Abstract

In this study, a biocellulose (BC) sheet containing Aloe vera gel extract (AE) was developed for application in healing chronic wounds, such as diabetic wounds. The BC sheet was produced by Acetobacter xylinum and then lyophilized to obtain dried sheets. A. vera gel was extracted by precipitation in 35% ammonium sulfate, lyophilized, dried, and incorporated into the BC sheet. The protein content of the AE was 12.32 ± 3.4% w/w, with a molecular weight of ∼20 kDa. The release of TNF-α from lipopolysaccharide-induced RAW264.7 cells was reduced by treatment with AE in a dose-dependent manner. The physicochemical and biological properties of the developed sheet were investigated. Morphological examination of the BC/AE sheet using scanning electron microscopy revealed the 3D construction of nanofibrils, which showed high porosity. The BC/AE sheet exhibited water absorption at 74%, and the release of proteins in the AE reached 97.23% at 4 h. The BC sheet incorporated with proteins in the AE at 283.78 ± 7.7 μg/cm2 can promote the wound healing in streptozotocin-induced diabetic rats. The recovering skin in diabetic wounds treated with the BC/AE sheet exhibited a normal cell arrangement without fibrosis, as revealed by histological staining. The research findings indicate that the BC/AE sheet has potential for applications in wound dressings.
1. Introduction
Diabetic wounds are common chronic wounds with high inflammation. During the healing process, particularly in patients with diabetes, it is essential that the wound retains moisture and remains free of excess exudate to support skin regeneration. A wound dressing is a barrier that prevents pathogenic infection and retains moisture while removing exudates. Many wound dressings have been developed, and some are available for clinical use1 with different advantages and disadvantages. A widely used dressing that is inexpensive and traditionally used is gauze. However, macroporous dressings allow wound exudates to be effectively drained but cannot prevent microorganism penetration or wound dehydration. The other dressings used are dressings with calcium alginate, nanoparticles, or hydrogels. Nevertheless, these dressings are expensive and may cause a smaller impact on the healing process or non-biocompatibility.2
Biocellulose (BC) is an interesting biomaterial for wound dressings. It is a purified extracellular polysaccharide composed of nanofibrils from natural sources. BC can be produced from various microorganisms, such as Aerobacter, Agrobacterium, Achromobacter, Rhizobium, and Acetobacter (Komagataeibacter). BC has been researched for several biomedical applications, such as artificial blood vessels, scaffolds for tissue engineering, drug delivery systems, and dental implants.3,4 BC has been identified as a promising material for wound dressings due to its fibrillar network structure, which is compatible with the human skin extracellular matrix, and its water-holding capacity, strength, biodegradability, biocompatibility, molding, and nontoxicity.2,5−8 However, BC naturally lacks antibacterial and anti-inflammatory properties, which is a limitation of biomedical applications. Many previous studies have been performed to overcome this problem by incorporating bioactive molecules or compounds with antibacterial or anti-inflammatory properties.9,10
Aloe vera (Aloe vera Linn.) is a tropical plant that grows easily in places with hot and dry climates, including Thailand. In Thailand, A. vera gel has been used to treat burns for a long time and is included in the list of herbal drugs used for various wound therapy.11 Previous studies reported the presence of many active compounds in A. vera gel, such as flavonoids, lectins, terpenoids, fatty acids, tannins, polysaccharides, enzymes, minerals, and vitamins.12−14 Several studies have reported the anti-microbial, anti-diabetic, and anti-inflammatory properties of A. vera, which contains whole gel and polysaccharide components.15−17 However, there are few publications on the effects of the protein components of A. vera gel, which is usually found as glycoprotein, particularly in diabetic wound healing. In addition, a study on protein extracted from various types of A. vera indicated that the extract from A. vera exhibited high levels of pharmacological and physiological activities.18 Therefore, in the present study, A. vera gel extract in part of the protein (glycoprotein) was proposed as an alternative bioactive compound to reduce inflammation and accelerate healing for diabetic wounds.
In the present study, we aimed to develop a BC sheet incorporated with the A. vera gel extract (AE) that was obtained from a protein salting-out process for potential use as an effective diabetic wound dressing. The physicochemical and biological properties of the developed BC/AE sheet were determined. Subsequently, an in vivo study was used to investigate the potential of the BC/AE sheet to promote wound healing in a diabetic rat model.
2. Materials and Methods
2.1. Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The procedure used in this study was approved by the Thai IACUC under the license of the University of Phayao (certificated no. 610104002).
2.2. Materials
Ammonium sulfate [(NH4)2SO4] was purchased from Merck, Darmstadt, Germany. Bovine serum albumin, lipopolysaccharide (LPS), phosphate buffer saline (PBS), and streptozotocin (STZ) were purchased from Sigma-Aldrich Co., St. Louis, Missouri, USA. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS) 0.25% trypsin/0.01 M ethylenediaminetetraacetic acid, and penicillin/streptomycin solution were purchased from Gibco, Invitrogen, USA. The 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay kit was purchased from Boehringer Mannheim, Mannheim, Germany. A tumor necrosis factor-α (TNF-α) enzyme-linked immunosorbent assay (ELISA) kit was purchased from eBioscience, Inc., California, USA. A detergent compatible (DC) protein assay kit was obtained from Bio-Rad Laboratories, Philadelphia, USA. d-Glucose, di-sodium hydrogen phosphate (Na2HPO4), citric acid, and sodium hydroxide (NaOH) were purchased from Ajax Finechem, New South Wales, Australia. Peptone and yeast extract were obtained from HiMedia Laboratories, Maharashtra, India. Hematoxylin and eosin staining dyes were obtained from C.V. Laboratories Co., Ltd., Bangkok, Thailand. Aerrene isoflurane, USP, was purchased from Baxter, USA.
2.3. Preparation of A. vera Gel Extract (AE)
A. vera (A. vera Linn.) leaves were collected from Muang, Phayao, Thailand. A carbohydrate-free A. vera gel extract was used in this study. Briefly, a colorless gel was isolated from A. vera leaves, homogenized, and centrifuged to collect the supernatant. The supernatant was precipitated in 35% (NH4)2SO4. The precipitant was then collected and dialyzed with a dialysis bag (MW cut off 6–8 kDa, Spectra/Por, Spectrumlab Inc.) against deionized water for 2 days. After lyophilizing, the content and molecular weight of protein in the AE were determined using a Bio-Rad DC protein assay kit and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad Laboratories, Philadelphia, USA), respectively.
2.4. Determination of the Anti-inflammatory Activity of AE
Briefly, RAW264.7 cells (TIB-71) in DMEM containing 10% FBS were seeded on 96-well plates at a density of 1 × 104 cells/well. After 24 h of culture, cells were treated with various concentrations of AE (0, 50, 100, 200, and 400 μg/mL; equivalent to 0, 6.16, 12.32, 24.64, and 49.28 μg/mL of the protein contained). After treatment with AE for 4 h, the cells were exposed to 1 μg/mL of LPS for 24 h.19 The cell-free supernatant was then collected to determine the content of the released TNF-α using a TNF-α ELISA kit. In addition, the viability of RAW264.7 cells was observed by XTT assay.20 All experiments were performed in triplicate.
2.5. Preparation of Biocellulose Sheet Incorporating Aloe Gel Extract (BC/AE)
Biocellulose (BC) sheet production was performed according to a previous study with some modifications.21Acetobacter xylinum (TISTR107, obtained from Thailand Institute of Scientific and Technological Research) was cultured in Hestrin and Schramm medium (HS medium) for 7 days.22 This medium (aqueous solution, pH 5) was composed of 2% w/v d-glucose, 0.5% w/v peptone, 0.5% w/v yeast extract, 0.27% w/v Na2HPO4 and 0.115% w/v citric acid. The culture conditions, including the container shape and size, inoculum volume, culture media volume, and incubation time, were designed to obtain the consistency of the sheet shape and thickness. After a cellulose sheet formed above the culture media, it was collected and neutralized by immersion in 0.25 M NaOH at room temperature for 48 h. The sheet was then rinsed with distilled water and lyophilized to obtain the dried BC sheet. To incorporate with the AE in the BC sheet, the simple soaking method was used. The dried BC sheet (1.5 cm × 1.5 cm × 0.1 cm) was soaked in 1 mL of aqueous solution containing 4.0 mg of AE (equivalent amount of protein, 650.44 ± 62.09 μg) at room temperature for 1 h. After 1 h, the soaked sheet was removed, and the unincorporated AE solution was measured for protein content to extrapolate the protein incorporated in the soaked sheet. The soaked sheet was dried at 40 °C to obtain the BC/AE sheet. The BC and BC/AE sheets were characterized for morphology by scanning electron microscopy (SEM), and their chemical characteristics were evaluated using Fourier transform infrared spectroscopy (FTIR spectrometer, Spectrum GX series, USA).
2.6. Determination of the Water Absorption Ability of the Prepared Sheets
The dried BC or the dried BC/AE sheet (1.5 cm × 1.5 cm × 0.1 cm) was weighed to determine its initial weight (Wi). After that, the weighed sheet was immersed in PBS pH 7.4 at room temperature for 24 h. At the end of the time intervals (1, 2, 4, 6, 8, 16, 18, and 24 h), the immersed sheets were taken to remove the excess water and weighed to obtain the wet weight (Wt). The experiment was performed in triplicate. The percent water absorption of the sheet was calculated as follows23
2.7. Determination of the AE Released from the BC/AE Sheet
The method used in the release study was modified from previous studies.24,25 In this method, the AE was released from all sides of the sheet and accumulated in the testing media. As the protein in the AE was used as a marker to indicate the release of the AE from the sheet, the total protein content in the collected media at each time interval was measured. Briefly, the tested sheets (1.5 cm × 1.5 cm × 0.1 cm) were immersed in 1000 μL of medium (PBS, pH 7.4) and maintained at 37 °C throughout the experiment of 48 h. At the time intervals, (1, 2, 4, 8, 16, 24, and 48 h), 20 μL of the medium was collected and immediately replaced with 20 μL of fresh PBS. The collected media were taken to determine the total protein content using the Bio-Rad DC protein assay kit. The accumulative protein released was calculated and presented as the percent protein released.
2.8. Determination of the Wound Healing Efficacy of the Sheets in STZ-Induced Diabetic Rats
A STZ-induced diabetic rat model was used according to our previous study.20 The procedure used in this study was approved by the Thai IACUC under the license of the University of Phayao (certificated no. 610104002). Male Sprague–Dawley rats aged 6 weeks and weighing approximately 250 g were purchased from Nomura Siam International Co., Ltd. (Bangkok, Thailand), housed at 23 ± 2 °C and 55 ± 10% relative humidity, and fed ad libitum for 1 week at the laboratory animal research center, University of Phayao. A single intraperitoneal injection of STZ (70 mg/kg) in 0.1 M citrate buffer, pH 4.0, was used to induce diabetes in rats. Glucose levels were measured at 3 days after STZ injection using a blood glucose meter and strip (GlucoSure AutoCode, ApexBio, Taiwan). The model animals used in this study had blood glucose levels greater than 200 mg/dL.
2.8.1. Wound Excision and Treatment
After 7 days of diabetes induction, rat skin was inflicted by the excision of pieces (1.5 cm × 1.5 cm) of skin from the dorsal surface. The animals were anesthetized with 3% aerrane isoflurane during excision. The 15 rats were classified into 5 groups, with 3 rats in each group as follows: (1) normal rats without any wound treatment (normal control), (2) diabetic rats without any wound treatment (negative control), (3) diabetic rats with AE treatment, (4) diabetic rats with BC sheet treatment, and (5) diabetic rats with BC/AE sheet treatment. After infliction, wounds were covered with gauze and the appropriate sheet with a size of 2 cm × 2 cm × 0.1 cm on the first day of wounding.
2.8.2. Wound Evaluation and Histological Study
The size of the wound was measured on days 0, 7, 14, and 21 using a Vernier caliper. On day 21 after wound size measurement, the rats were sacrificed by using carbon dioxide inhalation. Then, skin wound samples were excised and fixed in tissue-freezing medium for histological staining. The tissue samples were frozen at −20 °C, sectioned, and stained with hematoxylin–eosin (H&E) dyes.
2.9. Statistical Analysis
All data are presented as the means and standard deviations of three independent groups. One-way analysis of variance was used to test for between-group differences in percent cell viability, released TNF-α, and wound area, followed by Fisher’s least significant difference test in R4.1.1 to analyze the significance of differences between experimental groups. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Characterization of the AE
The physical morphology of the AE was cotton-like fibrils. From 1 kg of fresh A. vera gel, 1.2 g of the dried AE (0.12% yield) was extracted. The total protein content of the AE was 12.32 ± 3.4% of the lyophilized extract. Protein characteristics assessed using SDS-PAGE revealed a molecular weight of approximately 20 kDa (Figure 1a).
Figure 1.
Biological properties of A. vera gel extract. SDS-PAGE of protein extract from A. vera gel (a); left-lane is a protein marker, and the center and right lanes are precipitated protein from A. vera gel extract (batch no. 1 and 2). Percentage of cell viability after treatment with A. vera gel extract; n = 3 (b). Amount of TNF-α released from the LPS-induced RAW264.7 cell line after treatment with A. vera gel extract; n = 3 (c). **, p < 0.01 compared to the non-A. vera gel extract treatment group.
3.2. Anti-inflammatory Activity of the AE
The percent viability results implied that AE at the tested concentrations (50–400 μg/mL) was not toxic to RAW264.7 cells (Figure 1b). AE inhibited the production of TNF-α in a dose-dependent manner (Figure 1c). The release of TNF-α was significantly reduced in the sample treated with 100–400 μg/mL of AE (equivalent to 12.32–49.28 μg/mL of the protein contained) compared to the sample in the non-AE-treated group.
3.3. Physicochemical Properties of the BC and BC/AE Sheets
After lyophilization, the visual appearances of the BC and the BC/AE sheet were very similar. Both sheets appeared white with a rough surface (Figure 2a) and had a thickness of 0.1 ± 0.011 cm. The sheets were strong and flexible. Both BC and BC/AE sheets displayed a 3D nanofibril structure, although the BC/AE sheet showed differences in the electron densities of BC and AE with good dispersion between phases. However, the fibrils of the BC/AE sheet were composed of more tightly woven 3D nanofibrils according to the AE coating. The porosity of the BC/AE sheet was less than that of the BC sheet (Figure 2b).
Figure 2.
Physical properties of BC and BC/AE sheets. Visible appearance of the wet BC, dried BC, and dried BC/AE extract sheets (a). The surface (top, at magnification of 3k) and cross-sectional (bottom, at magnification of 5k) morphologies of dried BC and dried BC/AE sheets obtained by SEM (b). Percent water absorption of BC and BC/AE sheets in 24 h (c).
3.3.1. Release of the AE from the BC/AE Sheet
Water absorption of both sheets rapidly increased within the first 1 h and then slightly increased until a constant value was reached. After 2 h, the BC and BC/AE had a percentage of water absorption of 63 ± 0.57 and 65 ± 0.52%, respectively. The percentage of water uptake of both sheets in 24 h was approximately 74% (Figure 2c), and all tested sheets retained their shapes after immersion in PBS at room temperature for 24 h.
According to the soaking method used for incorporating AE into BC, the BC/AE sheet (1.5 cm × 1.5 cm × 0.1 cm) contained 638.51 ± 17.32 μg (283.78 ± 7.7 μg/cm2) of protein from AE.
3.3.2. FTIR Spectroscopy of AE, BC Sheet, and BC/AE Sheet
The infrared spectra of the AE, BC sheet, and BC/AE sheet are depicted in Figure 3. The Fourier transformed infrared (FTIR) spectra of the AE showed the characteristic peaks of −NH stretching and −OH stretching hydrogen bond at 3274 cm–1, −C=O stretching at 1635 cm–1, C=C stretching in the aromatic ring at 1514 cm–1, and C–O–C stretching at 1234 cm–1. For BC, a broad absorption band of −OH stretching with a hydrogen bond at approximately 3340 cm–1, −OH bending at 1646 cm–1, and a broad absorbed water (hydrogen-bonded) at 1640 cm–1, were observed. The characteristic peaks of BC/AE sheet show the combination of characteristic peaks of AE and BC with frequency shifts toward lower wavenumbers; a broad peak of −NH stretching and −OH stretching at 3338 cm–1 and a broad peaks combination of −C=O, −OH bending and absorbed water (hydrogen-bonded) at 1637 cm–1.
Figure 3.
Fourier transform infrared spectra of AE, BC sheet, and BC and BC/AE sheet.
3.3.3. Release of the AE from the BC/AE Sheet
The release of AE from the BC/AE sheet (1.5 cm × 1.5 cm × 0.1 cm) was observed by measuring the protein content in the media collected at each time interval and then calculating the percent protein released from the sheets. The released protein from the BC/AE sheet at 1 and 2 h was 55.11 ± 27.75 μg (19.42 ± 9.78%) and 246.22 ± 30.79 μg (86.765 ± 10.85%), respectively. Then, the release at 4 h reached 277.33 ± 35.27 μg (97.73 ± 12.43%), as shown in Figure 4.
Figure 4.
Percent protein release profile of AE incorporated in BC sheet immersed in PBS at 37 °C for 48 h (n = 3).
3.4. Wound Healing Efficacy of the Sheets in STZ-Induced Diabetic Rats
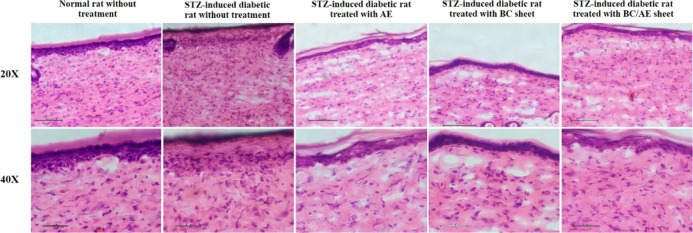
Figure 5a indicates that the untreated wounds in non-diabetic rats were completely healed within 14 days, whereas the untreated wounds in diabetic rats healed more slowly. The wound area in the diabetic rats treated with AE, BC sheet, or BC/AE sheet group was smaller than that in the untreated diabetic group. Interestingly, the wound areas in the group of diabetic rats treated with BC/AE sheet and the normal group were similar and significantly different from those in the diabetic non-treatment group (Figure 5b). Although the untreated diabetic wounds took 21 days to heal, a small dent still appeared due to an incomplete epithelialization, which is illustrated by the H&E staining results as shown in Figure 6. In diabetic wounds treated with only the AE and only the BC sheet, the epithelialization process was complete, but the fibrillary arrangement was looser than that in the normal group. Diabetic rat wounds treated with the BC/AE sheet were completely healed on day 14, and the epithelialization process was complete with the normal arrangement of dermal fibrils.
Figure 5.
Skin wound in a normal rat (a); normal rat without treatment, STZ-induced diabetic rat without treatment, STZ-induced diabetic rat treated with AE, BC sheet, and BC/AE sheet for the specified duration. Area of the rat wound at days 0, 7, and 14 after topical treatment with AE, BC sheet, and BC/AE sheet (b), n = 3. *, p < 0.05 and **, p < 0.01 compared to the untreated diabetic group.
Figure 6.
Histology of wounds stained with H&E on day 21 after wounding a normal rat without treatment, STZ-induced diabetic rat without treatment, STZ-induced diabetic rat treated with AE, BC sheet, and BC/AE sheet for the specified duration.
4. Discussion
In this study, a biocellulose (BC) sheet was constructed according to our preparation conditions. Its three-dimensional nanofibril structure coincides with results from previous studies, and this structure influenced its strength, flexibility, porosity, water sorption ability, and active carrier capacity.23,26 We found that the BC sheet provided the absorption capacity up to 74% water and retained its shape after water uptake. This ability of water uptake and shape retainability are important properties of the dressing sheet, as they are key to exudate or fluid absorption from the wound.2
To enhance wound healing capacity in diabetic wounds, AE with anti-inflammatory action was incorporated into the BC sheet. By the AE incorporation condition designed in this study, 90% (638.51 ± 17.32 μg) of the protein from the prepared AE solution (containing 650.44 ± 62.09 μg protein) could be absorbed. In this method, the loaded AE is thought to be contained in the BC by the adsorption process, as illustrated by the clear change in the surface sheet morphology from SEM after incorporation with the AE. However, the sheet provided high strength, flexibility, and water absorption ability (approximately 74%). Its shape was also retained under wet conditions. According to the FTIR spectra, we found that when comparing the BC sheet and AE to the BC/AE sheet, the frequency peaks of BC/AE sheet show spectra sum of BC sheet and AE with a broader band and frequency shift, in which the −OH stretching and −C=O stretching were slightly shifted from 3340 to 3338 cm–1 and from 1635 to 1633 cm–1, respectively. The reason for the broader band and peak shifts is because of a number of possible interaction mechanisms, such as H-bond, electrostatic charges, or surface energy between the −OH group of the BC and the amino acid group of AE through the adsorption processes.27 This causes a change in the electron cloud that alters the resonant frequency of that particular bond, resulting in different molecules having slightly different hydrogen bonding states, leading to different frequencies and a broad band.28 Chopra et al. reported that BC nanofibrils have a neutral electrostatic charge, which facilitates the incorporation of bioactive compounds with both positive and negative charges.9 In addition, the BC and AE molecules usually contained polar groups, hydroxyl groups in particular. Therefore, it was possible that both molecules bonded together via H-bonds.27 When the BC/AE is hydrated, the uptaken water binds to BC and the H-bonds hold BC and AE together breakdown along with other interactions leading to rapid release of the adsorbed AE (4 h).
According to Femenia et al., the content of AE gel is high in water (98.5–99.5%), solid polysaccharides (>60%), and some other components, such as proteins, lipids, dietary fiber, vitamins, and minerals.12−14,29 Focusing on the protein component in A. vera gel, it is usually combined with sugars as glycoproteins.18 Previous studies have reported that the proteins extracted from aloe are lectins and lectin-like substances.29,30 Some researchers have reported that the proteins extracted from A. vera gel in the molecular weight range of 20–100 kDa are anti-inflammatory substances because they can inhibit the action of bradykinin.29,31 In general, bradykinin can stimulate the release of TNF, an inflammatory cytokine. According to the protein molecular weight pattern of AE, we found a band at approximately 20 kDa.29 Moreover, FTIR spectra of the AE indicated the presence of O-acetyl ester at 1234 cm–1, which indicated that the presence of the acetyl group is necessary for the biological activity of crossing hydrophobic barriers in cells.32 The biological activity of AE was remarkably observed in LPS-induced RAW264.7 cells. We found that AE suppressed the release of TNF-α from LPS-induced RAW264.7 cells in a dose-dependent manner. The mechanism underlying the suppression of TNF-α release involved blocking LPS-toll-like receptor binding and/or interfering with transcription factor activity by the protein component(s) of the AE.19,33 The anti-inflammatory activity of AE was expected from the protein component contained in the extract. The release of protein from the BC/AE sheet was observed to clarify the delivery capacity of the sheet. The protein released from the BC/AE sheet reached to 97.73 ± 12.43% (277.23 mg protein by approximately) within 4 h of the release study. This released amount is sufficient to provide anti-inflammatory activity at the wound site. This is because, as per the LPS-induced macrophage study, TNF-α release suppression was observed in the macrophage cell line treated with 100 μg/mL of AE (containing 12.32 μg/mL protein). Generally, for diabetic wound care, the dressing should be changed frequently or daily to clean ulcers, prevent infection, and enhance healing.34 Therefore, attaining a rapid and maximal release of the active protein from the dressing is necessary for enhancing wound healing in the clinic.
An in vivo study in STZ-induced diabetic rats was conducted to observe the potential of the BC/AE sheet in wound treatment under hyperglycemic conditions. STZ is commonly known as a substance that can induce type 1 diabetes by destroying β-cells in rats.35 The results from this study suggested that wound contraction in STZ-induced diabetic rats was delayed as compared to that in normal rats. Hyperglycemic conditions lead to prolonged production of cytokines, thus disrupting fibroblast migration, proliferation, and transformation into myofibroblasts, performing wound contraction, and producing new connective tissues.33,36,37 To evaluate the healing efficacy of three treatment groups (1) AE, (2) BC, and (3), a combination of BC and AE on STZ-induced diabetic rat wounds was compared to that on the STZ-induced diabetic rat without treatment. The result indicated that the physical appearance of wounds was similar in all groups and the wounds were completely closed on day 14. However, the result of histological staining revealed that the skin fibrillar arrangement of STZ-induced diabetic rats treated with solely AE or BC was not complete. They exhibited loose fibrils compared to normal rat skin. In contrast, the fibrillar arrangement of STZ-induced diabetic rats treated with the BC combination with AE was close to normal rat skin. It was indicated that the wound healing efficacy of a combination of BC and AE was better than using AE or BC solely. Herein, it was probable that AE acted as an active compound that could undergo anti-inflammation by suppressing TNF-α expression, while BC acted as the barrier to maintain wound moisture and promote cell proliferation. Moreover, the BC was important in entrapping the AE and contributed to the specific area of wounds. Therefore, both BC and AE could co-promote the wound-healing process. The healing efficacy of solely tested BC was not better because it had only a barrier to prevent dehydration but not an active compound. Similarly, the healing efficacy of the solely tested AE was not better because it had only an active compound but not a barrier to prevent dehydration. BC sheet is a 3D flexible structure that provides essential functions such as being a physical barrier against pathogens and promoting tissue granulation. In addition, the structure of the BC sheet also provides a large amount of water, which prevents wound dehydration, promotes gas exchange, and absorbs wound exudates and consequently the fibroblasts to more rapidly migrate and enhance the process of granulation.34,38,39
Diabetes is a complex disease. In this study, high blood glucose in rats was chemically induced, and these hyperglycemic conditions mimicked one aspect of diabetic conditions in humans. The obtained results indicate the potential of BC/AE sheet for improving the wounds under hyperglycemic conditions. However, further in vivo studies including the expression of inflammatory cytokines and growth factors involved in wound healing and the formation of new connective tissues at the wound site should be conducted to clarify the healing mechanisms of the BC/AE sheet.
5. Conclusions
In summary, the BC and BC/AE sheets displayed 3D nanofibrils and high porosity, which suggested that they can carry active ingredients, such as antibiotics, protein, or other medications to the desired target. The high-porosity film could increase water absorption, which is a desirable property of a wound dressing because it aids in the absorption of wound exudate. It was indicated that the wound healing efficacy of a combination of BC and AE was better than using AE or BC solely. Therefore, the BC sheet belonging to AE presented the potential to improve wounds under hyperglycemic conditions and has the potential for applications as a wound dressing.
Acknowledgments
We would like to acknowledge the Research Grant for New Scholars (MRG6080037) and the Fundamental Found of University of Phayao (FF65-RIM110) and Global and Frontier Research University (grant number R2566C052), Naresuan University for financial support for this study. We are also thankful for the Center of Excellence for Innovation in Chemistry (PERCH-CIC), the Office of the Higher Education Commission and the Faculty of Pharmaceutical Sciences, Naresuan University for supporting facilities.
The authors declare no competing financial interest.
References
- Komesu M. C.; Tanga M. B.; Buttros K. R.; Nakao C. Effects of acute diabetes on rat cutaneous wound healing. Pathophysiology 2004, 11, 63–67. 10.1016/j.pathophys.2004.02.002. [DOI] [PubMed] [Google Scholar]
- de Amorim J. D. P.; da Silva Junior C. J. G.; de Medeiros A. D. M.; do Nascimento H. A.; Sarubbo M.; de Medeiros T. P. M.; Costa A. F. d. S.; Sarubbo L. A. Bacterial cellulose as a versatile biomaterial for wound dressing application. Molecules 2022, 27, 5580. 10.3390/molecules27175580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres F.; Commeaux S.; Troncoso O. Biocompatibility of bacterial cellulose-based biomaterials. J. Funct. Biomater. 2012, 3, 864–878. 10.3390/jfb3040864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshk S. M. A. S. Bacterial cellulose production and its industrial applications. J. Bioprocess. Biotech. 2014, 4, 150. 10.4172/2155-9821.1000150. [DOI] [Google Scholar]
- Czaja W.; Krystynowicz A.; Bielecki S.; Brownjr R. Microbial cellulose-the natural power to heal wounds. Biomaterials 2006, 27, 145–151. 10.1016/j.biomaterials.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Czaja W. K.; Young D. J.; Kawecki M.; Brown R. M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. 10.1021/bm060620d. [DOI] [PubMed] [Google Scholar]
- Sulaeva I.; Henniges U.; Rosenau T.; Potthast A. Bacterial cellulose as a material for wound treatment: Properties and modifications. a review. Biotechnol. Adv. 2015, 33, 1547–1571. 10.1016/j.biotechadv.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Cherng J. H.; Chou S. C.; Chen C. L.; Wang Y. W.; Chang S. J.; Fan G. Y.; Leung F. S.; Meng E. Bacterial cellulose as a potential bio-scaffold for effective re-epithelialization therapy. Pharmaceutics 2021, 13, 1592. 10.3390/pharmaceutics13101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra H.; Gandhi S.; Gautam R. K.; Kamal M. A. Bacterial nanocellulose based wound dressings: current and future prospects. Curr. Pharm. Des. 2022, 28, 570–580. 10.2174/1381612827666211021162828. [DOI] [PubMed] [Google Scholar]
- Mirmohammadsadegh N.; Shakoori M.; Moghaddam H. N.; Farhadi R.; Shahverdi A. R.; Amin M. Wound healing and anti-inflammatory effects of bacterial cellulose coated with Pistacia atlantica fruit oil. Daru 2022, 30, 1–10. 10.1007/s40199-021-00405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittichai N.; Picheansoonthon C.. Herbal Medicines Used in Primary Health Care in ASEAN; Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, WVO Office of Printing Mill: Bangkok, Thailand, 2014; pp 79–81. [Google Scholar]
- Sahu P. K.; Giri D. D.; Singh R.; Pandey P.; Gupta S.; Shrivastava A.; Kumar A.; Pandey K. D.; Pandey K. D. Therapeutic and medicinal uses of Aloe vera: a review. Pharmacol. Amp. Pharm. 2013, 04, 599–610. 10.4236/pp.2013.48086. [DOI] [Google Scholar]
- Rodrigues L.; de Oliveira A.; Tabrez S.; Shakil S.; Khan M. I.; Asghar M. N.; Matias B. D.; Batista J. M. A. D. S.; Rosal M. M.; de Lima M. M. D. F.; Gomes S. R. F.; de Carvalho R. M.; de Moraes G. P.; de Alencar M. V. O. B.; Islam M. T.; Melo-Cavalcante A. A. d. C. Mutagenic, antioxidant and wound healing properties of Aloe vera. J. Ethnopharmacol. 2018, 227, 191–197. 10.1016/j.jep.2018.08.034. [DOI] [PubMed] [Google Scholar]
- Hęś M.; Dziedzic K.; Górecka D.; Jędrusek-Golińska A.; Gujska E. Aloe vera (L.) Webb.: natural sources of antioxidants - a review. Plant Foods Hum. Nutr. 2019, 74, 255–265. 10.1007/s11130-019-00747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran S.; Sivagnanam K.; Ravi K.; Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J. Med. Food 2004, 7, 61–66. 10.1089/109662004322984725. [DOI] [PubMed] [Google Scholar]
- Habeeb F.; Shakir E.; Bradbury F.; Cameron P.; Taravati M. R.; Drummond A. J.; Gray A. I.; Ferro V. A. Screening methods used to determine the anti-microbial properties of Aloe vera inner gel. Methods 2007, 42, 315–320. 10.1016/j.ymeth.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Reuter J.; Jocher A.; Stump J.; Grossjohann B.; Franke G.; Schempp C. M. Investigation of the anti-inflammatory potential of Aloe vera gel (97.5%) in the ultraviolet erythema test. Skin Pharmacol. Physiol. 2008, 21, 106–110. 10.1159/000114871. [DOI] [PubMed] [Google Scholar]
- Park Y. I.; Son B. W.. Proteins in Aloe. In New Perspectives on Aloe; Park Y. I., Lee S. K., Eds.; Springer: Boston, 2006; pp 35–56. [Google Scholar]
- Yang J. Y.; Woo S. Y.; Lee M. J.; Kim H. Y.; Lee J. H.; Kim S. H.; Seo W. D. Lutonarin from Barley Seedlings Inhibits the Lipopolysacchride-Stimulated Inflammatory Response of RAW 264.7 Macrophages by Suppressing Nuclear Factor-κB Signaling. Molecules 2021, 26, 1571. 10.3390/molecules26061571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inpanya P.; Faikrua A.; Ounaroon A.; Sittichokechaiwut A.; Viyoch J. Effects of the blended fibroin/Aloe vera gel film on wound healing in streptozotocin-induced diabetic rats. Biomed. Mater. 2012, 7, 035008. 10.1088/1748-6041/7/3/035008. [DOI] [PubMed] [Google Scholar]
- Bautista-Pérez R.; Segura-Cobos D.; Vázquez-Cruz B. In vitro antibradykinin activity of Aloe barbadensis gel. J. Ethnopharmacol. 2004, 93, 89–92. 10.1016/j.jep.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Hestrin S.; Schramm M. Synthesis of cellulose by Acetobacter xylinum. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. 10.1042/bj0580345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Cai Z.; Lee H. S.; Choi G. S.; Lee D. H.; Jo C. Preparation and characterization of a bacterial cellulose/chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. 10.1007/s10965-010-9470-9. [DOI] [Google Scholar]
- Chandrasekaran A. R.; Jia C.; Theng C. S.; Muniandy T.; Muralidharan S.; Arumugam S. In vitro studies and evaluation of metformin marketed tablets-Malaysia. J. Appl. Pharm. Sci. 2011, 1, 214–217. [Google Scholar]
- Xue P.; Zhang X.; Chuah Y. J.; Wu Y.; Kang Y. Flexible PEGDA-based microneedle patches with detachable PVP-CD arrowheads for transdermal drug delivery. RSC Adv. 2015, 5, 75204–75209. 10.1039/c5ra09329e. [DOI] [Google Scholar]
- Kwak M. H.; Kim J. E.; Go J.; Koh E. K.; Song S. H.; Son H. J.; Kim H. S.; Yun Y. H.; Jung Y. J.; Hwang D. Y. Bacterial cellulose membrane produced by Acetobacter sp. A10 for burn wound dressing applications. Carbohydr. Polym. 2015, 122, 387–398. 10.1016/j.carbpol.2014.10.049. [DOI] [PubMed] [Google Scholar]
- Wohlert M.; Benselfelt T.; Wågberg L.; Furó I.; Berglund L. A.; Wohlert J. Cellulose and the role of hydrogen bonds: not in charge of everything. Cellulose 2022, 29, 1–23. 10.1007/s10570-021-04325-4. [DOI] [Google Scholar]
- Yooyod M.; Ross G. M.; Limpeanchob N.; Suphrom N.; Mahasaranon S.; Ross S. Investigation of silk sericin conformational structure for fabrication into porous scaffolds with poly (vinyl alcohol) for skin tissue reconstruction. Eur. Polym. J. 2016, 81, 43–52. 10.1016/j.eurpolymj.2016.05.023. [DOI] [Google Scholar]
- Femenia A.; Sanchez E.; Simal S.; Rossello C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr. Polym. 1999, 39, 109–117. 10.1016/s0144-8617(98)00163-5. [DOI] [Google Scholar]
- Suzuki I.; Saito H.; Inoue S.; Migita S.; Takahashi T. Purification and characterization of two lectins from Aloe arborescens Mill. J. Biochem. 1979, 85, 163–171. 10.1093/oxfordjournals.jbchem.a132306. [DOI] [PubMed] [Google Scholar]
- Talmadge J.; Chavez J.; Jacobs L.; Munger C.; Chinnah T.; Chow J. T.; Williamson D.; Yates K. Fractionation of Aloe vera L. inner gel, purification and molecular profiling of activity. Int. Immunopharmacol. 2004, 4, 1757–1773. 10.1016/j.intimp.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Chang X. L.; Chen B. Y.; Feng Y. M. Water-soluble polysaccharides isolated from skin juice, gel juice and flower of Aloe vera Miller. J. Taiwan Inst. Chem. Eng. 2011, 42, 197–203. 10.1016/j.jtice.2010.07.007. [DOI] [Google Scholar]
- Tan W. S.; Arulselvan P.; Ng S. F.; Mat Taib C. N.; Sarian M. N.; Fakurazi S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on sprague dawley rats. BMC Complementary Altern. Med. 2019, 19, 20. 10.1186/s12906-018-2427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitha K. V.; Tiwari S.; Purandare V. B.; Khedkar S.; Bhosale S. S.; Unnikrishnan A. G. Choice of wound care in diabetic foot ulcer: A practical approach. World J. Diabetes 2014, 5, 546–556. 10.4239/wjd.v5.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh A.; Norouzian D.; Mehrabi M. R.; Jamshidi Sh.; Farhangi A.; Verdi A. A.; Mofidian S. M.; Rad B. L. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. 10.1007/bf02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga-Acosta J.; Schultz G. S.; López-Mola E.; Guillen-Nieto G.; García-Siverio M.; Herrera-Martínez L. Glucose toxic effects on granulation tissue productive cells: the diabetics’ impaired healing. BioMed Res. Int. 2013, 2013, 256043. 10.1155/2013/256043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grada A.; Mervis J.; Falanga V. Research techniques made simple: animal models of wound healing. J. Invest. Dermatol. 2018, 138, 2095–2105.e1. 10.1016/j.jid.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Junker J. P.; Kamel R. A.; Caterson E. J.; Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv. Wound Care 2013, 2, 348–356. 10.1089/wound.2012.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aditya T.; Allain J. P.; Jaramillo C.; Restrepo A. M. Surface modification of bacterial cellulose for biomedical applications. Int. J. Mol. Sci. 2022, 23, 610. 10.3390/ijms23020610. [DOI] [PMC free article] [PubMed] [Google Scholar]