Abstract
Objective
Evaluate the impact of a targeted family communication intervention for mothers undergoing genetic counseling and testing (GCT) for BRCA gene alterations.
Methods
Following BRCA GCT, mothers (N = 204; M age = 45 y) were randomized to either a control condition (self-help print materials) or intervention (printed decision support guide, based on behavioral decision making theory in health care) for supporting choices about disclosing maternal genetic test results to children and adolescents. Behavioral assessments were administered prior to maternal GCT and after receipt of results: primary outcomes were maternal disclosure to children and parent-child communication quality.
Results
Mothers in the intervention were > 2x likely to disclose their BRCA test results to their children compared to those in the control condition (odds ratio [OR] = 2.33, 95% confidence interval [CI] = 1.06, 5.10; p = .04). This effect was moderated by children’s ages: mothers of preteens (<13 y) assigned to the intervention were >3x likely to disclose their results (OR = 3.74, 95% CI = 1.49, 9.41; p = .005). In adjusted models, intervention was also associated with favorable changes in the quality of parent-child communication (95% CI = 0.30, 9.00; p < .05).
Conclusion
Decision support improves parent-child communication outcomes about GCT for hereditary breast-ovarian cancer.
Innovation
This trial is among the first to empirically evaluate the outcomes of a behavioral intervention to support family communication of maternal BRCA risk information to children.
Keywords: Genetic counseling, Genetic testing, Family communication, Parents, Children
1. Introduction
Genetic counseling and testing (GCT) for susceptibility to hereditary breast-ovarian cancer (HBOC) is integral to the management of many women’s treatment decision-making, as well as prevention choices among those with a family history of these cancers [1]. Pathogenic variants (PVs) in the BRCA1 and BRCA2 (BRCA) genes account for most cases of HBOC [2]. A positive BRCA result is associated with a lifetime risk of breast cancer of at least 70% and a risk of ovarian cancer ranging between 17–44% [3]. First-degree relatives of individuals with a PV in BRCA have a 50% chance of testing positive. These at-risk relatives include minor-age children and adolescents who are not recommended to undergo testing until adulthood [4].
Mothers tested for BRCA often struggle with what genetic results mean for their own health and that of their children. One study observed that 81% of mothers with breast cancer were concerned about their children’s risk, 71% felt childhood was the most appropriate time to provide HBOC education, and 65% wanted professional assistance navigating this issue [5]. Parents with a known family history of cancer commonly report needing more information about how to talk with their children about hereditary risk [6]. Patient education resources exist for adults seeking cancer predisposition testing [7,8] as well as for children at increased risk of developing hereditary cancers in childhood (e.g., familial adenomatous polyposis) [9,10]. However, there are limited resources available for parents and children that focus on the decisions and implications of parental GCT for adult-onset cancers for minor-age offspring.
Parents in this situation must be educated and counseled about what the results mean for their own health, as well as that of their potentially at-risk relatives and including their children [11]. These implications include, but are not limited to, opportunities for family members (children and adults alike) to be more aware of cancer risk and engage in cancer-preventive behaviors, such as cascade genetic testing in adulthood [12]. Such outcomes are largely predicated on patients’ disclosure of their PV status, and more open communication styles and behavior patterns, within and among families at-risk--outcomes that are poorly understood, and contribute to the low uptake of GCT in these kindreds [13]. Early family communication about cancer and cancer risk may also help children and adults better understand their family’s health history and its role in their own health maintenance [14].
Prior research suggests parents with a family history of cancer often need more information on how to discuss hereditary cancer risks with their children [15], including guidance on deciding if, when, and how they should share their test results with their children [16]. Decision aids are resources that educate patients about care options and are known to reduce decisional conflict when individuals make choices in line with their preferences [17]. A number of decision support tools have been created to assist individuals with decisions surrounding HBOC risk management [18]. However, there is a lack of evidence-based tools available to help guide mothers regarding family communication about HBOC risk. Providing families with resources to facilitate these conversations is important because open conversations can improve the outcomes of hereditary cancer risk management [19].
To address this gap, we developed a decision support guide modeled after the principles of the Ottawa Decision Support Framework, and as an adjunct to standard GCT [20]. This intervention aims to facilitate patients undergoing GCT for BRCA as part of comprehensive HBOC risk management in reaching more fully-informed choices about their communication of adult-onset inherited cancer syndrome risk information to their children and adolescents. As part of a randomized controlled trial, we sought to determine if the intervention enhanced GCT outcomes (i.e., family communication) for this population.
2. Methods
2.1. Design
This research focused on improving psychosocial outcomes of GCT for BRCA, including mothers’ disclosure of test results to their children and adolescents. We conducted multivariable analyses of randomized controlled trial data comparing the relative effects of a targeted intervention (family communication decision support guide) or a comparison condition (patient education only).
2.2. Intervention development and delivery
A detailed description of the intervention development process and its contents is published elsewhere [20]. Briefly, the study team followed guidelines for the development of family-oriented health education materials [21], as well as informed decision-making interventions [22], and decision-making interventions in cancer control [23]. The patient decision aid was designed to help mothers think about whether, when, how, and in what detail to talk with their children and adolescents about their genetic test results and the associated implications for cancer control and prevention. Called “My Children, My Test Results”, it is intended to provide information and support to mothers across four steps: (1) clarify their family communication choices, (2) identify decision-making needs, (3) exploring these needs, and (4) developing and implementing a communication action plan as a next step. Composite quotations are included throughout the decision aid, representing a variety of viewpoints about communication decisions, concerns, and potential outcomes. These quotations were adapted from prior research, along with summary questions and tips.
2.3. Sample
Participants were recruited in-person, and by mail/phone, surrounding pre-test GCT from two comprehensive cancer research and treatment centers in the mid-Atlantic and northeast regions of the US. Study participants included female primary caretakers (age 21+) to at least one child (ages 8–17 years-old), who resided in the same home as the child for at least the past 6 months, and with intentions to continue living together for the next 6 months. Eligible participants were also able to comprehend English, underwent GCT for BRCA genes, and had consistent access to a telephone.
At enrollment, participants enumerated their children, including ages, dates of birth, genders, birth order, and birth relationship (e.g., biological child). For those with more than one child between the ages of 8–17 years-old, a random selection algorithm denoted the family’s index child of interest to alleviate parental selection, reduce bias, and maintain 1:1 parent-child dyads for statistical analysis. Using adaptive assignment, we stratified index children reported on by the sample of participants in two ways: by age as “younger” (8–12 years-old) or “older” children (ages 13–17 years-old), and by gender (female or male).
2.4. Procedures
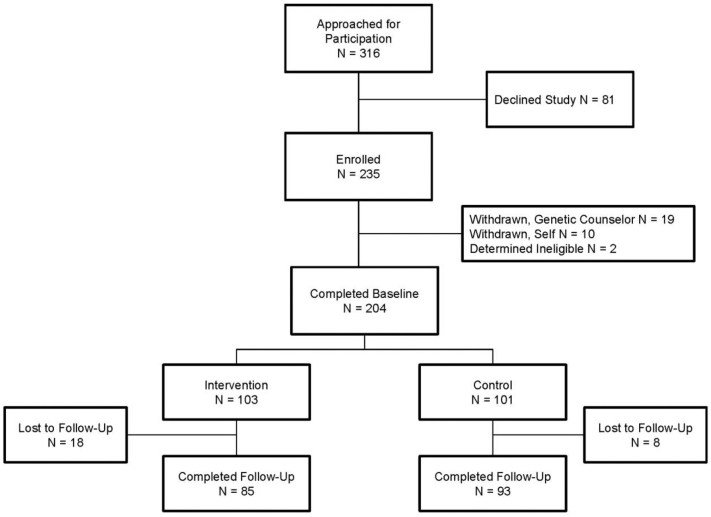
The trial’s recruitment, enrollment, and evaluation cascade is presented in Fig. A.1. A baseline assessment was conducted by telephone within seven days of pre-test genetic counseling. Following the baseline, participants were randomly allocated to either the intervention or control condition using a random digit table. In the control condition, participants were provided with a print guide offered by the National Cancer Institute at the National Institutes of Health (“Genetic Testing for Breast and Ovarian Cancer Risk: It’s Your Choice”). This comprehensive guide addressed how family history may contribute to HBOC, BRCA genes, risks and benefits of genetic testing, medical management options for carriers of PVs, and questions to consider when participating in GCT (including family communication, and informing one’s potentially at-risk relatives about HBOC risk). The intervention condition included providing participants with “My Children, My Test Results.” Follow-up assessments were conducted via telephone approximately 30 days after post-test genetic counseling. A $10 gift card was offered to participants upon the completion of each survey.
Fig. A.1.
Trial’s participant flow diagram, following the CONsolidated Standards of Reporting Trials (CONSORT).
2.5. Measures
Outcomes of GCT were assessed using reliable and valid self-report measures. Trial assessments consisted of a multi-item, multi-dimensional self-report survey lasting approximately 30 minutes.
2.5.1. Disclosure of maternal BRCA test results to children and adolescents
The trial’s assessment of whether mothers elected to share BRCA test results with their index child by the time of the 1-month follow-up included a single Yes/No item previously demonstrated to be sensitive to maternal HBOC and GCT contexts [24].
2.5.2. Quality of parent-child communication
Parent-child communication quality is a central aspect of parent-child psychosocial well-being [25]. The Parent-Adolescent Communication Scale was developed as a clinical research measure to assess this construct [26]. It has 20 items and a 5-point scale tapping both positive (e.g., openness) and negative (e.g., problems) features of parent-child verbal and nonverbal dyadic exchanges, with excellent psychometric properties.
2.5.3. General maternal psychosocial stress
To assess mothers’ self-reported levels of general psychosocial stress (e.g., anxiety and depression symptoms), we used a 12 item measure from the Brief Symptom Inventory [27] at baseline and follow-up. It measures psychological discomfort in reference to different statements (1=Not at all, 4=Extremely) and with adequate test-retest reliability.
2.5.4. General child psychosocial stress
We used a 16-item screening instrument specifically designed for mother-reported observations of the degree of presence (0=Not true, 2=Very true or often true) of stress- and worry-related behaviors in their children. These items are derived from the Child Behavior Checklist, which is among the most widely used parent report measures of behavioral symptoms in children [28]. The scale for stress/worry assesses children’s general dysphoria, traumatic stress, and behavior problems, and has been found reliable.
2.6. Data analysis
The analysis of the randomized controlled trial data was completed in multiple steps. First, descriptive statistics were generated about the characteristics of the entire sample, including maternal and child demographics and maternal clinical features. Next, psychosocial outcomes were described, followed by descriptive statistics for the intervention and control arms and a comparison between the baseline demographic characteristics of those in each arm of the trial: no significant differences were observed at baseline (see Table A.1). The effect of the intervention on maternal disclosure of BRCA test results at the 1-month follow-up time point was then evaluated, along with the putative effects of BRCA results themselves, maternal proband status, and the age and gender of the household’s index child. These effects were evaluated by logistic regression for the binary disclosure outcome, generating odds ratios and confidence intervals. Interactions were also examined, including where significant main effects existed, and stratified results are presented (Table B.1). Finally, an analysis of covariance (ANCOVA) model was run to test for possible differences in the adjusted means of the psychosocial outcomes by whether or not maternal disclosure had (disclosed) or had not (not disclosed) occurred, stratified by trial arm, and examined within. Adjustments were made based on the possibility that maternal, child, and site-specific differences may have confounded the associations of interest: estimates were provided with 95% confidence intervals (Table B.2). Baseline factors that were not significantly associated with the outcomes at the bivariate level were not retained in multivariable models.
Table A.1.
Baseline sample characteristics, overall and stratified by randomized controlled trial condition.
| Full sample |
Intervention condition |
Control condition |
|
|---|---|---|---|
| N = 204 | N = 103 | N = 101 | |
| Maternal Demographics | |||
| Age, years (M [SD]) | 44.6 (5.3) | 43.9 (5.4) | 45.2 (5.1) |
| Race | |||
| Non-Hispanic white | 70.6% (144) | 69.9% (72) | 71.3% (72) |
| Non-white | 29.4% (60) | 30.1% (29) | 28.7% (29) |
| Education | |||
| < College Education | 26.5% (54) | 28.2% (29) | 24.8% (25) |
| ≥ College Education | 73.5% (150) | 71.8% (74) | 75.2% (76) |
| Marital Status | |||
| Married/Living as Married | 79.4% (162) | 78.6% (81) | 80.2% (81) |
| Unmarried | 20.6% (42) | 21.4% (22) | 19.8% (20) |
| Maternal Clinical Characteristics | |||
| Proband Status | |||
| Yes | 82.8% (169) | 80.6% (83) | 85.1% (86) |
| No | 17.2% (35) | 19.4% (20) | 14.9% (15) |
| Number of First-Degree Relatives with Breast/Ovarian Cancer (M [SD]) | 0.56 (0.67) | 0.48 (0.67) | 0.65 (0.65) |
| Personal History of Breast/Ovarian Cancer | |||
| Yes | 52.5% (107) | 57.3% (59) | 47.5% (48) |
| No | 47.5% (97) | 42.7% (44) | 52.5% (53) |
| BRCA Genetic Test Results | |||
| Negative/Uninformative | 90.2% (184) | 91.3% (94) | 89.1% (90) |
| Positive | 9.8% (20) | 8.7% (9) | 10.9% (11) |
| Index Child Demographics | |||
| Age (M [SD]) | 12.5 (3.0) | 12.5 (3.0) | 12.6 (3.0) |
| <13 years | 48.5% (99) | 47.6% (49) | 49.5% (50) |
| ≥13 years | 51.5% (105) | 52.4% (54) | 50.5% (51) |
| Gender | |||
| Female | 52.0% (106) | 52.4% (54) | 51.5% (52) |
| Male | 48.0 % (98) | 47.6% (59) | 48.5% (49) |
| Psychosocial Outcomes | |||
| Parent-Child Communication (M [SD]) | 79.5 (9.7) | 80.0 (9.2) | 79.0 (10.3) |
| Maternal Psychosocial Stress (M [SD]) | 17.2 (5.6) | 17.4 (5.8) | 17.0 (5.3) |
| Child Psychosocial Stress (M [SD]) | 4.4 (3.9) | 4.4 (4.0) | 4.4 (3.8) |
| Trial Site | |||
| 1 | 66.7% (136) | 66.0% (68) | 67.3% (68) |
| 2 | 33.3% (68) | 34.0% (35) | 32.7% (33) |
Data display % (n) within a column unless otherwise indicated. M, Mean; SD, Standard deviation. There were no statistically significant differences between groups at baseline.
Table B.1.
Intervention effects on maternal disclosure of BRCA test results at follow-up by child age.
| Full sample (N = 178) |
Index children < 13 years-old (n = 86) |
Index children ≥ 13 years-old (n = 92) |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Trial Condition | .035 | .005 | .663 | |||
| Intervention | 2.33 (1.06, 5.10) | 3.74 (1.49, 9.41) | 0.71 (0.15, 3.34) | |||
| Control | Ref. | Ref. | Ref. | |||
| BRCA Test Result | .061 | .175 | .016 | |||
| Negative/Uninformative | 3.94 (0.94, 16.61) | 3.01 (0.61, 14.77) | 13.90 (1.65, 117.34) | |||
| Positive | Ref. | Ref. | Ref. | |||
| Proband | .183 | .900 | .023 | |||
| Yes | 2.09 (0.71, 6.18) | 1.09 (0.30, 3.92) | 7.99 (1.29, 46.65) | |||
| No | Ref. | Ref. | Ref. | |||
| Index Child Age | 1.56 (1.34, 1.82) | <.001 | -- | -- | -- | -- |
| Trial Site | .228 | .569 | .281 | |||
| 1 | 0.78 (0.51, 1.17) | 0.86 (0.52, 1.43) | 0.66 (0.31, 1.41) | |||
| 2 | Ref. | Ref. | Ref. | |||
OR = Odds ratio, 95% CI = 95% Confidence interval, Ref. = Reference group. Trial arm x child age interaction p = .025 in model with full sample.
Table B.2.
Adjusted means for psychosocial outcomes at follow-up by maternal disclosure of BRCA test results within each trial arm.
| Intervention condition |
Control condition |
|||||||
|---|---|---|---|---|---|---|---|---|
| Psychosocial outcome | Overall | Disclosed | Not disclosed | 95% CI difference | Overall | Disclosed | Not disclosed | 95% CI difference |
| Parent-Child Communication Quality | 78.2 (1.0) | 80.5 (1.1) | 75.9 (1.5) | 0.3, 9.0 | 78.4 (0.9) | 78.0 (1.2) | 78.8 (1.2) | −3.4, 5.0 |
| Maternal Psychosocial Stress | 16.1 (0.60) | 15.9 (0.66) | 16.3 (0.85) | −2.1, 2.9 | 16.2 (0.56) | 16.6 (0.72) | 15.8 (0.72) | −3.2, 1.8 |
| Child Psychosocial Stress | 4.3 (0.37) | 4.0 (0.41) | 4.7 (0.53) | −0.90, 2.2 | 4.3 (0.34) | 3.9 (0.45) | 4.7 (0.45) | −0.72, 2.3 |
Data are adjusted means (standard errors) from ANCOVAs. Models adjusted for child age, maternal BRCA test result, proband status, study site, and baseline measure of the psychosocial outcome to address potential confounding of the relationship between disclosure and the outcomes. Bolded text indicates means differed significantly at p < .05 after Bonferroni correction for multiple comparisons. 95% CI = 95% Confidence interval.
3. Results
3.1. Participant characteristics
A total of N = 316 mothers were approached during the trial’s 18-month enrollment period, and N= 235 of these mothers (74.4%) consented to participate. Of those who completed a baseline survey, N= 178 also completed a follow-up assessment (87.3%). Table A.1 presents baseline sociodemographic, clinical, and behavioral descriptions of the sample. Trial participants averaged 44.6 years-old (SD = 5.3), were mostly non-Hispanic white (n = 144, 70.6%), married or in a partnered relationship (n = 162, 79.4%), and college-educated (n = 150, 73.5%). Clinically, 52.5% had a personal history of breast or ovarian cancer: 90.2% of participants received negative or uninformative BRCA test results and the majority were family probands (n = 169, 82.8%). Of the index children, about half were female (n = 106, 52.0%) and at least 13 years-old (n = 105, 51.5%).
3.2. Effectiveness of the intervention on GCT outcomes
The effects of including a family communication decision-making support guide on maternal disclosure of BRCA test results to children and adolescents is summarized in Table B.2. Overall, those who received the targeted guide reported a more than doubling of the odds of discussing their genetic test results with their children compared to those in the control condition (OR = 2.33, 95% CI [1.06, 5.10]). This intervention effect was moderated by child age, where the odds of a mother sharing her BRCA results with a preteen were more than three times greater among patients in the intervention group than among controls (OR = 3.74, 95% CI [1.49, 9.41]). The mother’s genetic test result also had an effect on sharing results with older children, as mothers were close to 14 times more likely to share negative results with their children if the child was 13 or older, compared to when they received a positive test result (OR = 13.90, 95% CI [1.65, 117.34]). Mothers whose index child was 13 years or older were about 8 times more likely to disclose their test results if they had proband status than those who did not (OR = 7.99, 95% CI [1.29, 46.65]). All of these variables were statistically significantly associated with the intervention, with p-values less than 0.05.
3.3. Psychosocial GCT outcomes and disclosure of BRCA results
A one-way ANCOVA was conducted to compare the efficacy of the family communication decision support guide while controlling for parent-child communication at baseline. The adjusted means for psychosocial GCT outcomes evaluated at follow-up are reported in Table B.2. The intervention was successful at promoting more open and less conflictual communication behaviors between children and their mothers. Importantly, the intervention resulted in stronger parent-child communication patterns for mothers who disclosed their BRCA test result (adjusted mean = 80.5) compared to those who did not (adjusted mean = 75.9). These adjusted means differed significantly with p-values less than 0.05, even after Bonferroni corrections were applied for multiple comparisons. There was no statistically significant effect of the intervention on mother or child general psychosocial stress. However, mothers in the intervention who disclosed their BRCA status had lower stress scores than did those who disclosed in the control group (Table B.2).
4. Discussion and conclusion
4.1. Discussion
Family communication of BRCA genetic testing to adult at-risk relatives has been studied [29]. However, far fewer efforts have been made to understand the process and content of such communication with children, adolescents, and young adults [30]. As such, few tools exist to help parents consider the implications of their BRCA GCT with respect to whether, when, and how to disclose that information to their children, and these resources have not been evaluated in clinical trials to monitor their impact [21,31].
To address this gap, we conducted a randomized trial to assess the outcomes of a decision-making support guide on BRCA-tested mothers’ family communication choices with their 8–17 year-old children. We found that compared to mothers in a control condition, those who received the intervention were more than twice as likely to discuss their GCT and BRCA test results with children. The data revealed that mothers’ disclosure appeared to enrich the parent-child communication dynamic overall: mothers receiving the intervention were more likely to report that such discussions fostered open communication interactions with their children. Additionally, intervention participants who disclosed their genetic results were less distressed than mothers who shared their results but did not receive the intervention. Taken together, these findings reflect that such mothers may have experienced relief after disclosing their results and that subsequent discussions with their children about GCT, if any, did not add to stress. It also points to the fact that mothers were satisfied with their disclosure decision, and perhaps the process of achieving this outcome. Furthermore, it is likely that if disclosure had resulted in emotional challenges among their children, maternal stress levels would have mirrored this impact: we did not observe this. Prior research supporting this assertion has demonstrated that the disclosure of maternal BRCA GCT does not adversely affect the long-term quality of life in at-risk adolescents or young adult offspring [32], further bolstering its safety and efficacy.
This trial’s findings also indicate that mothers who received the intervention guide were three times more likely to share their results with preteen children than mothers in the comparison condition. This finding is particularly interesting because a majority of the participants had received BRCA negative or uninformative genetic test results. Under this scenario, mothers may have felt more knowledgeable about and empowered to share ʻgood news’ about GCT with their younger children without over-explaining medical findings. Conversely, mothers who were probands were more likely to disclose their BRCA results to their teenagers. Because those mothers had a personal history of cancer, such discussions may have been too complex for younger children to comprehend but older children were considered mature enough to appreciate the significance.
When the effect of genetic test results on maternal disclosure was examined, striking differences were found: mothers were far more likely to share negative vs. positive results with teenagers. This outcome is not surprising since revealing positive results to older children may entail an explanation of the parents’ cancer risks and medical follow-up, as well as the children’s cancer risks, risk of inheriting the pathogenic variant, and possible testing implications.
Although the number of BRCA PV carriers who received the intervention was modest, the fact that they were less likely to share their results with children suggests that these parents might benefit from additional and more targeted forms of patient education, guided decision-making support, and/or counseling. For example, they may benefit from obtaining formal support and guidance from healthcare professionals (e.g., genetic counselors) or informal strategies from peer supports (i.e., other mothers who carry PVs in BRCA and have navigated issues related to communicating with their children) [33]. In both scenarios, the outcomes of such efforts should be evaluated in clinical trials and the results of any such efforts remain to be seen.
4.1.1. Limitations
The study sample was predominantly non-Hispanic White, partnered, educated, and resided in or near urban areas. This limits the trial’s findings to those from underrepresented and more rural populations. Also, trial participants received comprehensive pre- and post-test genetic counseling with professional counselors at research-intensive cancer centers. This level of patient education and support may not be available to patients seen in community-based practices, and especially if they obtain BRCA testing through a direct-to-consumer company, primary care provider, or laboratory. These points underscore the importance of educating all patients about the importance of family communication early in the GCT process, including disclosure to at-risk relatives and children [33].
4.2. Innovation
Future research can assess whether supplementing the decision guide with alternate forms of education and support, especially among mothers carrying BRCA PVs, produces more uniformly beneficial outcomes. Other research could also include broader dissemination and evaluation in non-clinical and community-based settings with more diverse patient populations, including fathers. Subsequent iterations of the intervention could also be tailored to specific issues related to the disclosure of GCT for other high-risk genes, such as those associated with Lynch syndrome. The communication of positive test results to minor children may be especially important because these discussions may affect how they adapt to their risk status (e.g., are more aware of cancer risk and/or engage in cancer preventive behaviors), as well as make decisions and utilize cascade testing in adulthood [22]. Such testing may inform medical management choices that can potentially lead to early detection of cancer, cancer risk reduction, and decreased mortality, especially for females [34]. Individuals who test negative for the familial BRCA PV may obtain psychological relief and avoid unnecessary surveillance or risk-reduction measures [35]. For these reasons, it would be interesting to determine whether receiving education and support about communication with children could also prompt disclosure to adult relatives (or vice versa).
4.3. Conclusion
In conclusion, compared to standard print material, the decision guide resulted in a higher level of maternal disclosure of BRCA test results that appeared to be beneficial to mothers and their relationship with their children. This sets the stage for further dissemination of the intervention and its evaluation in broader populations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Institutes of Health (grant numbers HG002686, CA246589, and CA051008). We would like to acknowledge the contributions of the late Andrea Farkas Patenaude, PhD. We also wish to thank the participants and research staff who made this study possible.
References
- 1.US Preventive Services Task Force, Owens D.K., Davidson K.W., Krist A.H., Barry M.J., Cabana M., et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US preventive services task force recommendation statement. JAMA. 2019;322:652–665. doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- 2.LaDuca H., Polley E.C., Yussuf A., Hoang L., Gutierrez S., Hart S.N., et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet. Med. 2020;22:407–415. doi: 10.1038/s41436-019-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 4.Committee on Bioethics, Committee on Genetics, American College of Medical Genetics, Genomics Social, Ethical, Legal Issues Committee Ethical and policy issues in genetic testing and screening of children. Pediatrics. 2013;131:620–622. doi: 10.1542/peds.2012-3680. [DOI] [PubMed] [Google Scholar]
- 5.Miesfeldt S., Cohn W.F., Jones S.M., Ropka M.E., Weinstein J.C. Breast cancer survivors’ attitudes about communication of breast cancer risk to their children. Am. J. Med. Genet. C: Semin. Med. Genet. 2003;119C:45–50. doi: 10.1002/ajmg.c.10012. [DOI] [PubMed] [Google Scholar]
- 6.Huelsman K., Howell K., Parmar S., Pritzlaff M., Shanmugham A., White S., et al. 20th annual education conference, National Society of Genetic Counselors, Washington, DC. 2001. Literature needs for patients evaluated with hereditary cancer: development of 5 new brochures; p. 288. [Google Scholar]
- 7.Bacon J.A., Booker S.V., Brensinger J.D., et al. The Johns Hopkins guide for patients and families: Hereditary nonpolyposis colon cancer. 2000. www.hopkins-gi.org/multimedia/database/intro_83_HNPCC%20Booklet.pdf
- 8.Burton S., Haidle J.L., Hampel H., Eng C. Cowden syndrome: a guide for patients and their families. 2004. www.vh.org/pediatric/patient/cancercenter/cowden/index.html
- 9.Ghate S., Leininger A., Solomon C.H., Teed N., Trimbath J.B. National Society of Genetic Counselors; Wallingford, PA: 2004. FAP and me: A kid's guide to familial adenomatous polyposis. [Google Scholar]
- 10.Hibbs K. Graduate thesis submitted to the University of Minnesota; 2004. Assessment of educational materials for children at risk for inherited colon cancer. [Google Scholar]
- 11.Seven M., Shah L.L., Yazici H., Daack-Hirsch S. From probands to relatives: Communication of genetic risk for hereditary breast-ovarian cancer and its influence on subsequent testing. Cancer Nurs. 2022;45:E91–E98. doi: 10.1097/NCC.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 12.Bednar E.M., Sun C.C., McCurdy S., Vernon S.W. Assessing relatives’ readiness for hereditary cancer cascade genetic testing. Genet. Med. 2020;22:719–726. doi: 10.1038/s41436-019-0735-3. [DOI] [PubMed] [Google Scholar]
- 13.Frey M.K., Ahsan M.D., Bergeron H., et al. Cascade testing for hereditary cancer syndromes: Should we move toward direct elative contact? A systematic review and meta-analysis. J. Clin. Oncol. 2022;40:4129–4143. doi: 10.1200/JCO.22.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tercyak K.P., O’Neill S.C., Roter D.L., McBride C.M. Bridging the communication divide: a role for health psychology in the genomic era. Prof. Psychol. Res. Pr. 2012;43:568–575. doi: 10.1037/a0028971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner-Lin A., Merrill S.L., Brandt A.C., Barnett R.E., Matloff E.T. Talking with children about adult-onset hereditary cancer risk: a developmental approach for parents. J. Genet. Couns. 2018;27:533–548. doi: 10.1007/s10897-017-0191-7. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton J.G., Peshkin B.N., Mays D., DeMarco T.A., Patenaude A.F., Tercyak K.P. Maternal perceptions of BRCA genetic counseling communication processes about disclosing cancer risk information to children and adult relatives. Psychooncology. 2018;27:1825–1832. doi: 10.1002/pon.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leinweber K.A., Columbo J.A., Kang R., Trooboff S.W., Goodney P.P. A review of decision aids for patients considering more than one type of invasive treatment. J. Surg. Res. 2019;235:350–366. doi: 10.1016/j.jss.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krassuski L., Vennedey V., Stock S., Kautz-Freimuth S. Effectiveness of decision aids for female BRCA1 and BRCA2 mutation carriers: a systematic review. BMC Med. Inform. Decis Mak. 2019;19:154. doi: 10.1186/s12911-019-0872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seven M., Shah L.L., Daack-Hirsch S., Yazici H. Experiences of BRCA1/2 gene mutation-positive women with cancer in communicating genetic risk to their relatives. Cancer Nurs. 2021;44:E142–E150. doi: 10.1097/NCC.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 20.Peshkin B.N., Demarco T.A., Tercyak K.P. On the development of a decision support intervention for mothers undergoing BRCA1/2 cancer genetic testing regarding communicating test results to their children. Familial Cancer. 2010;9:89–97. doi: 10.1007/s10689-009-9267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson S., Bennett E. Children’s Hospital & Regional Medical Center; Seattle: 2005. Patient and family education materials development kit. [Google Scholar]
- 22.O’Connor A.M., Bennett C.L., Stacey D., Barry M., Col N.F., Eden K.B., et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2009 doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Bowen D.J., Allen J.D., Vu T., Johnson R.E., Fryer-Edwards K., Hart A. Theoretical foundations for interventions designed to promote informed decision making for cancer screening. Ann. Behav. Med. 2006;32:202–210. doi: 10.1207/s15324796abm3203_5. [DOI] [PubMed] [Google Scholar]
- 24.Tercyak K.P., Hughes C., Main D., Snyder C., Lynch J.F., Lynch H.T., et al. Parental communication of BRCA1/2 genetic test results to children. Patient Educ. Couns. 2001;42:213–224. doi: 10.1016/S0738-3991(00)00122-1. [DOI] [PubMed] [Google Scholar]
- 25.Foster S.L. Assessing and treating parent-adolescent conflict. Prog. Behav. Modif. 1994;29:53–72. [PubMed] [Google Scholar]
- 26.Barnes H.L., Olson D.H. Parent-Adolescent Communication Scale. In: Olson D.H., editor, et al. Family Inventories: Inventories Used in a National Survey of Families Across the Family Life Cycle. St. Paul: Family Social Science, University of Minnesota; 1982. p. 33–48.
- 27.Derogatis L.R., Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychol. Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 28.Achenbach T.M. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. Manual for the Child Behavior Checklist/4-18 and 1991 profile. [Google Scholar]
- 29.Conley C.C., Otto A.K., McDonnell G.A., Tercyak K.P. Multiple approaches to enhancing cancer communication in the next decade: translating research into practice and policy. Transl. Behav. Med. 2021;11:2018–2032. doi: 10.1093/tbm/ibab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Neill S.C., Hamilton J.G., Conley C.C., Peshkin B.N., Sacca R., McDonnell G.A., et al. Improving our model of cascade testing for hereditary cancer risk by leveraging patient peer support: a concept report. Hered Cancer Clin. Pract. 2021;19:40. doi: 10.1186/s13053-021-00198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santerre-Theil A., Bouchard K., St-Pierre D., Drolet A.-M., Chiquette J., Dorval M. Centre ROSE, Development of a tool to guide parents carrying a BRCA1/2 mutation share genetic results with underage children. J. Cancer Educ. 2018;33:569–575. doi: 10.1007/s13187-016-1127-x. [DOI] [PubMed] [Google Scholar]
- 32.McDonnell G.A., Peshkin B.N., DeMarco T.A., Peterson S.K., Arun B.K., Miesfeldt S., et al. Long-term adaptation among adolescent and young adult children to familial cancer risk. Pediatrics. 2022;150 doi: 10.1542/peds.2022-056339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patenaude A.F., Tung N., Ryan P.D., Ellisen L.W., Hewitt L., Schneider K.A., et al. Young adult daughters of BRCA1/2 positive mothers: what do they know about hereditary cancer and how much do they worry? Psychooncology. 2013;22:2024–2031. doi: 10.1002/pon.3257. [DOI] [PubMed] [Google Scholar]
- 34.Whitaker K.D., Obeid E., Daly M.B., Hall M.J. Cascade genetic testing for hereditary cancer risk: an underutilized tool for cancer prevention. JCO Precis Oncol. 2021;5:1387–1396. doi: 10.1200/PO.21.00163. [DOI] [PubMed] [Google Scholar]
- 35.Domchek S.M., Gaudet M.M., Stopfer J.E., Fleischaut M.H., Powers J., Kauff N., et al. Breast cancer risks in individuals testing negative for a known family mutation in BRCA1 or BRCA2. Breast Cancer Res. Treat. 2009;119:409. doi: 10.1007/s10549-009-0611-y. [DOI] [PubMed] [Google Scholar]