Abstract
Purpose
To evaluate rates of familial disclosure of hereditary cancer syndrome information.
Methods
A systematic review and meta-analysis was conducted in accordance with PRISMA guidelines (PROSPERO no.: CRD42020134276). Key electronic databases were searched to identify studies evaluating hereditary cancer syndrome cascade relative disclosure. Eligible studies were subjected to meta-analysis.
Results
Thirty-four studies met inclusion criteria. Among 11,711 included relatives, 70% (95% CI 60 - 78%) were informed of their risk of carrying a cancer-associated pathogenic variant; of 2,875 relatives informed of their risk who were evaluated for uptake of cascade testing, 43% (95% CI 27 - 61%) completed testing. Rates of disclosure were higher among female vs male relatives (79% [95% CI 73% - 84%] vs 67% [95% CI 57% - 75%]) and first-degree vs second-degree relatives (83% [95% CI 77% - 88%] vs 58% [95% CI 45 – 69%]).
Conclusion
Nearly one-third of at-risk relatives remain uninformed of their risk of carrying a cancer-associated pathogenic variant. Even among those informed, fewer than half subsequently complete genetic testing, representing a critical missed opportunity for precision cancer prevention.
Innovation
Five studies evaluating interventions to improve disclosure rates were generally ineffective. Urgent work is needed to elucidate barriers to relative disclosure by probands to develop targeted interventions that can optimize proband-mediated cascade genetic testing rates.
Keywords: Disclosure, Cascade genetic testing, Hereditary cancer syndromes, Lynch syndrome, Hereditary breast and ovarian cancer
Highlights
-
•
30% relatives are unaware of their risk of having a cancer associated pathogenic variant.
-
•
Female relatives and first-degree relatives are more likely to be informed.
-
•
Among those aware of their risk, only 43% complete genetic testing.
1. Introduction
Cascade genetic testing refers to the process of extending genetic testing to relatives of probands in whom germline pathogenic variants have been identified. In the context of cancer-predisposing pathogenic variants, cascade genetic testing offers the opportunity for cancer surveillance and risk-reduction strategies that can decrease cancer morbidity and mortality [[1], [2], [3], [4]]. There are several hereditary cancer syndromes with evidence-based surveillance guidelines to reduce cancer risk [5,6]. Furthermore, the Centers for Disease Control and Prevention Office of Public Health Genomics has designated cascade genetic testing for hereditary breast and ovarian cancer as well as Lynch syndrome as a tier one genomic application, defined as having significant potential for positive impact on public health [7]. Risk-reducing bilateral salpingo-oophorectomy and bilateral mastectomy are associated with a decreased risk of breast and ovarian cancer in individuals with BRCA1/2 pathogenic variants, with risk-reducing bilateral salpingo-oophorectomy associated with a significantly lower all-cause mortality rate in this population [2]. For individuals with Lynch syndrome, surveillance with colonoscopy has been shown to decrease the risk for colorectal cancer, prevent colorectal cancer deaths, and decrease overall mortality [3,4].
Approximately four million people currently living in the United States harbor a cancer-associated pathogenic variant; however, the majority of these individuals are not aware [[8], [9], [10]]. Prediction modeling suggests that genetic testing at time of cancer diagnosis combined with cascade genetic testing of 70% of first- and second-degree relatives could result in identification of all carriers in less than a decade [10,11]. However, the literature suggests that only 35% of at-risk relatives currently complete cascade genetic testing for cancer syndromes [12]. Low rates of cascade genetic testing represent a critical missed opportunity in oncology care. Additionally, the literature suggests that racial and ethnic minorities and those of low socioeconomic status experience even greater underutilization of all aspects of genetic services including cascade testing [12,13].
Although relatives can learn of the presence of a cancer-associated pathogenic variant in their family without being informed by the proband, for example via direct contact by healthcare providers, disclosure by probands is the most common method of information dissemination within families [11]. Disclosure of genetic risk information by probands to their relatives is the critical first step in initiating the process of proband-mediated cascade genetic testing. As such, we aimed to systematically review the literature about disclosure patterns among families with cancer-associated pathogenic variants and conduct a meta-analysis on the pooled rates of disclosure and uptake of cascade testing among relatives informed of their risk, which has not previously been reported.
2. Methods
2.1. Overview
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was preregistered with PROSPERO (registration no.: CRD42020134276) [14]. A comprehensive literature search was devised with the assistance of a librarian and conducted on July 23, 2021, using the following bibliographic databases with no limit on year of publication: Ovid MEDLINE (In-Process and Other Non-Indexed Citations and Ovid MEDLINE 1946 to present), Ovid EMBASE (1974 to present), and Cochrane Library (Wiley). No article type, date, or language restrictions were included in the search. Search concepts included: cascade screening, genetic counseling, and cancer. The full Ovid MEDLINE search strategy is available in Supplementary Table 1.
2.2. Inclusion and exclusion criteria
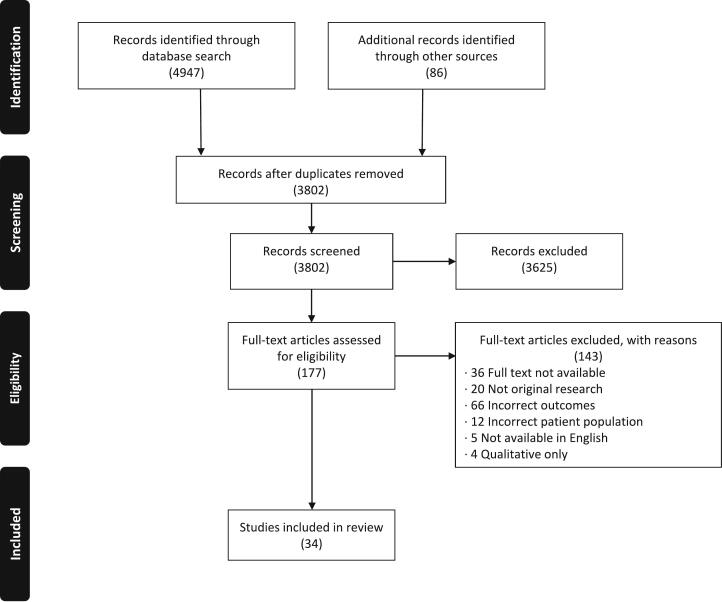
Eligible manuscripts included all primary English language studies that assessed cascade genetic testing for cancer-associated pathogenic gene variants with a focus on disclosure of genetic testing results to at-risk relatives. All non-primary research studies including commentaries, case reports, systematic reviews, and meta-analyses were excluded. A comprehensive review of reasons for exclusion of studies can be found in the PRISMA flow diagram (Figure 1).
Fig. 1.
PRISMA Flow Diagram.
2.3. Data extraction
All manuscripts were independently evaluated for inclusion by two reviewers and disagreements were discussed with a third reviewer. Data were independently extracted by two different reviewers, with a third reviewer checking the final extracted data for accuracy.
2.4. Risk of bias assessment
All included studies were evaluated for risk of bias in their design, conduct, and analysis using the Joanna Briggs Institute’s Critical Appraisal tools [15].
2.5. Statistical analysis
Meta-analyses for the proportion of probands that informed at least one at-risk relative, the proportion of at-risk relatives who were successfully informed and proportion of at-risk relatives who completed genetic testing among those informed were conducted using R software (Version 3.6.1[07/05/19], R Foundation for Statistical Computing, Vienna, Austria). Statistical heterogeneity was tested through the chi-square test (i.e., Cochrane Q test), and a P value < 0.2 was used to indicate the presence of heterogeneity. Statistical heterogeneity was also assessed by the inconsistency statistic (I2). A random effects analysis was used to calculate pooled proportions. The random effects analysis is more conservative and allows for more variability in the individual study proportion estimates when generating the pooled proportion. The pooled proportion was calculated using the Freeman-Tukey Double arcsine transformation, and the 95% CI was calculated using the Clopper-Pearson interval. The DerSimonian-Laird estimator was used to estimate the between-study variance. For the outcome proportions of interest, the results of each study were expressed as binary proportions with exact 95% CIs. For each meta-analysis, a funnel plot was constructed and reviewed, displaying the study proportion against study precision, estimated by the standard error, to assess for publication bias.
3. Results
3.1. Study characteristics
A total of 34 publications of original research were included in our systematic review, of which, data from 31 publications were included in our meta-analyses. Five studies evaluated interventions to improve disclosure rates among families. Although data from intervention arms of all five studies were excluded from our meta-analysis of non-intervention studies to avoid biasing results, two intervention studies utilized historical controls for comparison, data from which were included in our meta-analysis of non-intervention studies. Across all 34 included articles, study designs included 24 cross-sectional studies, 9 prospective studies, and 1 retrospective study. Study publication dates ranged from 2003-2020 and spanned 10 countries: United States (20), France (3), Australia (3), Netherlands (2), Belgium (1), Finland (1), Israel (1), Malaysia (1), Sweden (1), and the United Kingdom (1). Twenty studies evaluated disclosure rates among relatives at risk for hereditary breast and ovarian cancer only, 4 evaluated disclosure rates among those at risk for Lynch syndrome only, 1 among those at risk for hereditary pancreatic cancer only, and 9 included mixed hereditary cancer syndrome populations (Table 1).
Table 1.
| Study | Hereditary Cancer Type (Specific Genes) | Method of Obtaining Disclosure Information | No. of Probands/ Relatives (degree of relatives) |
Proband Age, years / Relative Age, years |
Proband Sex, No. / Relative Sex, No. |
Proband Cancer History, No. / Relative Cancer History, No. |
Proband Race and Ethnicity, No. / Relative Race and Ethnicity, No. |
|---|---|---|---|---|---|---|---|
| Aktan-Collan et al, 2011[37] | Lynch Syndrome | Proband Self Report (Questionnaire) | 248/0 (First – Children) |
Probands: Mean: 56.4 |
Probands: Female: 127 Male: 121 |
Probands: Yes: 133 |
|
| Alegre et al, 2019[47] | HBOC**, Lynch Syndrome | Proband Self Report (Interview) | 103/0 | Probands: Mean: 55.2 |
Probands: Female: 92 Men: 11 |
Probands: Yes: 98 No: 5 |
|
| Bednar et al, 2020[16] | HBOC**, Lynch Syndrome, Other (SDHB, SDHC, BAP1, PTEN, AXIN2, APC, NF1, TP53, VHL) | Proband Self Report (Survey) | 150/825 (First) |
Probands: Mean: 46.2 |
Probands: Female: 132 Male: 18 Relatives: Female: 380 Male: 445 |
Probands: White: 140 Black/African American: 2 American Indian/Alaska Native: 2 Asian Indian: 1 Chinese: 1 Others: 4 Non-Hispanic: 139 Hispanic: 10 Prefer not to answer: 1 |
|
| Blandy et al, 2003[17] | HBOC** | Proband Self Report (Interview) | 30/310 (First, Second, Third) | Probands: Mean: 52.0 |
Probands: Female: 30 Relatives: Female: 162 Male: 148 |
Probands: Yes: 30 (breast and ovarian) |
|
| Bradbury et al, 2007[28] | HBOC** | Proband Self Report (Interview) | 42/86 (First – Children) | Probands: Median: 45.0 Relatives: Median: 12 |
Probands: Female: 37 Male: 5 Relatives: Female: 53 Male: 33 |
Probands: Yes: 23 No: 19 |
Probands: White: 39 Black: 1 Hispanic: 2 |
| Bradbury et al, 2012[50] | HBOC** | Proband Self Report (Interview) | 253/505 (First – Children) | Probands: Median: 47.7 Relatives: Median: 17 |
Probands: Female: 241 Male: 12 Relatives: Female: 253 Male: 252 |
Probands: Yes: 169 No: 84 |
Probands: White: 232 Black: 13 Others: 8 |
| Brooks et al, 2004[18] | HBOC** | Not Reported | 0/384 (First, Second, Distant) | Relatives: Female: 202 Male: 182 |
|||
| Cheung et al, 2010[24] | HBOC** | Proband Self Report (Survey) | 1,103/0 | Probands: Female: 1,103 |
Probands: Yes: 776 No: 327 |
Probands: White: 948 Asian: 66 Latina: 61 African American: 28 |
|
| Conley et al, 2020[27] | HBOC** | Proband Self Report (Questionnaire) | 149/0 | Probands: Mean: 44.9 |
Probands: Female: 149 |
Probands: Black: 149 |
|
| Wagner Costalas et al, 2003[51] | HBOC** | Proband Self Report (Survey) | 162/444 (First) | Probands: Median: 50 Relatives: Median: 50 |
Probands: Female: 162 Relatives: Female: 204 Male: 240 |
Probands: White: 147 Unknown: 15 |
|
| Dilzell et al, 2014[25] | Lynch Syndrome | Proband Self Report (Questionnaire) | 50/0 (First, Second) | Probands: Mean: 47.0 |
Probands: Female: 33 Male: 9 |
Probands: White: 41 Native American: 2 African American: 1 Asian: 1 Hispanic: 0 Others: 0 Unknown: 5 Relatives: White: 20 Native American: 1 Hispanic: 1 Others: 0 Unknown: 2 |
|
| Eijzenga et al, 2018[38] | HBOC**, Hereditary Colorectal Cancer | Proband Self Report (Survey) | 305/0 | Probands: Intervention mean: 53.1 Control mean: 54.4 |
Probands: Female: 228 Male: 77 |
Probands: Yes: 216 No: 86 |
|
| Fehniger et al, 2013[42] | HBOC** | Proband Self Report (Interview) | 73/606 (First, Second) | Mean: 47.4 | Relatives: Female: 241 Male: 202 |
Probands: African American: 7 Asian/Pacific Islander: 14 Hispanic: 17 White: 32 Mixed: 3 Relatives: White: 135 African American: 53 Asian/Pacific Islander: 117 Hispanic: 123 Mixed: 15 |
|
| Finlay et al, 2008[19] | HBOC** | Proband Self Report (Questionnaire) | 115/655 (First, Second) | Probands: Female: 83 Male: 32 |
Probands: Ashkenazi Jewish: 28 Non-Ashkenazi/White: 79 Unknown/White: 7 Others: 1 |
||
| Forrest et al, 2008[30]* | HBOC**, Lynch Syndrome, MEN Type 1, Peutz-Jegher syndrome | Proband Self Report (Interview) | 19/131 | Probands: Intervention mean: 39.2 Control mean: 38.1 Relatives: Intervention mean: 49.4 Control mean: 42.0 |
Probands: Female: 12 Male: 7 Relatives: Female: 66 Male: 65 |
||
| Gaff et al, 2005[31] | Lynch Syndrome | 12/0 | |||||
| Garcia et al, 2020[32] | HBOC** | Proband Self Report (Questionnaire) | 40/0 | Probands: Preintervention cohort median: 63.0 Postintervention cohort: median 49.0 |
Probands: Female: 40 |
Probands: Yes (breast and ovarian) |
Probands: Preintervention: Non-Hispanic White: 18 Non-Hispanic Black: 2 Postintervention: Non-Hispanic White: 17 Non-Hispanic Black: 1 Hispanic: 1 Unknown: 1 |
| Griffin et al, 2020[20] | HBOC**, Lynch Syndrome (BRCA1, BRCA2, MLH1, MSH2, MSH6, PMS2, RAD51D) | Proband Self Report (Survey) | 64/1,955 | Probands: Mean: 53.0 |
Probands: Female: 60 Male: 4 |
Probands: White: 62 African American: 2 |
|
| Hall et al, 2018[36] | Non-BRCA 1/2, Non-Lynch Syndrome (MYH [Monoallelic, Biallelic], CHEK2, ATM, PALB2, APC, TP53, CDH1, NBN, RAD51C, PTEN, RAD51D, Other: SDHA, SDHB, CDKN24, MRE11A, RAD50, FLCN, FH, MEN1, RET, CEBPA, EGFR, BMPR1A, BARD1, NF2) | Proband Self Report (Survey) | 57/0 | Probands: Median: 52 |
Probands: Female: 47 Male: 10 |
Probands: Yes: 39 No: 18 |
Probandsc: Non-Hispanic/White: 38 Hispanic: 11 Asian: 7 Ashkenazi Jewish: 3 Native American: 2 African American: 1 Others: 2 |
| Hayat Roshanai et al, 2010[33] | HBOC**, Hereditary Colorectal Cancer | Proband Self Report (Interview) | 147/81 | Probands: Female: 133 Male: 14 Relatives: Female: 57 Male: 24 |
Probands: Yes: 54 No: 93 |
||
| Healey et al, 2017[34] | HBOC** | Proband Self Report (Interview) | 165/0 | Probands: Female: 138 Male: 27 |
|||
| Kardashian et al, 2012[39] | HBOC** | Proband Self Report (Survey) | 19/198 (First, Second, Cousins) | Probands: Female: 19 |
Probands: White: 14 Hispanic: 2 African American: 1 South Asian/Indian: 1 Asian/Pacific Islander: 1 Ashkenazi Jewish (as subset of above): 3 |
||
| Kegelaers et al, 2014[29] | HBOC** | Proband Self Report (Questionnaire) | 99/0 | Probands: Mean: 49 |
Probands: Female: 74 Male: 25 |
Probands: White or Ashkenazi Jewish: 99 |
|
| Landsbergen et al, 2005[35] | HBOC** | Proband Self Report (Questionnaire) | 50/0 | Probands: Mean at study: 49 Mean at testing: 44 |
|||
| Lieberman 2018[21] | HBOC** | Proband Self Report (Questionnaire) | 595/0 (First, Second) | Probands: Mean: 52.0 |
Probands: Ashkenazi Jewish: 595 |
||
| McGivern et al, 2004[22] | HBOC** | Proband Self Report (Survey) | 38/803 (First, Second, Third) | Probands: Mean: 48.1 |
Probands: Female: 38 |
Probands: White: 37 Native American: 1 |
|
| Montgomery et al, 2013[40] | HBOC** | Proband Self Report (Survey) | 345/1,046 (First) | Probands: Mean: 48.5 |
Probands: Female: 345 |
Probands: White: 328 Others: 17 |
|
| Patenaude et al, 2006[47] | HBOC** | Proband Self Report (Questionnaire) | 273/0 | Probands: Female: 273 |
Probands: White: 273 |
||
| Peters et al, 2019[49] | Pancreatic ductal adenocarcinoma (APC, ATM, BRCA1, BRCA2, CDKN2A, CHEK2, MUTYH, NF1, PALB2, RAD50, TP53) | Proband Self Report (Survey) | 104/466 | Probands: Median: 67 |
Probands: Female: 49 Male: 55 |
Probands: Yes: 104 |
Probands: White: 82 (Among 99 who completed MICRA) |
| Ricker et al, 2018[52] | HBOC**, Lynch Syndrome, Other Heritable Cancers (APC, BMPR1A, CDH1, CDK4, CDKN2A, GREM1, MUTYH, NBN, POLE, POLD1 PTEN, SMAD4, STK11, and TP53) | Proband Self Report (Questionnaire) | 136/0 | Probands: Mean: 52.4 |
Probands: Female: 105 Male: 31 |
Probands: Yes: 103 No: 33 |
Probands: Non-Hispanic/White: 63 Hispanic: 56 Asian: 14 Black: 2 Others: 1 |
| Stoffel et al, 2008[26] | Lynch Syndrome | Proband Self Report (Questionnaire) | 174/0 | Probands: Mean: 46.7 |
Probands: Female: 122 Male: 52 |
Probands: Yes: 106 No: 68 |
Probands: White: 157 Non-White: 16 Unknown/missing: 1 |
| Taber et al, 2015[23] | HBOC**, Lynch Syndrome | Proband Self Report (Survey) | 77/0 | Probands: Median: 54.5 |
Probands: Female: 58 Male: 17 |
Probands: Yes: 33 No: 44 (breast and colon) |
Probands: Non-Hispanic White: 40 Non-Hispanic Black: 15 Hispanic/Latino: 13 Others: 3 |
| Troian et al, 2020[48] | HBOC** | Proband Self Report (Questionnaire) | 230/465 (First – Children) | Probands: Female mean: 48.8 Male mean: 60.4 |
Probands: Female: 160 Male: 70 Relatives: Female: 249 Male: 216 |
Probands: Yes: 44 |
|
| Yoon et al, 2011[53] | HBOC** | Not Reported | 37/471 (First) | Probands: Median: 45.0 |
Probands: Female: 37 Relatives: Female: 227 Male: 244 |
Probands: Malaysian: 6 Indian: 8 Chinese: 23 Relatives: Malaysian: 11 Indian: 8 Chinese: 42 |
Data for X-linked conditions and balanced reciprocal translocations from Forrest 2008 were excluded from our review.
HBOC : Hereditary breast and ovarian cancer.
Reported as per original article.
3.2. Cumulative patient characteristics
A total of 3,779 probands and 11,711 relatives were evaluated for disclosure of genetic test results. The median age of probands across all studies was 50 years and the median age of relatives across all studies was 33.5 years. Among 31 studies that included information on proband biologic sex, 5,118 (83.9%) were female and 981 (16.1%) were male. Among 11 studies that included information on relative biologic sex, 2,094 (50.5%) were female and 2,051 (49.5%) were male.
Twenty-two studies reported information on probands’ race and ethnicity. Among the 2,136 probands in these studies, 1601 (75.0%) were White, 224 (10.5%) were Black, 170 (8.0%) were Hispanic, 125 (5.9%) were Asian and 6 (0.3%) were Native American. Of these probands, 626 (29.3%) were Ashkenazi Jewish.
3.3. Cumulative rates of results disclosure and cascade genetic testing
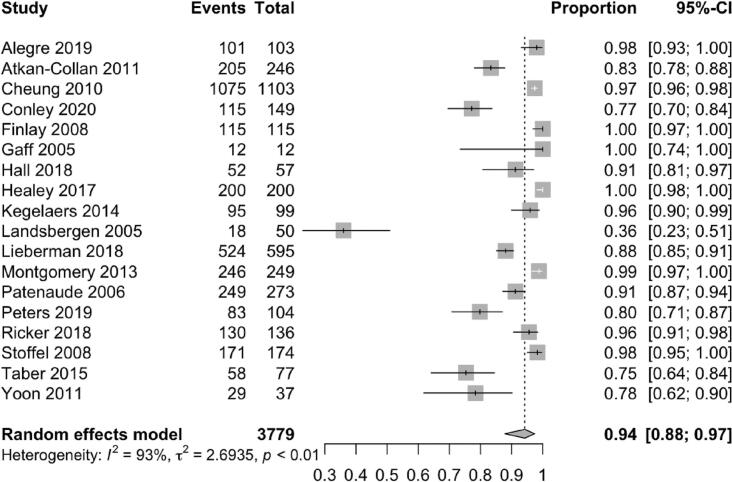
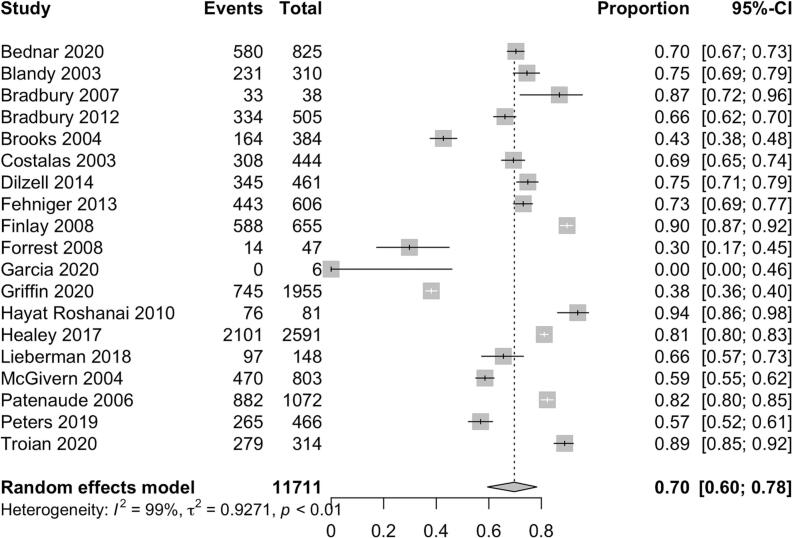
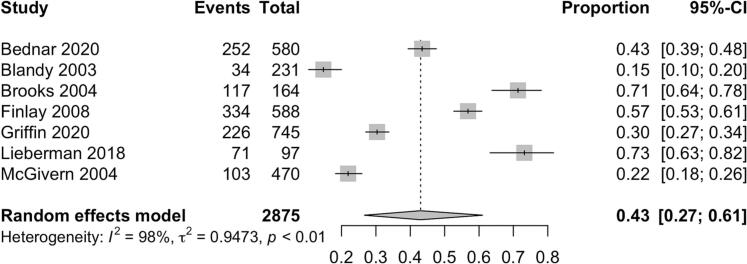
Among 3,779 probands, 94% (95% CI 88-97%) initiated the cascade of information about their pathogenic gene variant by disclosing to at least one relative (Figure 2). Among 11,711 relatives, 70% (95% CI 60 - 78%) received information about the hereditary cancer syndrome identified in their family (Figure 3). Female relatives were more likely to have genetic information disclosed to them as compared to male relatives (79% [95% CI 73% - 84%] vs 67% [95% CI 57% - 75%]). First-degree relatives were also more likely to be informed as compared to second-degree relatives (83% [95% CI 77% - 88%] vs 58% [95% CI 45% – 69%]). Among 2,875 relatives across 7 studies who were informed of their risk of carrying a pathogenic gene variant, 43% (95% CI 27 - 61%) eventually completed genetic testing (figure 4) [[16], [17], [18], [19], [20], [21], [22]].
Fig. 2.
Pooled proportion of probands who informed at least 1 at-risk relative.
Fig. 3.
Pooled proportion of at-risk relatives who were informed.
Fig. 4.
Pooled proportion of at-risk relatives who completed genetic testing among those informed.
3.4. Additional factors associated with disclosure
Other studies identified factors associated with disclosure of genetic test results, but the data were either too limited or heterogenous to be meta-analyzed. Four studies reported on the association between race and ethnicity and results disclosure. Taber et al. and Cheung et al. reported higher rates of disclosure among White vs non-White families, whereas two studies found no association [[23], [24], [25], [26]]. Conley et al. studied disclosure patterns among Black families only, finding that 77% of Black probands in their cohort disclosed their genetic test results to at least one relative [27].
Five studies evaluated the impact of proband education level on rates of disclosure. Two studies reported that probands who had at least a college education were more likely to disclose results to relatives when compared to probands whose highest level of education was high school [28,29]. Three studies reported no association between proband education level and disclosure rates [21,25,26].
Two studies evaluated the impact of proband socioeconomic status on disclosure rates. Taber et al. reported that probands with annual incomes higher than $35,000 were more likely to share their genetic test results with relatives when compared to probands with annual incomes lower than $35,000; Cheung et al. reported no association between income and disclosure rates of probands [23,24].
3.5. Barriers to disclosure
Sixteen studies reported on barriers that probands faced in disclosing to relatives. The most common barrier to disclosure reported by probands in 10 studies was not being in close contact with relatives, including being estranged and not having relatives’ contact details [[20], [21], [22],26,[30], [31], [32], [33], [34], [35]]. Nine studies reported probands’ fear of causing their relatives distress or anxiety as a barrier to disclosure [[19], [20], [21], [22],[26], [27], [28],30,35]. Six studies reported that probands felt their relatives were either too old or too young to learn of the familial pathogenic variant [17,20,28,32,33,36]. Five studies reported that probands either did not know why it was important to share information about the familial pathogenic variant with relatives or that they felt genetic information was too personal to share [19,20,22,28,30]. Four studies reported probands did not disclose to relatives because they found the topic too distressing to bring up [19,27,30,37]. Other less commonly reported barriers included inferring a lack of interest from relatives and not feeling comfortable sharing complex medical information [19,21,22].
3.6. Interventions
Five studies evaluated interventions to assist probands in disclosing information about a familial pathogenic variant to their relatives (Table 2). A meta-analysis of disclosure rates among intervention studies was not possible due to an inadequate number of studies evaluating sufficiently homogenous interventions and reported outcomes. Two studies evaluated telephone counselling interventions whereby a member of the clinical team counseled the proband via telephone regarding identification of at-risk relatives, underscoring the importance of disclosing results, and providing information about what content to disclose [30,38]. Two studies evaluated the provision of written educational materials such as information about their familial cancer syndrome, information about cost and insurance coverage of genetic testing and letters to share with relatives informing them of their risk [32,39]. One study evaluated in-person counseling of probands to provide them with strategies on how to disclose to relatives, prepare them for relatives’ emotional reactions and share genetic counseling resources with their relatives [40]. Of all these studies, only Forrest et al and Kardashian et al reported positive results of their telephone counseling and written educational resource interventions respectively [30,39].
Table 2.
Description of intervention studies.
| Study | Design | Description of Intervention | Control Group (Non-Randomized Studies) | Disclosure rate in intervention group | Disclosure rate in control group | p-value |
|---|---|---|---|---|---|---|
| Eijzenga, 2018[38] | Randomized controlled trial | Telephone counselling of probands to assist them in disclosing | N/A | 1st Degree Relatives: 82% 2nd Degree Relatives: 75% |
1st Degree Relatives: 83% 2nd Degree Relatives: 78% |
1st Degree Relatives: NS 2nd Degree Relatives: NS |
| Forrest, 2008[30] |
Non-randomized pre-post study | Telephone counselling of probands to assist them in disclosing | Historical controls | 61% | 36% | p=0.01 |
| Garcia, 2020[32] |
Non-randomized pre-post study | Provision of written educational materials to probands | Historical controls | 77% | 83% | p=0.26 |
| Kardashian, 2012[39] | Non-randomized pre-post study | Provision of written educational materials to probands | All eligible patients seen for genetic counselling prior to implementation of intervention | 1st Degree Relatives: 90% 2nd Degree Relatives: 75% Cousins: 63% |
1st Degree Relatives: 88% 2nd Degree Relatives: 38% Cousins: 40% |
1st Degree Relatives: p=1.00 2nd Degree Relatives: p=0.32 Cousins: p=0.86 |
| Montgomery, 2013[40] | Randomized controlled trial | In-person counselling of probands to help them in disclosing | N/A | Shared with at least one relative: 99.3% Shared with all FDR: 54.0% |
Shared with at least one relative: 99.2% Shared with all FDR: 52.7% |
Shared with at least one relative: p=0.59 Shared with all FDR: p=0.83 |
3.7. Risk of bias assessment
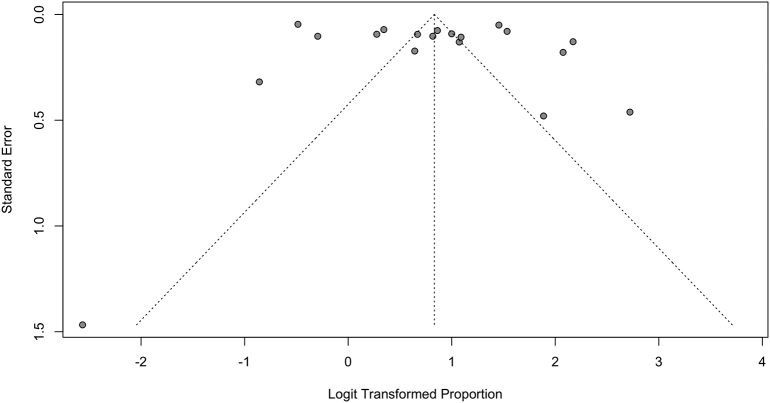
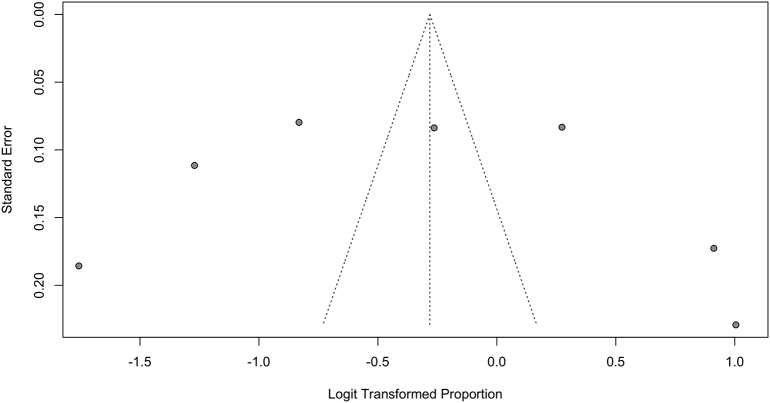
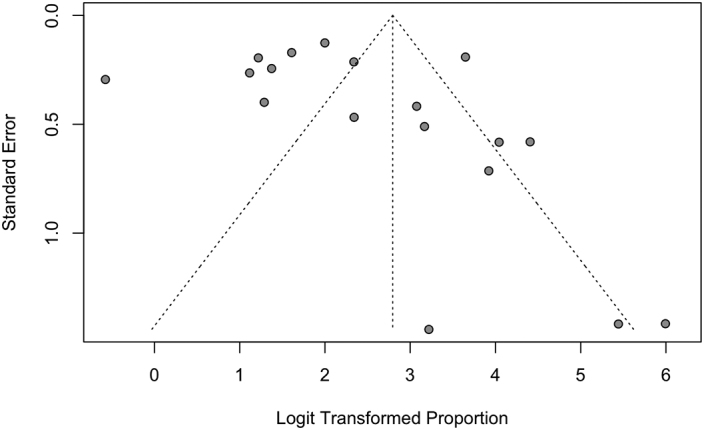
Studies were assessed for risk of bias using tools from the Joanna Briggs Institute. Although all studies suffered from risk of bias in at least one domain, most commonly in lack of identification and control of confounders, they were all deemed appropriate to include in this synthesis. The funnel plots for rates of disclosure and uptake of cascade genetic testing suggest underrepresentation of smaller studies (Supplementary Fig. 1, Supplementary Fig. 2, Supplementary Fig. 3).
Supplementary Fig. 1.
Funnel plot for pooled proportion of probands who Informed at least 1 at-risk relative
Supplementary Fig. 2.
Funnel plot for pooled proportion of at-risk relatives who were informed
Supplementary Fig. 3.
Funnel plot for pooled proportion of at-risk relatives who completed genetic testing among those informed
4. Discussion
We have systematically reviewed the available literature on disclosure of genetic test results by probands to their relatives in the context of cancer-associated pathogenic variants and found that up to 30% of relatives are not aware of the familial cancer risk. Disclosure of genetic testing results by probands is the obligate prerequisite to the process of proband-mediated cascade genetic testing that can result in early cancer detection and cancer prevention for at-risk relatives. With the majority of cancer-associated pathogenic gene variant carriers in the U.S. unaware of their risk, determining rates of disclosure as well as uptake of genetic testing among those relatives to whom disclosure was made, are essential to characterize the efficiency of cascade genetic testing when the process is mediated by probands [9]. Subsequently, this will enable the field to identify avenues for improvement. To the best of our knowledge, this is the first meta-analysis on rates of disclosure among probands identified to have cancer-associated pathogenic gene variants to their at-risk relatives. Although our prior systematic review and meta-analysis reported uptake rates of cascade genetic testing, uptake rates specifically among relatives who were informed of their risk of carrying a pathogenic gene variant were not reported, and to the best of our knowledge, this is also the first meta-analysis reporting this outcome [12].
Notably, our findings revealed that only 70% of at-risk relatives are aware of their increased risk of carrying a cancer-associated pathogenic gene variant. Furthermore, among those relatives who are successfully informed, only 43% successfully complete genetic testing to define their pathogenic variant status. Probands were more likely to disclose to female vs male relatives, a trend that has also been observed in uptake of cascade genetic testing [12]. Probands were more likely to disclose to first-degree vs more distant relatives, which has also been reported for cascade testing [12]. Limited literature suggested that probands who were non-White, with lower income and lower levels of completed education were less likely to disclose genetic risk information to their relatives; however, other studies found no association. These studies evaluated the impact of race, ethnicity, income and education level on disclosure rates as secondary outcomes and could thus be underpowered for these outcomes. This highlights the need for well-designed studies, specifically evaluating the influence of these factors on relative disclosure rates. Literature on the impact of race, ethnicity, income and education on cascade testing is also sparse; however, limited data suggest similar trends of lower uptake rates among racial and ethnic minorities, relatives with lower income, and those with lower levels of completed formal education [24,[41], [42], [43], [44]]. Notably, 75% of probands among all studies that reported on disclosure of genetic test results were identified as White. Lack of inclusion of racially and ethnically diverse populations is a critical issue in cancer genetics research and highlights the need to study disclosure patterns among minority and underserved populations [41,45].
This study should be viewed in light of important limitations. First, disclosure of genetic test results to relatives was reported by probands and not confirmed by relatives for all included studies, and thus the meta-analyzed data are subject to recall bias. Furthermore, funnel plots for both proportion of probands disclosing to at least one at-risk relative as well as proportion of at-risk relatives disclosed to demonstrate a skew towards higher disclosure rates among smaller studies. However, larger studies far outnumbered smaller studies in both these funnel plots, and thus the absence of smaller studies with lower disclosure rates is unlikely to be a significant contributor to publication bias. Finally, although all studies were deemed to be of sufficiently low risk of bias overall to be included in our synthesis, no study was entirely unbiased—every included study suffered from risk of bias in one or more domain, most commonly failing to identify and control for confounders.
4.1. Innovation
Interventions aimed to better equip probands to disclose genetic risk information by providing written or telephone resources were generally ineffective, highlighting the need for studies focused on proband barriers to disclosure so that targeted interventions can be developed.
Recent studies have focused on direct relative contact via clinicians or genetic testing clinical laboratories as a strategy to increase rates of cascade genetic testing [11,12]. Direct relative contact is promising in alleviating barriers such as strained relationships, lack of contact and distress in disclosing to relatives, which we found to be the most commonly reported barriers to disclosure. However, our review suggests that several other factors may contribute to an individual’s decision to disclose results to their at-risk relatives including biologic sex, degree of relation and family demographics. It is thus of vital importance to further characterize patterns in and barriers to disclosure to relatives of cancer genetic information in order to develop innovative interventions that can result in equitable improvement in familial disclosure and completion of cascade cancer genetic testing.
The following are the supplementary data related to this article.
Search Strategy
Funding support
Melissa K. Frey is supported by the following grant: NIH/NCATS Grant # KL2-TR-002385. Ravi N. Sharaf is supported by the following grants: National Cancer Institute Grant # K07CA216326 and R01CA211723 and Patient Centered Outcomes Research Institute Grant # IHS-2017C3-9211.
Paul J. Christos and Charlene Thomas are supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
Declaration of Competing Interest
Kevin Holcomb reports a relationship with Johnson & Johnson that includes: consulting or advisory.
References
- 1.Finch A.P., et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32(15):1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X., et al. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-analysis and Systematic Review. Clin Cancer Res. 2016;22(15):3971–3981. doi: 10.1158/1078-0432.CCR-15-1465. [DOI] [PubMed] [Google Scholar]
- 3.Engel C., et al. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol. 2010;8(2):174–182. doi: 10.1016/j.cgh.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 4.de Jong A.E., et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130(3):665–671. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Daly M.B., et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(1):77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 6.Weiss J.M., et al. NCCN Guidelines(R) Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 1.2021. J Natl Compr Canc Netw. 2021;19(10):1122–1132. doi: 10.1164/jnccn.2021.0048. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2020. People Who are at Higher Risk for Severe Illness.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html Available from: (Accessed on April 01, 2020) [Google Scholar]
- 8.Manchanda R., et al. Randomised trial of population-based BRCA testing in Ashkenazi Jews: long-term outcomes. BJOG. 2020;127(3):364–375. doi: 10.1111/1471-0528.15905. [DOI] [PubMed] [Google Scholar]
- 9.Drohan B., et al. Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol. 2012;19(6):1732–1737. doi: 10.1245/s10434-012-2257-y. [DOI] [PubMed] [Google Scholar]
- 10.Offit K., et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38(13):1398–1408. doi: 10.1200/JCO.19.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey M.K., et al. Prospective feasibility trial of a novel strategy of facilitated cascade genetic testing using telephone counseling. J Clin Oncol. 2020;38(13):1389–1397. doi: 10.1200/JCO.19.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey M.K., et al. Cascade testing for hereditary cancer syndromes: should we move toward direct relative contact? a systematic review and meta-analysis. J Clin Oncol. 2022;40(35):4129–4143. doi: 10.1200/JCO.22.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey M.K., et al. Genetic testing for all: overcoming disparities in ovarian cancer genetic testing. Am Soc Clin Oncol Educ Book. 2022;42:1–12. doi: 10.1200/EDBK_350292. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moola S., et al. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int J Evid Based Healthc. 2015;13(3):163–169. doi: 10.1097/XEB.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 16.Bednar E.M., et al. Assessing relatives' readiness for hereditary cancer cascade genetic testing. Genet Med. 2020;22(4):719–726. doi: 10.1038/s41436-019-0735-3. [DOI] [PubMed] [Google Scholar]
- 17.Blandy C., et al. Testing participation in BRCA1/2-positive families: initiator role of index cases. Genet Test. 2003;7(3):225–233. doi: 10.1089/109065703322537241. [DOI] [PubMed] [Google Scholar]
- 18.Brooks L., et al. BRCA1/2 predictive testing: a study of uptake in two centres. Eur J Hum Genet. 2004;12(8):654–662. doi: 10.1038/sj.ejhg.5201206. [DOI] [PubMed] [Google Scholar]
- 19.Finlay E., et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12(1):81–91. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin N.E., et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: An opportunity to improve cancer prevention. Gynecol Oncol. 2020;156(1):140–146. doi: 10.1016/j.ygyno.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman S., et al. Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med. 2018;20(11):1446–1454. doi: 10.1038/gim.2018.26. [DOI] [PubMed] [Google Scholar]
- 22.McGivern B., et al. Family communication about positive BRCA1 and BRCA2 genetic test results. Genet Med. 2004;6(6):503–509. doi: 10.1097/01.gim.0000144014.91237.a1. [DOI] [PubMed] [Google Scholar]
- 23.Taber J.M., et al. Prevalence and correlates of receiving and sharing high-penetrance cancer genetic test results: findings from the Health Information National Trends Survey. Public Health Genomics. 2015;18(2):67–77. doi: 10.1159/000368745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung E.L., et al. Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2211–2219. doi: 10.1158/1055-9965.EPI-10-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dilzell K., et al. Evaluating the utilization of educational materials in communicating about Lynch syndrome to at-risk relatives. Fam Cancer. 2014;13(3):381–389. doi: 10.1007/s10689-014-9720-9. [DOI] [PubMed] [Google Scholar]
- 26.Stoffel E.M., et al. Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol. 2008;6(3):333–338. doi: 10.1016/j.cgh.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conley C.C., et al. The big reveal: Family disclosure patterns of BRCA genetic test results among young Black women with invasive breast cancer. J Genet Couns. 2020;29(3):410–422. doi: 10.1002/jgc4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradbury A.R., et al. How often do BRCA mutation carriers tell their young children of the family’s risk for cancer? A study of parental disclosure of BRCA mutations to minors and young adults. J Clin Oncol. 2007;25(24):3705–3711. doi: 10.1200/JCO.2006.09.1900. [DOI] [PubMed] [Google Scholar]
- 29.Kegelaers D., et al. Disclosure pattern and follow-up after the molecular diagnosis of BRCA/CHEK2 mutations. J Genet Couns. 2014;23(2):254–261. doi: 10.1007/s10897-013-9656-5. [DOI] [PubMed] [Google Scholar]
- 30.Forrest L.E., et al. Increased genetic counseling support improves communication of genetic information in families. Genet Med. 2008;10(3):167–172. doi: 10.1097/GIM.0b013e318164540b. [DOI] [PubMed] [Google Scholar]
- 31.Gaff C.L., et al. Facilitating family communication about predictive genetic testing: probands’ perceptions. J Genet Couns. 2005;14(2):133–140. doi: 10.1007/s10897-005-0412-3. [DOI] [PubMed] [Google Scholar]
- 32.Garcia C., et al. Mechanisms to increase cascade testing in hereditary breast and ovarian cancer: Impact of introducing standardized communication aids into genetic counseling. J Obstet Gynaecol Res. 2020;46(9):1835–1841. doi: 10.1111/jog.14366. [DOI] [PubMed] [Google Scholar]
- 33.Hayat Roshanai A., et al. Disclosing cancer genetic information within families: perspectives of counselees and their at-risk relatives. Fam Cancer. 2010;9(4):669–679. doi: 10.1007/s10689-010-9364-3. [DOI] [PubMed] [Google Scholar]
- 34.Healey E., et al. Quantifying family dissemination and identifying barriers to communication of risk information in Australian BRCA families. Genet Med. 2017;19(12):1323–1331. doi: 10.1038/gim.2017.52. [DOI] [PubMed] [Google Scholar]
- 35.Landsbergen K., et al. Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer. 2005;4(2):115–119. doi: 10.1007/s10689-004-7991-2. [DOI] [PubMed] [Google Scholar]
- 36.Hall E.T., et al. Pathogenic Variants in Less Familiar Cancer Susceptibility Genes: What Happens After Genetic Testing? JCO Precis Oncol. 2018;2:1–10. doi: 10.1200/PO.18.00167. [DOI] [PubMed] [Google Scholar]
- 37.Aktan-Collan K.I., et al. Sharing genetic risk with next generation: mutation-positive parents' communication with their offspring in Lynch Syndrome. Fam Cancer. 2011;10(1):43–50. doi: 10.1007/s10689-010-9386-x. [DOI] [PubMed] [Google Scholar]
- 38.Eijzenga W., et al. How to support cancer genetics counselees in informing at-risk relatives? Lessons from a randomized controlled trial. Patient Educ Couns. 2018;101(9):1611–1619. doi: 10.1016/j.pec.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Kardashian A., et al. A Pilot study of the Sharing Risk Information Tool (ShaRIT) for Families with Hereditary Breast and Ovarian Cancer Syndrome. Hered Cancer Clin Pract. 2012;10(1):4. doi: 10.1186/1897-4287-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery S.V., et al. Preparing individuals to communicate genetic test results to their relatives: report of a randomized control trial. Fam Cancer. 2013;12(3):537–546. doi: 10.1007/s10689-013-9609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braley E.F., et al. Patient ethnicity and cascade genetic testing: a descriptive study of a publicly funded hereditary cancer program. Fam Cancer. 2022;21(3):369–374. doi: 10.1007/s10689-021-00270-0. [DOI] [PubMed] [Google Scholar]
- 42.Fehniger J., et al. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns. 2013;22(5):603–612. doi: 10.1007/s10897-013-9592-4. [DOI] [PubMed] [Google Scholar]
- 43.Sanz J., et al. Uptake of predictive testing among relatives of BRCA1 and BRCA2 families: a multicenter study in northeastern Spain. Fam Cancer. 2010;9(3):297–304. doi: 10.1007/s10689-009-9313-1. [DOI] [PubMed] [Google Scholar]
- 44.Holloway S.M., et al. Uptake of testing for BRCA1/2 mutations in South East Scotland. Eur J Hum Genet. 2008;16(8):906–912. doi: 10.1038/ejhg.2008.17. [DOI] [PubMed] [Google Scholar]
- 45.Lemke A.A., et al. Addressing underrepresentation in genomics research through community engagement. Am J Hum Genet. 2022;109(9):1563–1571. doi: 10.1016/j.ajhg.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alegre N., et al. Psychosocial and clinical factors of probands impacting intrafamilial disclosure and uptake of genetic testing among families with BRCA1/2 or MMR gene mutations. Psychooncology. 2019;28(8):1679–1686. doi: 10.1002/pon.5142. [DOI] [PubMed] [Google Scholar]
- 47.Patenaude A.F., et al. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24(4):700–706. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- 48.Troian J., et al. Parental disclosure of positive BRCA1/2 mutation status to children 10 years after genetic testing. Psychol Health Med. 2020;25(6):756–766. doi: 10.1080/13548506.2019.1659981. [DOI] [PubMed] [Google Scholar]
- 49.Peters M.L.B., et al. Family communication and patient distress after germline genetic testing in individuals with pancreatic ductal adenocarcinoma. Cancer. 2019;125(14):2488–2496. doi: 10.1002/cncr.32077. [DOI] [PubMed] [Google Scholar]
- 50.Bradbury A.R., et al. When parents disclose BRCA1/2 test results: their communication and perceptions of offspring response. Cancer. 2012;118(13):3417–3425. doi: 10.1002/cncr.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner Costalas J., et al. Communication of BRCA1 and BRCA2 results to at-risk relatives: a cancer risk assessment program’s experience. Am J Med Genet C Semin Med Genet. 2003;119C(1):11–18. doi: 10.1002/ajmg.c.10003. [DOI] [PubMed] [Google Scholar]
- 52.Ricker C.N., et al. Patient communication of cancer genetic test results in a diverse population. Transl Behav Med. 2018;8(1):85–94. doi: 10.1093/tbm/ibx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon S.Y., et al. Genetic counseling for patients and families with hereditary breast and ovarian cancer in a developing Asian country: an observational descriptive study. Fam Cancer. 2011;10(2):199–205. doi: 10.1007/s10689-011-9420-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategy