Abstract
Objective
Financial toxicity affects 30–50% of people with cancer in the US. Although experts recommend patients and physicians discuss treatment cost, cost discussions occur infrequently. We pilot-tested the feasibility, acceptability and influence on outcomes of the DIScussions of COst (DISCO) App, a multi-level communication intervention designed to improve cost discussions and related outcomes.
Methods
While waiting to see their physician, patients (n = 32) used the DISCO App on a tablet. Physicians were given a cost discussion tip sheet. Clinic visits were video recorded and patients completed pre- and post-intervention measures of self-efficacy for managing costs, self-efficacy for interacting with physicians, cost-related distress, and perceptions of the DISCO App. Coders observed the recordings to determine the presence of cost discussions, initiators, and topics.
Results
Most patients reported needing ≤15 min to use the DISCO App, and that it made it easier to ask cost-related questions. Findings showed increased self-efficacy for managing treatment costs (p = .02) and for interacting with physicians (p = .001). All visits included a cost discussion.
Conclusions
Prompting patients to discuss costs may improve cost treatment discussions and related outcomes.
Innovation
An app-based and tailorable treatment-cost communication intervention is feasible, acceptable, and demonstrates promise in prompting cost discussions and improving outcomes.
Trial registration: Clinical Trials.gov registration number: NCT03676920 (September 19, 2018).
Keywords: Financial toxicity, Patient active participation, Question prompt list, Cancer treatment cost discussion, App-based intervention
1. Introduction
Financial toxicity, the severe material and psychological burden of the cost of cancer treatment, affects an estimated 30–50% of patients with cancer in the United States, including people with health insurance [1]. As the cost of care increasingly shifts to patients, more patients must use up their savings, incur debt and file for bankruptcy [[2], [3], [4], [5], [6], [7]]. On average, cancer patients are responsible for $16,000 annually for direct and indirect out-of-pocket treatment costs [8]. Patients may also suffer great psychological harm, including significant, even catastrophic, levels of cost-related distress [[9], [10], [11], [12], [13]].
Cancer treatment cost and financial toxicity can influence treatment decisions, treatment adherence, and health outcomes, including an increased risk of mortality [1,[14], [15], [16], [17], [18], [19], [20]]. Treatment costs factor into patients' decisions about treatment [[21], [22], [23], [24]], including whether to participate in clinical trials [22,25]. For example, patients with lower incomes are more likely to choose treatments with lower costs even if those treatments have lower survival and higher toxicity [23]. To offset cost, patients may deviate from recommended treatment (including treatment for side effects) [15,26,27] and/or forgo treatment altogether [24]. A study of 254 patients being treated with either chemotherapy or hormonal therapy found that 20% took less than, partially filled, or avoided filling the prescribed medication due to the out-of-pocket costs [15]. Another study of 164 patients with solid tumors found that 45% were non-adherent to treatment due to cost [28]. A study of 1556 cancer survivors found that those who reported financial problems were more likely to delay (18.3% vs. 7.4%) or forgo treatment (13.8% vs. 5.0%) than respondents without financial problems [29].
Addressing financial toxicity requires policy changes at the national, state, and hospital levels. In the meantime, however, patient-physician treatment cost discussions early in diagnosis and treatment may help alleviate financial toxicity [10,14,[30], [31], [32], [33]] by improving patients' knowledge of and how to manage potential costs, and by connecting patients with vital economic support early in the treatment process [34]. Most patients (80%) and oncologists (80%) want to discuss treatment costs [35,36], and professional organizations such as the American Society of Clinical Oncology (ASCO) encourage oncologists to discuss treatment cost with patients. However, observational research has found that these discussions are infrequent [36,37]. In our previous observational study of video-recorded treatment discussions between patients with cancer and their oncologists (n = 103), we found that cost discussions occurred in only 45% of treatment discussions [38]. When cost was discussed, it was mostly patient-initiated (63%) and focused more on indirect costs (e.g., time off work) than on direct costs (e.g., copayments) [39]. Without a cost discussion early in treatment decision making, patients are unlikely to be referred for guidance or assistance in a timely manner, thereby missing out on early financial and psychological support, which are critical steps in reducing longer-term financial toxicity [15,34,40] and improving treatment adherence [10,28,41].
Question prompt lists (QPL) are simple communication tools that have been shown to prompt discussion about specific topics during clinical interactions. QPLs are composed of a list of questions provided to patients to encourage them to prepare for visits by considering questions they would like to ask their healthcare provider [[42], [43], [44]]. QPLs have been shown to improve communication quality (e.g., patient active participation in interactions [45], patient-oncologist information exchange [46], topics discussed [47]), patient psychological and cognitive outcomes (e.g., satisfaction, anxiety; information recall) [42], and patient role in treatment decisions and trust in their oncologist [42,44,48].
However, most current QPLs are limited in two ways: they do not adequately address treatment costs, and most are paper-based and static. Although a few previous QPLs and similar interventions have been tailorable, tailorable QPLs have been not previously been used in the context of treatment cost communication or financial toxicity [49]. An electronic, cost-focused QPL in the form of an application or “app” provided to patients in the clinic prior to meeting with their physician may overcome these limitations.
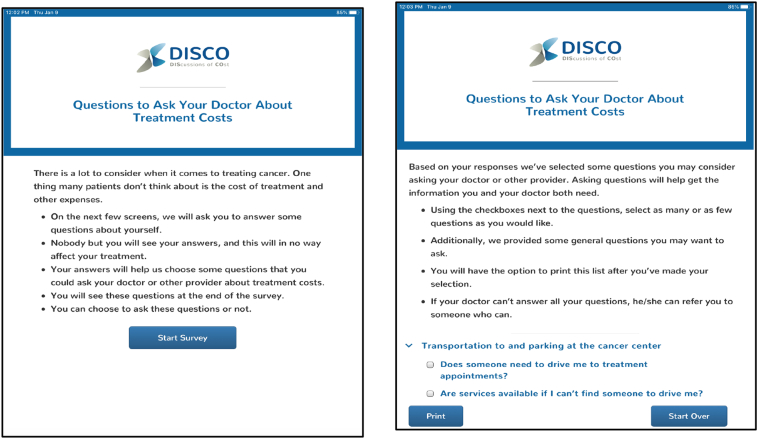
Building on our experience with testing QPLs in oncology outpatient clinics [45,50], we designed and built a novel multi-level communication intervention, including the DIScussion of COst Application for patients (DISCO App; Fig. 1) [51] and an accompanying treatment cost tip sheet for physicians (Fig. 2).
Fig. 1.
DISCO App QPL Introduction Screens.
Fig. 2.
Physician Tip Sheet.
The purpose of this study was to pilot-test the intervention in an oncology clinic setting to determine its feasibility, acceptability, and influence on outcomes of interest, including: patient-physician treatment cost discussions during treatment discussions, patients' self-efficacy for managing treatment costs, patients' self-efficacy for interacting with physicians, and patients' treatment cost-related distress.
2. Methods
2.1. Intervention
We designed a multi-level communication intervention to prompt treatment cost discussions between patients and physicians. Although the intervention was focused primarily on patients, research has suggested that physician attitudes and behavior may present barriers to cost discussions because physicians may be uncomfortable or unable to respond appropriately to patient questions about treatment cost [52,53]. To address this, at baseline, we provided physicians a treatment cost discussion “tip sheet.” The tip sheet emphasizes physicians' role in cost discussions (as recommended by ASCO) and suggests ways to overcome identified barriers to cost discussions [36,37,52,53]. The tip sheet also acknowledges the complexities of treatment costs by including statements such as “if a patient asks about cost and you do not know the answer, you can simply say: “I'm glad you brought this up, because it's important for me to know what concerns you have about your treatment. I'm not an expert in this area, but if you have questions about costs, I can arrange for you to meet with a social worker who can help after we're done here.”
For patients, the DISCO App (Fig. 1) [51] is displayed on an iPad provided just prior to their second interaction with their physician, in which they discuss and finalize treatment plans. The DISCO App opens and the QPL is introduced with text that explains that the DISCO App includes a short survey, which will lead to some cost-related questions the patient can consider asking the physician. This section asks patients to enter their demographic information and their financial circumstances. Specifically, patients respond to 17 questions, such as “How much do you know about your insurance coverage?”; “Are you currently employed?”; “Is there anyone who helps you when you're sick or need help of any kind?”. Based on patient responses, an individually-tailored QPL with up to 18 cost-related questions in 7 categories is generated (Table 1). For example, patients who indicate they are employed will be prompted to ask: “Can I schedule my treatment around my job?”; patients who indicate transportation concerns will be prompted to ask: “Are services available if I can't find someone to drive me?” Patients who indicate they are unfamiliar with their insurance coverage will be prompted to ask: “Is there someone I can talk to about my insurance and treatment cost questions?” All patients, regardless of their responses, are provided with four general questions about their diagnosis and have the option of adding in any of their own questions. Once they have completed the questions and received their individualized QPL, they can take the iPad or a printed question list into the meeting with the physician.
Table 1.
The DISCO App's Prompted Questions by Question Type.
| Cost of appointments and treatments |
|
|
|
|
| Help with understanding my treatment costs and what my insurance covers |
|
|
|
| Transportation to and parking at the cancer center |
|
|
|
| Living far from the cancer center |
|
|
| Working during treatment |
|
|
|
| Assistance programs |
|
|
| Family and living responsibilities |
|
| General questions about cancer and treatment (all patients will get these) |
|
|
|
|
2.2. Participants and setting
Data were collected in two outpatient community clinics affiliated with Wayne State University (WSU)/Karmanos Cancer Institute (KCI) located in Southeast, Michigan from August 2018 to December 2019.
Patients were eligible to participate if they were recently diagnosed with breast, lung, prostate, or colorectal cancer and were scheduled to see a participating radiation oncologist for an initial treatment discussion. We focused on these cancers due to their prevalence in the United States [54]. Radiation oncologists were eligible if they routinely treated patients for breast, lung, prostate, or colorectal cancer. We focused on radiation oncology because radiation treatment is disruptive, expensive, and includes both direct and indirect costs of treatment [55,56]. Given that this was a pilot test of an intervention, we recruited a small convenience sample, which is a common strategy for this type of clinic-based intervention pilot [57].
2.3. Procedure and measures
Upon consent into the study, physicians completed a one-time baseline questionnaire where they provided information on their sex and race/ethnicity, and then received the physician treatment cost discussion tip sheet. After patients were consented, they completed a baseline questionnaire that assessed sociodemographic characteristics and other personal attributes.
Patients arrived up to one hour before their scheduled appointment to complete self-report measures on an iPad through an electronic survey service (Qualtrics). After completing pre-interaction measures, a member of the research staff showed patients the DISCO App on an iPad and provided a brief explanation on how to use it. The iPad was connected to a printer on-site, allowing patients to print their individually-tailored QPL created from the DISCO App for note-taking.
Each examination room was equipped with unobtrusive digital audio and video devices that recorded the examination room during the clinical interaction. This recording system has been used by the study team for more than 15 years [28,41], and research has strongly suggested that video recording has little impact on participants' verbal or nonverbal behaviors [42] and provides enhanced validity compared with audio recording alone [43].
After the patient-physician treatment discussion, patients completed post-discussion self-report measures. The study was approved by WSU/KCI institutional review board. All patients and physicians provided consent as participants, which included specific permission to be video recorded.
2.4. Feasibility
To assess the feasibility of the DISCO App, patients reported how long it took them to use the DISCO App and research staff collected a copy of the printed question list to determine how many questions patients selected from the DISCO App to be printed for a discussion with their physician. The procedures would be considered feasible if the majority of patients were able to complete using the DISCO App in less than 15 min, and managed the DISCO App with little or no assistance. We also tracked whether any participant refused to be video recorded and/or left the study early.
2.5. Acceptability
To assess the acceptability of the DISCO App, after the clinical interactions, patients completed measures of their perceptions of the DISCO App (“the questions in the DISCO App were easy to understand”; “the DISCO App made it easier to ask my doctor cost-related questions”; “some of the questions in the DISCO App were useful to me as I was talking with my doctor”; “some of the questions in the DISCO App made me uncomfortable”) on a Likert scale (1 = strongly disagree to 5 = strongly agree). The DISCO App would be considered acceptable if the average response to the measures was at the mid-point or above.
2.6. Self-report measures of outcomes of interest
To assess the influence of the intervention on outcomes of interest, patients completed pre- and post-interaction measures of financial toxicity- and cost discussion-related outcomes including self-efficacy for managing treatment costs (e.g., how confident are you in your ability to find out how to pay for direct costs that arise with treatment?; 5 = very confident, 1 = not at all confident) [58]; self-efficacy for interacting with physicians (e.g., how confident are you in your ability to know what questions to ask a doctor?; 5 = very confident, 1 = not at all confident) [59], and cost-related distress (e.g., I am concerned about my ability to afford to pay for my cancer treatment; 5 = strongly agree, 1 = strongly disagree).
2.7. Physician and patient interaction coding
To further assess the influence of the intervention, we analyzed video-recorded patient-physician treatment cost discussions using methods from our previous research [38]. Patient-physician cost discussions were broadly defined as verbal discussion of topics related to a monetary expense for the patient for cancer treatment. This includes direct costs such as appointment copayments and indirect costs such as loss of income due to time off work.
Two trained coders observed all video-recorded interactions to identify the presence of cost discussions using a validated coding system, and, when these occurred, the initiator (patient or physician) and the cost topics raised, from a list identified from previous research, including insurance/copayment, out-of-pocket costs, time off from work, transportation/parking, lodging, social work/financial navigation, and scheduling treatment around patient's schedule [38].
Interrater reliability was assessed using 20% of the sample of video-recorded interactions. First, the coders identified the presence of cost discussions and reliability was determined using percent agreement, which was 87.5%. Second, the coders labeled each cost discussion's initiator and topic. Reliability for this phase was determined by Cohen's kappa (K = 1.0). Because the high K value suggested high intercoder reliability, the remaining video-recorded interactions were coded by one coder each.
2.8. Data analysis
Data included patient and physician self-report sociodemographics, patient perceptions of the DISCO App, patient self-reported outcome variables, and coder ratings of the video-recorded clinical interactions. We used descriptive statistics to describe the patient and physician participant sociodemographic characteristics, feasibility, acceptability and outcomes of interest. To describe feasibility, we determined how long patients reported using the DISCO App and how many questions patients selected from list the DISCO App created for them. We also determined how many patient and physician participants agreed to have their visits video recorded and if any patients dropped out of the study. To assess acceptability, we determined patients' perceptions of the DISCO App. To assess influence on outcomes of interest, we determined presence, initiator, and topics of any cost discussions that occurred during the video-recorded interactions. We used two-tailed paired samples t-tests to determine any pre- to post-intervention changes in patient self-report outcomes of self-efficacy for managing treatment costs, self-efficacy for interacting with physicians, and cost-related distress. For all analyses α was set at p < .05 (two-tailed).
3. Results
Thirty-two recently-diagnosed patients of two of the three participating physicians agreed to participate and are included in the final sample. Most (n = 30, 94%) interactions were video recorded (in two cases technical difficulties prevented recording) and lasted for an average of 25.7 min (SD = 5.1 min). The sociodemographic characteristics of patients and physicians are reported in Table 2. Most patients had breast cancer (84%).
Table 2.
Patient and physicians sociodemographics.
| Patients1 | Total n = 32 |
|---|---|
| Age | M = 61.48 (SD = 8.08) |
| Female | 31 (97%) |
| Race/Ethnicity | |
| Caucasian or White/Non Hispanic | 32 (100%) |
| Education | |
| < High School | 1 (3%) |
| Graduated High School | 9 (28%) |
| Some College | 9 (28%) |
| Graduated College | 11 (34%) |
| Post-graduate degree | 1 (3%) |
| Marital Status | |
| Married/Partnered | 20 (63%) |
| Divorced/Widowed/Separated | 9 (28%) |
| Single | 1 (3%) |
| Annual Household Income | |
| 0 - $19,999 | 4 (13%) |
| $20,000 - $39,999 | 6 (19%) |
| $40,000 - $59,999 | 14 (44%) |
| $60,000 – $79,999 | 8 (25%) |
| Employment | |
| Employed | 12 (38%) |
| Unemployed but looking for employment | 1 (3%) |
| Retired | 14 (44%) |
| Disabled/unemployed for another reason | 5 (16%) |
| Insurance | |
| Medicaid | 8 (25%) |
| Medicare/Supplement | 9 (28%) |
| Private insurance from employer | 13 (41%) |
| Private insurance the patient pays for | 2 (6%) |
| Primary Tumor Site | |
| Breast | 27 (84%) |
| Lung | 5 (16%) |
| Interaction Length (in Minutes) |
M = 25.7 SD = 5.1 |
| Physicians (n = 3) | |
| Male | 3(100%) |
| Race/Ethnicity | |
| Caucasian or White | 2 (50%) |
| Asian or Pacific Islander | 1 (50%) |
| Number of participating patients seen | |
| MD 1 | 25 (78%) |
| MD 2 | 7 (22%) |
1 Some data are missing because of omissions in patients' responses (education and marital status are not available for one and two patients, respectively).
3.1. Feasibility
All participants agreed to have their interactions video recorded and completed all procedures for the study. Most patients (84%) reported needing 15 min or less to use the DISCO App, while 13% needed 16 to 30 min, and 3% needed 31 to 45 min. All patients finished using the DISCO App without assistance during the time they were in the exam room and waiting to see their physician. On average, patients selected 6.5 questions from the individualized question prompt list to print, with a range of 1–18 selected questions. We emphasize that the list of questions was individually-tailored so not every patient was presented with all 18 possible questions.
3.2. Acceptability
Patients reported that the questions in the DISCO App were easy to understand (M = 4.5; SD = 0.8); the DISCO App made it easier to ask their doctor cost-related questions (M = 3.8; SD = 0.8); and that some of the questions in the DISCO App were useful to them as they were talking with the doctor (M = 3.8; SD = 0.7). They also reported that the questions in the DISCO App did not make them feel uncomfortable (M = 4.1; SD = 1.1; this was a reverse-coded item).
3.3. Influence on outcomes of interest
3.3.1. Observed cost discussions
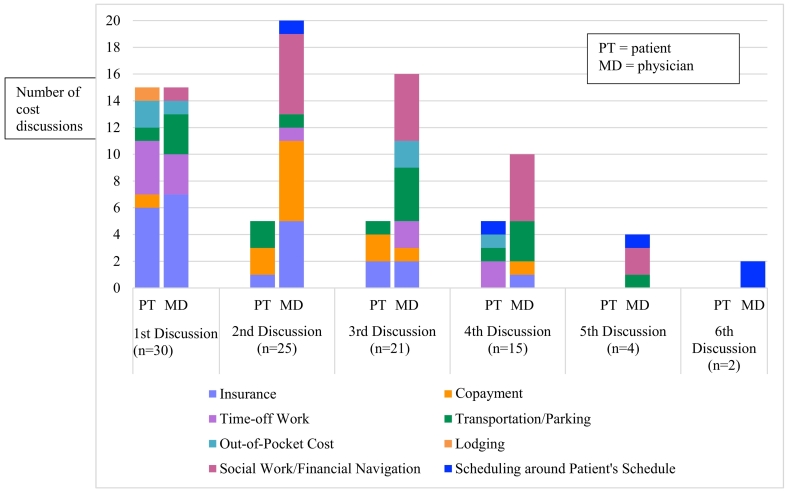
Cost discussions occurred in all 30 of the video-recorded interactions. The number of cost discussions per interaction ranged from 1 to 6, for a total of 97 individual cost discussions across all interactions. Patients and physicians were equally likely to initiate the first cost discussion during a clinical interaction, and the topic most-frequently discussed first was insurance. Physicians were more likely to initiate any subsequent cost discussions. The most frequent patient-initiated topics were insurance followed by time off from work. The most frequent physician-initiated topics were social work/financial navigation followed by insurance (Table 3).
Table 3.
Observed treatment cost discussion by initiator and topic.
3.3.2. Financial toxicity-related outcomes
We also examined whether the DISCO App influenced other financial toxicity- and cost discussion-related outcomes reported by patients before and after they used the DISCO App and subsequently met with their physician. There were significant pre- (M = 16.17, SD = 6.76) to post- (M = 18.69, SD = 5.07) intervention increases in patients' self-efficacy for managing treatment costs (t(31) = 2.38, p = .02) and in patients' self-efficacy for interacting with physicians (M = 40.78, SD = 6.69; M = 43.84, SD = 5.77; t(31) = 3.86, p < .001). There was also a non-significant decrease in pre- (M = 3.23, SD = 0.63) to post- (M = 3.09, SD = 0.77) DISCO App use in treatment cost-related distress (t(31) = 1.28, p = .21).
As a post-hoc sensitivity test, we conducted Levene's Test of Equality of Variances to determine if there was a physician effect on outcomes. Tests for self-efficacy for managing treatment costs (p = .15), self-efficacy for interacting with physicians (p = .95), and cost-related distress (p = .72) all showed equal variances in outcomes when we included physician.
4. Discussion and conclusion
4.1. Discussion
To our knowledge, this study is one of the first to investigate an intervention to prompt patient-physician treatment cost discussions, and to begin to determine if we see the improvements in financial toxicity-related outcomes for patients. In this pilot test, this multi-level intervention appears feasible in a clinic setting and acceptable to patients. Moreover, it appears to influence short-term outcomes, including prompting treatment cost discussions and increasing patient self-efficacy for interacting with physicians and managing costs, which is promising, as improving these may lead to improving longer-term outcomes like the experience of financial toxicity and treatment adherence. It is difficult to discern which of the two parts of the intervention was the most effective, but multi-level interventions are likely to be more effective given the dyadic nature of clinical communication [60,61].
Treatment cost discussions have been identified as one way to help mitigate the deleterious experience of financial toxicity due to cancer treatment by helping to connect patients to important resources earlier on in treatment, and by increasing patients' self-efficacy in managing their treatment costs [10,14,[30], [31], [32], [33], [34]]. In a previous observational study we conducted also using video-recorded treatment discussions, we observed that only 45% of interactions had a treatment cost discussion [38]. Here, we observed that 100% of video-recorded treatment visits had at least one treatment cost discussion, and 70% of treatment discussions had at least three treatment cost discussions. Interestingly, once an initial cost discussion occurred physicians were more likely to initiate subsequent cost discussions in a given interaction. Perhaps this demonstrates that when physicians are prompted by a patient that cost is a concern, they are more likely to continue the discussion. Surprisingly, we observed that physicians would sometimes ask patients for their list of questions and responded to their questions that way, rather than waiting for patients to verbalize their questions. This was not part of our planned analyses, but we made note of this as observational data were collected. Additional work is needed to better understand how the presence of the question list produced by the DISCO App influences both the content and quality of cost discussions, and patients' satisfaction with those discussions.
We also see improvements in some self-reported financial toxicity-related patient outcomes, including self-efficacy in managing treatment costs. Of course, our pilot study design does not allow us to conclude with certainty that the DISCO App or cost discussions prompted from it led to those improvements, but these data are encouraging enough to warrant further investigation into the longer-term effects of the DISCO App and treatment cost discussions for patients.
ASCO's Value of Cancer Treatment Options Framework [14] and its Patient-Clinician Communication Guideline [62] both encourage physicians to discuss treatment costs with their patients. Despite these guidelines, however, research has shown that cost discussions infrequently occur, and that physician engagement around cost concerns is an unmet patient need [63]. Many of the cost discussions we observed in the recorded data did not focus on treatment cost as ASCO defines it. ASCO's tools focus exclusively on direct out-of-pocket costs for patients such as copayments or insurance costs [62]. In this study, both patients and physicians initiated discussions about indirect costs for patients (e.g., taking time off from work, transportation to treatment appointments), which can often be just as detrimental to patients as the direct cost of treating cancer [[64], [65], [66], [67]]. We emphasize this here knowing that the distinction between direct and indirect treatment costs may not be experienced by patients, but that this may be evidence that physicians are well positioned to address the indirect cost of care, even if the precise direct cost of treatments remain elusive.
Concern that cost discussions may add time to clinic interactions has been identified as a barrier to discussing cost [68,69]. Recorded interactions in our study lasted an average of 25.7 min, and we did not have a comparison group to determine if the intervention added time to the treatment discussions. However, data from our own program of research testing similar paper-based QPLs indicated that QPLs do not add a significant amount of time to cancer treatment discussions [45].
Lack of knowledge of the cost of treatment has also been identified as a barrier to providers discussing treatment cost with patients [53]. Our findings, albeit from a small pilot, demonstrate that while both patients and physicians may initiate treatment cost-related discussions, physicians are more likely to follow up with additional topics and discussions. Perhaps this suggests that once a physician knows treatment cost is important to a patient and that the patient is open to discussing cost, the physician is more likely to follow up. Research has shown that, despite the guidelines from ASCO, treatment cost discussions do not occur regularly, perhaps these guidelines coupled with an individualized prompt from an intervention like the DISCO App just prior to meeting with physicians may help these discussions occur with more regularity for patients.
Findings must be considered within the limitations of the study. This was a descriptive, one-arm pilot test using pre-post patient-reported measures and recorded data to assess the DISCO App's feasibility, acceptability, and influence on short-term outcomes. Most patient participants were women with breast cancer, and we did not track patient refusal as a part of our protocol, raising questions about selection bias and limiting the generalizability of the findings [67,70]. Further study with a randomized controlled trial design is needed to assess short and longer-term outcomes, and this research is needed in a diverse patient population with regard to racial/ethnic background, gender, and cancer type.
4.2. Innovation
The DISCO App is innovative because it is among the first of its kind to adapt the QPL, an effective paper-based communication intervention, into an electronic, individually-tailorable, and highly scalable multi-level communication intervention. Designing a communication intervention in an electronic format is especially innovative as we aim to enhance scalability to diverse patient populations and begin to integrate into electronic medical records and patient portals. Additionally, our study is innovative in its methods which included evaluation of outcomes using rigorous, systematic analysis of video-recorded interactions of patient-physician treatment discussions. This work is contributing to our understanding of the mechanisms through which treatment cost discussions and other aspects of clinical communication improve short- and longer-term patient outcomes related to financial toxicity.
4.3. Conclusion
In conclusion, we found the DISCO App to be feasible and acceptable in a clinic setting and potentially effective at prompting treatment cost discussions between patients and physicians and improving other patient outcomes related to financial toxicity. We note here that these findings provided preliminary evidence supporting a larger test of the DISCO App. The effectiveness of an enhanced version of the DISCO App on short- and longer-term patient outcomes, including patient-physician treatment cost discussions, is currently being tested in a randomized controlled trial with a diverse patient population (RSG-20-026-01-CPHPS, Hamel, PI) [71].
Funding
This work was supported by an internal grant from the Karmanos Cancer Institute, Detroit, MI (Hamel, PI).
Declaration of Competing Interest
None.
Footnotes
A previous version of this study was presented as a poster at the American Society of Clinical Oncology) Annual Meeting (May/June 2020) and as a podium presentation to ASCO's virtual Quality Care Symposium (October 2020).
Contributor Information
Lauren M. Hamel, Email: hamell@karmanos.org.
David W. Dougherty, Email: david_dougherty@dfci.harvard.edu.
Theresa A. Hastert, Email: hastertt@karmanos.org.
Seongho Kim, Email: kimse@karmanos.org.
Hadeel Assad, Email: assadh@karmanos.org.
Jasminder Phalore, Email: phalorej@karmanos.org.
Roger Soulliere, Email: roger.soulliere@ppd.com.
Susan Eggly, Email: egglys@karmanos.org.
References
- 1.Altice C.K., Banegas M.P., Tucker-Seeley R.D., et al. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins S.R., Rasmussen P.W., Doty M.M., et al. Findings from the Commonwealth Fund Affordable Care Act Tracking Survey, March-May 2015. Issue Brief. Vol. 16. 2015. Americans’ experiences with marketplace and medicaid coverage; pp. 1–17. [PubMed] [Google Scholar]
- 3.PricewaterhouseCoopers Medical cost trend: Behind the numbers 2016. https://www.pwc.com/us/en/health-industries/behind-the-numbers/assets/pwc-hri-medical-cost-trend-chart-pack-2016.pdf2016
- 4.American Society of Clinical Oncology The state of cancer care in America, 2016: A report by the American Society of Clinical Oncology. J Oncol Pract. 2016;12:339–382. doi: 10.1200/JOP.2015.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagsi R., Pottow J.A., Griffith K.A., et al. Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol. 2014;32:1269–1276. doi: 10.1200/JCO.2013.53.0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsey S., Blough D., Kirchhoff A., et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Kaiser Family Foundation Employer Health Benefits Survey. 2015. http://kff.org/health-costs/report/2015-employer-health-benefits-survey/2015
- 8.Mariotto A.B., Yabroff K.R., Shao Y., et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenn K.M., Evans S.B., McCorkle R., et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332–338. doi: 10.1200/JOP.2013.001322. [DOI] [PubMed] [Google Scholar]
- 10.Bestvina C.M., Zullig L.L., Rushing C., et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10:162–167. doi: 10.1200/JOP.2014.001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharp L., Carsin A.E., Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22:745–755. doi: 10.1002/pon.3055. [DOI] [PubMed] [Google Scholar]
- 12.Chino F., Peppercorn J., Taylor D.H., Jr., et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19:414–420. doi: 10.1634/theoncologist.2013-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado-Guay M., Ferrer J., Rieber A.G., et al. Financial distress and its associations with physical and emotional symptoms and quality of life among advanced cancer patients. Oncologist. 2015;20:1092–1098. doi: 10.1634/theoncologist.2015-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnipper L.E., Davidson N.E., Wollins D.S., et al. Updating the American Society of Clinical Oncology Value Framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34:2925–2934. doi: 10.1200/JCO.2016.68.2518. [DOI] [PubMed] [Google Scholar]
- 15.Zafar S.Y., Peppercorn J.M., Schrag D., et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Cancer Society Cancer Action Network . 2010. A National Poll: Facing Cancer in the Health Care System. [Google Scholar]
- 17.Zafar S.Y., McNeil R.B., Thomas C.M., et al. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11:145–150. doi: 10.1200/JOP.2014.001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsey S.D., Bansal A., Fedorenko C.R., et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34:980–986. doi: 10.1200/JCO.2015.64.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastert T.A., Banegas M.P., Hamel L.M., et al. Race, financial hardship, and limiting care due to cost in a diverse cohort of cancer survivors. J Cancer Surviv. 2019;13:429–437. doi: 10.1007/s11764-019-00764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastert T.A., Kyko J.M., Reed A.R., et al. Financial hardship and quality of life among African American and white cancer survivors: the role of limiting care due to cost. Cancer Epidemiol Biomark Prev. 2019;28:1202–1211. doi: 10.1158/1055-9965.EPI-18-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meropol N.J., Wong Y.N., Albrecht T., et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34:469–478. doi: 10.1200/JCO.2015.63.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong Y.N., Schluchter M.D., Albrecht T.L., et al. Financial concerns about paarticipation in clinical trials among patients with cancer. J Clin Oncol. 2016;34:479–487. doi: 10.1200/JCO.2015.63.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong Y.N., Egleston B.L., Sachdeva K., et al. Cancer patients’ trade-offs among efficacy, toxicity, and out-of-pocket cost in the curative and noncurative setting. Med Care. 2013;51:838–845. doi: 10.1097/MLR.0b013e31829faffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markman M., Luce R. Impact of the cost of cancer treatment: an internet-based survey. J Oncol Pract. 2010;6:69–73. doi: 10.1200/JOP.091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weckstein D.J., Thomas C.A., Emery I.F., et al. Assessment of perceived cost to the patient and other barriers to clinical trial participation. J Oncol Pract. 2011;7:330–333. doi: 10.1200/JOP.2011.000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dusetzina S.B., Winn A.N., Abel G.A., et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32:306–311. doi: 10.1200/JCO.2013.52.9123. [DOI] [PubMed] [Google Scholar]
- 27.Neugut A.I., Subar M., Wilde E.T., et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zullig L.L., Peppercorn J.M., Schrag D. Financial distress, use of cost-coping strategies, and adherence to prescription medication among patients with cancer. J Oncol Pract. 2013;9:60s–63s. doi: 10.1200/JOP.2013.000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent E.E., Forsythe L.P., Yabroff K.R., et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710–3717. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ubel P.A., Abernethy A.P., Zafar S.Y. Full disclosure–out-of-pocket costs as side effects. N Engl J Med. 2013;369:1484–1486. doi: 10.1056/NEJMp1306826. [DOI] [PubMed] [Google Scholar]
- 31.Ubel P.A. Doctor, first tell me what it costs. New York Times. 2013;3:2013. [Google Scholar]
- 32.Blum D. How physicians can explain value to patients. ASCO Daily News May. 2015;29:2015. [Google Scholar]
- 33.Bath C. Disclosing medical costs can help avoid ‘financial toxicity’. ASCO Post. 2013;3 [Google Scholar]
- 34.Smith S.K., Nicolla J., Zafar S.Y. Bridging the gap between financial distress and available resources for patients with cancer: a qualitative study. J Oncol Pract. 2014;10:e368–e372. doi: 10.1200/JOP.2013.001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullock A.J., Hofstatter E.W., Yushak M.L., et al. Understanding patients’ attitudes toward communication about the cost of cancer care. J Oncol Pract. 2012;8:e50–e58. doi: 10.1200/JOP.2011.000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly R.J., Forde P.M., Elnahal S.M., et al. Patients and physicians can discuss costs of cancer treatment in the clinic. J Oncol Pract. 2015;11:308–312. doi: 10.1200/JOP.2015.003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim P. Cost of cancer care: the patient perspective. J Clin Oncol. 2007;25:228–232. doi: 10.1200/JCO.2006.07.9111. [DOI] [PubMed] [Google Scholar]
- 38.Hamel L.M., Penner L.A., Eggly S., et al. Do patients and oncologists discuss the cost of cancer treatment? An observational study of clinical interactions between African American patients and their oncologists. J Oncol Pract. 2017;13:e249–e258. doi: 10.1200/JOP.2016.015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander G.C., Casalino L.P., Meltzer D.O. Patient-physician communication about out-of-pocket costs. JAMA. 2003;290:953–958. doi: 10.1001/jama.290.7.953. [DOI] [PubMed] [Google Scholar]
- 40.Board T.A. Cancer patient financial navigation: Helping patients manage their costs while protecting program margins. Oncology Roundtable. 2014:1–112. [Google Scholar]
- 41.Zolnierek K.B., Dimatteo M.R. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandes K., Linn A.J., Butow P.N., et al. The characteristics and effectiveness of Question Prompt List interventions in oncology: a systematic review of the literature. Psychooncology. 2015;24:245–252. doi: 10.1002/pon.3637. [DOI] [PubMed] [Google Scholar]
- 43.Dimoska A., Tattersall M.H., Butow P.N., et al. Can a “prompt list” empower cancer patients to ask relevant questions? Cancer. 2008;113:225–237. doi: 10.1002/cncr.23543. [DOI] [PubMed] [Google Scholar]
- 44.Sansoni J.E., Grootemaat P., Duncan C. Question Prompt Lists in health consultations: A review. Patient Educ Couns. 2015;98:1454–1464. doi: 10.1016/j.pec.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Eggly S., Hamel L.M., Foster T.S., et al. Randomized trial of a question prompt list to increase patient active participation during interactions with black patients and their oncologists. Patient Educ Couns. 2017;100:818–826. doi: 10.1016/j.pec.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barton E., Moore T.F., Hamel L.M., et al. The influence of a question prompt list on patient-oncologist information exchange in an African-American population. Patient Educ Couns. 2020;103:505–513. doi: 10.1016/j.pec.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown R.F., Bylund C.L., Li Y., et al. Testing the utility of a cancer clinical trial specific Question Prompt List (QPL-CT) during oncology consultations. Patient Educ Couns. 2012;88:311–317. doi: 10.1016/j.pec.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henselmans I., de Haes H.C., Smets E.M. Enhancing patient participation in oncology consultations: a best evidence synthesis of patient-targeted interventions. Psychooncology. 2013;22:961–977. doi: 10.1002/pon.3099. [DOI] [PubMed] [Google Scholar]
- 49.Knerr S., Wernli K.J., Leppig K., et al. A web-based personalized risk communication and decision-making tool for women with dense breasts: Design and methods of a randomized controlled trial within an integrated health care system. Contemp Clin Trials. 2017;56:25–33. doi: 10.1016/j.cct.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eggly S., Hamel L.M., Heath E., et al. Partnering around cancer clinical trials (PACCT): study protocol for a randomized trial of a patient and physician communication intervention to increase minority accrual to prostate cancer clinical trials. BMC Cancer. 2017;17:807. doi: 10.1186/s12885-017-3804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamel L.M., Thompson H.S., Albrecht T.L., et al. Designing and testing apps to support patients with cancer: looking to behavioral science to lead the way. JMIR Cancer. 2019;5 doi: 10.2196/12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander G.C., Casalino L.P., Tseng C.W., et al. Barriers to patient-physician communication about out-of-pocket costs. J Gen Intern Med. 2004;19:856–860. doi: 10.1111/j.1525-1497.2004.30249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altomare I., Irwin B., Zafar S.Y., et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12:247–248. doi: 10.1200/JOP.2015.007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siegel R.L., Miller K.D., Fuchs H.E., et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 55.Smith G.L., Ganz P.A., Bekelman J.E., et al. Promoting the appropriate use of advanced radiation technologies in oncology: summary of a national cancer policy forum workshop. Int J Radiat Oncol Biol Phys. 2017;97:450–461. doi: 10.1016/j.ijrobp.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmer J.D., Patel T.T., Eldredge-Hindy H., et al. Patients undergoing radiation therapy are at risk of financial toxicity: a patient-based prospective survey study. Int J Radiat Oncol Biol Phys. 2018;101:299–305. doi: 10.1016/j.ijrobp.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Hertzog M.A. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 58.Peterson A.M., Harper F.W., Albrecht T.L., et al. Parent caregiver self-efficacy and child reactions to pediatric cancer treatment procedures. J Pediatr Oncol Nurs. 2014;31(1):18–27. doi: 10.1177/1043454213514792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maly R.C., Frank J.C., Marshall G.N., DiMatteo M.R., Reuben D.B. Perceived efficacy in patient-physician interactions (PEPPI): validation of an instrument in older persons. J Am Geriatr Soc. 1998;46(7):889–894. doi: 10.1111/j.1532-5415.1998.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 60.Taplin S.H., Anhang Price R., Edwards H.M., et al. Introduction: Understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stange K.C., Breslau E.S., Dietrich A.J., Glasgow R.E. State-of-the-art and future directions in multilevel interventions across the cancer control continuum. J Natl Cancer Inst Monogr. 2012;2012(44):20–31. doi: 10.1093/jncimonographs/lgs006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilligan T., Bohlke K., Baile W.F. Patient-clinician communication: American Society of clinical oncology consensus guideline summary. J Oncol Pract. 2018;14(1):42–46. doi: 10.1200/JOP.2017.027144. [DOI] [PubMed] [Google Scholar]
- 63.Jagsi R., Ward K.C., Abrahamse P.H., et al. Unmet need for clinician engagement regarding financial toxicity after diagnosis of breast cancer. Cancer. 2018;124(18):3668–3676. doi: 10.1002/cncr.31532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayadevappa R., Schwartz J.S., Chhatre S., Gallo J.J., Wein A.J., Malkowicz S.B. The burden of out-of-pocket and indirect costs of prostate cancer. Prostate. 2010;70(11):1255–1264. doi: 10.1002/pros.21161. [DOI] [PubMed] [Google Scholar]
- 65.Guy G.P., Jr., Ekwueme D.U., Yabroff K.R., et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31(30):3749–3757. doi: 10.1200/JCO.2013.49.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bradley C.J., Yabroff K.R., Dahman B., Feuer E.J., Mariotto A., Brown M.L. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst. 2008;100(24):1763–1770. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trogdon J.G., Liu X., Reeder-Hayes K.E., Rotter J., Ekwueme D.U., Wheeler S.B. Productivity costs associated with metastatic breast cancer in younger, midlife, and older women. Cancer. 2020;126:4118–4125. doi: 10.1002/cncr.33077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown G.D., Hunter W.G., Hesson A., et al. Discussing out-of-pocket expenses during clinical appointments: an observational study of patient-psychiatrist interactions. Psychiatr Serv. 2017;68(6):610–617. doi: 10.1176/appi.ps.201600275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hunter W.G., Zafar S.Y., Hesson A., et al. Discussing health care expenses in the oncology clinic: analysis of cost conversations in outpatient encounters. J Oncol Pract. 2017;13(11):e944–e956. doi: 10.1200/JOP.2017.022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trogdon J.G., Baggett C.D., Gogate A., et al. Medical costs associated with metastatic breast cancer in younger, midlife, and older women. Breast Cancer Res Treat. 2020;181(3):653–665. doi: 10.1007/s10549-020-05654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamel L.M., Dougherty D.W. DISCO App: Study protocol for a randomized controlled trial to test the effectiveness of a patient intervention to reduce the financial burden of cancer in a diverse patient population. BMC. Trials. 2021;22:636–653. doi: 10.1186/s13063-021-05593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]