Abstract
The ongoing COVID-19 pandemic has resulted in millions of deaths globally, highlighting the need to develop potent prophylactic and therapeutic strategies against SARS-CoV-2. Small molecule inhibitors (remdesivir, Paxlovid, and molnupiravir) are essential complements to vaccines and play important roles in clinical treatment of SARS-CoV-2. Many advances have been made in development of anti-SARS-CoV-2 inhibitors in China, but progress in discovery and characterization of pharmacological activity, antiviral mechanisms, and clinical efficacy are limited. We review development of small molecule anti-SARS-CoV-2 drugs (azvudine [approved by the NMPA of China on July 25, 2022], VV116 [approved by the NMPA of China on January 29, 2023], FB2001, WPV01, pentarlandir, and cepharanthine) in China and summarize their pharmacological activity, potential mechanisms of action, clinical trials and use, and important milestones in their discovery. The role of structural biology in drug development is also reviewed. Future studies should focus on development of diverse second-generation inhibitors with excellent oral bioavailability, superior plasma half-life, increased antiviral activity against SARS-CoV-2 and its variants, high target specificity, minimal side effects, reduced drug-drug interactions, and improved lung histopathology.
Keywords: COVID‐19, SARS‐CoV‐2, Azvudine, VV116, FB2001, Cepharanthine
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, which originated in Wuhan, China, has spread rapidly and caused 6.89 million deaths since December 2019 [1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, has evolved into multiple variants (e.g., Omicron BA.5) with enhanced transmissibility and immune escape capability. These variants present a challenge to the efficacy of current treatments (e.g., vaccination) [2,3]. Remdesivir (Gilead Sciences), Paxlovid (Pfizer), and molnupiravir (Merck) have been approved or authorized by the US FDA for treatment of COVID-19 [[4], [5], [6]]. Small molecule drugs are excellent complements to vaccines as prophylactic and post-exposure therapeutic agents.
However, current small molecule drugs suffer from poor efficacy. For example, the efficacy of intravenous remdesivir has varied among reports, which has limited its widespread use for treatment of COVID-19 [[7], [8], [9], [10]]. Paxlovid (nirmatrelvir plus ritonavir) is an oral inhibitor that can be dispensed at community pharmacies. However, Paxlovid is expensive (US $530 for each 5-day course) [11,12]. Nirmatrelvir is a selective covalent inhibitor that suffers from poor pharmacokinetics. Ritonavir, a metabolic enhancer, is used to slow metabolism of nirmatrelvir. Ritonavir has multiple drug-drug interactions that require specific evaluation prior to use [11,12]. Molnupiravir (US $700 per 5-day course), an oral inhibitor with moderate clinical efficacy, has mutagenic potential in human cells [13,14]. Significant progress has been made in treatment of COVID-19, but currently approved small molecule therapeutics have suffered from lack of global access, limited administration routes, and poor efficacy against SARS-CoV-2 variants.
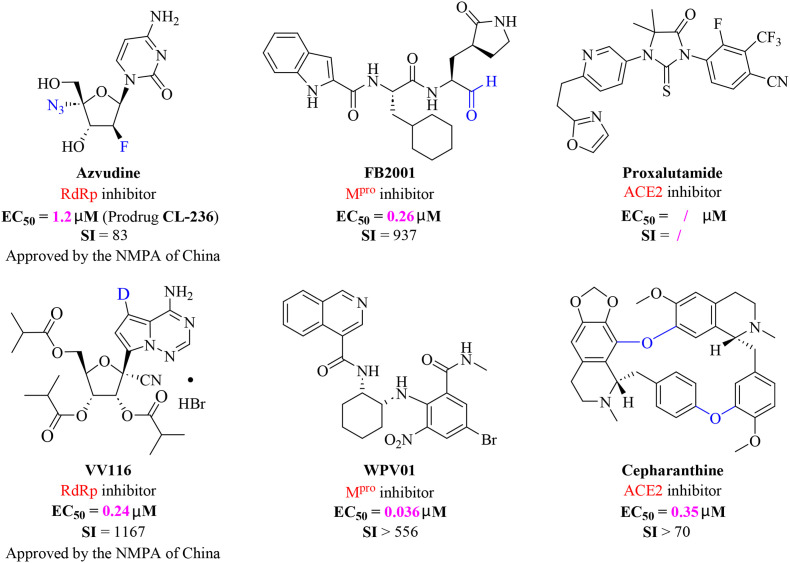
Development of novel small molecule drugs with efficacy against the Omicron variant is needed. China has made significant advances in therapeutics for treatment against COVID-19 in the past three years. In this manuscript, we review contributions made by China, including development of novel small molecule anti-SARS-CoV-2 drugs (azvudine [15], VV116 [16], proxalutamide [17], FB2001 [18], WPV01 [19], and cepharanthine [20]; Fig. 1 ). We summarize the discovery process, pharmacological parameters, potential mechanisms, and results of clinical trials. We also highlight in the contributions of structural biology in anti-SARS-CoV-2 drug design.
Fig. 1.
Chemical structures of representative SARS-CoV-2 inhibitors. Azvudine, a self-developed oral SARS-CoV-2 RdRp inhibitor, approved by the NMPA of China; VV116, the first deuterated oral RdRp candidate, approved by the NMPA of China; FB2001, China's first inhaled Mpro clinical candidate; WPV01, non-covalent Mpro inhibitor; Proxalutamide, ACE2 inhibitor, approved for emergency use in Uruguay; Cepharanthine, natural product-derived ACE2 inhibitor.
2. Use of structural biology to develop anti-SARS-CoV-2 drugs
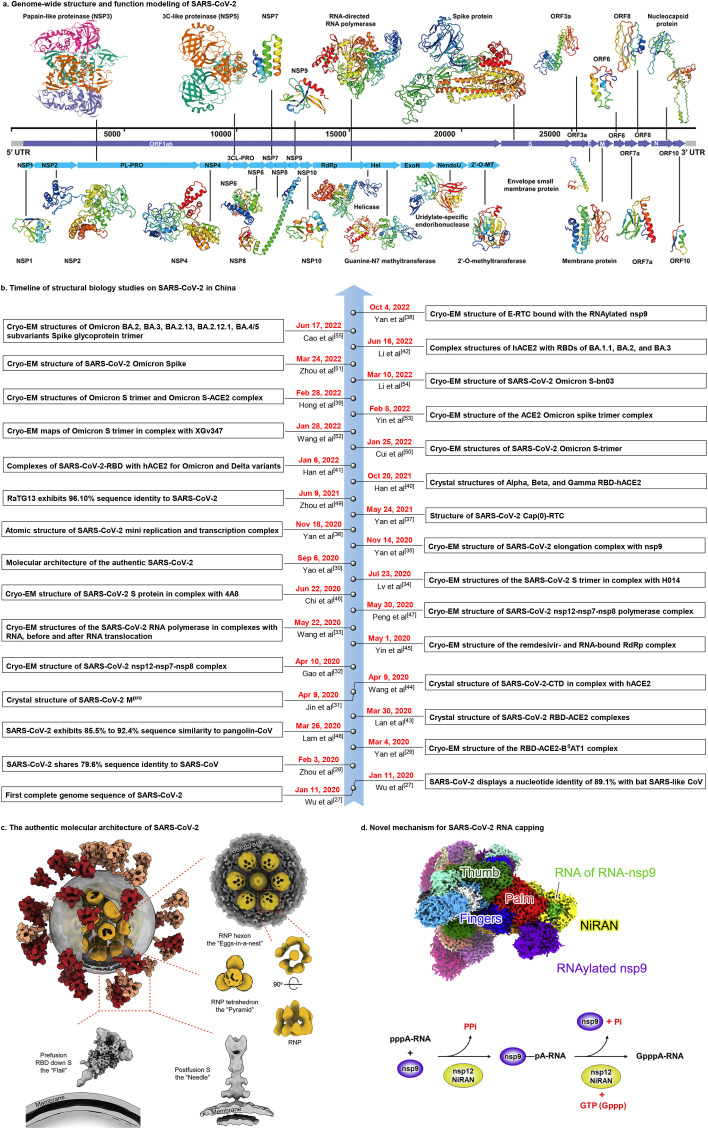
SARS-CoV-2 is a 29.9 kb single-stranded, positive-sense RNA virus that encodes 29 proteins involved in viral adsorption, replication, transcription, assembly, and release [21,22]. The genome structure and functional modeling of SARS-CoV-2 (https://seq2fun.dcmb.med.umich.edu/COVID-19/) is shown in Fig. 2 a. Structural biology studies have shown that papain-like protease (NSP3, PLpro), 3C-like protease (NSP5, 3CLpro), RNA-dependent RNA polymerase (RdRp), and spike (S) protein are promising drug targets [[23], [24], [25], [26]]. Structural biology is an important tool for determination of structure and function of potential drug targets, allowing for structure-based drug discovery and vaccine development.
Fig. 2.
SARS-CoV-2 genome structure and structural biology advances. (a) Genome-wide structure and function modeling of SARS-CoV-2 (https://seq2fun.dcmb.med.umich.edu/COVID-19/). (b) Timeline of structural biology studies on SARS-CoV-2 in China over the last three years. (c) The molecular architecture of SARS-CoV-2. Reproduced with permission. Copyright 2020, Elsevier Inc. (d) Novel mechanism of SARS-CoV-2 RNA capping. Reproduced with permission. Copyright 2022, Elsevier Inc.
Structural biology has allowed for three-dimensional visualization of SARS-CoV-2 structure, critical residues/mutations, and sites of molecular recognition, entry, and replication. Structural biology has been at the forefront of research since the initial COVID-19 outbreak (Fig. 2b). On January 11, 2020, Wu et al. [27] reported the genomic sequence of SARS-CoV-2. They found that SARS-CoV-2 had 89.1% sequence identity with bat SARS-like CoV, which indicated that bats may have been the original host of SARS-CoV-2. Notably, the amino acid sequence of SARS-CoV-2 RBD is highly similar to that of SARS-CoV, which indicated that SARS-CoV-2 could effectively use human ACE2 as a receptor to enter cells, thus providing a mechanism for human-to-human transmission. Zhou et al. [28] showed that SARS-CoV-2 shared 79.6% sequence identity with SARS-CoV, and also confirmed that SARS-CoV-2 uses ACE2 as a cellular entry receptor. These studies provided important information regarding how SARS-CoV-2 could enter human cells, which is critical for determination of how to block infection. On March 4, 2020, Zhou's team [29] reported cryo-electron microscopy (cryo-EM) structures of the full-length human ACE2-B0AT1 complex and RBD-ACE2-B0AT1 complex. This work clarified the structural and functional correlates of SARS-CoV-2 entry into target cells, and may play an important role in discovery and optimization of inhibitors that block cell entry. Li et al. [30] characterized the molecular structure of SARS-CoV-2 (Fig. 2c) at high resolution using cryo-ET and subtomogram averaging. This work showed how the virus packs its single-stranded RNA into the viral lumen.
Rao et al. [[31], [32], [33], [34], [35], [36], [37], [38]] systematically characterized the transcription and replication processes of SARS-CoV-2 and analyzed the three-dimensional structures of its key target and its replication and transcription complex. This study significantly increased understanding of the molecular mechanism (Fig. 2d) of SARS-CoV-2 and is critical for development of highly effective antiviral drugs. The SARS-CoV-2 variants exhibit striking immune evasion, resulting in resurgence of infections. Using cryo-EM, Hong et al. [39] visualized the Omicron S-ACE2 complex, which elucidated the structural basis of Omicron immune evasion and informed drug design. Furthermore, Gao et al. [[40], [41], [42]] systematically evaluated the crystal structures of the RBD-hACE2 complexes of multiple SARS-CoV-2 variants (Alpha, Beta, Gamma, and Omicron), and showed that structurally highly-conserved regions (with high‐affinity binding) and consensus sites of majority variants (with broad-spectrum protection) could be used to develop an ideal universal agent against SARS-CoV-2 variants.
Structural biology research has resulted in additional advances in China in the past three years (Fig. 2b). This progress includes characterization of the RBD-ACE2 complex [43], the CTD-ACE2 complex [44], the remdesivir- and RNA-bound RdRp complex [45], the S-4A8 complex [46], and the nsp12-nsp7-nsp8 polymerase complex [47]. Furthermore, other sequence similarity studies have been performed [48,49]. Recently, the structures of the Omicron spike [50,51], the Omicron S-XGv347 complex [52], the Omicron S-ACE2 complex [53], the Omicron S-bn03 complex [54], and the Omicron BA.2, BA.3, BA.2.13, BA.2.12.1, BA.4/5 subvariants spike glycoprotein trimer [55] were reported. These studies may aid in structure-based development of anti-SARS-CoV-2 drugs.
3. Azvudine—The first Chinese oral SARS-CoV-2 RdRp inhibitor
RdRp is a well-known conserved protein without a human equivalent that is a promising therapeutic target for SARS-CoV-2 treatment [56,57]. Azvudine (FNC, RO-0622), a first-in-class nucleoside-based prodrug developed by Henan Sincere Biotech Co., Ltd., was granted conditional marketing authorization by the National Medical Products Administration (NMPA) of China on July 25, 2022, and was the first Chinese oral SARS-CoV-2 RdRp inhibitor for treatment of adult patients with COVID-19 [58,59].
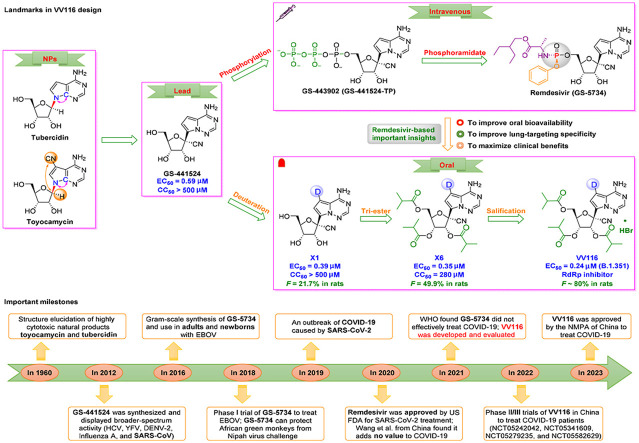
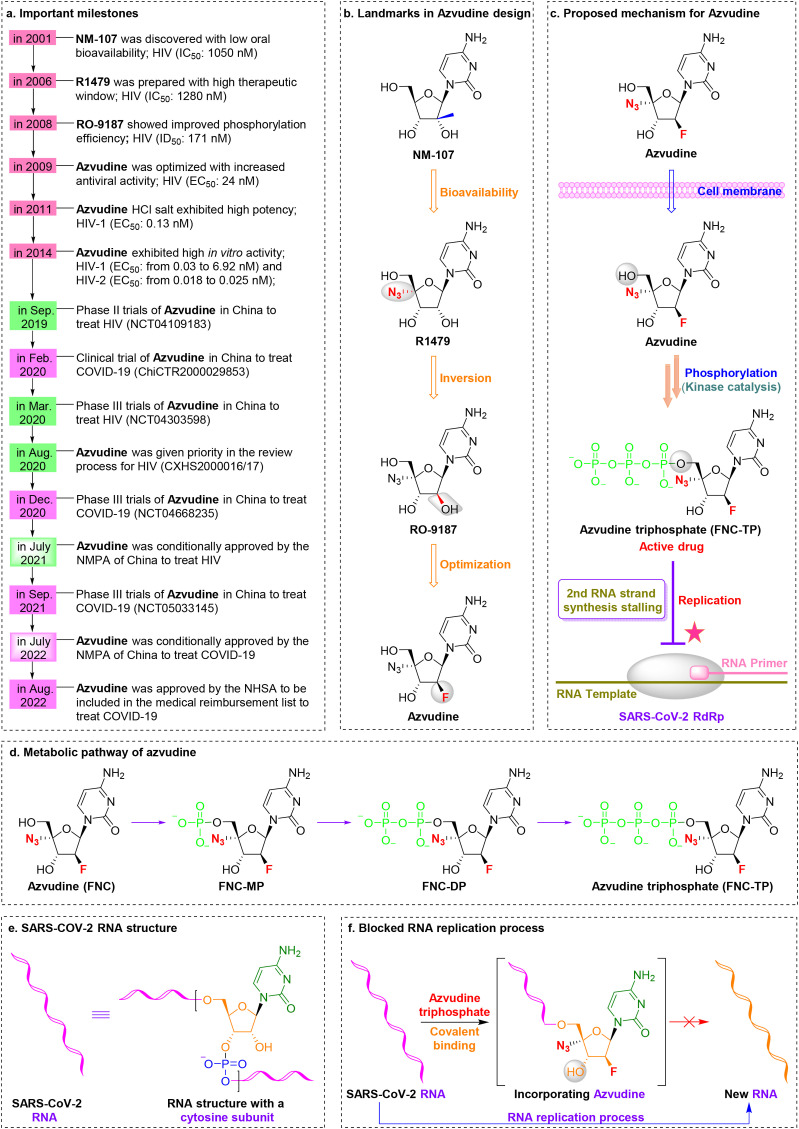
Synthesis of azvudine began with structural modification and optimization of the anti-HIV RdRp inhibitor RO-9187, which is derived from the potent nucleoside inhibitor NM-107 [60,61] (Fig. 3 a,3b). Studies showed that 4′-azido-substituted R1479 had remarkable activity against HIV in vitro (IC50 = 1.28 μM) with increased oral bioavailability and a greater therapeutic window [62,63]. Inversion of the 2′-hydroxy group to form RO-9187 resulted in increased potency against HIV in vitro (IC50 = 0.171 μM) [64,65]. Furthermore, RO-9187 showed increased phosphorylation efficiency, which is a rate-limiting step. Replacement of the 2′-β-hydroxyl group of RO-9187 by 2′-β-fluorine resulted in increased anti-HCV potency in vitro (EC50 = 0.024 μM) [66]. Formation of the hydrochloride salt of FNC resulted in superior antiviral activity against HIV (wild-type) in vitro (EC50 = 0.13 nM) [67]. Furthermore, FNC exhibited excellent in vitro activity against HIV-1 (EC50 from 0.03 to 6.92 nM) and HIV-2 (EC50 from 0.018 to 0.025 nM) with low cytotoxicity (selectivity index [SI] > 1000) [68]. The safety and efficacy of FNC have been evaluated in HIV cases since 2013 (such as ClinicalTrials.gov: NCT04109183, NCT04303598). The efficacy and safety of FNC have been documented in vitro, in vivo, and in clinical trials [15,69,70]. For example, FNC showed an excellent long-term safety profile in patients with AIDS during a 48-week oral treatment regimen [15]. FNC was conditionally approved by the NMPA of China to treat HIV on July 21, 2021 (XZXK-2021-214).
Fig. 3.
Discovery of the orally available SARS-CoV-2 RdRp covalent inhibitor azvudine. (a) Important milestones in azvudine discovery from NM-107 to the first-in-class HIV and SARS-CoV-2 dual inhibitor azvudine. (b) Medicinal chemistry efforts led to discovery of the oral RdRp inhibitor azvudine. (c) Proposed mechanism of action of the prodrug azvudine against SARS-CoV-2. (d) Metabolic pathway of azvudine. (e) Structure of SARS-CoV-2 RNA with a cytosine subunit. (f) A diagram showing the SARS-CoV-2 RNA replication process blocked by the active metabolite azvudine triphosphate through formation of a covalent bond.
FNC exhibits broad-spectrum antiviral properties in vitro (e.g., HIV-1 [EC50, from 0.03 to 6.92 nM], HIV-2 [EC50, from 0.018 to 0.025 nM], HCoV-OC43 [EC50 = 4.3 μM], and SARS-CoV-2 [EC50 = 1.2 μM]) [15,68] and favorable anti-SARS-CoV-2 activity in infected rhesus macaques [15]. Notably, FNC targeted the thymus in a rat SARS-CoV-2 model and showed excellent absolute oral bioavailability in a dog model (F = 82.7%) [71]. Clinical trials (ChiCTR2000029853, NCT04668235, and NCT05033145) have been initiated to evaluate the efficacy of FNC. Several clinical trials have shown that FNC inhibited SARS-CoV-2 replication and enhanced immune function in patients with COVID-19 [15,72]. A double-blind, multicenter phase III trial was conducted to evaluate the efficacy of a 7-day treatment regimen with FNC in 280 patients with moderate COVID-19. The results showed that COVID-19-related clinical symptoms were significantly improved in FNC group compared with those in the control group (39.43% [56 of 142] vs. 10.87% [15 of 138], p < 0.001) [15]. Based on the results of these trials, Sincere Biotech Co., Ltd. was able to achieve conditional approval for the oral SARS-CoV-2 RdRp inhibitor FNC by the NMPA of China to treat adult patients with COVID-19.
Studies of the molecular mechanisms of FNC (Fig. 3c-3f) showed that FNC can be efficiently converted to its active form (FNC-NTP) by cellular kinases in the thymus in vivo, after which FNC-NTP terminates RNA replication by embedding into SARSCoV-2 RNA [73]. Note that the immune system, especially the thymus, is the target organ of azvudine and its triphosphate (FNC-TP) [73]. The 4′-azide group of FNC plays an important role in inhibiting SARS-CoV-2 RNA replication through intramolecular hydrogen bonds with the 3′-hydroxyl group, thereby reducing the nucleophilicity of the 3′-hydroxyl group [73].
Studies have shown that FNC can cause mild and temporary dizziness and nausea in patients with COVID-19 (16.12% [5 of 31]) [15]. Therefore, the safety and efficacy of FNC will need extensive post-approval monitoring. Lower oral doses (5 mg/person, once daily, orally) of FNC may reduce the risk of clinical resistance in COVID-19 patients. Furthermore, FNC is relatively inexpensive (US $40 or CN ¥270) per course. FNC has potential to be an effective therapy against the Omicron variant.
3.1. VV116—China's first up-and-coming deuterated oral SARS-CoV-2 RdRp candidate
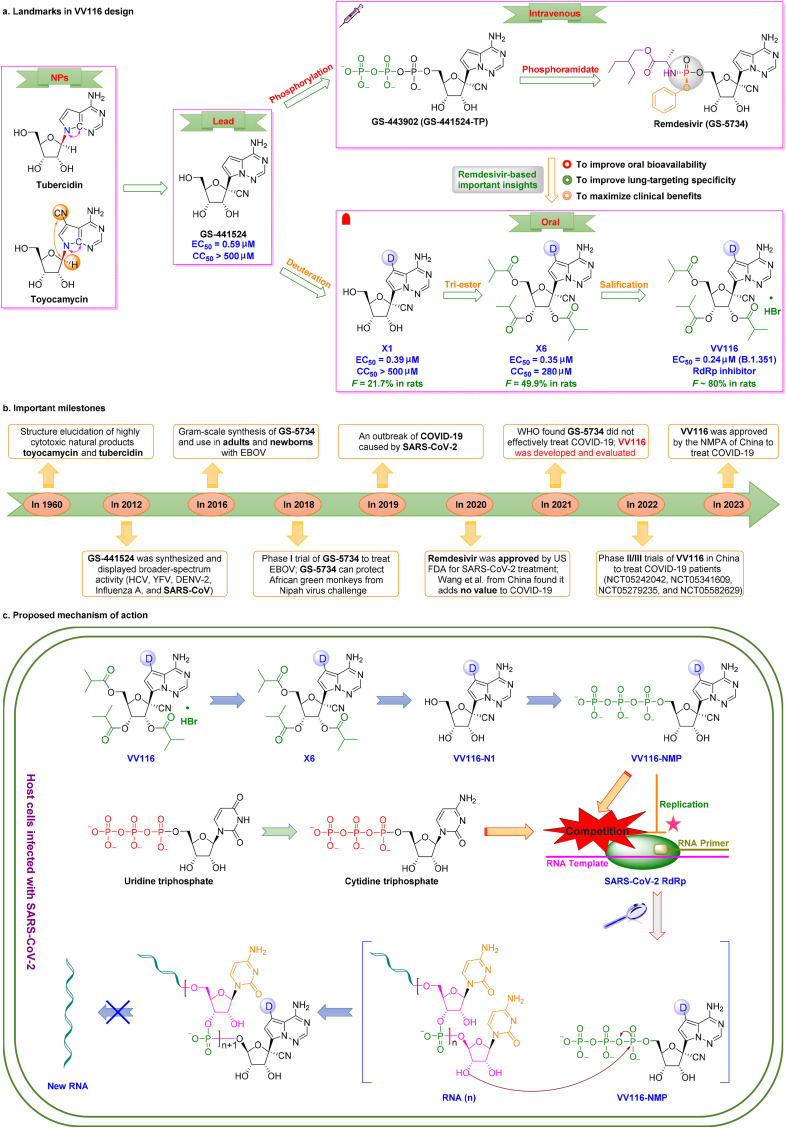
VV116 (Renmindevir, JT001), the first deuterated tri-isobutyrate ester prodrug developed by Junshi Biosciences Co., Ltd., was granted conditional marketing authorization by the NMPA of China on January 29, 2023, and exhibited satisfactory oral anti-SARS-CoV-2 efficacy in clinical trials through inhibition of RdRp replication [74,75]. As a deuterated version of Gilead's GS-62173, the carbon-deuterium bond (∼0.005 Å) of VV116 is more stable than the C–H bond during oxidative clearance, resulting in a blocked metabolic transformation and increased anti-SARS-CoV-2 activity.
Development of VV116 began with structural optimization of the remdesivir parent nucleoside GS-441524 derived from the natural nucleoside inhibitors tubercidin and toyocamycin [76,77] (Fig. 4 a,4b). Studies showed that GS-441524, a 1′–CN–substituted C-nucleoside, had broad-spectrum activity against HIV, YFV, DENV-2, influenza A, and SARS-CoV in vitro [78] and had a favoble safety profile. However, GS-441524 demonstrated poor oral bioavailability in rats (F = 16%), monkeys (F < 8.0%), and humans (F = 13%) [79]. GS-441524 exhibits low protein binding in plasma (62–78% plasma free fraction), and urinary excretion was the major route of elimination [80]. Studies have indicated that triphosphorylated GS-443902 is the active form of GS-441524 [81,82]. To obtain lead compounds with improved activity and drug-like properties, monophosphorylated remdesivir was synthesized in 2016. This derivative of GS-441524 had improved cellular uptake and phosphorylation efficiency [83,84]. Since 2016, remdesivir has been used to treat adults and newborns with EBOV [85,86]. GS-5734 showed broad-spectrum activity against EBOV (EC50 = 12 nM, SI = 303), RSV (EC50 = 21 nM, SI = 395), SARS-CoV (EC50 = 69 nM, SI > 100), MERS-CoV (EC50 = 25 nM, SI > 400), and SARS-CoV-2 (EC50 = 770 nM, SI > 130) [[87], [88], [89], [90]]. Remdesivir, the first intravenously administered SARS-CoV-2 RdRp inhibitor, was conditionally approved by US FDA for COVID-19 treatment on October 22, 2020 [91].
Fig. 4.
Discovery of the first up-and-coming deuterated oral SARS-CoV-2 RdRp covalent candidate VV116. (a) Medicinal chemistry efforts led to discovery of the first deuterated oral RdRp candidate, VV116. (b) Important milestones in VV116 discovery from natural nucleosides to VV116. (c) Proposed mechanism of action of the tri-isobutyrate ester prodrug VV116 against SARS-CoV-2. SARS-CoV-2 RNA replication was blocked by the active metabolite VV116-NMP through formation of a covalent bond.
However, some clinical studies have indicated that remdesivir did not effectively treat COVID-19 [[92], [93], [94], [95]]. To improve oral bioavailability and lung-targeting specificity, and to maximize clinical benefits, deuteration at the C7 position of GS-441524 resulted in derivative X1, which had excellent anti-SARS-CoV-2 potency and low cytotoxicity in vitro (EC50 = 0.39 μM, SI > 1282) [16]. Esterification at the 2′-, 3′-, and 5′-positions resulted in X6, which improved oral bioavailability (F = 50% in rats). Formulation of the hydrobromide salt of X6 resulted in VV116 [16]. VV116 displayed promising antiviral efficacy against the Omicron variant (EC90 = 0.30 μM), superior oral bioavailability (F = 90% in beagle dogs, F = 80% in rats), excellent chemical stability, excellent safety (single dose: >2.0 g/kg in rats), and effective tissue distribution (higher lungs-to-plasma concentration ratios in mice and rats) [16,96]. Deuteration may hinder metabolic conversion via inhibition of oxidation of the pyrrolotriazine moiety, resulting in improved bioavailability [97]. These findings indicated that VV116 may be a promising deuterated oral RdRp candidate for treatment of COVID-19.
The proposed bioconversion pathway of VV116 is shown in Fig. 4c. After intestinal absorption, VV116, a tri-isobutyrate ester prodrug, can be rapidly converted by cellular enzymes in host cells infected with SARS-CoV-2 to the plasma metabolite VV116–N1, which undergoes further phosphorylation to yield VV116-NTP, which interferes with RdRp to inhibit viral replication [98]. VV116-NTP and cytidine triphosphate compete to determine whether formation of new SARS-CoV-2 RNA occurs.
In a phase I study (86 healthy subjects, aged 18–45 years, NCT05221138, NCT05227768, and NCT05201690) by Qian et al. [98] in Shanghai, VV116 showed satisfactory safety and tolerability. In addition, prospective cohort study was conducted by Shen et al. [96] in Shanghai, China to evaluate the efficacy of VV116 (300 mg, twice daily, orally, 5 days) in 136 hospitalized patients infected with the Omicron variant with non-severe COVID-19. The results showed that the viral shedding time was significantly shorter in the VV116 group than that in the control group (8.56 vs. 11.13 days). However, some mild adverse events occurred. Cao et al. [99]onducted a phase III, noninferiority, observer-blinded trial (NCT05341609, ChiCTR2200057856) in Shanghai, China. The objective was to evaluate the efficacy of VV116 (600 mg every 12 h on day 1 followed by 300 mg every 12 h on days 2 through 5) in 384 adults with symptomatic mild-to-moderate COVID-19 who were infected with the Omicron variant. The study showed that VV116 was noninferior to Paxlovid and resulted in a shorter sustained clinical recovery time (4 days vs. 5 days) and fewer adverse events (67.4% vs. 77.3%) in adults with mild-to-moderate COVID-19. Moreover, three other clinical trials (NCT05582629, NCT05242042, and NCT05279235) of VV116 are currently in progress.
4. FB2001—China's first inhaled aerosolized SARS-CoV-2 Mpro candidate drug
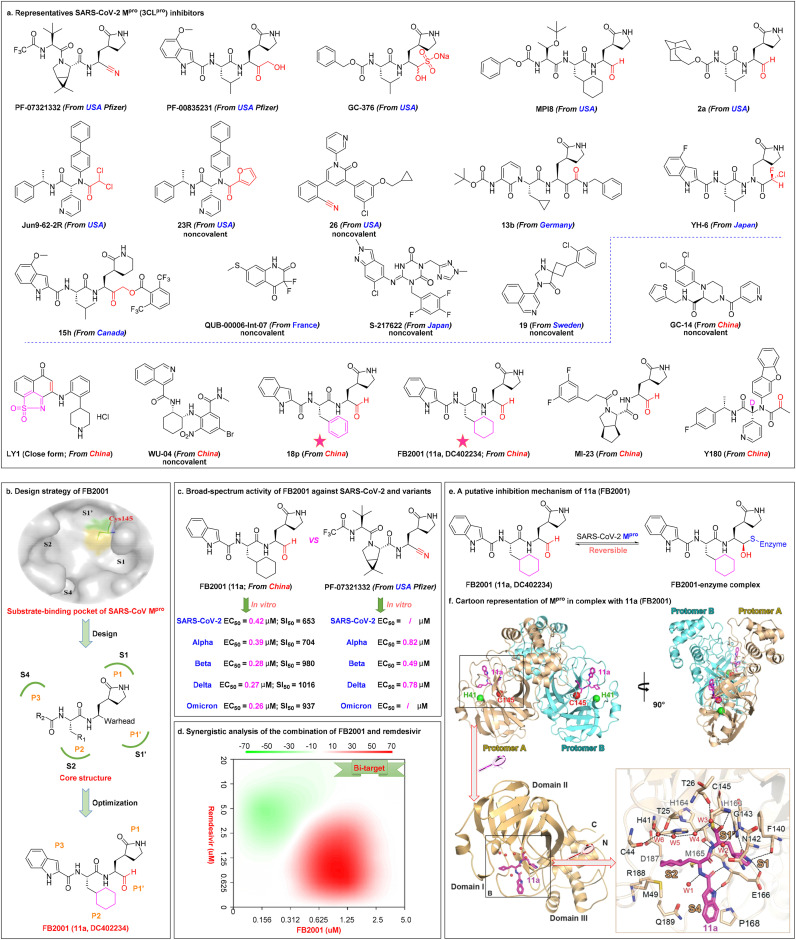
Mpro (sp5, 3CLpro), a validated high-profile antiviral without a human homolog, is a three-domain cysteine protease highly conserved throughout the subfamily Coronavirinae [100,101]. Studies have shown that SARS-CoV-2 Mpro inhibitors exerted potent antiviral activity in cell culture, animal models, and clinical trials (Fig. 5 a). The majority of existing SARS-CoV-2 Mpro inhibitors with strong antiviral effects are peptide-like covalent inhibitors with reactive functional groups such as nitriles (PF-07321332 [102,103]), ketones (PF-00835231 [104,105] and 15h [106]), aldehydes (GC-376 [103,[104], [105], [106], [107],108], MPI8 [109,110], and 2a [111]), haloacetamides (Jun9–62-2R [112] and YH-6 [113]), or α-ketoamides (13b [114,115]). Development of small molecule inhibitors targeting Mpro led to FDA approval of Pfizer's first orally available drug, Paxlovid (PF-07321332 + ritonavir), for emergency COVID-19 treatment on December 22, 2021 [116]. Hirose et al.[ 113] reported that YH-6 inhibited viral replication in SARS-CoV-2-infected cells, and its potency (Omicron BA.2, EC50 = 17.1 nM) was comparable to that of nirmatrelvir (Omicron BA.2, EC50 = 21.7 nM). However, the highly reactive functional groups may result in potential side effects. Note that non-selective Mpro inhibitors (such as GC-376, MP18, and 18p) may have off-target cytotoxic effects due to inhibition of host proteases, including cathepsin L and calpains [[117], [118], [119]]. Recently, noncovalent, nonpeptidic small molecule SARS-CoV-2 Mpro inhibitors (such as QUB-00006-Int-07 [120], S-217622 [121], 19 [122], 23R [123], and 26 [124]) have received increasing attention.
Fig. 5.
Representative SARS-CoV-2 Mpro(3CLpro) inhibitors and China's first inhaled aerosolized SARS-CoV-2 Mproclinical candidate FB2001. (a) Chemical structures of selected SARS-CoV-2 Mpro inhibitors (PF-07321332, PF-00835231, GC-376, MPI8, 2a, Jun9–62-2R, 23R, and 26 from USA; 15h from Canada; 13b from Germany; QUB-00006-Int-07 from France; YH-6 and S-217622 from Japan; 19 from Sweden; GC-14, LY1, WU-04, 18p, FB2001, MI-23, and Y180 from China) for COVID-19 treatment. (b) The design strategy of FB2001 through analysis of the substrate-binding pocket of SARS-CoV Mpro to guide design of SARS-CoV-2 Mpro inhibitors (based on the high degree of structural homology and similar substrate specificity of Mpro between SARS-CoV-2 and SARS-CoV). (c) Broad-spectrum activity of FB2001 against SARS-CoV-2 and variants, and comparison of activity between FB2001 with US FDA-approved PF-07321332. (d) Synergistic analysis of the combination of FB2001 and remdesivir. In cases where the synergy score <−10 the interaction was likely to be antagonistic, −10 < synergy score <10 indicated an additive effect, and synergy score >10 indicate a synergistic effect. (e) Schematic representation of the inhibitory mechanism of FB2001. The aldehyde carbon of FB2001 reacts reversibly with the nucleophilic sulfur atom of Cys145, thus forming a covalently bound tetrahedral complex. (f) The structure of SARS-CoV-2 Mpro in complex with FB2001. Cartoon representation of Mpro in complex with FB2001 in two different views. The catalytic dyad (His41 and Cys145) is indicated as green and red spheres, respectively. FB2001 is shown as magenta sticks. Reproduced with permission. Copyright 2022, Elsevier B.V.
Therapeutic advances have been made by China in the development of Mpro clinical candidates (GC-14 [125], LY1 [126], WU-04 [19], 18p [127], FB2001 [18], MI-23 [128], and Y180 [129]; Fig. 5a). Westlake University developed a highly potent oral inhibitor, WPV01 (WU-04), which non-covalently binds to the catalytic pocket of Mpro to inhibit SARS-CoV-2 replication. Hou et al. [19] reported that the anti-SARS-CoV-2 activity of WU-04 was comparable to that of PF-07321332 in Caco-2 cells (Omicron, EC50 = 24 nM for WU-04 vs EC50 = 33 nM for PF-07321332) and in K18-hACE2 mice (orally administered, 300 mpk, twice daily). WPV01 has shown excellent safety and efficacy. WPV01 was approved by the National Drug Control Center of China on September 6, 2022, to enter clinical trials. Novel dual-target inhibitors (such as LY1 [126], a promising dual covalent inhibitor against Mpro [IC50 = 0.12 μM] and PLpro [IC50 = 0.99 μM]) with high selectivity and low toxicity are attractive candidates for COVID-19 treatment. New mutations will emerge over time, and dual-target inhibitors may be less susceptible to viral resistance.
In early 2020, based on the mentioned-above strong mechanistic evidence [130], Dai et al. [131] analyzed the substrate-binding pocket of SARS-CoV Mpro to guide design of SARS-CoV-2 Mpro inhibitors, as shown in Fig. 5b. The active sites of multiple coronavirus Mpro consist of four subsites, S1', S1, S2, and S4. The thiol moiety of a cysteine residue in the S1′ site is the active center that can anchor Mpro inhibitors via covalent bond. Structure-based design showed that an aldehyde occupies the S1' site in P1', an (S)-γ-lactam ring occupies the S1 site in P1, a cyclohexyl moiety occupies the S2 site in P2, and an indole group forms hydrogen bonds with the S4 site in P3 in the optimal drug configuration, which led to discovery of FB2001 (11a, DC402234) [131].
The inhaled antiviral candidate FB2001 has been shown to be a promising broad-spectrum inhibitor to treat infections in vitro in Vero E6 cells with SARS-CoV-2 (EC50 = 0.42 μM, SI = 653) and multiple variants, including Alpha (EC50 = 0.39 μM, SI = 704), Beta (EC50 = 0.28 μM, SI = 980), Delta (EC50 = 0.27 μM, SI = 1016), and Omicron (EC50 = 0.26 μM, SI = 937; EC50 = 0.0.42 μM, when CP-100356 was added) without significant cytotoxicity [18], as shown in Fig. 5c. These results showed that FB2001 might be a more potent Mpro inhibitor than PF-07321332. In addition, Shang et al. [18] showed that FB2001 enhanced antiviral activity in SARS-CoV-2 Delta variant-infected K18-hACE2 mice, resulting in significantly reduced viral load in the lungs (200 mg/kg FB2001, reduced by 1.14 log10 copies/g on day 4) and brains (200 mg/kg FB2001, reduced by 5.26 log10 copies/g on day 4) of the mice in a dose-dependent manner. In addition, FB2001 showed excellent target distribution in the lungs. The EC50 for the Omicron variant was 0.042 μM, more than 132-fold lower than the predicted human lung total concentration [18]. Furthermore, the combination of FB2001 with remdesivir produced an additive effect in blocking SARS-CoV-2 replication through targeting Mpro and RdRp [18], as shown in Fig. 5d. To determine the inhibitory mechanism, Dai et al. [131] determined the 1.5 Å x-ray crystal structure of the FB2001-Mpro complex (PDB: 6LZE) and showed that the aldehyde of FB2001 forms a covalent bond with Cys145 of Mpro to form a reversible thioimidate adduct, as shown in Fig. 5e. Compared with PF-07321332, FB2001 exhibits more stable conjugation when combined with SARS-CoV-2 Mpro as demonstrated by recovery of enzymatic activity [131]. The catalytic dyad formed by Cys145 and His41 (Cys-His) in the active site of the enzyme Mpro is thought to be critical to its potency [131], as shown in Fig. 5f.
A randomized phase I trial (NCT05197179) was conducted by Frontier Biotechnologies Inc. to evaluate the safety, tolerability, and pharmacokinetics of FB2001 in 40 healthy adult subjects. The interim results showed that FB2001 has good safety and tolerability in healthy subjects. This study supported use of FB2001 for treatment of COVID-19. Another two-part, double-blind phase I/II trial (NCT04766931) was conducted by Frontier Biotechnologies Inc. to evaluate the tolerance, pharmacokinetics, and safety of FB2001 in healthy subjects and patients with moderate to severe COVID-19. This study was completed on November 28, 2022, and will be disclosed shortly. Currently, three approved phase I/II/III clinical trials (NCT05445934, NCT05415241, and NCT05583812) of FB2001 are in progress to assess its safety and efficacy profiles. Further investigation of FB2001 for COVID-19 treatment is needed.
5. Cepharanthine—Natural product-derived SARS-CoV-2 receptor binding inhibitor
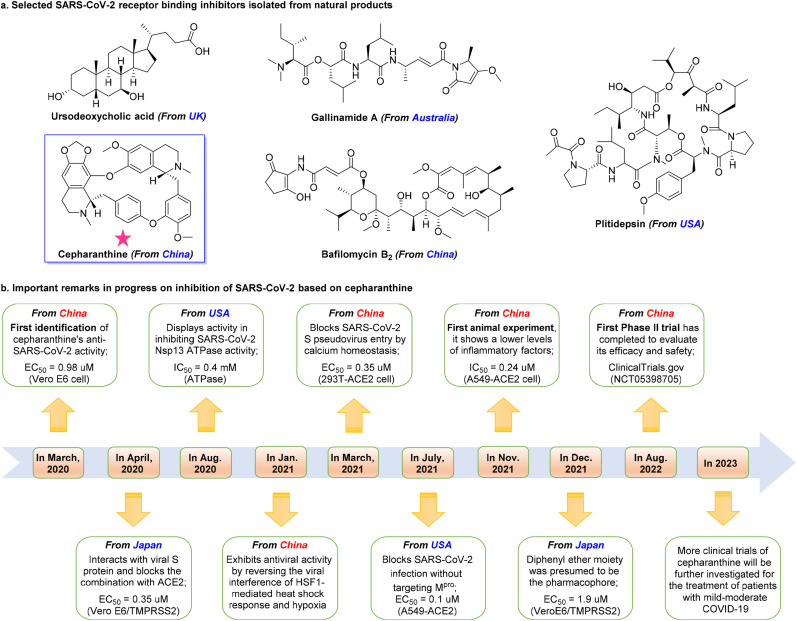
Natural products isolated from animals, plants, microorganisms, and marine, have aided in development of SARS-CoV-2 therapeutics [132,133]. Numerous SARS-CoV-2 inhibitors have been isolated from natural products (Fig. 6 a). Angiotensin-converting enzyme 2 (ACE2) plays an essential role in SARS-CoV-2 infection [134,135]. Brevini et al. [136] from the United Kingdom recently showed that ursodeoxycholic acid (UDCA), the only US FDA-approved natural animal product for treatment of primary biliary cholangitis, reduced SARS-CoV-2 infection by downregulating ACE2 expression in the lung, cholangiocytes, and intestinal organoids of humans, mice, and hamsters. Ursodeoxycholic acid may be promising for prophylaxis and treatment of SARS-CoV-2 infection by reducing entry of SARS-CoV-2 into host cells [136]. Cathepsin L, a promising drug target for viral entry, is a host cysteine protease that plays a crucial role in SARS-CoV-2 infection [137,138]. Ashhurst et al. [139] from the United States found that gallinamide A, a selective Cathepsin L inhibitor isolated from the marine-derived cyanobacterium Schizothrix sp., significantly reduced viral load in VeroE6 cells (IC90 = 0.088 μM) and potently inhibited SARS-CoV-2 entry (EC50 = 0.028 μM) in the nanomolar range. Host protein eukaryotic translation elongation factor 1A (eEF1A) is another promising drug target [140,141]. White et al. [142] from the United States found that plitidepsin (Aplidin®), an eEF1A inhibitor initially isolated from marine Aplidium albicans, exhibited potent inhibition of SARS-CoV-2 replication in Vero E6 cells (IC50 = 0.7 nM), hACE2-293T cells (IC50 = 0.73 nM), and pneumocyte-like cells (IC50 = 1.6 nM) at nanomolar concentrations.
Fig. 6.
Selected bioactive natural products in COVID-19 therapy and clinical candidate cepharanthine. (a) Chemical structures of selected SARS-CoV-2 inhibitors (Ursodeoxycholic acid, initial study from UK; Gallinamide A, initial study from Australia; Plitidepsin, initial study from USA; Bafilomycin B2 and Cepharanthine, initial studies from China) for COVID-19 treatment. (b) Important milestones in progress cepharanthine inhibition of SARS-CoV-2. This timeline shows the speed with which drugs can be developed with collaboration.
As shown in Fig. 6a, impressive advances in development of SARS-CoV-2 agents from natural products have been made in China. For example, Xie et al. [143] from Guangzhou, China, showed that bafilomycin B2, a feces-derived bafilomycin, inhibited SARS-CoV-2 infection at nanomolar concentrations (IC50 = 5.11 nM) in Vero E6 cells through inhibition of endosomal ATP-driven proton pumps [144], which are essential for cellular operation. However, the in vivo information and mechanisms of action of many natural products remain elusive, which is essential before considering clinical studies. Anyway, these studies showed that natural products are an excellent starting point for development of effective agents against SARS-CoV-2 infection.
Drug development is time-intensive and complex, and collaboration can speed drug discovery (as shown in Fig. 6b). Cepharanthine, an approved alkaloid with a good safety profile isolated from the medicinal plant Schizothrix sp., has been widely used to treat several diseases since 1951 [145]. Cepharanthine exerts broad-spectrum therapeutic effects against viruses in vitro, including against HIV-1, SARS-CoV, and HCoV-OC43, with IC50 values of 0.026 μM, 9.5 μg/mL, and 0.83 μM, respectively [[146], [147], [148]]. In March 2020, Fan et al. [149] from Beijing, China, showed that cepharanthine exerted anti-SARS-CoV-2 activity using the SARS-CoV-2 like pangolin coronavirus GX_P2V with an EC50 of 0.98 μM with minimal toxicity (SI > 40) in Vero E6 cells. Ohashi et al. [20,150] from Japan showed that cepharanthine potently inhibited SARS-CoV-2 replication in Vero E6/TMPRSS2 cells (EC50 = 0.35 μM, SI > 70). Cepharanthine can interact with SARS-CoV-2 S protein and block conjugation with ACE2 [20]. White et al. [151] from the United States showed that cepharanthine inhibited viral replication (IC50 = 0.4 mM) by targeting the SARS-CoV-2 Nsp13 (Helicase), which is a critical target due to high sequence conservation and essential role in viral replication [152]. In addition, Li et al. [153] from Beijing, China, showed that cepharanthine inhibited SARS-CoV-2 infection by reversing viral interference of HSF1-mediated heat shock response and hypoxia pathways.
The aforementioned potential mechanisms provide therapeutic evidence for cepharanthine-based COVID-19 treatment. Many research groups have shown the efficacy of cepharanthine against SARS-CoV-2. He et al. [154] from Chongqing, China, showed that cepharanthine potently inhibited SARS-CoV-2 replication (EC50 = 0.35 μM) in 293T-ACE2 cells by inhibiting SARS-CoV-2 S pseudovirus entry. Drayman et al. [155] from the United States reported that cepharanthine blocked SARS-CoV-2 infection (EC50 = 0.1 μM) in A549-ACE2 cells without targeting Mpro. Interestingly, cepharanthine has been shown to be more effective than remdesivir. Hijikata et al. [156] from Japan used computer simulations to show that the anti-SARS-CoV-2 pharmacophore of cepharanthine is the diphenyl ether moiety. They also reported that cepharanthine potently inhibited SARS-CoV-2 replication with an EC50 of 1.9 μM in VeroE6/TMPRSS2 cells [156]. Zhang et al. [157] from Beijing, China showed that cepharanthine inhibited SARS-CoV-2 B.1.351 variant activity in human lung A549-ACE2 cells (IC50 = 0.24 μM) and in Huh7.5.1 cells (IC50 = 0.06 μM). In an hACE2 transgenic mice model, cepharanthine significantly reduced viral load and lung injury as evidenced by reduced levels of inflammatory factors (TNFα and IL-6) [157]. The combination of cepharanthine and nelfinavir trifluoperazine (5 μM each) showed synergistic effects against B.1.351 in Huh7.5.1 cells (vRNA levels decreased to less than 0.01% compared with the control group, which was 50- and 1000-fold more potent than either cepharanthine or trifluoperazine alone) [157].
These findings showed that cepharanthine was an excellent drug candidate. A phase II trial (NCT05398705) conducted at Shanghai Jiao Tong University evaluated the efficacy and safety of high and low doses of cepharanthine in 450 non-hospitalized adults who were asymptomatic or had mild symptoms. The results showed that cepharanthine had good safety in patients with asymptomatic COVID-19 and could shorten disease course in patients with primary asymptomatic/mild COVID-19 (HR = 1.56, 95% CI, 1.03–2.37; p = 0.035). Large, double-blind, controlled phase II/III trials will be needed to verify the efficacy of cepharanthine. Approval for cepharanthine for clinical use may take some time. However, the findings regarding the safety and efficacy of cepharanthine show that development of natural product-based SARS-CoV-2 inhibitors can occur quickly through coordinated collaboration among natural product chemists, pharmacologists, clinicians, and pharmaceutical companies.
6. Other small molecule anti-SARS-CoV-2 candidates in development
Novel small molecule drugs are critical to mitigating the COVID-19 epidemic. Impressive progress has been made in China in developing SARS-CoV-2 agents. As shown in Table 1 , several small molecule anti-SARS-CoV-2 candidates are in development in China.
Table 1.
Current status of small molecule anti-SARS-CoV-2 drugs in development in China.
| Drug | R&D institution | Target | Delivery | Status | Characteristic |
|---|---|---|---|---|---|
| Azvudine (FNC) | Henan Sincere Biotech Co., Ltd./Zhengzhou University/Henan Normal University | RdRp | Oral | Gain conditional approval in China on July 25, 2022 | Repurposed HIV drug; first-in-class |
| VV116 (JT001) | Shanghai Junshi Biosciences Co., Ltd./Shanghai Institute of Materia Medica, CAS | RdRp | Oral | Gain conditional approval in China on January 29, 2023 | Optimized SARS-CoV-2 candidate prodrug |
| Xiannuoxin (Simnotrelvir [SIM0417] + Ritonavir) | Jiangsu Simcere Pharmaceutical Co., Ltd./Shanghai Institute of Materia Medica, Wuhan Institute of Virology, CAS | Mpro | Oral | Gain conditional approval in China on January 29, 2023 | Ritonavir can boost simnotrelvir's antiviral efficacy. |
| Shenosivir (SHEN26) | Kexing Biopharmaceutical Co., Ltd./Southern University of Science and Technology/Sun Yat-Sen University | RdRp | Oral | Phase I | Oral remdesivir prodrugs, including ATV006 |
| ASC10 | Ascletis Pharmaceuticals Co., Ltd. | RdRp | Oral | Phase I | Double prodrug of ASC10-A |
| CH2101 | Suzhou Jianghe Pharmaceutical Co., Ltd. | RdRp | Oral | Phase I | Innovative drug breaks through Merck's patent |
| RAY1216 | Guangdong RAYNOVENT Co., Ltd. | Mpro | Oral | Phase III | Broad-spectrum, wild, Omicron, and mutants |
| Pentarlandir™ UPPTA | SyneuRx International (Taiwan) Corp. | Mpro | Oral | Phase II | Natural products; ultrapure and potent tannic acids |
| VV993 | Shanghai Institute of Materia Medica, CAS/Wuhan Institute of Virology, CAS | Mpro | Oral | Preclinical | Approval to enter clinical trials |
| WPV01 (WU-04) | Westlake University/Chinese Academy of Agricultural Sciences | Mpro | Oral | Preclinical | First-in-class; approval to enter clinical trials; non-covalent inhibitor; |
| GS-00202 | Shanghai Anovent Pharmaceutical | Mpro | Oral | Preclinical | Approval to enter clinical trials |
| GST-HG171 | Fujian Cosunter Pharmaceutical Co., Ltd. | Mpro | Oral | Phase II/III | Broad-spectrum inhibitor |
| RAY003 | Guangdong RAYNOVENT Co., Ltd. | Mpro | Oral | Preclinical | Approval to enter clinical trials |
| FB2001 (DC402234) | Frontier Biotechnologies Inc./Shanghai Institute of Materia Medica, CAS | Mpro | Injection or inhalation | Phase II/III | The first inhaled aerosolized inhibitor in China |
| BE-60 | Shanghai Institute of Materia Medica, CAS | E protein | Intravenous injection | Preclinical | China's first SARS-CoV-2 ion channel inhibitor |
| STC3141 | Grand Medical Pty Ltd. | NETs | Intravenous injection | Phase II | First-in-class, Severe COVID-19 Pneumonia |
| GS221 | Grand Medical Pty Ltd. | Mpro | Oral | Phase II | Self-developed |
| Proxalutamide (GT0918) | Kintor Pharmaceutical Co., Ltd. | ACE2, TMPRSS2 | Oral | Phase III | Repurposed mCRPC drug |
| Carrimycin | Shenyang Tonglian Group Co., Ltd./Chinese Academy of Medical Sciences | Viral RNA, ACE2 | Oral | Phase III | Repurposed antibiotics; natural product; first-in-class |
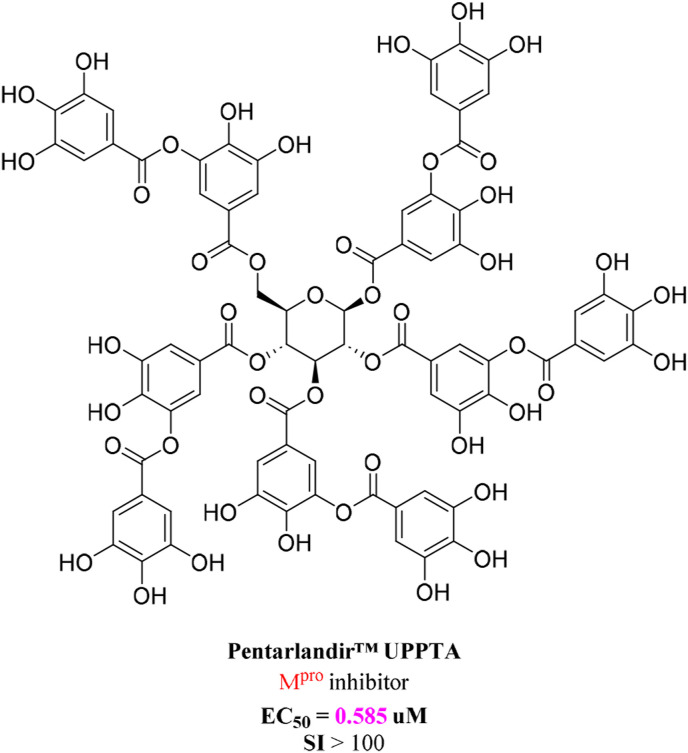
Shih et al.[ 158] from Taiwan, China showed that Pentarlandir™ UPPTA (Fig. 7 ), a naturally occurring polyphenol with antioxidant properties, potently inhibited SARS-CoV-2 infection by targeting 3CLpro in Vero E6 cells. The EC50, EC90, and EC99 were 0.585, 8.307, and 12.90 μM, respectively. Furthermore, Pentarlandir™ UPPTA showed excellent target distribution in the lungs, which is the primary site of SARS-CoV-2 infection [158]. A randomized phase II trial (NCT04911777) by SyneuRx International (Taiwan) Corp. is in progress to evaluate the pharmacokinetics, viral shedding, safety, and clinical effects of Pentarlandir™ UPPTA in 90 patients with mildly symptomatic early COVID-19. Many other candidate drugs are currently being investigated.
Fig. 7.
Chemical structures of Pentarlandir™ UPPTA.
7. Conclusion and perspectives
Small molecule inhibitors are essential tools for SARS-CoV-2 treatment. Development of novel small molecule anti-SARS-CoV-2 drugs is a rapidly evolving field in China, but continued discovery and development is necessary to deal with current and future variants. To provide maximal public-health benefits, several aspects of drug research could facilitate the drug development process.
First, focus should be placed on basic research and enhanced capacity for innovation. Basic research critical to drug innovation, and requires sustained accumulation of knowledge. Professor Zihe Rao has been working in the coronavirus field for 20 years since the SARS-CoV epidemic in 2003 [159]. Rao's team has systematically explored the life cycle of SARS-CoV-2, which has provided a framework for understanding the molecular mechanisms of SARS-CoV-2 and allowed for development of effective anti-SARS-CoV-2 drugs. Second, guidance for drug development is necessary. For example, the “Major New Drug Creation” special project, launched in 2008, has significantly improved China's pharmaceutical innovation capacity. Forty-one New class I drugs were created in China from 2008 to 2018, and 10, 12, and 15 were added in 2018, 2019, and 2020 respectively. In contrast, in the 23 years before the special project was launched, there were only 5 new class I drugs. The change is incredible and moving. Development of the anti-SARS-CoV-2 drugs azvudine, VV116, and carrimycin were supported by this special project. Third, strong collaborations between universities, institutes, and pharmaceutical companies need to continue to grow. These collaborations can improve the national drug innovation technology system. China's first oral SARS-CoV-2 RdRp inhibitor azvudine, inhaled host cathepsin L inhibitor FB2001, and carrimycin resulted from collaborative innovation.
Effective prevention and treatment approaches have not been developed. However, continued advances and multiple optimization measures (e.g., receptor/target-, structure-, property-, and mechanism-based drug design) may lead to more effective anti-SARS-CoV-2 drugs. Ideal drugs should have the following key characteristics: (i) superior oral bioavailability and greater plasma half-life; (ii) broad-spectrum activity against SARS-CoV-2 and its variants; (iii) sufficient safety, less drug-drug interactions, and reduced organ damage; (iv) manufacturable with a sustainable supply, reasonable price, and global availability to outpatients.
As new variants continue to evolve, further investigation of the impact of SARS-CoV-2 variants on immune escape, receptor binding, and efficacy of small molecule agents is needed. Therefore, it makes sense to focus our efforts on understanding catalytic residues/mutations, critical targets, conserved motifs/regions, molecular recognition, and replication mechanisms. This will help shed light on which mutations develop resistance and how, as well as understanding how certain mutations act as compensators, and help design more effective SARS-CoV-2 inhibitors. It is important to discuss extensively the drug resistance of promising drug targets (e.g., RdRp and Mpro) and the spectrum of antiviral activity. In reality, SARS-CoV-2 resistance to nirmatrelvir and remdesivir has been widely reported [[160], [161], [162], [163], [164]]. For example, Iketani et al. [160] demonstrated that in vitro high-level resistance to protease inhibitor nirmatrelvir does readily arise by SARS-CoV-2 via any one of several mutational pathways. Gandhi et al. [163] illustrated that the E802D mutation in the nsp12 RdRp conferres a ∼6-fold increase in remdesivir IC50 (0.7 μM for original SARS-CoV-2 vs 4.2 μM for E802D variant) in vitro. Drug combination therapy that targets multiple replication pathways of SARS-CoV-2, which can improve therapeutic efficacy and prevent the development of drug resistance, is a promising treatment approach. Zhou et al. [164] confirmed that remdesivir retains activity against nirmatrelvir-resistant variants, and combination with nirmatrelvir enhanced therapeutic efficacy compared to each individual inhibitor.
Similarly, we should also focus on SARS-CoV-2 proteins that have fewer mutations but are functionally important, including structural proteins (such as envelope protein) and relatively unfocused non-structural proteins (such as NSP16, which plays a crucial role in immune evasion). Furthermore, researchers should be encouraged to discover novel small molecule anti-SARS-CoV-2 drugs using potent broad-spectrum antiviral agents, dual-target inhibitors, inhalation administration, or combination therapeutic strategies as scaffolds. In parallel, novel inhibitors new sources, such as marine natural products, may treat SARS-CoV-2 infection and prevent drug resistance or insensitivity. In addition, some promising antiviral drugs against SARS-CoV-2 and its variants are subject to safety and efficacy limitations that need to be addressed prior to approval for clinical use.
Author contributions
LY: Conceptualization, writing-review & editing, funding acquisition. ZW: Conceptualization, collecting the literatures, writing original draft, writing-review & editing, visualization, funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Shandong Provincial Natural Science Foundation (ZR2022MH162, ZR2022QE202), the PhD Research Start-up Foundation of Qufu Normal University (614901, 615201).
Data availability
No data was used for the research described in the article.
References
- 1.World Health Organization WHO coronavirus (COVID-19) Dashboard. https://covid19.who.int/
- 2.Aggarwal A., Akerman A., Milogiannakis V., Silva M.R., Walker G., Stella A.O., et al. SARS-CoV-2 Omicron BA. 5: evolving tropism and evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. EBioMedicine. 2022;84 doi: 10.1016/j.ebiom.2022.104270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen R.M., Bang L.L., Tornby D.S., Madsen L.W., Holm D.K., Sydenham T.V., et al. Omicron BA. 5 neutralization among vaccine-boosted persons with prior Omicron BA. 1/BA. 2 infections. Emerg. Infect. Dis. 2022;28:2575–2577. doi: 10.3201/eid2812.221304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb R.L., Vaca C.E., Paredes R., Mera J., Webb B.J., Perez G., et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong C.K., Au I.C., Lau K.T., Lau E.H., Cowling B.J., Leung G.M. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400:1213–1222. doi: 10.1016/S0140-6736(22)01586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbel R., Wolff Sagy Y., Hoshen M., Battat E., Lavie G., Sergienko R., et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N. Engl. J. Med. 2022;387:790–798. doi: 10.1056/NEJMoa2204919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottlieb R.L., Vaca C.E., Paredes R., Mera J., Webb B.J., Perez G., et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Solidarity Trial Consortium Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas I.O., Diaz G., Gottlieb R.L., Lobo S.M., Robinson P., Hunter B.D., et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med. 2021;47:1258–1270. doi: 10.1007/s00134-021-06507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;2020(395):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. 10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burki T. The future of Paxlovid for COVID-19. Lancet Respir. Med. 2022;10:e68. doi: 10.1016/S2213-2600(22)00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal-Ré R., Becker S.L., Bottieau E., Holm S. Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach. Lancet Infect. Dis. 2022;22:e231–e238. doi: 10.1016/S1473-3099(22)00119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabinger F., Stiller C., Schmitzová J., Dienemann C., Kokic G., Hillen H.S., et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021;28:740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menéndez-Arias L. Decoding molnupiravir-induced mutagenesis in SARS-CoV-2. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J.L., Li Y.H., Wang L.L., Liu H.Q., Lu S.Y., Liu Y., et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Tar. 2021;6:414. doi: 10.1038/s41392-021-00835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y., Yin W., Zhang Y., Shang W., Wang Z., Luan X., et al. Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2. Cell Res. 2021;31:1212–1214. doi: 10.1038/s41422-021-00570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheema H.A., Rehman A.U., Elrashedy A.A., Mohsin A., Shahid A., Ehsan M., et al. Antiandrogens for the treatment of COVID-19 patients: a meta-analysis of randomized controlled trials. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28740. [DOI] [PubMed] [Google Scholar]
- 18.Shang W., Dai W., Yao C., Xu L., Tao X., Su H., et al. In vitro and in vivo evaluation of the main protease inhibitor FB2001 against SARS-CoV-2. Antivir. Res. 2022;208 doi: 10.1016/j.antiviral.2022.105450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou N., Shuai L., Zhang L., Xie X., Tang K., Zhu Y., et al. Development of highly potent noncovalent inhibitors of SARS-CoV-2 3CLpro. ACS Cent. Sci. 2023;9:217–227. doi: 10.1021/acscentsci.2c01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience. 2021;2021(24) doi: 10.1016/j.isci.2021.102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brant A.C., Tian W., Majerciak V., Yang W., Zheng Z.M. SARS-CoV-2: from its discovery to genome structure, transcription, and replication. Cell Biosci. 2021;11:136. doi: 10.1186/s13578-021-00643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone B., Urakova N., Snijder E.J., Campbell E.A. Structures and functions of coronavirus replication–transcription complexes and their relevance for SARS-CoV-2 drug design. Nat. Rev. Mol. Cell Biol. 2022;23:21–39. doi: 10.1038/s41580-021-00432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iketani S., Hong S.J., Sheng J., Bahari F., Culbertson B., Atanaki F.F., et al. Functional map of SARS-CoV-2 3CL protease reveals tolerant and immutable sites. Cell Host Microbe. 2022;30:1354–1362. doi: 10.1016/j.chom.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillen H.S., Kokic G., Farnung L., Dienemann C., Tegunov D., Cramer P. Structure of replicating SARS-CoV-2 polymerase. Nature. 2020;584:154–156. doi: 10.1038/s41586-020-2368-8. [DOI] [PubMed] [Google Scholar]
- 26.Ke Z., Oton J., Qu K., Cortese M., Zila V., McKeane L., et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588:498–502. doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao H., Song Y., Chen Y., Wu N., Xu J., Sun C., et al. Molecular architecture of the SARS-CoV-2 virus. Cell. 2020;183:730–738. doi: 10.1016/j.cell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 32.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q., Wu J., Wang H., Gao Y., Liu Q., Mu A., et al. Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell. 2020;182:417–428. doi: 10.1016/j.cell.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv Z., Deng Y.Q., Ye Q., Cao L., Sun C.Y., Fan C., et al. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020;369:1505–1509. doi: 10.1126/science.abc5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan L., Ge J., Zheng L., Zhang Y., Gao Y., Wang T., et al. Cryo-EM structure of an extended SARS-CoV-2 replication and transcription complex reveals an intermediate state in cap synthesis. Cell. 2021;184:184–193. doi: 10.1016/j.cell.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan L., Zhang Y., Ge J., Zheng L., Gao Y., Wang T., et al. Architecture of a SARS-CoV-2 mini replication and transcription complex. Nat. Commun. 2020;11:5874. doi: 10.1038/s41467-020-19770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan L., Yang Y., Li M., Zhang Y., Zheng L., Ge J., et al. Coupling of N7-methyltransferase and 3′-5′ exoribonuclease with SARS-CoV-2 polymerase reveals mechanisms for capping and proofreading. Cell. 2021;184:3474–3485. doi: 10.1016/j.cell.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan L., Huang Y., Ge J., Liu Z., Lu P., Huang B., et al. A mechanism for SARS-CoV-2 RNA capping and its inhibition by nucleotide analogue inhibitors. Cell. 2022;185:4347–4360. doi: 10.1016/j.cell.2022.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong Q., Han W., Li J., Xu S., Wang Y., Xu C., et al. Molecular basis of receptor binding and antibody neutralization of Omicron. Nature. 2022;604:546–552. doi: 10.1038/s41586-022-04581-9. [DOI] [PubMed] [Google Scholar]
- 40.Han P., Su C., Zhang Y., Bai C., Zheng A., Qiao C., Wang Q., Niu S., Chen Q., Zhang Y., et al. Molecular insights into receptor binding of recent emerging SARS-CoV-2 variants. Nat. Commun. 2021;12:6103. doi: 10.1038/s41467-021-26401-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han P., Li L., Liu S., Wang Q., Zhang D., Xu Z., et al. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185:630–640. doi: 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L., Liao H., Meng Y., Li W., Han P., Liu K., et al. Structural basis of human ACE2 higher binding affinity to currently circulating Omicron SARS-CoV-2 sub-variants BA. 2 and BA. 1.1. Cell. 2022;185:2952–2960. doi: 10.1016/j.cell.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368:1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng Q., Peng R., Yuan B., Zhao J., Wang M., Wang X., et al. Structural and biochemical characterization of the nsp12-nsp7-nsp8 core polymerase complex from SARS-CoV-2. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam T.T.Y., Jia N., Zhang Y.W., Shum M.H.H., Jiang J.F., Zhu H.C., et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhou H., Ji J., Chen X., Bi Y., Li J., Wang Q., et al. Identification of novel bat coronaviruses sheds light on the evolutionary origins of SARS-CoV-2 and related viruses. Cell. 2021;184:4380–4391. doi: 10.1016/j.cell.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui Z., Liu P., Wang N., Wang L., Fan K., Zhu Q., et al. Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron. Cell. 2022;185:860–871. doi: 10.1016/j.cell.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou T., Wang L., Misasi J., Pegu A., Zhang Y., Harris D.R., et al. Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B. 1.1. 529. Science. 2022;376 doi: 10.1126/science.abn8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang K., Jia Z., Bao L., Wang L., Cao L., Chi H., et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature. 2022;603:919–925. doi: 10.1038/s41586-022-04466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin W., Xu Y., Xu P., Cao X., Wu C., Gu C., et al. Structures of the Omicron Spike trimer with ACE2 and an anti-Omicron antibody. Science. 2022;375:1048–1053. doi: 10.1126/science.abn8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C., Zhan W., Yang Z., Tu C., Hu G., Zhang X., et al. Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific single-domain antibody. Cell. 2022;185:1389–1401. doi: 10.1016/j.cell.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao Y.R., Yisimayi A., Jian F., Song W., Xiao T., Wang L., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maio N., Lafont B.A., Sil D., Li Y., Bollinger J.M., Jr., Krebs C., et al. Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets. Science. 2021;373:236–241. doi: 10.1126/science.abi5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., Anirudhan V., Du R., Cui Q., Rong L. RNA‐dependent RNA polymerase of SARS‐CoV‐2 as a therapeutic target. J. Med. Virol. 2021;93:300–310. doi: 10.1002/jmv.26264. [DOI] [PubMed] [Google Scholar]
- 58.Yu B., Chang J. The first Chinese oral anti-COVID-19 drug Azvudine launched. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu B., Chang J. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct. Tar. 2020;5:236. doi: 10.1038/s41392-020-00351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sommadossi J.-P., La Colla P. Preparation of antiviral nucleosides and methods for treating hepatitis C virus. NoVirio Pharmaceuticals Limited, UniVersita Degli Studi Di Cagliari; WO 2001090121, CAN. 2001;136(6296):296. [Google Scholar]
- 61.Sommadossi J.-P., La Colla P. Methods and compositions using modified nucleosides for treating flaviviruses and pestiviruses. NoVirio Pharmaceuticals Limited, UniVersita Degli Studi Di Cagliari; WO 2001092282, CAN. 2001;136(590):302. [Google Scholar]
- 62.Klumpp K., Leveque V., Le Pogam S., Ma H., Jiang W.-R., Kang H., et al. The novel nucleoside analog R1479 (4′-azidocytidine) is a potent inhibitor of NS5B-dependent RNA synthesis and hepatitis C virus peplication in cell culture. J. Biol. Chem. 2006;281:3793–3799. doi: 10.1074/jbc.M510195200. [DOI] [PubMed] [Google Scholar]
- 63.Smith D.B., Martin J.A., Klumpp K., Baker S.J., Blomgren P.A., Devos R., et al. Design, synthesis and antiviral properties of 4′-substituted ribonucleosides as inhibitors of hepatitis C virus placation: the discovery of R1479. Bioorg. Med. Chem. Lett. 2007;17:2570–2576. doi: 10.1016/j.bmcl.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Klumpp K., Kalayanov G., Ma H., Le Pogam S., Leveque V., Jiang W.-R., et al. 2′-Deoxy-4′-azido nucleoside analogs are highly potent inhibitors of HCV replication despite the lack of 2′-R-hydroxyl groups. J. Biol. Chem. 2008;283:2167–2175. doi: 10.1074/jbc.M708929200. [DOI] [PubMed] [Google Scholar]
- 65.Smith D.B., Kalaynov G., Sund C., Winqvist A., Pinho P., Maltseva T., et al. The design, synthesis and antiviral activity of 4′-azidocytidine analogues against hepatitis C virus replication: the discovery of 4′-azidoarabinocytidine. J. Med. Chem. 2009;52:219–223. doi: 10.1021/jm800981y. [DOI] [PubMed] [Google Scholar]
- 66.Smith D.B., Kalayanov G., Sund C., Winqvist A., Maltseva T., Leveque V.J.P., et al. The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4′-azidocytidine against hepatitis C virus replication: the discovery of 4′-azido-2′-deoxy-2′-fluorocytidine and 4′-azido-2′-dideoxy-2′, 2′-difluorocytidine. J. Med. Chem. 2009;52:2971–2978. doi: 10.1021/jm801595c. [DOI] [PubMed] [Google Scholar]
- 67.Wang Q., Hu W., Wang S., Pan Z., Tao L., Guo X., et al. Synthesis of new 2′-deoxy-2′-fluoro-4′-azido nucleoside analogues as potent anti-HIV agents. Eur. J. Med. Chem. 2011;46:4178–4183. doi: 10.1016/j.ejmech.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang R.R., Yang Q.H., Luo R.H., Peng Y.M., Dai S.X., Zhang X.J., et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu N., Yang J., Zheng B., Zhang Y., Cao Y., Huan C., et al. The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses. J. Virol. 2020;94 doi: 10.1128/JVI.00204-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun L., Peng Y., Yu W., Zhang Y., Liang L., Song C., et al. Mechanistic insight into antiretroviral potency of 2′-deoxy-2′-β-fluoro-4′-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention. J. Med. Chem. 2020;63:8554–8566. doi: 10.1021/acs.jmedchem.0c00940. [DOI] [PubMed] [Google Scholar]
- 71.Peng Y., Cheng T., Dong L., Zhang Y., Chen X., Jiang J., et al. Quantification of 2′-deoxy-2′-β-fluoro-4′-azidocytidine in rat and dog plasma using liquid chromatography-quadrupole time-of-flight and liquid chromatography-triple quadrupole mass spectrometry: application to bioavailability and pharmacokinetic studies. J. Pharmaceut. Biomed. Anal. 2014;98:379–386. doi: 10.1016/j.jpba.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 72.Ren Z., Luo H., Yu Z., Song J., Liang L., Wang L., et al. A randomized, open‐label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID‐19, a pilot study. Adv. Sci. 2020;7 doi: 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang J. 4′-Modified nucleosides for antiviral drug discovery: achievements and perspectives. Acc. Chem. Res. 2022;55:565–578. doi: 10.1021/acs.accounts.1c00697. [DOI] [PubMed] [Google Scholar]
- 74.Pauly I., Singh A.K., Kumar A., Singh Y., Thareja S., Kamal M.A., et al. Current insights and molecular docking studies of the drugs under clinical trial as RdRp inhibitors in COVID-19 treatment. Curr. Pharmaceut. Des. 2022;28:3677–3705. doi: 10.2174/1381612829666221107123841. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z., Yang L., Song X.Q. Oral GS-441524 derivatives: next-generation inhibitors of SARS‐CoV‐2 RNA‐dependent RNA polymerase. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1015355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishimura H., Katagiri K., Satō K., Mayama M., Shimaoka N. Toyocamycin, a new anti-candida antibiotics. J. Antibiot. 1956;9:60–62. [PubMed] [Google Scholar]
- 77.Anzai K., Nakamura G., Suzuki S. A new antibiotic, tubercidin. J. Antibiot. 1957;10:201–204. [PubMed] [Google Scholar]
- 78.Wang Z., Yang L. GS-5734: a potentially approved drug by FDA against SARS-Cov-2. New J. Chem. 2020;44:12417–12429. [Google Scholar]
- 79.Rasmussen H.B., Thomsen R., Hansen P.R. Nucleoside analog GS‐441524: pharmacokinetics in different species, safety, and potential effectiveness against Covid‐19. Pharmacol. Res. Perspe. 2022;10 doi: 10.1002/prp2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang A.Q., Hagen N.R., Padilha E.C., Yang M., Shah P., Chen C.Z., et al. Preclinical pharmacokinetics and in vitro properties of GS-441524, a potential oral drug candidate for COVID-19 treatment. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.918083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie J., Wang Z. Can remdesivir and its parent nucleoside GS-441524 be potential oral drugs? An in vitro and in vivo DMPK assessment. Acta Pharm. Sin. B. 2021;11:1607–1616. doi: 10.1016/j.apsb.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y., Cao L., Li G., Cong F., Li Y., Sun J., et al. Remdesivir metabolite GS-441524 effectively inhibits SARS-CoV-2 infection in mouse models. J. Med. Chem. 2022;65:2785–2793. doi: 10.1021/acs.jmedchem.0c01929. [DOI] [PubMed] [Google Scholar]
- 83.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., Zhang L., et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo [2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 85.Jacobs M., Rodger A., Bell D.J., Bhagani S., Cropley I., Filipe A., et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388:498–503. doi: 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dörnemann J., Burzio C., Ronsse A., Sprecher A., De Clerck H., Van Herp M., et al. First newborn baby to receive experimental therapies survives Ebola virus disease. J. Infect. Dis. 2017;2017(215):171–174. doi: 10.1093/infdis/jiw493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McMullan L.K., Flint M., Chakrabarti A., Guerrero L., Lo M.K., Porter D., et al. Characterisation of infectious Ebola virus from the ongoing outbreak to guide response activities in the Democratic Republic of the Congo: a phylogenetic and in vitro analysis. Lancet Infect. Dis. 2019;19:1023–1032. doi: 10.1016/S1473-3099(19)30291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tchesnokov E.P., Raeisimakiani P., Ngure M., Marchant D., Götte M. Recombinant RNA-dependent RNA polymerase complex of Ebola virus. Sci. Rep. 2018;8:3970. doi: 10.1038/s41598-018-22328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsu J. Covid-19: what now for remdesivir? BMJ. 2020;371:m4457. doi: 10.1136/bmj.m4457. [DOI] [PubMed] [Google Scholar]
- 92.Kaka A.S., MacDonald R., Greer N., Vela K., Duan-Porter W., Obley A., Wilt T.J. Major update: remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians practice points. Ann. Intern. Med. 2021;174:663–672. doi: 10.7326/M20-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.WHO Solidarity Trial Consortium Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399:1941–1953. doi: 10.1016/S0140-6736(22)00519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ader F., Bouscambert-Duchamp M., Hites M., Peiffer-Smadja N., Poissy J., Belhadi D., et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022;22:209–221. doi: 10.1016/S1473-3099(21)00485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rochwerg B., Agarwal A., Zeng L., Leo Y.S., Appiah J.A., Agoritsas T., et al. Remdesivir for severe covid-19: a clinical practice guideline. BMJ. 2020;370:m2924. doi: 10.1136/bmj.m2924. [DOI] [PubMed] [Google Scholar]
- 96.Shen Y., Ai J., Lin N., Zhang H., Li Y., Wang H., et al. An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants. Emerg. Microb. Infect. 2022;11:1518–1523. doi: 10.1080/22221751.2022.2078230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gajula S.N.R., Nadimpalli N., Sonti R. Drug metabolic stability in early drug discovery to develop potential lead compounds. Drug Metab. Rev. 2021;53:459–477. doi: 10.1080/03602532.2021.1970178. [DOI] [PubMed] [Google Scholar]
- 98.Qian H.J., Wang Y., Zhang M.Q., Xie Y.C., Wu Q.Q., Liang L.Y., et al. Safety, tolerability, and pharmacokinetics of VV116, an oral nucleoside analog against SARS-CoV-2, in Chinese healthy subjects. Acta Pharmacol. Sin. 2022;43:3130–3138. doi: 10.1038/s41401-022-00895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao Z., Gao W., Bao H., Feng H., Mei S., Chen P., et al. VV116 versus nirmatrelvir–ritonavir for oral treatment of Covid-19. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2208822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Günther S., Reinke P.Y., Fernández-García Y., Lieske J., Lane T.J., Ginn H.M., et al. X-ray screening identifies active site and allosteric inhibitors of SARS-CoV-2 main protease. Science. 2021;372:642–646. doi: 10.1126/science.abf7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Z., Yang L., Zhao X.E. Co-crystallization and structure determination: an effective direction for anti-SARS-CoV-2 drug discovery. Comput. Struct. Biotechnol. J. 2021;19:4684–4701. doi: 10.1016/j.csbj.2021.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Owen D.R., Allerton C.M., Anderson A.S., Aschenbrenner L., Avery M., Berritt S., et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 103.Wang Z., Yang L. In the age of Omicron variant: Paxlovid raises new hopes of COVID‐19 recovery. J. Med. Virol. 2022;94:1766–1767. doi: 10.1002/jmv.27540. [DOI] [PubMed] [Google Scholar]
- 104.Hoffman R.L., Kania R.S., Brothers M.A., Davies J.F., Ferre R.A., Gajiwala K.S., et al. Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J. Med. Chem. 2020;63:12725–12747. doi: 10.1021/acs.jmedchem.0c01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boras B., Jones R.M., Anson B.J., Arenson D., Aschenbrenner L., Bakowski M.A., et al. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19. Nat. Commun. 2021;12:6055. doi: 10.1038/s41467-021-26239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bai B., Belovodskiy A., Hena M., Kandadai A.S., Joyce M.A., Saffran H.A., et al. Peptidomimetic α-acyloxymethylketone warheads with six-membered lactam P1 glutamine mimic: SARS-CoV-2 3CL protease inhibition, coronavirus antiviral activity, and in vitro biological stability. J. Med. Chem. 2021;65:2905–2925. doi: 10.1021/acs.jmedchem.1c00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma C., Sacco M.D., Hurst B., Townsend J.A., Hu Y., Szeto T., et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xia Z., Sacco M., Hu Y., Ma C., Meng X., Zhang F., et al. Rational design of hybrid SARS-CoV-2 main protease inhibitors guided by the superimposed cocrystal structures with the peptidomimetic inhibitors GC-376, telaprevir, and boceprevir. ACS Pharmacol. Transl. Sci. 2021;4:1408–1421. doi: 10.1021/acsptsci.1c00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao W., Cho C.C.D., Geng Z.Z., Shaabani N., Ma X.R., Vatansever E.C., et al. Evaluation of SARS-CoV-2 main protease inhibitors using a novel cell-based assay. ACS Cent. Sci. 2022;8:192–204. doi: 10.1021/acscentsci.1c00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma X.R., Alugubelli Y.R., Ma Y., Vatansever E.C., Scott D.A., Qiao Y., et al. MPI8 is potent against SARS‐CoV‐2 by inhibiting dually and selectively the SARS‐CoV‐2 main protease and the host cathepsin L. ChemMedChem. 2022;17 doi: 10.1002/cmdc.202100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dampalla C.S., Kim Y., Bickmeier N., Rathnayake A.D., Nguyen H.N., Zheng J., et al. Structure-guided design of conformationally constrained cyclohexane inhibitors of severe acute respiratory syndrome coronavirus-2 3CL protease. J. Med. Chem. 2021;64:10047–10058. doi: 10.1021/acs.jmedchem.1c00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma C., Xia Z., Sacco M.D., Hu Y., Townsend J.A., Meng X., et al. Discovery of di-and trihaloacetamides as covalent SARS-CoV-2 main protease inhibitors with high target specificity. J. Am. Chem. Soc. 2021;143:20697–20709. doi: 10.1021/jacs.1c08060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hirose Y., Shindo N., Mori M., Onitsuka S., Isogai H., Hamada R., et al. Discovery of chlorofluoroacetamide-based covalent inhibitors for severe acute respiratory syndrome coronavirus 2 3CL protease. J. Med. Chem. 2022;65:13852–13865. doi: 10.1021/acs.jmedchem.2c01081. [DOI] [PubMed] [Google Scholar]
- 114.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumari A., Mittal L., Srivastava M., Asthana S. Binding mode characterization of 13b in the monomeric and dimeric states of SARS-CoV-2 main protease using molecular dynamics simulations. J. Biomol. Struct. Dyn. 2021;40:9287–9305. doi: 10.1080/07391102.2021.1927844. [DOI] [PubMed] [Google Scholar]
- 116.Cokley J.A., Gidal B.E., Keller J.A., Vossler D.G. PaxlovidTM information from FDA and guidance for AES members. Epilepsy Curr. 2022;22:201–204. doi: 10.1177/15357597221088415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sacco M.D., Ma C., Lagarias P., Gao A., Townsend J.A., Meng X., et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monica G.L., Bono A., Lauria A., Martorana A. Targeting SARS-CoV-2 main protease for treatment of COVID-19: covalent inhibitors structure–activity relationship insights and evolution perspectives. J. Med. Chem. 2022;65:12500–12534. doi: 10.1021/acs.jmedchem.2c01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan B., Joyce R., Tan H., Hu Y., Wang J. SARS-CoV-2 main protease drug design, assay development, and drug resistance studies. Acc. Chem. Res. 2023;56:157–168. doi: 10.1021/acs.accounts.2c00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.El Khoury L., Jing Z., Cuzzolin A., Deplano A., Loco D., Sattarov B., et al. Computationally driven discovery of SARS-CoV-2 M(pro) inhibitors: from design to experimental validation. Chem. Sci. 2022;13:3674–3687. doi: 10.1039/d1sc05892d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Unoh Y., Uehara S., Nakahara K., Nobori H., Yamatsu Y., Yamamoto S., et al. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J. Med. Chem. 2022;65:6499–6512. doi: 10.1021/acs.jmedchem.2c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luttens A., Gullberg H., Abdurakhmanov E., Vo D.D., Akaberi D., Talibov V.O., et al. Ultralarge virtual screening identifies SARS-CoV-2 main protease inhibitors with broad-spectrum activity against coronaviruses. J. Am. Chem. Soc. 2022;144:2905–2920. doi: 10.1021/jacs.1c08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kitamura N., Sacco M.D., Ma C., Hu Y., Townsend J.A., Meng X., et al. Expedited approach toward the rational design of noncovalent SARS-CoV-2 main protease inhibitors. J. Med. Chem. 2022;65:2848–2865. doi: 10.1021/acs.jmedchem.1c00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang C.H., Stone E.A., Deshmukh M., Ippolito J.A., Ghahremanpour M.M., Tirado-Rives J., et al. Potent noncovalent inhibitors of the main protease of SARS-CoV-2 from molecular sculpting of the drug perampanel guided by free energy perturbation calculations. ACS Cent. Sci. 2021;7:467–475. doi: 10.1021/acscentsci.1c00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao S., Sylvester K., Song L., Claff T., Jing L., Woodson M., et al. Discovery and crystallographic studies of trisubstituted piperazine derivatives as non-covalent SARS-CoV-2 main protease inhibitors with high target specificity and low toxicity. J. Med. Chem. 2022;65:13343–13364. doi: 10.1021/acs.jmedchem.2c01146. [DOI] [PubMed] [Google Scholar]
- 126.Yu W., Zhao Y., Ye H., Wu N., Liao Y., Chen N., et al. Structure-Based Design of a Dual-targeted covalent inhibitor against papain-like and main proteases of SARS-CoV-2. J. Med. Chem. 2022;65:16252–16267. doi: 10.1021/acs.jmedchem.2c00954. [DOI] [PubMed] [Google Scholar]
- 127.Dai W., Jochmans D., Xie H., Yang H., Li J., Su H., et al. Design, synthesis, and biological evaluation of Peptidomimetic aldehydes as broad-Spectrum inhibitors against enterovirus and SARS-CoV-2. J. Med. Chem. 2021;65:2794–2808. doi: 10.1021/acs.jmedchem.0c02258. [DOI] [PubMed] [Google Scholar]
- 128.Qiao J., Li Y.S., Zeng R., Liu F.L., Luo R.H., Huang C., et al. SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science. 2021;371:1374–1378. doi: 10.1126/science.abf1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Quan B.X., Shuai H., Xia A.J., Hou Y., Zeng R., Liu X.L., et al. An orally available Mpro inhibitor is effective against wild-type SARS-CoV-2 and variants including Omicron. Nat. Microbiol. 2022;7:716–725. doi: 10.1038/s41564-022-01119-7. [DOI] [PubMed] [Google Scholar]
- 130.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3:e324. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Z., Wang N., Yang L., Song X.Q. Bioactive natural products in COVID-19 therapy. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.926507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang L., Wang Z. Natural products, alone or in combination with FDA-approved drugs, to treat COVID-19 and lung cancer. Biomedicines. 2021;9:689. doi: 10.3390/biomedicines9060689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Medina-Enríquez M.M., Lopez-León S., Carlos-Escalante J.A., Aponte-Torres Z., Cuapio A., Wegman-Ostrosky T. ACE2: the molecular doorway to SARS-CoV-2. Cell Biosci. 2020;10:148. doi: 10.1186/s13578-020-00519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Y., Zhou W., Yang L., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brevini T., Maes M., Webb G.J., John B.V., Fuchs C.D., Buescher G., et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2023;615:134–142. doi: 10.1038/s41586-022-05594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao M.M., Yang W.L., Yang F.Y., Zhang L., Huang W.J., Hou W., et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Tar. 2021;6:134. doi: 10.1038/s41392-021-00558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ashhurst A.S., Tang A.H., Fajtová P., Yoon M.C., Aggarwal A., Bedding M.J., et al. Potent anti-SARS-CoV-2 activity by the natural product gallinamide A and analogues via inhibition of Cathepsin L. J. Med. Chem. 2021;65:2956–2970. doi: 10.1021/acs.jmedchem.1c01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dinda B., Dinda M., Dinda S., Chakraborty M. Some natural compounds and their analogues having potent anti-SARS-CoV-2 and anti-proteases activities as lead molecules in drug discovery for COVID-19. Eur. J. Med. Chem. Rep. 2022;6 doi: 10.1016/j.ejmcr.2022.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sachse M., Tenorio R., de Castro I.F., Muñoz-Basagoiti J., Perez-Zsolt D., Raïch-Regué D., et al. Unraveling the antiviral activity of plitidepsin against SARS-CoV-2 by subcellular and morphological analysis. Antivir. Res. 2022;200 doi: 10.1016/j.antiviral.2022.105270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.White K.M., Rosales R., Yildiz S., Kehrer T., Miorin L., Moreno E., et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science. 2021;371:926–931. doi: 10.1126/science.abf4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xie X., Lu S., Pan X., et al. Antiviral bafilomycins from a feces-inhabiting Streptomyces sp. J. Nat. Prod. 2021;84:537–543. doi: 10.1021/acs.jnatprod.0c01243. [DOI] [PubMed] [Google Scholar]
- 144.Li G.F., An X.X., Yu Y., Jiao L.R., Canarutto D., Yu G., et al. Do proton pump inhibitors influence SARS-CoV-2 related outcomes? A meta-analysis. Gut. 2021;70:1806–1808. doi: 10.1136/gutjnl-2020-323366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailly C. Cepharanthine: an update of its mode of action, pharmacological properties and medical applications. Phytomedicine. 2019;62 doi: 10.1016/j.phymed.2019.152956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Okamoto M., Ono M., Baba M. Potent inhibition of HIV type 1 replication by an antiinflammatory alkaloid, cepharanthine, in chronically infected monocytic cells. AIDS Res. Hum. Retrovir. 1998;14:1239–1245. doi: 10.1089/aid.1998.14.1239. [DOI] [PubMed] [Google Scholar]
- 147.Zhang C.H., Wang Y.F., Liu X.J., Lu J.H., Qian C.W., Wan Z.Y., et al. Antiviral activity of cepharanthine against severe acute respiratory syndrome coronavirus in vitro. Chin. Med. J. 2005;118:493–496. [PubMed] [Google Scholar]
- 148.Kim D.E., Min J.S., Jang M.S., Lee J.Y., Shin Y.S., Song J.H., et al. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9:696. doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fan H.H., Wang L.Q., Liu W.L., An X.P., Liu Z.D., He X.Q., et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin. Med. J. 2020;133:1051–1056. doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., et al. Multidrug treatment with nelfinavir and cepharanthine against COVID-19. bioRxiv. 2020 doi: 10.1101/2020.04.14.039925. [DOI] [Google Scholar]
- 151.White M.A., Lin W., Cheng X. Discovery of COVID-19 inhibitors targeting the SARS-CoV-2 Nsp13 helicase. J. Phys. Chem. Lett. 2020;11:9144–9151. doi: 10.1021/acs.jpclett.0c02421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Perez-Lemus G.R., Menéndez C.A., Alvarado W., Byléhn F., de Pablo J.J. Toward wide-spectrum antivirals against coronaviruses: molecular characterization of SARS-CoV-2 NSP13 helicase inhibitors. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abj4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li S., Liu W., Chen Y., Wang L., An W., An X., et al. Transcriptome analysis of cepharanthine against a SARS-CoV-2-related coronavirus. Briefings Bioinf. 2021;22:1378–1386. doi: 10.1093/bib/bbaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He C.L., Huang L.Y., Wang K., Gu C.J., Hu J., Zhang G.J., et al. Identification of bis-benzylisoquinoline alkaloids as SARS-CoV-2 entry inhibitors from a library of natural products. Signal Transduct. Tar. 2021;6:131. doi: 10.1038/s41392-021-00531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Drayman N., DeMarco J.K., Jones K.A., Azizi S.A., Froggatt H.M., Tan K., et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science. 2021;373(6557):931–936. doi: 10.1126/science.abg5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hijikata A., Shionyu‐Mitsuyama C., Nakae S., Shionyu M., Ota M., Kanaya S., et al. Evaluating cepharanthine analogues as natural drugs against SARS‐CoV‐2. EBS Open Bio. 2022;12:285–294. doi: 10.1002/2211-5463.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang S., Huang W., Ren L., Ju X., Gong M., Rao J., et al. Comparison of viral RNA–host protein interactomes across pathogenic RNA viruses informs rapid antiviral drug discovery for SARS-CoV-2. Cell Res. 2022;32:9–23. doi: 10.1038/s41422-021-00581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shih P.C., Mao Y.W., Hu J.W., Hsieh H.Y., Shih T.M., Lu L.P., et al. Development of ultrapure and potent tannic acids as a pan-coronal antiviral therapeutic. ACS Pharmacol. Transl. Sci. 2022;5(2022):400–412. doi: 10.1021/acsptsci.1c00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang H., Yang M., Ding Y., Liu Y., Lou Z., Zhou Z., et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Iketani S., Mohri H., Culbertson B., Hong S.J., Duan Y., Luck M.I., et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature. 2023;613:558–564. doi: 10.1038/s41586-022-05514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Focosi D., Maggi F., McConnell S., Casadevall A. Very low levels of remdesivir resistance in SARS-COV-2 genomes after 18 months of massive usage during the COVID19 pandemic: a GISAID exploratory analysis. Antivir. Res. 2022;198 doi: 10.1016/j.antiviral.2022.105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hu Y., Lewandowski E.M., Tan H., Zhang X., Morgan R.T., Zhang X., et al. Naturally occurring mutations of SARS-CoV-2 main protease confer drug resistance to nirmatrelvir. 2022. [DOI] [PMC free article] [PubMed]
- 163.Gandhi S., Klein J., Robertson A.J., Peña-Hernández M.A., Lin M.J., Roychoudhury P., et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. Nat. Commun. 2022;13:1547. doi: 10.1038/s41467-022-29104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou Y., Gammeltoft K.A., Ryberg L.A., Pham L.V., Tjørnelund H.D., Binderup A., et al. Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system. Sci. Adv. 2022;8 doi: 10.1126/sciadv.add7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.