In Brief
This is the largest published study of tethered cord surgery (TCS) among people with myelomeningocele. Objectives were to describe the temporal incidence of TCS and variance between institutions and test the hypothesis that tethering is related to longitudinal spine growth by comparing the relationship between sex-specific spine growth rates and TCS. The authors found no sex-specific differences in TCS timing. This challenges traditional concepts that tethering is solely related to longitudinal spine growth.
Keywords: spina bifida, myelomeningocele, neural tube defect, tethered spinal cord syndrome, hydrocephalus, Chiari malformation type II, congenital, spine
ABBREVIATIONS : MMC = myelomeningocele, NSBPR = National Spina Bifida Patient Registry, TCR = tethered cord release
Abstract
OBJECTIVE
The aims of this study were to review the National Spina Bifida Patient Registry (NSBPR) data set to study the rates of tethered spinal cord release (TCR) among patients with myelomeningocele and variability between centers, to compare TCR rates between males and females, and to study the relationships between TCR rates and other condition-specific characteristics.
METHODS
The NSBPR registry was queried to identify all patients with myelomeningocele. TCR rates were calculated over time using survival analyses; rates between centers and between males and females were compared. Cox proportional hazards models were constructed to identify relationships between TCR rates and sex, functional lesion level, ambulation status, treated hydrocephalus, and prior Chiari decompression.
RESULTS
Of 6339 patients with information about their operations, 1366 (21.5%) underwent TCR, with significant variability between centers. The majority (75.8%) underwent a single TCR. The annual TCR rate was linear between birth and 13 years (1.8%/year) but declined sharply from 14 to 21 years (0.7%/year). There was no period of time at which the TCR rate accelerated. There were no significant differences in TCR rates between males and females. TCR rate was not related to functional lesion level but was lower among nonambulators compared with community ambulators (p = 0.005) and among those with treated hydrocephalus (HR 0.30, p < 0.001), and higher among those having prior Chiari decompression (HR 1.71, p < 0.001).
CONCLUSIONS
These results extend the results of prior single-institution studies, demonstrate significant treatment variability between institutions, and challenge the traditional concept that tethering is related to spinal cord stretching due to spinal growth.
Spinal cord tethering is a significant complication of myelomeningocele (MMC). Spinal cord untethering procedures are performed in approximately 10%–32% of this population, second in frequency only to operations for hydrocephalus.1–5 Sensorimotor decline; worsening of urological or bowel function; back, leg, or groin pain; and/or progressive orthopedic deformities are all a result of symptomatic spinal cord tethering. The mechanism of deterioration is thought to involve mechanical stretching of the distal spinal cord with continued growth of the spinal column, leading to a shift from oxidative to anaerobic metabolism and metabolic failure.6–8
The Centers for Disease Control and Prevention established the National Spina Bifida Patient Registry (NSBPR) in 2008 to facilitate research and improve clinical care for children and adults with spina bifida.9,10 The NSBPR began in 2009 with 10 participating spina bifida clinics and expanded to include a total of 19 clinics and 35 clinic sites by 2017. The NSBPR collects data longitudinally, using both family/patient reports and medical record review at the time of routine clinic visits. It includes multiple checks on data integrity and quality, as has been reported previously.10 The majority of participants in the NSBPR have MMC. The goal of the present study was to use data collected in the NSBPR to better understand the treatment of spinal cord tethering among individuals with MMC. The NSBPR represents the largest repository of information about individuals with MMC in the world; at the time of this study, there were 35 participating clinics (including both adults and pediatric clinics) involving 6768 individuals.
We used NSBPR registry data to 1) examine the time course for tethered cord release (TCR) among individuals with MMC; 2) establish the variability in the time course for TCR among each of the 35 participating clinics; 3) probe the relationship between functional lesion level, ambulation status, prior treatment for hydrocephalus, and prior treatment for Chiari malformation on the time course for TCR; and 4) examine the influence of sex on age-related rates for TCR, the hypothesis being that if tethering is related to linear spine growth, then girls, who enter puberty on average 2 years prior to boys, would undergo TCR earlier than boys.
Methods
All patients with the primary diagnosis of MMC attending participating clinics for whom consent had been given were eligible to participate in the NSBPR. The details of enrollment have previously been described.11 All data are entered at each clinic by a data registrar at the time of initial enrollment and annually or at each clinic visit thereafter, both by review of medical records and direct interviews with enrollees. Data quality checks are performed monthly. Surgery for initial repair and initial treatment for hydrocephalus are documented at the time of enrollment. TCR and decompression of Chiari II malformation are documented also at the time of enrollment and later if subsequent surgeries are performed after initial registry enrollment. The data for this serial cross-sectional study were collected from patients as they enrolled in the registry and completed annual follow-up visits.
After approval of the data analysis proposal by the NSBPR Committee on Science and Publication and the institutional review board at Penn State College of Medicine, the NSBPR was queried for all patients with a diagnosis of MMC enrolled between January 2009 and December 2017 inclusive. Data for each patient were extracted and collated for analyses. The variables in the data set included age, sex, clinic site, height (including sitting or standing height and/or arm span length), number of TCR operations and age at each TCR, best ambulatory status and functional level prior to the first TCR, and prior treatment of hydrocephalus (shunt and/or endoscopic procedure) and Chiari decompression.
Analyses were performed both for the entire group and for the group who were children (i.e., < 21 years of age). For the entire group, calculated TCR rates were expressed as the number of patients at each site who underwent TCR divided by the total number of patients (those with and those without TCR) at each site. For children < 21 years, the calculated TCR rates were expressed as the number of patients at each site who underwent TCR before the age of 21 years divided by the total number of patients younger than 21 years at that site.
Survival curves were constructed for the entire group, as well as for males and females independently, using time to first TCR as the primary endpoint. Survival curves were also constructed individually for each participating clinic site to examine the degree of variability among sites. The influence of ambulation status, functional level, and prior surgical treatments for hydrocephalus and/or Chiari malformation on age-related TCR rates for the first TCR was examined.
Means, medians, and standard deviations are presented for continuous variables, and frequencies and percentages for categorical variables. For those patients who underwent TCR, the time to the first TCR (in months) was calculated based on surgery date and date of birth. We also conducted a subgroup analysis that included only children younger than 21 years. Within this subgroup analysis, patients who experienced their first TCR at ≥ 21 years of age (i.e., as adults) were treated as censoring and the censoring time as 21 years, and those who never underwent TCR were also treated as censoring with the time to the last visit date or 21 years, whichever was earlier, considered the censoring time. Kaplan-Meier curves stratified by factors (e.g., facility) were plotted for the time to the first TCR with log-rank tests for stratified group comparison of survival curves. Variability between clinics was calculated after adjustment for mean clinic age (since clinics having older patient populations have higher TCR rates). In the first analysis, the percentage of TCR at any age was calculated as the number of patients at each site with TCR at the time of last clinic follow-up divided by the total number of clinic patients (those with and those without TCR). In the second analysis, the percentage of TCR in childhood (< 21 years) was calculated as the number of patients with TCR before the age of 21 years divided by all clinic patients, both with and without TCR before 21 years.
Cox proportional hazards models were constructed to further evaluate the effects of 5 variables (sex, highest recorded functional lesion level [using upper lumbar as reference] and best ambulation status [using community ambulation as reference] prior to the first TCR, prior [shunt or endoscopic] treatment for hydrocephalus, and prior Chiari decompression) on the risk of experiencing the first TCR. Hazard ratio estimates with 95% confidence intervals as well as adjusted p values based on Wald tests were obtained. All hypothesis tests were two-sided with a significance level of 0.05. Data were analyzed using R statistical software (version 3.6.1).
Results
At the time of the study, the NSBPR contained the records of 6768 patients with MMC; of these records, 6339 included information on unique patients regarding their neurosurgical operations. These patients form the basis for this study. Of this group, 1366 (21.5%) unique patients underwent at least one TCR procedure, including 652 males (47.7%) and 714 females (52.3%). The majority (1035/1366; 75.8%) underwent a single TCR, 233 (17.1%) had 2 TCRs, 73 (5.3%) had 3 TCRs, 23 (1.7%) had 4 procedures, and 1 patient each had 5 and 6 TCRs. The calculated retethering rates following each TCR were therefore as follows: following the first TCR, the retethering rates for the second, third, fourth, and fifth TCRs were 331 of 1366 (24.2%), 98 of 331 (29.6%), 25 of 98 (25.5%), 2 of 25 (8.0%), and 1 of 2 (50%), respectively. For the 47 adults, the majority (32/47; 68%) underwent a single TCR, 11 (23.4%) had 2 TCRs, 3 (6.4%) had 3 TCRs, and 1 (2.1%) had 4 TCRs. Similarly, for adults, the calculated retethering rates following each TCR were as follows: following the first TCR, the retethering rates for the second, third, and fourth TCRs were 15 of 47 (31.9%), 4 of 11 (36.4%), and 1 of 3 (33%), respectively.
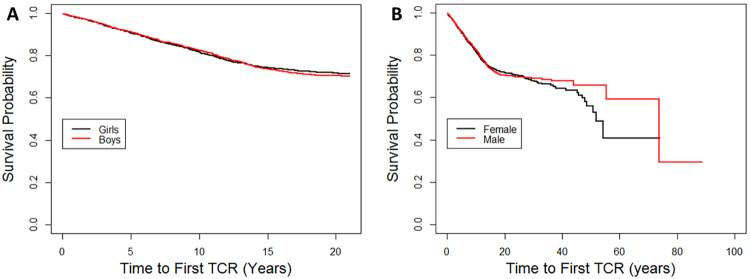
Survival curves are constructed in Fig. 1 for those undergoing the first TCR. For 87 patients, the age at the time of first TCR was not available; among the remaining 1279 patients, the mean age at the time of first TCR was 7.8 years (median 6 years, range 0–74 years, SD 7.22 years). The mean age to first TCR was 7.5 years (median 6, SD 6.54) among males and 8.1 years (median 6, SD 7.78) among females (p = 0.11). For children younger than 21 years, the mean age to first TCR was 6.7 years (median 6, SD 4.70) among males and 6.9 years (median 6, SD 4.58) among females (p = 0.48). The annual TCR rate remained relatively linear (1.8%/year) between 0 and 13 years of age, after which it dropped to 0.7%/year between 14 and 21 years of age; there was no time period during which the TCR rate accelerated. Annual TCR rates were compared with annual spine growth rates extracted from normal growth curves;12 there was no correlation between annual TCR rates and spine growth for either boys or girls (Pearson’s correlation coefficients 0.35 [p = 0.15] and 0.38 [p = 0.14], respectively). When stratified by sex, Kaplan-Meier curves for the time to first TCR (Fig. 1) and second TCR (data not shown) were not significantly different either among all patients (p = 0.7) or for children younger than 21 years (p = 0.9).
FIG. 1.
Kaplan-Meier curves for time to first TCR by sex among individuals younger than 21 years (log-rank comparison p = 0.9; A) and among all individuals (log-rank comparison p = 0.7; B). Figure is available in color online only.
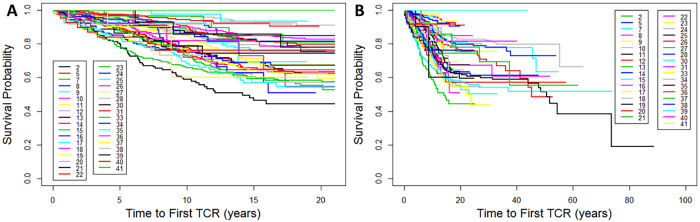
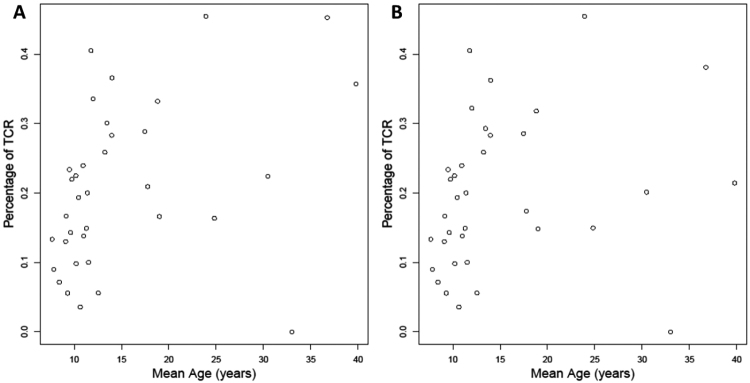
Kaplan-Meier curves for time to first TCR were constructed for each of the 35 clinic sites (Fig. 2), and regression analyses were performed to assess the degree of variability after controlling for the mean age at last follow-up for patients at each clinic. As expected, the TCR rate rose when plotted against the age at last follow-up, but there was significant variability between clinics even after adjusting for age (Fig. 3) (Spearman’s correlation coefficient for all subjects regardless of age at TCR = 0.497, p = 0.003; Spearman’s correlation coefficient for children undergoing TCR at < 21 years = 0.430, p = 0.011).
FIG. 2.
Kaplan-Meier curves for time to first TCR by site among 35 clinics (each labeled by clinic code) for individuals younger than 21 years (log-rank comparison p < 0.001; A) and for all individuals (log-rank comparison p < 0.001; B). Figure is available in color online only.
FIG. 3.
Percentage of first TCR versus mean age of each of 35 clinics as plotted against mean clinic age at last follow-up for individuals younger than 21 years (A) and for all individuals (B).
The TCR rate was 17.2% among patients with and 39.9% among those without prior treatment for hydrocephalus. The TCR rate was 30.9% among patients with and 20.7% among those without prior Chiari decompression.
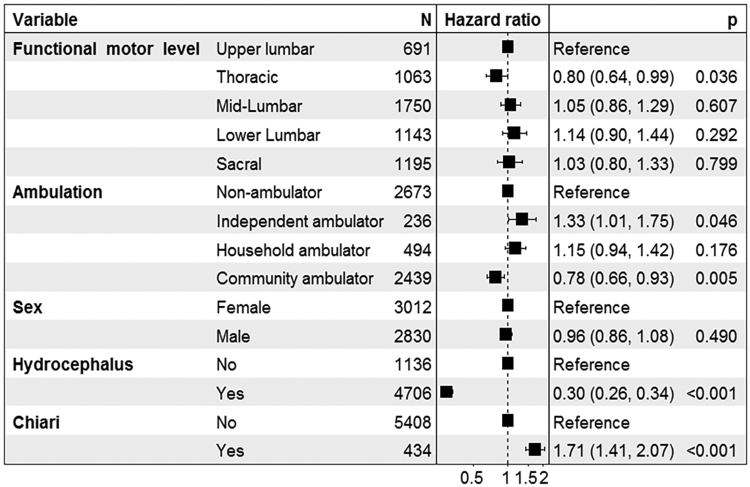
The Cox proportional hazards model is provided in Fig. 4. There was no consistent effect of lesion level on TCR rates (using upper lumbar level as reference). Relative to nonambulators, independent ambulators were significantly more likely, and community ambulators less likely, to undergo TCR. There were highly significant effects on TCR for both prior treatment for hydrocephalus (HR 0.30, 95% CI 0.26–0.34; p < 0.001) and prior Chiari decompression (HR 1.71, 95% CI 1.41–2.07; p < 0.001).
FIG. 4.
Cox proportional hazards regression analysis on time to first TCR. Functional motor levels were referenced to upper lumbar rather than to thoracic levels, as subjects with thoracic-level lesions, lacking any significant motor function, would not be able to exhibit motor deterioration.
Discussion
This is the largest analysis of TCR among patients with MMC ever reported; our analyses confirm and extend the results of prior studies.1,2,5,13–24 Both the overall 21.5% rate of TCR and mean age of 7.8 years at the time of TCR in the present study are similar to the 10%–45% incidence and average age of 6–13 years reported in prior, smaller studies.1,4,13,20,25–27 However, there remained significant variability in TCR rates between institutions after correcting for the average age of each clinic’s population (Figs. 2 and 3). The reason(s) for this variability is not clear, although the diligence with which clinical deterioration is sought and treated and the accepted clinical indications for TCR are obvious potential factors that might vary between clinics. For example, some clinics commonly perform TCR in advance of corrective scoliosis surgery; in one study, 40% of TCRs were performed for progressive scoliosis (although in only 11% was this the only indication).2 Other clinics may have more restrictive indications for TCR. Unfortunately, the indications for TCR among the majority of our study patients were unknown. A second possibility is variability among clinics in the rigor with which shunt malfunction was ruled out before performing TCR.
The results of the present study are also consistent with reoperation rates for second, third, fourth, and fifth TCRs. Among Bowman and colleagues’ series of 502 patients who underwent MMC closure and follow-up at Ann and Robert H. Lurie Children’s Hospital, 81% of those who underwent a TCR had only a single TCR, 17% a second TCR, 10% a third TCR, and 3% a fourth TCR.1
The age distribution of TCR was not linear in a prior study by Al-Holou et al.,13 which has supported a widely held belief among pediatric neurosurgeons that symptomatic tethering occurs more frequently during periods of greater spinal growth, particularly during early adolescence, due to greater spinal cord traction. However, to what degree increased somatic growth is due to vertebral column versus leg elongation is not clear. The annual growth of the vertebral column among normal children is greatest during the 1st postnatal year, decelerates somewhat during the first 5 years of life, remains stable until early adolescence, accelerates again for about 2–3 years during the adolescent growth spurt, and declines again thereafter (Fig. 5).12 Females, who normally enter puberty on average 2 years earlier than males, undergo accelerated vertebral column growth 2 years earlier than males (Fig. 5).12,28–31 However, the growth rate among normal individuals may not be applicable to the MMC population; a study by Duval-Beaupère and Soulignac found no sex-specific differences in the growth rate of either the spine or lower extremities in children with lower-extremity paralysis.32
FIG. 5.
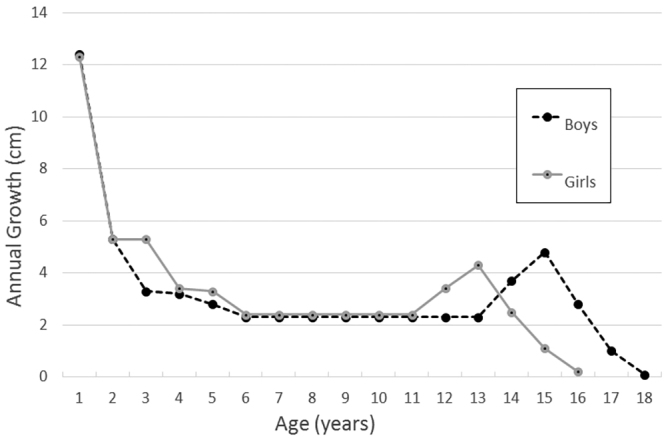
Linear growth of sitting height (representing spine growth) during childhood in girls and boys. Figure is based on data from DiMeglio et al.12
However, the somatic growth of individuals with spina bifida is influenced by a number of other factors. First, half of individuals with spina bifida have short stature and growth hormone deficiency33–35 that leads to shortened growth of both the spine and lower extremities, but with a greater proportion attributed to shortened lower extremities. Short stature is also greater among those with higher-level lesions (at least during the first 7 years of life and perhaps later as well).32–34,36 With advancing age, somatic growth among individuals with MMC lags further behind age-matched controls; one study found greater disparities in vertebral column length over time compared with leg length,32 although another study demonstrated appropriate vertebral column growth.34 People with spina bifida also have spinal deformities (scoliosis, kyphosis and/or lordosis), and others have vertebral abnormalities, which may impact spine length measurements. Precocious puberty occurs in nearly 20% of persons with spina bifida (much more commonly in girls)33,37,38 and may alter the growth trajectory in this population.37,39,40 Finally, tethering may itself influence the rate of somatic growth.41
One of the most significant findings of the present study is that there was no accelerated frequency of TCR at any time during childhood and, in particular, during periods in which accelerated somatic growth would be expected, such as the 1st postnatal year and the adolescent growth spurt. Moreover, the TCR rate among girls was no earlier than among boys, despite girls entering puberty 2 years earlier; this is in keeping with results of an earlier study by Duval-Beaupère and Soulignac, who found no sex-specific differences in the growth rate of either the spine or lower extremities in children with lower-extremity paralysis.32 In contrast to the results of Al-Holou et al.,13 the annual TCR rate in the present study was actually linear up to early adolescence and declined rather abruptly beginning after the age of 13 years, when accelerated spine growth would be expected to occur. The lack of an increased TCR rate during the first few years of life when somatic growth is greatest could be due to a protective effect of prophylactic untethering at the time of initial MMC closure. It is also possible that variability in pubertal onset among the study population could mask the adolescent growth spurt. However, our findings, along with the fact that symptomatic retethering occurs in adults after spine growth is completed, challenge the concept that tethered cord is solely a function of spinal cord stretching due to spine growth. Additional pathophysiological mechanisms may also contribute, including repeated microtrauma from stretching associated with dynamic spine movements, spinal cord compression, ischemia related to other causes, and potentially others. Unfortunately, our results are hampered by a lack of information about somatic growth and pubertal development among the study population.
The present study also did not find any consistent relationship between functional lesion level and TCR rates, in contrast to the results of Al-Holou et al.13 and Marreiros et al.,20 who found higher TCR rates for people with lower-level lesions, but consistent with the results of Bowman and colleagues,1 who also found no relationship between functional lesion level and TCR rates. On the other hand, we did find a relationship between ambulatory status and TCR rates; with reference to the TCR rate among community ambulators, household ambulators were significantly more likely, and nonambulators less likely, to undergo TCR. One reason for the reduced TCR rate among nonambulators is that sensorimotor deterioration in this group may be less likely to be discovered and/or treated as aggressively in the absence of other symptoms such as pain or bowel/bladder dysfunction. Another reason may be that TCR may be less commonly performed for progressive scoliosis in this group compared with those who are ambulatory. It is not clear why household ambulators would be more likely to undergo TCR than community ambulators.
Finally, the results of this study demonstrated two other interesting and perhaps nonintuitive findings. The first, not previously reported to our knowledge, is that individuals with treated hydrocephalus are significantly less likely to undergo TCR than those without treated hydrocephalus. In contrast, those who had previously undergone a Chiari decompression are more likely to undergo subsequent TCR. This second result was also noted previously by Kellogg and colleagues.5 The increased incidence of TCR among those without treated hydrocephalus might reflect the possibility that hydrocephalus is rarely considered as a cause of deterioration among this group, whereas it would commonly be considered (and addressed first) among those with previously treated hydrocephalus. As for the impact of previous Chiari decompression, we have no ready explanation other than the possibility of a correlation between a more aggressive (or proactive) approach to neurosurgical interventions (including both Chiari decompressions and TCR) among some clinic sites. We plan to investigate this further by comparing rates of the three neurosurgical interventions (shunt operations, Chiari decompressions, and TCR) among sites in the NSBPR.
There are multiple limitations to this study. First, the NSBPR is a registry of patients who are followed in spina bifida clinics and, as such, may not represent the MMC population at large. In particular, those who are followed in a spina bifida clinic may represent a more complicated group than those who are not. However, unlike individuals with occult dysraphic malformations, many of whom are followed outside of a standard spina bifida clinic, proportionately more (at least most children) with MMC are more likely to be followed in a multidisciplinary clinic. Second, this study involves only those who gave consent to be in the registry, and, although some information is sought from those who did not give consent to determine comparability, it is possible that these results could be altered if the remaining spina bifida clinic patients were to be included. Third, although neurosurgical operations are considered “if ever” procedures in the registry, it is possible that some procedures performed before enrollment, and their dates, are incorrect. Fourth, the indications for various neurosurgical procedures are not included in this study, as they have only recently been documented. Fifth, we do not have sufficient information on somatic growth in general, vertebral column growth in particular, nor the pubertal status of individuals in the registry so that we cannot correlate TCR rates with these parameters and instead had to rely on normative data on pubertal development as a proxy to compare TCR rates among males and females.
Conclusions
This, the largest study of TCR among patients with MMC, confirms and expands on the results of prior, smaller and single-institutional studies in terms of the frequency of TCR (21.5%), the average age at first TCR (7.8 years), and the frequency of repeated TCR. One significant difference between this and prior studies is that the frequency of TCR in the present study was linear throughout early childhood up to about 13 years of age and actually declined during adolescence, in contrast to the widespread belief that TCR increases during a presumed growth spurt during preadolescence and early puberty. We also found no difference in the TCR rates for males and females, despite the fact that females enter puberty 2 years earlier than males. Together, these findings challenge the widely held assumption that TCR is related to somatic growth, although much more information is needed on vertebral growth and pubertal development in this population to better address the reason(s) for these findings. We also found wide and significant variability among clinic sites in the frequency of TCR; moreover, we demonstrated a relative protective effect of prior hydrocephalus treatment and a deleterious effect of prior Chiari decompression on subsequent TCR rates; the NSBPR is presently seeking the indication(s) for neurosurgical operations, which may increase our understanding as to why this is so.
Acknowledgments
This study was supported by a grant from the Centers for Disease Control and Prevention (National Spina Bifida Patient Registry [NSBPR] DD-19-001). We acknowledge the assistance of Dr. Vernon Chinchilli, Chair of the Department of Public Health Outcomes at Penn State College of Medicine, and Sandeep Pradhan, MD, MPH, and Paddy Ssentongo, MMBS, from the Department of Public Health Outcomes, in verifying the statistical methodologies used.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Dias, Rizk. Acquisition of data: Dias, Rizk, Bowman, Partington, Blount, Rocque, Hopson, Ettinger, Lee, Walker. Analysis and interpretation of data: all authors. Drafting the article: Dias. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Dias. Statistical analysis: Wang. Administrative/technical/material support: Dias, Hopson, Ettinger. Study supervision: Dias, Rizk, Lee.
Supplemental Information
Current Affiliations
Dr. Partington: Department of Neurosurgery, Children’s Mercy Hospital and University of Kansas School of Medicine, Kansas City, KS.
References
- 1. Bowman RM, McLone DG, Grant JA, et al. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001;34(3):114–120. doi: 10.1159/000056005. [DOI] [PubMed] [Google Scholar]
- 2. Bowman RM, Mohan A, Ito J, et al. Tethered cord release: a long-term study in 114 patients. J Neurosurg Pediatr. 2009;3(3):181–187. doi: 10.3171/2008.12.PEDS0874. [DOI] [PubMed] [Google Scholar]
- 3. Begeer JH, Meihuizen de Regt MJ, HogenEsch I, et al. Progressive neurological deficit in children with spina bifida aperta. Z Kinderchir. 1986;41(suppl 1):13–15. doi: 10.1055/s-2008-1043387. [DOI] [PubMed] [Google Scholar]
- 4.Bowman RM, McLone DM. In: Spina Bifida Management and Outcome. Ozek MM, Cinalli G, Maixner WJ, editors. Springer-Verlag; 2008. Tethered cord in children with spina bifida; pp. 267–274. [Google Scholar]
- 5. Kellogg R, Lee P, Deibert CP, et al. Twenty years’ experience with myelomeningocele management at a single institution: lessons learned. J Neurosurg Pediatr. 2018;22(4):439–443. doi: 10.3171/2018.5.PEDS17584. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Iacono RP, Yamada BS. In: Tethered Cord Syndrome. Yamada S, editor. American Association of Neurological Surgeons; 1996. Pathophysiology of the tethered spinal cord; pp. 29–48. [Google Scholar]
- 7. Yamada S, Won DJ, Yamada SM. Pathophysiology of tethered cord syndrome: correlation with symptomatology. Neurosurg Focus. 2004;16(2):E6. doi: 10.3171/foc.2004.16.2.7. [DOI] [PubMed] [Google Scholar]
- 8. Yamada S, Zinke DE, Sanders D. Pathophysiology of “tethered cord syndrome.”. J Neurosurg. 1981;54(4):494–503. doi: 10.3171/jns.1981.54.4.0494. [DOI] [PubMed] [Google Scholar]
- 9. Sawin KJ, Liu T, Ward E, et al. The National Spina Bifida Patient Registry: profile of a large cohort of participants from the first 10 clinics. J Pediatr. 2015;166(2):444–50.e1. doi: 10.1016/j.jpeds.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thibadeau JK, Ward EA, Soe MM, et al. Testing the feasibility of a National Spina Bifida Patient Registry. Birth Defects Res A Clin Mol Teratol. 2013;97(1):36–41. doi: 10.1002/bdra.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alabi NB, Thibadeau J, Wiener JS, et al. Surgeries and health outcomes among patients with spina bifida. Pediatrics. 2018;142(3):e20173730. doi: 10.1542/peds.2017-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DiMeglio A, Canavese F, Charles P. Growth and adolescent idiopathic scoliosis: when and how much? J Pediatr Orthop. 2011;31(1)(suppl):S28–S36. doi: 10.1097/BPO.0b013e318202c25d. [DOI] [PubMed] [Google Scholar]
- 13. Al-Holou WN, Muraszko KM, Garton HJ, et al. The outcome of tethered cord release in secondary and multiple repeat tethered cord syndrome. J Neurosurg Pediatr. 2009;4(1):28–36. doi: 10.3171/2009.2.PEDS08339. [DOI] [PubMed] [Google Scholar]
- 14. Mehta VA, Bettegowda C, Ahmadi SA, et al. Spinal cord tethering following myelomeningocele repair. J Neurosurg Pediatr. 2010;6(5):498–505. doi: 10.3171/2010.8.PEDS09491. [DOI] [PubMed] [Google Scholar]
- 15. Phuong LK, Schoeberl KA, Raffel C. Natural history of tethered cord in patients with meningomyelocele. Neurosurgery. 2002;50(5):989–995. doi: 10.1097/00006123-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 16. Selber P, Dias L. Sacral-level myelomeningocele: long-term outcome in adults. J Pediatr Orthop. 1998;18(4):423–427. [PubMed] [Google Scholar]
- 17. Tamaki N, Shirataki K, Kojima N, et al. Tethered cord syndrome of delayed onset following repair of myelomeningocele. J Neurosurg. 1988;69(3):393–398. doi: 10.3171/jns.1988.69.3.0393. [DOI] [PubMed] [Google Scholar]
- 18. Shurtleff DB, Duguay S, Duguay G, et al. Epidemiology of tethered cord with meningomyelocele. Eur J Pediatr Surg. 1997;7(1)(suppl 1):7–11. doi: 10.1055/s-2008-1071200. [DOI] [PubMed] [Google Scholar]
- 19. Hertzler DA, II, DePowell JJ, Stevenson CB, Mangano FT. Tethered cord syndrome: a review of the literature from embryology to adult presentation. Neurosurg Focus. 2010;29(1):E1. doi: 10.3171/2010.3.FOCUS1079. [DOI] [PubMed] [Google Scholar]
- 20. Marreiros H, Loff C, Calado E. Who needs surgery for pediatric myelomeningocele? A retrospective study and literature review. J Spinal Cord Med. 2015;38(5):626–640. doi: 10.1179/2045772314Y.0000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kural C, Solmaz I, Tehli O, et al. Evaluation and management of lumbosacral myelomeningoceles in children. Eurasian J Med. 2015;47(3):174–178. doi: 10.5152/eurasianjmed.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balasubramaniam C, Laurent JP, McCluggage C, et al. Tethered-cord syndrome after repair of meningomyelocele. Childs Nerv Syst. 1990;6(4):208–211. doi: 10.1007/BF01850974. [DOI] [PubMed] [Google Scholar]
- 23.McLone DG, Naidich TP. In: Pediatric Neurosurgery. McLaurin RL, Shut L, Venes JL, Epstein F, editors. Saunders; 1989. Myelomeningocele: outcome and late complication; pp. 53–70. [Google Scholar]
- 24. Winston K, Hall J, Johnson D, Micheli L. Acute elevation of intracranial pressure following transection of non-functional spinal cord. Clin Orthop Relat Res. 1977;(128):41–44. [PubMed] [Google Scholar]
- 25. Herman JM, McLone DG, Storrs BB, Dauser RC. Analysis of 153 patients with myelomeningocele or spinal lipoma reoperated upon for a tethered cord. Presentation, management and outcome. Pediatr Neurosurg. 1993;19(5):243–249. doi: 10.1159/000120739. [DOI] [PubMed] [Google Scholar]
- 26. Kirollos RW, Van Hille PT. Evaluation of surgery for the tethered cord syndrome using a new grading system. Br J Neurosurg. 1996;10(3):253–260. doi: 10.1080/02688699650040106. [DOI] [PubMed] [Google Scholar]
- 27. McLone DG, La Marca F. The tethered spinal cord: diagnosis, significance, and management. Semin Pediatr Neurol. 1997;4(3):192–208. doi: 10.1016/s1071-9091(97)80037-x. [DOI] [PubMed] [Google Scholar]
- 28. Dimeglio A, Canavese F. The immature spine: growth and idiopathic scoliosis. Ann Transl Med. 2020;8(2):22–28. doi: 10.21037/atm.2019.11.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang H, Sucato DJ, Nurenberg P, McClung A. Morphometric analysis of vertebral body growth Using magnetic resonance imaging in the normal skeletally immature spine. Spine (Phila Pa 1976) 2018;43(2):133–140. doi: 10.1097/BRS.0b013e3181c80ec5. [DOI] [PubMed] [Google Scholar]
- 30. DiMeglio A. Growth of the spine before age 5 years. J Pediatr Orthop B. 1993;1(1):102–107. [Google Scholar]
- 31. Dede O, Büyükdoğan K, Demirkıran HG, et al. Thoracic spine growth revisited. How accurate is the Demiglio data? Spine (Phila Pa 1976) 2017;42(12):917–920. doi: 10.1097/BRS.0000000000002104. [DOI] [PubMed] [Google Scholar]
- 32. Duval-Beaupère G, Soulignac G. Premature pubarche and the growth of the trunk in paralysed children. Ann Hum Biol. 1975;2(1):69–80. doi: 10.1080/03014467500000581. [DOI] [PubMed] [Google Scholar]
- 33. Trollmann R, Dörr HG, Strehl E, et al. Growth and pubertal development in patients with meningomyelocele: a retrospective analysis. Acta Paediatr. 1996;85(1):76–80. doi: 10.1111/j.1651-2227.1996.tb13894.x. [DOI] [PubMed] [Google Scholar]
- 34. Charney EB, Rosenblum M, Finegold D. Linear growth in a population of children with myelomeningocele. Z Kinderchir. 1981;34(4):415–419. doi: 10.1055/s-2008-1063385. [DOI] [PubMed] [Google Scholar]
- 35. Hochhaus F, Butenandt O, Schwarz HP, Ring-Mrozik E. Auxological and endocrinological evaluation of children with hydrocephalus and/or meningomyelocele. Eur J Pediatr. 1997;156(8):597–601. doi: 10.1007/s004310050672. [DOI] [PubMed] [Google Scholar]
- 36. Rotenstein D, Adams M, Reigel DH. Adult stature and anthropomorphic measurements of patients with myelomeningocele. Eur J Pediatr. 1995;154(5):398–402. doi: 10.1007/BF02072114. [DOI] [PubMed] [Google Scholar]
- 37. Greene SA, Frank M, Zachmann M, Prader A. Growth and sexual development in children with meningomyelocele. Eur J Pediatr. 1985;144(2):146–148. doi: 10.1007/BF00451900. [DOI] [PubMed] [Google Scholar]
- 38. Elias ER, Sadeghi-Nejad A. Precocious puberty in girls with myelodysplasia. Pediatrics. 1994;93(3):521–522. [PubMed] [Google Scholar]
- 39. Rotenstein D, Reigel DH. Growth hormone treatment of children with neural tube defects: results from 6 months to 6 years. J Pediatr. 1996;128(2):184–189. doi: 10.1016/s0022-3476(96)70387-6. [DOI] [PubMed] [Google Scholar]
- 40. Rotenstein D, Reigel DH. The endocrine control of growth for patients with myelomeningocele. Endocrinologist. 1998;8(4):260–264. [Google Scholar]
- 41. Rotenstein D, Reigel DH, Lucke JF. Growth of growth hormone-treated and nontreated children before and after tethered spinal cord release. Pediatr Neurosurg. 1996;24(5):237–241. doi: 10.1159/000121045. [DOI] [PubMed] [Google Scholar]