Significance
Gender inequality is associated with worse mental health and academic achievement in women. Using a dataset of 7,876 MRI scans from healthy adults living in 29 different countries, we here show that gender inequality is associated with differences between the brains of men and women: cortical thickness of the right hemisphere, especially in limbic regions such as the right caudal anterior cingulate and right medial orbitofrontal, as well as the left lateral occipital, present thinner cortices in women compared to men only in gender-unequal countries. These results suggest a potential neural mechanism underlying the worse outcome of women in gender-unequal settings, as well as highlight the role of the environment in the brain differences between women and men.
Keywords: gender inequality, structural brain MRI, sex differences
Abstract
Gender inequality across the world has been associated with a higher risk to mental health problems and lower academic achievement in women compared to men. We also know that the brain is shaped by nurturing and adverse socio-environmental experiences. Therefore, unequal exposure to harsher conditions for women compared to men in gender-unequal countries might be reflected in differences in their brain structure, and this could be the neural mechanism partly explaining women’s worse outcomes in gender-unequal countries. We examined this through a random-effects meta-analysis on cortical thickness and surface area differences between adult healthy men and women, including a meta-regression in which country-level gender inequality acted as an explanatory variable for the observed differences. A total of 139 samples from 29 different countries, totaling 7,876 MRI scans, were included. Thickness of the right hemisphere, and particularly the right caudal anterior cingulate, right medial orbitofrontal, and left lateral occipital cortex, presented no differences or even thicker regional cortices in women compared to men in gender-equal countries, reversing to thinner cortices in countries with greater gender inequality. These results point to the potentially hazardous effect of gender inequality on women’s brains and provide initial evidence for neuroscience-informed policies for gender equality.
Gender inequality profoundly impacts the society by creating an environment that significantly harms women. Women experience discrimination across many domains, including in education, the workplace, and in public office, and are disproportionately impacted by unpaid care work (1). However, gender inequality varies across countries as quantified using measures related to health, political representation, educational attainment, and labor market participation (2, 3). Such metrics have allowed to uncover country-level gender inequality associations with women’s worse mental health (4) and lower educational attainment (5).
Research on gender differences in brain structure could clarify possible reasons for gender differences in mental health problems (6). This would extend prior studies focused on endocrine or genetic contributions to gender differences in mental health problems (7, 8). Many studies find larger total intracranial volume in men, but other results are less consistent. This includes features of specific brain areas and findings for multiple morphometric properties such as thickness or surface area (9). Other work links brain structure to social and environmental factors. Such factors could differentially relate to brain structures across genders, contributing to inconsistency in studies of gender differences in brain structure. For example, exposure to early stimulation might increase gray matter cortical volumes in ways that persist in adulthood (10). Similarly, adverse childhood experiences could influence cortical surface area, thickness, and hippocampal volumes (11). Such adverse experiences might include exposure to hostile environments associated with stigma directed toward minority groups (12) or exposure to poverty (13). Leading hypotheses link these associations to stress physiology (14), accelerated aging process (15, 16), levels of environmental enrichment (17), and nutrition and health care (18). Women living in countries with high levels of gender inequality experience many of these same factors that are linked in prior research to brain structure. These adverse experiences include exposure to violence (19) as well as insufficient exposure to education and appropriate health care, both of which are considered indicators of gender inequality (2, 3). Therefore, it is also possible that brain structure is vulnerable to gender inequality: Women living in societies with high levels of gender inequality experience greater adversity, and this could negatively impact their brain development. Consistent with this perspective, a previous study based in 17 states in the United States found a trend-level association between hippocampal volume in 10-y-old girls and views on gender within the state (12). An international approach with increased variance in gender inequality could have more power to examine a potential effect. Gender inequality as an aggregate of these adverse factors, if presenting associations with brain structure, would link an important social determinant of health to the brain, which in turn might help explain gender-related differences in psychopathology (20). Such a study might also inform public policy in similar ways to studies examining other social correlates of brain structure (21).
To examine this possibility, differences in brain structure between healthy adult men and women from samples obtained in 29 countries were entered into a random-effects meta-analysis including a meta-regression, in which country-level gender inequality acted as an explanatory variable for the observed differences. Based on the findings from previous imaging studies on environmental factors, we explored associations with hemispheric and regional cortical thickness and surface area, as well as hippocampal volume, all measured using MRI. Cortical thickness and surface area have been widely used in previous multicenter studies (22). They are genetically and developmentally distinct (23, 24), and arguably provide a cleaner metric than volume, which is a composite of the two. Moreover, they relate in different ways to psychopathology across age groups and distinct forms of diagnosis (25, 26). For our metric of gender inequality, we combined the two most widely used national-level gender inequality metrics: the Gender Gap Index (2) and the Gender Inequality Index (3). We hypothesized that we would observe few structural differences in the brains of men and women in gender-equal countries, with differences appearing with higher levels of gender inequality.
Results
This study included 139 samples from 29 different countries, totaling 7,876 MRI scans from 4,078 women and 3,798 men (Figs. 1 and 2 and Dataset S1). Nearly 35.26% of the participants lived in low- and middle-income countries. The median mean age across samples was 24.19 y (range 18.83 to 31.69 y). To account for a potential effect of age within samples, we regressed the linear effect of age in each sample.
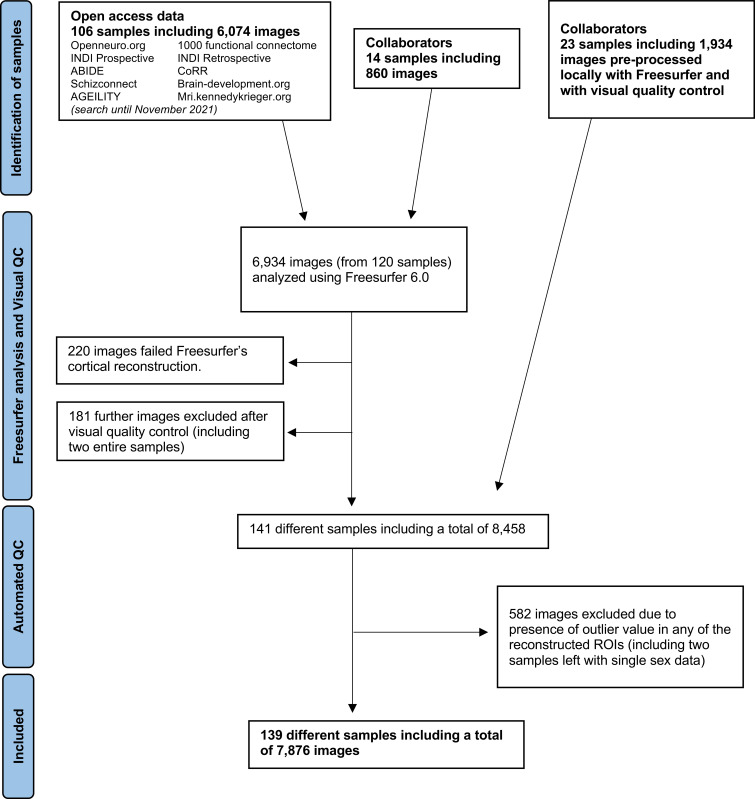
Fig. 1.
Flowchart of sample selection.
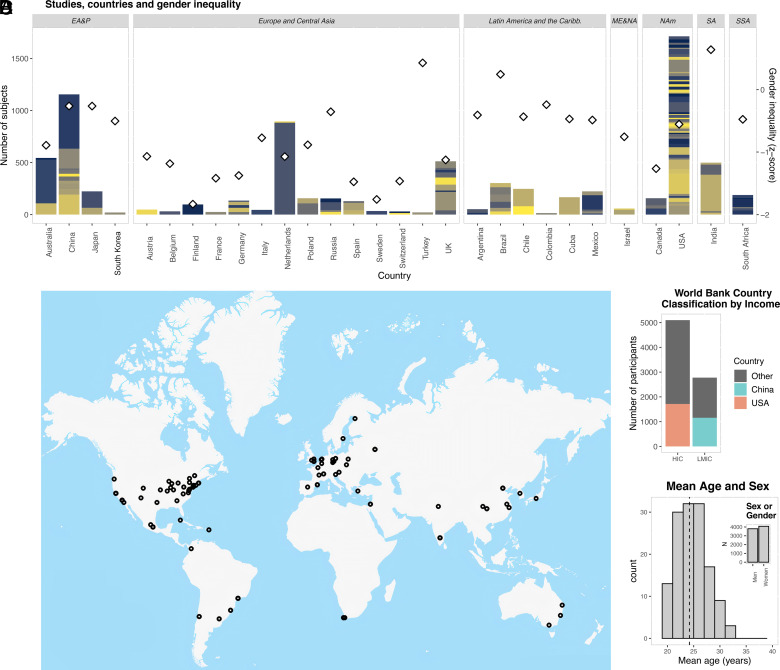
Fig. 2.
Demographic characteristics of samples included. (A) Number of participants included in each country (bars), with different colors denoting different studies/samples. The right Y axis and diamonds describe gender inequality Z-score, where higher values denote higher inequality. (B) Map showing the location of the main institutions that performed the studies included. (C) Number of participants from high-income countries (HIC) and low- and middle-income countries (LMIC), highlighting participants from China and the United States. (D) Histogram with mean age and sex within and across the samples, respectively. EA&P = East Asia and Pacific; ME&NA = Middle East and North Africa; NAm = North America; SA = South Asia; SSA = Sub-Saharan Africa.
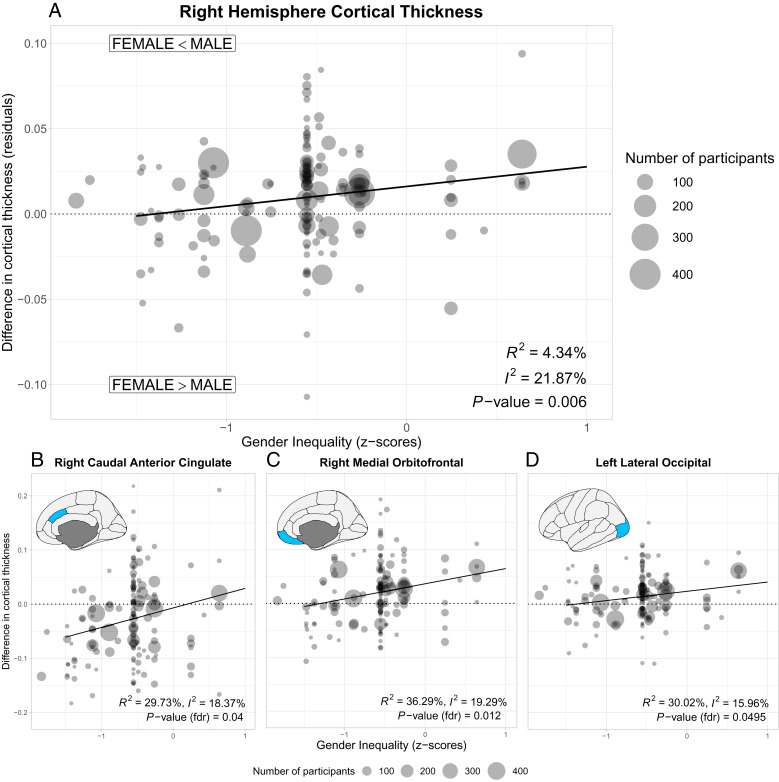
Analyses of hemisphere-wide average cortical thickness revealed a significant association with gender inequality in the right (Fig. 3A, beta 0.012 (95% CI 0.0033 to 0.020), P = 0.006, with an R2 = 4.34%), but not left hemisphere (beta 0.008 (95% CI −0.001 to 0.0155), P = 0.07; SI Appendix, Fig. S1). Countries with greater gender equality showed practically no differences in cortical thickness in the right hemisphere between the sexes. Sex differences emerged in countries with greater gender inequality, with men having thicker cortices than women. Reliability analyses showed that this result was not driven by a single sample (SI Appendix, Fig. S2). National gender inequality indices are associated with economic development (27). Our result remained significant after controlling for the logarithm of the per capita gross domestic product [beta 0.015 (95% CI 0.004 to 0.026), P = 0.0065]. Analyses looking at the association between cortical thickness with gender inequality in women only found that cortical thickness in the right hemisphere tended to decrease with higher gender inequality, albeit not significantly [SI Appendix, Fig. S3, beta −0.022 (95% CI −0.047 to 0.0036), P = 0.093]. We found no evidence of an association in men [beta −0.0075 (95% CI −0.036 to 0.021), P = 0.6].
Fig. 3.
Associations between country-level gender inequality and the average difference of the cortical thickness between women and men. (A) Right hemisphere. Circles represent the thickness difference between men and women in a specific sample; their size represents the number of participants. Negative values of the gender inequality index describe a higher equality between men and women. Solid line represents fit of main analysis. (B–D) Significant associations between gender inequality and regional cortical thickness after controlling for multiple comparisons.
Analyses on brain regions of interest (68 subregions tested) showed that cortical thickness differences in three brain regions correlated significantly with gender inequality after correcting for multiple testing using false-discovery rate: right caudal anterior cingulate gyrus [beta 0.036 (95% CI 0.014 to 0.058), PFDR = 0.040], right orbitofrontal gyrus [beta 0.028 (95% CI 0.014 to 0.043), PFDR = 0.012], and left lateral occipital cortex [beta 0.017 (95% CI 0.006 to 0.028), PFDR = 0.0495; Fig. 3 B–D]. These three areas shared the same pattern: There were no differences (or even thicker regional cortices in the case of the caudal anterior cingulate gyrus) in women in countries with greater gender equality, reversing to thinner cortices in countries with greater gender inequality. The association with the right caudal anterior cingulate gyrus also remained significant after controlling for economic development [beta 0.055 (95% CI 0.027 to 0.084), PFDR = 0.0098], but was no longer significant for the right medial orbitofrontal gyrus (PFDR = 0.064) or the left lateral occipital cortex (PFDR = 0.13).
Further analyses demonstrated that our cortical thickness results were consistent across several methodological variations. One could hypothesize that the association was driven by samples from China and the United States, the two countries that contributed the greatest number of images. Our results were not modified substantially when we excluded studies from those two countries (SI Appendix, Fig. S4). The results were also consistent when considering the individual noncombined inequality indices (SI Appendix, Fig. S5), including only studies performed on 3T MRI scanners (SI Appendix, Fig. S6), excluding very small studies (SI Appendix, Fig. S7), or exclusively analyzing those with visual quality checks performed by the same two researchers (SI Appendix, Fig. S8).
There were no significant associations between gender inequality and hemispheric or regional surface area (SI Appendix, Fig. S9), hippocampal volumes (SI Appendix, Fig. S10), or with total intracranial volume (SI Appendix, Fig. S11).
Discussion
The results show that country-level gender inequality is related to the average structural brain differences between women and men in cortical thickness. The effect seen was a global one, significant in the cortical thickness of the right hemisphere.
Gender inequality indices are composite measures that incorporate diverse experiences that might be mediating their effect on the brain through different biological mechanisms. However, we could hypothesize about the predominant underlying mechanisms based on the localized brain regions in which a significant association was found, namely, the anterior cingulate gyrus and orbitofrontal gyrus. These regions have been related to several aspects of emotional control, including resilience to adversity (28), responses to inequity (29), or negative social comparisons (30). Changes in these regions have also been found in pathological conditions where stress is considered a central mechanism, including thinning in depression (25), or reduced volume in posttraumatic stress disorder (31). Stress would lead to these macroscopic changes through dendritic remodeling and synaptic pruning, possibly mediated by stress hormones (32). Overall, the observed association may result from exposure to an adverse environment and subsequent stress response throughout life. This would imply that sex differences in the thickness of those regions would be smaller in early development and increase during aging. This resonates with evidence highlighting the role of gender inequality in the higher prevalence of depression in girls which appears in adolescence (33). Other data support this hypothesis about the timing of the observed changes. For example, stress in the adult brain seems to correlate most consistently with cortical thickness rather than cortical surface area (34, 35); similarly, hippocampal volume relates most consistently to early life stress (32). However, other mechanisms could contribute to such changes. Women could have lower access to beneficial, enriched environments, which could alter their brain structure through higher dendritic branching and increased synapse formation (17). Indeed, the composite indices of gender inequality incorporate the lower educational opportunities of women compared to men. The observed associations could also relate to very early disturbances in development, particularly since cortical thickness peaks early in brain maturation (23). Our study could not examine further which of these mechanisms were involved, since many types of adverse experiences coexist across societies (36). New studies looking at specific populations in which they are not as correlated might inform about the underlying mechanisms. Further insights could come from studies examining differences between cohorts that have been exposed to changing levels of adversity over time, particularly since some domains might improve earlier or be subject to specific policy interventions (such as improving perinatal care). A longitudinal temporal view would also strengthen the case for a causality mechanism in the observed association.
While analyses performed on small, nonrandom samples may not be representative of the population, we included multiple studies and from different cities in each country when possible, to increase representativeness. Neuroimaging is still an expensive tool, and despite our data including more than a third of participants from low- and middle-income economies, only India represented the low- and lower-middle-income groups. By focusing on the difference between the brains of women and men within each site included, our analyses were less likely to be biased by factors related to the MRI scanner and sequence used (37), or the ethnic and socioeconomic background of the population studied (13, 38).
These results highlight the relevance of the macrosocial environment where sex differences in brain structure are manifested. Future studies will need to examine the mechanisms involved, their moderating factors, and their timing, providing new opportunities for neuroscience-informed policies (21) to promote gender equality.
Materials and Methods
We included samples that reported structural MRI data (T1 weighted) acquired on 1.5T and 3T scanners from healthy adults aged 18 to 40 y (inclusive). Although “gender” is related to the individual expression of identity, gender inequality measured across countries is usually reduced to biological sex, which is also collected in many research data. We therefore use the term sex of participants, acknowledging incomplete overlap with gender identity. Data were obtained both from open-access platforms and collaborators across the world (Fig. 1), and they all had local institutional ethical approval. Images were analyzed with FreeSurfer, focusing on cortical thickness and surface area from 68 regions of the Desikan–Killiany’s template and the two hemispheres, as well as the hippocampal volumes. Age was linearly regressed out from each sample (23). Following previous studies focusing on sex differences beyond brain size (9), total intracranial volume was also controlled for in the surface area and hippocampal volume analyses (SI Appendix, Fig. S12). When examining localized (regional) associations, we corrected results for multiple testing using false discovery rate (FDR). Further details of the methods can be found in SI Appendix. Group-level data and the script with the main analyses can be downloaded from https://github.com/zugmana/CLGI. Dataset S1 provides detailed information on how to gain access to individual-subject data from the different sites included.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (DOCX)
Acknowledgments
We are grateful to all researchers who shared their data on different open-access platforms. The list of references acknowledging their work is found in Dataset S1. This work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). Funding from the different studies included can be found in SI Appendix.
Author contributions
N.A.C. designed research; A.Z., L.M.A., V.M., R.A.I.B., J.S., A.M.W., D.S.P., S.E.-L., and N.A.C. performed research; A.Z., L.M.A., V.M., C.A., A.A., L.A., M. Bellgrove, V.B., M. Bernardo, P.B., J.B.-B., R.B., G.F.B., M.N.C., T.C.-A., A.C., M.C., L.C., P.D., C.d.l.F.-S., M.D.F., C.M.D.-C., A.M.D.-Z., S.D.P., F.L.S.D., S.F., A.F., N.B.F., A.G., C.S.G., R.G., C.G.-R., C.G.C., A.G.-V., S.G., B.H., A.I., D.I., A.J., P.L.-O., C.L., C.L.-J., H.L., R. Massuda, P.M., J.M., R. Mizrahi, R. Murray, A.O., P.M.P., M.P., L.P., J.P.R.-M., R.R., T.R.M., F.R.-M., A.R., P.R., G. Salum, F.S., G. Schumann, M.S., D.J.S., A.T., J.T., T.U., J.U., E.A.U., P.V.-S., I.V., M.V., T.T.W.-B., N.Y., F.Z., M.V.Z., c.V., and N.A.C. contributed new reagents/analytic tools; A.Z., G.R., and N.A.C. analyzed data; L.M.A., V.M., and N.A.C. collated open-access data; and A.Z., L.M.A., V.M., R.A.I.B., J.S., G.R., C.A., A.A., L.A., M. Bellgrove, V.B., M. Bernardo, P.B., J.B.-B., R.B., G.F.B., M.N.C., T.C.-A., A.C., M.C., L.C., P.D., C.d.l.F.-S., M.D.F., C.M.D.-C., A.M.D.-Z., S.D.P., F.L.S.D., S.F., A.F., N.B.F., A.G., C.S.G., R.G., C.G.-R., C.G.C., A.G.-V., S.G., B.H., A.I., D.I., A.J., P.L.-O., C.L., C.L.-J., H.L., R. Massuda, P.M., J.M., R. Mizrahi, R. Murray, A.O., P.M.P., M.P., L.P., J.P.R.-M., R.R., T.R.M., F.R.-M., A.R., P.R., G. Salum, F.S., G. Schumann, M.S., D.J.S., A.T., J.T., T.U., J.U., E.A.U., P.V.-S., I.V., M.V., T.T.W.-B., N.Y., F.Z., M.V.Z., A.M.W., D.S.P., S.E.-L., and N.A.C. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. D.M.B. is a guest editor invited by the Editorial Board.
Contributor Information
Nicolas A. Crossley, Email: ncrossley@uc.cl.
Collaborators: Pratima Murthy, Amit Chakrabarti, Debasish Basu, B.N. Subodh, Lenin Singh, Roshan Singh, Kartik Kalyanram, Kamakshi Kartik, Kalyanaraman Kumaran, Ghattu Krishnaveni, Rebecca Kuriyan, Sunita Simon Kurpad, Gareth J. Barker, Rose D. Bharath, Sylvane Desrivieres, Meera Purushottam, Dimitri P. Orfanos, Eesha Sharma, Matthew Hickman, Jon Heron, Mireille B. Toledano, and Nilakshi Vaidya
Data, Materials, and Software Availability
Group data have been deposited in Github (https://github.com/zugmana/CLGI) (39). Individual participant data can be accessed from different sources as detailed in Dataset S1. Some datasets require consent from principal investigators named there.
Supporting Information
References
- 1.United Nations Entity for Gender Equality and the Empowerment of Women (UN Women), “Turning promises into action: gender equality in the 2030 agenda for sustainable development” (2018).
- 2.Crotti R., Pal K. K., Ratcheva V., Zahidi S., “Global Gender Gap Report 2021” (World Economic Forum, Switzerland, 2021). [Google Scholar]
- 3.Gaye A., Klugman J., Kovacevic M., Twigg S., Zambrano E., Measuring key disparities in human development: The gender inequality index. Human development research paper 2010/46 (2010).
- 4.Yu S., Uncovering the hidden impacts of inequality on mental health: A global study. Transl. Psychiatry 8, 98 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiso L., Monte F., Sapienza P., Zingales L., Culture, gender, and math. Science 320, 1164–1165 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Afifi M., Gender differences in mental health. Singapore Med. J. 48, 385 (2007). [PubMed] [Google Scholar]
- 7.Mallard T. T., et al. , X-chromosome influences on neuroanatomical variation in humans. Nat. Neurosci. 24, 1216–1224 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Paus T., Sex differences in the human brain: A developmental perspective. Prog. Brain Res. 186, 13–28 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Eliot L., Ahmed A., Khan H., Patel J., Dump the “dimorphism”: Comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci. Biobehav. Rev. 125, 667–697 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Farah M. J., et al. , Randomized manipulation of early cognitive experience impacts adult brain structure. J. Cogn. Neurosci. 33, 1197–1209 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Gehred M. Z., et al. , Long-term neural embedding of childhood adversity in a population-representative birth cohort followed for 5 decades. Biol. Psychiatry 90, 182–193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatzenbuehler M. L., et al. , Smaller hippocampal volume among black and latinx youth living in high-stigma contexts. J. Am. Acad. Child Adolesc. Psychiatry 61, 809–819 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble K. G., et al. , Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 18, 773–778 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen B. S., Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Ann. N. Y. Acad. Sci. 1204, 38–59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joos C. M., Wodzinski A. M., Wadsworth M. E., Dorn L. D., Neither antecedent nor consequence: Developmental integration of chronic stress, pubertal timing, and conditionally adapted stress response. Dev. Rev. 48, 1–23 (2018). [Google Scholar]
- 16.Tooley U. A., Bassett D. S., Mackey A. P., Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 22, 372–384 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nithianantharajah J., Hannan A. J., Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Eryilmaz H., et al. , Association of prenatal exposure to population-wide folic acid fortification with altered cerebral cortex maturation in youths. JAMA Psychiatry 75, 918–928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yodanis C. L., Gender inequality, violence against women, and fear: A cross-national test of the feminist theory of violence against women. J. Interpers. Violence 19, 655–675 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Mauvais-Jarvis F., et al. , Sex and gender: Modifiers of health, disease, and medicine. Lancet 396, 565–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farah M. J., Socioeconomic status and the brain: Prospects for neuroscience-informed policy. Nat. Rev. Neurosci. 19, 428–438 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Thompson P. M., et al. , The ENIGMA Consortium: Large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 8, 153–182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bethlehem R. A. I., et al. , Brain charts for the human lifespan. Nature 604, 525–533 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler A. M., et al. , Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53, 1135–1146 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmaal L., et al. , Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22, 900–909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boedhoe P. S. W., et al. , Subcortical brain volume, regional cortical thickness, and cortical surface area across disorders: Findings from the ENIGMA ADHD, ASD, and OCD working groups. Am. J. Psychiatry 177, 834–843 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayachandran S., The roots of gender inequality in developing countries. Annu. Rev. Econ. 7, 63–88 (2015). [Google Scholar]
- 28.Parvizi J., Rangarajan V., Shirer W. R., Desai N., Greicius M. D., The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron 80, 1359–1367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao X., et al. , Distinguishing neural correlates of context-dependent advantageous-and disadvantageous-inequity aversion. Proc. Natl. Acad. Sci. U.S.A. 115, E7680–E7689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindner M., et al. , Neural patterns underlying social comparisons of personal performance. Soc. Cogn. Affect. Neurosci. 10, 569–576 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., et al. , Cortical volume abnormalities in posttraumatic stress disorder: An ENIGMA-psychiatric genomics consortium PTSD workgroup mega-analysis. Mol. Psychiatry 26, 4331–4343 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupien S. J., McEwen B. S., Gunnar M. R., Heim C., Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Bone J. K., Lewis G., The role of gender inequalities in adolescent depression. Lancet Psychiatry 7, 471–472 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Wrocklage K. M., et al. , Cortical thickness reduction in combat exposed US veterans with and without PTSD. Eur. Neuropsychopharmacol. 27, 515–525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kremen W. S., et al. , Salivary cortisol and prefrontal cortical thickness in middle-aged men: A twin study. Neuroimage 53, 1093–1102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes K., et al. , The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Heal. 2, e356–e366 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Schnack H. G., et al. , Mapping reliability in multicenter MRI: Voxel-based morphometry and cortical thickness. Hum. Brain Mapp. 31, 1967–1982 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holla B., et al. , A series of five population-specific Indian brain templates and atlases spanning ages 6–60 years. Hum. Brain Mapp. 41, 5164–5175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crossley N. A., Zugman A., Group-level data and code for International Collaboration on the brain effects of gender inequality. CLGI. https://github.com/zugmana/CLGI5. Deposited 8 February 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (DOCX)
Data Availability Statement
Group data have been deposited in Github (https://github.com/zugmana/CLGI) (39). Individual participant data can be accessed from different sources as detailed in Dataset S1. Some datasets require consent from principal investigators named there.