Abstract
Objective
To examine the feasibility, acceptability, and effects of a self-stigma reduction program for patients with type 2 diabetes mellitus1 (T2DM).
Methods
We adopted a within-subjects pre–post study design, measuring self-stigma among T2DM patients who received treatment at a tertiary-level hospital.
Results
Of the 17 participants, 11 participants completed the program (mean age: 54.36 ± 8.58 years; women: 63.6%; mean T2DM duration: 12.09 ± 10.41 years). Participants experienced reduced levels of self-stigma between the pre- and post-study time points (mean pre-study score: 35.82 ± 16.26; mean post-study score: 25.55 ± 16.91). The difference in self-stigma was not significant (effect size: d = 0.8, χ2 = 3.6, p = 0.057). Overall, participants who completed the program were satisfied except for the duration of each session.
Conclusion
The self-stigma reduction program was relatively feasible and acceptable. Although due to the small sample size our results were not statistically significant, a large reduction of self-stigma was found in those who completed the program, which is promising. Future studies with larger sample sizes are needed to measure the program’s long-term effects on the reduction of self-stigma.
Innovation
This program is innovative as the researchers and healthcare professionals collaborated with patients who contributed their narratives.
Keywords: Type 2 diabetes mellitus, Feasibility study, Patient education, Stigma, Patient activation
Highlights
-
•
T2DM self-help psychoeducational self-stigma reduction program used patient stories.
-
•
A theory-based approach, integrating behavior change techniques was used.
-
•
The study examines feasibility, acceptability, and effects of the program for T2DM.
-
•
The program was acceptable, although its feasibility can be strengthened.
-
•
Results show that the intervention could help reduce levels of self-stigma.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a major medical global issue [1]. The worldwide prevalence of diabetes among adults aged 20−79 years in 2019 was 8.3% [1], and it continues to rapidly increase. In Japan, the prevalence of diabetes has increased in recent years. In 2019, it was 7.9% [2]. By 2030, it is estimated to reach 9.8%, affecting 9.7 million Japanese individuals [2]. Diabetes is not curable but is treatable. Diabetes treatment attains sufficient glycemic control to prevent further diabetes-related complications [3]. Many complications may be prevented through good quality medical care, patients’ adherence to treatment and daily self-care practices [4,5]. However, T2DM greatly affects patients’ psychosocial health (e.g., diabetes distress, depression). This may hinder their daily self-care practices required for optimal treatment outcomes [[6], [7], [8], [9]].
Stigma is one of the psychosocial issues encountered by individuals with T2DM [8,9]. Individuals with T2DM often have negative stereotypes of their condition, as it may be perceived as a lifestyle disease [8,9]. Some patients may blame themselves for their illness [8,9]. Self-stigma greatly affects the self-care practice behaviors of individuals with T2DM [[10], [11], [12]]. Self-stigma is defined as a state whereby individuals develop negative attitudes toward themselves because of their condition. This is caused by either experiencing or perceiving social stigma [9,11,[13], [14], [15]]. Self-stigma may be an obstacle for treatment adherence in individuals with T2DM. For example, self-stigmatized individuals with T2DM may try to prevent social stigma by avoiding prescribed food and not taking medications and/or insulin injections in the presence of others. They also may not disclose their illness [9].
Our previous study used path analysis to investigate the mechanisms whereby self-stigma undermines patient activation for self-care among individuals with T2DM [16]. This shows the direct and indirect effects (by reducing self-esteem and self-efficacy) of self-stigma on lowering patient activation for self-care [16]. Individuals with a higher level of self-stigma and lower willingness to change their self-care behaviors are more likely to experience suboptimal treatment outcomes [10,11,16]. Based on the mechanisms whereby self-stigma may affect patient activation for self-care [16], a program that promotes self-esteem and self-efficacy to modify patient behavior may be the most effective approach. Unfortunately, no studies on diabetes to date have sought to develop a self-stigma reduction program. However, core evidence from the field of psychiatry may be a useful reference and applicable to individuals with T2DM.
For individuals with mental illnesses, literature reviews have indicated that the most common types of interventions to reduce the degree of self-stigma were either psychoeducational, cognitive-behavioral, narrative communication interventions, or a combination thereof [[17], [18], [19], [20], [21], [22]]. In many Asian countries, psychoeducation is the most applied intervention to reduce self-stigma among individuals with mental illnesses [23]. While T2DM and mental illnesses are not the same, it is possible to learn from and refer to some core components of the research methods used in the psychiatry field when developing a self-stigma reduction program for individuals with T2DM. Psychoeducation is defined as an educational intervention that does not focus on structured and didactic knowledge of an illness, diagnosis, treatment, prescription, or cure. It centers on goal setting, skill-teaching, goal achievement, and/or satisfaction. It integrates emotional and motivational aspects to enable patients to cope with their illness [24]. In addition, narrative communication may change individuals’ beliefs and attitudes, and motivate their behaviors [25,26]. Narrative communication is defined as “a representation of connected events and characters that have an identifiable structure, is bound in space and time, and contains implicit or explicit messages about the topic being addressed” [25]. It contains patients’ knowledge and experiences as gained from their day-to-day management of their illnesses. It is compelling and counteracts disagreement. This is when the characters in the narrative are familiar, identifiable, and convincing [26,27].
We therefore developed a psychoeducational self-stigma reduction program for individuals with T2DM by using narrative communication. This was underpinned by a theory-based approach, as provided in our previous study [16]. This study’s purpose was to test the feasibility, acceptability, and effects of this program by using a pre–post study design. To the best of our knowledge, this is the first study to examine the feasibility, acceptability, and effects of a psychoeducational program; using narrative communication to reduce self-stigma among individuals with T2DM.
2. Materials and methods
2.1. Development of the self-stigma reduction program
Our previous study established a theoretical framework to explain how self-stigma affects patient activation for self-care. It consequently leads to suboptimal treatment outcomes among individuals with T2DM [16]. Following this framework [16], we formulated three intervention targets to reduce stigmatizing beliefs and attitudes: self-stigma, self-esteem, and self-efficacy. The theory of planned behavior was used to reduce negative attitudes (self-stigma), increase perceived control (self-efficacy) over self-care behaviors, and reduce perceived subjective norms regarding the social unacceptability of T2DM [28]. In the self-esteem modules, we focused on three elements of facilitating the recovery and maintenance of self-worth identified in our previous qualitative study: 1) gaining “control” over the illness, 2) discovering a positive aspect of T2DM, and 3) discovering a new sense of self-worth [29]. The contents of the self-esteem and self-efficacy modules depended on the constructs of social cognitive theory (e.g., goal setting, self-monitoring of behaviors, coping planning, problem-solving, and planning social support) [30]. These are the mediating components of self-stigma to enhance the readiness for change in patients’ self-care behaviors.
The proposed self-stigma reduction program contained 10 weekly sessions that integrated the three intervention targets indirectly through psychoeducation: 1) self-stigma directly, 2) self-esteem and 3) self-efficacy. It was 10 weeks long and involved watching a video at home weekly. Each session of the program was designed to help individuals with T2DM adopt new diabetes-related behaviors [30]. Each video session lasted approximately 10 minutes. The program’s duration (10 weekly sessions for 10 weeks) was determined based on previous studies [[18], [19], [20], [21], [22]]. The entire structure of the program was developed as psychoeducational content. The weekly videos contained other patients’ stories in various social contexts. The narrator of the videos simply read out the stories without editorializing or changing the patients’ words. The other patients’ stories were real narratives collected in our previous qualitative study [29], so they were familiar and relatable. Watching videos was connected to doing “homework” related to each session (e.g., goal setting, action planning, and coping planning). Table 1 illustrates the program’s contents. All of the program’s content was reviewed by diabetes specialist physicians, a psychosomatic medicine physician, a psychiatric nurse, a clinical psychologist, and patients with T2DM. These individuals reviewed the program contents using their respective professional positions to ensure that program contents did not contradict general treatment for diabetes.
Table 1.
Contents of the psychoeducational self-stigma reduction program for individuals with T2DM.
| Sessions | Targets | Modules |
|---|---|---|
| 1. Diagnosis | Self-stigma | -information on the emotional and social consequences -reducing subjective norms |
| 2. Diet | Self-efficacy | -goal setting/action planning -behavior self-monitoring -graded tasks -efforts to reinforce self-care behaviors |
| 3. Exercise | Self-efficacy | -goal setting/action planning -behavior self-monitoring -graded tasks -efforts to reinforce self-care behaviors |
| 4. Reducing stress | Self-efficacy | -stress management -planning social support |
| 5. Support from family and friends | Self-stigma | -general communication skills -coping planning |
| 6. Treatment | Self-stigma | -problem-solving skills -coping planning |
| 7. Adapting to the illness | Self-esteem | -reframing incompatible beliefs -focusing on past successes -gaining “control” over the illness |
| 8. Accepting the illness | Self-esteem | -reframing incompatible beliefs -focusing on past successes -discovering a positive aspect of T2DM |
| 9. Acceptance of the self | Self-esteem | -valuing self-identity -providing information about others’ approval -discovering a new sense of self-worth |
| 10. Round-up | Self-stigma/self-esteem/self-efficacy | -dealing with emotional aspects -building upon strengths -cultivating resilience |
The program contained the following contents (Table 1): 1) Self-stigmatized individuals with T2DM have irrational ideas regarding their illness; including diagnosis, treatment, and prognosis [8,9]. They first need to receive accurate information about their illness and its onset. Developing the ability to deal with emotional aspects of their condition may reconstruct their understanding of the illness and encourage a fair self-appraisal. The self-stigma module included information about emotional and social consequences, as well as information on reducing subjective norms. Along with support from family and friends, developing general communication and problem-solving skills may also help normalize their social lives. It thus facilitates the development of coping skills for their illness and stigmatized social situations [31].
Second, self-stigmatized individuals underestimate their own abilities in general and show a passive readiness for change [9]. They need to develop positive attitudes and behaviors toward themselves and sustain their achieved changes [9]. Promoting behavioral modifications may help them progress toward the action stage. This includes changing their self-care behaviors, such as diet and exercise; setting realistic individual goals (i.e., graded tasks; allowing them to progress from elementary tasks to complex but achievable tasks toward performing the prescribed behaviors), gaining a sense of control in daily life, living with their illness without being too impeded by it, and imagining a positive future for themselves. Thus, the self-efficacy module included goal setting or action planning, behavior self-monitoring, graded tasks, efforts to reinforce self-care behaviors, stress management, and planning social support [31].
Third, self-stigmatized individuals have irrational ideas regarding their self-concept [9]. They tend to believe that they do not deserve to be valued or respected by others because of their illness. This undermines their meaningful life roles, such as being a parent, spouse, romantic partner, friend, manager, or employee [9]. They need to develop the ability to manage their condition, while addressing these emotional aspects to develop self-acceptance [29]. This includes adapting to their condition; developing self-compassion, which may help them acquire realistic and objective perceptions of their illness; developing their strengths; cultivating resilience; and ultimately assimilating the illness into their identity. Thus, the self-esteem module included: reframing incompatible beliefs, focusing on past successes, valuing their self-identity, providing information about others’ approval [31], gaining “control” over the illness, gaining positive insights on T2DM, and discovering an improved sense of self-worth [29].
2.2. Participants
The self-stigma reduction program was a follow-up to a hospitalization program. We used maximum variation sampling by recruiting inpatients with T2DM. They had participated in a structured diabetes education program at a university hospital in Tokyo, between March and July 2018. The participants were recruited by research staff. After the study’s purpose was explained, inpatients who provided informed consent were enrolled as the study’s participants. The inclusion criteria were as follows: 1) diagnosed with T2DM, 2) aged 20−65 years, 3) not undergoing treatment for psychiatric and/or eating disorders, 4) fluent in the Japanese language, and 5) having a self-stigma level more than zero. All the participants received bookstore gift cards (net value 2,000 Japanese yen) as an incentive. This study was approved by the Research Ethics Committee of the University of Tokyo Graduate School of Medicine and Faculty of Medicine (Approval No. 11728).
2.3. Data collection and measures
This study adopted a within-subjects 10-week pre–post study design, by using a pen-and-paper self-report questionnaire. The sociodemographic data collected were: gender, age, education (in years), and marital status. The clinical data collected were: their body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), T2DM duration (in years), primary treatment (lifestyle, oral hypoglycemic agent, insulin injection, and/or other injectable medication), diabetes-related complications, and hemoglobin A1c (HbA1c) level (measured on the day when the participants completed the pre- and post-study questionnaires). HbA1c is a blood test that shows the average levels of blood glucose over the past 8–12 weeks.
The participants completed the questionnaires at baseline and watched the program’s first session at the hospital. The remaining sessions were streamed live on vimeo.com by participants at home. By using a real-time video tracking function on vimeo.com, the research staff recorded each participant’s actual video watching duration at home. After watching all 10-week sessions, the participants completed the same questionnaires at home and returned them to the research staff. Each participant’s graded tasks (e.g., Diet, Exercise) were confirmed in the questionnaires by the research staff. Administration of the Patient Activation Measure (PAM-13) is recommended every 3–6 months (12–24 weeks) to track program effectiveness [37]. Watching all videos in the program took 10 weeks. Therefore, for the purpose of safety, we intentionally chose these two time points for the pre- and post-study, which was the shortest study design period. Thus, the participants’ physicians were able to monitor the participants at their clinic visits immediately in the event of any possible adverse event caused by the program. The participants also completed the following instruments:
2.3.1. Self-stigma
The Self-Stigma Scale (SSS-J) measures self-stigma levels [13]. The original scale was developed by Mak et al. [32]. The 39-item Japanese version of the SSS-J was validated by Kato et al [13]. Items are rated on a 4-point Likert scale (0 = strongly disagree, 1 = disagree, 2 = agree, and 3 = strongly agree). The total score ranges from 0 to 117 and is treated as a continuous variable. Higher scores indicate higher levels of self-stigma. The SSS-J’s internal consistency (Cronbach’s alpha) was 0.96 in this study.
2.3.2. Self-esteem
The Rosenberg Self-Esteem Scale measures self-esteem [33,34]. Self-esteem was measured as a relevant variable to self-stigma, which was the primary outcome of interest in this study. The original scale was developed by Rosenberg [33]. Its Japanese version was developed by Mimura et al. [34]. It has a high reliability and validity and is a widely accepted scale [33,34]. It consists of 10 items scored on a 4-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = agree, and 4 = strongly agree). After the scores for five negative items are reversed, all the scores are summed. The Rosenberg Self-Esteem Scale’s internal consistency was 0.84 in this study.
2.3.3. Self-efficacy
The General Self-Efficacy Scale measures the strengths in general self-efficacy across diverse settings in everyday life. Self-efficacy was measured as a relevant variable to self-stigma, which was the primary outcome of interest in this study. The original scale was developed in Japanese by Sakano et al [35]. It consists of 16 items in a dichotomous response format. It has a high reliability and validity and is commonly used to measure self-efficacy in Japan. The total score ranges from 0 to 16 and is treated as a continuous variable. Higher scores indicate higher self-efficacy. The General Self-Efficacy Scale’s internal consistency was 0.79 in this study.
2.3.4. Patient activation
The Patient Activation Measure (PAM-13) assesses the patient’s activation level for engaging in self-care [[36], [37], [38]]. The original scale was developed by Hibbard et al [38]. The Japanese version of the scale was validated by Fujita et al [36]. The PAM-13 considers not only single instances of general health self-management behaviors (e.g., exercising or eating properly during a given week) but also the respondent’s knowledge, skills, beliefs, confidence, and engagement in disease-specific self-management behaviors required to manage their illness (e.g., keeping a written diary of glucose levels, taking diabetes medication as recommended by a personal physician) [37,39]. Previous studies showed that the scale could predict not only dietary and exercise behaviors but also comprehensive behaviors including disease-specific self-management behaviors (e.g., glucose monitoring, taking medications) [37,39]. The PAM-13 has a high reliability and validity and is used in clinical settings. It consists of 13 items scored on a 4-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = agree, and 4 = strongly agree). The total score ranges from 13 to 52 and is converted to an interval scale (0–100) according to a scoring algorithm [37]. Higher scores indicate a more positive attitude toward the behavioral changes necessary for generating significant self-care intervention outcomes. The scores can be converted to “activation levels” according to the scale scoring algorithm [37]. The scores are categorized by the following four activation level segments. Level 1 (“disengaged and overwhelmed”) refers to individuals who do not feel in charge of their own health and care and may not yet believe that their role as a patient is important. Level 2 (“becoming aware but still struggling”) refers to individuals who have some knowledge about their illness but still have large gaps in their understanding. Level 3 (“taking action and gaining control”) refers to individuals who still strive to engage in best practice behaviors but are goal-oriented, while building self-management skills. Level 4 (“maintaining behaviors and pushing further”) refers to individuals who have made most of the necessary behavior changes but may have difficulty maintaining behaviors during times of stress.
The Japanese version of the PAM-13 was used without the words “mental health,” as requested by the scale’s developer. The PAM-13’s internal consistency was 0.86 in this study.
2.4. Feasibility and acceptability of the program
We asked participants to rate the feasibility and acceptability of each session of the program quantitatively via a questionnaire, by rating seven items (Table 4) on a 5-point scale (1 = strongly disagree, 5 = strongly agree). They also answered three open-ended questions (“Tell us anything that you disagreed with in the video’s narratives,” “Tell us about your personal experiences that are useful for other patients, if any, besides the video’s narratives,” and “Tell us about your thoughts and opinions on the videos”).
Table 4.
Participants’ assessment of the program’s feasibility and acceptability (n = 11).
| Rating score |
|
|---|---|
| Mean ± SD: all 10-week sessions (range) | |
| I liked the length. | 2.7 ± 0.24 (1–4) |
| I liked the voice. | 4.2 ± 0.19 (3–5) |
| It was easy to read the screen. | 2.9 ± 0.17 (1–5) |
| The information was easy to comprehend. | 3.9 ± 0.26 (2–5) |
| It was easy to complete assigned homework. | 3.7 ± 0.23 (2–5) |
| The session was helpful. | 4.1 ± 0.30 (2–5) |
| I would recommend the session to others. | 4.0 ± 0.27 (2–5) |
Abbreviation: SD = standard deviation.
2.5. Statistical analysis
Regarding the descriptive statistics, we calculated the mean and standard deviation (SD) for all numerical variables, and raw numbers (frequency) for the categorical variables. For all the instruments, the internal consistency was measured by using Cronbach’s alpha.
Due to the small sample size, we used non-parametric tests. We performed the Wilcoxon signed-rank test for the numerical variables to investigate the differences in the rank totals for the whole study group between the pre- and post-study scores. We also conducted the Friedman rank sum test to explore the differences in the rank totals between each participant’s pre- and post-study scores. A two-tailed p-value < 0.05 indicated the statistical significance. We performed a post-hoc power test to determine the power of our analysis.
Regarding the program’s feasibility and acceptability, we calculated the rating scores means and SDs for each session. For all the statistical analyses, we used R: A language and environment for statistical computing [40].
2.6. Patient involvement
Patients were not involved in recruiting participants, designing, or conducting this study.
3. Results
Of the 22 inpatients, 17 inpatients consented to participate in the program, yielding a participation rate of 77.3%. Five individuals declined participation, due to a lack of access to mobile devices or feeling overwhelmed by the information about the T2DM treatment already provided by their doctors. Of the 17 participants, 11 completed the 10-week program, while six participants dropped out of the study. The retention rate was 64.7%.
3.1. Participants’ characteristics
Table 2 presents the participants’ descriptive statistics. In total, we examined 11 participants, whose mean (SD) age was 54.36 (8.58) years. Seven participants were female, and four participants were male. Seven were married and four were single. The mean (SD) duration of having diabetes was 12.09 (10.41) years. As their primary treatment, five participants reported using “oral hypoglycemic agents,” three “other than insulin injectable medications,” one “insulin injections,” one “insulin injections and oral hypoglycemic agents,” and one “lifestyle improvements.” Additionally, regarding diabetes-related complications, two patients experienced “nephropathy” and one “retinopathy.” There were no missing data.
Table 2.
Participants’ sociodemographic and clinical characteristics (n = 11).
| Participants’ characteristics | Frequency or Mean ± SD |
|---|---|
| Gender: | |
| Male | 4 |
| Female | 7 |
| Age (years): | 54.36 ± 8.58 |
| Duration of diabetes (years): | 12.09 ± 10.41 |
| Primary treatment: | |
| Lifestyle | 1 |
| Oral hypoglycemic agents | 5 |
| Insulin injections | 1 |
| Insulin injections and oral hypoglycemic agents | 1 |
| Other injectable medications (other than insulin) | 3 |
| Diabetes-related complications: | |
| None | 8 |
| Retinopathy | 1 |
| Nephropathy | 2 |
| Education: | |
| High school | 2 |
| Technical/junior college | 3 |
| Bachelor’s degree or higher | 6 |
| Marital Status: | |
| Married | 7 |
| Unmarried/Divorced/Bereaved | 4 |
Abbreviations: SD = standard deviation.
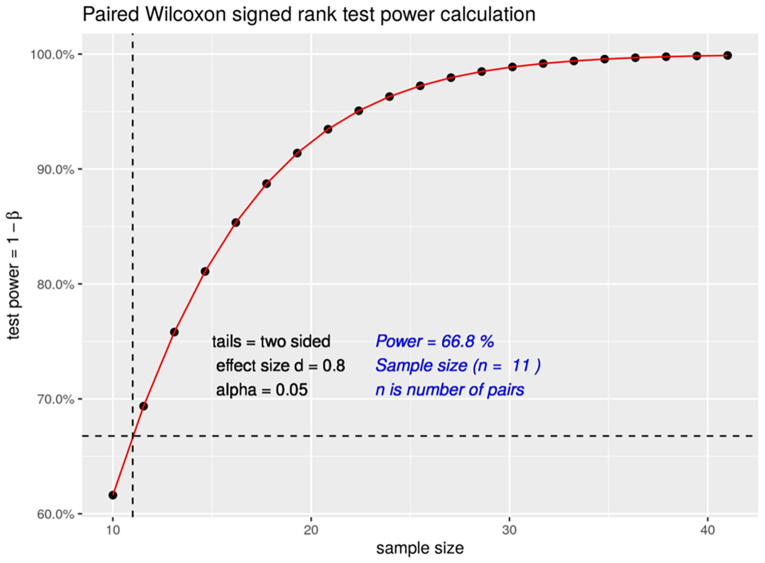
3.2. Power of the analysis
We determined the statistical power of our analysis to be 66.8 for a significance level of less than 0.05 and a large effect size (d = 0.8) (Supplementary Fig. 1). Therefore, we consider the results as indicative of the self-stigma reduction program’s effects.
Supplementary Fig. 1.
Paired Wilcoxon signed-rank test power calculation.
3.3. Primary outcomes
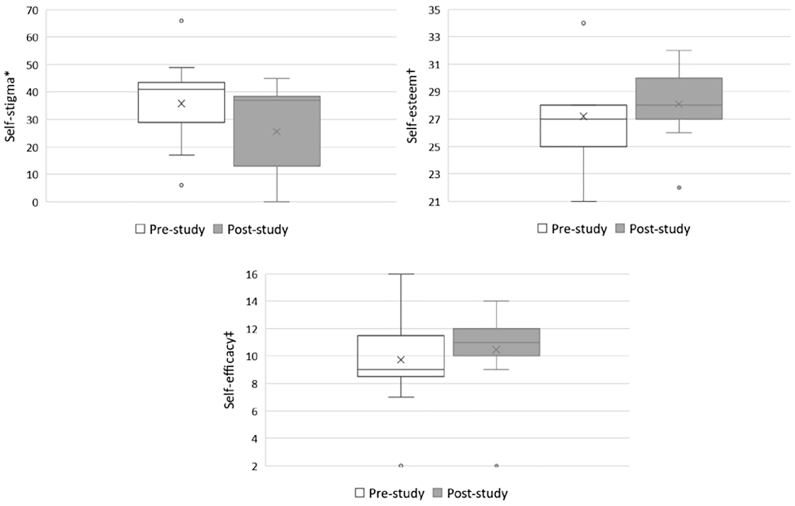
We performed a Wilcoxon signed-rank test to detect changes in self-stigma, self-esteem, and self-efficacy levels between the pre- and post-study time points. Table 3 shows that the mean self-stigma score at pre-study was 35.82 and decreased to 25.55 at post-study. Thus, the mean change was 10.27 (V = 44, p = 0.102). In the Friedman sum rank test that examined the differences between each participant’s pre- and post-study scores for self-stigma, the difference was not significant (χ2 = 3.6, p = 0.057). There were no significant changes in self-esteem or self-efficacy (V = 19, p = 0.721 and V = 7.5, p = 0.307, respectively).
Table 3.
Pre–post study differences in primary and secondary outcomes (n = 11).
| Pre-study |
Post-study |
Wilcoxon signed-rank test |
Friedman rank sum test |
|||
|---|---|---|---|---|---|---|
| Mean ± SD | V | p-value | χ2 | p-value | ||
| Primary outcomes | ||||||
| Self-stigma⁎ | 35.82 ± 16.26 | 25.55 ± 16.91 | 44 | 0.102 | 3.6 | 0.057 |
| Self-esteem† | 27.18 ± 4.14 | 28.09 ± 2.81 | 19 | 0.721 | 0.1 | 0.738 |
| Self-efficacy‡ | 9.73 ± 3.69 | 10.45 ± 3.14 | 7.5 | 0.307 | 1.2 | 0.256 |
| Secondary outcomes | ||||||
| PAM-13 score§ | 54.77 ± 11.66 | 58.42 ± 11.82 | 14.5 | 0.373 | 1 | 0.317 |
| PAM-13 level | Level 2 (n = 2) | Level 3 (n = 5) | ||||
| BMI (kg/m2) | 27.73 ± 6.23 | 26.55 ± 5.85 | 45 | 0.006 | 9 | 0.002 |
| HbA1c (%) | 8.18 ± 1.89 | 6.82 ± 0.87 | 41.5 | 0.023 | 5.4 | 0.019 |
Abbreviations: SD = standard deviation. BMI = body mass index. HbA1c = glycated hemoglobin.
Japanese version of the Self-Stigma Scale (SSS-J).
Rosenberg Self-Esteem Scale.
General Self-Efficacy Scale.
Patient Activation Measure (PAM-13).
3.4. Secondary outcomes
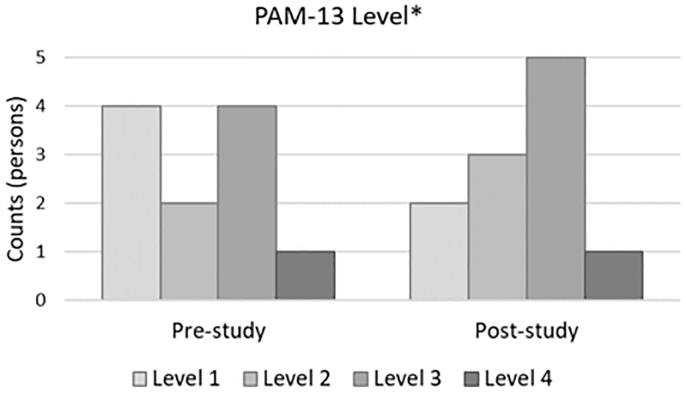
Table 3 shows that the mean patient activation score at pre-study was 54.77, which increased to 58.42 post-study. Thus, the mean change was 3.65, which was not statistically significant (V = 14.5, p = 0.373). However, when patient activation scores were converted to “activation levels” according to the scale algorithm, the results showed that Level 3 of the patient activation at post-study was higher than Level 2 at the pre-study. Three out of 11 participants increased in their activation levels (one from Level 1 to Level 2, one from Level 1 to Level 3, and one from Level 3 from Level 4). Seven out of 11 participants remained at the same activation levels (two at Level 1, two at Level 2, and three at Level 3). Participants’ BMI and HbA1c significantly decreased after the program (V = 45, p = 0.006 and V = 41.5, p = 0.023, respectively). Fig. 1, Fig. 2 indicate changes in self-stigma, self-esteem, self-efficacy, and patient activation levels between the pre- and post-study time points.
Fig. 1.
Changes in self-stigma, self-esteem, and self-efficacy levels between the pre- and post-study time points.
⁎ Japanese version of the Self-Stigma Scale (SSS-J).
† Rosenberg Self-Esteem Scale.
‡ General Self-Efficacy Scale.
Fig. 2.
Frequency of the changes in participants’ patient activation levels between the pre- and post-study time points.
⁎ Patient Activation Measure (PAM-13).
3.5. Feasibility and acceptability
Table 4 shows the quantitative results from the questionnaire evaluating the program’s feasibility and acceptability. Overall, those who completed the program reported that it was helpful (rating score: 4.1/5.0) and that they would recommend it to others (rating score: 4.0/5.0). Regarding the qualitative assessment, many participants liked the program (e.g., “I was able to think back about my diabetes throughout this program,” “I was able to realize what my diabetes has given me in my life because of this program,” and “I was able to start to face up to my diabetes thanks to this program”). However, regarding the barriers to completing the program, some participants reported experiencing time constraints in continuing mid-course and wanting to drop out of the program because it required them to watch 10-minute videos for 10 weeks over the weekend. This was combined with doing “homework” related to each session (e.g., goal setting, action planning, coping planning). This was especially difficult for those who worked both outside and inside the house (e.g., caregiving for older parents, childcare) because of their time and physical stamina constraints. This barrier is shown quantitatively in Table 4 under the item on the program’s length (“I like the length”).
4. Discussion and conclusion
4.1. Discussion
This study found that individuals with T2DM, who completed the psychoeducational program using narrative communication, experienced reduced levels of self-stigma immediately after the program. Although the difference was not significant (p = 0.057), we did observe a 10.27 point change in self-stigma (pre-study score [35.82 ± 16.26]; post-study score [25.55 ± 16.91]). The program therefore shows a preliminary signal toward reducing self-stigma. Even though the findings of this study are not statistically significant due to the small sample size, the results still show similarities with those of previous studies on individuals with mental illnesses [41,42] which reported that psychoeducation, either using a brochure or the internet, led to a significant decrease in self-stigma in the post-study. Because of these similarities, we believe that the previous studies are applicable to our current findings, and that a future study with a larger sample should show statistically significant support for those previous studies. In addition, the use of a group approach may be the most common and beneficial for individuals with mental illnesses. However, our findings support the premise that an individual approach may also make a positive contribution to reducing self-stigma. This aligns with other studies that reported a decline in self-stigma in programs using an individual approach [18,20]. In our study, a relatively early-medium self-stigma reduction program for individuals with T2DM (e.g., the mean duration of T2DM was 12 years) may provide valuable benefits regarding developing positive beliefs and attitudes toward themselves and their illness.
Although patient activation scores were not statistically significant, this study showed that individuals with T2DM who completed the program experienced increased levels of patient activation for self-care (from Level 2 to Level 3). This result could help support the theory of planned behavior [28]. This theory aims to help individuals with T2DM not only reduce their self-stigma (i.e., negative attitudes) but also adopt new diabetes-related behaviors. This can be explained by increased levels of behavioral intention for change through decreased levels of self-stigma (i.e., negative attitudes). This is achieved by normalizing the beliefs and attitudes of individuals with T2DM about their illness. It thus influences their behavioral intention and enhances their readiness for change. Additionally, the significantly decreased levels of BMI and HbA1c were more likely the result of increased levels of patient activation for self-care, rather than the direct effects of the self-stigma reduction program. In particular, the HbA1c change was large (from 8.18% to 6.82%). This effect could be explained by the effect of the participants’ inpatient stay as well. We did not determine the exact factors. However, we have discussed and agreed that this large amount of change could not have been achieved by only inpatient stay and medication therapy, but that everyday efforts of the participants, including diet and exercise after returning home, contributed.
Overall, participants who completed the program were satisfied with the entire 10-week program and provided favorable ratings for each session. However, since six out of 17 participants dropped out of the program, the feasibility of the program can be strengthened.
This study has some limitations: First, there was a high dropout rate. The research incentive failed to further motivate participants to continue their participation in the study. Future studies may shorten each session’s duration while continuing to build on the basis of the theory of planned behavior and the theoretical framework established in our previous study, and integrating online peer support into the current individual self-help-type intervention. Due to the characteristics of diabetes treatment (e.g., clinic visits every four–six weeks), it is not realistic to have a face-to-face group intervention for individuals with T2DM in Japan. However, online peer support is expected to help decrease the number of dropouts by encouraging participants and providing opportunities for interaction with other patients. Based on the social learning theory [43], such interactions in a social context are expected to help improve self-efficacy through observational learning and social comparison. Additionally, our future intervention program should allow participants to determine when they are ready to start the program within a certain time during study period.
Second, this study was a feasibility pilot study and was not powered to detect statistical differences in reducing self-stigma due to its limited power. However, these results were preliminary and showed overall positive, acceptable results. Future studies will need to increase their statistical power according to the effect size that resulted from this study. This can be done by recruiting larger sample sizes and obtaining larger effects of this self-stigma reduction program for individuals with T2DM. In this study, we did not observe a significant improvement in the components of the self-concept, namely self-stigma, self-esteem, and self-efficacy. This may be due to the small sample size and insufficient power necessary to detect changes between the pre- and post-study time points. Additionally, individuals may require a longer duration to show observable improvements in these components of the self-concept over time after the program. Future studies could be designed to measure the long-term effects (e.g., six- and 12-month follow-ups after the program). This would generate additional valuable information about the effects of this program. Moreover, it would allow validation of how the program can reframe and sustain positive views of illness and self-concept (e.g., self-esteem and self-efficacy) and reduce self-stigma over time, as compared to a control group. Thus, these results would add to the developing body of knowledge about self-stigma in individuals with T2DM from a psychosocial care perspective.
4.2. Innovation
The self-stigma reduction program is innovative as it is a collaboration between researchers, healthcare professionals, and the real narratives of T2DM patients. The patients were not involved in designing this study or developing the self-stigma reduction program. Researchers and healthcare professionals worked together to develop the program by integrating the other patients’ real narratives into behavior change techniques grounded in theoretical approaches. The contribution of the other patients’ real narratives was essential for the program. Additionally, the self-stigma reduction program is a cutting-edge program in response to the demands of the times, as it is an individual self-help program that can be observed anytime at home, outside of clinical visits. Nowadays, in line with the times, more Japanese individuals with T2DM desire to have access to online individual self-help-type intervention supports that are available anytime from their own home (not by clinic visit). The self-stigma reduction program has the potential to help individuals with T2DM reduce stigmatizing beliefs and attitudes toward their illness and thus to be proactive in their daily diabetes self-management.
4.3. Conclusion
The self-stigma reduction program was relatively feasible and acceptable. Although due to the small sample size our results were not statistically significant, a large reduction of self-stigma was found in those who completed the program, which is promising. Future studies with larger sample sizes are needed to measure the program’s long-term effects on the reduction of self-stigma. Nevertheless, this pilot study’s findings will help us determine whether the contents of our program were appropriate. The findings will also assist with research design and protocols for a full-scale research leading to a randomized intervention trial in the future.
The following are the supplementary data related to this article.
Ethics statement
This study was conducted with prior approval from the Research Ethics Committee of the University of Tokyo Graduate School of Medicine and Faculty of Medicine (Approval No. 11728). This study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Statement patient identifiers
We confirm that all patient and personal identifiers have been removed or disguised, so that the patient(s) or person(s) described are not identifiable and cannot be identified through the details of this manuscript.
Data statement
The data that support the study’s findings are available from the corresponding author upon reasonable request.
Funding
This work was supported by a grant from JSPS KAKENHI (grant number JP17K12240.
CRediT authorship contribution statement
Asuka Kato: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Project administration, Resources, Funding acquisition. Kazuhiro Yoshiuchi: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing, Project administration, Supervision. Hideki Hashimoto: Formal analysis, Investigation, Methodology, Validation, Writing – review & editing, Project administration, Supervision, Resources. Ryo Suzuki: Writing – review & editing, Project administration, Resources, Supervision. Toshimasa Yamauchi: Formal analysis, Validation, Writing – review & editing, Resources. Takashi Kadowaki: Conceptualization, Formal analysis, Validation, Writing – review & editing, Project administration, Resources, Supervision.
Declaration of Competing Interest
We declare that none of the authors have any competing interests in the completion and submission of this research.
Acknowledgments
We are grateful to the funder of the study (JSPS KAKENHI Grant Number JP17K12240) and to all the study participants.
Footnotes
T2DM: type 2 diabetes mellitus.
Contributor Information
Asuka Kato, Email: asukakato-tky@umin.ac.jp, asukakato@m.u-tokyo.ac.jp.
Kazuhiro Yoshiuchi, Email: kyoshiuchi@m.u-tokyo.ac.jp.
Hideki Hashimoto, Email: hidehashim@m.u-tokyo.ac.jp.
Ryo Suzuki, Email: suzukir@tokyo-med.ac.jp.
Toshimasa Yamauchi, Email: tyamau@m.u-tokyo.ac.jp.
Takashi Kadowaki, Email: t-kadowaki@toranomon.kkr.or.jp.
References
- 1.International Diabetes Federation Diabetes Atlas. 9th ed. IDF; Brussels: 2019. [Google Scholar]
- 2.Charvat H., Goto A., Goto M., et al. Impact of population aging on trends in diabetes prevalence: A meta-regression analysis of 160,000 Japanese adults. J. Diabetes Investig. 2015;6:533–542. doi: 10.1111/jdi.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Japan Diabetes Society . 2020. Tonyobyo Chiryo Gaido 2020–2021 [Diabetes Care Guide 2020–2021], Bunkodo, Tokyo. [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial Research Group, Nathan D.M., Genuth S., et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Ducat L., Philipson L.H., Anderson B.J. The mental health comorbidities of diabetes. JAMA. 2014;312:691–692. doi: 10.1001/jama.2014.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher L., Skaff M.M., Mullan J.T., et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- 8.Browne J.L., Ventura A., Mosely K., Speight J. ‘I call it the blame and shame disease’: a qualitative study about perceptions of social stigma surrounding type 2 diabetes. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato A., Fujimaki Y., Fujimori S., et al. A qualitative study on the impact of internalized stigma on type 2 diabetes self-management. Patient Educ. Couns. 2016;99:1233–1239. doi: 10.1016/j.pec.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Kato A., Fujimaki Y., Fujimori S., et al. Association between self-stigma and self-care behaviors in patients with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res. Care. 2016;4 doi: 10.1136/bmjdrc-2015-000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato A., Fujimaki Y., Fujimori S., et al. Psychological and behavioural patterns of stigma among patients with type 2 diabetes: a cross-sectional study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puhl R.M., Himmelstein M.S., Hateley-Browne J.L., Speight J. Weight stigma and diabetes stigma in US adults with type 2 diabetes: associations with diabetes self-care behaviors and perceptions of health care. Diabetes Res. Clin. Pract. 2020;168:1–9. doi: 10.1016/j.diabres.2020.108387. [DOI] [PubMed] [Google Scholar]
- 13.Kato A., Takada M., Hashimoto H. Reliability and validity of the Japanese version of the self-stigma scale in patients with type 2 diabetes. Health Qual. Life Outcomes. 2014;12:179. doi: 10.1186/s12955-014-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrigan P.W., Watson A.C., Barr L. The self-stigma of mental illness: implications for self-esteem and self-efficacy. J. Soc. Clin. Psychol. 2006;25:875–884. doi: 10.1521/jscp.2006.25.8.875. [DOI] [Google Scholar]
- 15.Rüsch N., Angermeyer M.C., Corrigan P.W. Mental illness stigma: concepts, consequences, and initiatives to reduce stigma. Eur. Psychiatry. 2005;20:529–539. doi: 10.1016/j.eurpsy.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Kato A., Fujimaki Y., Fujimori S., et al. How self-stigma affects patient activation in persons with type 2 diabetes: a cross-sectional study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-034757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal D., Sullivan G., Chekuri L., et al. Empirical studies of self-stigma reduction strategies: a critical review of the literature. Psychiatr. Serv. 2012;63:974–981. doi: 10.1176/appi.ps.201100459. [DOI] [PubMed] [Google Scholar]
- 18.MacInnes D.L., Lewis M. The evaluation of a short group programme to reduce self-stigma in people with serious and enduring mental health problems: Self-stigma reduction programme. J. Psychiatr. Ment. Health Nurs. 2008;15:59–65. doi: 10.1111/j.1365-2850.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 19.Yanos P.T., Roe D., Lysaker P.H. Narrative enhancement and cognitive therapy: a new group-based treatment for internalized stigma among persons with severe mental illness. Int. J. Group Psychother. 2011;61:577–595. doi: 10.1521/ijgp.2011.61.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucksted A., Drapalski A., Calmes C., et al. Ending self-stigma: pilot evaluation of a new intervention to reduce internalized stigma among people with mental illnesses. Psychiatr. Rehabil. J. 2011;35:51–54. doi: 10.2975/35.1.2011.51.54. [DOI] [PubMed] [Google Scholar]
- 21.Sibitz I., Provaznikova K., Lipp M., et al. The impact of recovery-oriented day clinic treatment on internalized stigma: preliminary report. Psychiatry Res. 2013;209:326–332. doi: 10.1016/j.psychres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Knight M.T.D., Wykes T., Hayward P. Group treatment of perceived stigma and self-esteem in schizophrenia: a waiting list trial of efficacy. Behav. Cogn. Psychother. 2006;34:305–318. doi: 10.1017/S1352465805002705. [DOI] [Google Scholar]
- 23.Xu Z., Huang F., Kösters M., Rüsch N. Challenging mental health related stigma in China: systematic review and meta-analysis. II. Interventions among people with mental illness. Psychiatry Res. 2017;255:457–464. doi: 10.1016/j.psychres.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Authier J. The psychoeducation model: definition, contemporary roots, and content. Can. J. Couns. Psychother. 1977;12:1. [Google Scholar]
- 25.Green M.C. Narratives and cancer communication. J. Commun. 2006;56(Suppl. 1):S163–S183. doi: 10.1111/j.1460-2466.2006.00288.x. [DOI] [Google Scholar]
- 26.Moyer-Gus E., Nabi R.L. Explaining the effects of narrative in an entertainment television program: overcoming resistance to persuasion. Hum. Commun. Res. 2010;36:26–52. doi: 10.1111/j.1468-2958.2009.01367.x. [DOI] [Google Scholar]
- 27.Kreuter M.W., Green M.C., Cappella J.N., et al. Narrative communication in cancer prevention and control: a framework to guide research and application. Ann. Behav. Med. 2007;33:221–235. doi: 10.1007/BF02879904. [DOI] [PubMed] [Google Scholar]
- 28.Ajzen I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991;50:179–211. doi: 10.1016/0749-5978(91)90020-T. [DOI] [Google Scholar]
- 29.Kato A., Yoshiuchi K., Fujimaki Y., et al. Understanding the experiences of long-term maintenance of self-worth in persons with type 2 diabetes in Japan: a qualitative study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-034758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandura A. Human agency in social cognitive theory. Am. Psychol. 1989;44:1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 31.Michie S., Rechardson M., Johnston M., et al. The behavior change technique taxonomy, 1 of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 32.Mak W.W.S., Cheung R.Y.M. Self-stigma among concealable minorities in Hong Kong: conceptualization and unified measurement. Am. J. Orthopsychiatry. 2010;80:267–281. doi: 10.1111/j.1939-0025.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg M. Princeton University Press; Princeton, New Jersey: 1965. Society and Adolescent Self-Image. [Google Scholar]
- 34.Mimura C., Griffiths P. A Japanese version of the Rosenberg Self-Esteem Scale: translation and equivalence assessment. J. Psychosom. Res. 2007;62:589–594. doi: 10.1016/j.jpsychores.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Sakano Y., Tohjoh M. The General Self-Efficacy Scale (GSES): scale development and validation. Jpn. J. Behav. Ther. 1986;12:73–82. [Google Scholar]
- 36.Fujita E., Kuno E., Kato D., et al. Development and validation of the Japanese version of the Patient Activation Measure 13 for Mental Health. Seishin Igaku [Clin. Psychiatry]. 2010;52:765–772. doi: 10.11477/mf.1405101678. [DOI] [Google Scholar]
- 37.Hibbard J.H., Mahoney E.R., Stockard J., Tusler M. Development and testing of a short form of the Patient Activation Measure (PAM) Health Serv. Res. 2005;40:1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hibbard J.H., Stockard J., Mahoney E.R., Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv. Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibbard J.H., Mahoney E.R., Stock R., Tusler M. Do increases in patient activation result in improved self-management behaviors? Health Serv. Res. 2007;42:1443–1463. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 41.Hammer J.H., Vogel D.L. Men’s help seeking for depression: the efficacy of a male-sensitive brochure about counseling. Couns. Psychol. 2010;38:296–313. doi: 10.1177/0011000009351937. [DOI] [Google Scholar]
- 42.Griffiths K.M., Christensen H., Jorm A.F., et al. Effect of web-based depression literacy and cognitive-behavioural therapy interventions on stigmatizing attitudes to depression: randomized controlled trial. Br. J. Psychiatry. 2004;185:342–349. doi: 10.1192/bjp.185.4.342. [DOI] [PubMed] [Google Scholar]
- 43.Bandura A. Prentice Hall; Englewood Cliffs, New Jersey: 1977. Social Learning Theory. [Google Scholar]