In Brief
Researchers evaluated the feasibility of collecting bone marrow–derived human mesenchymal stem cells (BM-hMSCs) from previously treated recurrent malignant glioma patients for the delivery of oncolytic viruses. BM-hMSCs were successfully obtained from 5 patients with glioma; when loaded with Delta-24-RGD oncolytic virus, these patient-derived BM-hMSCs effectively eradicated human gliomas in vitro and extended survival in an in vivo mouse model. These findings provide a foundation for future clinical studies using autologous patient-derived BM-hMSCs for malignant glioma.
Keywords: mesenchymal stem cells, glioblastoma, brain tumor, oncolytic virus, oncology
ABBREVIATIONS : BM-hMSC = bone marrow–derived human MSC, DNX2401 = clinical grade Delta-24-RGD, FBS = fetal bovine serum, GBM = glioblastoma, GFP = green fluorescent protein, HD-BM-hMSC = healthy donor–derived BM-hMSC, ISCT = International Society for Cellular Therapy, MEM = minimum essential medium, MOI = multiplicity of infection, MSC = mesenchymal stem cell, PBS = phosphate-buffered saline, PD-BM-hMSC = patient-derived BM-hMSC, PD-BM-MSC-D24 = PD-BM-hMSCs loaded with Delta-24-RGD, PFU = plaque-forming units
Abstract
OBJECTIVE
Delta-24-RGD is an oncolytic adenovirus that is capable of replicating in and killing human glioma cells. Although intratumoral delivery of Delta-24-RGD can be effective, systemic delivery would improve its clinical application. Bone marrow–derived human mesenchymal stem cells (BM-hMSCs) obtained from healthy donors have been investigated as virus carriers. However, it is unclear whether BM-hMSCs can be derived from glioma patients previously treated with marrow-toxic chemotherapy or whether such BM-hMSCs can deliver oncolytic viruses effectively. Herein, the authors undertook a prospective clinical trial to determine the feasibility of obtaining BM-hMSCs from patients with recurrent malignant glioma who were previously exposed to marrow-toxic chemotherapy.
METHODS
The authors enrolled 5 consecutive patients who had been treated with radiation therapy and chemotherapy. BM aspirates were obtained from the iliac crest and were cultured to obtain BM-hMSCs.
RESULTS
The patient-derived BM-hMSCs (PD-BM-hMSCs) had a morphology similar to that of healthy donor–derived BM-hMSCs (HD-BM-hMSCs). Flow cytometry revealed that all 5 cell lines expressed canonical MSC surface markers. Importantly, these cultures could be made to differentiate into osteocytes, adipocytes, and chondrocytes. In all cases, the PD-BM-hMSCs homed to intracranial glioma xenografts in mice after intracarotid delivery as effectively as HD-BM-hMSCs. The PD-BM-hMSCs loaded with Delta-24-RGD (PD-BM-MSC-D24) effectively eradicated human gliomas in vitro. In in vivo studies, intravascular administration of PD-BM-MSC-D24 increased the survival of mice harboring U87MG gliomas.
CONCLUSIONS
The authors conclude that BM-hMSCs can be acquired from patients previously treated with marrow-toxic chemotherapy and that these PD-BM-hMSCs are effective carriers for oncolytic viruses.
Glioblastoma (GBM) is the most common malignant brain tumor in adults. Despite improvements in standard therapy, the median survival time of patients with GBM is only 14 months.1 Treating GBM patients with biological agents such as oncolytic viruses has shown promising results in preclinical animal studies and in the clinic.2–4 Oncolytic viruses are replication-competent, tumor-selective viruses that cause glioma cells to lyse and are subsequently released into the microenvironment. Numerous oncolytic viruses have been tested in clinical trials in a variety of cancers, either as single agents or in combination with chemotherapy or radiation therapy.3,5 We have developed the oncolytic adenovirus Delta-24-RGD as a virotherapy agent to treat GBM. In a phase I clinical trial, Delta-24-RGD was given intratumorally to patients with recurrent malignant glioma (clinicaltrials.gov identifier NCT00805376);3 analysis of posttreatment specimens demonstrated that Delta-24-RGD replicated in tumor cells and induced an antiglioma immune response. Furthermore, outcome data showed potential efficacy, as 12% of patients had complete and durable (> 3.5 years) responses. However, the efficacy of these intratumorally delivered biological therapies may be limited by their inefficient distribution to the majority of the tumor.6,7 Thus, there is an urgent need to develop new delivery methods for Delta-24-RGD.
To further improve reagent delivery, stem cell carriers have been explored. Specifically, bone marrow–derived human mesenchymal stem cells (BM-hMSCs) have been used as cellular carriers of antitumoral agents, including oncolytic viruses,8,9 because BM-hMSCs are known to have the ability to migrate to a variety of solid tumors after systemic injection.10 Over the past 10 years, we and others have demonstrated the feasibility of using healthy donor–derived BM-hMSCs (HD-BM-hMSCs) to deliver antitumoral agents to tumors in mouse models of glioma.11 Specifically, we were the first to show that HD-BM-hMSCs localize to human gliomas after systemic (intraarterial) delivery.12 We also identified a causal role for platelet-derived growth factor–BB13 and transforming growth factor–β14 in the localization of HD-BM-hMSCs to gliomas. Most importantly, we were the first to show that the intravascular delivery of HD-BM-hMSCs carrying either interferon-β or Delta-24-RGD resulted in improved survival and even cures in a subset of animals harboring human gliomas,12,15 establishing HD-BM-hMSCs loaded with antiglioma agents as a powerful therapeutic agent for the treatment of gliomas.
The basic premise of the described studies is consistent with the allogeneic BM-hMSC strategy. BM-hMSCs can be acquired from healthy donors and used as allogeneic transplants. On the other hand, BM-hMSCs can be acquired from patients and delivered back as autologous transplants, thus avoiding the allo-immune barrier and the possibility of rejection.16,17 Although autologous BM-hMSCs have advantages, the extent to which BM-hMSCs can be acquired from the BM of GBM patients who have been treated with radiation therapy and myelosuppressive chemotherapy remains unclear. In addition, even if autologous BM-hMSCs can be acquired, it is unclear whether they are capable of homing to and delivering a therapeutic agent like Delta-24-RGD to brain tumors. To address this gap in knowledge, we isolated BM-hMSCs from patients with recurrent high-grade gliomas and evaluated their ability to infect and eradicate gliomas after they had been loaded with Delta-24-RGD.
Methods
Preparation of BM-hMSCs
Patients who had been treated with radiation therapy and chemotherapeutic agents (procarbazine, lomustine, vincristine, or temozolomide) signed informed consent and were enrolled in a prospective clinical protocol (Table 1). Prior to undergoing craniotomy for their recurrent tumors, patients were put under general anesthesia and BM aspirates were obtained from the iliac crest; BM-hMSCs were isolated and cultured from these aspirates, as previously described.18 In brief, mononuclear cells were collected from the interface generated after Histopaque (Sigma-Aldrich) density gradient separation and washed once in MSC culture medium: α-minimum essential medium (MEM; Mediatech) supplemented with 100 U/ml of penicillin, 100 mg/ml of streptomycin sulfate (Flow Laboratories), 2 mM of L-glutamine (Mediatech), and 20% (v/v) fetal bovine serum (FBS; Lonza). The mononuclear cells from 10 ml of BM aspirate were cultured in a 75-cm2 culture flask at a concentration of 1–5 × 106/ml at 37°C. After 2–3 days, nonadherent (hematopoietic) cells were removed and adherent cells were cultured in MSC culture medium until the cells achieved approximately 70% confluence. Adherent cells were released by trypsin digestion (1 × trypsin-EDTA, Invitrogen) and subcultured into new 75-cm2 culture flasks. The BM-hMSCs were maintained as confluent monolayers in 175-cm2 tissue culture flasks and were subcultured at least 3 times before the cells were placed in frozen storage, freezing to ensure the removal of residual hematopoietic cells. The cells used in the described experiments were from cell passages 3–6. As a positive control, clinical grade BM-hMSCs isolated from the iliac crest of a healthy male donor who had never received chemotherapy were obtained from the Department of Stem Cell Transplantation at MD Anderson.
TABLE 1.
Characteristics of patients from whom BM-hMSCs were derived
| Case No. | Age (yrs) | Sex | Pathological Dx | Previous Treatment | Time from Chemo to Harvest | Hb (g/dl) | WBCs (K/μl) | Platelets (K/μl) |
|---|---|---|---|---|---|---|---|---|
| 001 |
58 |
M |
GS |
S, RT, TMZ |
2 mos |
14.8 |
10.8 |
222 |
| 002 |
51 |
F |
AA |
S, RT, PCV |
18 yrs |
13.3 |
4.6 |
182 |
| 003 |
52 |
M |
AA |
S, RT, PCV, TMZ |
3 mos |
14.3 |
5.7 |
224 |
| 004 |
38 |
F |
GBM |
S, RT, TMZ |
7 yrs |
14.6 |
9.5 |
283 |
| 005 | 46 | F | AO | S, RT, TMZ | 14 yrs | 11.6 | 4.4 | 334 |
AA = anaplastic astrocytoma; AO = anaplastic oligodendroglioma; Dx = diagnosis; GS = gliosarcoma; PCV = procarbazine, lomustine, and vincristine; RT = radiotherapy; S = surgery; TMZ = temozolomide; WBC = white blood cell.
Characterization of BM-hMSCs
Isolated cells were characterized as BM-hMSCs using the criteria developed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT), namely 1) adherence to tissue culture plastic; 2) positive expression of CD105, CD73, and CD90 but lacking expression of CD34, CD45, and CD11b; and 3) capacity to differentiate into osteoblasts, adipocytes, and chondroblasts under standard in vitro differentiation conditions.19,20 Expression markers were analyzed at passages 3–4 by flow cytometry for CD105, CD73, CD90, CD34, CD45, and CD11b.
Differentiation Protocols
For adipogenesis, 105 cells were plated in a 12-well culture plate, and when the cells reached 100% confluence, adipogenic induction medium (Lonza) was added and changed every 3 days. After 3 weeks, cells were stained with Oil Red O. For osteogenesis, 5 × 104 cells were seeded in a 12-well culture plate. After 24 hours, differentiation was induced with osteogenic induction medium (Lonza), and the medium was changed every 3 days. After 3 weeks, cells were stained with Alizarin Red. For chondrogenesis, cell pellets were prepared by pelleting 3 × 105 cells in 15-ml polypropylene tubes and were supplemented with complete chondrogenic induction medium (Lonza). Cell pellets were fed with fresh complete chondrogenic medium every 3 days. After 4 weeks, pellets were fixed with 10% formalin and paraffin embedded; 5-μm sections were mounted on slides and stained for glycosaminoglycans using Safranin O.
Tumor Cells
U87MG cells were obtained from American Type Culture Collection. The cells were grown in MEM containing 10% FBS and 1% penicillin-streptomycin. U87MG-LucNeo was provided by B.S. Carter (Massachusetts General Hospital) and grown in U87MG medium containing 0.5 mg/ml of zeocin, as described previously.21
Electron Microscopy
Cell samples were fixed with a solution containing 3% glutaraldehyde plus 2% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.3) for 1 hour. After fixation, the samples were washed and treated with 0.1% Millipore-filtered cacodylate-buffered tannic acid, then postfixed for 1 hour with 1% buffered osmium tetroxide, and finally stained with 1% Millipore-filtered uranyl acetate (Sigma-Aldrich). The samples were dehydrated in ethanol and embedded in Spurr’s low-viscosity medium. They were then polymerized in an oven for 2 days at 70°C. Ultrathin sections were cut with a Leica Ultracut microtome, stained with uranyl acetate and lead citrate in a Leica electron microscopy stainer, and examined using a JEM-1010 transmission electron microscope (JEOL USA Inc.) at an accelerating voltage of 80 kV. Digital images were obtained using imaging system AMT software (Advanced Microscopy Techniques).
MSC Labeling and Infection With Delta-24-RGD
For in vivo studies, BM-hMSCs were transduced with green fluorescent protein (GFP) using replication-incompetent Ad5/F35-CMV-GFP (adenovirus type 5 containing in its E3 region the GFP gene driven by the cytomegalovirus promotor; Vector Development Laboratory, Baylor College of Medicine), as previously described.15 BM-hMSC monolayers were treated with 50 multiplicities of infection (MOIs) in 3 ml of serum-free MSC medium and were shaken every 10 minutes at 37°C. After 1 hour, MSC medium containing 10% FBS was added.
For infection with Delta-24-RGD, 10 to 100 plaque-forming units (PFU)/cell of viral stock solution were added to 3 ml of serum-free medium.
In Vitro Efficacy Testing
Transwell assays were performed using 0.4-μm inserts (Greiner Bio-One). BM-hMSCs were infected with Delta-24-RGD at 50 MOIs. Twenty-four hours after infection, cells were collected, washed, and replated at various densities of cells/well in the upper wells and placed over the lower wells containing 104 U87MG cells. After 7 days, the numbers of viable U87MG cells were counted using Vi-Cell XR (version 1.01, Beckman Coulter Inc.).
Intracranial Injection
Animals (n = 95) were housed and cared for as previously described in accordance with Institutional Animal Care and Use Committee guidelines.22 For glioma xenograft implantation, U87MG or U87MG-LucNeo cells were grafted via cranial guide screws, as described previously.23 Mice were injected with 5 × 105 cells via a Hamilton syringe inserted to a depth of 5 mm. Cells were simultaneously implanted in 10 mice via a microinfusion syringe pump (0.5 μl/min, Harvard Apparatus), as described previously.12 For intracranial virus injection, mice were injected twice with 5 μl of Delta-24-RGD (108 PFU) via guide screws, as described previously.23 A summary of all animal model experiments is depicted in Supplementary Fig. 1.
Internal Carotid Artery Injection of BM-hMSCs
BM-hMSCs were trypsinized, centrifuged (1500 rpm, 5 minutes), and resuspended in MSC medium with 10% FBS at 106 cells per 100 μl. Injection into the right internal carotid artery of the mice was performed using a 30-gauge needle, as described previously.24
Detection of Delta-24-RGD in Tumor
Twenty-four hours after plating, BM-hMSCs (106) were infected with Delta-24-RGD at 50 MOIs for 1 hour. Twenty-four hours after infection, BM-hMSCs were trypsinized and collected by centrifugation. Resuspended BM-hMSCs (5 × 104) were plated on a Lab-Tek chamber slide (Thermo Fisher Scientific Life Sciences). Forty-eight hours after infection, chamber slides were fixed with 2% paraformaldehyde for 10 minutes at room temperature, treated with 5% bovine serum albumin, and then stained with the following antibodies to detect Delta-24-RGD: mouse antihexon (1:500 dilution, Santa Cruz Biotechnology) and rabbit anti-E1A (1:400 dilution, Santa Cruz Biotechnology) antibodies. Primary antibodies were incubated overnight at 4°C. Immunohistochemical analyses were performed using the Vectastain ABC-HRP kit (Vector Laboratories) according to the manufacturer’s protocol.
Detection and Quantification of BM-hMSCs in Tumors
Three days after injection of GFP-transduced BM-hMSCs, the mice were humanely killed via intracardiac perfusion of phosphate-buffered saline (PBS) and 4% paraformaldehyde. Their brains were removed, fixed in 10% formalin for 24 hours, embedded in paraffin, and cut into 5-μm sections. For immunofluorescence of GFP-labeled BM-hMSCs, paraffin sections were stained with rabbit anti-GFP antibodies (1:200 dilution, Abcam). Immunofluorescence was detected using an Alexa Fluor 488–conjugated secondary antibody (1:200 dilution, Invitrogen) according to the manufacturer’s protocol. H & E staining or 4′,6-diamidino-2-phenylindole staining (Vectashield H-1200, Vector Laboratories) was performed for visualization of the tumor.
The tumor was outlined within each section, and the tumor area was determined using Olympus cellSens imaging program version 1.15 (Olympus Scientific Solutions Americas Inc.). The number of GFP-positive cells in the tumor area was counted using a fluorescence microscope (40×); the number of cells per mm2 of tumor was calculated for each section and averaged among all sections.
Bioluminescence Imaging
Mice harboring U87MG-LucNeo cells were given 4 mg of D-luciferin by intraperitoneal injection and imaged with the IVIS-200 system (Xenogen Corp.). Bioluminescence images were overlaid on grayscale photographic images using Living Image 3.0 software (Xenogen Corp.).
Statistical Analysis
Cell counts were expressed as the mean ± standard deviation of at least 3 replicate determinations for each experiment. Comparisons were made using one-way ANOVA. Survival curves were compared using the log-rank test. Analyses were performed using GraphPad Prism 6.05. A p value < 0.05 was considered statistically significant.
Results
Derivation of BM-hMSCs From Glioma Patients
Five patients with high-grade glioma at recurrence, including gliosarcoma, anaplastic astrocytoma, GBM, and anaplastic oligodendroglioma, were enrolled in the study (Table 1). All patients underwent successful iliac crest aspiration while under general anesthesia. The time between their last chemotherapy and the cell harvest ranged from 2 months to 18 years, and no patients had evidence of myelosuppression on examination of their blood cell counts at the cell harvest. Cells from the aspirates were successfully grown as adherent cultures from all 5 patients using the protocol for isolating BM-hMSCs, as described in Methods. Cells from the aspirate of a healthy male donor were also grown using the same methods.
Characterization of BM-hMSCs
Cells from all 5 patients were isolated and numerically expanded to large-scale cultures. Consistent with the definitions of BM-hMSCs developed by the ISCT20 and with the characteristics of BM-hMSCs from healthy donors (HD-BM-hMSCs), adherent cells from all 5 patients exhibited fibroblast-like morphology and formed homogeneous colonies at passage 3 (Fig. 1A). Cells at passages 3 and 4 were assayed for the expression of canonical BM-hMSCs markers. Flow cytometry revealed that more than 97% of cells in all 5 cell lines expressed CD105, CD73, and CD90 and were negative for CD34, CD45, and CD11b (Table 2), consistent with the surface marker characteristics of BM-hMSCs. In addition, all 5 cell lines underwent adipogenesis, osteogenesis, and chondrogenesis using the respective culture conditions for these processes and successfully differentiated into adipocytes, osteoblasts, or chondrocytes, respectively (Fig. 1B). These results collectively demonstrated that the cells met the histological, surface marker, and differentiation criteria for BM-hMSCs and are henceforth referred to as “patient-derived BM-hMSCs” (PD-BM-hMSCs).
FIG. 1.
Characterization of BM-hMSCs. A: Morphological features of cells from 5 different patients. Similar to the cells from the healthy donor (HD-BM-hMSCs), all of these cells showed fibroblast-like morphology and formed homogeneous colonies at passage 3. Original magnification ×100. B: Differentiation of BM-hMSCs. All 5 PD-BM-hMSC cell lines could successfully differentiate into adipocytes, osteoblasts, or chondrocytes. For osteogenic and adipogenic differentiation, original magnification ×100 from inverted microscope. For chondrogenic differentiation, bar = 100 μm from upright brightfield microscope.
TABLE 2.
Flow cytometric analysis of cell surface markers in PD-BM-hMSC lines
| MSCs | CD105 | CD73 | CD90 | CD105/73/90 | CD45 | CD34 | CD11b |
|---|---|---|---|---|---|---|---|
| PD-BM-MSC-001 |
99.4 |
99.8 |
99.2 |
98.7 |
0 |
0 |
0 |
| PD-BM-MSC-002 |
99.7 |
99.9 |
99.4 |
98.8 |
0.12 |
0 |
0 |
| PD-BM-MSC-003 |
99.5 |
99.8 |
99.2 |
97.1 |
0.46 |
0 |
0 |
| PD-BM-MSC-004 |
99.1 |
99.9 |
99.8 |
98.6 |
0.22 |
0 |
0 |
| PD-BM-MSC-005 | 99.5 | 99.9 | 99.6 | 98.6 | 0 | 0 | 0 |
Delta-24-RGD Infection and Replication in PD-BM-hMSCs In Vitro
The ability of Delta-24-RGD to infect and replicate in PD-BM-hMSCs was explored. Forty-eight hours after infection with 50 MOIs of Delta-24-RGD, PD-BM-hMSCs were analyzed by immunohistochemistry for adenoviral E1A and hexon proteins (Fig. 2A). Viral protein expression for both E1A and hexon was seen only after infection with Delta-24-RGD. Electron microscopy revealed viral replication within PD-BM-hMSCs (Fig. 2B). Virions were observed in the nucleus after 24 hours and in the cytoplasm surrounding the inclusion body after 48 hours.
FIG. 2.
Delta-24-RGD infection and replication within PD-BM-hMSCs in vitro. A: Viral E1A and hexon proteins were seen in PD-BM-hMSCs. The PD-BM-hMSCs were infected with Delta-24-RGD (50 MOIs, 1 hour), trypsinized, and replated on chamber slides, as described in Methods. Forty-eight hours after infection, slides were immunostained with anti-E1A or antihexon antibodies. Representative PD-BM-hMSCs, PD-BM-MSC-001, showed viral E1A protein and hexon protein in cells. B: Representative electron micrographs showed viral replication in PD-BM-MSC-001. PD-BM-hMSCs were infected with Delta-24-RGD at 50 MOIs and examined at 24 and 48 hours after infection, as indicated in the figure. Virions were seen in the nucleus after 24 hours and in the cytoplasm surrounding the inclusion body (arrow) after 48 hours. h = hours.
In Vitro Efficacy Testing
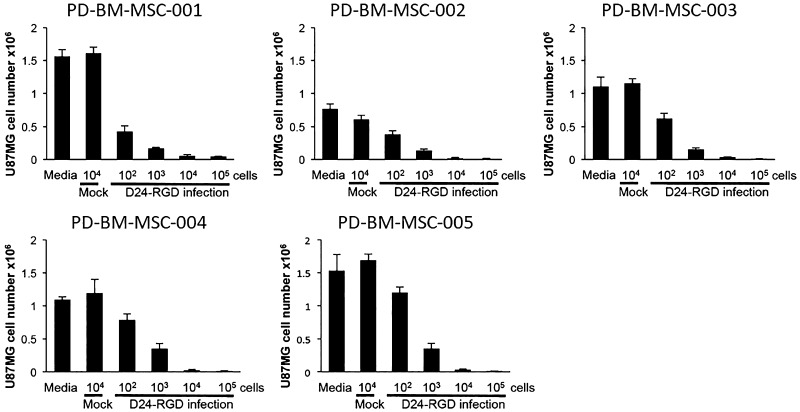
To assess the capacity of PD-BM-hMSCs loaded with Delta-24-RGD (PD-BM-MSC-D24) to release, infect, and lyse human gliomas, in vitro Transwell expirations were undertaken. PD-BM-MSC-D24 cells were placed in the upper wells at increasing doses (102–105 cells/well), and U87MG cells (104) were placed in the lower wells. PD-BM-hMSCs not infected with Delta-24-RGD (mock) were used as controls. Quantitative analyses of the viability of U87MG cells after 7 days demonstrated that PD-BM-hMSCs release viable Delta-24-RGD that is capable of infecting and killing U87MG cells in a dose-dependent manner (Fig. 3), which was observed for all PD-BM-MSC-D24 cell lines.
FIG. 3.
Transwell assay. PD-BM-hMSCs were infected with Delta-24-RGD at 50 MOIs and then washed and plated in the upper wells of a Transwell plate at varying numbers of cells/well (ranging from 102 to 105 cells), as shown in this figure. U87MG cells were plated in the bottom wells (104 cells per well) and incubated for 7 days, after which they were counted. Killing occurred in a dose-response manner in all PD-BM-MSC-D24 cell lines.
PD-BM-hMSCs Localize to Human Gliomas After Intracarotid Delivery
To determine the extent to which PD-BM-hMSCs are capable of selectively localizing to human gliomas after intraarterial delivery in vivo, U87MG cells (5 × 105) were implanted in the frontal lobes of nude mice (n = 3 for each PD-BM-hMSC). The PD-BM-hMSCs were transduced with Ad5/F35-CMV-GFP (50 MOIs) and injected into the right internal carotid artery (106 cells/100 μl of medium) of mice harboring 7-day-old xenografts. As a control, HD-BM-hMSCs were also injected into tumor-bearing mice (n = 3). The mice were humanely killed 3 days later. H & E and immunofluorescence staining with anti-GFP antibodies demonstrated PD-BM-hMSCs within the tumors for each of the 5 PD-BM-hMSCs (Fig. 4A). Virtually no PD-BM-hMSCs were seen elsewhere in the brain. To quantify these results, at least 5 tissue sections were counted per mouse, and the number of PD-BM-hMSCs per area of tumor was determined. Analyses across the 5 PD-BM-hMSCs revealed some variation in the homing capacities of PD-BM-hMSCs from the different donors, but there were no significant differences among these PD-BM-hMSCs compared with HD-BM-hMSCs, according to ANOVA (p = 0.07, one-way ANOVA; Fig. 4B). This result indicates that PD-BM-hMSCs maintain their capacity to home to gliomas despite prior exposure to chemotherapy and radiation.
FIG. 4.
PD-BM-hMSCs localize to human gliomas after intracarotid delivery. A: Representative mouse microscopic section showing U87MG xenografts; PD-BM-MSC-001 labeled with GFP was injected into the mouse via the carotid artery. One million GFP-labeled PD-BM-hMSCs were injected into the right carotid artery on day 7, and the mouse brain was extracted for histological examination on day 10. Bar = 500 μm (left). Immunofluorescence microscopy was used to track PD-BM-hMSCs to U87MG xenografts after intracarotid injection. Sections were cut and immunostained with FITC-labeled anti-GFP antibody (green). Sections were also stained with 4′,6-diamidino-2-phenylindole (DAPI). The boxed area was studied by immunofluorescence microscopy using anti-GFP antibodies to reveal the distribution of PD-BM-hMSCs within the tumor. Bar = 200 μm (right). B: Density of GFP-positive cells within U87MG xenografts from each of the injected PD-BM-hMSC cell lines. Cell density was determined as described in Methods. As compared with HD-BM-hMSCs, significant differences were not observed among these PD-BM-hMSC cell lines (n = 3 for each PD-BM-hMSC cell line; p = 0.069, one-way ANOVA).
PD-BM-MSC-D24 Inhibit Xenograft Growth and Improve Survival
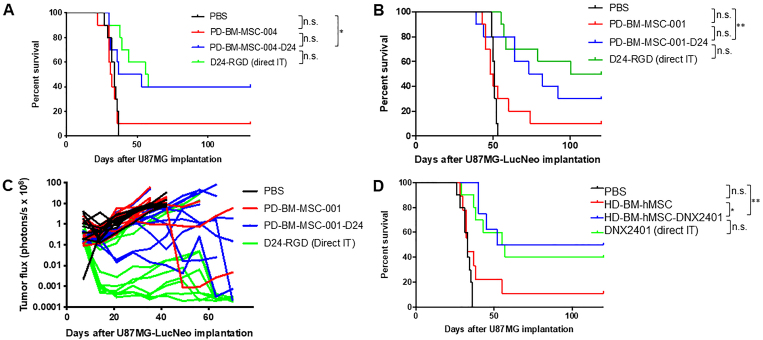
We then examined the extent to which PD-BM-MSC-D24 are efficacious at eradicating gliomas in vivo. U87MG cells were implanted (5 × 105 cells) in the brains of nude mice, and 4 and 18 days later, the mice (n = 10/group) were treated with PBS, PD-BM-MSC-004 (1.5 × 106 cells/100 μl), or PD-BM-MSC-004 loaded with Delta-24-RGD (PD-BM-MSC-004-D24; 1.5 × 106 cells/100 μl) via internal carotid artery injection (Supplementary Fig. 2A). A separate group of mice (n = 10) received two direct intratumoral injections of Delta-24-RGD (108 PFU) at 4 and 8 days after implantation (Supplementary Fig. 2B). Survival analyses showed significant improvement in median survival from 34 days after treatment with PBS to 45 days after treatment with PD-BM-MSC-004-D24, with 40% of mice treated with PD-BM-MSC-004-D24 living long term (> 100 days) as compared with 0% after treatment with PBS (p < 0.05, log-rank test; Fig. 5A). Treatment with PD-BM-MSC-004-D24 resulted in improved survival compared to that with PD-BM-MSC-004 (uninfected), although the difference between the two did not reach significance because of one outlier in the PD-BM-MSC-004 group (p = 0.05). There was no significant difference in survival between those injected with PD-BM-MSC-004-D24 and two direct injections of Delta-24-RGD (p = 0.66).
FIG. 5.
PD-BM-MSC-D24 inhibits xenograft growth and improves survival. Survival data corresponding to two independent experiments in which nude mice were implanted with U87MG (A) and U87MG-LucNeo (B). In each experiment, mice underwent treatments of intraarterial injection with PBS, with uninfected PD-BM-hMSCs (1.5 × 106 cells, PD-BM-MSC-004 [A] or PD-BM-MSC-001 [B]), or with PD-BM-MSC-D24 (1.5 × 106 cells, 50 MOIs × 24 hours, PD-BM-MSC-004-D24 [A] or PD-BM-MSC-001-D24 [B]). A group of mice received two direct intratumoral injections of Delta-24-RGD (108 PFU). n = 10 mice/group. Using cells from donor 004 (A), the median survival time was 45 days in PD-BM-MSC-004-D24–treated mice compared with 34 days in PBS controls and 31.5 days in mice given uninfected PD-BM-MSC-004. After treatment with PD-BM-MSC-004-D24, 40% of animals lived long term (> 100 days). Using cells from donor 001 (B), the median survival time was 77.5 days in PD-BM-MSC-001-D24–treated mice compared with 51 days in PBS controls and 49 days in mice given uninfected PD-BM-MSC-001. After treatment with PD-BM-MSC-001-D24, 30% of animals lived long term (> 100 days). Photon flux of PD-BM-MSC-001 xenografts (C). Total photonic flux was measured from fixed regions of interest encompassing the entire mouse head. Each line represents efflux from a single animal. Slower growth is seen with PD-BM-MSC-001-D24 treatment and intratumoral injection treatment. Survival data (D) using clinical grade healthy donor MSCs (HD-BM-hMSCs) and clinical grade Delta-24-RGD (DNX2401) in which nude mice were implanted with U87MG. The median survival time was 86 days in HD-BM-hMSC-DNX2401–treated mice compared with 33 days in PBS controls and 33 days in mice given uninfected HD-BM-hMSCs (n = 9 mice for HD-BM-hMSCs, n = 8 mice for HD-BM-hMSC-DNX2401, and n = 10 mice/group for the other groups). D24-RGD = Delta-24-RGD; IT = intratumoral injection; n.s. = not significant. *p < 0.05 and **p < 0.01, log-rank test.
The experiment was repeated using different PD-BM-hMSCs (PD-BM-MSC-001; Fig. 5B). In addition, in this experiment, U87MG-LucNeo cells were employed to allow bioluminescence tracking of tumor growth. Briefly, U87MG-LucNeo cells were implanted (5 × 105 cells) in the brains of nude mice, and after 4 and 18 days, mice (n = 10/group) were treated with PBS, PD-BM-MSC-001 (1.5 × 106 cells/100 μl), or PD-BM-MSC-001-D24 (1.5 × 106 cells/100 μl) via carotid artery injection. Survival analyses showed a significant improvement in median survival from 51 to 77.5 days (p < 0.01; Fig. 5B), with 30% of mice treated with PD-BM-MSC-001-D24 living long term (> 100 days) compared with 0% after treatment with PBS. Treatment with PD-BM-MSC-001-D24 resulted in improved survival compared to that with PD-BM-MSC-001 (uninfected), although the difference between the two did not reach significance because of one outlier in the PD-BM-MSC-001 group (p = 0.08). There was no significant difference in survival between those injected with PD-BM-MSC-001-D24 and direct injection of Delta-24-RGD (p = 0.39). Bioluminescence imaging revealed marked differences in photon flux among the groups by 4 weeks (Supplementary Fig. 3). Total photonic flux was measured from fixed regions of interest encompassing the entire mouse head. Quantitative analyses of each animal showed slower growth with the PD-BM-MSC-D24 and Delta-24-RGD direct injection groups compared with the other groups (Fig. 5C).
In order to provide a comparison of PD-BM-hMSCs with HD-BM-hMSCs, an independent survival study was performed using clinical, good manufacturing practices grade HD-BM-hMSCs and clinical grade Delta-24-RGD (DNX2401; n = 9 mice for HD-BM-hMSCs, n = 8 mice for HD-BM-hMSC-DNX2401, n = 10 mice/group for the other groups). Survival analyses showed a significant improvement in median survival from 33 days after treatment with PBS to 86 days after treatment with HD-BM-hMSC-DNX2401 (p < 0.01; Fig. 5D). Furthermore, there were significant differences between those injected with noninfected HD-BM-hMSCs and those injected with HD-BM-hMSC-DNX2401 (p = 0.02). Interestingly, there was one outlier in the noninfected HD-BM-hMSCs group, which is similar to what was observed in the PD-BM-hMSC-D24 survival studies (Fig. 5A and B). Comparing these results using HD-BM-hMSCs (from a healthy donor) with the outcomes of the independent experiments using PD-BM-hMSCs obtained from patients previously treated with radiation and chemotherapy (Fig. 5A and B), we noted that the PD-BM-hMSCs perform similarly to the HD-BM-hMSCs.
Discussion
Here we show that BM-hMSCs can be successfully isolated from patients with recurrent high-grade gliomas who had been previously treated with radiation therapy and chemotherapy, which are therapies that can cause BM toxicity. In 5 consecutive cases, these PD-BM-hMSCs were similar to those from healthy donors, meeting the definition of BM-hMSCs based on ISCT criteria in terms of morphology, surface markers, and differentiation ability. PD-BM-hMSCs were easily expanded numerically in vitro and effectively homed to intracranial glioma xenografts in mice after intraarterial injection. Importantly, PD-BM-MSC-D24 were able to eradicate human gliomas in vitro and in vivo just as effectively as were clinical grade HD-BM-hMSCs. Specifically, two intracarotid injections of PD-BM-MSC-D24 into U87MG glioma-bearing mice resulted in significantly increased survival compared with that in controls.
We and others have explored using various cell types as carriers of oncolytic viruses, including HD-BM-hMSCs, neural stem cells, and T cells.25 To be a suitable cell carrier for oncolytic viruses, several conditions must be met: 1) the virus must be able to infect the cell carrier; 2) the kinetics of viral replication and release must be favorable; and 3) the cell carrier must be able to travel from the site of injection to the tumor location. This study demonstrated that Delta-24-RGD infects and replicates in PD-BM-hMSCs, that PD-BM-hMSCs effectively deliver Delta-24-RGD to the tumors, and that the kinetics of infection and release are favorable for tumor eradication. Therefore, our studies support the notion that BM-hMSCs obtained from patients previously treated with radiation and chemotherapy (i.e., PD-BM-hMSCs) are capable of delivering functional Delta-24-RGD to malignant gliomas.
Originally, allogeneic MSCs were used for translational and clinical studies11 because the methods for acquiring MSCs from healthy donors are well established. Furthermore, BM-hMSCs are known to express a low level of major histocompatibility complex class I molecules and do not express major histocompatibility complex class II antigens on the cell surface.26 However, a recent study showed that hMSCs may not be immune privileged.16 Therefore, using autologous BM-hMSCs has an advantage in terms of avoiding an unfavorable immune reaction. Currently, several clinical trials have evaluated, or are in the process of evaluating, autologous hMSCs, either from BM or from other sources, as oncolytic virus carriers. For example, a phase I/II trial at the Mayo Clinic (Principal Investigator E. Galanis, M.D.) is underway to determine the safety, dosage, and clinical effects of using adipose tissue–derived autologous hMSCs infected with an oncolytic measles virus to treat patients with relapsed ovarian cancer via intraperitoneal injection (clinicaltrials.gov identifier NCT02068794). Nevertheless, questions remain regarding whether BM-hMSCs can be acquired from patients treated with chemoradiation and whether these hMSCs retain the ability of homing to and delivering an oncolytic virus to brain tumors. Here we have shown the feasibility of acquiring BM-hMSCs from patients with high-grade glioma who have undergone toxic chemotherapy. Although the time between the last chemotherapy and BM-hMSCs harvest varied from 2 months to 18 years in our cohort, all of the PD-BM-hMSCs showed a similar ability to load and release Delta-24-RGD in vitro and to home to brain tumors in vivo compared with HD-BM-hMSCs. Moreover, even though PD-BM-MSC-001 cells were isolated just 2 months after temozolomide chemotherapy, they were capable of being expanded numerically, and PD-BM-MSC-001-D24 cells retained their ability to kill human glioma cells in vitro and in vivo.
Upon analysis of tumor size by bioluminescence imaging, intratumoral injection of naked DNX2401 (without BM-hMSCs) appeared to be superior to intraarterial injection of BM-MSCs loaded with DNX2401 (Fig. 5C), even though there was not a significant difference in animal survival (Fig. 5D). A possible explanation for this difference is that the intratumoral treatment group was given injections on days 4 and 8 after tumor implantation, whereas the intraarterial injection group was treated on days 4 and 18, with a delayed second injection to allow greater vascularization of the tumor after implantation. We posit that intratumoral injection initially appears somewhat better based on bioluminescence analyses than with intraarterial injection in this experiment because the tumor is smaller at the time of treatment in the intratumoral injection cohort than in the intraarterial group. The survival curves of the two groups are nearly identical because the day 18 intraarterial injection effectively reduces the size of a larger tumor. In the clinical setting, our studies support the use of both strategies, and one can envision that smaller tumors may be treated with direct intratumor injection, whereas intraarterial injection may be best suited for larger tumors.
It was of interest that in each of our in vivo efficacy experiments, at least 1 animal in the BM-hMSC group (no Delta-24-RGD) lived long term. Bioluminescence analysis in one experiment showed a loss of signal consistent with eradication of the tumor, but histological study showed that a fibrotic mass had replaced the tumor. These results suggest that on occasion BM-hMSCs may be capable of inducing transdifferentiation of the tumor cells into fibroblastic cells. Although this phenomenon needs to be explored further, it was not observed when Delta-24-RGD was loaded into the MSCs. Both PD-BM-MSC-D24 and HD-BM-MSC-D24 cells are able to completely eradicate human gliomas.
Conclusions
Our results confirm that BM-hMSCs derived from high-grade glioma patients (PD-BM-hMSCs) can be loaded with Delta-24-RGD and can be used in treating GBM patients. This study provides the foundation for future clinical studies aimed at using autologous PD-BM-hMSCs to deliver Delta-24-RGD to the site of brain tumors via intraarterial injection.
Acknowledgments
We are grateful to Ann Sutton, Department of Scientific Publications, and David Wildrick, PhD, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, for their editorial assistance.
This study was supported by the National Cancer Institute (grants nos. 1R01CA214749, 1R01CA247970, P30CA016672, and 2P50CA127001), The University of Texas MD Anderson Moon Shots Program, The Broach Foundation for Brain Cancer Research, The Elias Family Fund, The Priscilla and Jason Hiley Fund, The Baumann Family/CureFest Fund, The Jim and Pam Harris Fund, The Gene Pennebaker Brain Cancer Fund, The Schneider Memorial Cancer Research Fund, The Sweet Family Cancer Research Fund, The Dr. Marnie Rose Foundation, The Gold Family Memorial Fund, and The Sorenson Foundation (all to F.F.L.).
Disclosures
Drs. Lang, Fueyo, and Gomez-Manzano are patent holders of Delta-24-RGD, which has been licensed to DNATrix, Inc. Drs. Fueyo and Gomez-Manzano are consultants for, receive royalties from, and hold patents with DNATrix, Inc. Dr. Lang holds a patent with DNATrix, Inc.
Author Contributions
Conception and design: Shimizu, Gumin. Acquisition of data: Shimizu, Gumin, Gao, Yang, Ledbetter. Analysis and interpretation of data: Shimizu, Gumin, Hossain. Drafting the article: Lang, Shimizu, Hossain, Kondo, Parker Kerrigan. Critically revising the article: Lang, Parker Kerrigan. Reviewed submitted version of manuscript: Lang, Shpall, Kondo, Parker Kerrigan, Yang, Ledbetter, Fueyo, Gomez-Manzano. Approved the final version of the manuscript on behalf of all authors: Lang. Administrative/technical/material support: Gumin, Shpall, Fueyo, Gomez-Manzano. Study supervision: Lang.
Supplemental Information
- Supplementary Figs. 1–3. https://thejns.org/doi/suppl/10.3171/2021.3.JNS203045.
Previous Presentations
These data were previously presented as a poster at the 23rd Annual Meeting and Education Day of the Society for Neuro-Oncology held on November 15–18, 2018, in New Orleans, Louisiana.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2. Jiang H, Gomez-Manzano C, Lang FF, et al. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9(5):422–427. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer J. 2012;18(1):69–81. doi: 10.1097/PPO.0b013e31824671c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaufmann JK, Chiocca EA. Glioma virus therapies between bench and bedside. Neuro Oncol. 2014;16(3):334–351. doi: 10.1093/neuonc/not310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaichana KL, Pinheiro L, Brem H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Ther Deliv. 2015;6(3):353–369. doi: 10.4155/tde.14.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ciavarella S, Dominici M, Dammacco F, Silvestris F. Mesenchymal stem cells: a new promise in anticancer therapy. Stem Cells Dev. 2011;20(1):1–10. doi: 10.1089/scd.2010.0223. [DOI] [PubMed] [Google Scholar]
- 9. Nakashima H, Kaur B, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21(2-3):119–126. doi: 10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–3608. [PubMed] [Google Scholar]
- 11. Parker Kerrigan BC, Shimizu Y, Andreeff M, Lang FF. Mesenchymal stromal cells for the delivery of oncolytic viruses in gliomas. Cytotherapy. 2017;19(4):445–457. doi: 10.1016/j.jcyt.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 13. Hata N, Shinojima N, Gumin J, et al. Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas. Neurosurgery. 2010;66(1):144–157. doi: 10.1227/01.NEU.0000363149.58885.2E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shinojima N, Hossain A, Takezaki T, et al. TGF-β mediates homing of bone marrow-derived human mesenchymal stem cells to glioma stem cells. Cancer Res. 2013;73(7):2333–2344. doi: 10.1158/0008-5472.CAN-12-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yong RL, Shinojima N, Fueyo J, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Δ24-RGD to human gliomas. Cancer Res. 2009;69(23):8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Griffin MD, Ryan AE, Alagesan S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91(1):40–51. doi: 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- 17. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37(4):359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21. Szentirmai O, Baker CH, Lin N, et al. Noninvasive bioluminescence imaging of luciferase expressing intracranial U87 xenografts: correlation with magnetic resonance imaging determined tumor volume and longitudinal use in assessing tumor growth and antiangiogenic treatment effect. Neurosurgery. 2006;58(2):365–372. doi: 10.1227/01.NEU.0000195114.24819.4F. [DOI] [PubMed] [Google Scholar]
- 22. Lang FM, Hossain A, Gumin J, et al. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro Oncol. 2018;20(3):380–390. doi: 10.1093/neuonc/nox152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lal S, Lacroix M, Tofilon P, et al. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92(2):326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- 24. Fidler IJ, Schackert G, Zhang RD, et al. The biology of melanoma brain metastasis. Cancer Metastasis Rev. 1999;18(3):387–400. doi: 10.1023/a:1006329410433. [DOI] [PubMed] [Google Scholar]
- 25. Kim J, Hall RR, Lesniak MS, Ahmed AU. Stem cell-based cell carrier for targeted oncolytic virotherapy: translational opportunity and open questions. Viruses. 2015;7(12):6200–6217. doi: 10.3390/v7122921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- Supplementary Figs. 1–3. https://thejns.org/doi/suppl/10.3171/2021.3.JNS203045.