Abstract
We have identified genes in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 that are involved with redox control of photosynthesis and pigment-related genes. The genes, rppA (sll0797) and rppB (sll0798), represent a two-component regulatory system that controls the synthesis of photosystem II (PSII) and PSI genes, in addition to photopigment-related genes. rppA (regulator of photosynthesis- and photopigment-related gene expression) and rppB exhibit strong sequence similarity to prokaryotic response regulators and histidine kinases, respectively. In the wild type, the steady-state mRNA levels of PSII reaction center genes increased when the plastoquinone (PQ) pool was oxidized and decreased when the PQ pool was reduced, whereas transcription of the PSI reaction center genes was affected in an opposite fashion. Such results suggested that the redox poise of the PQ pool is critical for regulation of the photosystem reaction center genes. In ΔrppA, an insertion mutation of rppA, the PSII gene transcripts were highly up-regulated relative to the wild type under all redox conditions, whereas transcription of phycobilisome-related genes and PSI genes was decreased. The higher transcription of the psbA gene in ΔrppA was manifest by higher translation of the D1 protein and a concomitant increase in O2 evolution. The results demonstrated that RppA is a regulator of photosynthesis- and photopigment-related gene expression, is involved in the establishment of the appropriate stoichiometry between the photosystems, and can sense changes in the PQ redox poise.
Bacteria are very adaptable organisms that can survive in a wide variety of environmental conditions. One way in which bacteria control their response to changing environmental conditions is through the mechanism of the two-component regulatory system, which consists of a sensor kinase and a response regulator (10, 26, 34). The sensor kinase has sensor and histidine phosphotransferase domains. The sensor domain recognizes the signal and autophosphorylates a histidine residue of the phosphotransferase domain. The phosphoryl group is subsequently transferred to the aspartate residue of the cognate response regulator, which is activated by the phosphoryl group (22). The output domain of a response regulator usually is a DNA-binding module, so that the regulator functions as a transcription factor (40). The Asp phosphorylation serves to control the ability to either bind its target DNA sequence or interact with other components of the transcription machinery (26). Some two-component systems utilize more than one histidine kinase protein or response regulator, and some single proteins encompassed both of the two-component elements (40, 41).
We have used unicellular cyanobacteria with high-frequency transformation systems as model organisms to study oxygenic photosynthesis. One such organism that has become a key model system is Synechocystis sp. strain PCC 6803 (54), which has been used for many mutagenesis, molecular biological, and biophysical studies. Most importantly, the entire genome of Synechocystis sp. strain PCC 6803 has been sequenced, and we can now utilize the rapidly expanding field of genomics to study the way in which this cyanobacterium controls photosynthesis and other metabolic processes (28). The genomic sequence reveals that Synechocystis sp. strain PCC 6803 has 80 genes that are potential two-component signal transducers within the total of 3,168 potential proteins. This includes 26 genes for sensory kinase proteins that contain both the transmitter and receiver domains (34).
In oxygenic photosynthetic organisms, light-induced charge separation is carried by two photosystems, I and II (PSII and PSI), which are major pigment-protein complexes in the thylakoid membrane (5). The D1 protein of PSII is a key element in oxygenic electron transport and in light acclimation processes. D1 and the related D2 protein form a heterodimer that bind all of the cofactors essential for the transfer of electrons from the water-splitting complex to the plastoquione (PQ) pool (5). In cyanobacteria, the phycobilisome (PBS) is the major light-harvesting, multiprotein complex and is attached extrinsically to the photosynthetic membrane (25).
It has been shown that genes encoding some photosynthesis proteins are under redox control in both cyanobacteria and higher plants (1, 2, 42). The redox status of the PQ pool has been implicated as a signal which regulates gene expression during long-term acclimation to light intensity. This hypothesis proposes that the signal transduction pathway is initiated via the PQ pool redox status or the excitation pressure on PSII, thereby coupling cellular regulatory pathways controlling gene expression and enzyme activation to utilize light energy (21). The redox state of the PQ pool seems to play a pivotal role in sensing cellular status and in regulating photosynthetic capacity. The signal of the PQ redox poise may be transduced through a redox-sensing protein kinase, which then activates the response regulator by phosphorylation or dephosphorylation. The activated regulator will then, either directly or indirectly, regulate the expression of the target genes (19).
We are particularly interested in the way in which photosynthesis and other metabolic processes are controlled by the redox poise of the PQ pool (15, 33). In photosynthetic bacteria, a two-component system called RegB-RegA (Rhodobacter capsulatus) or PrrB-PrrA (R. sphaeroides) had been demonstrated to be a global signal transduction system involved in the anaerobic induction of many physiological processes. These include the synthesis of the light-harvesting, reaction center, and cytochrome components of the bacterial photosystem and the assimilation of carbon dioxide and nitrogen (6, 18, 20, 38, 43, 49). The sensor RegB (PrrB) is believed to detect changes in oxygen levels, by responding to change in either the flow of reductant or a redox carrier, and then activates the response regulator RegA (PrrA) (39). Using sequence comparisons, we have identified genes in Synechocystis sp. strain PCC 6803 that are similar to the photosynthesis response regulator and kinase genes, regA (prrA) and regB (prrB). These genes were cloned, and knockout mutants were constructed by either insertion or deletion; all of the mutants grew under photoautotrophic conditions. These mutants were analyzed under a variety of environmental conditions that lead to changes in the PQ redox poise, including photoautotrophic (light-grown cells) and photomixotrophic (light plus 5 mM glucose) conditions. We have also used photosynthetic inhibitors, such as 3-(3,4-dichlorophenyl)-1,1-dimethyl-urea (DCMU) and 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB), to alter the PQ redox poise. Through this analysis, we found one of the two-component systems, rppA (regulator of photosynthesis- and photopigment-related genes) and rppB, to be most interesting. Here we report the effects of the rppA mutation on cellular growth, photosynthetic activity, the transcriptional regulation of photosynthesis- and photopigment-related gene expression and the accumulation of PSII reaction center proteins.
MATERIALS AND METHODS
Strains, culture conditions, and pigment analysis.
Synechocystis sp. strain PCC 6803 wild type and mutants were cultured at 30°C, 40 microeinsteins (μE) m−2 s−1, in a modified BG-11 medium with 5 mM NaNO3 as a basal medium. This growth condition is referred to photoautotrophic or control. For photomixotrophic growth, 5 mM glucose was added to the basal medium. For different redox conditions, DCMU and DBMIB were added to the photoautotrophic growth culture at 10 μM (final concentration), and cells were treated for 6 h before harvesting. For different illumination experiments, cells were grown in low light (LL; 40 μE m−2 s−1) until mid- to late log phase (5 × 107 to 10 × 107 cells ml−1) and then transferred to the dark for 6 h, to medium light (ML; 200 μE m−2 s−1) for 2 days, or to high light (HL; 1000 μE m−2 s−1) for 3 h. When needed, the protein synthesis inhibitor chloramphenicol (50 μg ml−1) was added to the media.
The cell density of the cultures was determined using a Coulter Counter (Coulter Electronics Inc., Hialeah, Fla.). Chlorophyll (Chl) and phycocyanin (PC) concentrations were quantified by spectrophotometry as previously described (16, 33).
Construction of rppA and rppB mutants.
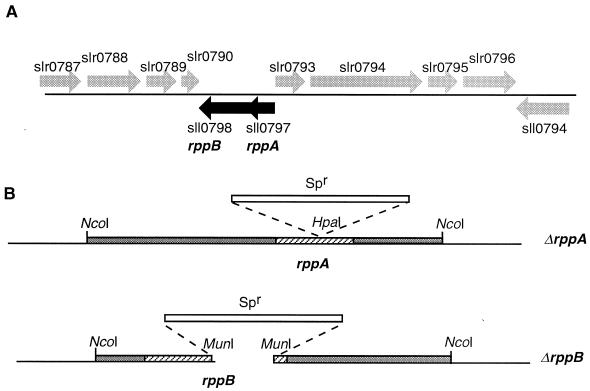
A 4.0-kb NcoI DNA fragment was cloned into plasmid pUC19 from cosmid CS1377 (a kind gift from Nakamura Yasukazu, Kazusa DNA Research Institute). The ΔrppA mutation was constructed by inserting a 2.0-kb spectinomycin resistance cassette (from plasmid pRL453) in the HpaI site. rppB was inactivated by replacing a 789-bp MunI fragment with the 2.0-kb spectinomycin resistance cassette (Fig. 1B). Wild-type Synechocystis sp. strain PCC 6803 was transformed with these plasmid constructs, and transformants were selected on plates containing the antibiotic spectinomycin (40 μg ml−1). Segregation was confirmed by Southern blotting and PCR. Characterization of the mutant was repeated at least three times for each of the parameters measured.
FIG. 1.
Structure of the rpp region and mutations of rppA and rppB: genes of Synechocystis sp. strain PCC 6803. (A) Genomic organization of the response regulator gene rppA (locus sll0797) and the adjacent sensor kinase gene rppB (locus s110798). (B) Genetic construction of ΔrppA and ΔrppB. The rppA gene was interrupted by insertion of a spectinomycin resistance (Spr) cassette in the HpaI site; the rppB gene was inactivated by replacement of a 789-bp MunI-MunI fragment with the spectinomycin resistance cassette.
Determination of oxygen evolution rate.
Photosynthesis activity was determined by measuring O2 production and consumption using a Clark electrode (model 5331; Yellow Springs Instruments, Yellow Springs, Ohio), with an amplifier in a cuvette thermostatted at 30°C. Actinic light was provided by a fiber-optic illuminator filtered through a CS 2-63 red filter to minimize photoinhibition. The cell suspension was supplemented with 10 mM NaHCO3 (final concentration) as the terminal electron acceptors before measurement. The rate of O2 production was obtained by adding the rates of O2 evolution in the light and O2 consumption in the dark due to photorespiration.
RNA isolation and Northern analysis.
Total RNA was extracted and purified using phenol-chloroform extraction and CsCl2 gradient purification as previously described (16, 44); 10 μg of total RNA was fractionated by electrophoresis on a 1.0% agarose gel with 0.6 M formaldehyde. RNA was transferred to a nylon membrane as previously described (46) and fixed by baking at 80°C for 2 h in a vacuum oven. The blots were hybridized with α-32P-labeled DNA probes prepared by random primer labeling using a Ready-To-Go kit (Pharmacia Biotech, Piscataway, N.J.). Hybridization was performed at 41 to 42°C with 50% formamide. The equal loading of total RNA was standardized by hybridization with a Synechocystis sp. strain PCC 6803 rRNA probe after stripping the previously hybridized probe. The Northern blot experiments were repeated at least twice for each gene with mRNA isolated from two separate experiments.
DNA probes and primers.
The following homologous probes from Synechocystis sp. strain PCC 6803 were used: 0.73-kb EcoRI fragment of psbB (plasmid pSL158), 0.44-kb BstEII fragment of psbD (plasmid pKW1344), 0.70-kb EcoRI fragment of psaC (plasmid TOPO-psaC), 0.75-kb EcoRI fragment of psaD (plasmid TOPO-psaD), 0.68-kb ApaI-HindIII fragment of psaLI (plasmid TOPO-psaLI), 0.98-kb HindIII fragment of apcABC (plasmid TOPO-apcABC), and 0.44-kb HincII fragment of nblA (plasmid TOPO-nblA). The three heterologous probes used were a 0.60-kb BstEII fragment of psbA from Synechococcus sp. strain PCC 7942 (plasmid pSG200), a 2.8-kb EcoRI-BglII fragment of psaAB from Synechococcus sp. strain PCC 7002 (plasmid pAQPR80), and a 1.2-kb SmaI-XhoI fragment of cpcBA from Synechococcus sp. strain PCC 7002 (plasmid pAQPR1).
The following primers were used for cloning genes by PCR from Synechocystis sp. strain PCC 6803: psaC forward (5′-GCCTAGCTTTGGTCGAAAATCG-3′) and reverse (5′-CGCCGCCAGTCTAACTTTTG-3′), psaD forward (5′-CACAGAAGTCCCCATAATCTCCTTG-3′) and reverse (5′-CCAACATTGAAAGAGCGAACTGTC-3′), psaLI forward (5′-CGTGCGTAAAATGGGGACTAAAG-3′) and reverse (5′-CGAATCGGTTCAGTCATCTTGC-3′), apcABC forward (5′-TTACGGGGGCAGTGTAATCAGG-3′) and reverse (5′-TGGAGCAAAACGGTTGGACG-3′), and nblA forward (5′-CCCAGAGCAACAACAAGAGTTACTG-3′) and reverse (5′-CAGGTAAGATCAAGTTTGCGGC-3′). All primers were synthesized by Integrated DNA Technologies, Inc. PCR products were cloned into pCR2.1-TOPO vector (Invitrogen).
Thylakoid membrane isolation and Western blot analysis.
Cells were grown under photomixotrophic conditions and harvested at mid- to late log phase. Thylakoid membranes were isolated as previously described (55, 56), with some modifications. Briefly, cell pellets were resuspended in 1 ml of inhibitor buffer (50 mM morpholine ethanesulfonic acid-NaOH [pH 6.5], 50 mM CaCl2, 0.3 M sucrose, 2 mM ɛ-aminocaproic acid, 2 mM phenylmethylsulfonyl fluoride). About 0.5 ml of glass beads (0.1-mm diameter) was added after transfer of the cyanobacterial cells to a 2-ml microcentrifuge tube. Cells were broken in a Braun homogenizer by four bursts of 30-s duration at high speed and centrifuged at 1,600 × g for 10 min to remove cell debris. The supernatant was centrifuged at 13,000 × g for 20 min to pellet the thylakoid membrane. The pellet was resuspended in inhibitor buffer, and 5 μg of Chl of each sample was solubilized on ice for 10 min, loaded into a lithium dodecyl sulfate (LDS)–10 to 20% polyacrylamide gradient gel, and separated by polyacrylamide gel electrophoresis (PAGE) at 1.5 W at 4°C for 16 to 18 h.
Protein pulse-chase experiment with [35S]methionine.
Pulse-labeling was performed to detect the synthesis of D1 protein in wild-type and ΔrppA cells. Cells were grown under LL and photomixotrophic conditions and harvested at the mid- to late logarithmic growth stage by centrifugation at 1,200 × g for 10 min at room temperature. The cell pellet was resuspended in BG-11 medium without sulfate. [35S]Met was added to the medium to a final concentration of 1 μCi ml−1, and the culture was incubated under LL for 30 min. An aliquot of cells was rapidly harvested by centrifugation, and the remainder of the culture was used for the pulse-chase experiment.
Cells were spun down at room temperature and resuspended in BG-11 medium without SO42−. Unlabeled Met was added to the cells (final concentration of 1.0 mM) and transferred to HL. Cells were chilled on ice and harvested at 0.5, 1.0, 2.0, 3.0, 4.0, and 5.0 h after HL exposure. The isolation of thylakoid membranes and PAGE were the same as described above. The gel was dried at 80°C for 3 h before X-ray autoradiography.
Quantification.
Quantification of Northern blots, Western blots, and pulse-chase gels was performed with IP Lab Gel (Signal Analytics, Vienna, Va.) after scanning the information into an Apple Macintosh 9500 computer. In all cases, a relatively short exposure autoradiogram or a lightly stained gel was scanned into the computer to ensure linearity. The figures were darkened for publication to permit visualization of the lighter bands. The protein sequences were analyzed by MacVector 5.0 (Genetics Computer Group, Madison, Wis.).
RESULTS
The organization of the Synechocystis sp. strain PCC 6803 genome in the vicinity of rppA (sll0797) and rppB (sll0798) is shown in Fig. 1A. The gene map is from the Cyanobase web site (http://www.kazusa.or.jp/cyano/cyano.html), which contains the complete nucleotide sequence of Synechocystis sp. strain PCC 6803. The relative positions of 11 open reading frames near rppA and rppB are displayed. RppA is the response regulator (234 amino acids), and RppB is the cognate histidine kinase (454 amino acids). The rppA and rppB genes were inactivated by insertion of and replacement with a spectinomycin resistance cassette, respectively, as shown in Fig. 1B. The constructed plasmids were used to transform Synechocystis wild-type cells. Knockout mutants were demonstrated by Southern blots and PCR (data not shown). The growth characteristics, photosynthetic activity, and gene expression under different redox conditions had been examined in wild-type, ΔrppA, and ΔrppB cells. Since most of the detected features of the ΔrppB mutant were similar to those of the wild type, we present only results for the wild type and ΔrppA mutant. We also constructed a knockout mutant of sll0789 which had a primary sequence similar to that of RppA. The properties of this mutant was also similar to those of the wild type, thus providing a useful control.
Sequence analysis of RppA.
Amino acid alignment among RppA and several response regulators was performed by the ClustW program (Fig. 2). These response regulators have been identified as transcriptional regulators for genes involved in various metabolic responses under different stress conditions. RegA and PrrA activate the transcription of puf and puh genes in anaerobic conditions in photosynthetic bacteria (20, 49). NblR activates transcription of the nblA gene, which codes for a small polypeptide that triggers the complete degradation of PBSs in cells grown under nitrogen and sulfur deficiency (14, 48).
FIG. 2.
Alignment of RppA with other response regulators. The sequences are as follows: RppA and sll0789, Synechocystis sp. strain PCC 6803; RegA, Rhodobacter capsulatus (GenBank accession no. M64976); PrrA, R. sphaeroides (L25895); PhoB, Synechococcus sp. WH7803 (U38917); OmpR, Escherichia coli (J01656); NblR, Synechococcus sp. strain PCC 7942 (AF049128); CheY, E. coli (M13463). Identical residues are shaded in black, conserved residues are shaded in dark gray, and similar residues are shaded in light gray. Arrowheads denote the highly conserved aspartic acid (Asp8 and Asp53), threonine (Thr81), and lysine (Lys103) residues in RppA, which correspond to Asp13, Asp57, Thr87, and Lys109 in CheY, as well as to Asp20, Asp63, Thr91, and Lys113 in PrrA/RegA. Stars denote the two conserved prolines in RppA, s110789, RegA, and PrrA.
RppA demonstrated ∼35% similarity and ∼20% identity to PrrA/RegA. More importantly, the highly conserved residues Asp8, Asp53, Thr81, and Lys103 in RppA corresponded to Asp13, Asp57, Thr87, and Lys109 in CheY, as well as to Asp20, Asp63, Thr91, and Lys113 in PrrA/RegA. CheY is the response regulator of bacterial chemotaxis; a detailed three-dimensional structure and site-directed mutagenesis studies of this protein have shown that Asp13 is essential for phosphorylation and dephosphorylation (51) and that Asp57 is the phosphorylation site (47). Thr87 and Lys109 are important for the phosphorylation-induced conformational change (3, 31). This suggests that RppA has the characteristics appropriate for a bacterial response regulator.
A recent analysis has demonstrated a remarkable similarity among six RegA homologues from four photosynthetic bacteria and two nitrogen-fixing bacteria (32). The proteins from the nitrogen fixers are regulator proteins thought to be involved in sensing low pH or in the control of nitrogen fixation-associated genes. These comparisons established some key attributes of these proteins. In addition to the conserved amino acids mentioned above (Fig. 2), there are two important structural features: (i) a series of four prolines (in positions 133 to 136 in RegA), two of which are retained in the ActR from Rhizobium meliloti (52); and (ii) an extremely well conserved C-terminal region that contains a helix-turn-helix motif. The RppA protein from Synechocystis sp. strain PCC 6803 retains two of the conserved prolines, but they are separated by a 23-amino-acid segment with a third Pro in the center. Secondary structure analysis indicated that this region in both RppA and RegA models as a random coil plus some extended strand. Thus, this region in RppA may function similarly to that of RegA, despite the modified sequence. Last, our analysis using MacVector confirmed the helix-turn-helix motif in RegA as well as in NblR and indicated the possibility of a similar motif in RppA.
Physiological characterization of ΔrppA.
Synechocystis sp. strain PCC 6803 wild type and ΔrppA cells grow differently under photoautotrophic and photomixotrophic (with 5 mM glucose) conditions (Table 1). Under photoautotrophic conditions, liquid cultures of ΔrppA grew at almost the same growth rate (half-life [t1/2] ∼20 h) and had less green color than wild-type cells, a phenomenon that could also be detected directly on plates (data not shown). When glucose was present in the media, the ΔrppA strain grew faster than the wild type (t1/2 ∼9.5 versus 12 h), and cell numbers increased at least 1.5-fold over wild-type levels by the late logarithmic growth phase. Interestingly, light microscopic observations indicated that more than 90% of the wild-type cells were doublets, whereas most of the ΔrppA cells were seen as single cells. Without taking these doublets into account, ΔrppA appeared to have threefold more cells than the wild type. The measurement of cellular biomass showed that photomixotrophic ΔrppA cultures, in the mid- to late log growth phase, had approximately 25 to 70% greater wet weight than did wild-type cultures (data not shown). The whole-cell spectra showed somewhat lower Chl and PC concentrations per cell in the ΔrppA mutant. However, the PC/Chl ratio of the ΔrppA mutant is higher than that of the wild type under both growth conditions, which suggests that the mutant has a higher PSII/PSI ratio or less Chl per photosystem. The oxygen evolution activity in ΔrppA cells was slightly higher than the wild-type level under photoautotrophic growth conditions. However, when cells were cultured in LL with glucose, the photosynthesis activity of ΔrppA was about 50% higher than the wild-type level (Table 1). This difference cannot be accounted for merely on the basis of the difference in Chl concentration per cell (4.7 versus 3.9 μg of Chl/10−8 cells) and must represent an actual increase in the specific activity for PSII of approximately one-third.
TABLE 1.
Characteristics of Synechocystis sp. strain PCC 6803 wild-type and ΔrppA cellsa
| Strain | Growth conditions | Growth rate (t1/2, h) | μg of Chl/108 cells | μg of PC/108 cells | PC/Chl | O2 evolution [μmol of O2 (mg of Chl)−1 h−1] |
|---|---|---|---|---|---|---|
| Wild type | Photoautotrophic | 19 | 3.8 | 29.8 | 7.2 | 209 |
| Photomixotrophic | 12 | 4.7 | 37.8 | 8.1 | 265 | |
| ΔrppA | Photoautotrophic | 21 | 3.1 | 26.3 | 7.9 | 229 |
| Photomixotrophic | 9.5 | 3.9 | 33.6 | 8.5 | 398 |
Cells were grown under LL conditions until mid- to late log phase. Values are averages from four separate experiments.
Transcriptional regulation of PSII genes.
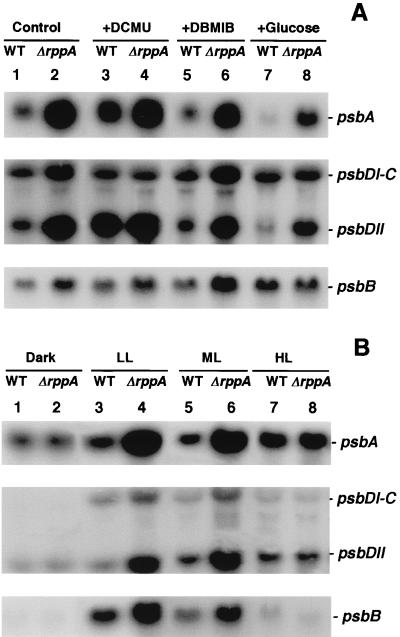
The impact of ΔrppA on transcription and on redox regulation of transcription was tested using various DNA probes against wild-type and ΔrppA total mRNAs. The electron transfer inhibitors DCMU and DBMIB were used to generate oxidizing and reducing states of the PQ pool. The cells were grown for 6 h in the presence of these compounds so that we could analyze long-term effects on cellular growth and physiology relative to transcriptional patterns. Northern blots of PSII genes against wild-type mRNA demonstrated that psbA was obviously regulated by the PQ redox conditions: up-regulated when the PQ pool was oxidized by DCMU (Fig. 3A, psbA, lanes 3 versus 1) and down-regulated when the PQ pool was reduced by DBMIB or glucose (lanes 5 and 7 versus 1). The transcription of psbA was dramatically increased in ΔrppA cells compared to the wild type. In ΔrppA cells, the psbA mRNA level was about twofold higher than the wild-type level when the PQ pool was oxidized (lanes 4 versus 3) and 5- to 10-fold higher when the PQ pool was reduced (lanes 6 versus 5 and 8 versus 7). It should be noted that the psbA family in Synechocystis sp. strain PCC 6803 consists of three genes, psbA-1, psbA-2, and psbA-3, although psbA-1 is not expressed (27). The nucleotide sequences of psbA-2 and psbA-3 are more than 99% identical, the transcription sizes are also identical, and psbA-2 accounts for the majority (>90%) of the psbA transcripts under normal growth conditions (37). In this study, the psbA signal in Fig. 3A represents the mRNA of both psbA-2 and psbA-3.
FIG. 3.
Northern blot analysis of PSII gene expression in Synechocystis sp. strain PCC 6803 wild type (WT) and ΔrppA cells. RNA was isolated from both wild-type (lanes 1, 3, 5, and 7) and ΔrppA (lanes 2, 4, 6, and 8) cells after treatment with different redox (A) and illumination (B) conditions as described in Materials and Methods. The sizes of psbA, psbDI-C, psbDII, and psbB are 1.2, 2.5, 1.2, and 2.0 kb, respectively.
We also analyzed the transcriptional activity of the psbD gene, which codes for the other PSII reaction center protein, D2. In Synechocystis sp. strain PCC 6803, there are two copies of the psbD gene, psbDI and psbDII. The psbDI is cotranscribed with psbC, which encodes the CP43 polypeptide of the PSII complex (35). Two different-sized bands were seen on the Northern blots: the upper band (∼2.5 kb) represents the transcripts of psbDI-psbC, whereas the lower band (∼1.2 kb) is the psbDII transcript. The transcriptional patterns of psbDI-psbC and psbDII under different redox conditions were not the same. In the wild type, the steady-state mRNA level of psbDI-psbC did not change much under various PQ redox conditions and thus more closely followed the pattern of psbB, the other antenna protein (see below). psbDII responded like the psbA gene and was up-regulated when the PQ pool was oxidized (Fig. 3A, psbDII, lanes 3 versus 1) and down-regulated when the PQ pool was reduced (lanes 5 and 7 versus 1). In ΔrppA cells, psbDI-psbC transcripts were higher than in the wild type only under photoautotrophic (control) growth conditions and in DBMIB-treated cells (Fig. 3A, psbDI-psbC, lanes 2 versus 1 and 6 versus 5). The presence of DCMU and glucose did not change the psbDI-psbC transcriptional pattern. The psbDII gene is highly expressed in ΔrppA under all examined conditions (Fig. 3A, psbDII, lanes 2, 4, 6, and 8). Since the psbA and psbD genes encode the two PSII reaction center proteins, D1 and D2, it is reasonable to speculate that they are under similar transcriptional control.
The transcriptional pattern of psbB, which encodes the CP47 polypeptide of the PSII complex, was different from that of psbA and psbDII. In the wild type, the steady-state mRNA level of psbB was slightly increased in both oxidized and reduced conditions (Fig. 3A, psbB, lanes 3, 5, and 7 versus 1). When glucose was present in the growth media, the psbB mRNA level was about threefold higher than the control level (lanes 7 versus 1), whereas psbA and psbDII levels were very low under this condition. In the ΔrppA strain, the accumulation of psbB transcripts was higher than in the wild type, especially when DBMIB was added (lanes 6 versus 5). At the same time, growth in the presence of glucose led to no difference in psbB transcription between wild-type and ΔrppA cells (lanes 8 versus 7).
We also used different illumination conditions in wild type and ΔrppA strains and demonstrated that the excessive up-regulation of PSII genes in ΔrppA cells was restricted to LL and ML conditions (Fig. 3B). When cells were transferred to the dark for 6 h or exposed to high light intensity for 3 h, the steady-state mRNA levels of psbA and psbDII were the same in wild type and ΔrppA cells (Fig. 3B, psbA and psbDII, lanes 2 versus 1 and 8 versus 7). Compared to the other PSII genes, psbA and psbDII transcripts are more stable in the dark and HL, which is consistent with previous studies (35, 36). The higher levels of psbA and psbDII transcripts under HL illumination were required to meet an accelerated turnover of the D1 and D2 proteins. The differences in transcriptional control of psbDI and psbDII were reflected in their different responses to illumination. The steady-state mRNA level of psbDI-psbC is much lower than that of psbDII in wild-type and ΔrppA cells. The expression pattern of psbDI-psbC under different illumination conditions was the same as for the psbB gene: the transcripts are almost undetectable in the dark, and there were very low levels under HL, suggesting that the mRNA has a short half-life under these extreme light conditions.
Transcriptional regulation of PSI genes.
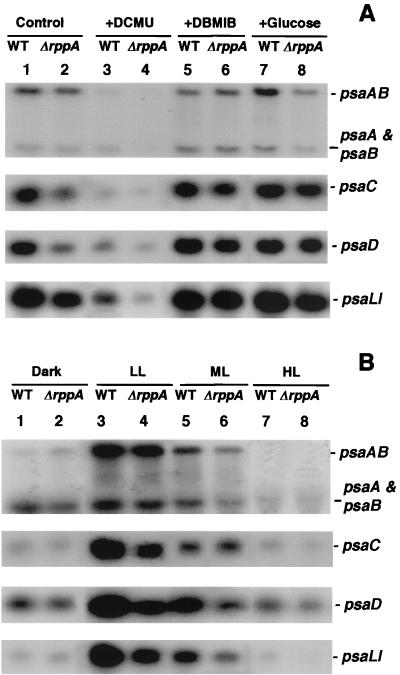
The PSI reaction center proteins, PsaA and PsaB, are encoded in an operon by the psaA and psaB genes. Northern blot analysis (Fig. 4) showed two distinct transcripts, a 5.5-kb transcript, which represented the entire gene cluster, and a 2.5-kb transcript, which corresponds to psaA and psaB (50). In contrast to the PSII reaction center genes, psaAB transcription in wild-type cells was repressed under oxidizing conditions (Fig. 4A, psaAB, lanes 3 versus 1). Interestingly, the psaA-psaB transcripts became more prevalent under reducing conditions and were induced relative to the control (Fig. 4A, psaA + psaB, lanes 5 and 7 versus 1). In the wild type, psaAB mRNA levels increased when glucose was present (Fig. 4A, psaAB, lanes 7 versus 1) but not in the presence of DBMIB. In ΔrppA cells, the mRNA levels of psaAB and psaA-psaB were very similar to the wild-type level in control and DBMIB-treated cells (Fig. 4A, psaAB and psaA + psaB, lanes 2 versus 1 and 6 versus 5) but decreased when the cells were treated with DCMU or when glucose was present in the media (lanes 4 versus 3 and 8 versus 7). The genes encoding proteins that are attached to the PSI reaction center (psaC, psaD, and psaLI) (11, 24) showed very similar transcriptional patterns under different redox conditions: they were all down-regulated under oxidized conditions (Fig. 4A, psaC, psaD, and psaLI, lanes 3 versus 1) but were at the same levels when the PQ pool was reduced by either DBMIB or glucose (lanes 5 and 7 versus 1). In ΔrppA cells, the psaC, psaD, and psaLI transcripts were under the same control: they were all decreased two- to fivefold compared to the wild type under photoautotrophic (control) and PQ-oxidized conditions (lanes 2 versus 1 and 4 versus 3) but only slightly reduced or unchanged when PQ was reduced (lanes 6 versus 5 and 8 versus 7).
FIG. 4.
Northern blot analysis of PSI gene expression in Synechocystis sp. strain PCC 6803 wild-type (WT) and ΔrppA cells. RNA was isolated from both wild-type (lanes 1, 3, 5, and 7) and ΔrppA (lanes 2, 4, 6, and 8) cells after treatment with different redox (A) and illumination (B) conditions as described in Materials and Methods. The sizes of psaAB, psaA and psaB, psaC, psaD, and psaLI are 5.5, 2.5, 0.6, 0.6, and 1.0 kb, respectively.
When cells were kept in the dark for 6 h, the abundance of full-length psaAB transcripts was dramatically decreased, but the psaA-psaB transcripts continued to accumulate to high levels in both wild-type and ΔrppA cells (Fig. 4B, psaAB and psaA + psaB, lanes 1 and 2). This result suggested that the full-length psaAB transcripts were less stable in the dark and produced transcripts that encode only psaA and psaB after processing in the intergenic region. The steady-state mRNA levels of psaAB and psaA-psaB decreased as the light intensity increased. When cells were exposed to high light for 3 h, psaAB transcripts were undetectable in both wild-type and ΔrppA cells (Fig. 4B, psaAB, lanes 7 and 8). psaC, psaD, and psaLI showed the same transcriptional patterns under all illumination conditions: highly expressed under LL and ML conditions but depressed under both dark and HL conditions. This decline can be caused either by a lower transcriptional rate or faster mRNA degradation. In the ΔrppA strain, PSI gene expression was very similar to that in the wild type under both dark and HL conditions (Fig. 4B, lanes 2 versus 1 and 8 versus 7).
Transcriptional regulation of PBS-related genes.
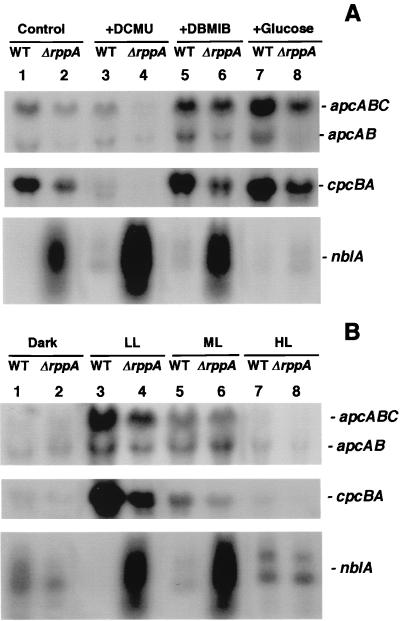
In Synechocystis sp. strain PCC 6803, the genes encoding the PBS core subunits, allophycocyanin α and β, and a small core linker protein form an operon, apcABC. Northern blots revealed two transcripts of 1.8 and 1.5 kb (Fig. 5). The larger band contains the apcA, apcB, and apcC transcripts, whereas the smaller contains the apcA and apcB transcripts. Transcription of the apc operon in the wild type was repressed under PQ-oxidizing conditions (Fig. 5A, apcABC, lanes 3 versus 1) and induced under PQ-reducing conditions (lanes 5 and 7 versus 1). In ΔrppA cells, the apcABC transcripts were down-regulated under all detected conditions compared to the wild type (lanes 2 versus 1, 4 versus 3, 6 versus 5, and 8 versus 7). Interestingly, in ΔrppA cells, the larger transcript (apcABC) decreased mainly under oxidizing conditions (lanes 4 versus 3), whereas the smaller transcript (apcAB) is strikingly decreased under reducing conditions (Fig. 5A, apcAB, lanes 6 versus 5 and 8 versus 7), especially when cells were grown in the presence of glucose. The transcript of the cpcBA operon, which encode α and β subunits of PC, showed the same expression pattern as the apc operon (Fig. 5A).
FIG. 5.
Northern blot analysis of PBS-related gene expression in Synechocystis sp. strain PCC 6803 wild-type (WT) and ΔrppA cells. RNA was isolated from both wild-type (lanes 1, 3, 5, and 7) and ΔrppA (lanes 2, 4, 6, and 8) cells after treatment with different redox (A) and illumination (B) conditions as described in Materials and Methods. The sizes of apcABC, apcAB, cpcBA, and nblA are 1.8, 1.4, 1.5, and 0.25 to 1.0 kb, respectively.
NblA is a small polypeptide, first identified in Synechococcus sp. strain PCC 7942 (14), that is involved with the complete degradation of light-harvesting phycobiliproteins in nutrient-deprived cyanobacteria. Two copies of the nblA gene (ssl0452 and ssl0453) were identified in the Synechocystis sp. strain PCC 6803 genomic sequence. Since we are interested in RppA regulation of photosynthesis- and photopigment-related gene expression, we examined the nblA transcript levels under different redox conditions. Northern analysis indicated that the nblA gene was extremely highly expressed in ΔrppA under photoautotrophic and both PQ-oxidized and PQ-reduced conditions (Fig. 5A, nblA, lanes 2 versus 1, 4 versus 3, and 6 versus 5). Unexpectedly, growth in the presence of glucose almost completely repressed the high expression of nblA in ΔrppA cells (lanes 8 versus 7). Such results suggested a correlation between the transcriptional regulation activity of RppA and high rates of respiration which lead to reduction of the PQ pool.
In wild-type and ΔrppA cells, the apc and cpc transcripts are dramatically decreased under dark and HL exposure (Fig. 5B, apcABC, apcAB, and cpcBA, lanes 1, 2, 7, and 8). In ΔrppA cells, the apc transcript was down-regulated under LL (Fig. 5B, apcABC and apcAB, lanes 4 versus 3), and cpc transcription was repressed under both LL and ML compared to the wild type (Fig. 5B, cpcBA, lanes 4 versus 3 and 6 versus 5). These results agreed with the absorbance spectral data showing that under LL and photoautotrophic growth conditions, the ΔrppA strain contained less PC per cell than the wild type. The nblA transcripts were differently expressed from the apc and cpc operons under different illumination conditions. In the wild type, nblA transcription was higher under dark, ML, and HL conditions than under LL conditions (Fig. 5B, nblA, lanes 1, 5, and 7 versus 3), consistent with the phycobiliprotein degradation process under these nonoptimal light conditions. In ΔrppA cells, the steady-state mRNA levels of nblA were lower in dark and HL than in LL and ML conditions (Fig. 5B, nblA, lanes 2 and 8 versus 4 and 6).
Photosynthesis activity and half-life of D1 protein in wild-type and ΔrppA cells.
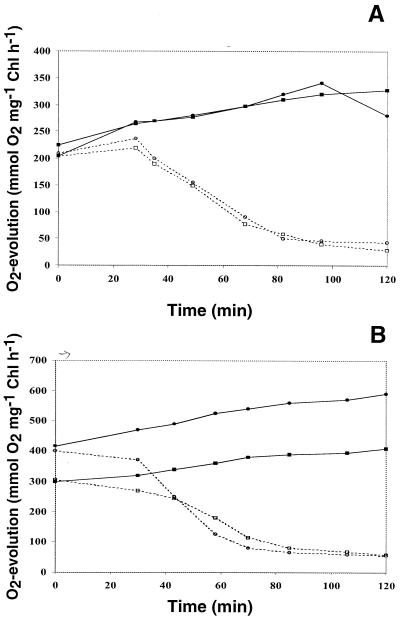
Under photoautotrophic, LL growth conditions, the O2 evolution rate of ΔrppA cells is very close to that of the wild type. After 2 h of HL exposure, the photosynthesis activity of wild-type and ΔrppA cells increased about 40% ± 4% (n = 3) (Fig. 6A). When cells were cultured in LL conditions with glucose, the photosynthesis activity of the ΔrppA mutant was much higher than the wild-type level (Fig. 6B, t = 0 h), as shown in Table 1 for LL conditions. After 2 h of HL treatment, the photosynthesis activity of wild-type and ΔrppA cells also increased about 40% ± 4% (n = 3).
FIG. 6.
Oxygen evolution activities of Synechocystis sp. strain PCC 6803 wild-type and ΔrppA cells under high light intensity (1,000 μE m−2 s−1). Cells were grown in LL without (A) or with (B) glucose until mid- to late log phase and then transferred to HL. Samples were taken at different time points after exposure to HL. The protein synthesis inhibitor chloramphenicol was added at 0 h to a final concentration of 50 μg ml−1. The oxygen evolution activity was measured as described in Materials and Methods. Samples: wild type without (■) and with (□) chloramphenicol; ΔrppA without (●) and with (○) chloramphenicol.
The half-life of the D1 protein under HL was examined by adding the protein synthesis inhibitor chloramphenicol to the culture. Results showed that under photoautotrophic, HL conditions, the D1 half-lives of wild-type and ΔrppA cells were quite similar (∼60 min) (Fig. 6A). In photomixotrophic, HL conditions, ΔrppA showed a slightly higher D1 degradation rate than the wild type (45 versus 60 min) (Fig. 6B). These results suggested that one factor for the higher O2 evolution activity of ΔrppA could be the somewhat faster de novo turnover of the D1 protein. The next step was to determine how much of this turnover was due to de novo synthesis.
Translational regulation of the D1 protein.
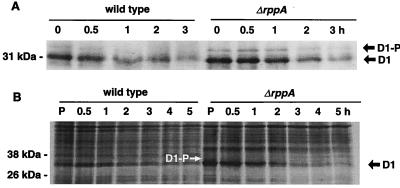
To clarify why the oxygen evolution activity in ΔrppA cells is higher than in the wild type under photomixotrophic growth conditions, we analyzed protein accumulation by immunoblotting (Fig. 7A) and protein synthesis with pulse-chase experiments (Fig. 7B). For the immunoblot experiment, cells were grown in LL until mid- to late log phase, chloramphenicol was added, and the cells were exposed to HL for different time intervals. The thylakoid membranes were isolated, and membranes containing 5 μg of Chl were loaded in each lane. As shown in Fig. 7A (t = 0 h), the steady-state D1 protein level in ΔrppA cells was about 1.5-fold higher than in the wild type. When cells were exposed to HL, degradation of the D1 protein in the ΔrppA strain was faster than in the wild type. Notably, we observed a band above the mature D1 protein band that was previously designated the D1 precursor (55, 56). The abundance of this band was higher in ΔrppA than in wild-type cells, especially within the first hour of chloramphenicol treatment. This result indicated that the rate of D1 synthesis in the ΔrppA strain increased to keep up with the high photosynthesis activity and to compensate for the rapid D1 degradation. However, the processing rate may not have been altered, and the new precursor would accumulate to higher levels in ΔrppA than in wild-type cells.
FIG. 7.
(A) Immunoblot analysis of D1 protein of Synechocystis sp. strain PCC 6803 wild-type and ΔrppA cells. Thylakoid proteins were isolated from cells which had been grown under LL with glucose until mid- to late log phase, chloramphenicol was added, and the cells were exposed to high light intensity for different time intervals. Thylakoids (5 μg of Chl per lane) were loaded, separated by LDS-PAGE, blotted onto a nitrocellulose membrane, and incubated with D1 antisera. The D1 precursor (D1-P) that migrates more slowly than the mature D1 protein was observed in both wild-type and ΔrppA cells. (B) Pulse-chase labeling of thylakoid proteins from Synechocystis sp. strain PCC 6803 wild-type and ΔrppA cells. Cells were labeled with [35S]Met in vivo under LL with glucose for 30 min (P). The radioactivity was subsequently chased for 0.5, 1, 2, 3, 4, and 5 h under HL (see Materials and Methods). The D1 precursor (D1-P; white arrow) that migrates more slowly than the mature D1 protein was observed in ΔrppA cells. Labeled proteins were detected by autoradiography.
Pulse-labeling experiments showed that the D1 protein synthesis in ΔrppA was faster than in the wild type. Quantification of the labeled band showed a twofold increase in the amount of D1 in ΔrppA over wild-type cells after 30 min of labeling (Fig. 7B, lane P). During the chase under HL conditions, D1 turned over more rapidly in ΔrppA than in wild-type cells. Again, a protein band with a slightly slower mobility than the mature D1 protein was observed at 0 h in ΔrppA cells, which indicated faster D1 protein synthesis in the mutant. From both Western blot and pulse-chase experiments, we conclude that the PSII reaction center protein D1 was synthesized more rapidly and accumulated at a higher steady-state level in photomixotrophically grown ΔrppA cells than in the wild type. The rapid degradation of the D1 protein is balanced by an enhancement of gene expression that compensated for the loss of these proteins and maintained active PSII complexes. Thus, the high level of PSII reaction center gene transcription is physiologically relevant and is seen in a higher level of de novo synthesis of D1.
DISCUSSION
Overall characterization of photosynthesis gene transcription in ΔrppA.
Figures 3 to 5 demonstrate that the transcription of photosynthesis genes was profoundly affected in ΔrppA. In general, transcription of genes coding for PSII proteins was enhanced in ΔrppA cells compared to the wild type, as seen for psbA (Fig. 3A). Conversely, transcription of genes encoding for PSI proteins and phycobiliproteins was somewhat decreased, as seen for psaC (Fig. 4A) or apcABC (Fig. 5A). These changes were not seen when cells were grown in the dark or in HL, as demonstrated for psbA (Fig. 3B). A major exception to the above generality was transcription of nblA, which was vastly increased (∼100-fold) in ΔrppA compared to wild-type cells (Fig. 5A), whereas the PBS structural genes were down-regulated. From these results, we tentatively concluded that the RppA protein is involved in regulating the stoichiometry of the photosystems by depressing transcription of PSII and increasing the transcription of PSI. At the same time, it increases transcription of PBS genes and inactivates a system for destruction of PBS. A more detailed analysis of the RppA regulation follows, including the effects of redox poise and light on transcription.
Redox state of the PQ pool controls photosynthesis and PBS-related gene expression.
Changes in the redox state of components of the electron transport chain have been implicated in controlling the transcriptional activators of photosynthetic gene expression in cyanobacteria (1, 2). To test if RppA is a regulator that responds to redox poise, we have treated Synechocystis sp. strain PCC6803 wild-type and ΔrppA cultures under different light conditions and with two specific inhibitors of electron transport, DCMU and DBMIB. DCMU blocks electron transfer from the PSII primary acceptor QA to the PQ pool, and DBMIB prevents the electron transfer from PQ pool to cytochrome b6/f. Our results indicated that in wild-type cells, the steady-state mRNA levels of PSII genes increased when the PQ pool was oxidized by DCMU, decreased when the PQ pool was reduced by DBMIB, and were extremely depressed when glucose was present (Fig. 3A). In contrast, transcription of the PSI reaction center operon was depressed when the PQ pool was oxidized, and net accumulation of the psaA-psaB transcripts increased when the PQ pool was reduced (Fig. 4A). The inverse effects of DCMU and DBMIB strongly suggest that the balance between the reduced and oxidized forms of the PQ pool is involved in the signal transduction of PS gene expression. Compared to the wild type, expression of photosynthesis genes in ΔrppA, especially the PSII reaction center genes, was less sensitive to PQ redox variation (Fig. 3A). We conclude that the rppA gene is normally involved in the establishment of the appropriate stoichiometry between the photosystems and can sense changes in the PQ redox poise.
Our results relate to a number of important areas of photosynthesis research, including redox control of gene expression and the effect of photoinhibition on PSII gene expression. Similar results in regard to redox control of chloroplast gene expression were obtained recently by Pfannschmidt et al. (42). Their paper supported and expanded on the model of Allen (2) that relates the transcriptional activity of PSI and PSII genes to the redox poise of the PQ pool. Overall, their results indicated that when either photosystem becomes rate limiting for photosynthesis, transcription of genes for its specific reaction center proteins becomes induced. Their results for chloroplasts from mustard seedlings and ours for cyanobacteria are virtually identical in this regard. Another detailed study of redox control of psbA expression in Synechocystis (1) also concluded that such transcription is under redox control. These authors specifically emphasized that accumulation of QA− activates psbA transcription. We have explicitly not tried to differentiate between QA− and PQ pool redox state or the thiol redox state at this early stage of our studies, since it is difficult to determine which would be the actual signal. It is possible that QA− could be involved directly with the mechanism to indicate that PSII centers need to be replaced. However, it is less certain if this is the direct signal for the transcriptional regulation of PSI genes or those involved with PBSs. For simplicity, we have referred just to the redox poise of the PQ pool as we begin the process of sorting out the control mechanism in the ΔrppA, as well as in other mutants.
Another very fruitful line of investigation has been initiated by Grossman and colleagues (14, 17, 48), who isolated mutants of Synechococcus sp. strain PCC 7942 that were defective in the degradation of PBSs during sulfur- or nitrogen-limited growth. They identified NblA, a small polypeptide that is critical for PBS degradation during this nutrient deprivation (14). In Synechococcus sp. strain PCC 7942, nblA transcripts were very low in nutrient-replete cells, and their abundance increased about 50-fold during sulfur or nitrogen deprivation (14). The present study showed that in Synechocystis sp. strain PCC 6803, RppA strongly depressed nblA transcription. The steady-state mRNA level of nblA was dramatically increased when RppA was absent. This may be one reason why ΔrppA cells contained less PC than the wild type, since over expression of nblA could trigger the PBS-degradative process. Interestingly, nblA transcription is also up-regulated in the RppB mutant, but to a lesser degree than in ΔrppA cells (data not shown). These results suggest that phosphorylation is essential for RppA activation, and RppA could be phosphorylated by other kinases in addition to RppB. In ΔrppA cells, the accumulation of nblA transcripts increased greatly under both PQ oxidizing and reducing conditions (Fig. 5A). The regulation of RppA on nblA transcription was eliminated when glucose was present and when cells were transferred to dark or to HL. These data implied that nblA transcription is under different controls under diverse environmental stress conditions. NblR was identified to be an essential inducer of nblA expression in Synechococcus sp. strain PCC 7942 under nitrogen and sulfur deprivation conditions (48). NblR had all of the characteristics of a response regulator that is controlled by the intracellular redox state. Based on these data, we currently conclude that RppA and NblR work differently toward controlling nblA expression, although they may have overlapping functions. We will soon be able to study their related functions by constructing double mutants of Synechocystis sp. strain PCC 6803 that are deficient in both RppA and NblR.
In cyanobacteria, the light-harvesting antenna consists of the PBSs and Chl. The majority of Chl molecules are associated with PSI, whereas the PBSs are generally the major light-harvesting antenna for PSII. The state transitions are associated with the movement of the PBSs from PSII to PSI when PSII has been provided too much excitation energy (45). Our results are very similar to those of Alfonso et al. (1) in that PSI- and PBS-related genes were not under the same level of redox control as the PSII reaction center genes. Our results indicated that in the wild type, transcription of PSI- and PBS-related genes decreased in the presence of DCMU and increased in the presence of DBMIB and glucose. It is of interest that regulation of the PBS-related genes is closer to that of PSI than to those of PSII. This suggests that the main function of the redox-regulated signal is to allow for the degradation and resynthesis of PSII. Under these circumstances, new transcription of PBS-related genes and PBS synthesis could complicate the repair mechanism and would be more appropriate at a later time. In ΔrppA cells, transcription of the PBS structural genes (apcABC and cpcBA) was significantly reduced under both oxidizing and reducing conditions, especially the presence of glucose. Once again, the high rate of PSII synthesis may require that the transcription of the light-harvesting proteins be reduced significantly.
Light conditions affect transcription and translation of the PSII reaction center components.
Exposure of oxygenic photosynthetic organisms to high light intensity causes photoinhibition of photosynthesis. Photoinhibition is associated with an inactivation of PSII electron transport and subsequent degradation of the PSII reaction center proteins (4, 29). In Synechococcus sp. strain PCC 7942, there are two forms of D1 protein, D1:1 and D1:2. It has been demonstrated that PSII reaction centers containing D1:2 have a higher intrinsic resistance to photoinhibition and are more photochemically efficient than PSII centers with D1:1 (9, 12, 13, 30). In Synechocystis sp. strain PCC 6803, only one form of D1 has been identified. The rapid degradation of D1, and possibly D2, is balanced by an induction of gene expression at the high light intensity that compensates for the loss of these proteins and maintains a functional PSII. In Synechocystis sp. strain PCC 6803, the accumulation of psbA and psbDII transcripts was enhanced by a shift to HL conditions (Fig. 3B). Like in Synechococcus, the primary function of the monocistronic psbDII locus in Synechocystis may be to produce extra D2 protein to maintain a functional PSII at high light intensity without increasing synthesis of the more stable psbC gene product (8). The oxygen evolution of both Synechocystis sp. strain PCC 6803 wild type and ΔrppA mutant increased 35 to 45% in photoautotrophic and photomixotrophic growth conditions, respectively, under HL for 2 h (Fig. 6). The protein synthesis inhibitor chloramphenicol caused the O2 evolution activity to be lost completely within 2 h under high light irradiation, indicating that rapid de novo protein synthesis is required to maintain PSII activity. Fast D1 degradation and synthesis were confirmed by the pulse-chase and immunoblot experiments. Both experiments indicated that D1 synthesis was faster and the steady-state level was higher in ΔrppA cells than in the wild type. This phenomenon was more obvious when cells were grown in the presence of glucose. It is important to note that although D1 and D2 synthesis was enhanced, the synthesis of CP43 and CP47 was not, especially in ΔrppA cells. This suggests that there can be more PSII centers but with less antenna Chl on average. Thus, the O2 evolution per milligram of Chl in ΔrppA cells would appear higher than the wild type (Table 1 and Fig. 6).
ACKNOWLEDGMENTS
We thank Hsiao-Yuan Tang for help in construction of the mutants, Don Tucker, Kim Hirsh, and Ruth Falwell for technical assistance, and Abhay Singh for helpful discussions.
This research was supported by grant DE-FG-02-99ER20342 from the Department of Energy.
REFERENCES
- 1.Alfonso M, Perewoska I, Constant S, Kirilovsky D. Redox control of psbA expression in cyanobacteria Synechocystis strains. J Photochem Photobiol B: Biol. 1999;48:104–113. [Google Scholar]
- 2.Allen J F. Thylakoid protein phosphorylation, state 1-state 2 transitions, and photosystem stoichiometry adjustment: redox control at multiple levels of gene expression. Physiol Plant. 1995;93:196–205. [Google Scholar]
- 3.Appleby J L, Bourret R B. Proposed signal transduction role for conserved CheY residue Thr87, a member of the response regulator active-site quintet. J Bacteriol. 1998;180:3563–3569. doi: 10.1128/jb.180.14.3563-3569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 5.Barber J. Photosynthetic reaction centers: a common link. Trends Biochem Sci. 1987;12:321–326. [Google Scholar]
- 6.Bird T H, Du S, Bauer C E. Autophosphorylation, phosphotransfer, and DNA-binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus. J Biol Chem. 1999;274:16343–16348. doi: 10.1074/jbc.274.23.16343. [DOI] [PubMed] [Google Scholar]
- 7.Borkovich K A, Kaplan N, Hess J F, Simon M I. Transmembrane signal transduction in bacterial chemotaxis involves ligand-dependent activation of phosphate group transfer. Proc Natl Acad Sci USA. 1989;86:1208–1212. doi: 10.1073/pnas.86.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustos S A, Golden S S. Light-regulated expression of the psbD gene family in Synechococcus sp. strain PCC 7942: evidence for the role of duplicated psbD genes in cyanobacteria. Mol Gen Genet. 1992;232:221–230. doi: 10.1007/BF00280000. [DOI] [PubMed] [Google Scholar]
- 9.Campbell D, Zhou G, Gustafsson P, Oquist G, Clarke A K. Electron transport regulation exchange of two forms of photosystem II D1 protein in the cyanobacterium Synechococcus. EMBO J. 1995;14:5457–5466. doi: 10.1002/j.1460-2075.1995.tb00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C, Stewart R C. The two-component system regulation of diverse signaling pathways in prokaryotes and eukaryotes. Plant Physiol. 1998;117:723–731. doi: 10.1104/pp.117.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitnis V P, Chitnis P R. PsaL subunit is required for the formation of photosystem I trimers in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 1993;336:330–334. doi: 10.1016/0014-5793(93)80831-e. [DOI] [PubMed] [Google Scholar]
- 12.Clarke A K, Hurry V M, Gustafsson P, Öquist G. Two functionally distinct forms of the photosystem II reaction-center protein D1 in the cyanobacterium Synechococcus sp. PCC 7942. Proc Natl Acad Sci USA. 1993;90:11985–11989. doi: 10.1073/pnas.90.24.11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke A K, Soitamo A, Gustafsson P, Öquist G. Rapid interchange between two distinct forms of cyanobacterial photosystem II reaction-center protein D1 in response to photoinhibition. Proc Natl Acad Sci USA. 1993;90:9973–9977. doi: 10.1073/pnas.90.21.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collier J L, Grossman A R. A small polypeptide triggers complete degradation of light-harvesting phycobiliproteins in nutrient-deprived cyanobacteria. EMBO J. 1994;13:1039–1047. doi: 10.1002/j.1460-2075.1994.tb06352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colón-López M S, Sherman L A. Transcriptional and translational regulation of photosystem I and II genes in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J Bacteriol. 1998;180:519–526. doi: 10.1128/jb.180.3.519-526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colón-López M S, Sherman D M, Sherman L A. Transcriptional and translational regulation of nitrogenase in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J Bacteriol. 1997;179:4319–4327. doi: 10.1128/jb.179.13.4319-4327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolganov N, Grossman A R. A polypeptide with similarity to phycocyanin α-subunit phycocyanobilin lyase involved in degradation of phycobilisome. J Bacteriol. 1999;181:610–617. doi: 10.1128/jb.181.2.610-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du S, Kouadio J L, Bauer C E. Regulated expression of a highly conserved regulatory gene cluster is necessary for controlling photosynthesis gene expression in response to anaerobiosis in Rhodobacter capsulatus. J Bacteriol. 1999;181:4334–4341. doi: 10.1128/jb.181.14.4334-4341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durnford D G, Falkowski P G. Chloroplast redox regulation of nuclear gene transcription during photoacclimation. Photosyn Res. 1997;53:229–241. [Google Scholar]
- 20.Eraso J M, Kaplan S. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escoubas J-M, Lomas M, Laroche J, Falkowski P G. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabret C, Feher V A, Hoch J A. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J Bacteriol. 1999;181:1975–1983. doi: 10.1128/jb.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forst S, Comeau D, Norioka S, Inouye M. Localization and membrane topology of EnvZ, a protein involved in osmoregulation of OmpF and OmpC in Escherichia coli. J Biol Chem. 1987;262:16433–16438. [PubMed] [Google Scholar]
- 24.Fromme P. Structure and function of photosystem I. Curr Opin Struct Biol. 1996;6:473–484. doi: 10.1016/s0959-440x(96)80112-6. [DOI] [PubMed] [Google Scholar]
- 25.Grossman A R, Schaefer M R, Chiang G G, Collier J L. Environmental effects on the light-harvesting complex of cyanobacteria. J Bacteriol. 1993;175:575–582. doi: 10.1128/jb.175.3.575-582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakenbeck R, Stock J B. Analysis of two-component signal transduction systems involved in transcriptional regulation. Methods Enzymol. 1996;273:281–300. doi: 10.1016/s0076-6879(96)73026-4. [DOI] [PubMed] [Google Scholar]
- 27.Jansson C, Debus R J, Osiewacz H D, Gurevitz M, McIntosh L. Construction of an obligate photoheterotrophic mutant of the cyanobacterium Synechocystis 6803. Plant Physiol. 1987;85:1021–1025. doi: 10.1104/pp.85.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 29.Kanervo E, Maenpaa P, Aro E M. D1 protein degradation and psbA transcription levels in Synechocystis PCC 6803 during photoinhibition in vivo. J Plant Physiol. 1993;142:669–675. [Google Scholar]
- 30.Kulkarni R D, Golden S S. Form II of D1 is important during transition from standard to high light intensity in Synechocystis sp. strain PCC 7942. Photosyn Res. 1995;46:435–443. doi: 10.1007/BF00032298. [DOI] [PubMed] [Google Scholar]
- 31.Lukat G S, Lee B H, Mottonen J M, Atock A M, Stock J B. Roles of the highly conserved aspartate and lysine residues in the response regulator of bacterial chemotaxis. J Biol Chem. 1991;266:8348–8354. [PubMed] [Google Scholar]
- 32.Masuda S, Matsumoto Y, Nagashima K V P, Shimada K, Inoue K, Bauer C E, Matsuura K. Structural and functional analyses of photosynthetic regulatory genes regA and regB from Rhodovulum sulfidophilum, Roseobacter dentrificans, and Rhodobacter capsulatus. J Bacteriol. 1999;181:4205–4215. doi: 10.1128/jb.181.14.4205-4215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meunier P C, Colón-López M S, Sherman L A. Temporal changes in state transition and photosystem organization in the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. Plant Physiol. 1997;115:991–1000. doi: 10.1104/pp.115.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno T, Kaneko T, Tabata S. Compilation of all genes encoding bacterial two-component signal transducers in the genome of the cyanobacterium, Synechocystis sp. strain PCC 6803. DNA Res. 1996;3:407–414. doi: 10.1093/dnares/3.6.407. [DOI] [PubMed] [Google Scholar]
- 35.Mohamed A, Jansson C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1989;13:693–700. doi: 10.1007/BF00016024. [DOI] [PubMed] [Google Scholar]
- 36.Mohamed A, Jansson C. Photosynthetic electron transport controls degradation but not production of psbA transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1991;16:891–897. doi: 10.1007/BF00015080. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed A, Eriksson J, Osiewacz H D, Jansson C. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol Gen Genet. 1993;238:161–168. doi: 10.1007/BF00279543. [DOI] [PubMed] [Google Scholar]
- 38.O'Gara J P, Eraso J M, Kaplan S. A redox-responsive pathway for aerobic regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1998;180:4044–4050. doi: 10.1128/jb.180.16.4044-4050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouchane S, Kaplan S. Topological analysis of the membrane-localized redox-responsive sensor kinase PrrB from Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1999;274:17290–17296. doi: 10.1074/jbc.274.24.17290. [DOI] [PubMed] [Google Scholar]
- 40.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 41.Perraud A-L, Weiss V, Gross R. Signalling pathways in two-component phosphorelay systems. Trends Microbiol. 1999;7:115–120. doi: 10.1016/s0966-842x(99)01458-4. [DOI] [PubMed] [Google Scholar]
- 42.Pfannschmidt T, Nilsson A, Allen J F. Photosynthetic control of chloroplast gene expression. Nature. 1999;397:625–628. [Google Scholar]
- 43.Qian Y, Tabita F R. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J Bacteriol. 1996;178:12–18. doi: 10.1128/jb.178.1.12-18.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy K J, Webb R, Sherman L A. Bacterial RNA isolation with one hour centrifugation in a table-top ultracentrifuge. BioTechniques. 1990;8:250–251. [PubMed] [Google Scholar]
- 45.Rögner M, Boekema E J, Barber J. How does photosystem 2 split water? The structural basis of efficient energy conversion. Trends Biochem Sci. 1996;21:44–49. doi: 10.1016/s0968-0004(96)80177-0. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 47.Sander D A, Gilliece-Castro B L, Strock A M, Burlingame A L, Koshland D E Jr. Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J Biol Chem. 1989;264:21770–21778. [PubMed] [Google Scholar]
- 48.Schwarz R, Grossman A R. A response regulator of cyanobacteria integrates diverse environmental signals and is critical for survival under extreme conditions. Proc Natl Acad Sci USA. 1998;95:11008–11013. doi: 10.1073/pnas.95.18.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sganga M W, Bauer C. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell. 1992;68:945–954. doi: 10.1016/0092-8674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- 50.Smart L B, McIntosh L. Expression of photosynthesis genes in the cyanobacterium Synechocystis sp. PCC 6803: psaA-psaB and psbA transcripts accumulate in dark-grown cells. Plant Mol Biol. 1991;17:959–971. doi: 10.1007/BF00037136. [DOI] [PubMed] [Google Scholar]
- 51.Stock A M, Martinez-Hackert E, Rasmussen B F, West A H, Stock J B, Ringe D, Petsko G A. Structure of the Mg2+-bound form of CheY and mechanism of phosphoryl transfer in bacterial chemotaxis. Biochemistry. 1993;32:13375–13380. doi: 10.1021/bi00212a001. [DOI] [PubMed] [Google Scholar]
- 52.Tiwari R P, Reeve W G, Dilworth M J, Glenn A R. Acid tolerance in Rhizobium meliloti strain WSM419 involves a two-component sensor-regulator system. Microbiology. 1996;142:1693–1704. doi: 10.1099/13500872-142-7-1693. [DOI] [PubMed] [Google Scholar]
- 53.Watson G M, Scanlan D J, Mann N H. Characterization of the genes encoding a phosphate-regulated two component sensory system in the marine cyanobacterium Synechococcus sp. WH7803. FEMS Microbiol Lett. 1996;142:105–109. doi: 10.1111/j.1574-6968.1996.tb08415.x. [DOI] [PubMed] [Google Scholar]
- 54.William J G K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988;167:766–778. [Google Scholar]
- 55.Yu J, Vermaas W F J. Transcript levels and synthesis of photosystem II components in cyanobacterial mutants with inactivated photosystem II genes. Plant Cell. 1990;2:315–322. doi: 10.1105/tpc.2.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu J, Vermaas W F J. Synthesis and turnover of photosystem II reaction center polypeptides in cyanobacterial D2 mutants. J Biol Chem. 1993;268:7407–7413. [PubMed] [Google Scholar]