Abstract
Background:
The National Institute on Aging-Alzheimer’s Association Research Framework proposes defining Alzheimer’s disease by grouping imaging and fluid biomarkers by their respective pathologic processes. The AT(N) structure proposes several neurodegenerative fluid biomarkers (N) including total tau (t-tau), neurogranin (Ng), and neurofilament light chain (NfL). However, pathologic drivers influencing each biomarker remain unclear.
Objective:
To determine whether CSF-neurodegenerative biomarkers (N) map differentially to Alzheimer’s pathology measured by Aβ42 (an indicator of amyloidosis, [A]), p-tau (an indicator of tau deposition, [T]) and MRI vascular pathology indicators (measured by white-matter integrity, infarcts, and microbleeds [V]).
Methods:
Participants were from Mayo Clinic Study of Aging (MCSA) with CSF measures of NfL, Ng, t-tau, Aβ42 and p-tau and available MRI brain imaging. Linear models assessed associations between CSF neurodegeneration (N) markers, amyloid markers (A), tau (T), and vascular pathology (V).
Results:
Participants (n=408) had a mean age of 69.2±10.7; male, 217 (53.2%); cognitively unimpaired, 359 (88%). All three neurodegeneration biomarkers correlated with age (p<0.001 for NfL and t-tau, p=0.018 for Ng). Men had higher CSF-NfL levels; women had higher Ng (p<0.001). NfL and t-tau levels correlated with infarcts (p=0.009, p=0.034 respectively); no biomarkers correlated with white-matter integrity. N biomarkers correlated with p-tau levels (T, p<0.001). Higher Aβ42 levels associated with higher N-biomarker levels but only among cognitively unimpaired (A, p<0.001).
Conclusions:
The influence of vascular pathology in the general population on CSF (N) biomarkers is modest, with greater influence of infarcts than white-matter disruption. Neurodegeneration markers more closely correlated with tau than amyloid markers.
Keywords: Alzheimer’s, amyloid, cerebrospinal fluid, neurodegeneration, neurofilament light chain, neurogranin, total tau, white matter integrity
Introduction
The AT(N) system of the National Institute on Aging-Alzheimer’s Association Research Framework [1] includes markers of neurodegeneration. By definition, these markers are non-specific to Alzheimer’s disease but are a measure of severity in that they are more proximate in time and causality to cognitive decline. Multiple pathologies can impact each marker of neurodegeneration. In this study, we examined three commonly investigated fluid biomarkers of neurodegeneration (N) = total tau (t-tau), neurogranin (Ng), and neurofilament light chain (NfL). It has been shown that these three markers correlate and are elevated in Alzheimer’s Disease (AD) [2, 3], suggesting that they reflect some common aspects of neurodegeneration. CSF t-tau is a marker of neurodegeneration thought to reflect the intensity of neuronal damage (N), whereas CSF p-tau, a marker of the phosphorylated tau found in neurofibrillary tangles, is an estimate of tau pathology (T). Ng is a neuronal-specific postsynaptic protein and marker of synaptic integrity and function [4]. While CSF t-tau and p-tau levels are highly correlated, t-tau has also been shown to be elevated in non-Alzheimer’s pathologies [5, 6], highlighting the need to identify different pathological influences on CSF biomarkers. Among the (N) biomarkers, t-tau and Ng, are thought to be more closely associated with AD-related synaptic and neuronal degeneration [7–10]. On the other hand, NfL, a useful neurodegeneration marker reflecting axonal degeneration and white matter disruption [11], is considered non-specific and independent of amyloid pathology [7, 12, 13].
Data on associations between N CSF biomarkers and vascular impairment vary, depending on the type of vascular pathology investigated, the disease severity, and the population analyzed [14]. Compared to other N biomarkers, NfL has been found to be more closely related to vascular risk factors, small vessel disease and stroke history [12, 15]. Previous studies demonstrated higher levels of CSF t-tau in association with vascular impairment, multiple microbleeds [16] and after acute strokes [17] but without any obvious link to white matter hyperintensities [7, 18, 19]. Although CSF Ng levels correlate with the volume of acute cerebral ischemia [20], Ng is considered a promising marker of synaptic dysfunction rather than vascular pathology [21].
Focusing on the overlap of AD and cerebrovascular disease, the objective of this study was to determine the associations between AD (A, T) and vascular pathologies (V) with different (N) markers in a community-based sample. We hypothesized that NfL levels were more influenced by vascular brain changes compared to t-tau and Ng.
Methods
The study, which was done in accord with the Helsinki Declaration of 1975, was approved by Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Written informed consent was obtained from all participants.
Participants
We included participants from the Mayo Clinic Study of Aging (MCSA). The MCSA is a population-based, longitudinal study examining the epidemiology of cognitive decline and risk of mild cognitive impairment (MCI) among residents living in Olmsted County, Minnesota, which began enrolling in 2004. Details of the study and its design were previously described [22]. In short, the MCSA includes 15-month interval evaluations done by a study coordinator, a physician, and a psychometrist. Final clinical diagnoses are established by consensus using previously published criteria [23, 24]. The current study included 408 participants with available MRI data as well as cerebrospinal fluid (CSF) measures.
CSF analyses
CSF samples were collected via lumbar puncture. The collected CSF was transported to the lab in polypropylene tubes, aliquoted, and stored at −80°C without any additional freeze-thaw cycles until use. Aβ42, t-tau, and hyperphosphorylated tau- 181 (p-tau) were analyzed using Elecsys β-amyloid (1–42) CSF, Elecsys Total-Tau CSF, and Elecsys Phospho-Tau (181P) CSF electrochemiluminescence immunoassays (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) [25–27]; these assays are approved for clinical use in countries that accept the Conformité Européenne (CE) mark. These immunoassays are for investigational purposes and are not yet approved for clinical use in the United States. As previously described, the quality control process included a validation method of precision and accuracy prior to beginning the analysis, as well as using Elecsys PreciControl samples throughout the trial to monitor the quality of the results [28]. In-house developed enzyme-linked immunosorbent assays (ELISAs) were used to measure CSF NfL and Ng concentrations; assay characteristics and methods have been described elsewhere [29–31].
Imaging measures
Structural MRI was acquired using standardized magnetization-prepared, rapid-gradient echo (MPRAGE) sequences on 3-Tesla GE scanners (GE Medical Systems). FreeSurfer version 5.3 was run on the MPRAGE scans. Diffusion-tensor imaging sequences were processed and analyzed for fractional anisotropy of the genu of the corpus callosum (FA-GCC) using standardized processing that was previously published [32, 33]. In addition to its low measurement variability, the anterior part (genu) of the corpus callosum provides information about diffusion directionality and may be more sensitive to small-vessel disease as a cerebrovascular biomarker [33]. Two-dimensional FLAIR image was used to detect WMH using a semi-automated method, as explained previously [34, 35]. In short, FLAIR images were used to identify possible WMH voxels using a clustering method, and information from MPRAGE image was incorporated to reduce false positives. Total WMH estimated volume was then edited by a trained imaging analyst for accuracy and scaled as the percentage of total intracranial volume (TIV). Recognition and grading of infarcts was done as previously described [36] using FLAIR MRI co-registered to the MPRAGE scan. Cerebral microbleeds (CMB) were recognized and computed as previously described [37] in agreement to consensus criteria [38, 39] as homogeneous hypointense lesions in the gray or white matter, which are distinct from vessel flow voids on T2* GRE images. All possible infarcts and CMB were initially identified by trained image analysts and confirmed by a vascular neurologist to whom all clinical information was masked.
Assessment of vascular pathology (V)
The (V) component was based on MRI markers of cerebrovascular disease (CVD), as previously described [32]. In brief, we used a previously validated principle component analyses, with imaging assessments of CVD from T2* GRE, FLAIR, and diffusion MRI including FAGCC and WMH. As a quantitative indicator of white-matter microstructure degeneration, the PC1 variable is a weighted sum of the deep and periventricular regional WMH score and White Matter Integrity (FAGCC) score. An increase in WMH as well as a decrease in FAGCC was reflected by a higher PC1 score. The continuous number of microbleeds and continuous number of infarctions were binned into two components (PC2 and PC3). PC2 was calculated based on the total number of deep and lobar microbleeds. PC3 corresponded to the number of cortical and subcortical infarcts recognized on imaging as described above.
Statistical analyses
Continuous demographic and clinical data were summarized using means and standard deviations; categorical variables were summarized as counts and percentages. NfL measures were transformed using a natural logarithm transformation to normalize the distributions. One outlier with an extreme CSF NfL value (>30,000 pg/mL) was excluded from the analyses. Univariate and multivariate linear models were conducted to associate outcomes with each predictor of interest (N biomarkers: t-tau, Nfl, Ng). All multivariate models were adjusted for age and sex. To address the curvature in p-tau CSF levels, quadratic terms were included as necessary in the final models to account for non-linear relationships (p-tau2). Parsimonious models were produced through a backward-elimination process.
Results
Participants
The characteristics of the 408 participants with CSF measures and available MRI scans are shown in Table 1. The mean age was 69.19 (±10.71); there were 217 men (53.19%). A total of 359 (87.99%) participants were cognitively unimpaired, 46 (11.27%) had MCI, and three (0.74%) had dementia. There were 210 (51.4%) participants with CSF Aβ42<1026 pg/mL, and 130 (31.9%) participants with elevated CSF p-tau levels (p-tau>21 pg/mL). Sixty-four (15.69%) participants had infarcts; 50 (12.2%) had evidence on neuroimaging of cerebral microbleeds and 59 (14.46%) had high WMH burden (WMH/TIV >1.7% [40], correlative to Fazekas 3+).
Table 1.
Participant Demographics by Cognitive Status
| Cognitively Unimpaired, N = 359 | Cognitively Impaired, N = 49 | P-value | |
|---|---|---|---|
|
| |||
| Age, yrs. | 68.03 (10.57) | 77.45 (7.69) | <0.001 |
| Male | 191.00 (53.20%) | 26.00 (53.06%) | >0.9 |
| Education, yrs. | 14.70 (2.47) | 13.43 (3.56) | 0.009 |
| CSF Aβ42 (pg/mL) | 1,077.46 (409.97) | 839.14 (463.97) | <0.001 |
| CSF T-tau (pg/mL) | 218.11 (83.45) | 275.54 (108.06) | <0.001 |
| CSF P-tau (pg/mL) | 18.89 (7.62) | 24.55 (11.65) | <0.001 |
| CSF NfL (pg/mL) | 6.19 (0.63) | 6.61 (0.66) | <0.001 |
| Neurogranin (pg/mL) | 175.80 (65.33) | 196.08 (63.66) | 0.036 |
| Vascular measures* | |||
| PC1 | −0.87 (0.63) | −0.30 (0.55) | <0.001 |
| PC2 | −0.30 (1.39) | −0.48 (1.18) | 0.12 |
| PC3 | 0.28 (0.85) | 0.36 (0.77) | 0.2 |
| WMH | 0.01 (0.01) | 0.02 (0.01) | <0.001 |
| Any Infarction | 53 (15%) | 11 (22%) | 0.2 |
| Cortical Infarction | 0.07 (0.41) | 0.14 (0.46) | 0.037 |
| Subcortical Infarction | 0.22 (0.68) | 0.22 (0.51) | 0.4 |
| FA GCC | 0.61 (0.04) | 0.57 (0.06) | <0.001 |
Mean (SD); n (%)
Vascular measures were calculated as follows: PC1 is a weighted sum of the WMH score and White Matter Integrity (FA-GCC) score; PC2 is based on total number of deep and lobar microbleeds; PC3 is based on total number of infarcts. WMH is presented as percentage of total intracranial volume (TIV).
Abbreviations: MCI, Mild Cognitive Impairment; t-tau, total tau; p-tau, phosphorylated tau; NfL, neurofilament light chain; FA GCC, Fractional anisotropy for the genu of Corpus Collosum.
Associations of CSF N markers with AD CSF biomarkers and vascular pathology
Results from univariate linear models are shown in Table 2. The associations between demographic variables and markers of amyloid (Aβ42), tau (p-tau), vascular pathology (PC1 and PC3), and CSF N biomarkers are shown in Table 3. Additional analyses, including a vascular component comprised of microbleeds (PC2), did not reveal significant associations and was therefore excluded from the presented models.
Table 2.
Linear regression models of CSF neurodegenerative biomarkers predicting Alzheimer’s and vascular pathology
| NfL | Neurogranin | T-Tau | |
|---|---|---|---|
| Aβ42 | <0.001, p =0.729 | 0.044, p <0.001 | 0.0311, p =0.003 |
| P-Tau | 0.0315, p <0.001 | 6.108, p <0.001 | 10.272, p <0.001 |
| PC1* | 0.324, p <0.001 | 7.107, p =0.153 | 34.945, p <0.001 |
| PC3† | 0.185, p <0.001 | 7.278, p =0.059 | 14.533, p =0.005 |
Coefficients presented are regression coefficients from univariate models.
PC1 is a weighted sum of WMH score and White Matter Integrity (FA-GCC) score.
PC3 is based on total number of infarcts.
Table 3.
Associations between CSF neurodegeneration biomarkers and Alzheimer’s and vascular pathology biomarkers using full linear high order and parsimonious models
| NfL | Neurogranin | T-Tau | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Models | Parsimonious Models | Full Models | Parsimonious Models | Full Models | Parsimonious Models | |||||||||||||
| Predictors | Coefficient | P | Partial R 2 | Coefficient | P | Partial R 2 | Coefficient | P | Partial R 2 | Coefficient | P | Partial R 2 | Coefficient | P | Partial R 2 | Coefficient | P | Partial R 2 |
|
| ||||||||||||||||||
| (Intercept) | 4.27 | <0.001 | 4.29 | <0.001 | 196.34 | <0.001 | 212.29 | <0.001 | 192.01 | <0.001 | 181.62 | <0.001 | ||||||
| Age | 0.02 | <0.001 | 0.071 | 0.02 | <0.001 | 0.12 | −0.57 | 0.018 | 0.014 | −0.76 | <0.001 | 0.040 | 0.24 | 0.049 | 0.010 | 0.36 | <0.001 | 0.033 |
| Male | 0.23 | <0.001 | 0.045 | 0.23 | <0.001 | 0.043 | −14.46 | <0.001 | 0.041 | −13.60 | <0.001 | 0.038 | −0.30 | 0.862 | <0.001 | |||
| Aβ42 | 0.00 | 0.107 | 0.006 | 0.02 | <0.001 | 0.064 | 0.02 | <0.001 | 0.071 | 0.02 | <0.001 | 0.14 | 0.02 | <0.001 | 0.13 | |||
| PTAU | 0.02 | <0.001 | 0.071 | 0.02 | <0.001 | 0.078 | 1089.51 | <0.001 | 0.67 | 1090.84 | <0.001 | 0.67 | 1697.55 | <0.001 | 0.95 | 1696.59 | <0.001 | 0.95 |
| PTAU2 | −270.01 | <0.001 | −273.84 | <0.001 | −110.86 | <0.001 | −109.15 | <0.001 | ||||||||||
| PC1* | 0.05 | 0.412 | 0.002 | −5.15 | 0.162 | 0.005 | 3.03 | 0.097 | 0.007 | |||||||||
| PC3† | 0.09 | 0.009 | 0.017 | 0.10 | 0.002 | 0.023 | 1.28 | 0.550 | <0.001 | 2.26 | 0.034 | 0.011 | 2.50 | 0.018 | 0.014 | |||
| PC3*Male | 4.30 | 0.035 | 0.011 | |||||||||||||||
|
| ||||||||||||||||||
| N | 408 | 408 | 408 | 408 | 408 | 408 | ||||||||||||
| R2 | 0.341 | 0.336 | 0.727 | 0.726 | 0.963 | 0.963 | ||||||||||||
PC1 is a weighted sum of WMH score and White Matter Integrity (FA-GCC) score.
PC3 is based on total number of infarcts.
Abbreviations: NfL, neurofilament light chain; t-tau, total tau. P-tau, phosphorylated tau.
NfL
In the univariate linear models (Table 2), higher CSF NfL levels correlated with higher p-tau levels (p<0.001) but not with CSF Aβ42 levels. An association was observed between CSF NfL levels and the two vascular variables, PC1 and PC3 (p<0.001). In the multiple-regression models (Table 3), CSF NfL levels were associated with age (β=0.02, p<0.001, Partial R2=0.071) and were higher in men than women (β=0.23, p<0.001, Partial R2=0.045). CSF NfL levels were associated with CSF p-tau levels (β=0.02, p<0.001, Partial R2=0.071), but no association was found with Aβ42 (Table 3). A relationship between NfL and vascular pathology was significant for infarcts (PC3, β=0.09, p=0.009, Partial R2=0.017), but not for white-matter damage (PC1, p=0.412).
T-tau
In the univariate linear models (Table 2), CSF t-tau levels positively correlated with CSF Aβ42 levels (p=0.003), CSF p-tau (p<0.001), and both PC1 and PC3 (p<0.001, p=0.005 respectively). Multiple-regression models (Table 3) showed that CSF t-tau levels were associated with age (β= 0.24, p=0.049, Partial R2=0.010). Increased CSF t-tau levels were associated with higher CSF p-tau levels (T, β=1697.55, p<0.001, Partial R2=0.95). A weak association was observed between higher Aβ42 levels and CSF t-tau levels (A, β=0.02, p<0.001, Partial R2=0.14). Finally, higher CSF t-tau levels were associated with more infarcts (PC3, β=2.26, p=0.034, Partial R2=0.011) (Table 3).
Ng
Higher Ng levels were associated with higher p-tau levels (Table 2, p<0.001) but also with higher CSF Aβ42 level (p<0.001). There was no association between Ng levels and number of infarcts (PC3, p=0.059), or other vascular variables. Subsequent multiple-regression analyses (Table 3) showed that CSF Ng levels correlated with age (β=−0.57, p=0.018, Partial R2=0.018) and were higher in women than men (β=−14.46, p<0.001, Partial R2=0.041). Higher CSF Ng levels were closely associated with higher CSF p-tau levels (T, β=1089.51, p<0.001, Partial R2=0.67), but an association with higher CSF Aβ42 levels (A, β=0.02, p<0.001, Partial R2=0.064) was also observed. No associations were found between Ng and the measures of vascular pathology (Table 3).
As shown in Table 3, significant associations for all three biomarkers survived parsimonious model analyses involving backwards elimination of predictors until only significant predictors remain. Exclusion of the three participants diagnosed with dementia from the analyses did not change the observed associations.
Predictive plots for average male and female participants
Predictive plots based on the full regression models (Table 3) according to sex are shown in Figure 1. The predicted plots of CSF N biomarkers by sex followed similar trajectories, yet at baseline males had consistently higher CSF NfL values while females had higher Ng levels. A significant interaction was observed for infarcts (PC3) as a predictor of CSF t-tau, where an increased infarct score predicted higher t-tau levels for males but not for females (p=0.035).
Figure 1. Predicted plots of CSF N biomarkers with sex interactions.
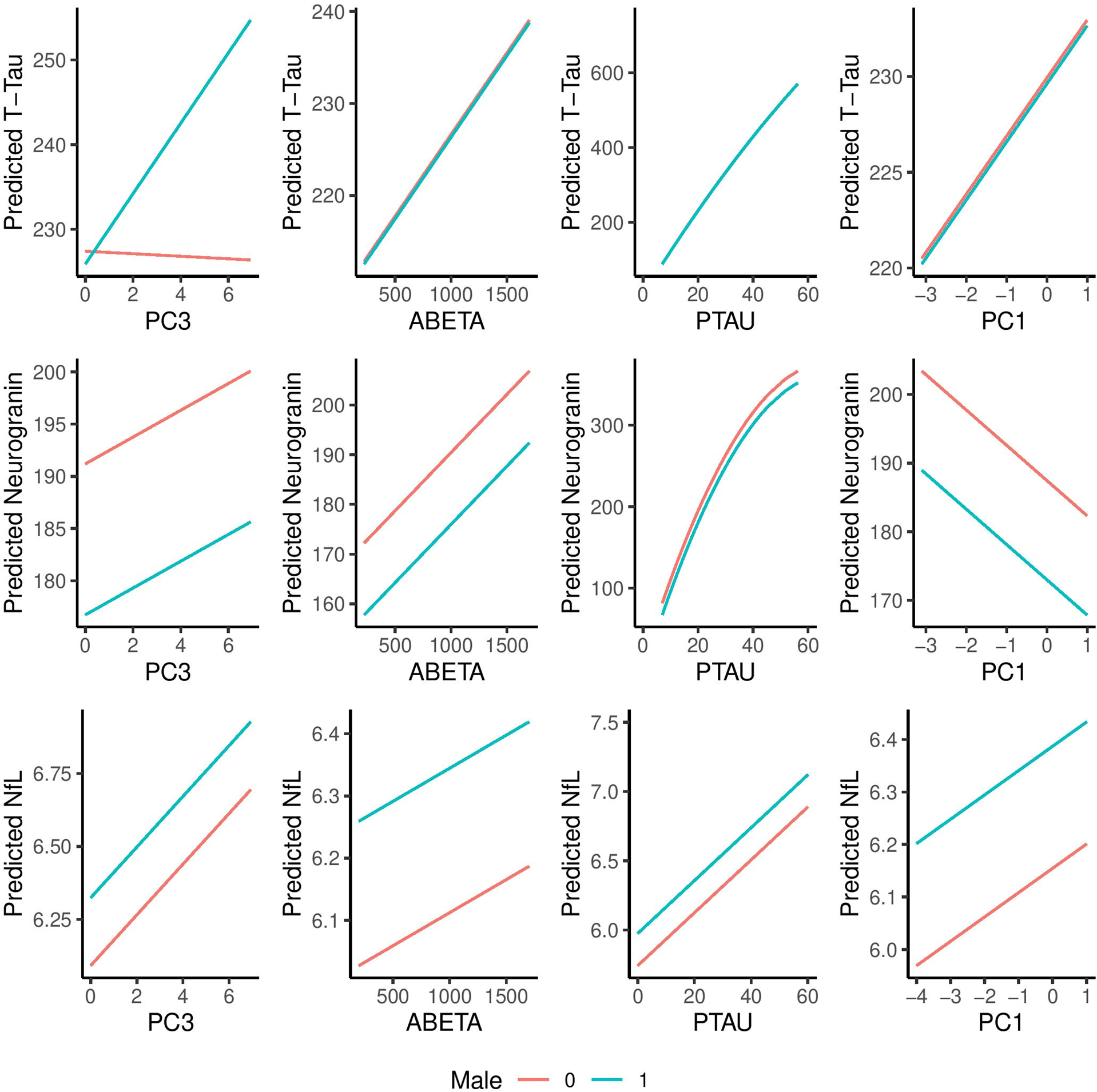
Prediction plots are based on the full linear higher order models. The curvature in the P-tau covariate reflects the higher order term (x2) in the T-tau and neurogranin models. Units for t-tau, neurogranin, NfL, ABETA, p-tau are pg/mL. PC1 is a weighted sum of the WMH score and White Matter Integrity (FA-GCC) score. PC3 is based on total number of infarcts.
Abbreviations: t-tau, total tau; p-tau, phosphorylated tau; NfL, neurofilament light chain
Associations between CSF Aβ42 and N biomarkers
To further explore the unexpected positive associations of the N markers with CSF Aβ42 levels, additional models stratified by cognitive impairment status were conducted, controlling for age (Table 4). The observed association remains in the cognitively unimpaired participants only. For cognitively impaired participants, the direction of the association between CSF AB42 levels and NfL and t-tau levels changed to negative, although this was not significant in this small subgroup.
Table 4.
Cognitive impairment-stratified models (Aβ42 age-adjusted)
| Neurogranin (CU) | Neurogranin (Impaired) | NfL (CU) | NfL (Impaired) | T-Tau (CU) | T-Tau (Impaired) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Coefficient | P | Coefficient | P | Coefficient | P | Coefficient | P | Coefficient | P | Coefficient | P |
|
| ||||||||||||
| (Intercept) | 8.06 | 0.730 | 13.72 | 0.890 | 3.85 | <0.001 | 5.13 | <0.001 | −95.33 | 0.001 | −75.06 | 0.647 |
| Aβ42 | 0.06 | <0.001 | 0.01 | 0.620 | 0.0003 | <0.001 | −0.0004 | 0.080 | 0.07 | <0.001 | −0.02 | 0.606 |
| Age | 1.48 | <0.001 | 2.26 | 0.070 | 0.03 | <0.001 | 0.02 | 0.063 | 3.55 | <0.001 | 4.74 | 0.022 |
|
| ||||||||||||
| Observations | 359 | 49 | 359 | 49 | 359 | 49 | ||||||
| R2 | 0.182 | 0.070 | 0.256 | 0.159 | 0.265 | 0.128 | ||||||
CU (n=359), Impaired (n=49)
Abbreviations: CU, cognitively unimpaired; NfL, neurofilament light chain; t-tau, total tau.
Discussion
In the present study our aim was to determine how CSF N biomarkers, NfL, t-tau and Ng differentially associate with CSF measures of Aβ42 (A) and p-tau (T) and imaging measures of vascular pathology (V). All three biomarkers increased with age; NfL levels were higher in males whereas Ng levels were higher in females. We found that cerebrovascular pathology modestly influences NfL and t-tau levels but not Ng, with infarcts having a greater impact on neurodegeneration biomarkers in comparison to white-matter disruption. All three biomarkers were associated with p-tau levels (T). Among the cognitively unimpaired participants, CSF N biomarkers were positively associated with CSF Aβ42 levels (A).
According to our findings, vascular pathology has a limited impact on CSF N biomarkers in this mostly cognitively unimpaired cohort. It appears that of the two components of V investigated, the first composed of white-matter hyperintensity and integrity scores (PC1) and the second of neuroimaging evidence of infarctions (PC3), infarctions drove the observed associations while there was no association with microbleeds (PC2). NfL is a sensitive but non-specific, amyloid-independent marker of white-matter integrity and axonal injury[13, 15], more closely associated with vascular risk factors, small vessel disease and history of stroke compared to other N biomarkers [12, 15]. Our results support these findings, with NfL levels showing the strongest association with neuroimaging evidence of infarctions. However, no association was observed between PC1 and NfL levels in mostly cognitively unimpaired individuals. In studies where participants are cognitively impaired or have greater burden of symptomatic cerebrovascular disease and, therefore, greater white-matter damage, there is an association between WMH and CSF NfL [13, 15, 41, 42]. These findings suggest that CSF NfL better captures vascular brain changes in populations with symptomatic cerebrovascular disease or more severe cognitive impairment. Similarly, CMB (PC2) were previously linked with cognitive impairment and dementia in patients with significant cerebrovascular disease [43–45], but our cohort of mostly cognitively unimpaired individuals showed no association with N biomarkers, suggesting a correlation may occur in a more severely affected population or a population where cerebrovascular disease is over-represented.
In previous studies, t-tau and Ng were better predictors of amyloid status than NfL[7, 46], but our results show an inverse relationship between these N biomarkers and CSF Aβ42 levels. Ng, a post synaptic protein localized in dendritic spines of association cortex neurons, was previously found to be elevated in AD patients as opposed to other neurodegenerative illnesses [46, 47], and elevated CSF t-tau was found to be relevant for AD diagnosis, severity, and prediction of disease progression [48, 49]. Our cohort consisted of mostly cognitively unimpaired individuals (88%). It appears that the inverse relationship observed between CSF Aβ42, Ng, and t-tau levels is driven by the large, cognitively unimpaired subgroup, as seen in the cognition-stratified analysis. In clinically or pathologically advanced populations, these two N biomarkers may be more accurate for predicting amyloid status.
As opposed to CSF NfL, the relationship between small-vessel disease and CSF t-tau has shown mixed results, with some studies showing that WMH burden serves as a modifier of atrophy rate and t-tau increase in patients with MCI [50] and others not finding these relationships [7, 18, 19]. Our results did not show an association between PC1 (WMH and white-matter integrity summation) and CSF t-tau (p=0.097), although this may reflect the low number of participants with MCI and, therefore, high Braak neurofibrillary tangle stage.
Using CSF T-tau as a marker of (N) is complicated by the fact that levels are highly correlated with CSF p- tau, a marker of (T), as previously shown [12, 51, 52]. Because CSF p-tau is considered to be specific to amyloid pathology [12], it’s possible that CSF t-tau is a N biomarker more closely related to AD. However, additional studies with a larger number of cognitively impaired individuals with more severe vascular and amyloid pathologies are needed to further elucidate the value of CSF t-tau for diagnosis or prognosis in AD.
Growing evidence indicates that cerebrovascular and AD neuropathology risk factors vary by gender [53], affecting resilience and vulnerability, and leading to complex interactions. In most studies, CSF biomarkers are usually adjusted for sex, but only a few explored sex differences [53]. These sex-specific differences are thought to be reflected in ante-mortem measurement of CSF biomarkers, with higher NfL levels in men, thought to represent vascular burden [12, 54, 55], higher measurement of t-tau and p-tau in women[56–58], and increased Ng levels in women. Even though these differences are considered more pronounced in advance disease stages [57], our results indicate a significant interaction sex effect when PC3 (infarcts count) is used as a predictor of t-tau levels in a mostly cognitively unimpaired sample, revealing an association only in males. Sex may modulate CSF t-tau elevation influenced by vascular insults.
Limitations of the study warrant consideration. First, the MCSA is a community-based study, more likely representative of the general population than other clinic-based studies, but predominantly of European ancestry, which might limit generalizability. A second limitation is the lack of CSF measures of Aβ40; therefore, our definition of (A) is based solely on Aβ42, and not on the Aβ42/40 ratio, which was shown to be a superior estimate of brain amyloid [59, 60]. Given the exploratory nature of this study, no multiple comparison correction was applied to the models.
The CSF N measures will most likely be used as measures of disease progression than for a clinical diagnosis. Our findings suggest that the CSF N biomarker changes are associated with advanced neuropathology and clinical status. CSF NfL levels may begin to be influenced at lower levels of vascular pathology compared to other N biomarkers, specifically by infarcts, and this should be considered in the clinical evaluation. Longitudinal studies with serial CSF measures are needed to better determine the relationships between CSF N measures, CSF Ab42 (A), CSF p-tau (T), and vascular pathology (V) over time.
Acknowledgements
Funding
This study was supported by funding from the National Institutes of Health (RF1 AG069052-01A1, U01 AG006786, R37 AG011378, R01 041851, R01 NS097495, and P30 AG062677) and the GHR Foundation. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676.
Footnotes
Conflict of Interest
M.M. Mielke served as a consultant to Brain Protection Company, Biogen, and LabCorp and receives research support from the National Institutes of Health and the Department of Defense. She is a Senior Associate Editor for Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association. She is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
D.S. Knopman serves on a Data Safety Monitoring Board for Biogen (fee paid to institution), the DIAN-TU study (receives personal consulting fees), Agenbio (unpaid), and an endovascular carotid reconstruction study (unpaid). He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the Alzheimer’s Disease Cooperative Study, and receives research support from the National Institutes of Health and philanthropic funds. He is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
Drs. Knopman and Mielke are Editorial Board Members of this journal but were not involved in the peer-review process nor had access to any information regarding its peer-review.
P. Vemuri received speaking fees from Miller Medical Communications, LLC, and receives research support from the National Institutes of Health.
J. Graff-Radford receives NIH funding and serves on the editorial board for Neurology. He has received payment for speaking at the American Academy of Neurology Annual meeting.
C.R. Jack serves on an independent data monitoring board for Roche, but he receives no personal compensation from any commercial entity. He receives research support from the National Institutes of Health, the GHR Foundation and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic.
R.C. Petersen is a consultant for Roche, Inc., Merck, Inc., Biogen, Inc., and Eisai, Inc. He has received payment for serving on a Data Safety Monitoring Board for Genentech, receives royalties from Oxford University Press and UpToDate, and receives research support from the National Institutes of Health.
V. J. Lowe consults for Bayer Schering Pharma, Piramal Life Sciences, Life Molecular Imaging, Eisai Inc., AVID Radiopharmaceuticals, and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI). A. Algeciras-Schimnich participates in advisory boards for Roche Diagnostics, Fujirebio Diagnostics and Siemens.
All other authors have no conflicts to report.
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author.
References
- [1].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Van Hulle C, Jonaitis EM, Betthauser TJ, Batrla R, Wild N, Kollmorgen G, Andreasson U, Okonkwo O, Bendlin BB, Asthana S, Carlsson CM, Johnson SC, Zetterberg H, Blennow K (2021) An examination of a novel multipanel of CSF biomarkers in the Alzheimer’s disease clinical and pathological continuum. Alzheimers Dement 17, 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Merluzzi AP, Vogt NM, Norton D, Jonaitis E, Clark LR, Carlsson CM, Johnson SC, Asthana S, Blennow K, Zetterberg H, Bendlin BB (2019) Differential effects of neurodegeneration biomarkers on subclinical cognitive decline. Alzheimers Dement (N Y) 5, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kvartsberg H, Portelius E, Andreasson U, Brinkmalm G, Hellwig K, Lelental N, Kornhuber J, Hansson O, Minthon L, Spitzer P, Maler JM, Zetterberg H, Blennow K, Lewczuk P (2015) Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer’s disease patients and healthy controls. Alzheimers Res Ther 7, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Knopman DS, Haeberlein SB, Carrillo MC, Hendrix JA, Kerchner G, Margolin R, Maruff P, Miller DS, Tong G, Tome MB, Murray ME, Nelson PT, Sano M, Mattsson N, Sultzer DL, Montine TJ, Jack CR Jr., Kolb H, Petersen RC, Vemuri P, Canniere MZ, Schneider JA, Resnick SM, Romano G, van Harten AC, Wolk DA, Bain LJ, Siemers E (2018) The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: Perspectives from the Research Roundtable. Alzheimers Dement 14, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Engelborghs S, De Vreese K, Van de Casteele T, Vanderstichele H, Van Everbroeck B, Cras P, Martin JJ, Vanmechelen E, De Deyn PP (2008) Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging 29, 1143–1159. [DOI] [PubMed] [Google Scholar]
- [7].Mielke MM, Przybelski SA, Lesnick TG, Kern S, Zetterberg H, Blennow K, Knopman DS, Graff-Radford J, Petersen RC, Jack CR Jr, Vemuri P (2021) Comparison of CSF neurofilament light chain, neurogranin, and tau to MRI markers. Alzheimers Dement 17, 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dulewicz M, Kulczyńska-Przybik A, Mroczko B (2020) Neurogranin and VILIP-1 as Molecular Indicators of Neurodegeneration in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Visser PJ, Reus LM, Gobom J, Jansen I, Dicks E, van der Lee SJ, Tsolaki M, Verhey FRJ, Popp J, Martinez-Lage P, Vandenberghe R, Lleó A, Molinuevo JL, Engelborghs S, Freund-Levi Y, Froelich L, Sleegers K, Dobricic V, Lovestone S, Streffer J, Vos SJB, Bos I, Smit AB, Blennow K, Scheltens P, Teunissen CE, Bertram L, Zetterberg H, Tijms BM (2022) Cerebrospinal fluid tau levels are associated with abnormal neuronal plasticity markers in Alzheimer’s disease. Mol Neurodegener 17, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Janelidze S, Hertze J, Zetterberg H, Landqvist Waldö M, Santillo A, Blennow K, Hansson O (2016) Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann Clin Transl Neurol 3, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zetterberg H, Skillbäck T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K (2016) Association of Cerebrospinal Fluid Neurofilament Light Concentration With Alzheimer Disease Progression. JAMA Neurol 73, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Skoog I, Vemuri P, Machulda MM, Graff-Radford J, Knopman DS, Jack CR Jr., Petersen RC, Kern S (2019) Comparison of variables associated with cerebrospinal fluid neurofilament, total-tau, and neurogranin. Alzheimers Dement 15, 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90, 870–881. [DOI] [PubMed] [Google Scholar]
- [14].Llorens F, Schmitz M, Ferrer I, Zerr I (2016) CSF biomarkers in neurodegenerative and vascular dementias. Prog Neurobiol 138–140, 36–53. [DOI] [PubMed] [Google Scholar]
- [15].Meeker KL, Butt OH, Gordon BA, Fagan AM, Schindler SE, Morris JC, Benzinger TLS, Ances BM (2022) Cerebrospinal fluid neurofilament light chain is a marker of aging and white matter damage. Neurobiol Dis 166, 105662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goos JD, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P, van der Flier WM (2009) Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke 40, 3455–3460. [DOI] [PubMed] [Google Scholar]
- [17].Radanovic M, Stella F, Silva LG, Talib LL, Forlenza OV (2017) Increased CSF levels of total Tau in patients with subcortical cerebrovascular pathology and cognitive impairment. Dement Neuropsychol 11, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walsh P, Sudre CH, Fiford CM, Ryan NS, Lashley T, Frost C, Barnes J (2020) CSF amyloid is a consistent predictor of white matter hyperintensities across the disease course from aging to Alzheimer’s disease. Neurobiol Aging 91, 5–14. [DOI] [PubMed] [Google Scholar]
- [19].van Waalwijk van Doorn LJC, Ghafoorian M, van Leijsen EMC, Claassen J, Arighi A, Bozzali M, Cannas J, Cavedo E, Eusebi P, Farotti L, Fenoglio C, Fortea J, Frisoni GB, Galimberti D, Greco V, Herukka SK, Liu Y, Lleó A, de Mendonça A, Nobili FM, Parnetti L, Picco A, Pikkarainen M, Salvadori N, Scarpini E, Soininen H, Tarducci R, Urbani A, Vilaplana E, Meulenbroek O, Platel B, Verbeek MM, Kuiperij HB (2021) White Matter Hyperintensities Are No Major Confounder for Alzheimer’s Disease Cerebrospinal Fluid Biomarkers. J Alzheimers Dis 79, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].De Vos A, Bjerke M, Brouns R, De Roeck N, Jacobs D, Van den Abbeele L, Guldolf K, Zetterberg H, Blennow K, Engelborghs S, Vanmechelen E (2017) Neurogranin and tau in cerebrospinal fluid and plasma of patients with acute ischemic stroke. BMC Neurol 17, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Manniche C, Simonsen AH, Hasselbalch SG, Andreasson U, Zetterberg H, Blennow K, Høgh P, Juhler M, Hejl AM (2020) Cerebrospinal Fluid Biomarkers to Differentiate Idiopathic Normal Pressure Hydrocephalus from Subcortical Ischemic Vascular Disease. J Alzheimers Dis 75, 937–947. [DOI] [PubMed] [Google Scholar]
- [22].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA (2008) The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256, 183–194. [DOI] [PubMed] [Google Scholar]
- [24].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rozga M, Bittner T, Höglund K, Blennow K (2017) Accuracy of cerebrospinal fluid Aβ1–42 measurements: evaluation of pre-analytical factors using a novel Elecsys immunosassay. Clin Chem Lab Med 55, 1545–1554. [DOI] [PubMed] [Google Scholar]
- [26].Lifke V, Kollmorgen G, Manuilova E, Oelschlaegel T, Hillringhaus L, Widmann M, von Arnim CAF, Otto M, Christenson RH, Powers JL, Shaw LM, Hansson O, Doecke JD, Li QX, Teunissen C, Tumani H, Blennow K (2019) Elecsys(®) Total-Tau and Phospho-Tau (181P) CSF assays: Analytical performance of the novel, fully automated immunoassays for quantification of tau proteins in human cerebrospinal fluid. Clin Biochem 72, 30–38. [DOI] [PubMed] [Google Scholar]
- [27].Bittner T, Zetterberg H, Teunissen CE, Ostlund RE Jr., Militello M, Andreasson U, Hubeek I, Gibson D, Chu DC, Eichenlaub U, Heiss P, Kobold U, Leinenbach A, Madin K, Manuilova E, Rabe C, Blennow K (2016) Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement 12, 517–526. [DOI] [PubMed] [Google Scholar]
- [28].van Harten AC, Wiste HJ, Weigand SD, Mielke MM, Kremers WK, Eichenlaub U, Dyer RB, Algeciras-Schimnich A, Knopman DS, Jack CR Jr., Petersen RC (2022) Detection of Alzheimer’s disease amyloid beta 1–42, p-tau, and t-tau assays. Alzheimers Dement 18, 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kern S, Syrjanen JA, Blennow K, Zetterberg H, Skoog I, Waern M, Hagen CE, van Harten AC, Knopman DS, Jack CR Jr., Petersen RC, Mielke MM (2019) Association of Cerebrospinal Fluid Neurofilament Light Protein With Risk of Mild Cognitive Impairment Among Individuals Without Cognitive Impairment. JAMA Neurol 76, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kvartsberg H, Lashley T, Murray CE, Brinkmalm G, Cullen NC, Höglund K, Zetterberg H, Blennow K, Portelius E (2019) The intact postsynaptic protein neurogranin is reduced in brain tissue from patients with familial and sporadic Alzheimer’s disease. Acta Neuropathol 137, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gaetani L, Höglund K, Parnetti L, Pujol-Calderon F, Becker B, Eusebi P, Sarchielli P, Calabresi P, Di Filippo M, Zetterberg H, Blennow K (2018) A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vemuri P, Graff-Radford J, Lesnick TG, Przybelski SA, Reid RI, Reddy AL, Lowe VJ, Mielke MM, Machulda MM, Petersen RC, Knopman DS, Jack CR Jr. (2021) White matter abnormalities are key components of cerebrovascular disease impacting cognitive decline. Brain Commun 3, fcab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vemuri P, Lesnick TG, Przybelski SA, Graff-Radford J, Reid RI, Lowe VJ, Zuk SM, Senjem ML, Schwarz CG, Gunter JL, Kantarci K, Machulda MM, Mielke MM, Petersen RC, Knopman DS, Jack CR Jr. (2018) Development of a cerebrovascular magnetic resonance imaging biomarker for cognitive aging. Ann Neurol 84, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Rabinstein AA, Gunter JL, Senjem ML, Przybelski SA, Lesnick T, Ward C, Mielke MM, Lowe VJ, Petersen RC, Kremers WK, Kantarci K, Jack CR, Vemuri P (2019) White matter hyperintensities: relationship to amyloid and tau burden. Brain 142, 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, Senjem ML, Gunter JL, Vemuri P, Jack CR Jr., Miller VM, Kantarci K (2013) Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology 80, 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Graff-Radford J, Aakre JA, Knopman DS, Schwarz CG, Flemming KD, Rabinstein AA, Gunter JL, Ward CP, Zuk SM, Spychalla AJ, Preboske GM, Petersen RC, Kantarci K, Huston J, Jack CR Jr., Mielke MM, Vemuri P (2020) Prevalence and Heterogeneity of Cerebrovascular Disease Imaging Lesions. Mayo Clin Proc 95, 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Graff-Radford J, Botha H, Rabinstein AA, Gunter JL, Przybelski SA, Lesnick T, Huston J, 3rd, Flemming KD, Preboske GM, Senjem ML, Brown RD Jr., Mielke MM, Roberts RO, Lowe VJ, Knopman DS, Petersen RC, Kremers W, Vemuri P, Jack CR Jr., Kantarci K (2019) Cerebral microbleeds: Prevalence and relationship to amyloid burden. Neurology 92, e253–e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM (2009) Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12, 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Scharf EL, Graff-Radford J, Przybelski SA, Lesnick TG, Mielke MM, Knopman DS, Preboske GM, Schwarz CG, Senjem ML, Gunter JL, Machulda M, Kantarci K, Petersen RC, Jack CR Jr., Vemuri P (2019) Cardiometabolic Health and Longitudinal Progression of White Matter Hyperintensity: The Mayo Clinic Study of Aging. Stroke 50, 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mattsson N, Andreasson U, Zetterberg H, Blennow K (2017) Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 74, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jonsson M, Zetterberg H, van Straaten E, Lind K, Syversen S, Edman A, Blennow K, Rosengren L, Pantoni L, Inzitari D, Wallin A (2010) Cerebrospinal fluid biomarkers of white matter lesions - cross-sectional results from the LADIS study. Eur J Neurol 17, 377–382. [DOI] [PubMed] [Google Scholar]
- [43].Gyanwali B, Shaik MA, Venketasubramanian N, Chen C, Hilal S (2019) Mixed-Location Cerebral Microbleeds: An Imaging Biomarker for Cerebrovascular Pathology in Cognitive Impairment and Dementia in a Memory Clinic Population. J Alzheimers Dis 71, 1309–1320. [DOI] [PubMed] [Google Scholar]
- [44].Li X, Yuan J, Qin W, Yang L, Yang S, Li Y, Hu W (2021) Cerebral Microbleeds Are Associated with Impairments in Executive Function and Processing Speed. J Alzheimers Dis 81, 255–262. [DOI] [PubMed] [Google Scholar]
- [45].Li X, Yuan J, Yang L, Qin W, Yang S, Li Y, Fan H, Hu W (2017) The significant effects of cerebral microbleeds on cognitive dysfunction: An updated meta-analysis. PLoS One 12, e0185145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wellington H, Paterson RW, Portelius E, Törnqvist U, Magdalinou N, Fox NC, Blennow K, Schott JM, Zetterberg H (2016) Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology 86, 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hall S, Janelidze S, Zetterberg H, Brix B, Mattsson N, Surova Y, Blennow K, Hansson O (2020) Cerebrospinal fluid levels of neurogranin in Parkinsonian disorders. Mov Disord 35, 513–518. [DOI] [PubMed] [Google Scholar]
- [48].Blennow K, Zetterberg H (2018) Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med 284, 643–663. [DOI] [PubMed] [Google Scholar]
- [49].Pedrero-Prieto CM, García-Carpintero S, Frontiñán-Rubio J, Llanos-González E, Aguilera García C, Alcaín FJ, Lindberg I, Durán-Prado M, Peinado JR, Rabanal-Ruiz Y (2020) A comprehensive systematic review of CSF proteins and peptides that define Alzheimer’s disease. Clin Proteomics 17, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tosto G, Zimmerman ME, Hamilton JL, Carmichael OT, Brickman AM (2015) The effect of white matter hyperintensities on neurodegeneration in mild cognitive impairment. Alzheimers Dement 11, 1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, Wahl S, Benzinger TLS, Holtzman DM, Morris JC, Fagan AM (2018) Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimers Dement 14, 1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mattsson N, Scholl M, Strandberg O, Smith R, Palmqvist S, Insel PS, Hagerstrom D, Ohlsson T, Zetterberg H, Jogi J, Blennow K, Hansson O (2017) (18)F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer’s disease. EMBO Mol Med 9, 1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mielke MM, Aggarwal NT, Vila-Castelar C, Agarwal P, Arenaza-Urquijo EM, Brett B, Brugulat-Serrat A, DuBose LE, Eikelboom WS, Flatt J, Foldi NS, Franzen S, Gilsanz P, Li W, McManus AJ, van Lent DM, Milani SA, Shaaban CE, Stites SD, Sundermann E, Suryadevara V, Trani JF, Turner AD, Vonk JMJ, Quiroz YT, Babulal GM (2022) Consideration of sex and gender in Alzheimer’s disease and related disorders from a global perspective. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xue M, Sun FR, Ou YN, Shen XN, Li HQ, Huang YY, Dong Q, Tan L, Yu JT (2020) Association of cerebrospinal fluid neurogranin levels with cognition and neurodegeneration in Alzheimer’s disease. Aging (Albany NY) 12, 9365–9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, Alvarez-Cermeño JC, Andreasson U, Axelsson M, Bäckström DC, Bartos A, Bjerke M, Blennow K, Boxer A, Brundin L, Burman J, Christensen T, Fialová L, Forsgren L, Frederiksen JL, Gisslén M, Gray E, Gunnarsson M, Hall S, Hansson O, Herbert MK, Jakobsson J, Jessen-Krut J, Janelidze S, Johannsson G, Jonsson M, Kappos L, Khademi M, Khalil M, Kuhle J, Landén M, Leinonen V, Logroscino G, Lu CH, Lycke J, Magdalinou NK, Malaspina A, Mattsson N, Meeter LH, Mehta SR, Modvig S, Olsson T, Paterson RW, Pérez-Santiago J, Piehl F, Pijnenburg YAL, Pyykkö OT, Ragnarsson O, Rojas JC, Romme Christensen J, Sandberg L, Scherling CS, Schott JM, Sellebjerg FT, Simone IL, Skillbäck T, Stilund M, Sundström P, Svenningsson A, Tortelli R, Tortorella C, Trentini A, Troiano M, Turner MR, van Swieten JC, Vågberg M, Verbeek MM, Villar LM, Visser PJ, Wallin A, Weiss A, Wikkelsø C, Wild EJ (2019) Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol 76, 1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD (2012) Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci 32, 8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Babapour Mofrad R, Tijms BM, Scheltens P, Barkhof F, van der Flier WM, Sikkes SAM, Teunissen CE (2020) Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology 95, e2378–e2388. [DOI] [PubMed] [Google Scholar]
- [58].Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Bennett DA, Schneider JA, Jefferson AL (2018) Sex-Specific Association of Apolipoprotein E With Cerebrospinal Fluid Levels of Tau. JAMA Neurol 75, 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Janelidze S, Zetterberg H, Mattsson N, Palmqvist S, Vanderstichele H, Lindberg O, van Westen D, Stomrud E, Minthon L, Blennow K, Hansson O (2016) CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 3, 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Campbell MR, Ashrafzadeh-Kian S, Petersen RC, Mielke MM, Syrjanen JA, van Harten AC, Lowe VJ, Jack CR Jr., Bornhorst JA, Algeciras-Schimnich A (2021) P-tau/Aβ42 and Aβ42/40 ratios in CSF are equally predictive of amyloid PET status. Alzheimers Dement (Amst) 13, e12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author.


