 1).8 There were some missing data on specific measures due to examiner error, lack of cooperation, or inability to understand directions for some tasks.
FIG. 1.
Enrollment flowchart.
For this study, we excluded 4 participants who received a CSF shunt despite not meeting the study criteria and 6 participants who underwent shunt implantation after the MOMS study was completed at 30 months, thereby leaving a total sample of 151 children. These participants were excluded because we wanted to assess children who met predetermined criteria for shunting (thus excluding the 4 cases) and because the 6 participants who subsequently underwent shunt implantation had symptomatic hydrocephalus and did not clearly fit into the groups. Of these 151 children, 1 child was missing Vineland Adaptive Behavior Scales data and all neuropsychological measures, leaving a final cohort of 150 children for these secondary analyses (76 in the prenatal group and 74 in the postnatal surgery group). To create the groups for the primary analysis, we combined children across the prenatal and postnatal groups into groups categorized as no hydrocephalus (n = 22), hydrocephalus and no shunt (i.e., unshunted hydrocephalus) (n = 31), and shunted hydrocephalus (n = 97). Four children who underwent third ventricle ventriculostomy were placed in the shunted group. Within the shunted group, 59% of children required a shunt revision. Comparing these patients with those who underwent no revisions and 1–6 revisions revealed no significant differences in neurodevelopmental outcomes (p > 0.05). There was a trend for the group that received a shunt revision to score lower than the shunted group. Fisher’s exact test revealed no significant difference in the frequencies of shunt revisions between the prenatal (46%) and postnatal (65%) surgery groups (p = 0.089).
Because the MOMS2 follow-up study took place over several years, older children were evaluated first. We tried to evaluate all children when they were 6 years or older, but we had to evaluate some just before 6 years (youngest was 5 years 9 months of age). Participation consisted of a single comprehensive study visit at one of the clinical centers or at home for a partial evaluation (neuropsychological and physical evaluations) if the parent/caregiver did not wish to travel. Although the center visit was comprehensive and included urological, physical, and radiological evaluations over a 2-day period, the focus of this paper is the neurodevelopmental evaluation completed by study-designated psychologists at the clinical center or at home. The psychologist also completed the Vineland Adaptive Behavior Scales semi-structured interview with the parent/caregiver at the center or by telephone for home visits. For quality control, all psychologists were trained centrally and certified by submitting videotapes to the central site. Videotapes of study participants were randomly solicited and reviewed to maintain certification. All psychologists were blinded to the treatment group, and parents/caregivers were instructed not to identify the type of surgery.
Neurodevelopmental Measures
The primary outcome was the composite score from the Vineland Adaptive Behavior Scales, Second Edition, interview schedule.14 Adaptive behavior represents habitual performance of activities needed for communication, socialization, and daily living in the everyday environment. Secondary neuropsychological outcomes included the composite IQ score from the Kaufman Brief Intelligence Test, Second Edition (KBIT-2);15 Woodcock-Johnson Tests of Achievement, Third Edition (WJ-III), reading and math subtests;16 California Verbal Learning Test for Children (CVLT-C), a multi-trial list verbal learning and memory test;17 Children’s Memory Scale (CMS) nonverbal memory subtests (dot learning and faces);18 Beery-Buktenica Developmental Test of Visual-Motor Integration;19 Purdue Pegboard Test, a measure of fine motor dexterity;20 and a word generation test.21 To evaluate sensorimotor and lower-limb improvements, items from the Functional Rehabilitation Evaluation of Sensori-Neurological Outcomes (FRESNO) scale were included.22,23 These items evaluated the amount of assistance needed by the child to complete motor activities related to daily living and advanced motor skills. Parents completed rating scales for attention-deficit/hyperactivity disorder (ADHD) behavior (Swanson, Nolan and Pelham–IV [SNAP-IV] rating scale)24 and the Behavior Rating Inventory of Executive Function (BRIEF) to assess executive functions.25
Statistical Analysis
For the Vineland, neuropsychological, and the BRIEF evaluations, we calculated mean ± SD according to study group by using the norm-referenced scores. For the Purdue Pegboard test, the number of pegs with the preferred hand, nonpreferred hand, and pairs of pegs with both hands were separately converted into z-scores by using normative data and the average z-score of the three conditions reported. The FRESNO score was the percentage of the maximum possible score, while the SNAP score was the raw score averages for the inattention and hyperactivity-impulsivity items. Lower scores indicated fewer problems in the rated domains.
Each outcome variable was separately analyzed. We initially compared the two groups with no hydrocephalus and unshunted hydrocephalus in terms of neurodevelopmental outcomes to determine whether these groups could be combined. These comparisons yielded no significant results. In addition, to evaluate whether prenatal surgery had an effect on neurodevelopmental outcomes even if shunting was required, we compared the prenatal and postnatal groups with the shunted hydrocephalus groups. There were no significant differences except in terms of FRESNO scores, so the shunted groups were combined, thereby leaving two groups for comparison (no/unshunted vs shunted hydrocephalus). In the subsequent analysis, we maintained randomization and compared the prenatal groups with the no hydrocephalus, unshunted hydrocephalus, and shunted hydrocephalus. Nominal two-sided p values less than 0.05 were considered to indicate statistical significance; no adjustments were made for multiple comparisons because it was more important in this secondary analysis to detect a potentially significant difference than to miss a difference (i.e., more protection for type II errors than type I errors).
Results
Demographic and Clinical Characteristics
The group with no hydrocephalus was less likely to be female and had lower-level spinal lesions, lower frequency of callosal hypogenesis, less cerebellar herniation, and smaller ventricles than the other unshunted and shunted hydrocephalus groups (Table 2). There were no significant differences in age, ethnicity (White vs other), or gestational age at birth.
TABLE 2.
Demographic and baseline clinical characteristics (n = 150)
| Characteristic | No Hydrocephalus (n = 22) | Unshunted Hydrocephalus (n = 31) | Shunted Hydrocephalus (n = 97) |
|---|---|---|---|
| Age at MOMS2 visit*
|
8,3 ± 1.2 |
7,6 ± 1.1 |
7,8 ± 1.3 |
| Female sex†
|
6 (27) |
20 (65) |
54 (56) |
| Gestational age at birth, wks |
35.1 ± 2.8 |
35.5 ± 2.3 |
36.2 ± 2.2 |
| Gestational age at surgery‡§
|
|
|
|
| ≤23 wks |
10 (48) |
14 (70) |
7 (21) |
| ≥24 wks |
11 (52) |
6 (30) |
27 (79) |
| Child race or ethnic group¶
|
|
|
|
| White non-Hispanic |
21 (95) |
29 (94) |
84 (87) |
| Black non-Hispanic |
0 (0) |
0 (0) |
1 (1) |
| Hispanic |
0 (0) |
0 (0) |
5 (5) |
| Other/not reported |
1 (5) |
2 (6) |
7 (7) |
| Anatomic lesion level†
|
|
|
|
| Thoracic |
0 (0) |
1 (3) |
1 (1) |
| L1–2 |
0 (0) |
5 (16) |
17 (18) |
| L3–4 |
12 (55) |
15 (48) |
50 (52) |
| L5–S2 |
10 (45) |
10 (33) |
29 (30) |
| Cerebellar herniation level at 12 mos‡**
|
9 (41) |
19 (62) |
79 (83) |
| Corpus callosum dysgenesis at 12 mos††
|
4 (18)†
|
19 (63)‡
|
69 (72)‡
|
| Ventricle size on prenatal ultrasound, mm†
|
|
|
|
| <10 |
16 (73) |
10 (32) |
29 (30) |
| 10–15 |
6 (27) |
17 (55) |
50 (52) |
| ≥15 | 0 | 4 (13) | 18 (19) |
Data are presented as mean ± SD or number (%) unless indicated otherwise.
Age is shown as mean (in years, months) ± SD (years).
The proportion of patients with no hydrocephalus was signficiantly less than the proportion with unshunted hydrocephalus, which was equal to the proportion with shunted hydrocephalus (p < 0.05).
The proportion of patients with no hydrocephalus was equal to the proportion with unshunted hydrocephalus and less than the proportion with shunted hydrocephalus (p < 0.05).
The postnatal group was set to missing.
Race or ethnic group was self-reported. The statistical comparison was between White and all other groups.
C1, C2, or below C2 vs normal.
Yes vs no.
Neurodevelopmental Outcomes
Hydrocephalus Groups
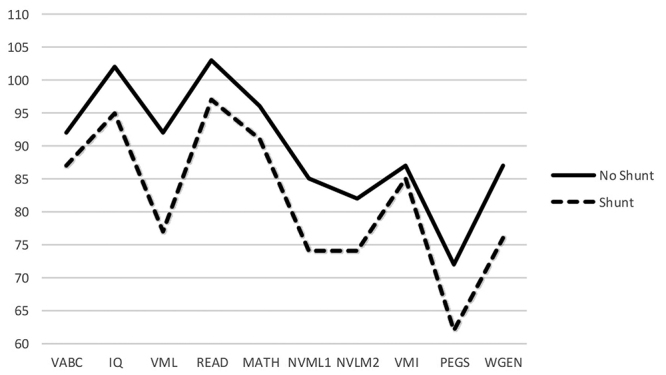
There was a consistent pattern showing higher performance for the combined no/unshunted hydrocephalus group compared with that of the shunted hydrocephalus group (Table 3, Fig. 2). These differences were statistically significant for the Vineland composite score (p = 0.002). On the neuropsychological tests, the group with no/unshunted hydrocephalus, compared with the shunted group, had higher IQ scores (p = 0.01), stronger verbal learning and memory (p = 0.001) and nonverbal learning and memory (p = 0.031), better reading scores (p = 0.026), better fine motor coordination (p = 0.006), and better ability to generate words to a category (p = 0.028). On the SNAP-IV ADHD scale, the combined no/unshunted group had lower inattention scores (less inattention) than the shunted group (p = 0.021), but the difference on the hyperactivity-impulsivity scale was not significant. FRESNO scores showed better sensorimotor skills and walking in the combined no/unshunted hydrocephalus group than the shunted group (p = 0.001).
TABLE 3.
Mean ± SD values and results of significance tests for neurodevelopmental outcomes by hydrocephalus groups (combined prenatal and postnatal groups)
| Characteristic | No Hydrocephalus (n = 22) |
Unshunted Hydrocephalus (n = 31) |
p Value (No vs Unshunted Hydrocephalus) | Shunted Hydrocephalus (n = 97) |
p Value (Shunted vs No/Unshunted) | |||
|---|---|---|---|---|---|---|---|---|
| NT | Value | NT | Value | NT | Value | |||
| Vineland Adaptive Behavior Scales II*
|
22 |
92.4 ± 10.6 |
31 |
92.2 ± 9.8 |
0.918 |
96 |
86.7 ± 10.8 |
0.002 |
| Communication |
22 |
95.9 ± 12.1 |
31 |
100.8 ± 13.1 |
|
96 |
91.2 ± 13.4 |
|
| Daily living |
22 |
92.0 ± 11.8 |
31 |
89.1 ± 9.5 |
|
96 |
84.2 ± 11.7 |
|
| Socialization |
22 |
97.5 ± 12.9 |
31 |
98.7 ± 10.9 |
|
96 |
95.3 ± 11.6 |
|
| KBIT-2*
|
|
|
|
|
|
|
|
|
| Composite score |
22 |
100.5 ± 15.4 |
30 |
103.5 ± 15.5 |
0.494 |
93 |
94.6 ± 17.7 |
0.010 |
| Verbal learning |
22 |
102.2 ± 14.5 |
30 |
105.8 ± 14.3 |
|
93 |
98.7 ± 16.9 |
|
| Nonverbal reasoning |
22 |
98.3 ± 15.7 |
30 |
99.9 ± 16.3 |
|
93 |
91.3 ± 17.7 |
|
| CVCLT-2 (trials 1–5)†
|
22 |
45.1 ± 9.5 |
30 |
46.3 ± 10.5 |
0.511 |
87 |
38.8 ± 9.7 |
<0.001 |
| WJ-III*
|
|
|
|
|
|
|
|
|
| Reading composite‡
|
22 |
100.8 ± 14.8 |
30 |
105.3 ± 17.3 |
0.333 |
92 |
97.1 ± 16 |
0.026 |
| Math calculations |
22 |
92.6 ± 21.3 |
30 |
98.1 ± 18.6 |
0.330 |
90 |
90.6 ± 21.6 |
0.161 |
| CMS§
|
|
|
|
|
|
|
|
|
| Dot learning |
22 |
8.6 ± 3 |
30 |
8.5 ± 2.8 |
0.912 |
90 |
7.4 ± 2.9 |
0.031 |
| Faces immediate |
22 |
7.5 ± 2.6 |
30 |
8.6 ± 3.3 |
0.223 |
80 |
7.4 ± 3.4 |
0.190 |
| Beery Visual-Motor Integration*
|
22 |
83.8 ± 12.1 |
30 |
89.3 ± 7.4 |
0.068 |
92 |
84.8 ± 11 |
0.254 |
| Purdue Pegboard total pegs¶
|
22 |
−1.8 ± 1.2 |
30 |
−1.9 ± 0.9 |
0.852 |
91 |
−2.6 ± 1.3 |
<0.001 |
| NEPSY-II word generation§
|
22 |
8.6 ± 2.3 |
30 |
8.8 ± 2.6 |
0.721 |
92 |
7.6 ± 2.9 |
0.028 |
| SNAP—inattention**
|
22 |
1.0 ± 0.7 |
30 |
0.8 ± 0.5 |
0.328 |
93 |
1.2 ± 0.6 |
0.021 |
| SNAP—hyperactivity/ impulsivity**
|
22 |
0.5 ± 0.5 |
30 |
0.5 ± 0.4 |
0.771 |
93 |
0.6 ± 0.5 |
0.102 |
| BRIEF—global executive composite†
|
20 |
51.7 ± 11.6 |
30 |
51.4 ± 9.0 |
0.591 |
93 |
54.2 ± 10.9 |
0.169 |
| FRESNO percentage | 22 | 94.1 ± 10.0 | 30 | 93.8 ± 7.1 | 0.086 | 91 | 86.4 ± 14.8 | 0.001 |
NEPSY = A Developmental Neuropsychological Assessment; NT = number tested.
Values are shown as number or mean ± SD unless indicated otherwise.
Normative mean ± SD 100 ± 15.
Normative mean ± SD 50 ± 10.
Average of the Letter Word Identification and Passage Comprehension tests.
Normative mean ± SD 10 ± 3.
z-score average.
Raw score.
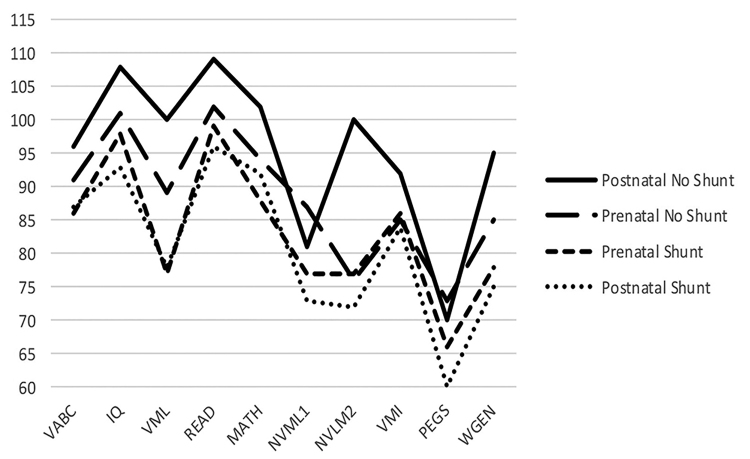
FIG. 2.
Plot of the mean values for the unshunted (no hydrocephalus/unshunted hydrocephalus) and shunted hydrocephalus groups, showing neurodevelopmental outcomes. All scores were standardized to a common normative mean ± SD 100 ± 15. IQ = Kaufman Brief Intelligence Test composite; MATH = Woodcock-Johnson math computations; NVML1 = Children’s Memory Test Dot Learning; NVML2 = Children’s Memory Test Faces; PEGS = Purdue Pegboard; READ = Woodcock-Johnson reading composite; VABC = Vineland Adaptive Behavior Scales composite; VMI = Beery Visual-Motor Integration; VML = California Verbal Learning Test; WGEN = word generation.
Prenatal Group
To help understand these results relative to the original randomization into surgical groups, the prenatal group was divided into no hydrocephalus (n = 21), unshunted hydrocephalus (n = 20), and shunted hydrocephalus groups (n = 35) (Table 4). There were no significant differences between the prenatal no hydrocephalus and unshunted hydrocephalus groups, so these groups were combined. Statistical comparisons within the prenatal group between no/unshunted hydrocephalus patients and those with a shunt showed significantly better Vineland composite (p = 0.035) and verbal learning (p = 0.012) test scores among those with no/unshunted hydrocephalus.
TABLE 4.
Means ± SD values and results of significance tests for neurodevelopmental outcomes by hydrocephalus groups (prenatal group only)
| Assessment | No Hydrocephalus (n = 21) |
Unshunted Hydrocephalus (n = 20) |
p Value (No vs Unshunted Hydrocephalus) | Shunted Hydrocephalus (n = 35) |
p Value (Shunted vs No/Unshunted Hydrocephalus) | |||
|---|---|---|---|---|---|---|---|---|
| NT | Value | NT | Value | NT | Value | |||
| Vineland Adaptive Behavior Scales II*
|
21 |
92.5 ± 10.9 |
20 |
89.7 ± 9 |
0.379 |
34 |
86.4 ± 8.9 |
0.035 |
| Communication |
21 |
95.9 ± 12.4 |
20 |
98.4 ± 12.3 |
|
34 |
91.4 ± 11.8 |
|
| Daily living |
21 |
92.4 ± 11.9 |
20 |
88 ± 9.5 |
|
34 |
84.4 ± 10.4 |
|
| Socialization |
21 |
97.3 ± 13.2 |
20 |
96.7 ± 9.1 |
|
34 |
94.4 ± 10.4 |
|
| KBIT-2*
|
|
|
|
|
|
|
|
|
| Composite score |
21 |
99.9 ± 15.5 |
19 |
101.5 ± 15.2 |
0.749 |
35 |
97.8 ± 17.3 |
|
| Verbal learning |
21 |
101.7 ± 14.7 |
19 |
104.1 ± 13.4 |
|
35 |
100.3 ± 16.3 |
|
| Nonverbal reasoning |
21 |
97.7 ± 15.8 |
19 |
98 ± 16.6 |
|
35 |
95.0 ± 19 |
|
| CVCLT-2 (trials 1–5)†
|
21 |
44.6 ± 9.5 |
19 |
44.4 ± 11.7 |
|
34 |
38.3 ± 10.1 |
0.012 |
| WJ-III*
|
|
|
|
|
|
|
|
|
| Reading composite‡
|
21 |
100.7 ± 15.1 |
19 |
103.2 ± 17.6 |
0.633 |
35 |
99.1 ± 20.5 |
|
| Math calculations |
21 |
92.6 ± 21.9 |
19 |
95.5 ± 20.9 |
0.676 |
35 |
88.7 ± 25.6 |
|
| CMS§
|
|
|
|
|
|
|
|
|
| Dot learning |
21 |
8.6 ± 3.1 |
19 |
8.8 ± 2.9 |
0.818 |
35 |
7.7 ± 3 |
|
| Faces immediate |
21 |
7.7 ± 2.5 |
19 |
7.5 ± 2.7 |
0.774 |
30 |
7.7 ± 2.7 |
|
| Beery Visual-Motor Integration*
|
21 |
83.4 ± 12.3 |
19 |
87.7 ± 6.8 |
0.174 |
35 |
86.2 ± 11.9 |
|
| Purdue Pegboard total pegs¶
|
21 |
−1.8 ± 1.2 |
19 |
−1.8 ± 1.1 |
0.957 |
35 |
−2.3 ± 1.3 |
|
| NEPSY-II word generation§
|
21 |
8.4 ± 2.2 |
19 |
8.6 ± 2.9 |
0.807 |
34 |
7.8 ± 2.9 |
|
| SNAP—inattention**
|
21 |
1.0 ± 0.6 |
19 |
1.0 ± 0.6 |
0.765 |
35 |
1.3 ± 0.7 |
|
| SNAP—hyperactivity/impulsivity**
|
21 |
0.5 ± 0.5 |
19 |
0.6 ± 0.4 |
0.731 |
35 |
0.7 ± 0.5 |
|
| BRIEF—global executive composite†
|
19 |
52.0 ± 11.8 |
19 |
53.6 ± 8.8 |
0.245 |
34 |
56.7 ± 10.6 |
|
| FRESNO percentage | 21 | 94.3 ± 10.2 | 19 | 92.9 ± 7.7 | 0.635 | 33 | 90.2 ± 9.4 | |
Normative mean ± SD 100 ± 15.
Normative mean ± SD 50 ± 10.
Average of the Letter Word Identification and Passage Comprehension tests.
Normative mean ± SD 10 ± 3.
z-score average.
Raw score.
These two prenatal groups and the shunted (n = 64) and unshunted (n = 12) postnatal groups were compared in Fig. 3. There was a consistent trend for the postnatal unshunted group to score higher than the other groups, but we did not test for differences because the sample size of the unshunted postnatal group was small and highly selected and any comparison was not motivated by a hypothesis. The mean values of the two shunted groups (prenatal shunted and postnatal shunted) paralleled one another even more closely than shown in Table 3 and Fig. 2, reflecting the influence of the higher scores of the postnatal unshunted group.
FIG. 3.
Plot of the mean values for the unshunted and shunted prenatal and postnatal hydrocephalus groups for neurodevelopmental outcomes. All scores were standardized to a common normative mean ± SD 100 ± 15.
Analysis of lateral ventricle volumes based on MRI measurements demonstrated that the prenatal and postnatal surgery groups with unshunted hydrocephalus had significantly larger ventricles (p < 0.001) than the shunted groups (Fig. 4) and the prenatal group with no hydrocephalus. This finding supports the hydrocephalus grouping.
FIG. 4.
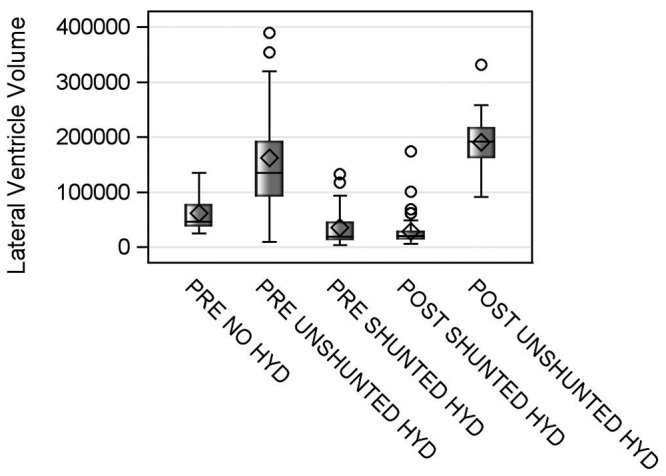
Lateral ventricle volumes for the prenatal and postnatal groups with no hydrocephalus, unshunted hydrocephalus, and shunted hydrocephalus (HYD). The median is represented by the straight horizontal line, and the mean is represented by the diamond (both located within the rectangle). The whiskers around the mean indicate variability outside the upper and lower quartiles (based on Tukey’s fencing rule for outlier detection). The bubbles represent univariate outliers (i.e., children with very enlarged ventricles).
Discussion
This secondary analysis permitted a more nuanced interpretation of the results of the MOMS trial in relation to the neurodevelopmental outcomes assessed at school age. In contrast to the largely null results for adaptive behavior and cognitive functions based on the original randomization into the prenatal and postnatal repair groups,8 the outcomes of the children with no hydrocephalus and unshunted hydrocephalus (analyzed as a single group because of the null results in the statistical comparisons) showed stronger adaptive behavior (the primary outcome) and cognitive skills compared with the children in the combined prenatal and postnatal groups who required a CSF diversionary procedure. In addition, the results show that in comparison with the outcomes of a traditionally treated postnatal group,9,13 neurobehavioral outcomes were generally higher in the unshunted groups. Because children with myelomeningocele who did not receive a CSF shunt were infrequently studied prior to the advent of prenatal surgery, these comparisons show that outcomes are better if the child does not meet the clinical criteria or judgment for shunting. It will be important to continue to study outcomes in this unique group of children with myelomeningocele.
In children with myelomeningocele who develop hydrocephalus, the mechanism is widely understood to be the development of the Chiari II malformation, which obstructs the flow of CSF and necessitates a diversionary procedure.26–28 In the McLone-Knepper model,26 Chiari II malformation results from an open neural tube that leads to a spectrum of embryological events that then lead to disturbances of ventricle growth, reduction in the size of the posterior fossa, and exposure of the fetus to CSF. Prenatal surgery is designed to prevent these events and to prevent or reduce the impact of Chiari II malformation.1 This should result in reduced hindbrain herniation and the spectrum of anomalies associated with Chiari II malformation. Reductions in the incidence and severity of hydrocephalus, and therefore in the need for shunting, should also stem from prenatal surgery.27,28
From the original school-age follow-up, it could not be concluded that prenatal surgery per se resulted in improved adaptive behavior and cognition.6 The present analyses show that hydrocephalus and shunting were associated with poorer adaptive behavior and cognition in both the prenatal and postnatal groups. However, because prenatal surgery reduced the likelihood and severity of hindbrain herniation and Chiari II malformation, there was an indirect effect on adaptive behavior and cognition. The prenatal group with no/unshunted hydrocephalus had stronger adaptive behavior and verbal learning and memory compared with the shunted prenatal group (Table 4).
Stronger adaptive behavior is most likely related to better semantic language and reading, as well as daily living skills, in the unshunted groups. Although language and reading are typical strengths associated with spina bifida,12 better daily living skills are likely related to the stronger gross and fine motor skills associated with prenatal surgery in general.8 The results were more pronounced in the unshunted prenatal groups relative to both shunted groups, suggesting better ambulation as well as practical skills involving buttoning, zipping, and use of utensils. This improvement in motor ability did not extend to perceptual motor skills, as evidenced by similar low average performance across groups on the Beery test.
In addition to adaptive behavior, the largest differences favoring the no/unshunted group were the results on the fine motor skills and the verbal learning tests. The primary paper reported that fine motor dexterity (Purdue Pegboard) was greater in the prenatal surgery group.6 In the present secondary analysis, fine motor dexterity was stronger in the no/unshunted group. We hypothesize that stronger fine motor dexterity was related to reduced cerebellar impairment secondary to reduced hindbrain herniation.
Studies of verbal learning typically show significant impairment in patients with myelomeningocele and hydrocephalus.8,9,29,30 The spectrum of abnormalities associated with hydrocephalus often leads to displacement and dysmorphology of the hippocampi, which are key structures for verbal and nonverbal learning and memory.30,31 This process may have been reduced by prenatal surgery because of the reduction of hindbrain herniation. Chiari II malformation may extend to the midbrain, a critical area for the control of involuntary attention (arousal and alerting). In large studies of children with myelomeningocele, most of whom underwent shunt implantation, about one-third met the rating scale criteria on SNAP for ADHD (predominantly inattentive type).13 The lower scores on the inattention scale of the unshunted group are consistent with the beneficial effects on the posterior attention system, which is impaired in many patients with myelomeningocele and shunted hydrocephalus.31,32 The absence of differences on the hyperactivity-impulsivity scale is also consistent with these prior studies.13
It would be inappropriate to attribute the current results to the presence of shunting. The improved outcomes associated with the absence of a shunt despite enlarged ventricles do seem to support the practice of monitoring hydrocephalus prior to shunt insertion at some centers.10 An assessment from the National Spina Bifida Patient Registry showed that about 80% of children with myelomeningocele across 28 centers identified between 2009 and 2016 underwent shunt implantation or other diversionary procedure in the 1st year of life.2,33 However, it is unclear to what extent ventriculomegaly should be permitted before shunting.34 In the present cohort, about 48% of the prenatal surgery sample received a shunt. Prenatal surgery reduced hindbrain herniation in all prenatal groups, although the reduction tended to be greater in the no hydrocephalus group than the unshunted hydrocephalus group. In general, the group with no hydrocephalus was at lower risk for adverse neurobehavioral outcomes. At prenatal MRI screening, this group had no cases with ventricular size > 15 mm and most were less than 10 mm, with no cases involving spinal lesion levels above L3. On MRI evaluations of school-age children, ventricular volumes were greatest in the unshunted groups with hydrocephalus. Despite larger ventricle volumes, the prenatal and postnatal groups with unshunted hydrocephalus had similar outcomes as the prenatal hydrocephalus group. These data do not provide information on how or when the decision to implant a shunt or not was made, which was left to the clinicians caring for the child.
The subgroup with no hydrocephalus (all but 1 case was in the prenatal group) also had fewer cases of callosal hypogenesis at 12 months than any other group. This finding is important because it may indicate less injury from the primary deficit in neurulation. Previous studies found that over half of traditionally treated children with myelomeningocele had callosal hypogenesis,13 consistent with the proportion (66%) reported in Table 2. The corpus callosum develops in the first 20 weeks of gestation in a rostral to caudal direction beginning with the genu; the most anterior portion of the corpus callosum (rostrum) develops around 18–20 weeks. This sequence implies prolonged disruption of neural development in spina bifida myelomeningocele.2 The absence of callosal hypogenesis and the lower lesion levels may indicate less disruption of neurulation and could be a predictor of outcomes associated with prenatal surgery. The overall picture is that it is not shunting per se that is related to outcomes, but the severity of the initial disease manifested by ventricular dilation, cerebellar herniation, and callosal hypogenesis. The need for a CSF shunt may reflect the greater severity of disease in those with a shunt.
Limitations
The limitations of this study include the small sample sizes for the hydrocephalus subgroups and the decision not to aggressively control for type I errors in the secondary analysis. We felt that it was more important to not miss a clinically important difference given the small sample sizes. As such, the results should be seen through a hypothesis-generating lens.
The sample of the MOMS study was highly selected and more stringent than the criteria of many current clinical centers, so the results may not generalize to other centers, especially if participants have higher-level lesions and identify as other ethnicities for whom spina bifida is prevalent and are subject to varying genetic and environmental contributors (e.g., individuals of Hispanic origin).
There are important domains that were not formally tested because of the need for a short assessment battery. In particular, attention and executive functions were not assessed with performance tasks.12 These domains generally require experimental tasks that would be difficult to implement in a multicenter study but would be fruitful for future studies. More assessments of the risk factors, especially from prenatal data, that may predict neurobehavioral outcomes are important for future studies.
There was a subgroup of children with hydrocephalus who did not undergo CSF diversion despite meeting predetermined clinical criteria for shunting. Patients with unshunted hydrocephalus met predefined criteria for hydrocephalus and some met criteria for shunting, but we recognize that there may have been overlap with varying degrees of ventriculomegaly. There is no information on the nature of the decision-making process between clinicians and parents, or the decision to use third ventricle ventriculostomy instead of a shunt. However, there is also no evidence of adverse cognitive outcomes associated with these decisions.
Conclusions
The primary assessment of school-age outcomes comparing the prenatal and postnatal groups showed no significant differences between the prenatal and postnatal groups in terms of the primary outcome, adaptive behavior, and no robust evidence of stronger cognitive functions. However, there were better sensorimotor, fine motor, and orthopedic outcomes in the prenatal surgery group. This secondary analysis shows that hydrocephalus and shunting were associated with poorer adaptive behavior and cognitive skills in the shunted groups (both prenatal and postnatal). However, it is unlikely that shunting per se explains the better outcomes. Rather, disease severity and dynamic changes in hydrocephalus status seem to be the primary factor in the development of hydrocephalus and the need for shunting,6 which are the major determinants of adaptive behavior and cognitive outcomes after prenatal surgery.
Acknowledgments
We acknowledge the assistance of Paulina Kulesz, PhD, and Pamela K. Burrows. Dr. Houtrow receives study-related clinical or research support from NIH. This study was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (no. U01 HD06854: follow-up of the Management of Myelomeningocele Study). Dr. Elizabeth A. Thom died on December 8, 2021.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Fletcher, Houtrow, MacPherson, Thomas, Adzick, Thom. Acquisition of data: Fletcher, Houtrow, Thomas, Adzick, Thom. Analysis and interpretation of data: Fletcher, Houtrow, MacPherson, Gupta, Adzick, Thom. Drafting the article: Fletcher, Houtrow, MacPherson, Thomas, Gupta. Critically revising the article: Fletcher, Houtrow, MacPherson, Thomas, Gupta, Adzick. Reviewed submitted version of manuscript: Fletcher, Houtrow, MacPherson, Thomas, Gupta, Adzick. Approved the final version of the manuscript on behalf of all authors: Fletcher. Statistical analysis: MacPherson, Thom.
References
- 1. Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nat Rev Dis Primers. 2015;1:15007. doi: 10.1038/nrdp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barkovich AJ. Analyzing the corpus callosum. AJNR Am J Neuroradiol. 1996;17(9):1643–1645. [PMC free article] [PubMed] [Google Scholar]
- 3. Dewan MC, Wellons JC. Fetal surgery for spina bifida. J Neurosurg Pediatr. 2019;24(2):105–114. doi: 10.3171/2019.4.PEDS18383. [DOI] [PubMed] [Google Scholar]
- 4. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meuli M, Meuli-Simmen C, Hutchins GM, et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med. 1995;1(4):342–347. doi: 10.1038/nm0495-342. [DOI] [PubMed] [Google Scholar]
- 6. Tulipan N, Wellons JC, III, Thom EA, et al. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J Neurosurg Pediatr. 2015;16(6):613–620. doi: 10.3171/2015.7.PEDS15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farmer DL, Thom EA, Brock JW, III, et al. The Management of Myelomeningocele Study: full cohort 30-month pediatric outcomes. Am J Obstet Gynecol. 2018;218(2):256.e1–256.e13. doi: 10.1016/j.ajog.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Houtrow AJ, Thom EA, Fletcher JM, et al. Prenatal repair of myelomeningocele and school-age functional outcomes. Pediatrics. 2020;145(2):e20191544. doi: 10.1542/peds.2019-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hampton LE, Fletcher JM, Cirino PT, et al. Hydrocephalus status in spina bifida: an evaluation of variations in neuropsychological outcomes. J Neurosurg Pediatr. 2011;8(3):289–298. doi: 10.3171/2011.6.PEDS10584. [DOI] [PubMed] [Google Scholar]
- 10. Bowman RM, McLone DG. Neurosurgical management of spina bifida: research issues. Dev Disabil Res Rev. 2010;16(1):82–87. doi: 10.1002/ddrr.100. [DOI] [PubMed] [Google Scholar]
- 11. Houtrow AJ, Burrows PK, Thom EA. Comparing neurodevelopmental outcomes at 30 months by presence of hydrocephalus and shunt status among children enrolled in the MOMS trial. J Pediatr Rehabil Med. 2018;11(4):227–235. doi: 10.3233/PRM-170481. [DOI] [PubMed] [Google Scholar]
- 12. Dennis M, Barnes MA. The cognitive phenotype of spina bifida meningomyelocele. Dev Disabil Res Rev. 2010;16(1):31–39. doi: 10.1002/ddrr.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fletcher JM, Copeland K, Frederick JA, et al. Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity. J Neurosurg. 2005;102(3 suppl):268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- 14.Sparrow S, Cichetti D, Balla D. AGS Publishing; 2005. Vineland Adaptive Behavior Scales-2. [Google Scholar]
- 15.Kaufman A, Kaufman N. Pearson; 2013. Kaufman Brief Intelligence Test-2. [Google Scholar]
- 16.Woodcock RW, Johnson MB. Riverside Publishing; 1989. Woodcock-Johnson Psycho-Educational Battery—III. [Google Scholar]
- 17.Delis D, Kramer JH, Kaplan E, Ober BA. Psychological Corporation; 1994. California Verbal Learning Test—Children’s Version. [Google Scholar]
- 18.Cohen MJ. Psychological Corporation; 1997. Children’s Memory Scale. Administration Manual. [Google Scholar]
- 19. Beery K, Buktenika NA, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration. 2002 ed. Pearson; [Google Scholar]
- 20. Gardner RA, Broman M. The Purdue Pegboard: normative data on 1334 school children. J Clin Child Psychol. 1979;8(3):156–162. [Google Scholar]
- 21. Korkman M, Kirk U, Kemp S. 2007 doi: 10.1207/S15326942DN2001_2. NEPSY-II. Pearson. [DOI] [PubMed] [Google Scholar]
- 22. Houtrow AJ, MacPherson C, Jackson-Coty J, et al. Prenatal repair and physical functioning among children with myelomeningocele: a secondary analysis of a randomized clinical trial. JAMA Pediatr. 2021;175(4):e205674. doi: 10.1001/jamapediatrics.2020.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roberts SD, Wells RD, Brown IS, et al. The FRESNO: a pediatric functional outcome measurement system. J Rehabil Outcomes Meas. 1999;3:11–99. [Google Scholar]
- 24.Swanson J. SNAP-IV Teacher and Parent Rating Scale. http://www.myadhd.com/snap-iv-6160-18sampl.html Accessed January 27, 2023.
- 25.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Psychological Assessment Resources; 2000. Behavior Rating Inventory of Executive Function. [Google Scholar]
- 26. McLone DG, Dias MS. The Chiari II malformation: cause and impact. Childs Nerv Syst. 2003;19(7-8):540–550. doi: 10.1007/s00381-003-0792-3. [DOI] [PubMed] [Google Scholar]
- 27. McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 1989;15(1):1–12. doi: 10.1159/000120432. [DOI] [PubMed] [Google Scholar]
- 28. Bouchard S, Davey MG, Rintoul NE, Walsh DS, Rorke LB, Adzick NS. Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. J Pediatr Surg. 2003;38(3):451–458. doi: 10.1053/jpsu.2003.50078. [DOI] [PubMed] [Google Scholar]
- 29. Scott MA, Fletcher JM, Brookshire BL, et al. Memory functions in children with early hydrocephalus. Neuropsychology. 1998;12(4):578–589. doi: 10.1037//0894-4105.12.4.578. [DOI] [PubMed] [Google Scholar]
- 30. Yeates KO, Enrile BG, Loss N, Blumenstein E, Delis DC. Verbal learning and memory in children with myelomeningocele. J Pediatr Psychol. 1995;20(6):801–815. doi: 10.1093/jpepsy/20.6.801. [DOI] [PubMed] [Google Scholar]
- 31. Treble-Barna A, Juranek J, Stuebing KK, Cirino PT, Dennis M, Fletcher JM. Prospective and episodic memory in relation to hippocampal volume in adults with spina bifida myelomeningocele. Neuropsychology. 2015;29(1):92–101. doi: 10.1037/neu0000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams VJ, Juranek J, Stuebing K, Cirino PT, Dennis M, Fletcher JM. Examination of frontal and parietal tectocortical attention pathways in spina bifida meningomyelocele using probabilistic diffusion tractography. Brain Connect. 2013;3(5):512–522. doi: 10.1089/brain.2013.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim I, Hopson B, Aban I, et al. Treated hydrocephalus in individuals with myelomeningocele in the National Spina Bifida Patient Registry. J Neurosurg Pediatr. 2018;22(6):646–651. doi: 10.3171/2018.5.PEDS18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29(5):245–249. doi: 10.1159/000028730. [DOI] [PubMed] [Google Scholar]