Abstract
Background
Periodontitis-related attachment loss is accompanied by mucosal bleeding and inflammatory lesions. Dietary vitamin K and fibre intake are known to be correlation factors of haemostasis and anti-inflammation, respectively.
Objective
To explore the association between severe periodontal attachment loss and vitamin K or fibre intake in American adults.
Methods
A cross-sectional analysis was conducted including 2747 males and 2218 females in the National Health and Nutrition Examination Surveys (NHANES) from 2009 to 2014. The number of teeth with severe periodontal attachment loss (above 5 mm attachment loss) was used as the dependent variable. The main independent variables included the intake of vitamin K and dietary fibre. The association among variables was examined using multivariable linear regression models, hierarchical regression, fitted smoothing curves, and generalized additive models.
Results
Based on the indicators of 4965 subjects, we found that severe attachment loss tended to occur in elderly individuals or males and was accompanied by less intake of vitamin K or dietary fibre, as well as lower educational qualification. Vitamin K intake was stably negatively associated with attachment loss progression in each multivariable linear regression model. In subgroup analyses, a negative association between fibre intake and attachment loss progression was identified in all races except blacks (β = 0.0005, 95% CI: -0.0005 to 0.0016). The relationship between fibre intake and attachment loss progression was a broad U-shaped curve (inflection point: 753.4 mg), which especially manifested in males (inflection point: 967.5 mg).
Conclusion
There was an inverse association between vitamin K intake and the progression of periodontal attachment loss in American adults, while dietary fibre should be moderate in intake (below 753.4 mg), especially in males (below 967.5 mg).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-023-02929-9.
Keywords: Periodontitis, Attachment loss, Vitamin K, Dietary fibre, NHANES
Introduction
Periodontitis is a widely distributed and high-incidence oral inflammatory disease caused by complex factors [1]. Dental plaque is the initiating factor of periodontitis [2], and the severity of periodontitis depends on environmental and host risk factors, including diabetes, smoking, obesity, genetic factors, and undernutrition [3]. The initial manifestations of progressive periodontitis are bleeding gums and local inflammation [4]. As inflammation develops, chewing pressure exceeds the periodontal load, leading to alveolar bone destruction and tooth loss [5, 6]. Meanwhile, vitamin K and fibre intake are known to be correlation factors of haemostasis [7] and anti-inflammatory effects [8], respectively. Previous studies have shown that periodontitis is closely related to nutritional intake [9], dietary habits [10], and lifestyle [11]. However, whether dietary intake acts as an accurate indicator for periodontitis progression remains unclear.
Vitamin K is a fat-soluble clotting vitamin [12], and its deficiency can lead to massive bleeding [13], bone dysplasia [14], osteoporosis [15], and cardiovascular diseases [16]. Vitamin K prevents gingival bleeding through posttranslational modification of glutamate residues in coagulation factors and high binding to phospholipid membrane regions [17, 18]. In addition, fibre consumption, such as whole-grain intake, may reduce the risk of periodontitis by increasing insulin sensitivity and reducing oxidative stress and cytokine production [19]. Furthermore, fibre can be fermented by gut microbes to form short-chain fatty acids, which have anti-inflammatory properties [20]. Interestingly, dietary fibre intake is closely related to the absorption of vitamin K in humans, especially vitamin K1 [21]. To indicate the occurrence and progression of severe periodontitis, it is necessary to explore the potential relationship between vitamin K or dietary fibre intake and periodontal attachment loss progression.
In this study, we hypothesized that the intake of vitamin K or dietary fibre was associated with periodontal attachment loss progression. A cross-sectional study was conducted based on the National Health and Nutrition Examination Surveys (NHANES) dataset from 2009–2014. Multiple linear regression and hierarchical regression were used to examine the association. To evaluate the nonlinear relationship, smooth curve fitting and threshold analysis were performed. This study sought to shed light on the potential food and nutrition facts of periodontitis prevention.
Method
Study participants
As a cross-sectional survey of nationally representative samples from 2009 to 2014, NHANES collected information about the nutritional and health status of noninstitutionalized individuals in the United States. The study was approved by the Ethics Review Committee of the National Center for Health Statistics (NCHS), and written informed consent was obtained from each participant. Adults who were over 30 years of age, had more than 1 natural tooth (except the third molar), and did not have a health condition requiring prophylactic antibiotics before periodontal probing were eligible for a full-mouth periodontal examination [22]. After excluding participants with missing data on vitamin K or dietary fibre intake (n = 729), 4965 respondents who were finally included in the analysis underwent a complete NHANES oral health periodontal examination, and their nutritional status and periodontal examination data were analysed. All measurements were recorded as required by the periodontal classification algorithm, and complete data from the NHANES questionnaire were obtained.
Periodontal examination
Periodontal attachment loss was identified by dental examiners who were dentists (D.D.S./D.M.D.) licenced in at least two U.S. states. All oral health assessments took place in a designated room at the mobile examination centre (MEC). According to the periodontitis guidelines in 2018, stage I periodontitis was defined as attachment loss (AL) ≤ 2 mm and ≥ 1 mm. Stage II was defined as AL ≤ 4 mm and ≥ 3 mm. Stage III and IV were defined as AL ≥ 5 mm. Attachment loss involving ≥ 30% of teeth was defined as generalized periodontitis attachment loss. In this study, a CPI periodontal probe was used for periodontal health assessment. Measurements were made at 6 sites around each tooth (mesio-, mid-, and distobuccal; mesio-, mid-, and distolingual) for all teeth, excluding third molars. Two measurements were made at each periodontal site, one for gingival recession, the distance from the free gingival margin to the cementoenamel junction, and the second for pocket depth (PD), the distance from the free gingival margin to the bottom of the sulcus or periodontal pocket. Clinical attachment loss (AL) was calculated as PD minus gingival recession. The number of teeth with severe periodontal attachment loss (≥ 5 mm) was used as the dependent variable [22, 23].
Other variables
We comprehensively screened confounding factors related to periodontitis, vitamin K and dietary fibre as covariates, including age, sex, body mass index, education, and race [24–26]. In addition, the intake of vitamin K and dietary fibre were assessed by two consecutive days of dietary recalls.
Statistical analysis
We performed all statistical analyses by using R (http://www.R-project.org), with statistical significance set at P < 0.05. All estimates were calculated by using sample weights following the analytical guidelines edited by the NCHS because the goal of the NHANES is to produce data representative of the civilian noninstitutionalized US population. The multivariate linear regression model and subgroup analysis were utilized to examine the relationship between vitamin K and dietary fibre intake and periodontal attachment loss. Three multiple linear regression models were constructed: Model 1, which did not adjust for covariates; Model 2, which adjusted for age, sex, and race; and Model 3, which adjusted for all covariables listed in Table 1. The nonlinear relationship was solved by smooth curve fitting and threshold analysis.
Table 1.
Characteristics of the participants
| Quartiles of PERIO | Q1 (n = 967) | Q2 (n = 1073) | Q3 (n = 1658) | Q4 (n = 1267) | P value |
|---|---|---|---|---|---|
| Age (years) | 42.3413 ± 10.6764 | 54.5396 ± 14.2871 | 57.7376 ± 13.6435 | 57.8587 ± 12.3326 | < 0.001 |
| BMI | 28.8750 ± 6.5105 | 29.9104 ± 6.4273 | 29.6258 ± 6.8878 | 29.0708 ± 6.6225 | < 0.001 |
| Vitamin K | 5.9959 ± 1.1846 | 5.7870 ± 1.309864 | 5.7358 ± 1.2727 | 5.6458 ± 1.2683 | < 0.001 |
| Fibre | 7.3291 ± 2.3188 | 6.8323 ± 2.4048 | 6.7402 ± 2.4201 | 6.5978 ± 2.4502 | < 0.001 |
| Gender | < 0.001 | ||||
| Male | 416 (43.0196%) | 550 (51.2582%) | 925 (55.7901%) | 856 (67.5612%) | |
| Female | 551 (56.9804%) | 523 (48.7418%) | 733 (44.2099%) | 411 (32.4388%) | |
| Race | < 0.001 | ||||
| Mexican American | 101 (10.4447%) | 167 (15.5638%) | 260 (15.6815%) | 254 (20.0474%) | |
| Other Hispanics | 74 (7.6525%) | 125 (11.6496%) | 172 (10.3739%) | 109 (8.6030%) | |
| Non-Hispanic Whites | 551 (56.9804%) | 431 (40.1678%) | 688 (41.4958%) | 452 (35.6748%) | |
| Non-Hispanic Blacks | 117 (12.0993%) | 240 (22.3672%) | 397 (23.9445%) | 335 (26.4404%) | |
| Other races | 124 (12.8232%) | 110 (10.2516%) | 141 (8.5042%) | 117 (9.2344%) | |
| Education | < 0.001 | ||||
| Below Grade 9 | 36 (3.7736%) | 101 (9.6651%) | 174 (10.6880%) | 183 (14.7343%) | |
| Grades 9–11 | 47 (4.9266%) | 140 (13.3971%) | 278 (17.0762%) | 238 (19.1626%) | |
| High school graduate | 130 (13.6268%) | 235 (22.4880%) | 402 (24.6929%) | 349 (28.0998%) | |
| College/AA degree | 279 (29.2453%) | 286 (27.3684%) | 443 (27.2113%) | 298 (23.9936%) | |
| College degree or above | 460 (48.2180%) | 281 (26.8900%) | 320 (19.6560%) | 170 (13.6876%) | |
| Refused to answer | 1 (0.1048%) | 0 (0.0000%) | 3 (0.1843%) | 0 (0.0000%) | |
| Do not know | 1 (0.1048%) | 2 (0.1914%) | 8 (0.4914%) | 4 (0.3221%) |
BMI Body mass index, vitamin K (log2-transformed), fibre (log2-transformed)
Mean ± SD for continuous variables: P value was calculated by the weighted linear regression model
% for categorical variables: P value was calculated by weighted chi-square test
Results
The baseline characteristics of the subjects (2747 males and 2218 females) are shown in Table 1. All participants were separated into four groups according to the quartile of severe attachment loss teeth counts (Q1: 0, Q2: 1, Q3: 2 to 5, and Q4: 6 to 27). Generalized periodontal attachment loss tended to occur in elderly individuals or males and was accompanied by less intake of vitamin K and dietary fibre, as well as lower educational qualification. From Q1 to Q4, the proportion of Mexican Americans and blacks gradually increased.
The results of the multivariate regression analyses are presented in Tables 2 and 3. In Model 1, vitamin K (β = -0.2477, 95% CI: -0.3312 to -0.1643, p < 0.000001) and fibre intake (β = -0.2812, 95% CI: -0.3641 to -0.1984, p < 0.000001) were negatively correlated with attachment loss progression. After adjustment for confounders, those positive associations were stable in Model 2 (P < 0.000001) and Model 3 (P < 0.01). In the subgroup analysis stratified by sex and race, the negative association between vitamin K and attachment loss progression was stable. However, fibre intake was negatively associated with attachment loss progression in all races except blacks.
Table 2.
Association between vitamin K intake and periodontal attachment loss progression
| Model 1 β (95% CI) P value |
Model 2 β (95% CI) P value |
Model 3 β (95% CI) P value |
|
|---|---|---|---|
| Vitamin K | -0.0014 (-0.0026, -0.0002) 0.022052 | -0.0016 (-0.0028, -0.0005) 0.006438 | -0.0009 (-0.0021, 0.0002) 0.123073 |
| Quartiles of Vitamin K | |||
| Q1 (0.75–31.20 mg) | 0 | 0 | 0 |
| Q2 (31.30–53.10 mg) | -0.0388 (-0.1223, 0.0446) 0.361802 | -0.0686 (-0.1441, 0.0068) 0.074658 | -0.0271 (-0.1010, 0.0468) 0.472478 |
| Q3 (53.15–93.35 mg) | -0.1991 (-0.2825, -0.1157) 0.000003 | -0.1894 (-0.2649, -0.1138) < 0.000001 | -0.1058 (-0.1800, -0.0316) 0.005237 |
| Q4 (93.45–1605.50 mg) | -0.2477 (-0.3312, -0.1643) < 0.000001 | -0.2342 (-0.3100, -0.1584) < 0.000001 | -0.1181 (-0.1930, -0.0433) 0.001982 |
| P trend | < 0.001 | < 0.001 | 0.010 |
| Stratified by gender | |||
| Male | -0.0012 (-0.0028, 0.0005) 0.173894 | -0.0012 (-0.0028, 0.0004) 0.146590 | -0.0005 (-0.0021, 0.0011) 0.531508 |
| Female | -0.0023 (-0.0040, -0.0007) 0.005283 | -0.0023 (-0.0040, -0.0007) 0.004573 | -0.0014 (-0.0030, 0.0002) 0.084140 |
| Stratified by race | |||
| Mexican American | -0.0020 (-0.0075, 0.0034) 0.462763 | -0.0026 (-0.0079, 0.0027) 0.338067 | 0.0016 (-0.0070, 0.0038) 0.557883 |
| Other Hispanics | -0.0032 (-0.0073, 0.0009) 0.123363 | -0.0022 (-0.0061, 0.0018) 0.286774 | -0.0009 (-0.0048, 0.0031) 0.669704 |
| Non-Hispanic Whites | -0.0013 (-0.0030, 0.0004) 0.143690 | -0.0015 (-0.0032, 0.0002) 0.083340 | -0.0002 (-0.0019, 0.0015) 0.806674 |
| Non-Hispanic Blacks | -0.0006 (-0.0029, 0.0017) 0.592771 | -0.0014 (-0.0037, 0.0008) 0.199793 | -0.0017 (-0.0039, 0.0005) 0.131520 |
| Other races | -0.0017 (-0.0053, 0.0020) 0.369406 | -0.0016 (-0.0052, 0.0020) 0.386952 | -0.0003 (-0.0039, 0.0032) 0.851076 |
Model 1: No covariates were adjusted
Model 2: Age, gender, and race were adjusted
Model 3: Adjusted for all variables listed in Table 1
Table 3.
Relationship between dietary fibre intake and periodontal attachment loss progression
| Model 1 β (95% CI) P value |
Model 2 β (95% CI) P value |
Model 3 β (95% CI) P value |
|
|---|---|---|---|
| Fibre | -0.0009 (-0.0014, -0.0005) 0.000008 | -0.0008 (-0.0012, -0.0004) 0.000050 | -0.0008 (-0.0012, -0.0004) 0.000190 |
| Quartiles of fibre | |||
| Q1 (0.00–19.35 mg) | 0 | 0 | 0 |
| Q2 (19.40–238.65 mg) | -0.0288 (-0.1123, 0.0546) 0.498731 | -0.0448 (-0.1204, 0.0308) 0.245495 | -0.0080 (-0.0815, 0.0656) 0.832138 |
| Q3 (238.70–469.55 mg) | -0.1536 (-0.2374, -0.0697) 0.000333 | -0.1545 (-0.2308, -0.0781) 0.000075 | -0.1249 (-0.1996, -0.0502) 0.001051 |
| Q4 (470.25–2428.85 mg) | -0.2812 (-0.3641, -0.1984) < 0.000001 | -0.2170 (-0.2924, -0.1415) < 0.000001 | -0.1695 (-0.2429, -0.0960) 0.000006 |
| P trend | < 0.001 | < 0.001 | < 0.001 |
| Stratified by gender | |||
| Male | -0.0012 (-0.0018, -0.0007) 0.000009 | -0.0010 (-0.0015, -0.0005) 0.000298 | -0.0010 (-0.0015, -0.0004) 0.000374 |
| Female | -0.0008 (-0.0014, -0.0002) 0.008639 | -0.0005 (-0.0011, 0.0001) 0.116644 | -0.0004 (-0.0010, 0.0002) 0.232311 |
| Stratified by race | |||
| Mexican American | -0.0012 (-0.0023, -0.0001) 0.029996 | -0.0012 (-0.0023, -0.0002) 0.020879 | -0.0012 (-0.0023, -0.0002) 0.023186 |
| Other Hispanics | -0.0009 (-0.0020, 0.0003) 0.134178 | -0.0008 (-0.0019, 0.0002) 0.128930 | -0.0008 (-0.0018, -0.0003) 0.159892 |
| Non-Hispanic Whites | -0.0013 (-0.0019, -0.0008) 0.000002 | -0.0012 (-0.0017, -0.0007) 0.000014 | -0.0012 (-0.0018, -0.0007) 0.000010 |
| Non-Hispanic Blacks | 0.0004 (-0.0007, 0.0014) 0.480656 | 0.0003 (-0.0007, 0.0013) 0.543631 | 0.0005 (-0.0005, 0.0016) 0.293950 |
| Other races | -0.0006 (-0.0022, 0.0011) 0.503325 | -0.0005 (-0.0021, 0.0011) 0.568293 | -0.0003 (-0.0019, 0.0013) 0.714169 |
Model 1: No covariates were adjusted
Model 2: Age, gender, and race were adjusted
Model 3: Adjusted for all variables listed in Table 1
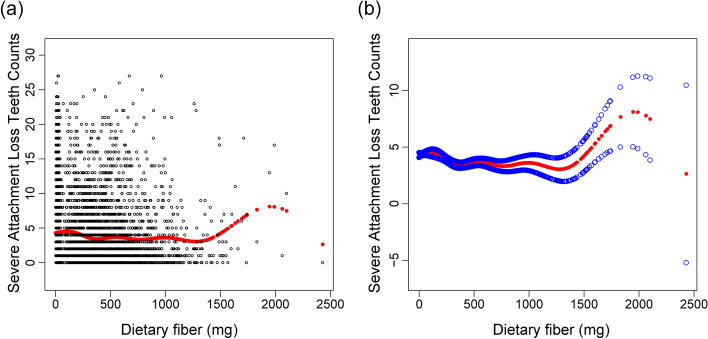
In addition, the weighted generalized additive models and smooth curve fitting showed the nonlinear relationship between dietary fibre intake and attachment loss progression. More details of vitamin K and attachment loss progression are shown in Figures S1 and S2. By two-piecewise linear regression models, the inflection point of dietary fibre intake was 753.4 mg (Table 4). For dietary fibre intake < 753.4 mg, every 1 mg increase in dietary fibre intake was correlated with 0.0016 (95% CI:—0.0021 to—0.0010) fewer severe attachment loss teeth. By comparison, for individuals with dietary fibre intake > 753.4 mg, every 1 mg increase in vitamin K or dietary fibre intake was significantly associated with 0.0015 (95% CI: 0.0001 to 0.0029) more severe attachment loss teeth. In addition, the relationship between fibre intake and attachment loss progression showed a broad U-shaped curve (Fig. 1), especially for males (inflection point: 967.5 mg) (Fig. 2, Table 5).
Table 4.
Two-piecewise regression analysis of the effect of dietary fibre intake on the progression of attachment loss
| Attachment loss progression | Adjusted β (95% CI), P value |
|---|---|
| Fitting by the standard linear model | -0.0009 (-0.0014, -0.0005) < 0.0001 |
| Fitting by the two-piecewise linear model | |
| Inflection point | 753.4 mg |
| Dietary fibre < 753.4 (mg) | -0.0016 (-0.0021, -0.0010) < 0.0001 |
| Dietary fibre > 753.4 (mg) | 0.0015 (0.0001, 0.0029) 0.0362 |
| Log likelihood ratio | < 0.001 |
Fig. 1.
Association between dietary fibre intake and loss of periodontal attachment. a Each black dot represents a sample. b The solid line represents the smooth curve fitting between variables. The blue bands represent the 95% confidence intervals of the fit
Fig. 2.
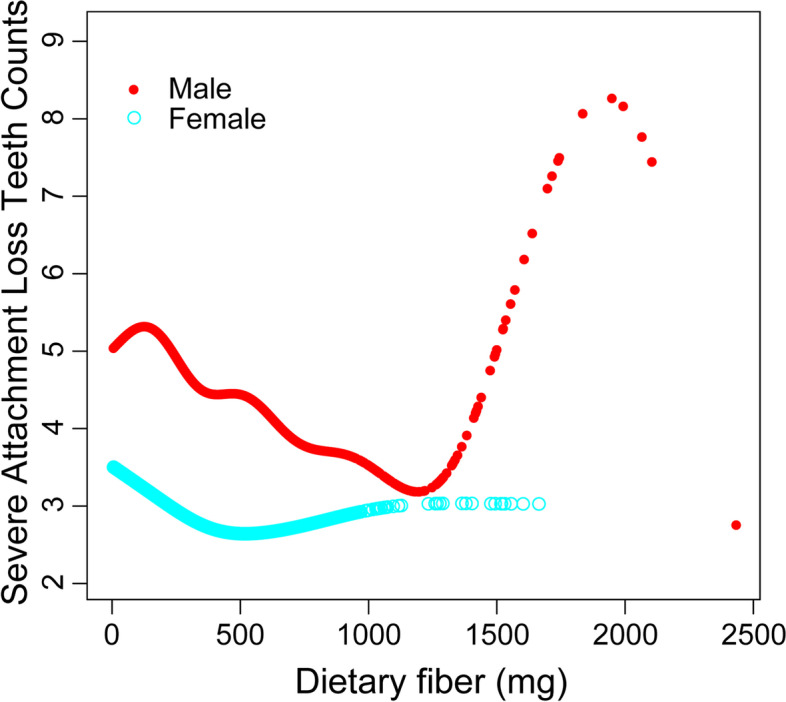
Association between dietary fibre intake and loss of periodontal attachment, stratified by sex. Adjusted for all variables listed in Table 1
Table 5.
Two-piecewise regression analysis of the effect of dietary fibre intake on the progression of attachment loss in males and females
| Attachment loss progression | Males β (95% CI), P value |
Females β (95% CI), P value |
|---|---|---|
| Fitting by the standard linear model | -0.0012 (-0.0018, -0.0007) < 0.0001 | -0.0008 (-0.0014, -0.0002) 0.0086 |
| Fitting by the two-piecewise linear model | ||
| Inflection point | 967.5 mg | 18.05 mg |
| Dietary fibre < Inflection point | -0.0018 (-0.0024, -0.0011) < 0.0001 | -0.1180 (-0.1743, -0.0618) < 0.0001 |
| Dietary fibre > Inflection point | 0.0022 (-0.0003, 0.0047) 0.0863 | -0.0002 (-0.0008, 0.0005) 0.6687 |
| Log likelihood ratio | 0.006 | < 0.001 |
Discussion
As the sixth most prevalent disease in the world, periodontitis causes alveolar bone resorption and tooth loss in adults [27]. Severe alveolar bone loss adds to the challenge of implant restoration and financial burden [28]. Therefore, exploring the risk factors for periodontitis is crucial to alveolar bone health and tooth survival [29]. Previous studies focused on the mechanism and treatment of severe periodontitis, including genetics [30], protein expression [31], and immune response [32]. For example, IL-23-dependent IL-17 is a powerful proinflammatory mediator that leads to bacterial overgrowth that causes periodontal disease [33]. Another study found that the modified MCPIP-1 and MALT-1 response and protein expression induced by periodontitis-related bacteria may be part of the pathogenesis of periodontitis [34]. A recent study found that circRNA forms a circRNA–miRNA‒mRNA network during the osteogenic differentiation of periodontal stem cells, which has the prospect of synchronous periodontal diagnosis and regeneration [30]. Multivariate logistic regression analyses indicated that abundant vitamin K and moderate dietary fibre are beneficial to periodontal health.
In addition to the haemostatic effects [13], vitamin K can also prevent bone health by taking in carboxylated vitamin K-dependent proteins, such as osteocalcin and stromal glass protein [15]. The lack of a balanced diet rich in vitamins and minerals predisposes individuals to inflammatory diseases, such as obesity, diabetes, and periodontitis [35]. It has been suggested that vitamin K2, a cofactor of vitamin D and calcium, can support bone health by promoting osteoblast differentiation and upregulating transcription [36]. It can also regulate immune cells that cause inflammation by reducing the production of inflammatory markers and therefore reducing inflammation [37]. A previous study showed that adequate vitamin K administration reduces osteoclast and alveolar bone resorption in patients with experimental periodontitis [38]. Multivariable linear regression results also suggested that vitamin K intake was stably negatively associated with attachment loss progression.
Healthy eating habits, especially increased dietary fibre intake, may improve periodontitis markers by reducing serum CRP levels [39]. Recent research identified that higher levels of fibre intake were inversely associated with periodontitis [40]. However, the study showed that dietary fibre intake was negatively correlated with periodontal attachment loss, and the threshold reverse effect occurred when a certain value was reached (inflection point = 753.4 mg). Due to the degeneration of periodontal membrane sensory function in patients with periodontitis, excessive intake of dietary fibre leads to pressure concentration, tooth abrasion and alveolar bone resorption [41]. The inflection point of fibre intake in the females was 18.05 mg, and the inflection point for the males was 967.5 mg. Due to the more powerful chewing function of males, an increased risk of attachment loss occurs with excessive fibre intake [42].
Although the current study demonstrated the association of vitamin K, dietary fibre intake and the progression of periodontal attachment loss, there are also several limitations. First, as a cross-sectional study, it was impossible to ascertain temporality between exposure and outcomes. Second, as we focused on the potential food and nutrition facts of periodontitis prevention, the intake of vitamin K was not equal to circulatory vitamin K, and we will further explore the association of circulatory vitamin K and periodontal attachment loss. Moreover, due to the occlusion function of participants being unknown, the relevant potential factors affecting mastication should be distinguished in future studies. Third, vitamin K and dietary fibre intake in this study was determined by a two-day dietary review interview with participants, which may be subject to recall bias. Fourth, the types of dietary fibre are diverse and need to be further divided in the future.
Conclusion
Vitamin K intake was inversely associated with the progression of periodontal attachment loss among American adults. Meanwhile, a broad U-shaped relationship (inflection point, 753.4 mg) between fibre intake and attachment loss progression was identified, particularly in males (inflection point, 967.5 mg). The study guides the potential role of dietary habits in community oral health prevention.
Supplementary Information
Additional file 1: Figure S1. Association between Vitamin K intake and loss of periodontal attachment. Each black dot represents a sample. The solid line represents the smooth curve fitting between variables. The blue bands represent the 95% confidence intervals of the fit.
Additional file 2: Figure S2. Association between vitamin K and loss of periodontal attachment, stratified by sex. Adjusted for all variate lists in Table 1.
Acknowledgements
Not applicable.
Authors’ contributions
C.Y. collected the data and wrote the manuscript; D.B. and H.M. assisted L.X. with data analyses. H.M. performed statistical analyses. W.Y. conceived of this study. Z.H. analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Basic and Clinical Cooperative Research Promotion Program of Anhui Medical University (2021xkjT038) and the 2022 Disciplinary Construction Project in the School of Dentistry, Anhui Medical University (2022xkfyhz09).
Availability of data and materials
Publicly available datasets were analysed in this study. Data used for this study are available on the NHANES website: https://wwwn.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
The studies involving human participants were approved by the institutional review board of the National Center for Health Statistics, CDC, and were conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuanyuan Chuai, Bichong Dai and Xiaoyun Liu contributed equally to this work.
Contributor Information
Yuanyin Wang, Email: wyy1970548@sohu.com.
Hengguo Zhang, Email: zhanghengguo@ahmu.edu.cn.
References
- 1.Cassiano LS, Peres MA, Motta JVS, Demarco FF, Horta BL, Ribeiro CC, Nascimento GG. Periodontitis is associated with consumption of processed and ultra-processed foods: findings from a population-based study. Nutrients. 2022;14(18):3735. doi: 10.3390/nu14183735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 3.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62(1):59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 4.Lang NP, Schätzle MA, Löe H. Gingivitis as a risk factor in periodontal disease. J Clin Periodontol. 2009;36(Suppl 10):3–8. doi: 10.1111/j.1600-051X.2009.01415.x. [DOI] [PubMed] [Google Scholar]
- 5.Barbe AG, Javadian S, Rott T, Scharfenberg I, Deutscher HCD, Noack MJ, Derman SHM. Objective masticatory efficiency and subjective quality of masticatory function among patients with periodontal disease. J Clin Periodontol. 2020;47(11):1344–1353. doi: 10.1111/jcpe.13364. [DOI] [PubMed] [Google Scholar]
- 6.Li XY, Wen MZ, Liu H, Shen YC, Su LX, Yang XT. Dietary magnesium intake is protective in patients with periodontitis. Front Nutr. 2022;9:976518. doi: 10.3389/fnut.2022.976518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halder M, Petsophonsakul P, Akbulut AC, Pavlic A, Bohan F, Anderson E, Maresz K, Kramann R, Schurgers L. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int J Mol Sci. 2019;20(4):896. doi: 10.3390/ijms20040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, Bohn T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki M, Taylor GW, Moynihan P, Yoshihara A, Muramatsu K, Watanabe R, Miyazaki H. Dietary ratio of n-6 to n-3 polyunsaturated fatty acids and periodontal disease in community-based older Japanese: a 3-year follow-up study. Prostaglandins Leukot Essent Fatty Acids. 2011;85(2):107–112. doi: 10.1016/j.plefa.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 10.McGuire S. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Departments of Agriculture and Health and Human Services, 2015. Adv Nutr. 2016;7(1):202–204. doi: 10.3945/an.115.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchbender M, Schlee N, Kesting MR, Grimm J, Fehlhofer J, Rau A. A prospective comparative study to assess the risk of postoperative bleeding after dental surgery while on medication with direct oral anticoagulants, antiplatelet agents, or vitamin K antagonists. BMC Oral Health. 2021;21(1):504. doi: 10.1186/s12903-021-01868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowd P, Ham SW, Naganathan S, Hershline R. The mechanism of action of vitamin K. Annu Rev Nutr. 1995;15:419–440. doi: 10.1146/annurev.nu.15.070195.002223. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez Gossweiler A, Martinez-Mier EA. Chapter 6: vitamins and oral health. Monogr Oral Sci. 2020;28:59–67. doi: 10.1159/000455372. [DOI] [PubMed] [Google Scholar]
- 14.Schurgers LJ, Teunissen KJ, Hamulyák K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109(8):3279–3283. doi: 10.1182/blood-2006-08-040709. [DOI] [PubMed] [Google Scholar]
- 15.Fusaro M, Cianciolo G, Brandi ML, Ferrari S, Nickolas TL, Tripepi G, Plebani M, Zaninotto M, Iervasi G, La Manna G, et al. Vitamin K and osteoporosis. Nutrients. 2020;12(12):3625. doi: 10.3390/nu12123625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol. 2009;297(6):C1358–1367. doi: 10.1152/ajpcell.00216.2009. [DOI] [PubMed] [Google Scholar]
- 17.Nelsestuen GL, Suttie JW. Mode of action of vitamin K. Calcium binding properties of bovine prothrombin. Biochemistry. 1972;11(26):4961–4964. doi: 10.1021/bi00776a013. [DOI] [PubMed] [Google Scholar]
- 18.Schurgers LJ, Spronk HM. Differential cellular effects of old and new oral anticoagulants: consequences to the genesis and progression of atherosclerosis. Thromb Haemost. 2014;112(5):909–917. doi: 10.1160/th14-03-0268. [DOI] [PubMed] [Google Scholar]
- 19.Merchant AT, Pitiphat W, Franz M, Joshipura KJ. Whole-grain and fiber intakes and periodontitis risk in men. Am J Clin Nutr. 2006;83(6):1395–1400. doi: 10.1093/ajcn/83.6.1395. [DOI] [PubMed] [Google Scholar]
- 20.Hills RD, Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11(7):1613. doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr. 1998;128(5):785–788. doi: 10.1093/jn/128.5.785. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen SJ, Trak-Fellermeier MA, Joshipura K, Dye BA. Dietary fiber intake is inversely associated with periodontal disease among US adults. J Nutr. 2016;146(12):2530–2536. doi: 10.3945/jn.116.237065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaud DS, Fu Z, Shi J, Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39(1):49–58. doi: 10.1093/epirev/mxx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim EH, Nam S, Park CH, Kim Y, Lee M, Ahn JB, Shin SJ, Park YR, Jung HI, Kim BI, et al. Periodontal disease and cancer risk: a nationwide population-based cohort study. Front Oncol. 2022;12:901098. doi: 10.3389/fonc.2022.901098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walther C, Spinler K, Borof K, Kofahl C, Heydecke G, Seedorf U, Beikler T, Terschüren C, Hajek A, Aarabi G. Evidence from the Hamburg City Health Study - association between education and periodontitis. BMC Public Health. 2022;22(1):1662. doi: 10.1186/s12889-022-14096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebersole JL, Al-Sabbagh M, Dawson DR., 3rd Heterogeneity of human serum antibody responses to P. gingivalis in periodontitis: Effects of age, race/ethnicity, and sex. Immunol Lett. 2020;218:11–21. doi: 10.1016/j.imlet.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balta MG, Papathanasiou E, Blix IJ, Van Dyke TE. Host modulation and treatment of periodontal disease. J Dent Res. 2021;100(8):798–809. doi: 10.1177/0022034521995157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel RM. Dental implants for patients with periodontitis. Prim Dent J. 2020;8(4):54–61. doi: 10.1308/205016820828463898. [DOI] [PubMed] [Google Scholar]
- 29.Lin P, Niimi H, Ohsugi Y, Tsuchiya Y, Shimohira T, Komatsu K, Liu A, Shiba T, Aoki A, Iwata T, et al. Application of ligature-induced periodontitis in mice to explore the molecular mechanism of periodontal disease. Int J Mol Sci. 2021;22(16):8900. doi: 10.3390/ijms22168900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao K, Walsh LJ, Ivanovski S, Han P. The emerging regulatory role of circular RNAs in periodontal tissues and cells. Int J Mol Sci. 2021;22(9):4636. doi: 10.3390/ijms22094636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Båge T, Kats A, Lopez BS, Morgan G, Nilsson G, Burt I, Korotkova M, Corbett L, Knox AJ, Pino L, et al. Expression of prostaglandin E synthases in periodontitis immunolocalization and cellular regulation. Am J Pathol. 2011;178(4):1676–1688. doi: 10.1016/j.ajpath.2010.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almubarak A, Tanagala K, Papapanou PN, Lalla E, Momen-Heravi F. Disruption of monocyte and macrophage homeostasis in periodontitis. Front Immunol. 2020;11:330. doi: 10.3389/fimmu.2020.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20(14):3394. doi: 10.3390/ijms20143394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firatli Y, Firatli E, Loimaranta V, Elmanfi S, Gürsoy UK. Regulation of gingival keratinocyte monocyte chemoattractant protein-1-induced protein (MCPIP)-1 and mucosa-associated lymphoid tissue lymphoma translocation protein (MALT)-1 expressions by periodontal bacteria, lipopolysaccharide, and interleukin-1β. J Periodontol. 2023;94(1):130–140. doi: 10.1002/JPER.22-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapple IL, Bouchard P, Cagetti MG, Campus G, Carra MC, Cocco F, Nibali L, Hujoel P, Laine ML, Lingstrom P, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S39–S51. doi: 10.1111/jcpe.12685. [DOI] [PubMed] [Google Scholar]
- 36.Akbari S, Rasouli-Ghahroudi AA. Vitamin K and bone metabolism: a review of the latest evidence in preclinical studies. Biomed Res Int. 2018;2018:4629383. doi: 10.1155/2018/4629383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB, Sr, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Kathiresan S, Keaney JF, Jr, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167(3):313–320. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aral K, Alkan BA, Saraymen R, Yay A, Şen A, Önder G. Therapeutic effects of systemic vitamin k2 and vitamin d3 on gingival inflammation and alveolar bone in rats with experimentally induced periodontitis. J Periodontol. 2015;86(5):666–673. doi: 10.1902/jop.2015.140467. [DOI] [PubMed] [Google Scholar]
- 39.Kondo K, Ishikado A, Morino K, Nishio Y, Ugi S, Kajiwara S, Kurihara M, Iwakawa H, Nakao K, Uesaki S, et al. A high-fiber, low-fat diet improves periodontal disease markers in high-risk subjects: a pilot study. Nutr Res. 2014;34(6):491–498. doi: 10.1016/j.nutres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz N, Kaye EK, Nunn ME, Spiro A, 3rd, Garcia RI. High-fiber foods reduce periodontal disease progression in men aged 65 and older: the veterans affairs normative aging study/Dental Longitudinal Study. J Am Geriatr Soc. 2012;60(4):676–683. doi: 10.1111/j.1532-5415.2011.03866.x. [DOI] [PubMed] [Google Scholar]
- 41.Alkan A, Keskiner I, Arici S, Sato S. The effect of periodontitis on biting abilities. J Periodontol. 2006;77(8):1442–1445. doi: 10.1902/jop.2006.060025. [DOI] [PubMed] [Google Scholar]
- 42.Kato S, Ekuni D, Kawakami S, Mude AH, Morita M, Minagi S. Relationship between severity of periodontitis and masseter muscle activity during waking and sleeping hours. Arch Oral Biol. 2018;90:13–18. doi: 10.1016/j.archoralbio.2018.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Association between Vitamin K intake and loss of periodontal attachment. Each black dot represents a sample. The solid line represents the smooth curve fitting between variables. The blue bands represent the 95% confidence intervals of the fit.
Additional file 2: Figure S2. Association between vitamin K and loss of periodontal attachment, stratified by sex. Adjusted for all variate lists in Table 1.
Data Availability Statement
Publicly available datasets were analysed in this study. Data used for this study are available on the NHANES website: https://wwwn.cdc.gov/nchs/nhanes/.