Abstract
White-matter injury in sickle-cell disease (SCD) includes silent cerebral infarction diagnosed by diffusion tensor imaging (DTI), a complication associated with cognitive dysfunction in children with SCD. The link between white-matter injury and cognitive dysfunction has not been fully elucidated. The goal of this study was to define whether cerebrovascular lesions and cognitive function in SCD are linked to neuroaxonal damage and astrocyte activation in humanized Townes’ SCD mice homozygous for human sickle hemoglobin S (SS) and control mice homozygous for human normal hemoglobin A (AA). Mice underwent MRI with DTI and cognitive testing, and histology sections from their brains were stained to assess microstructural tissue damage, neuroaxonal damage, and astrocyte activation. Fractional anisotropy, showing microstructural cerebrovascular abnormalities identified by DTI in the white matter, was significantly associated with neuronal demyelination in the SS mouse brain. SS mice had reduced learning and memory function with a significantly lower discrimination index compared with AA control mice in the novel object recognition tests. Neuroaxonal damage in the SS mice was synchronously correlated with impaired neurocognitive function and activation of astrocytes. The interplay between astrocyte function and neurons may modulate cognitive performance in SCD.
Keywords: sickle-cell disease, white-matter injury, cognitive dysfunction, astrocytes
Introduction
In sickle-cell disease (SCD), cerebrovascular complications including overt stroke, silent cerebral infarction (SCI), and cognitive dysfunction contribute to significant morbidity and mortality (1). Cognitive dysfunction in SCD includes poor performance in learning and memory, language and attention, intellectual, executive, and adaptive functioning, and is associated with stroke and SCI (2). MRI with diffusion tensor imaging (DTI) has revealed widespread white-matter abnormalities in the brains of patients with SCD (3). Pathological findings have shown that cerebrovascular lesions primarily include neuroaxonal damage in cortical and frontoparietal deep white-matter border areas (4). It is not clear how cerebrovascular lesions lead to cognitive dysfunction. Astrocytes—the glial cells connecting cerebral microvessels and neuronal cells—maintain neuronal integrity (5) and preserve tissue plasticity and memory function (6). There is evidence from other diseases that episodic or sustained ischemia triggers astrocyte activation, leading to neuroaxonal damage and cognitive dysfunction (7). In this study, we investigated a novel mechanism of white-matter neuroaxonal damage and cognitive dysfunction in sickle mice that emphasizes the role of pathological astrocyte activation.
Results
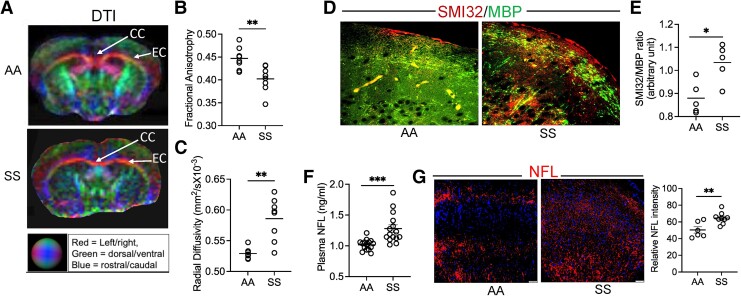
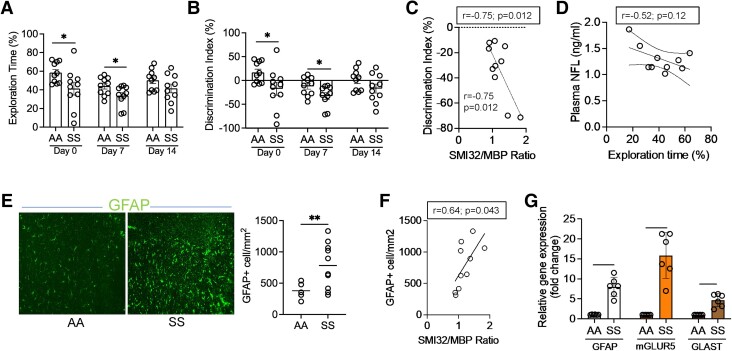
We performed DTI on the SS and AA mouse brains. Fractional anisotropy (FA), a marker of microstructural integrity of neuroaxonal fibers based on water diffusion directionality, was substantially lower, while radial diffusivity (RD), associated with changes in neuroaxonal diameter and density (8), was significantly elevated in SS mice compared with AA mice (P < 0.01, n = 8; Fig. 1A–C). We then confirmed white-matter injury histologically by measuring the expression of nonphosphorylated neurofilament H (SMI32) and myelin basic protein (MBP) and found an increased SMI32/MBP ratio in SS mice compared with AA controls (P < 0.05; n = 5; Fig. 1D and E). Neurofilament light chain (NFL), a neuron-specific protein component, is released following neuroaxonal damage and diffuses easily through brain parenchyma (8). There was a notable accumulation of NFL in SS mice plasma and brain when compared with controls, further confirming disruption of neuroaxonal integrity (Fig. 1F and G). Since increased accrual of NFL in the brain is associated with the activation of astrocytes, loss of neuronal plasticity, and impaired memory function in several diseases, including Alzheimer's, Parkinson's, and multiple sclerosis (9), we compared the neuroaxonal damage in SS mice with their neurocognitive responses and astrocyte activation. To test their differences in neurocognitive responses, SS and AA mice underwent Y-maze and novel object recognition (NOR) testing. The Y-maze test measures the willingness of rodents to explore new environments. The frequency of spontaneous alternations—a measure of spatial working memory—indicates the tendency of the mice to alternate the exploration of different arms of a Y-shaped maze (10). We found that the SS mice stayed longer in a new arm of the Y-maze, while the percent of spontaneous alternations between the three arms of the maze was reduced in the SS mice compared with AA mice (Fig. S1A and B). In the NOR study, the SS mice spent significantly less time exploring the novel object than their AA counterparts (Fig. 2A). The discrimination index, indicating the difference between the time spent exploring the familiar object and the time spent with the novel object, was significantly lower in the SS mice compared with the AA mice (Fig. 2B). We found a negative correlation (Pearson r = −0.75; P = 0.012) between the discrimination index in the NOR test and the ratio of SMI32/MBP staining intensity, demonstrating an association between white-matter integrity and cognitive function in the SS mice (Fig. 2C). Moreover, plasma NFL concentration was inversely correlated with the percentage of the novel object exploration time, suggesting that lower cognitive function is associated with increased plasma NFL in the SS mice (Fig. 2D). Activated astrocytes, phenotyped by a higher expression of glial fibrillary acidic protein (GFAP), colocalize with loss of neurons, demyelination, and abnormal FA after brain injury (11). We found a significant increase (>3.6-fold) in GFAP-positive (GFAP+)-activated astrocytes in the SS mice (Fig. 2E). The SMI32/MBP staining intensity ratio was positively correlated with the number of GFAP+ astrocytes in the SS mice (Pearson r = 0.64; P = 0.043; Fig. 2F). The astrocytes isolated from the SS mouse brain showed an upregulation of the GFAP gene expression (∼8-fold) and concurrent increases in the relative expression of metabolic glutamate receptor subtype 5 (mGLUR5; 17-fold) and glutamate aspartate transporter (GLAST; 4.5-fold) genes compared with AA astrocytes (Fig. 2G).
Fig. 1.
White-matter injury is linked to neuroaxonal damage in SCD mice. (A–C) Representative DTI images from AA and SS mice and quantitation of fractional anisotropy and RD (n = 8). The corpus callosum (CC) and external capsule (EC) represent the white-matter border area in the brain. (D and E) Elevated SMI32 accumulation compared with MBP and quantitation of staining intensity (SMI32/MBP ratio), indicating white-matter injury in the SS mice compared with AA mice (n = 5). (F) Plasma concentration of NFL in AA and SS mice (n = 15). (G) Representative immunofluorescence microscopic images and quantification of NFL staining intensity showing the expression of NFL in AA and SS mice (n = 6–9) brain with nuclear counterstain (DAPI). *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 2.
Cognitive dysfunction in sickle mice is associated with neuroaxonal damage and activation of cerebral astrocytes. (A and B) The AA and SS mice were tested for NOR at baseline on three different days as indicated (day 0, day 7, and day 14). Quantitation of percent exploration time and discrimination index showed lower NOR ability of the SS mice compared with the AA (n = 10). (C) Correlation of SMI32/MBP ratio and the Discrimination Index obtained from the same cohort of mice showing increased white-matter injury associated with poor NOR ability in the SS mice (n = 10). (D) Association of plasma NFL concentration with percentage of exploration time showed that poor NOR ability is associated with increased plasma NFL in the SS mice. (E) Cerebral tissue sections from AA and SS mice were stained for GFAP+ astrocytes. Increased number of GFAP+ astrocytes indicated astrocyte activation in the SS mice. (F) Correlation of SMI32/MBP staining intensity ratio (neuroaxonal damage indicator) and the number of GFAP+ astrocytes in the same cohort of SS mouse brain (n = 10; Pearson r = 0.64; P = 0.043). (G) Relative expression of GFAP, mGLUR5, and GLAST genes in astrocytes isolated from AA and SS mice brain tissue. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
We recapitulated studies in patients with SCD showing reduced FA in the subcortical white-matter border areas and higher RD in widespread regions of the brain (12), in humanized SS mice. Cerebral white matter primarily consists of neuronal axons, and hence the data from immunofluorescence-based histopathological staining showing an increased SMI32/MBP ratio as well as accumulation of NFL confirms disruption of neuroaxonal integrity in the SS brain tissue. The negative correlation between the discrimination index in the NOR test and the ratio of SMI32/MBP staining intensity, demonstrated an association between white-matter integrity and cognitive function in the SS mice. Decline in learning and memory function is an age-dependent phenomenon and it is generally evident in mice at older age (>6 months). Interestingly, we found cognitive deficit in sickle transgenic mice at a much younger age (∼3 months). In SCD, due to the underlying hemolytic as well as oxidative stress and chronic hypoxia, the tissue compartments including the brain suffer from subtle ischemic damage at an earlier age (3). As a result, many organs (including the brain, kidneys, and heart), and the immune system in SCD individuals show morphological and functional changes that are ordinarily seen in the elderly in the general population. Therefore, SCD is often considered as an accelerated aging syndrome (13). Our observation on learning and memory deficit in 12- to 14-week-old homozygous sickle mice (SS) might be due to the elevated intrinsic oxidative and inflammatory stresses, characteristics of SCD. Moreover, in a different study involving same strain of sickle mice (Townes'), deficits in cognitive learning were observed at an average age of 13.6 weeks using water T-maze test (14). Our findings in mice are also consistent with prior evidence showing that neuroinflammation with activated microglia was associated with cognitive deficits detected by NOR in sickle mice (15). These results are important because they mimic findings in children with SCD, where the presence of silent infarcts detected by DTI is associated with cognitive dysfunction (3).
In summary, we found that MRI-detectable white-matter abnormalities in Townes’ sickle mice correspond to neuroaxonal damage detected by histology, and cognitive dysfunction, thereby linking neuroradiological imaging to brain pathology and behavioral phenotype. Astrocyte activation is mediated by rapid intracellular calcium accumulation, and increased intracellular calcium in astrocytes has been found to alter synaptic plasticity by activation of type 5 metabotropic glutamate receptors (mGLUR5), and the glutamate transporter (GLAST) (16). It will be important to test whether astrocyte signaling via the mGLUR5 receptor is mechanistically linked to brain injury in SCD.
This shows that white-matter injury with demyelination of neuronal axons is associated with cognitive dysfunction in sickle mice. In addition, we found that activation of astrocytes may play a pivotal role in the development of neuroaxonal damage and cognitive dysfunction in SCD. In particular, astrocyte signaling via the mGLUR5 receptor may represent a novel pathway of brain injury in SCD. Further research into the mechanisms of astrocyte activation and the consequent molecular signaling pathways are warranted to elucidate the pathogenesis of cognitive dysfunction in SCD.
Materials and methods
The detailed information on methods are provided as Supplementary Information.
Animals
The knock in Townes’ sickle-cell mice expressing human HbS (SS) and the control mice expressing human HbA (AA) were purchased from The Jackson Laboratory (Strain# 013071) and studied at the University of Pittsburgh. All animal experiments were performed following Institutional Animal Care and Use Committee approval (Protocols #22010095, #21048805, and #21120288). Both male and female mice were used. All SS and AA mice were 12–14 weeks old at the time of experiments.
In vivo diffusion tensor imaging
In vivo noninvasive DTI was performed on AA and SS mice using a Bruker AV3HD 9.4T scanner, an 86 mm Tx coil, and four-channel mouse brain receiver array, running ParaVision 6.0.1 (Bruker BioSpin, Billerica MA, USA).
Immunofluorescence
Brain tissue sections were prepared from the same cohort of SS and AA mice following DTI. The sections were stained using the antibodies for MBP, SMI32, NFL, or GFAP. All images were analyzed using an Olympus AX70 microscope and the staining intensity was determined using ImageJ.
Real-time PCR for gene expression in isolated cerebral astrocytes
Primary astrocytes were isolated from AA and SS mouse brains from single-cell suspensions using microbeads labeled with the astrocyte-specific anti-ACSA-1 antibody (Miltenyi Biotec; #130-095-826) to evaluate the mRNA expression of GFAP, mGLUR5, and GLAST.
In vivo cognitive assessment
The SS and AA mice were placed in a Y-shaped maze to assess their spatial memory function, while the NOR test was used to evaluate short-term memory deficits. The alternations in Y-maze and the time spent with new and familiar objects in NOR test were recorded using the Anymaze system (17).
Supplementary Material
Acknowledgments
The authors thank Danielle Crosby and Diane Lenhart for their technical assistance with multiple experiments. This study was made possible by support from the Pittsburgh Heart, Lung, and Blood Vascular Medicine Institute, the Hemophilia Center of Western Pennsylvania, and Vitalant, Pittsburgh, PA (R.H.; 2021–2023), a Winters Foundation grant (R.H.; 2020), NIH grant R01DK124426-01A1 (S.G.), R01 HL127107-01A1 (E.M.N.), and VA grant I01 BX003651 (X.H.).
Contributor Information
Rimi Hazra, Department of Medicine, Pittsburgh Heart Lung and Blood Vascular Medicine Institute, University of Pittsburgh, 200 Lothrop Street, Pittsburgh, PA 15261, USA.
Hongjian Pu, Department of Neurology, University of Pittsburgh, 3471 Fifth Avenue, Pittsburgh, PA 15213, USA.
Lesley M Foley, Animal Imaging Center, McGowan Institute of Regenerative Medicine, University of Pittsburgh, 450 Technology Drive, Pittsburgh, PA 15219, USA.
Lynda Little-Ihrig, Department of Medicine, Pittsburgh Heart Lung and Blood Vascular Medicine Institute, University of Pittsburgh, 200 Lothrop Street, Pittsburgh, PA 15261, USA.
T Kevin Hitchens, Animal Imaging Center, McGowan Institute of Regenerative Medicine, University of Pittsburgh, 450 Technology Drive, Pittsburgh, PA 15219, USA.
Samit Ghosh, Department of Medicine, Pittsburgh Heart Lung and Blood Vascular Medicine Institute, University of Pittsburgh, 200 Lothrop Street, Pittsburgh, PA 15261, USA.
Solomon F Ofori-Acquah, Department of Medicine, Pittsburgh Heart Lung and Blood Vascular Medicine Institute, University of Pittsburgh, 200 Lothrop Street, Pittsburgh, PA 15261, USA.
Xiaoming Hu, Department of Neurology, University of Pittsburgh, 3471 Fifth Avenue, Pittsburgh, PA 15213, USA.
Enrico M Novelli, Department of Medicine, Pittsburgh Heart Lung and Blood Vascular Medicine Institute, University of Pittsburgh, 200 Lothrop Street, Pittsburgh, PA 15261, USA.
Supplementary Material
Supplementary material is available at PNAS Nexus online.
Author contributions
R.H. developed the ideas, designed the study, performed experiments, analyzed data, and wrote the manuscript. H.P. and L.L.-I. performed cognitive experiments and edited the manuscript. L.M.F. and T.K.H. performed MRI experiments and analyzed the data. S.G. designed experiments, interpreted data, and edited the manuscript. S.F.O.-A. reviewed the manuscript. E.M.N. shared materials and data, and E.M.N. and X.H. critically reviewed and edited the manuscript. All authors read and approved the manuscript.
Data availability
All data are included in the manuscript and/or supporting information.
References
- 1. Bernaudin F, et al. . 2015. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 125:1653–1661. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong FD, et al. . 1996. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics. 97:864–870. [PubMed] [Google Scholar]
- 3. Kawadler JM, et al. . 2015. White matter damage relates to oxygen saturation in children with sickle cell anemia without silent cerebral infarcts. Stroke. 46:1793–1799. [DOI] [PubMed] [Google Scholar]
- 4. Adams RJ, et al. . 1988. Cerebral infarction in sickle cell anemia: mechanism based on CT and MRI. Neurology. 38:1012–1017. [DOI] [PubMed] [Google Scholar]
- 5. Marina N, et al. . 2020. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat Commun. 11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siracusa R, Fusco R, Cuzzocrea S. 2019. Astrocytes: role and functions in brain pathologies. Front Pharmacol. 10:1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee HS, et al. . 2014. Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A. 111:E3343–E3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alirezaei Z, et al. . 2020. Neurofilament light chain as a biomarker, and correlation with magnetic resonance imaging in diagnosis of CNS-related disorders. Mol Neurobiol. 57:469–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bridel C, et al. . 2019. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 76:1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraeuter AK, Guest PC, Sarnyai Z. 2019. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 1916:105–111. [DOI] [PubMed] [Google Scholar]
- 11. Lee JK, et al. . 2021. Fractional anisotropy from diffusion tensor imaging correlates with acute astrocyte and myelin swelling in neonatal swine models of excitotoxic and hypoxic-ischemic brain injury. J Comp Neurol. 529:2750–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Issar P, Nehra M, Singh G, Issar SK. 2018. Conventional and advanced brain MR imaging in patients with sickle cell anemia. Indian J Radiol Imaging. 28:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Idris IM, Botchwey EA, Hyacinth HI. 2022. Sickle cell disease as an accelerated aging syndrome. Exp Biol Med (Maywood). 247:368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, et al. . 2016. Cognitive and behavior deficits in sickle cell mice are associated with profound neuropathologic changes in hippocampus and cerebellum. Neurobiol Dis. 85:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hardy RA, Rached NA, Jones JA, Archer DR, Hyacinth HI. 2021. Role of age and neuroinflammation in the mechanism of cognitive deficits in sickle cell disease. Exp Biol Med (Maywood). 246:106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shigetomi E, Saito K, Sano F, Koizumi S. 2019. Aberrant calcium signals in reactive astrocytes: a key process in neurological disorders. Int J Mol Sci. 20:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pu H, et al. . 2021. Intranasal delivery of interleukin-4 attenuates chronic cognitive deficits via beneficial microglial responses in experimental traumatic brain injury. J Cereb Blood Flow Metab. 41:2870–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and/or supporting information.