Abstract
The transcriptional organization and heat inducibility of the major heat shock genes hrcA, dnaK, dnaJ, groEL, and htpG were analyzed on the transcriptional level in Helicobacter pylori strain 69A. The strongly heat-induced dnaK operon was found to be tricistronic, consisting of the genes hrcA, grpE, and dnaK. The dnaJ gene specified one monocistronic mRNA which was also heat inducible. The genes groES and groEL were transcribed as one strongly heat-inducible bicistronic mRNA which exhibited exactly the same induction kinetic as the dnaK operon. Surprisingly, transcription of the monocistronic htpG gene was switched off after heat shock. The data presented are discussed with regard to the different mechanisms regulating expression of heat shock genes in H. pylori
Helicobacter pylori is a gram-negative, spiral-shaped pathogenic bacterium that specifically colonizes the gastric epithelium of primates and is the causative agent of chronic, active type B gastritis in humans (3). The following genetic determinants contribute to the successful colonization of the gastric mucosa: a urease neutralizing the bacterial microenvironment by producing ammonia from the urea present in mucosal sections (9, 10, 14); motility in the gastric mucus and adhesion to the mucosal cell membrane, enabling H. pylori to avoid the extremely low pH of the gastric lumen (11, 24, 35); and the low-pH-induced synthesis of H. pylori gene products inhibiting acid secretion by mucosal cells (26). As has been demonstrated for all other bacterial species examined so far, H. pylori elicits a heat shock response. Thermoregulation plays an important role in virulence gene expression in pathogenic bacteria including Escherichia coli, Salmonella spp., Shigella spp., and Yersinia spp. Given the importance of the heat shock response in the pathogenesis of other enteric pathogens, this stress response may also play an important role in pathogenesis of H. pylori-mediated gastritis.
The major heat shock proteins GroES/GroEL and DnaK have been identified in H. pylori. The amount of GroEL increases after heat shock and acid shock (22, 45). It has been proposed that GroEL may participate in protection and activity regulation of urease (12, 36). An increase in the amount of DnaK after acid shock has also been observed (22). Furthermore, DnaK was reported to be involved in the modulation of glycolipid binding specificity of H. pylori at low pH (21).
The aim of this study was to analyze expression of the major H. pylori heat shock genes at the transcriptional level under unstressed conditions and after heat shock. The availability of the published complete H. pylori genome sequence (38) made it possible to generate highly sensitive RNA probes allowing the detection of mRNA specified by the classical heat shock genes hrcA, dnaK, dnaJ, groEL, and htpG.
Recently, Spohn and Scarlato demonstrated the negative regulation of the promoters preceding the dnaK and groE operons by the HspR/HAIR (HspR-associated inverted repeat) repressor/operator system in H. pylori G27 (34). Surprisingly, this work failed to detect inducibility of the two operons after temperature upshift, whereas the groE promoter was induced by osmotic stress. Our results demonstrate that the dnaK operon of H. pylori strain 69A is tricistronic, consisting of the genes hrcA, grpE, and dnaK. Transcription of the operon was strongly induced by heat shock at the single promoter upstream of hrcA, which was demonstrated to be negatively regulated by the HspR repressor protein by Spohn and Scarlato (34). This promoter is furthermore preceded by a CIRCE-like operator sequence, suggesting dual control of the dnaK operon by the HspR/HAIR and HrcA/CIRCE regulatory systems. The amount of a monocistronic dnaJ transcript also increased after heat shock, but in this case, no obvious regulatory cis element is present upstream of the gene. The groESL operon of H. pylori 69A specified a typical bacterial bicistronic mRNA which was heat inducible to the same extent and exhibited the same kinetic as the dnaK operon. Here, no CIRCE-like element is present in the putative promoter region of this operon, indicating regulation by HspR and HAIR only. Surprisingly, the monocistronic mRNA specified by the htpG gene disappeared after thermal upshift, demonstrating that htpG is not heat inducible in H. pylori 69A.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
E. coli DH10B (Gibco BRL) grown in Luria broth full medium supplemented with ampicillin (200 μg ml−1) was used as host strain in all plasmid cloning procedures. H. pylori strain 69A (17, 29), obtained from the Institute of Medical Microbiology, University of Amsterdam, The Netherlands, was cultivated in 100 ml of brucella broth (Difco, Detroit, Mich.) supplemented with 5% horse serum (Sigma Aldrich, Deisenhofe, Germany) at 37°C under microaerobic conditions (5% air, 10% CO2, 85% N2).
DNA manipulations and analysis.
Large-scale plasmid DNA purification was carried out using QIAGEN (Hilden, Germany) columns. Minipreps were performed as described by Holmes and Quigley (18). PCR products were generated with Deep Vent DNA polymerase (New England Biolabs, Schwalbach, Germany). PCR primers were obtained from ARK Scientific GmbH Biosystems (Darmstadt, Germany). PCR products were purified using a QIAGEN PCR-purification kit. Cloning procedures were carried out by standard procedures (28). For ligation, we used a Fast-Link DNA ligase kit (Biozym, Hess. Oldendorf, Germany).
Construction of plasmids.
The PCR primers HPHRCA5′ (GGCCATGGATCCATGGTGATTGACGAGATTTTTCAA) and HPHRCA3′ (GGCCATGGATCCTTATTCCTCCTCAGAAATCGTTG) were used to amplify the complete coding region of the H. pylori 69A hrcA gene (831 bp). Using the primers HPDNAK5′ (GGCCATGGATCCAAACTCACTAGGGCTAAATTTGAA) and HPDNAK3′ (GGCCATGGATCCACTCCACTTCCGCATCAATCACAT), the 3′-terminal 985 bp of the H. pylori 69A dnaK gene were amplified. To generate a PCR fragment containing the complete coding sequence of the H. pylori 69A dnaJ gene (1,110 bp), we used the primers HPDNAJ5′ (GGCCATGGATCCGTGGAATTGAGTTATTATGAAATT) and HPDNAJ3′ (GGCCATGGATCCTTATTTGAACCAGTCTTTAATTTT). PCR performed with primers HPPERM5′ (GGCCATGGATCCATGCATGAGTTTCTAAAAGCTTTT) and HPPERM3′ (GGCCATGGATCCTTAGGGATTAAAAAAAGCCTTTTC) generated a DNA fragment containing the coding regions (1,028 bp) of the two genes downstream of dnaJ. The 791 5′-terminal bp of the H. pylori 69A groEL gene were amplified using the primers HPGROEL5′ (GGCCATGGATCCATGGCAAAAGAAATCAAATTTTCA) and HPGROEL3′ (GGCCATGGATCCTTCACCACTAGAGTCGTTAAAGCT). Using the primer pair HPHTPG5′ (GGCCATGGATCCTCGTTTGCGCATGATAACAGCGAA) and HPHTPG3′ (GGCCATGGATCCCTACAACGCTTTCAATAGCACGCT), a 3′-terminal fragment (1,097 bp) of the H. pylori 69A htpG gene was obtained. Finally, combination of the PCR primers HPHRCA3′ and PHPHRCAPEX5′ (GGCCATGGATCCGCTGTCAATGCCTCTTGTGTGTGT) generated a fragment of 1,253 bp containing the complete coding sequence of hrcA and 422 bp of the upstream region. In all cases, chromosomal DNA of H. pylori 69A was used as template. All PCR primers carried BamHI restriction sites close to their 5′ ends (GGATCC, underlined in the primer sequences shown above). After digestion with BamHI, the different PCR products were inserted in BamHI-linearized pBluescript SKII+ vector (Stratagene). The resulting plasmids were named pHPhrcARPT3, pHPdnaKRPT3, pHPdnaJRPT3, pHPpermRPT3, pHPgroELRPT3, pHPhtpGRPT3, and pHPhrcAPEX, in the order described for the corresponding PCRs above. In all plasmids, transcription of the inserts using the pBluescript T3 promoter leads to the synthesis of an RNA complementary to the mRNAs specified by the different H. pylori genes.
Preparation of RNA; slot blot and Northern blot analyses.
Total RNA of H. pylori 69A was prepared by the acid phenol method (42), with the modifications described elsewhere (19). Plasmids pHPhrcARPT3, pHPdnaKRPT3, pHPdnaJRPT3, pHPpermRPT3, pHPgroELRPT3, and pHPhtpGRPT3 were linearized by EcoRI and subsequently used as templates for in vitro transcription reactions using T3 RNA polymerase as instructed by the manufacturer (DIG [digoxigenin]-RNA labeling kit; Roche). Decreasing amounts of total RNA were transferred onto a positively charged nylon membrane (Pall) by slot blotting. After baking at 120°C for 1 h, filters were prehybridized, hybridized with DIG-labeled RNA probes, and washed, and hybridization signals were detected as instructed by the manufacturer (Roche), using Fuji RX films. The hybridization signals of the chemiluminographs were quantified using WinCam software version 2.1 (Cybertech, Berlin, Germany). The induction ratios were calculated by dividing the volumes of the signals obtained from RNA isolated from stressed cells by the volume of the signals obtained from RNA isolated from the controls (37°C, exponential growth). For Northern blot analysis, samples of total RNA were separated under denaturing conditions in 1.2% agarose–2.1 M formaldehyde-morpholinepropanesulfonic acid gels, stained with ethidium bromide, and transferred to a positively charged nylon membrane (Pall) by vacuum blotting. Hybridization and detection were carried out as described for slot blots. Sizes of the RNA molecular weight standard (Gibco BRL) used to determine the transcript sizes were 9.49, 7.46, 4.40, 2.37, 1.35, and 0.24 kb.
Primer extension analysis.
The primer extension experiment was carried out using the 32P-labeled primer HPHRCAPEX (GTCCGCCAACTCTTTTAAGCG) as outlined before (43). DNA sequencing reactions were carried out with the same primer and plasmid pHPhrcAPEX as template, and the sequencing products were separated on the same gel.
RESULTS
The dnaK operon of H. pylori 69A is tricistronic and strongly heat inducible.
To obtain highly sensitive molecular probes for the detection of the mRNAs specified by the H. pylori dnaK locus, we constructed DIG-labeled RNA probes with specificity for the first (hrcA) and the last (dnaK) genes of the postulated tricistronic operon derived from the genome sequence (38) and proposed by Spohn and Scarlato (34) as described in Materials and Methods. First, total RNA isolated from H. pylori 69A grown at 37°C and at different time points after upshift of the culture to 48°C was analyzed by Northern hybridization using the dnaK probe. Two distinct specific transcripts of approximately 3.4 and 3.6 kb were detected, a third band at 4.2 kb was also visible besides the typical signal clouds at the positions of the 16S and 23S rRNAs where degradation products of larger RNA molecules were being trapped (data not shown).
Calculated from the start codon of hrcA to the stop codon of dnaK, a minimal length of 3,290 nucleotides is postulated for the tricistronic operon. Due to the fact that transcription most probably starts not exactly at the hrcA start codon but further upstream and ends not exactly at the dnaK stop codon but further downstream, all three detected mRNA species are very well within the predicted range. This result strongly argued for the postulated tricistronic structure of the H. pylori 69A dnaK operon. No smaller dnaK mRNA species were detected. There was no increase in the amount of mRNA after the heat shock. All three mRNA species decreased weakly after the upshift to 48°C (data not shown). This observed lack of heat inducibility could be due to the extreme heat shock temperature, which might represent unphysiological, perhaps lethal, conditions. This high temperature was chosen to exert a strong induction effect.
Subsequently, the experiment was repeated by shifting the cells from 37 to 42°C. Under these conditions, we observed a strong heat induction of all transcripts (Fig. 1A) whereby the 3.4- and 3.6-kb mRNAs were more strongly induced compared to the faint 4.2-kb transcript.
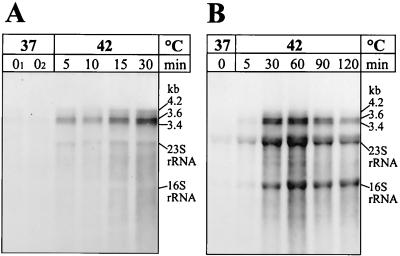
FIG. 1.
Northern blot analysis of the H. pylori 69A dnaK gene. Cells were first grown at 37°C and then heat shocked by upshift to 42°C. RNA was isolated before the upshift (0, 01, and 02 min, where 01 and 02 represent two independently withdrawn probes) and at the indicated times after application of stress. Note that the sampling times were different in panels A and B. Total RNA was separated by electrophoresis in 1.2% gels; after blotting, the nylon membranes were hybridized to a dnaK-specific RNA probe. The amounts of RNA applied per lane were 2 μg (A) and 10 μg (B).
Due to the very strong signals after the heat shock, we had to expose the X-ray films only for a short time to avoid overexposure. This is the reason for the lack of signals before heat shock which were also visible after longer exposure. These data clearly prove that the dnaK operon is heat inducible. We could not detect any shutoff of the heat shock response up to 30 min after temperature upshift, in contrast to many other bacterial species. In most known cases, turnoff occurs at the transcriptional level 10 to 20 min after heat induction. To identify the time point for the onset of shutoff of the H. pylori 69A dnaK operon, we repeated the experiment using a wider time period of up to 120 min after the upshift. The resulting Northern blot revealed that transcription of the dnaK operon was turned off between 60 and 90 min after heat induction (Fig. 1B). Here, we used 10 μg of RNA per lane, which turned out to be the optimal amount to detect dnaK transcripts but led to stronger signals at the position of the rRNAs due to the large RNA amount loaded per lane. Like in Fig. 1A, the 4.2-kb mRNA was visible as only a very faint band. It has to be emphasized that longer exposure times of the X-ray films led to a clear and unambiguous visualization of this signal. These extended exposure times resulted in a strong overexposure of the other signals and a strong black background rendering these images unsuitable for presentation.
Finally, the results concerning the operon structure obtained using the dnaK probe were verified by using the hrcA probe. This probe led to the detection of, besides the described mRNA species, a further specific transcript approximately 500 nucleotides in length (data not shown). Most probably this signal is generated by hybridization of the hrcA probe to a 5′-terminal degradation product of the tricistronic mRNA. In this experiment, we used an RNA preparation different from the one used for Fig. 1B, and the signal pattern obtained showed an intensity peak in mRNA amount 90 min after the temperature upshift, with a subsequent slight decrease (data not shown). Therefore, the time course of the transient heat shock response differed from the one described for Fig. 1B. In summary, the Northern blot experiments showed that the dnaK operon of H. pylori 69A is tricistronic and can be strongly induced by a heat shock from 37 to 42°C. The exact kinetic of its transcription is still unclear.
Quantitative analysis of the mRNA heat induction profile of the dnaK operon.
To quantify the exact extent of the transient heat shock response of the dnaK operon at the transcriptional level, we performed quantitative slot blot analyses. Again, the hrcA- and dnaK-specific probes were used in these hybridization experiments. The calculated results of these analyses are presented in Fig. 2. Both genes were approximately fivefold induced at the transcriptional level. Induction reached a maximum at around 90 min after heat shock, followed by a decline in the amount of mRNA. Because (i) the slot blot analysis was carried out several times using three different RNA preparations and (ii) this method is less prone to pipetting errors compared to the Northern experiments, we consider those data more relevant in terms of exact quantification.
FIG. 2.
Quantitative slot blot analysis of hrcA- and dnaK-specific mRNA before and after upshift from 37 to 42°C. Indicated are calculated mRNA heat induction profiles as determined by three independent experiments using different preparations of RNA.
Mapping of the heat-inducible promoter upstream of the dnaK operon.
Spohn and Scarlato characterized and mapped an HspR/HAIR-controlled promoter upstream of the dnaK operon of H. pylori G27 (34). As this promoter was described to be not inducible by heat shock, we performed primer extension experiments to identify the heat shock promoter responsible for induction of the H. pylori 69A dnaK operon after temperature upshift. Total RNA isolated before and at different times after heat shock was hybridized with the 32P-labeled oligonucleotide HRCA-PEX, complementary to the mRNA at the 5′ end of the hrcA gene. The oligonucleotide was then extended with reverse transcriptase. One single 5′ end identical to the 5′ end determined by Spohn and Scarlato (34), was clearly determined, indicating that no different start site was activated after heat shock in H. pylori 69A (data not shown). As determined by PhosphorImager analysis, the signal increased after heat shock by the same extent as measured before by slot blot analysis. Figure 3 indicates the position of the mapped 5′ end, which is separated by 6 bp from a perfect −10 promoter hexamer (TATAAT). A clear −35 promoter region cannot be identified in the allowed distance range of 17 or 18 bp upstream of the −10 region, a phenomenon often described for H. pylori (15, 33). As already mentioned by Spohn and Scarlato (34), a CIRCE-like element overlaps with the position of the potential −35 region, which strongly argues for the physiological significance of this palindromic element. Binding of HrcA to CIRCE could, in addition to the described regulation by HspR and HAIR, prevent initiation of transcription at the promoter by steric exclusion. As described for the Lactococcus lactis dnaJ gene (40), CIRCE itself is not transcribed as part of the mRNA. The combined data of Northern and slot blot analyses and primer extension allowed the development of the structural model of the H. pylori 69A dnaK operon presented in Fig. 4, which will be discussed below.
FIG. 3.
Promoter region of the H. pylori 69A dnaK operon. The start codon of hrcA is in boldface; the −10 promoter hexamer and the heat-inducible transcriptional start site mapped by primer extension are in boldface and underlined. The postulated CIRCE operator is marked by arrowheads above the sequence.
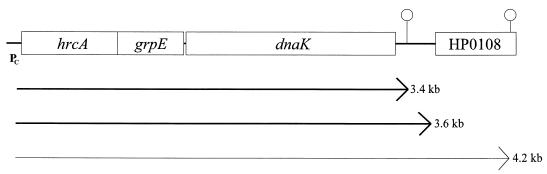
FIG. 4.
Transcriptional organization of the H. pylori 69A dnaK operon. The lengths of the transcripts deduced from Northern blot analysis are indicated, and the thickness of the arrows represents their relative abundance within the cell. PC indicates the heat shock promoter upstream of the operon which was demonstrated to be negatively regulated by the HAIR/HspR operator/repressor system (34) and which most probably is furthermore negatively regulated by the CIRCE/HrcA operator/repressor system. Potential stem-loop structures are indicated. The open reading frame HP0180 located immediately downstream of dnaK is transcribed in the opposite direction to the dnaK operon.
Transcription of H. pylori 69A dnaJ is also heat induced.
The DnaK chaperone machine is composed of three components: the Hsp70 chaperone DnaK, which binds to and releases partial unfolded or uncompletely folded substrate proteins in an ADP/ATP-dependent manner; the cochaperone GrpE, mediating the ADP-ATP exchange of DnaK; and the cochaperone DnaJ, which tags substrates for binding by DnaK and activates the latter by stimulating its ATPase activity. Consequently, after heat shock all three proteins are needed in larger amounts and all three genes must be heat induced at the transcriptional level (7). Therefore, it was obvious to analyze transcription of the dnaJ gene under heat shock conditions as well.
Analysis of the genome sequence led to the assumption of a tricistronic operon consisting of dnaJ and the two downstream genes named HP1331 and HP1330 by the H. pylori 26695 genome sequencing project (38). The dnaJ stop codon is separated from the start codon of HP1331 by only 10 nucleotides which contain the HP1331 ribosome binding site (AAAGG), and the stop codon of HP1331 (UGA) is located within the coding sequence of HP1330 separated by one nucleotide from the start codon of this last gene of the postulated operon. The HP1330 ribosome binding site (AGGA) is localized within the coding region of HP1331. The proteins encoded by HP1331 and HP1330 exhibit significant homologies to branched-chain amino acid transport proteins of Bacillus subtilis (2): HP1331 shows 65.07% similarity and 36% identity to AzlC, and HP1331 shows 67% similarity and 43% identity to AzlD. As the genes upstream of dnaJ (HP1333) and downstream of HP1330 (HP1329) are transcribed in the opposite direction, it was obvious to assume a tricistronic operon which should encompass approximately 2.2 kb.
However, Northern blot experiments using a dnaJ-specific RNA probe allowed only the detection of one transcript 1.1 kb in length (Fig. 5). This mRNA corresponds to a monocistronic dnaJ transcriptional unit. It is unclear if this mRNA is generated by transcriptional termination immediately downstream of dnaJ or by ribonucleolytic processing of the postulated tricistronic transcript. We could detect no secondary structure downstream of dnaJ which could function as a rho-independent transcriptional terminator or/and 3′ mRNA stabilizer. No specific transcript was detected using a second riboprobe complementary to the transcript specified by HP1330 and HP1331 (data not shown), thereby confirming the result obtained with the dnaJ probe.
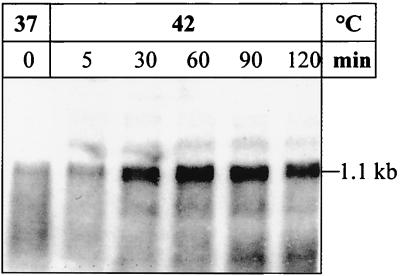
FIG. 5.
Northern blot analysis of H. pylori 69A dnaJ. Cells were first grown at 37°C and then heat shocked by upshift to 42°C. RNA was isolated before the upshift (0) and at the indicated times after heat shock. Total RNA (10 μg per lane) was separated by electrophoresis in a 1.2% gel; after blotting, the nylon membrane was hybridized to a dnaJ-specific RNA probe.
The monocistronic dnaJ mRNA clearly increased after a heat shock from 37 to 42°C (Fig. 5), confirming the expected heat inducibility of the third component of the DnaK chaperone machine. In this context, it must be mentioned that the demonstration of the dnaJ mRNA represented a serious technical problem and that only the most intact RNA preparations which showed not even partial RNA degradation allowed its detection, indicating a very low stability of this mRNA. Surprisingly, no obvious regulatory cis element (CIRCE or HAIR) was found in the putative promoter region upstream of dnaJ, suggesting that this gene is possibly controlled by some unknown heat-shock regulation mechanism.
The H. pylori 69A groESL operon specifies a bicistronic mRNA which is strongly heat induced.
Spohn and Scarlato (34) demonstrated negative control of the groE promoter mapped the first time by Suerbaum et al. (36) by the HspR/HAIR repressor/operator system in H. pylori G27. As in the case of the dnaK operon, this study failed to demonstrate inducibility of the promoter by heat shock (34). On the other hand, an increase in the amount of GroEL protein after heat shock has been demonstrated previously (45). Therefore, heat induction of the GroE synthesis was assumed to be regulated posttranscriptionally (34). We decided to analyze the heat inducibility of the groESL mRNA of H. pylori 69A by Northern hybridization. Furthermore, the operon structure which most probably corresponds to a classical bacterial bicistronic groESL operon could be verified by this method.
The Northern hybridization using a groEL-specific RNA probe led to the detection of a single mRNA of 2.1 kb, most probably representing a bicistronic groESL transcript (Fig. 6A). This mRNA was strongly induced after the temperature upshift, revealing heat shock regulation at the transcriptional level (Fig. 6A). Besides the 16S rRNA signal cloud, further weak signals most probably represent mRNA degradation intermediates. The derived model of the transcriptional organization is presented in Fig. 6B. Interestingly, the bicistronic mRNA exhibited a pattern of induction and shutoff similar to that of the dnaK operon (Fig. 1B). To analyze this induction pattern in more detail, a slot blot analysis was performed. The calculated mRNA heat induction profile is presented in Fig. 6C. The induction reached a maximum at around 90 min after thermal upshift, and shutoff started between 90 and 120 min after stress application. The maximal induction was about sixfold, slightly stronger than that of the dnaK operon. It can be inferred that the mechanisms regulating heat induction of the groESL and the dnaK operon are functionally coordinated and synchronized.
FIG. 6.
Transcriptional organization of the H. pylori 69A groESL operon. (A) Northern blot analysis. Cells were first cultivated at 37°C and then heat shocked by upshift to 42°C. RNA was isolated before application of heat stress (0) and at the indicated times after the upshift. RNA samples (200 ng per lane) were separated by electrophoresis in a 1.2% gel and vacuum blotted to a nylon membrane. Hybridization was performed using a groEL-specific RNA probe. (B) Schematic drawing of groESL operon and the detected bicistronic mRNA. The putative rho-independent transcriptional terminator downstream of the operon is indicated. (C) Quantitative slot blot analysis of groEL-specific mRNA before and after upshift from 37 to 42°C. The calculated mRNA heat induction profile as determined by three independent experiments using different preparations of RNA is shown.
Transcription of the monocistronic H. pylori 69A htpG gene is switched off after thermal upshift.
Whereas DnaK and GroEL represent members of the Hsp70 and Hsp60 families of heat shock proteins, identification of an HtpG-encoding gene in the H. pylori genome also indicated a member of the Hsp90 family in this organism (38). HtpG is the bacterial homologue of the Hsp90 family. These proteins also function as molecular chaperones (6, 44), and expression of the encoding genes in bacteria is strongly heat induced (1, 30). Surprisingly, htpG knockout mutants of E. coli and B. subtilis exhibit no distinct phenotypes (3, 41), which is in strong contrast to dnaK knockout mutants, which show a temperature-sensitive phenotype (27, 31), while mutations leading to functional inactivation of the GroEL chaperone machine are absolutely lethal (13, 25). Analysis of the H. pylori htpG promoter region revealed the absence of an obvious regulatory cis element. Therefore, we speculated that H. pylori htpG could be heat regulated by the same unknown mechanism as dnaJ. To verify this assumption, we performed a Northern blot analysis of the htpG gene, again using a specific RNA probe.
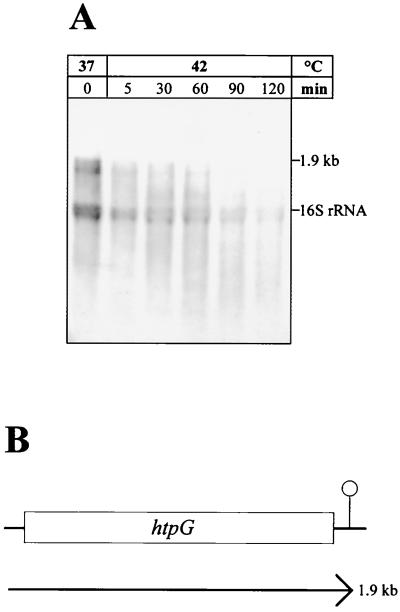
The Northern blot led to the detection of a transcript of about 1.9 kb (Fig. 7A), corresponding to the length of the htpG gene, demonstrating a monocistronic transcriptional unit as described for E. coli (1) and B. subtilis (30). Very surprisingly, this transcript was not inducible by heat shock from 37 to 42°C; on the contrary, the mRNA amount started to decrease already 5 min after heat shock (Fig. 7A). This unexpected result was reproduced several times using different RNA preparations. Similar results were obtained by shifting the cells from 37 to 48°C (data not shown). Therefore, we concluded that htpG is indeed not a heat-inducible gene in H. pylori. The transcriptional organization of H. pylori htpG is shown in Fig. 7B.
FIG. 7.
Transcriptional organization of the H. pylori 69A htpG gene. (A) Northern blot analysis. Cells were grown at 37°C and heat shocked by upshift to 42°C. RNA (10 μg per lane) isolated before thermal upshift (0) and at the indicated times after heat shock was separated by electrophoresis in a 1.2% gel and blotted to a nylon membrane. Hybridization was performed using an htpG-specific RNA probe. (B) Schematic representation of the htpG gene and the detected monocistronic mRNA. The putative rho-independent transcriptional terminator downstream of htpG is indicated.
DISCUSSION
Three mRNA species specified by the dnaK operon of H. pylori 69A were detected in Northern hybridization experiments, and it was demonstrated by primer extension that their transcription is initiated at one single promoter upstream of hrcA. Consequently, the three different mRNAs have to be generated by termination of transcription at different sites. Immediately downstream of dnaK, a stable stem-loop structure (ΔG = −13.8 kcal/mol) can be derived from the DNA primary sequence. Most probably, this structure is located at the 3′ end of the 3.4-kb mRNA. It remains unclear if this secondary structure functions as a rho-independent transcriptional terminator (the inverted repeat is not followed by a poly[T] stretch); alternatively, it might represent a 3′ mRNA stabilizer at which 3′ exoribonucleases are being stalled. The 3.6-kb mRNA may be generated by rho-dependent termination further downstream because no other significant secondary structures can be derived from the DNA sequence in the appropriate distance. Most probably, the less abundant 4.2-kb mRNA is generated by unspecific partial readthrough at the termination site(s) downstream of dnaK. At the 5′-most end of the downstream-localized HP0108, which is transcribed in the opposite direction, a significant stem-loop structure can be identified (ΔG = −10.1 kcal/mol). This structure could function as terminator or/and 3′ stabilizer.
The CIRCE-like element upstream of H. pylori hrcA is not transcribed as part of the mRNA as described in the case of the dnaJ gene of L. lactis (40). If CIRCE is part of the mRNA and localized close to the ribosome binding site of the following gene, it decreases the stability of mRNA by impairing ribosome binding and consequently deprotecting the 5′ end of mRNA against 5′-binding RNases (20, 32, 46). This destabilizing mechanism is not realized in the case of the H. pylori dnaK operon. Therefore, the question arises as to how H. pylori regulates the relative amount of the different proteins encoded by the dnaK operon. The proteins DnaK and GrpE as components of the Hsp70 chaperone machinery are needed in larger amounts than the repressor protein HrcA. In B. subtilis, the primary transcripts of the dnaK operon are processed and the hrcA-containing processing product carrying the destabilizing CIRCE at its 5′ end is rapidly degraded, thereby ensuring a 100-fold lower concentration of HrcA protein than of DnaK (20). No comparable mechanism was observed in H. pylori. However, no obvious ribosome binding site can be identified upstream of hrcA, and the resulting low translational efficiency could ensure an adequate low concentration of HrcA in H. pylori.
Our data concerning the transcriptional organization of the dnaK operon are in conflict with results presented by Huesca et al. (22), who postulated a monocistronic transcriptional unit of dnaK and a transcriptional start site immediately upstream of the gene. In fact, the Northern blot presented in their paper as evidence for the monocistronic mRNA shows mainly a smear which typically appears if the RNA preparation used is heavily degraded. If the same RNA preparation had been used in the primer extension experiment presented in the same paper, this could explain the determined 5′ end as the 5′ end of an mRNA degradation intermediate instead of a bona fide transcriptional start site. The postulated monocistronic mRNA which should be initiated at the postulated promoter was not detectable in our Northern blots. However, it should be emphasized here that Huesca and coworkers used strain HP439 and we used another strain, H. pylori 69A. The region located upstream of HP439 dnaK revealed a groEL-like gene 500 bp upstream of the dnaK sequence (22). In the strains used in the genome sequencing projects, which exhibit the same dnaK operon structure as strain 69A used here, the grpE gene is located in the corresponding region. Therefore, the data obtained using HP439 may not be comparable to our results.
Altogether, the structure of the H. pylori dnaK operon resembles the organization of the operon in Campylobacter jejuni (37). In this organism, besides the same gene order hrcA-grpE-dnaK, the dnaJ gene is also localized at another position of the bacterial chromosome. In H. pylori, there is also an increase in the amount of the dnaJ-specific mRNA after temperature upshift. This heat inducibility is not surprising because all three components of the DnaK chaperone machinery are required in larger amounts after a heat shock to cope with the stress. However, no obvious regulatory element (HAIR or CIRCE) is located upstream of this gene, indicating a further heat shock regulation mechanism in H. pylori. While in silico analysis of the DNA sequence suggested a tricistronic dnaJ operon, only a monocistronic mRNA was detected in Northern experiments. The origin of this monocistronic transcriptional unit is unclear.
The H. pylori groESL operon was demonstrated to specify a typical bacterial bicistronic groESL transcriptional unit. This verified the in silico predictions: downstream of groESL, a significant stable stem-loop structure (ψG = −10.30 kcal/mol) can be derived from the primary sequence which most probably represents a transcriptional terminator or/and 3′ mRNA stabilizer whereby the upstream gene dnaG is transcribed in the opposite direction.
Using DNase I footprinting experiments, Spohn and Scarlato demonstrated binding of the repressor protein HspR to the promoter regions upstream of the dnaK and groE operon of H. pylori G27 (34). Furthermore, binding of HspR to the promoter region of a third putative tricistronic operon encoding the genes cbpA (encoding a DnaJ-like protein [39]), hspR (encoding the HspR repressor), and orf (encoding a helicase-like protein) was proven (34). Analysis of an hspR null mutant revealed constitutive high levels of mRNA initiated at the promoters preceding groES, cbpA, and hrcA, indicating derepression in the absence of HspR (34). Binding of the HspR repressor to a consensus sequence named HAIR was described for Streptomyces coelicolor (4, 5, 16), and distinct HAIR sequences are indeed present in the promoter regions of H. pylori groE and cbpA, whereas no distinct HAIR sequence can be derived in the HspR binding region of hrcA (34). But in contrast to S. coelicolor, where the HspR/HAIR-regulated dnaK operon and the clpB gene are strongly heat inducible (4, 5, 16), Spohn and Scarlato detected no heat inducibility of the promoters preceding hrcA, groES, and cbpA (34). However, the promoters upstream of groES and cbpA responded to osmotic stress (34). The reason for this discrepancy between our results and those presented by Spohn and Scarlato (34) concerning heat inducibility is not clear.
As described for dnaK and htpG, we also analyzed the inducibility of the dnaJ and groESL operons after the upshift from 37 to 48°C, and in all cases, no heat induction occurred (data not shown). Most of the heat shock experiments presented by Spohn and Scarlato were carried out by shifting the cells from 37 to 45°C; this could possibly explain the lack of heat induction in these cases by a too high final temperature preventing heat induction as in our experiments to 48°C. On the other hand, Spohn and Scarlato also described the non-heat inducibility of the groELS operon after heat shock from 37 to 42°C (34). Our analyses demonstrated a clear induction of the operon under these conditions (Fig. 6A). Therefore, most probably, methodical differences in RNA analysis procedures are responsible for the observed discrepancy. Furthermore, strain-specific differences in the heat-shock behavior cannot be ruled out, as Spohn and Scarlato used H. pylori 69A. On the other hand, this seems unlikely for such a fundamental physiological mechanism as the heat shock response.
The dnaK operon seems to be under double negative control by the HrcA and HspR repressors. Such control by two different repressors has already been postulated for the dnaK operon of Staphylococcus aureus (8). Here, the operon is most probably regulated by HrcA and another repressor designated CtsR. Furthermore, the close proximity of the HspR binding site mapped by Spohn and Scarlato (34) and the CIRCE-like element in the upstream region of the H. pylori hrcA promoter may indicate a physical interaction of the two repressor proteins. This dual control could explain the differences in behavior of the hrcA promoter compared to the cbpA and groE promoters under conditions of osmotic stress (34).
Surprisingly, the htpG gene was found to be not heat induced in H. pylori by upshift from 37 to 42 or 48°C. The gene specifies a monocistronic mRNA which is most probably terminated at an obvious secondary structure (ΔG = −10.5 kcal/mol) immediately downstream of the coding sequence. After heat shock, the transcript disappears. This can be explained by faster degradation of the mRNA or by switching off of the htpG transcription. The lack of heat inducibility calls into question the physiological importance of the gene product in H. pylori. In all cases described so far, the htpG gene coding for a molecular chaperone of the Hsp90 family is transcriptionally induced after temperature upshift. Therefore, it will be of interest to examine the expression pattern of htpG under other stress conditions leading to protein damage, e.g., acid or oxidative stress.
In conclusion, this work should be considered a first step toward characterizing the expression patterns of the major H. pylori heat shock genes after temperature upshift, with the long-term goal of defining the underlying regulatory mechanisms in detail. Knowledge about these signal response networks will be useful to understand how H. pylori manages the different stress conditions which emerge in the course of infection. Huesca et al. (22) demonstrated induction of GroEL and DnaK proteins in H. pylori HP439 after acid shock. Consequently, induction of chaperones could also be essential in H. pylori 69A for survival under low-pH conditions. In this context, it is noteworthy that a dnaJ knockout mutant of C. jejuni was unable to colonize newly hatched Leghorn chickens (23). Experiments to analyze the transcriptional induction patterns of the major H. pylori 69A heat shock genes after acid shock are in progress.
ACKNOWLEDGMENTS
This work was financially supported by grants to D.K. from the BMBF and to W.S. from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Bardwell J C A, Craig E A. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sci USA. 1987;84:5177–5181. doi: 10.1073/pnas.84.15.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belitsky B, Gustafsson M C, Sonenshein A L, von Wachenfeld C. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J Bacteriol. 1997;179:5448–5457. doi: 10.1128/jb.179.17.5448-5457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 4.Bucca G, Ferina G, Puglia A M, Smith C P. The dnaK operon of Streptomyces coelicolor encodes a novel heat-shock protein which binds to the promoter region of the operon. Mol Microbiol. 1995;17:663–674. doi: 10.1111/j.1365-2958.1995.mmi_17040663.x. [DOI] [PubMed] [Google Scholar]
- 5.Bucca G, Hindle Z, Smith C P. Regulation of the dnaK operon of Streptomyces coelicolor A3(2) is governed by HspR, an autoregulatory repressor protein. J Bacteriol. 1997;179:5999–6004. doi: 10.1128/jb.179.19.5999-6004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchner J. Hsp90 & Co.—a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- 7.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 8.Derré I, Rapoport G, Msadek T. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in Gram-positive bacteria. Mol Microbiol. 1999;31:117–131. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 9.Dunn B E, Cambell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 10.Eaton K A, Brooks C L, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D G, Karjalainen T K, Evans D J, Graham D Y, Lee C-H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175:674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans D J, Evans D G, Engstrand L, Graham D. Urease-associated heat shock protein of Helicobacter pylori. Infect Immun. 1992;60:2125–2127. doi: 10.1128/iai.60.5.2125-2127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fayet O, Ziegelhoffer T, Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171:1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrero R L, Lee A. The importance of urease in acid protection for the gastric-colonising bacteria Helicobacter pylori and Helicobacter felis. Microb Ecol Health Dis. 1991;4:121. [Google Scholar]
- 15.Forsyth M H, Cover T L. Mutational analysis of the vacA promoter provides insight into gene transcription in Helicobacter pylori. J Bacteriol. 1999;181:2261–2266. doi: 10.1128/jb.181.7.2261-2266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandvalet C, de Crécy-Lagard V, Mazodier P. The ClpB ATPase of Streptomyces albus G belongs to the HspR heat shock regulon. Mol Microbiol. 1999;31:521–532. doi: 10.1046/j.1365-2958.1999.01193.x. [DOI] [PubMed] [Google Scholar]
- 17.Haas R, Mayer T F, Jos P, van Putten M. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol Microbiol. 1993;8:753–760. doi: 10.1111/j.1365-2958.1993.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 18.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 19.Homuth G, Masuda S, Mogk A, Kobayashi Y, Schumann W. The dnaK operon of Bacillus subtilis is heptacistronic. J Bacteriol. 1997;179:1153–1164. doi: 10.1128/jb.179.4.1153-1164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homuth G, Mogk A, Schumann W. Post-transcriptional regulation of the Bacillus subtilis dnaK operon. Mol Microbiol. 1999;32:1183–1197. doi: 10.1046/j.1365-2958.1999.01428.x. [DOI] [PubMed] [Google Scholar]
- 21.Huesca M, Borgia S, Hoffman P, Lingwood C A. Acidic pH changes receptor binding of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect Immun. 1996;64:2643–2648. doi: 10.1128/iai.64.7.2643-2648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huesca M, Goodwin A, Bhagwansingh A, Hoffman P, Lingwood C A. Characterization of an acidic-pH-inducible stress protein (hsp70), a putative sulfatide binding adhesin, from Helicobacter pylori. Infect Immun. 1998;66:4061–4067. doi: 10.1128/iai.66.9.4061-4067.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konkel M E, Kim B J, Klena J D, Young C R, Ziprin R. Characterization of the thermal stress response of Campylobacter jejuni. Infect Immun. 1998;66:3666–3672. doi: 10.1128/iai.66.8.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leying H, Suerbaum S, Geis G, Haas R. Cloning and genetic characterization of a Helicobacter pylori flagellin gene. Mol Microbiol. 1992;6:2863–2874. doi: 10.1111/j.1365-2958.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Wong S-L. Cloning and characterization of the groESL operon from Bacillus subtilis. J Bacteriol. 1992;174:3981–3992. doi: 10.1128/jb.174.12.3981-3992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGowan C C, Cover T L, Blaser M J. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterology. 1996;110:926–938. doi: 10.1053/gast.1996.v110.pm8608904. [DOI] [PubMed] [Google Scholar]
- 27.Peak K-H, Walker G C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987;169:283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Schmitt W, Odenbreit S, Heuermann D, Haas R. Cloning of the Helicobacter pylori recA gene and functional characterization of its product. Mol Gen Genet. 1995;248:563–572. doi: 10.1007/BF02423452. [DOI] [PubMed] [Google Scholar]
- 30.Schulz A, Schwab S, Versteeg S, Schumann W. The htpG gene of Bacillus subtilis belongs to class III heat shock genes and is under negative control. J Bacteriol. 1997;10:3103–3109. doi: 10.1128/jb.179.10.3103-3109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz A, Tzschaschel B, Schumann W. Isolation and analysis of mutants of the dnaK operon of Bacillus subtilis. Mol Microbiol. 1995;15:421–429. doi: 10.1111/j.1365-2958.1995.tb02256.x. [DOI] [PubMed] [Google Scholar]
- 32.Segal G, Ron E Z. Heat shock activation of the groESL operon of Agrobacterium tumefaciens and the regulatory roles of the inverted repeat. J Bacteriol. 1996;178:3634–3640. doi: 10.1128/jb.178.12.3634-3640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spohn G, Beier D, Rappuoli R, Scarlato V. Transcriptional analysis of the divergent cagAB genes encoded by the pathogenicity island of Helicobacter pylori. Mol Microbiol. 1997;26:361–372. doi: 10.1046/j.1365-2958.1997.5831949.x. [DOI] [PubMed] [Google Scholar]
- 34.Spohn G, Scarlato V. The autoregulatory HspR repressor protein governs chaperone gene transcription in Helicobacter pylori. Mol Microbiol. 1999;34:663–674. doi: 10.1046/j.1365-2958.1999.01625.x. [DOI] [PubMed] [Google Scholar]
- 35.Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suerbaum S, Thiberge J-M, Kansau I, Ferrero R L, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol Microbiol. 1994;14:959–974. doi: 10.1111/j.1365-2958.1994.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 37.Thies F L, Karch H, Hartung H P, Giegerich G. Cloning and expression of the dnaK gene of Campylobacter jejuni and antigenicity of heat shock protein 70. Infect Immun. 1999;67:1194–1200. doi: 10.1128/iai.67.3.1194-1200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L X, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzgerald L M, Lee N, Adams M D. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 39.Ueguchi C, Kakeda M, Yamada H, Mizuno T. An analogue of the DnaJ molecular chaperone in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:1054–1058. doi: 10.1073/pnas.91.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Asseldonk M, Simons A, Visser H, De Vos W M, Simons G. Cloning, nucleotide sequence, and regulatory analysis of the Lactococcus lactis dnaJ gene. J Bacteriol. 1993;175:1637–1644. doi: 10.1128/jb.175.6.1637-1644.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Versteeg S, Mogk A, Schumann W. The Bacillus subtilis htpG gene is not involved in thermal stress management. Mol Gen Genet. 1999;261:582–588. doi: 10.1007/s004380051004. [DOI] [PubMed] [Google Scholar]
- 42.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 43.Wetzstein M, Völker U, Dedio J, Löbau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992;174:3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiech H, Buchner J, Zimmermann R, Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992;358:169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- 45.Yokota K, Hirai Y, Haque M, Hayashi S, Isogai H, Sugiyama T, Nagamachi E, Tsukada Y, Fujii N, Oguma K. Heat shock protein produced by Helicobacter pylori. Microbiol Immunol. 1994;38:403–405. doi: 10.1111/j.1348-0421.1994.tb01799.x. [DOI] [PubMed] [Google Scholar]
- 46.Yuan G, Wong S-L. Regulation of groE expression in Bacillus subtilis: the involvement of the ςA-like promoter and the roles of the inverted repeat sequence (CIRCE) J Bacteriol. 1995;177:5427–5433. doi: 10.1128/jb.177.19.5427-5433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]