Abstract
In Escherichia coli, the intrinsic levels of resistance to multiple antimicrobial agents are produced through expression of the three-component multidrug efflux system AcrAB-TolC. AcrB is a proton-motive-force-dependent transporter located in the inner membrane, and AcrA and TolC are accessory proteins located in the periplasm and the outer membrane, respectively. In this study, these three proteins were expressed separately, and the interactions between them were analyzed by chemical cross-linking in intact cells. We show that AcrA protein forms oligomers, most probably trimers. In this oligomeric form, AcrA interacts specifically with AcrB transporter independently of substrate and TolC.
AcrAB is a major, constitutively expressed, multidrug efflux system from Escherichia coli (8). It recognizes and expels from the cells an unusually broad range of antimicrobial agents (9). The acrAB operon codes for two proteins, the efflux transporter AcrB (110 kDa) and the periplasmic lipoprotein AcrA (41 kDa without the attached lipid moiety), both of which are essential for drug efflux. Genetic studies have provided evidence that the multifunctional outer membrane channel TolC is the third component of this transporter, since mutations in tolC completely abolish the AcrAB-dependent multidrug resistance phenotype of E. coli (2). AcrAB-TolC was suggested to be similar in structure to the well-characterized tripartite complexes such as hemolysin A transporter HlyBD-TolC (5): the inner-membrane-associated transporter AcrB is thought to form a complex with the periplasmic component AcrA, which is anchored to the outer surface of the inner membrane through its lipid moiety and with the outer membrane channel TolC. Such organization is believed to provide the structural means for the transport of substrates from the cells directly into the medium, bypassing the periplasmic space (10). However, no evidence was presented for the interaction between these three components.
Chemical cross-linking in intact cells has been used extensively to define specific interactions in the membrane transport complexes (1, 13, 15). In this study, we have used such cross-linking along with the immunological detection to provide evidence for an interaction between AcrA and AcrB.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
All strains and plasmids used in this study are listed in Table 1. E. coli strains were grown at 37°C in L broth (10 g of Difco Yeast Extract, 10 g of Difco Tryptone Peptone, and 5 g of NaCl per liter). Antibiotics were added, when required, to the following final concentrations: kanamycin, 34 μg/ml; ampicillin, 100 μg/ml; and chloramphenicol, 15 μg/ml.
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| E. coli strains | ||
| AG100 | argE3 thi-1 rpsL xyl mtl galK supE441 Δ(gal-uvrB) λ− | 3, 11 |
| AG102MB | Kanr, same as AG100 but marR1 acrB::kan | S. Ohsuka and H. Nikaido, unpublished data |
| AG100A | Kanr, same as AG100 but ΔacrAB::kan | 11 |
| ZK796 | Tetr, same as MC4100 but tolC::Tn10 | 4, 5 |
| Plasmids | ||
| pBAD/Myc-HisA | Ampr, cloning vector for dosage-dependent expression under arabinose-inducible PBAD promoter | Invitrogen |
| pACYC184 | Cmr Tetr, cloning vector | New England Biolabs |
| pUC151A | Ampr, pUC19 derivative carrying acrA and acrB | 8 |
| pAB | Cmr, pACYC184 derivative carrying acrA and acrB under PBAD promoter | This study |
| pUCBPA | Ampr, pUC18 derivative carrying acrB under native promoter | Q. Wang et al., unpublished data |
| pEZ4 | Ampr, pET21d(+) derivative carrying ompA-acrA-his6 under T7 promoter | 16 |
| pARA | Ampr, pUC18 derivative carrying araC and polycloning site from pBAD/Myc-HisA | This study |
| pOA | Cmr, pACYC184 derivative carrying ompA-acrA-his6 under PBAD promoter | This study |
Plasmid pAB was constructed by cloning an NdeI-NsiI fragment, encoding acrA and acrB, from pUC151A (8) into NcoI and PstI sites of the polycloning region of pBAD/Myc-HisA (Invitrogen). Plasmid pAB expressed the AcrA and AcrB proteins under the dosage-dependent, arabinose-inducible promoter PBAD. Because NsiI cuts downstream from the termination codon of acrB, an intact AcrB without the Myc-His tag was expressed from the vector. To construct plasmid pOA, the SphI-BglI fragment from pBAD/Myc-HisA containing the activator gene araC, the promoter PBAD, and polycloning region was cloned into SphI and HincII sites of the polycloning region of pUC18, generating plasmid pARA. Then the NcoI-BlpI fragment from pEZ4 (16) encoding ompA-acrA-his6 was inserted into NcoI and BglII sites of pARA. The resulting plasmid, named pARAOmpA-AcrA, served as a donor of SphI-SmaI DNA fragment to be cloned into SphI and HincII sites of pACYC184. The resultant plasmid pOA expressed the AcrA protein, whose own signal sequence was replaced by the OmpA signal peptide and which in addition contained a C-terminal fusion of six histidine residues, under the control of PBAD promoter.
Chemical cross-linking in intact cells.
In general, E. coli cells were grown in 5 ml of Luria-Bertani medium supplemented with appropriate antibiotics to an optical density at 600 nm of 0.7 to 1.0, harvested by centrifugation, and washed with sodium phosphate buffer (pH 7.5). Cells were resuspended in 0.5 ml of the same buffer, and dithiobis(succinimidylpropionate) (DSP; Pierce) or disuccinimidyl glutarate (DSG; Pierce) was added to 0.4 or 0.2 mM, respectively. Cross-linking was carried out for 30 min, with DSP at 37°C or with DSG on ice, and was terminated with the addition of Tris-HCl (pH 7.5) to 20 mM (15). Cells were harvested by centrifugation and sonicated on ice for 1 to 2 min using the microtip of a Gallenkamp Soniprep 150 sonicator. Membranes were collected by centrifugation at 100,000 × g for 30 min.
Immunoblot analysis.
The membrane proteins were solubilized at room temperature for 20 min in 1% sodium dodecyl sulfate (SDS)–1 mM EDTA–10 mM Tris-HCl (pH 8.0)–1 mM phenylmethylsulfonyl fluoride and then separated by electrophoresis on an 8% polyacrylamide gel or 5 to 13% gradient polyacrylamide gel containing 0.1% SDS (SDS-PAGE). The proteins were transferred to an Immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore) overnight at 30 V, using the transfer buffer described in reference 17. The PVDF membranes were probed with the anti-AcrA and anti-AcrB antibodies as described elsewhere (16, 17).
Purification and analysis of the AcrA-His6-containing protein complexes.
After cross-linking with DSP, membrane-associated AcrA-His6 was purified by metal chelation chromatography using His-Bind Quick 300 cartridges (Novagen). For this purpose, cell membranes were isolated as described above and solubilized for 20 min on ice in loading buffer containing 100 mM Tris-HCl (pH 8.0), 500 mM NaCl, 5 mM imidazole, 1% Triton X-100, and 0.2% sarcosyl. Insoluble material was removed by centrifugation at 100,000 × g for 30 min, and membrane extract was loaded on His-Bind 300 cartridges. After extensive washing with loading buffer supplemented with 30 mM imidazole, bound proteins were eluted with solution containing 100 mM Tris-HCl (pH 8.0), 0.4 M imidazole, and 2% SDS.
To analyze the composition of cross-linked complexes, proteins eluted from the His-Bind 300 cartridges were separated by SDS-PAGE (5 to 13% gradient gel) and then further resolved by the second-dimension SDS-PAGE: a 1-cm lane of the first-dimension gel with the protein complexes was excised, dipped into 1% mercaptoethanol to cleave DSP cross-links, and placed on the top of an SDS–10% polyacrylamide gel as described previously (14). Proteins were visualized by the Coomassie blue staining or by immunoblotting with an anti-AcrA antibody.
RESULTS AND DISCUSSION
Major complexes containing both AcrA and AcrB are immunodetected after cross-linking in intact cells.
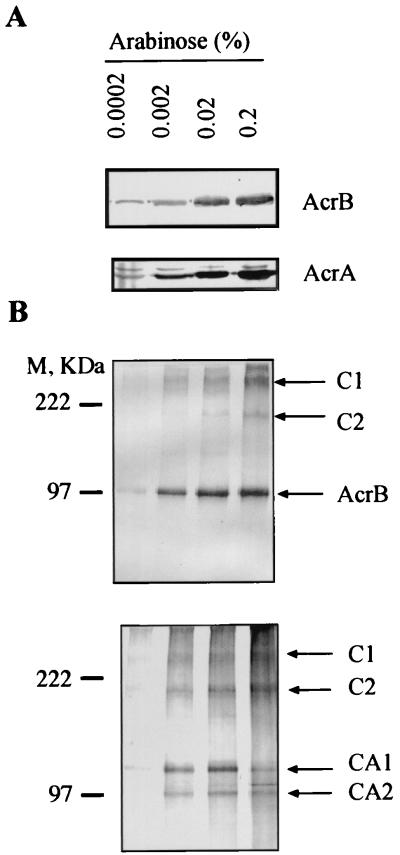
The AcrA and AcrB proteins were expressed in AcrAB-deficient E. coli strain AG100A (11) from plasmid pAB carrying acrA and acrB under the tightly controlled arabinose-inducible promoter PBAD. The expression of both AcrA and AcrB proteins from this construct was dosage dependent and increased with increasing concentrations of arabinose in the medium (Fig. 1A).
FIG. 1.
Immunoblotting analysis of AcrB and AcrA produced from pAB before (A) and after (B) cross-linking with DSP. Cross-linked complexes (B) were separated by SDS-PAGE (8% gel) and analyzed by Western blotting with anti-AcrB (top) and anti-AcrA (bottom) antibodies. Positions of AcrB- and AcrA-specific complexes are indicated by arrows. M, size markers.
To stabilize protein complexes, intact E. coli cells were treated with DSP, which has a spacer arm of 12 Å and a disulfide bond that can be cleaved with reducing agents. After treatment with DSP, membrane proteins from AG100A/pAB were analyzed by SDS-PAGE followed by immunoblotting with antibody against the AcrB protein (17) (Fig. 1B). In addition to the AcrB protein band, we observed two bands, C1 and C2, which had apparent molecular weights greater than that of AcrB protein alone. Confirming the specificity of these complexes, their amounts paralleled the amounts of AcrB protein (Fig. 1B, top), and these bands disappeared upon treatment of the membrane preparation with dithiothreitol (100 mM) at 37°C (data not shown). Parallel experiments with antibody against AcrA (16) (Fig. 1B, bottom) showed that two complexes with mobilities very similar to those of C1 and C2 were also present, suggesting that these complexes contained both AcrA and AcrB. From the Western blots, the apparent molecular masses of C1 and C2 were found to be >250 and about 180 kDa, respectively. In addition to complexes C1 and C2, the anti-AcrA antibody revealed two other complexes, CA1 and CA2, with apparent molecular masses of 132 and 100 kDa, respectively.
AcrA forms oligomers.
The secondary structure predictions for AcrA (7, 12) suggest that AcrA and its homologs from the membrane fusion protein family have extended α-helical regions with a high probability to form coiled coils. Such structures are often involved in protein oligomerization; at least for one membrane fusion protein, HlyD, a trimeric structure was suggested based on cross-linking experiments (15).
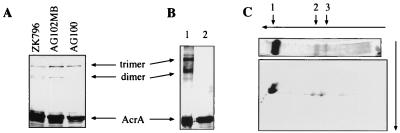
To assess the oligomeric state of AcrA, we used a similar approach. Cross-linking was performed in intact cells with the noncleavable cross-linker DSG, which has a spacer arm shorter (7.7 Å) than that of DSP and introduces cross-links only to proteins that are very close to each other. After cross-linking of AG100A/pUC151A with DSG, the membranes were purified and protein interactions were analyzed by SDS-PAGE and immunoblotting (Fig. 2A). In addition to the monomeric form of AcrA, migrating at 45 kDa, two high-molecular-weight forms of AcrA, migrating at 132 and 100 kDa, were identified. These AcrA forms were found also when the chromosomal copy of acrA gene was expressed in the AcrB-deficient E. coli strain AG102MB and the TolC-deficient strain ZK796 (4, 6) (Fig. 2A). AcrA thus might form a trimer when in association with the inner membrane of E. coli, even in the absence of the inner membrane transporter AcrB and the outer membrane channel TolC. The mobilities of these complexes were identical to those of CA1 and CA2 in Fig. 1B, suggesting that they also represent the trimeric and dimeric forms of AcrA, respectively. Finally, the results with ZK796 and AG102MB show that oligomerization occurs with the expression of a chromosomal copy of acrA and that it is not an artifact of acrA overexpression.
FIG. 2.
Inner-membrane-associated AcrA is a trimer. (A) DSG cross-linking experiments demonstrating that oligomerization of AcrA is independent of AcrB and TolC. AcrA was stained with anti-AcrA antibody. (B) Cross-linked oligomeric AcrA after purification on an Ni2+ affinity column. AcrA was expressed as OmpA-AcrA-His6 fusion protein from pOA. After cross-linking with DSP, AcrA-His6 was purified and analyzed by Western blotting with the anti-AcrA antibody. Treatment with dithiothreitol (lane 2), which reverses DSP cross-links, confirmed that the purified AcrA forms are products of cross-linking. (C) Analysis of the cross-linked AcrA by two-dimensional SDS-PAGE demonstrating that AcrA (arrow 1) is the only component of complexes CA1 (arrow 3) and CA2 (arrow 2). The proteins were stained with Coomassie blue.
To identify the components of these complexes, we transformed E. coli AG100A with plasmid pOA, which expresses OmpA-AcrA-His6 (16) under the PBAD promoter. Here, the OmpA-AcrA-His6 is a fusion product of the signal peptide from OmpA and the signal peptide-deficient AcrA with a hexahistidine tag at its C terminus. After induction with 0.2% arabinose, AG100A/pOA produced a large amount of AcrA-His6 protein, which was shown to be mostly located in the periplasm (16). After cross-linking with DSP in intact cells, membrane-associated AcrA-His6 was solubilized in 1% Triton X-100–0.2% sarcosyl (Materials and Methods) and purified by metal chelation chromatography. Three bands corresponding to monomeric AcrA-His6, CA1, and CA2 were detected by Coomassie blue staining and also by immunoblotting of the SDS-polyacrylamide gels with the protein fractions eluted from the Ni2+ column (Fig. 2B). All three forms of AcrA-His6 were further analyzed by a second-dimension SDS-PAGE after reductive cleavage of DSP cross-links (Fig. 2C). Complexes CA1 and CA2 were found to contain only one protein with an apparent mobility of 45 kDa corresponding to monomeric AcrA. CA1 and CA2 thus correspond to the trimeric and dimeric forms of AcrA. We cannot rule out the possibility that AcrA might exist in the cells as a higher-order oligomer, although the recovery of only the trimeric and dimeric forms of AcrA indicates that AcrA exists as trimers in cells. We note also that AcrA-His6 does not contain the N-terminal cysteine, the site for lipid modification (16). Oligomerization of these lipid-deficient AcrA derivatives indicates that AcrA forms dimers and trimers through specific interactions, not through nonspecific aggregation driven by the lipid moiety. Furthermore, the AcrA-His6 fraction, which is apparently not associated with the membranes and is recovered in the periplasmic/cytosolic fraction after the sedimentation of membranes by centrifugation at 100,000 × g, also appeared to form oligomers (data not shown). Thus, the interaction with the membrane is not required for AcrA oligomerization.
Oligomeric AcrA forms complexes with the AcrB transporter in the absence of the outer membrane channel TolC.
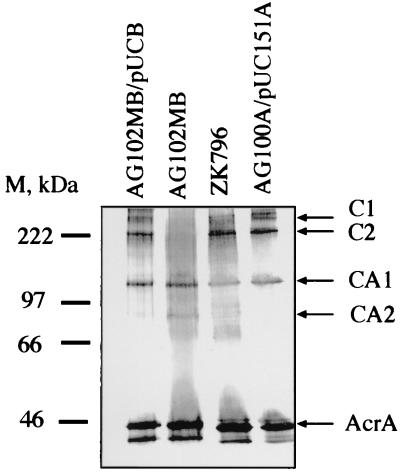
The molecular masses of AcrA and AcrB calculated from the amino acid composition are 41 and 110 kDa, respectively, and the respective migration rates in SDS-PAGE corresponded to 45 and 97 kDa. Accordingly, complexes C1 and C2 reacting with both anti-AcrA and anti-AcrB antibodies might correspond to an AcrB monomer associated with three and two molecules of AcrA, respectively. To test this hypothesis, cross-linking with DSP was performed on intact cells of AG102MB (acrB::kan). In this strain, complexes C1 and C2 were not detected with the anti-AcrA antibody (Fig. 3, lane 2). Complementation experiments were carried out with AG102MB transformed with plasmid pUCBPA, producing AcrB. The expression of AcrB from pUCBPA restored the formation of complexes C1 and C2 on Western blots (Fig. 3, lane 1). It is possible that a single AcrB molecule is cross-linked to two or three monomeric AcrA molecules in these complexes. However, the absence of a complex containing one AcrB molecule and one AcrA molecule suggests that only oligomeric forms of AcrA can interact with AcrB and that C1 and C2 are AcrB monomers cross-linked to trimers and dimers, respectively, of AcrA. These complexes were also identified in the TolC-deficient strain ZK796 (Fig. 3, lane 3), suggesting that TolC is not present in either C1 or C2 and that these complexes are formed without overexpression of AcrA and AcrB. Thus, AcrA and AcrB exist in a stable complex in association with the inner membrane of E. coli, and this complex can assemble independently of the outer membrane channel TolC.
FIG. 3.
Immunoblotting analysis of AcrA in the AcrB- and TolC-deficient cells cross-linked with DSP in intact cells. Western blot analysis with the anti-AcrA antibody showed that complexes C1 and C2 were absent in the acrB::kan strain AG102MB but were present in the tolC mutant ZK796. This finding is consistent with the conclusion that these complexes contain both AcrA and AcrB but not TolC. M, size markers.
To identify complexes containing the TolC protein, we used a polyclonal anti-TolC antibody, a gift from R. Misra. However, even with the use of a plasmid that produces an increased level of TolC, so far we have not been able to find cross-linked products containing TolC. The reason for this failure can be trivial. For example, outer membrane proteins usually contain few exposed lysine residues and are usually difficult to cross-link with reagents that use the ɛ-amino group of lysine as targets. In fact, TolC (471 amino acid residues) contains only 16 lysine residues. Another reason could be the low avidity and low specificity of the anti-TolC serum used. Furthermore, the complex containing three AcrA, one AcrB, and one TolC is expected to have a size of about 350 kDa, and it may be difficult to transfer such a large complex to PVDF membranes by electrophoresis. On the other hand, the reason for the failure may be nontrivial. With the type I protein secretion system, the recruitment of TolC into the secretion complex takes place only after the substrate, the exported protein, has assembled the rest of the machinery, including the transporter and the AcrA homolog, HlyD (15). Similarly, the recruitment of TolC into the efflux complex may take place on a transient basis, only when the substrate molecule is about to be exported.
In conclusion, we showed that the periplasmic lipoprotein AcrA interacts specifically with the inner-membrane-associated efflux pump AcrB and that this complex is assembled even in the absence of the outer membrane component TolC. It appears that only oligomeric forms of AcrA form complexes with the AcrB protein. Oligomerization of AcrA itself, however, does not require AcrB.
ACKNOWLEDGMENT
This study was supported in part by Public Health Service grant AI-09644.
REFERENCES
- 1.Derouiche R, Benedetti H, Lazzaroni J-C, Lazdunski C, Lloubes R. Protein complex within Escherichia coli inner membrane. J Biol Chem. 1995;270:11078–11084. doi: 10.1074/jbc.270.19.11078. [DOI] [PubMed] [Google Scholar]
- 2.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George A M, Levy S B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J Bacteriol. 1983;155:531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilson L, Mahanty H K, Kolter R. Genetic analysis of an MDR-like export system: the secretion of colicin V. EMBO J. 1990;9:3875–3894. doi: 10.1002/j.1460-2075.1990.tb07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland I B, Kenny B, Blight M. Haemolysin secretion from E. coli. Biochimie. 1990;72:131–141. doi: 10.1016/0300-9084(90)90138-7. [DOI] [PubMed] [Google Scholar]
- 6.Hwang J, Zhong X, Tai P C. Interactions of dedicated export membrane proteins of the colicin V secretion system: CvaA, a member of the membrane fusion protein family, interacts with CvaB and TolC. J Bacteriol. 1997;179:6264–6270. doi: 10.1128/jb.179.20.6264-6270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson J M, Church G M. Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J Mol Biol. 1999;287:695–715. doi: 10.1006/jmbi.1999.2630. [DOI] [PubMed] [Google Scholar]
- 8.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 10.Nikaido H. The role of outer membrane and efflux pumps in the resistance of Gram-negative bacteria: can we improve drug access? Drug Resist Updates. 1998;1:93–98. doi: 10.1016/s1368-7646(98)80023-x. [DOI] [PubMed] [Google Scholar]
- 11.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimenta A, Blight M, Clarke D, Holland I B. The gram-negative cell envelope ‘springs’ to life: coiled-coli trans-envelope proteins. Mol Microbiol. 1996;19:643–645. doi: 10.1046/j.1365-2958.1996.t01-3-442924.x. [DOI] [PubMed] [Google Scholar]
- 13.Prossnitz E, Nikaido K, Ulbrich S J, Ames G F-L. Formaldehyde and photoactivatable cross-linking of the periplasmic binding protein to a membrane component of the histidine transport system of Salmonella typhimurium. J Biol Chem. 1988;263:17917–17920. [PubMed] [Google Scholar]
- 14.Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 15.Thanabalu T, Koronakis E, Hudges C, Koronakis V. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 1998;17:6487–6496. doi: 10.1093/emboj/17.22.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zgurskaya H I, Nikaido H. AcrA from Escherichia coli is a highly asymmetric protein capable of spanning the periplasm. J Mol Biol. 1999;285:409–420. doi: 10.1006/jmbi.1998.2313. [DOI] [PubMed] [Google Scholar]
- 17.Zgurskaya H I, Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:7190–7195. doi: 10.1073/pnas.96.13.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]