In Brief
Resections in language-dominant ventral occipitotemporal cortex carry the risk of causing reading impairment. Here, the authors demonstrate that eloquent cortex in ventral visual cortex can be rapidly mapped intraoperatively using electrocorticography recordings. This method guides high-probability targets for stimulation, with limited patient participation, and can be used to avoid iatrogenic dyslexia following surgery.
Keywords: electrocorticography, reading, language, awake craniotomy, cortical stimulation, epilepsy, surgical technique
ABBREVIATIONS : BGA = broadband gamma activity, CSM = cortical stimulation mapping, ECoG = electrocorticography, FCD = focal cortical dysplasia, fMRI = functional MRI, SDE = subdural electrode, sEEG = stereo-electroencephalography, vOTC = ventral occipitotemporal cortex, VWFA = visual word form area
Abstract
OBJECTIVE
Resective surgery in language-dominant ventral occipitotemporal cortex (vOTC) carries the risk of causing impairment to reading. Because it is not on the lateral surface, it is not easily accessible for intraoperative mapping, and extensive stimulation mapping can be time-consuming. Here the authors assess the feasibility of using task-based electrocorticography (ECoG) recordings intraoperatively to help guide stimulation mapping of reading in vOTC.
METHODS
In 11 patients undergoing extraoperative, intracranial seizure mapping, the authors recorded induced broadband gamma activation (70–150 Hz) during a visual category localizer. In 2 additional patients, whose pathologies necessitated resections in language-dominant vOTC, task-based functional mapping was performed intraoperatively using subdural ECoG alongside direct cortical stimulation.
RESULTS
Word-responsive cortex localized using ECoG showed a high sensitivity (72%) to stimulation-induced reading deficits, and the confluence of ECoG and stimulation-positive sites appears to demarcate the visual word form area. Intraoperative task-based ECoG mapping was possible in < 3 minutes, providing a high signal quality, and initial intraoperative data analysis took < 3 minutes, allowing for rapid assessment of broad areas of cortex. Cortical areas critical for reading were mapped and successfully preserved, while also enabling pathological tissue to be completely removed.
CONCLUSIONS
Eloquent cortex in ventral visual cortex can be rapidly mapped intraoperatively using ECoG. This method acts to guide high-probability targets for stimulation with limited patient participation and can be used to avoid iatrogenic dyslexia following surgery.
Ventral occipitotemporal cortex (vOTC) contains several cortical regions dedicated to higher-level visual processing of scenes, faces, objects, and words that follow a well-characterized, medial-to-lateral topography with regard to their preferred tuning.1–3 Lesions of any of these category-selective regions can result in isolated deficits in a categorical domain, such as prosopagnosia or scene agnosia.4,5 In particular, the visual word form area (VWFA), which is located in the lateral aspect of language-dominant vOTC, displays category selectivity to written words, as demonstrated by functional imaging and direct cortical stimulation,6–9 and lesions that result in alexia.10,11 Given the critical role of reading in modern life, it is imperative to localize and preserve reading sites in vOTC to enable safe resective procedures in this region.
Direct cortical stimulation during an awake craniotomy is the widely accepted gold standard for localizing and preserving critical language regions.12,13 This enables optimal resections while preserving cognitive function.14,15 However, performing intraoperative stimulation of the ventral surface of the temporo-occipital region is challenging given its limited access, additional intraoperative time, and the high level of patient engagement required for comprehensive mapping of reading function.16 Electrocorticography (ECoG) has been widely used in the extraoperative setting to localize seizure foci in patients with drug-resistant epilepsy and for basic insight into cognitive functions. Previous studies have shown that extraoperative ECoG and cortical stimulation can colocalize language processes,17–20 including for naming,21 speech,22 and reading8,9 tasks, in reliable and concordant fashion. Furthermore, mapping category selectivity of word-selective cortex with these tasks can be performed rapidly and does not necessitate subject participation.23 This suggests that task-based ECoG may be an effective method of guiding and increasing efficiency of intraoperative stimulation of vOTC. Here we assess the use of extra- and intraoperative recordings alongside stimulation mapping to identify and preserve eloquent reading cortex in lateral vOTC while enabling resection of pathological tissue.
Methods
Participants
A total of 13 patients (6 men, 18–47 years old, all right-handed, IQ 94 ± 8, age at epilepsy onset 18 ± 9 years [mean ± SD]) received semipermanent implants (1–4 weeks) of intracranial electrodes for seizure localization of pharmacoresistant epilepsy. Eleven patients participated in an extraoperative visual category localizer task and underwent extraoperative cortical stimulation mapping (CSM) (Table 1). Two patients (cases 1 and 2), detailed below, performed an intraoperative category localizer task and underwent direct cortical stimulation during an awake resective craniotomy. All participants gave written informed consent and all experimental procedures were reviewed and approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston. Consent was obtained from participants for whom identifiable images are presented.
TABLE 1.
Demographic data in 11 patients with epilepsy who underwent extraoperative ECoG mapping
| Case No. | Sex | Age (yrs) | Electrode Type | Seizure Focus |
|---|---|---|---|---|
| E1 |
F |
42 |
SDE |
Lt inferior parietal |
| E2 |
F |
22 |
SDE |
Lt ventral temporal |
| E3 |
M |
43 |
sEEG |
Rt ventral occipital |
| E4 |
F |
42 |
sEEG |
Lt superior temporal |
| E5 |
F |
22 |
sEEG |
Lt ventral temporal |
| E6 |
M |
26 |
sEEG |
Bilat medial temporal |
| E7 |
F |
37 |
sEEG |
Lt PVNH |
| E8 |
M |
35 |
sEEG |
Lt medial temporal |
| E9 |
M |
37 |
sEEG |
Lt medial temporal |
| E10 |
M |
28 |
sEEG |
Bilat medial temporal |
| E11 | F | 47 | sEEG | Lt temporal pole |
E = extraoperative CSM group; PVNH = periventricular nodular heterotopia.
Functional Imaging
Task-based functional MRI (fMRI) data were acquired using a gradient-recalled echo planar imaging sequence consisting of 33 axial slices with 3-mm thickness and in-plane resolution of 2.75 mm isotropic (TE 30 msec, TR 2015 msec, flip angle 90°).
Category Localizer
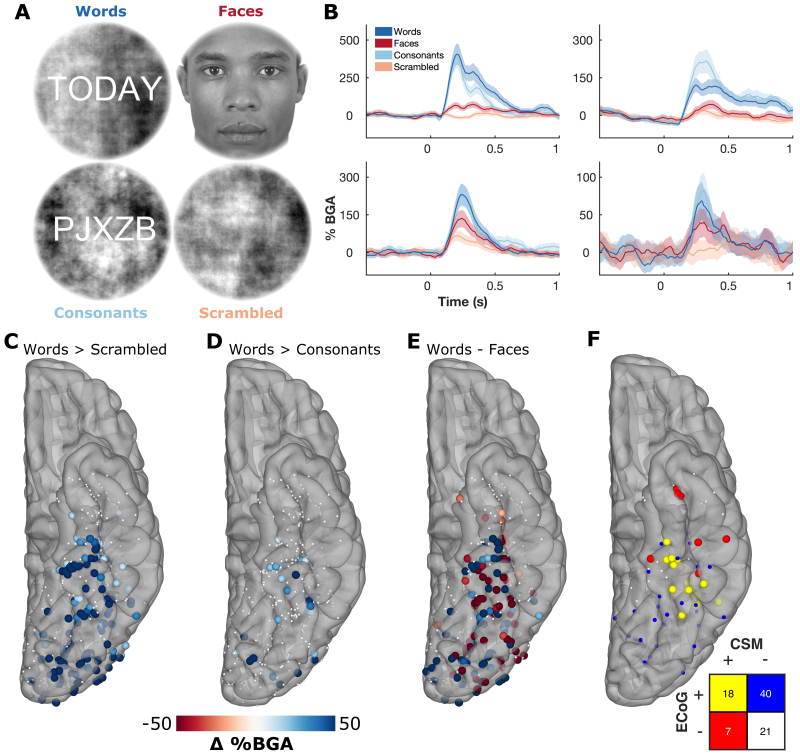
Stimuli were presented on a liquid crystal display (LCD) screen positioned at eye level, approximately 50 cm from the patient. A total of 60 (case 1, extraoperative) or 40 (case 2) each of 1) words; 2) faces;24 3) consonant strings; and 4) phase-scrambled faces were presented in pseudorandom order at an approximate size of 9° visual angle, the outer 0.5° of which was smoothly ramped using a cosine function (Fig. 1A).23 Lettered stimuli had a phase-scrambled background to minimize variance of overall visual complexity and visual field coverage between categories. All stimuli were presented in grayscale and were approximately matched for mean luminance and contrast. Stimuli were presented using Psychophysics Toolbox in MATLAB (MathWorks).25 Each image was displayed for 750 msec (case 1, extraoperative) or 500 msec (case 2) with an interstimulus interval of 500 msec. Stimulus timings were selected based on prior ECoG paradigms probing word and face processing in vOTC.1,3,9 Total stimulus presentation time for case 1 was < 7 minutes and for case 2 it was < 3 minutes. To ensure attention, 20 images were presented twice in a row, which the patients indicated by either pressing a button (extraoperative) or saying "repeat" aloud (cases 1 and 2).23 Repeat trials were not included in the analysis.
FIG. 1.
Extraoperative ECoG category selectivity. A: Examples of stimuli from the visual category localizer. B: Time course of BGA for representative stimulation-positive electrodes from 4 patients. C–E: Contrasts of words > scrambled (C), words > consonants (D), and words—faces (E) in the window 100–400 msec after stimulus onset. Thresholded by electrodes with a significant difference (2-sample t-test, p < 0.01). F: Concordance between task-related activation (words > scrambled; ECoG+) and CSM sites in vOTC, leading to reading disruption (CSM+). CSM was performed between neighboring electrode pairs. Pairs were considered ECoG positive if either electrode was significant.
Signal Analysis
Extraoperative data were acquired from either subdural electrodes (SDEs) arranged in grids (2 patients) or stereotactically placed depth electrodes (i.e., stereo-electroencephalography [sEEG]; 9 patients). The SDEs were platinum-iridium electrodes embedded in a silicone elastomer sheet (PMT Corp.; top-hat design, 3-mm-diameter cortical contact) and were surgically implanted via a craniotomy.26,27 Stereotactic probes were implanted using a robotic surgical assistant (Medtech).28,29 Each probe (PMT Corp.) was 0.8 mm in diameter and had 8–16 electrode contacts. Each contact was a platinum-iridium cylinder that was 2.0 mm in length and was separated from the adjacent contact by 1.5–2.43 mm.
For the intraoperative cases, ECoG electrodes were subdural platinum–iridium electrodes embedded in a silicone elastomer sheet (PMT Corp.; top-hat design, 3-mm-diameter cortical contact, 0.66-cm interelectrode spacing). Electrodes were implanted in standard fashion as previously described.26,27,30 Areas on the ventral temporal surface were sampled using a mixture of grids and strips, depending on the access yielded by the venous anatomy. Electrodes were temporarily secured in place by suturing the connection leads to the dural edges. Data were digitized at 2 kHz using the NeuroPort recording system (Blackrock Microsystems).
In the extraoperative cases, signals were re-referenced to the common average of the clean channels, and for the intraoperative cases signals were referenced to an electrode on the lateral cortical surface, away from visually responsive regions. Trials contaminated by interictal epileptic spikes were discarded. Broadband gamma activity (BGA; 70–150 Hz) was extracted with a frequency domain bandpass Hilbert transform (paired sigmoid flanks with half-width 1.5 Hz) and the analytical amplitude was smoothed (Savitzky-Golay finite impulse response, third order, frame length of 201 msec). BGA is presented here as percentage change from baseline level, defined as the period from −500 to −100 msec before stimulus presentation. Initial data analysis and visualization for intraoperative cases was performed in the operating room, on a laptop running a MATLAB-based pipeline, in < 3 minutes. Extraoperative electrode localization was performed by colocalizing a preoperative MRI sequence with the postimplantation CT.30 For postsurgical visualization of intraoperative cases, electrodes were localized manually in AFNI (Analysis of Functional NeuroImages) software, based on intraoperative photographs and localizations using a surgical navigation system (StealthStation S8; Medtronic). Cortical surface reconstruction was performed using FreeSurfer and imported into AFNI, in which the electrode positions were mapped onto the cortical surface.30,31
Cortical Stimulation Mapping
Direct cortical stimulation was carried out using a Nihon Kohden PE-210A stimulator through the ECoG electrodes (extraoperative), or with an OCS2 handheld stimulator (5-mm electrode spacing; Integra LifeSciences) (cases 1 and 2). A 50-Hz, 500-µsec square pulse stimulation was administered with a current between 5 and 10 mA depending on an established baseline that did not result in afterdischarges.26 Testing included reading of standardized passages (i.e., the "Grandfather," "Rainbow," and “North Wind” passages).9 Intraoperatively, visual stimuli were presented on the same screen used for the ECoG task stimuli, and auditory stimuli were delivered orally by the surgeon.
Results
Extraoperative Reading Mapping
We used 3 different ECoG contrasts to predict stimulation-induced reading deficits: 1) words > scrambled (72% sensitivity); 2) words > consonants (24% sensitivity); and 3) words—faces (56% sensitivity) (Fig. 1C–E). The words > scrambled contrast showed the greatest overlap with the CSM-positive sites, and the region of greatest concordance between ECoG and CSM was located within midfusiform cortex—the predicted location of the VWFA (Fig. 1F).
Within midfusiform cortex, discordant results between ECoG and CSM commonly occurred at electrodes neighboring true-positive electrodes. This probably reflects the lower resolution of stimulation, given that mostly nonoverlapping pairs of electrodes were stimulated. CSM-positive, ECoG-negative electrodes in the anterior temporal lobe were notably present in patients with epileptic foci in proximity to the site of stimulation. Dizziness, confusion, and aura-like symptoms were reported during stimulation of these sites.
Intraoperative Category Localizer
Two patients scheduled to undergo awake resective craniotomies in the language-dominant vOTC were recruited for intraoperative task-based ECoG mapping in addition to clinical standard language mapping.
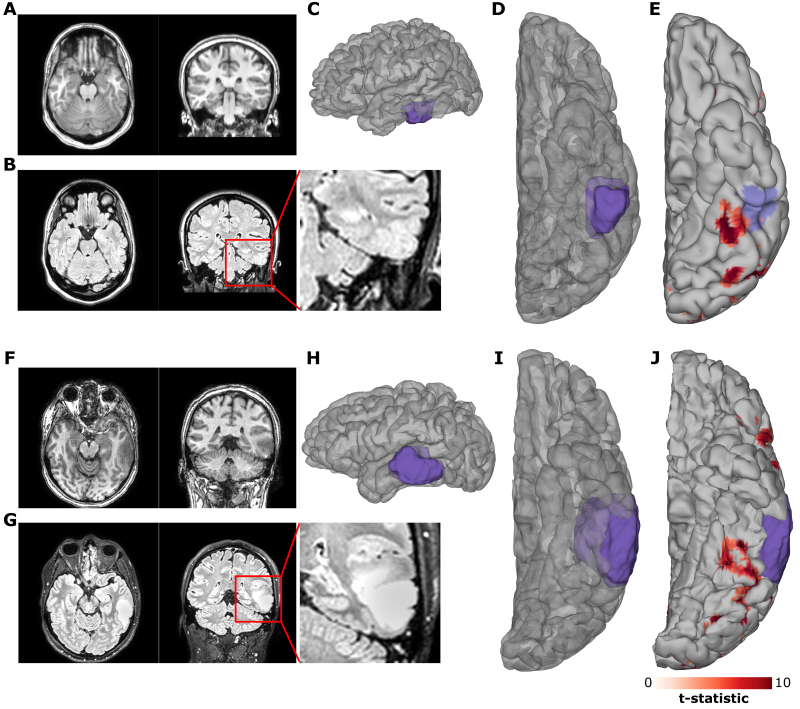
The patient in case 1 was an 18-year-old right-handed woman with medication-resistant focal epilepsy, in whom treatment with lacosamide and lamotrigine had failed. Seizures started as a loss of awareness with subsequent asymmetrical tonic limb posturing, a right-sided figure of 4 sign, and right-sided facial twitching. This evolved into right hemibody clonus and then a generalized tonic-clonic seizure. Her MRI revealed a lesion in the left inferior temporal gyrus consistent with a focal cortical dysplasia (FCD) (Fig. 2A–D). EEG showed interictal spikes and seizure onset localized to the left posterior temporal lobe. Clinical onset of seizures preceded electrographic manifestations on EEG. Magnetoencephalographic source reconstruction localized interictal discharges in the vicinity of the FCD.
FIG. 2.
A, B, F, and G: Preoperative imaging. Preoperative T1-weighted (A, F) and FLAIR (B, G) MRI for case 1 (A, B) and case 2 (F, G). C, D, H, and I: Lateral (C, H) and ventral (D, I) views of 3D reconstructions of the patients’ cortical surfaces and the FCD (case 1 [C, D]) and tumor (case 2 [H, I]), as determined from the preoperative MRI. E and J: fMRI contrast of words > fixation for case 1 (E) and case 2 (J) with the predicted pial involvement of the relevant pathology highlighted. fMRI thresholded at t > 5.
The patient in case 2 was a 34-year-old right-handed man presenting with new-onset seizures resulting in aphasia. His MRI revealed a left temporal lobe tumor spanning across the inferior, middle, and superior temporal gyri (Fig. 2F–I).
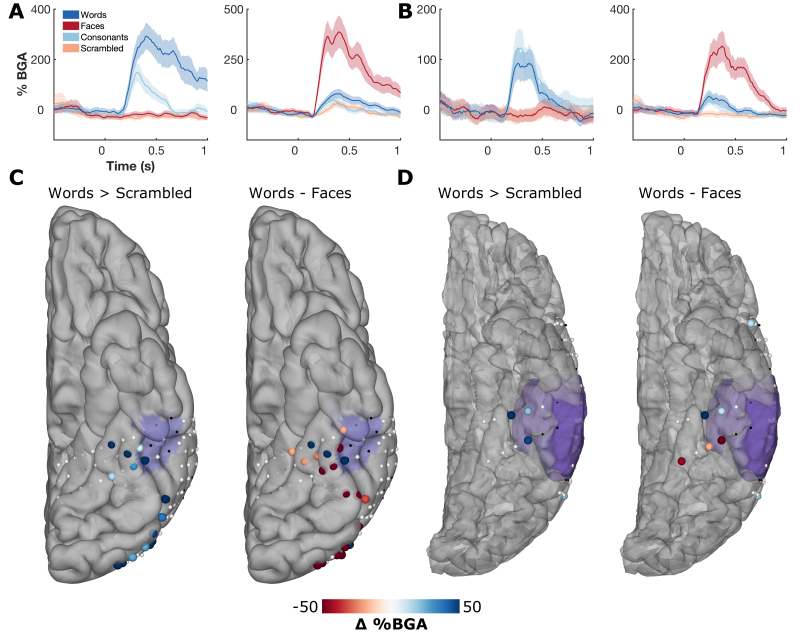
Left hemispheric dominance of language function was confirmed with fMRI in both patients, and both had word-responsive fMRI clusters in close proximity to their pathological tissue (Fig. 2E and J). Multiple electrodes demonstrated strong BGA selectivity for either words or faces (Fig. 3A and B). Contrasts of words and faces demonstrated spatially contiguous clusters of strong selectivity in vOTC (Fig. 3C and D). In both patients, functional BGA responses were observed around the borders of the pathological tissue, as defined by the MRI. Signal quality was comparable to that seen in the extraoperative cases, and there were no notable differences in the quality of neural responses between the < 7-minute (case 1) and < 3-minute (case 2) versions of the paradigm.
FIG. 3.
ECoG category selectivity. A and B: Example responses of word- and face-selective sites from case 1 (A) and case 2 (B). C and D: Spatial map of contrasts of words > scrambled, and words—faces in the window 100–400 msec after stimulus onset for case 1 (C) and case 2 (D). Thresholded by electrodes with a significant difference (2-sample t-test, p < 0.01). Electrodes in black were excluded due to excessive interictal spikes. Preoperative MRI-derived mask of pathological tissue is highlighted in purple.
Cortical Stimulation Mapping
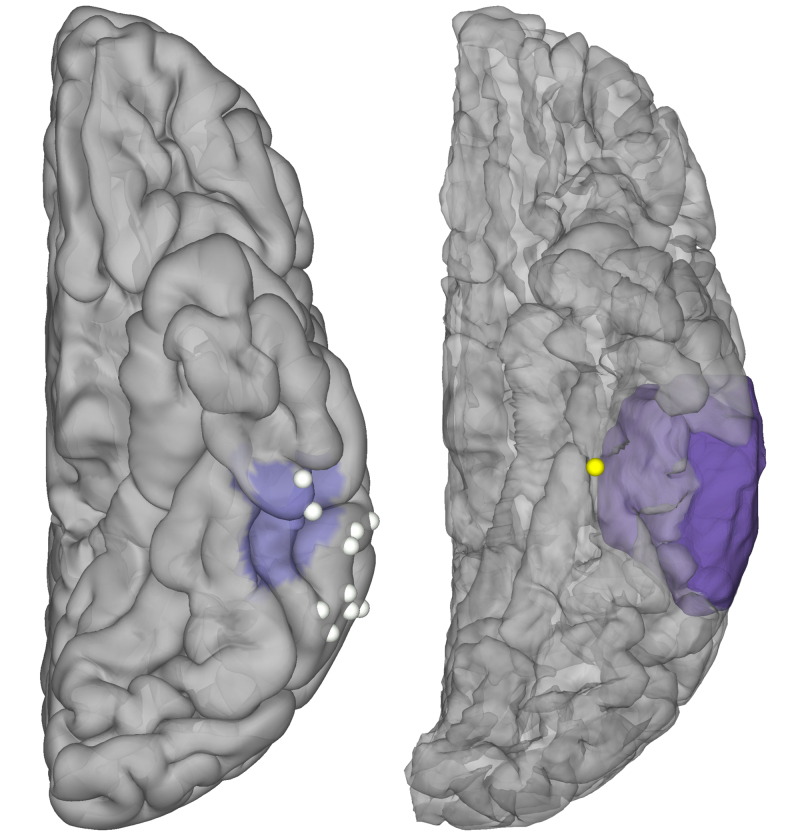
In the patient in case 1, 10 distinct sites across lateral inferior temporal gyrus and over the FCD were tested using a handheld stimulator. All of these sites were CSM and ECoG negative.
In the patient in case 2, the cortical patch that showed greater BGA to words than faces was stimulated using a handheld stimulator, leading to sustained reading disruption (Fig. 4 right, Video 1).
VIDEO 1. Intraoperative stimulation: case 2. Stimulation of word-selective cortex, as determined from intraoperative task-based ECoG activation. Copyright Nitin Tandon. Published with permission. Click here to view.
FIG. 4.
Intraoperative stimulation. Stimulation testing during reading in case 1 (left) and case 2 (right), highlighting CSM-positive (yellow) and CSM-negative (white) sites. MRI-derived mask of pathological tissue is highlighted in purple.
The patient’s subjective reports during reading disruptions were comparable to those from previous studies of stimulation in vOTC:9 “I see the words but they’re not coming out of my mouth.”
During the resection both patients were periodically tested without stimulation, using the same behavioral tasks, and displayed no noticeable deficits at any point.
Postoperative Follow-Up
In the intraoperative patients, standardized testing of reading ability performed following resection confirmed no clinically significant deficits in reading ability (Table 2). Both patients were confirmed to be seizure free at their 6-month follow-up.
TABLE 2.
Assessments of reading fluency in 2 patients with epilepsy who underwent awake resective craniotomy with intraoperative task-based ECoG mapping
| Preop |
Postop |
|||||
|---|---|---|---|---|---|---|
| Raw Score | AE | GE | Raw Score | AE | GE | |
| Case 1 |
|
|
|
|
|
|
| Word attack |
>27 |
>30 |
>12.9 |
>27 |
>30 |
>12.9 |
| Sentence reading fluency |
64 |
8–13 |
8.2 |
60 |
6–12 |
7.1 |
| Passage comprehension |
35 |
6–10 |
5.1 |
38 |
5–12 |
7.0 |
| Spelling |
44 |
8–16 |
11.2 |
44 |
8–16 |
11.2 |
| Case 2 |
|
|
|
|
|
|
| Word attack |
ND |
ND |
ND |
>27 |
>30 |
>12.9 |
| Sentence reading fluency |
ND |
ND |
ND |
88 |
>30 |
>12.9 |
| Passage comprehension |
ND |
ND |
ND |
49 |
>30 |
>12.9 |
| Spelling | ND | ND | ND | 53 | >30 | >12.9 |
AE = age equivalent; GE = grade equivalent; ND = not done.
Assessments of literacy were performed using the Woodcock-Johnson Tests of Cognitive Abilities IV. The patient in case 1 was tested 6 weeks postoperatively, and the patient in case 2 was tested 4 months postoperatively.
Discussion
Prior studies investigating the correspondence of task-based ECoG with cortical stimulation have primarily focused on higher-level language networks underlying visual naming across the lateral cortical surface.18–20 Here we show that task-based ECoG is also of utility for intraoperative mapping of ventral visual cortex, which contains several critical language sites that are mostly underappreciated in the clinical literature. Here we present evidence that intraoperative ECoG recordings in vOTC are robust, reliable, and repeatable. This stimulus-induced activity can act as a rapid method of mapping function in vOTC with little active patient participation required, which further reduces the risk of functional deficits following surgery. Although direct cortical stimulation is relatively time-consuming, its combination with task-based ECoG increases efficiency, reduces the need for active patient participation, and provides an additional tool to localize eloquent cortex.
In the extraoperative cohort we observed a subset of sites in ventral anterior temporal lobe that resulted in stimulation-induced reading disruption but no significant ECoG activity. These sites were all anterior to regions associated with long-term reading or naming deficits as predicted by lesion studies,32 and may be more reflective of spreading effects caused by stimulation within pathological tissue. Additionally, we observed that stimulation through the ECoG electrodes appeared to disrupt a broader area of cortex than is highlighted by ECoG activations. There are several likely explanations for this, including the following. 1) ECoG activation was derived for individual electrodes; however, stimulation was performed on neighboring bipolar pairs. Individual electrodes can be isolated using bipolar stimulation if they result in disruption as part of multiple tested pairs;19 however, given time constraints, it was not possible to stimulate all electrode pairs in these cases, and testing consisted primarily of nonoverlapping electrode pairs. 2) The effects of stimulation probably affect broader areas of cortex than the ECoG signals are recorded from.33 It is well known that at high currents, stimulation can cause nonlocal effects in functionally connected regions.12
Despite prior work linking lesions in this region to permanent reading disruption,8,10,11 in our patients reading-associated tissue was spared, and therefore it cannot be definitively confirmed whether removal of the tissue identified by the ECoG or stimulation mapping would result in acquired dyslexia. However, previous case studies suggest that surgical destruction of word-selective cortex can result in long-lasting postresection reading deficits, characterized by letter-by-letter reading and severely slowed word-reading times.8 Indeed, it is therefore prudent to recognize the underappreciated role of the ventral temporal lobe in language and protect it from iatrogenic injury.34,35
Cortical areas selective to scenes, faces, objects, and words have previously been identified in vOTC.1–3,7,36,37 The current paradigm could easily be expanded to include further stimulus categories to provide a more extensive map of the topography of category selectivity, and may further refine the measured selectivity of each category.
Conclusions
Given that ECoG activations are correlational rather than causal, they do not act as a replacement for traditional stimulation methods. However, this method can act as an effective broad mapping tool to prioritize high-probability functional regions and can serve as an additional tool when mapping eloquent cortex. Although many patients would benefit from mapping techniques that require less patient participation, this mostly passive method would be of particular utility in situations in which an extended stimulation mapping session is impractical, such as in patients experiencing delirium, agitation, or confusion while emerging from sedation, or within pediatric populations, who often have difficulty participating in long, repetitive, and highly structured tasks.17,18 Additionally, this method could be used in patients with seizure-onset zones located in close proximity to functional tissue, given that stimulation-induced disruptions may reflect the induction of a localized seizure rather than direct disruption of eloquent cortex. Altogether our results support the conclusion that the confluence of ECoG activation and stimulation-induced deficits acts as a localizer of the VWFA in midfusiform cortex.9 As such, the use of ECoG and cortical stimulation in combination to localize brain regions essential for reading in both extra- and intraoperative settings is a useful approach to identify functional tissue in order to minimize postoperative reading deficits following resection.10,11
Acknowledgments
We express our gratitude to all the patients who participated in this study; to the neurologists at the Texas Comprehensive Epilepsy Program who participated in the care of these patients; and to the nurses and technicians in the Epilepsy Monitoring Unit at Memorial Hermann Hospital who helped make this research possible. This work was supported by the National Institute of Neurological Disorders and Stroke, grant no. NS098981.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Tandon, Woolnough. Acquisition of data: Woolnough, Morse, McCarty. Analysis and interpretation of data: Woolnough. Drafting the article: Woolnough. Critically revising the article: Tandon, Woolnough, Snyder. Reviewed submitted version of manuscript: all authors. Statistical analysis: Woolnough. Administrative/technical/material support: all authors. Study supervision: Tandon.
Supplemental Information
Video 1. https://vimeo.com/678670345.
Preprint Server
An earlier version of this article can be found on a preprint server.
Preprint server name: medRxiv.
Preprint DOI: 10.1101/2021.11.11.21266202.
References
- 1. Kadipasaoglu CM, Conner CR, Whaley ML, Baboyan VG, Tandon N. Category-selectivity in human visual cortex follows cortical topology: a grouped icEEG study. PLoS One. 2016;11(6):e0157109. doi: 10.1371/journal.pone.0157109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacques C, Witthoft N, Weiner KS, et al. Corresponding ECoG and fMRI category-selective signals in human ventral temporal cortex. Neuropsychologia. 2016;83:14–28. doi: 10.1016/j.neuropsychologia.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woolnough O, Rollo PS, Forseth KJ, Kadipasaoglu CM, Ekstrom AD, Tandon N. Category selectivity for face and scene recognition in human medial parietal cortex. Curr Biol. 2020;30(14):2707–2715.e3. doi: 10.1016/j.cub.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duchaine B, Yovel G. A revised neural framework for face processing. Annu Rev Vis Sci. 2015;1(1):393–416. doi: 10.1146/annurev-vision-082114-035518. [DOI] [PubMed] [Google Scholar]
- 5. Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122(Pt 9):1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 6. Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 7. Yeatman JD, White AL. Reading: the confluence of vision and language. Annu Rev Vis Sci. 2021;7(1):487–517. doi: 10.1146/annurev-vision-093019-113509. [DOI] [PubMed] [Google Scholar]
- 8. Hirshorn EA, Li Y, Ward MJ, Richardson RM, Fiez JA, Ghuman AS. Decoding and disrupting left midfusiform gyrus activity during word reading. Proc Natl Acad Sci U S A. 2016;113(29):8162–8167. doi: 10.1073/pnas.1604126113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woolnough O, Donos C, Rollo PS, et al. Spatiotemporal dynamics of orthographic and lexical processing in the ventral visual pathway. Nat Hum Behav. 2021;5(3):389–398. doi: 10.1038/s41562-020-00982-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pflugshaupt T, Gutbrod K, Wurtz P, et al. About the role of visual field defects in pure alexia. Brain. 2009;132(Pt 7):1907–1917. doi: 10.1093/brain/awp141. [DOI] [PubMed] [Google Scholar]
- 11. Tsapkini K, Rapp B. The orthography-specific functions of the left fusiform gyrus: evidence of modality and category specificity. Cortex. 2010;46(2):185–205. doi: 10.1016/j.cortex.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borchers S, Himmelbach M, Logothetis N, Karnath HO. Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nat Rev Neurosci. 2011;13(1):63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- 13.Mandonnet E, Herbet G, Mandonnet E, Herbet G. Springer International Publishing; 2021. Intraoperative Mapping of Cognitive Networks. [Google Scholar]
- 14. Motomura K, Chalise L, Ohka F, et al. Neurocognitive and functional outcomes in patients with diffuse frontal lower-grade gliomas undergoing intraoperative awake brain mapping. J Neurosurg. 2019;132(6):1683–1691. doi: 10.3171/2019.3.JNS19211. [DOI] [PubMed] [Google Scholar]
- 15. Racine CA, Li J, Molinaro AM, Butowski N, Berger MS. Neurocognitive function in newly diagnosed low-grade glioma patients undergoing surgical resection with awake mapping techniques. Neurosurgery. 2015;77(3):371–379. doi: 10.1227/NEU.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 16.Zemmoura I, Mandonnet E, Cohen L. In: Mandonnet E, Herbet G, eds. Intraoperative Mapping of Cognitive Networks. Springer International Publishing; 2021. Reading; pp. 115–125. [Google Scholar]
- 17. Roland JL, Hacker CD, Leuthardt EC. A review of passive brain mapping techniques in neurological surgery. Neurosurgery. 2020;88(1):15–24. doi: 10.1093/neuros/nyaa361. [DOI] [PubMed] [Google Scholar]
- 18. Arya R, Wilson JA, Fujiwara H, et al. Electrocorticographic high-gamma modulation with passive listening paradigm for pediatric extraoperative language mapping. Epilepsia. 2018;59(4):792–801. doi: 10.1111/epi.14029. [DOI] [PubMed] [Google Scholar]
- 19. Arya R, Horn PS, Crone NE. ECoG high-gamma modulation versus electrical stimulation for presurgical language mapping. Epilepsy Behav. 2018;79:26–33. doi: 10.1016/j.yebeh.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Hays MA, Coogan C, et al. Spatial-temporal functional mapping combined with cortico-cortical evoked potentials in predicting cortical stimulation results. Front Hum Neurosci. 2021;15:661976. doi: 10.3389/fnhum.2021.661976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forseth KJ, Kadipasaoglu CM, Conner CR, Hickok G, Knight RT, Tandon N. A lexical semantic hub for heteromodal naming in middle fusiform gyrus. Brain. 2018;141(7):2112–2126. doi: 10.1093/brain/awy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forseth KJ, Hickok G, Rollo PS, Tandon N. Language prediction mechanisms in human auditory cortex. Nat Commun. 2020;11(1):5240. doi: 10.1038/s41467-020-19010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kay KN, Yeatman JD. Bottom-up and top-down computations in word- and face-selective cortex. eLife. 2017;6:e22341. doi: 10.7554/eLife.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma DS, Correll J, Wittenbrink B. The Chicago face database: a free stimulus set of faces and norming data. Behav Res Methods. 2015;47(4):1122–1135. doi: 10.3758/s13428-014-0532-5. [DOI] [PubMed] [Google Scholar]
- 25. Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s new in psychtoolbox-3? Perception. 2007;36(14):1–16. [Google Scholar]
- 26.Tandon N. In: Yoshor D, Mizrahi E, eds. Clinical Brain Mapping. McGraw Hill Education; 2012. Mapping of human language; pp. 203–218. [Google Scholar]
- 27. Tong BA, Esquenazi Y, Johnson J, Zhu P, Tandon N. The brain is not flat: conformal electrode arrays diminish complications of subdural electrode implantation, a series of 117 cases. World Neurosurg. 2020;144:e734–e742. doi: 10.1016/j.wneu.2020.09.063. [DOI] [PubMed] [Google Scholar]
- 28. Rollo PS, Rollo MJ, Zhu P, Woolnough O, Tandon N. Oblique trajectory angles in robotic stereo-electroencephalography. J Neurosurg. 2021;135(1):245–254. doi: 10.3171/2020.5.JNS20975. [DOI] [PubMed] [Google Scholar]
- 29. Tandon N, Tong BA, Friedman ER, et al. Analysis of morbidity and outcomes associated with use of subdural grids vs stereoelectroencephalography in patients with intractable epilepsy. JAMA Neurol. 2019;76(6):672–681. doi: 10.1001/jamaneurol.2019.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pieters TA, Conner CR, Tandon N. Recursive grid partitioning on a cortical surface model: an optimized technique for the localization of implanted subdural electrodes. J Neurosurg. 2013;118(5):1086–1097. doi: 10.3171/2013.2.JNS121450. [DOI] [PubMed] [Google Scholar]
- 31. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 32. Purcell JJ, Shea J, Rapp B. Beyond the visual word form area: the orthography-semantics interface in spelling and reading. Cogn Neuropsychol. 2014;31(5-6):482–510. doi: 10.1080/02643294.2014.909399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCarty MJ, Woolnough O, Mosher JC, Seymour J, Tandon N. The listening zone of human electrocorticographic field potential recordings. doi: 10.1101/2021.10.22.465519. bioRxiv. Preprint posted online October 24, 2021.doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Snyder KM, Forseth KJ, Donos C, et al. The critical role of the ventral temporal lobe in word retrieval. doi: 10.1101/2021.11.18.469030. bioRxiv. Preprint posted online November 19, 2021.doi: [DOI] [Google Scholar]
- 35. Binder JR, Tong JQ, Pillay SB, et al. Temporal lobe regions essential for preserved picture naming after left temporal epilepsy surgery. Epilepsia. 2020;61(9):1939–1948. doi: 10.1111/epi.16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aguirre GK, Zarahn E, D’Esposito M. An area within human ventral cortex sensitive to "building" stimuli: evidence and implications. Neuron. 1998;21(2):373–383. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 37. Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. https://vimeo.com/678670345.