Highlights
-
•
UA effectively inhibits MSRV proliferation in vitro.
-
•
UA has a protective effect on MSRV-infected fish.
-
•
UA is a valuable agent for MSRV in the industry.
Keywords: Ursolic acid, Antiviral activity, Micropterus salmoides rhabdovirus (MSRV), Largemouth bass
Abstract
Micropterus salmoides rhabdovirus (MSRV) has a high mortality rate and causes huge economic losses to the aquaculture industry. In this study, we identified that ursolic acid (UA) had antiviral efficacy against MSRV in vitro and in vivo. The results showed that UA inhibited MSRV replication in grass carp ovary (GCO) cells with a half-maximal inhibitory concentration (IC50) of 5.55 μM, reduced viral titers and decreased cytopathic effects (CPE). Mechanistically, UA does not directly damage viral particles. On the other hand, UA inhibits MSRV replication by altering viral binding and release. Furthermore, pre- and post-treatment assays revealed that UA had preventive and therapeutic effects. For in vivo studies, UA could enhance the survival rate of MSRV-infected largemouth bass. Similarly, UA reduced the viral load of MSRV in the heart, spleen and brain at 3, 5 and 7 d post-infection. In conclusion, UA is an effective inhibitor of rhabdovirus in aquaculture.
1. Introduction
Micropterus salmoides rhabdovirus (MSRV) belongs to the Vesiculovirus genus in the Rhabdoviridae family (Gao and Chen, 2018). MSRV is bullet-shaped, measured 115–143 nm in length and 62–78 nm in diameter (Ma et al., 2013). MSRV contains a ∼11 kb negative-sense ssRNA that encodes nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G) and RNA-dependent RNA polymerase (L) (Lyu et al., 2019). Since MSRV was isolated from largemouth bass in China, it has spread rapidly and caused several rounds of outbreaks (Ma et al., 2013). A well-known study reported that MSRV-infected largemouth bass died in large numbers within two days, with mortality rate of up to 40% (Ma et al., 2013). Regrettably, no authorized medicines exist to manage the epidemic. As a result, there is value in identifying effective therapeutic strategies to combat MSRV.
Currently, vaccines are the most effective means of prevention on MSRV. For example, subunit vaccines loaded with carbon nanotubes enhanced immune protective rate by 30.6% (Guo et al., 2020). Furthermore, one kind of live vaccine for MSRV via intraperitoneal injection provides 100% immune protection (Zhang et al., 2018). On the other hand, vaccines are restricted due to high labor and production costs (Plant et al., 2011). As a result, like supplements, antiviral medications are equally important in combat epidemics.
Natural products have critical roles in both drug development and chemical biology. For instance, Lonicera japonica Thunb. and its components could effectively inhibit grouper iridovirus in vitro and in vivo (Liu et al., 2020). In addition, two compounds (1, 5 - Anhydro - D - glucitol and 3, 4, 5 - trimethoxy cinnamic acid) isolated from Polygala tenuifolia could inhibit proliferation of grass carp reovirus (GCRV) in ctenopharyngodon idella kidney (CIK) cells. More importantly, arctigenin and its derivatives have inhibitory effect on multifarious rhabdovirus (Shen et al., 2020). Overall, this evidence indicated that it is feasible to identify novel inhibitors from natural products to combat MSRV.
Ursolic acid (UA) (as shown in Fig. 1A) has been isolated as a natural product from herbs such as Rosemarinus officinalis L.(Chen et al., 1992), Origanum majorana L. (Vagi et al., 2005) and Fructus Ligustri Lucidi (Kong et al., 2013). UA is a pentacyclic triterpenoid with ursane that exhibits antiviral (Xu et al., 1996), anti-inflammatory (Ikeda et al., 2008), anticancer (Tan et al., 2011) and antibacterial activities (Fontanay et al., 2008). Li et al. (2019) demonstrated that UA could inhibit the proliferation of infectious hematopoietic necrosis virus (IHNV), another rhabdovirus of fish, in epithelioma papulosum cyprinid (EPC) cells while increasing the survival rate of infected fish.
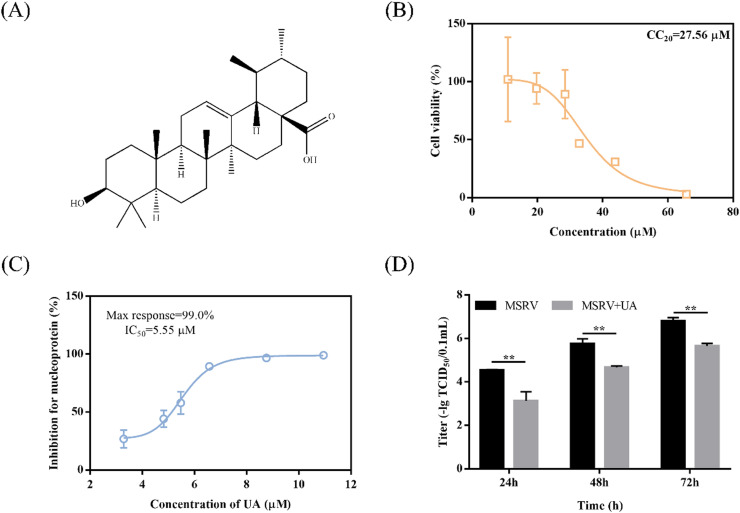
Fig. 1.
The anti-MSRV effects of ursolic acid (UA) in GCO cells. (A) The UA molecule's chemical structure. (B) In vitro cytotoxicity of UA. Six-point dose-response curves for UA in GCO cells are depicted in orange. The 20% cytotoxic concentration (CC20) of UA was indicated. The data were shown as mean ± SEM. (C) UA has an inhibitory impact on MSRV in GCO cells. Antiviral activity of UA was studied using six-point dose-response curve in GCO cells. The percent inhibition of UA in the MSRV of nucleoprotein (N) expression assay was shown in blue. The maximum percent inhibition observed (Max response) of MSRV was indicated. Data were shown as mean ± SEM. (D) UA reduced the titers of MSRV in GCO cells. Data were shown as mean ± SEM. The p value for each study was determined by Student's t-tests. **p < 0.01; *p < 0.05.
Herein, we confirmed the anti-MSRV activity of UA in GCO cells via real-time quantitative PCR (RT-qPCR), titer test and cytopathic effects (CPE) reduction assay. In vitro inhibition of UA was further confirmed by ultracentrifugation assays, viral binding and releasing assays, preventive and therapeutic effects assays. In addition, the antiviral activity of UA was investigated by viability assay and RT-qPCR in infected largemouth bass. Our results further demonstrated the application of ursane against rhabdovirus in the aquaculture industry.
2. Materials and methods
2.1. Chemicals
Ursolic acid (UA) purchased from Aladdin (Shanghai, China), CAS Number: 77-52-1. UA was dissolved in dimethylsulfoxide (DMSO) before being used and prepared as stocking solution (20 mg/mL). The stocking solution was kept at -20 °C for a long time.
2.2. Cell, virus and largemouth bass
Grass carp ovary (GCO) cell lines were gifted from Zhejiang Institute of Freshwater Research (Zhejiang, China). The cell suspension (in Medium 199 (Hyclone, USA) with 10% fetal bovine serum (FBS) (ZETA LIFE, USA)) was seeded into 25 cm2 culture flasks at a density of 1 × 105 cells and grown in a humidified incubator with 5% CO2 at 25 °C.
Micropterus salmoides rhabdovirus (MSRV) (FJ985) was originally isolated from largemouth bass and propagated on GCO cells at 25 °C with 5% CO2 (Yang et al., 2021). Complete genome of MSRV was uploaded to the NCBI database (GenBank: MT818233.1).
Juvenile largemouth bass (n = 2000, total length of 4.36 ± 0.24 cm, bodyweight of 0.94 ± 0.12 g.) were purchased from Yuxi Aquatic Products Company (Chongqing, China). Juvenile were maintained in condition which was a static water system of six 300 L aquarium ponds at 25 °C, pH of 6.6–8.6, dissolved oxygen of above 5 mg/L, and fed with commercial feed (Fuxing Organism Feed Co., Ltd) twice a day prior to the beginning of experiments. The fish were humanely euthanized with tricaine methanesulfonate (MS-222) at the final concentration of 40 μg/mL, and frozen at −80 °C until processing. All experiments were performed according to the Experimental Animal Management Law of China and approved by the Animal Ethics Committee of Northwest Agriculture & Forestry University.
2.3. Cell survival assay
The cell survival assay was performed using the MTT method. Briefly, culture supernatants were removed and exchanged with medium containing 0.5 mg/mL MTT. Then, the cells were incubated for 4 h at 37 °C in darkness, followed by the removal of the medium and adding 100 μL DMSO. The absorbance at 570 nm was detected and the data were expressed as the mean percentage of absorbance in treated vs. control cells. The value of the control was set at 100%.
2.4. Antiviral activity against MSRV in GCO cells
GCO cells with a density of 1 × 104/well were inoculated on 12-well plates for 24 h and cultured in a monolayer. Then, the media was removed. MSRV (1 × 103 TCID50/0.1 mL) was applied to a culture of GCO cells for 2 h. After infection, GCO cells were rinsed 3–5 times with 0.1 M phosphate buffer saline (PBS). UA was diluted to six concentrations (3.28, 4.82, 5.47, 6.57, 8.76 and 10.95 μM) by cell culture medium. GCO cells were incubated in medium containing UA at 25 °C for 48 h. Subsequently, GCO cells were collected, RNA extracted and real-time PCR analysis (MSRV nucleoprotein (N)).
2.5. Cytopathic effect reduction and titer assay
Cytopathic effect (CPE) and viral titer assays were performed as described in previous studies (Yang et al., 2021). Briefly, GCO cells were cultured to a monolayer and infected with gradient viral dilution for 2 h. It was then treated with 13.14 μM UA for 48 h. The viral titer was calculated by the Reed-Muench method (Reed and Muench, 1938). Each sample was directly observed and photographed under an inverted microscope.
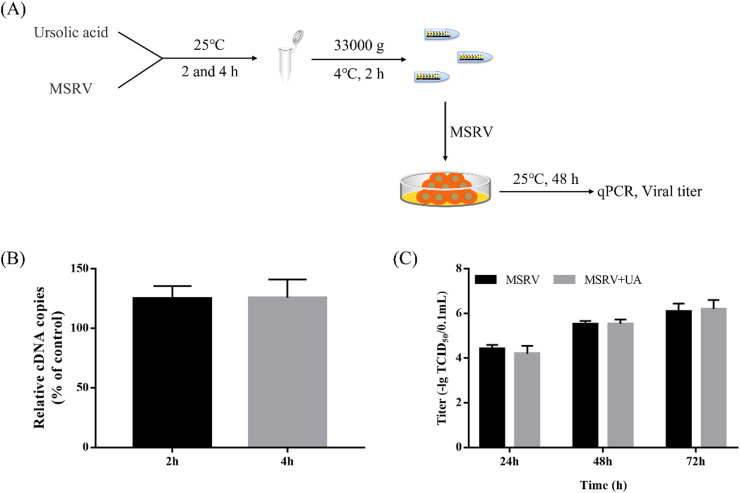
2.6. Ultracentrifugation assay
MSRV (1 × 103 TCID50/0.1 mL) and 13.14 μM UA were incubated at 25 °C for 2 and 4 h. Subsequently, each sample was centrifuged at 33,000 g for 2 h at 4 °C. The supernatant was replaced by 5% FBS M199 and re-suspended. GCO cells were infected with treated supernatant. After 48 h infection, the samples were investigated by viral titer assay and real-time PCR analysis of MSRV glycoprotein (G) gene (refer to Sections 2.4 and 2.5).
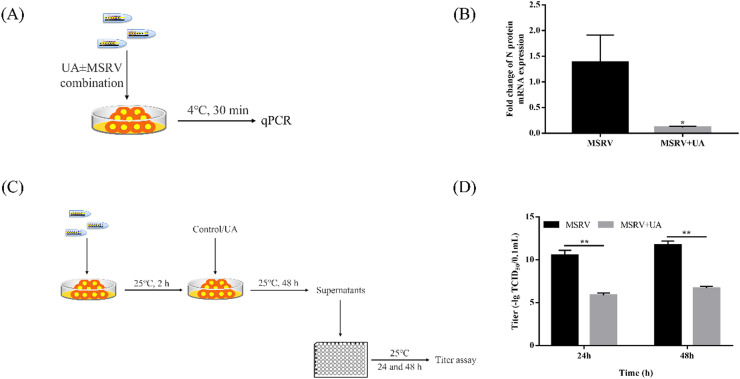
2.7. Viral binding assay
Viral binding assay refers to previous methods (Shen et al., 2020). GCO cells with a density of 1 × 104/well were inoculated into 25 cm2 culture dish. When GCO cells were cultured to a monolayer, the cell culture medium was changed to MSRV (1 × 103 TCID50/0.1 mL) with or without UA (13.14 μM). The infection process was performed for 30 min at 4 °C. Then, the supernatant was discarded and GCO cells were washed with PBS 3 times. By means of qRT-PCR, the expression of MSRV G on the surface of the infected cells (viral binding) was evaluated.
2.8. Viral releasing assay
GCO cells were inoculated in 12-well plates and cultured to a monolayer. Then, the supernatant was exchanged into MSRV and incubated for 2 h. After infection, GCO cells were treated with UA for 48 h. The supernatant was collected and the viral titer was detected (refer to Section 2.5).
2.9. Preventive effects of UA
The workflow of the experimental design is followed in Fig. 5A. UA was incubated with monolayer GCO cells for 4 h. After repeatedly washing with PBS, MSRV was added for 2 h. The viral suspension was exchanged for cell maintenance. After 48 h, cell samples were collected and the expression of MSRV N gene was detected by qRT-PCR.
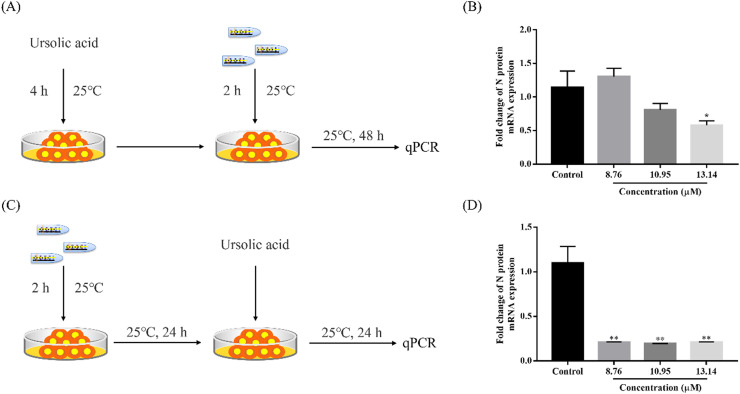
Fig. 5.
UA can be used to prevent and treat MSRV infection. (A) Workflow of the experimental design followed in (B). (B) qRT-PCR analysis of the N protein encoding gene. GCO cells were pretreated with different concentrations of UA for 4 h. Subsequently, GCO cells were infected with MSRV for 2 h. Viral load was determined by qRT-PCR. Data were shown as mean ± SEM. The p value for each study was determined by Student's t-tests. **p < 0.01; *p < 0.05. (C) Workflow of the experimental design followed in (D). (D) qRT-PCR analysis of the N protein encoding gene. GCO cells were infected with MSRV for 2 h. Subsequently, GCO cells were incubated for 24 h. Finally, GCO cells were treated with different concentrations of UA. Viral load was determined by qRT-PCR. Data were shown as mean ± SEM. The p value for each study was determined by Student's t-tests. **p < 0.01; *p < 0.05.
2.10. Therapeutic effect of UA
The workflow of the experimental design is followed in Fig. 5C. MSRV (1 × 103 TCID50/0.1 mL) was inoculated in 12-well plates grown into a monolayer and cultured for 2 h. The viral suspension was exchanged for maintenance and continued culture for 24 h. Subsequently, PBS was used to wash the GCO cells 2-3 times. After GCO cells were treated with cell medium contained UA (8.76, 10.95 and 13.14 μM) for 24 h, cell samples were collected for qRT-PCR detection (MSRV N gene).
2.11. Antiviral of UA in vivo
The workflow of the experimental design is followed in Fig. 6A.
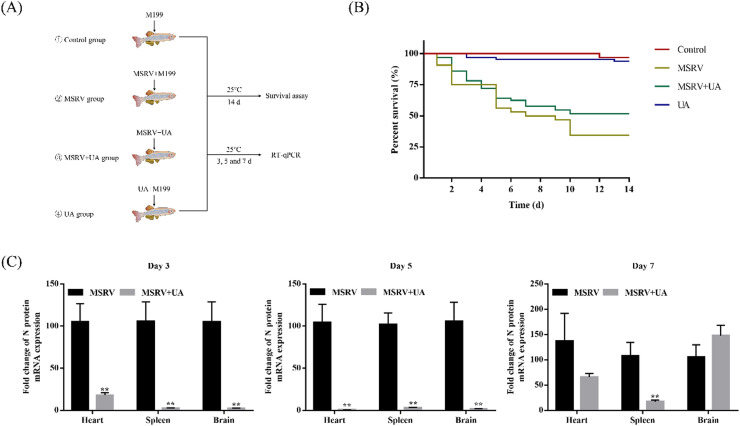
Fig. 6.
UA has protective effect on MSRV-infected largemouth bass. (A) Workflow of the experimental design followed in (B, C). (B) Kaplan-Meier survival curves of fish intraperitoneally injected with MSRV and UA. (C) Expression of MSRV N in heart, spleen and brain at 3, 5 and 7 post-infected. Data were shown as mean ± SEM. The p value for each study was determined by Student's t-tests. **p < 0.01; *p < 0.05.
2.11.1. Toxicity assay
Referring to previous studies, largemouth bass were divided into 6 groups (one tank per group, n = 30): control group (no treatment), M199 group (intraperitoneal injection of M199) and UA treatment group (intraperitoneal injection of 10, 20, 40, 80 mg/L UA). Each fish was injected with 20 μL mixture. After 14 days, survival rates for each group were counted.
2.11.2. Survival rate assay
Healthy largemouth bass were randomly divided into 4 groups and treated as follows: (1) control group (n = 64, intraperitoneal injection of M199); (2) MSRV group (n = 64, intraperitoneal injection of MSRV (1 × 103 TCID50/0.1 mL)); (3) MSRV+UA group (n = 64, intraperitoneal injection of MSRV+UA (80 mg/L)); (4) UA group (n = 64, intraperitoneal injection of UA). The injection volume was 20 μL. Survival rates of largemouth bass in each group were counted every 24 h until 14 d. Cumulative survival rates were counted every 24 h up to 14 d.
2.11.3. Gene expression in brain, spleen and heart of largemouth bass
Largemouth bass were treated according to Section 2.11.2. Samples (n = 3) were collected on 3, 5 and 7 post-infection and the expression of MSRV N gene in the brain, spleen and heart was detected the expression of MSRV N gene by qRT-PCR.
2.12. RNA extraction and real-time PCR analysis
Quantitative real-time PCR was performed using CFX96 Real-Time PCR Detection System (Bio-Rad, USA). The total RNA was isolated using an RNAex Pro reagent (Accurate Biology) (Hunan, China). The total RNA was reverse transcribed at 50 °C for 15 min and 85 °C for 2 min using HiScript Q Select RT SuperMix for qPCR (+gDNA wiper) (Vazyme) (Nanjing, China) to obtain the cDNA. The program for the PCR reactions was: 95 °C for 5 min followed by 39 cycles of 95 °C for 15 s, 60 °C for 60 s. The primers for real-time PCR are presented in Table 1. At the end of the real-time PCR, the CT value of each reaction was provided and the changes in the transcriptional level of the target genes normalized to β-actin were calculated by the following formula: Relative mRNA level of target gene (folds of control) = 2−ΔΔCT.
Table 1.
Primers used for the analysis of mRNA expression by qRT-PCR.
| Genes | Primer sequences (from 5’ to 3’) | Refs. | |
|---|---|---|---|
| MSRV nucleoprotein (N) | Forward | GCCCACATCGCATCATTCAC | Shen et al. (2020) |
| Reverse | GTGGCAGAGTAAGGGGACAC | ||
| MSRV glycoprotein (G) | Forward | TGTCAATGTGCGGAGAGGTG | Yang et al. (2021) |
| Reverse | TGTGATACGTAGCTGAGCCG | ||
| β-actin (GCO cells) | Forward | GATGATGAAATTGCCGCACTG | Yang et al. (2021) |
| Reverse | ACCGACCATGACGCCCTGATGT | ||
| β-actin (Largemouth bass) | Forward | CCACCACAGCCGAGAGGGAA | Yang et al. (2021) |
| Reverse | TCATGGTGGATGGGGCCAGG |
2.13. Statistical analysis
Data were subjected to analysis using SPSS 20.0 software (SPSS Inc., USA). The significance of the data was checked by unpaired, two-tailed Student's t-tests or nonparametric tests. The survival curve was constructed using the Kaplan-Meier method. Data were statistically analyzed by GraphPad Prism 6 (GraphPad Software, USA), using the Log-rank (Mantel-Cox) test to determine significance.
3. Results
3.1. Anti-MSRV activity of UA in GCO cells
The cytotoxicity of UA was assessed via MTT assay before antiviral activity assay. The results indicated that the cell viability was >80% ranging from 10.94 μM to 28.46 μM (as shown in Fig. 1B). Previous studies indicated that agents were considered safe if cell viability values were greater than 80% (Li et al., 2019). MTT data on cell activity showed that the 48 h 20% cytotoxic concentration (CC20) of UA was 27.56 μM. In order to investigate the antiviral effect of UA against MSRV, the five-point dose-response curve of RT-qPCR was matched with the expression of MSRV N gene. Fig. 1C shows that UA inhibits the expression of MSRV N gene in GCO cells, with a maximum inhibition rate of 99.00 ± 0.08%. The 48 h IC50 of UA on MSRV N gene was 5.55 μM. Consistent with RT-qPCR assay, 13.14 μM UA significantly decreased the titer of MSRV (as shown in Fig. 1D).
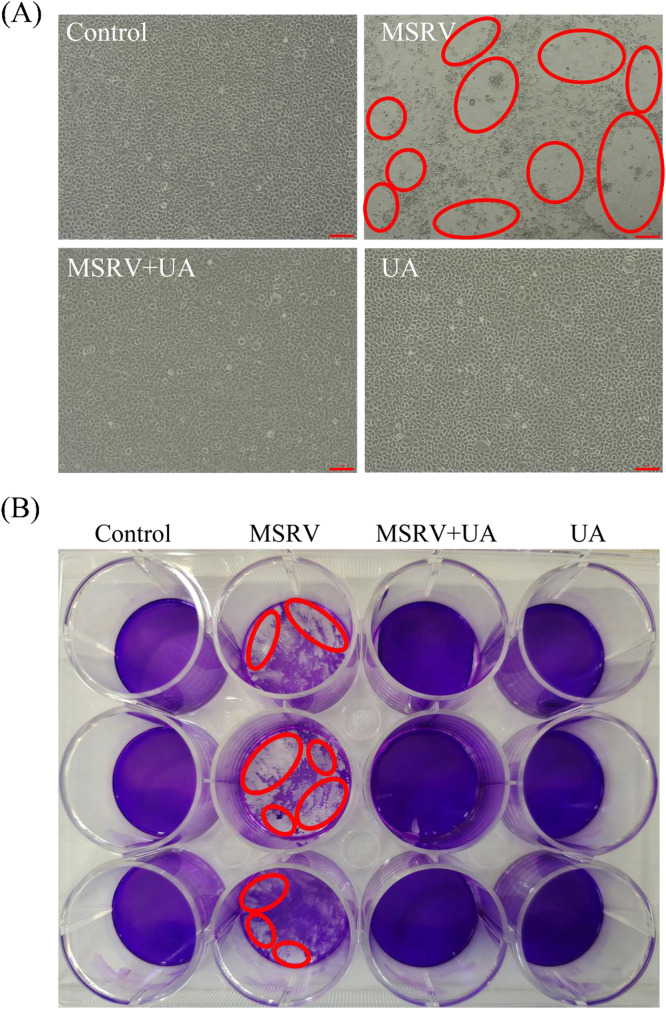
MSRV titer were 104.55 (24 h post-infection (p.i.)), 105.74 (48 h p.i.), 106.80 (72 h p.i.) TCID50/0.1 mL. Whereas MSRV titers were 103.13 (24 h p.i.), 104.67 (48 h p.i.) and 105.65 (72 h p.i.) TCID50/0.1 mL in the UA-treated group. Similarly, UA effectively reduced the CPE and protected cell morphology (as shown in Fig. 2A). In addition, crystalline violet staining demonstrated the remarkable reduction in the amounts of viable cells 48 h post-infection (as shown in Fig. 2B). In contrast, UA-treated group maintained natural cell morphology 48 h post-infection. The above results demonstrated that UA exhibited promising anti-MSRV activity in vitro.
Fig. 2.
Morphological protective effect of UA on MSRV in GCO cells. GCO cells cultured in 12-well plates were exposed to 103 TCID50/0.1 mL MSRV for 2 h and then the medium with MSRV was exchanged for fresh medium containing UA. After incubation periods, cytopathic effects were observed by an inverted microscope (A) and crystal violet staining (B). The cytopathic effects were highlighted in red.
3.2. UA could not damage the viral particle directly
To investigate whether UA could damage viral particles, the workflow of the experimental design is followed in Fig. 3A. We have confirmed that UA-treated virus particles maintain infectivity in GCO cells by centrifugation in vitro (Fig. 3B). As shown in Fig. 3C, there was no significant reduction in the titer of UA-treated MSRV. Mechanically speaking, UA could not directly damage MSRV and take the edge off its infectivity.
Fig. 3.
UA does not directly damage MSRV particles. (A) Workflow of the experimental design followed in (B-C). MSRV (1 × 103 TCID50/0.1 mL) and 13.14 μM UA were incubated at 25 °C for 2 and 4 h. Subsequently, qPCR assay and viral titer were performed. (B) qRT-PCR analysis of MSRV glycoprotein (G) encoding gene. The data were shown as mean ± SEM. (C) The titers of MSRV in GCO cells. Data were shown as mean ± SEM.
3.3. UA repressed viral particles bind and release
To probe into the antiviral mechanism of UA, we further analyzed whether UA could interfere with virus adsorption and release to GCO cells. The workflow of the experimental design is followed in Fig. 4A (viral binding) and Fig. 4C (viral releasing). Fig. 4B showed that the number of viral particles adhered to UA-treated cells was significantly lower than that in the non-treated cells, assessed by qRT-PCR analysis on MSRV G protein expression. The results suggested that UA could affect the adsorption of virus particles. Moreover, we further investigated whether UA treatment could reduce MSRV release. As shown in Fig. 4D, supernatants of MSRV-infected cells and UA-treated cells were collected to infect normal GCO cells and detected titer. The results showed that the UA-treated titer decreased significantly at 24 and 48 h post-infection and UA treatment was able to decrease the viral particles release.
Fig. 4.
UA has antiviral activity by affecting the binding and release of MSRV particles in GCO cells. (A) Workflow of the experimental design followed in (B). (B) qRT-PCR analysis of the G gene. Data were shown as mean ± SEM. The p value for each study was determined by Student's t-tests. **p < 0.01; *p < 0.05. (C) Workflow of the experimental design followed in (D). (D) The titers in the supernatant were measured by TCID50. Data were shown as mean ± SEM. The p value for each study was determined by Student's t-tests. **p < 0.01; *p < 0.05.
3.4. UA could be used for prevention and post-treatment
To investigate whether UA can be used to prevent MSRV infection, UA was utilized to pretreat GCO cells for 4 h. Subsequently, GCO cells were infected with MSRV and qRT-PCR analysis was performed at 48 h post-infection. GCO cells were pretreated with 13.14 μM UA for 3 h showed the certain preventive effect (as shown in Fig. 5B), but not at lower concentrations (8.76 μM and 10.95 μM). In contrast, GCO cells post-treated with UA showed significant down-regulation of the expression of MSRV N gene (Fig. 5D). It suggested that UA could be used as a therapeutic agent after infection.
3.5. UA increased survival rates and reduced viral load in infected fish
To evaluate the therapeutic effect of UA, we first performed the toxicity assays in largemouth bass before antiviral activity assays (as shown in Fig. S1). Briefly, intraperitoneal injection of UA (80 mg/L) could not cause significant mortality (< 10%) in largemouth bass. Hence, we considered 80 mg/L UA to be appropriate. To estimate the antiviral activity of UA in largemouth bass, the survival assay was administered after UA treatment. The results in Fig. 6B shown that MSRV-infected largemouth bass has the mortality rate of about 65.62%, while intraperitoneal administration of 80 mg/L UA improves largemouth bass survival by 12.50%. In addition, UA could reduce the viral load in MSRV-infected largemouth bass, and the expression of MSRV N gene in heart, spleen, and brain was significantly down-regulated at 3 and 5 d post-infection. At 7 d post-infection, MSRV replication was inhibited only in the spleen. Collectively, UA has anti-MSRV activity in largemouth bass. Taken together, intraperitoneal injection of UA can protect largemouth bass free from MSRV.
4. Discussion
As a common viral pathogen, rhabdovirus has caused irreversible losses to aquaculture industry. In recent years, MSRV, a novel rhabdovirus, has been a serious threat and attracted the attention of researchers. In order to combat MSRV, researchers have carried out in-depth studies and achieved valuable results. Recent studies have reported the inhibitory effect of five quinolines on MSRV with IC50 of 4.66, 8.92, 3.04, 4.83 and 5.63 μM (Li et al., 2022). In addition, Yang et al. (2021) reported that ribavirin has anti-MSRV activity in vivo and in vitro (IC50 = 1.21 μM). As an arctigenin derivative, BOA was identified as an effective inhibitor of MSRV replication in GCO cells with an IC50 of 0.45 μM (Shen et al., 2020). In this study, we demonstrated that UA can effectively inhibit MSRV replication (IC50 = 5.55 μM) and reduce cytopathic effects on host cells. Previous studies have reported that UA inhibits the up-regulation of mitogen-induced activation markers and co-stimulatory molecules in T and B cells, which suggested that UA had anti-inflammatory effects (Rahul et al., 2012). In addition, UA could perform anti-inflammatory effects by inhibiting NF-κB (Takada et al., 2010). These results suggested that UA is a potential immunosuppressant. Similarly, several immunosuppressants have been reported to have antiviral effects. For example, there are two known immunosuppressants, rapamycin (Bell et al., 2017; Ko et al., 2017) and dexamethasone (Bourinbaiar et al., 1995; Moreno et al., 2003), which have been reported antiviral effects as well as anti-inflammatory effects. Therefore, we speculate that the antiviral mechanism of UA may be similar to that of dexamethasone or rapamycin, which requires future validation.
According to previous studies, we evaluated whether UA affects the infectivity by directly damaging viral particles. After ultracentrifugation, viral titers and viral load assays showed that UA could not directly damage viral particles. It has been reported that UA does not directly destroy rotavirus particles (Tohme et al., 2019), however, virus binding assays indicated that UA affected the adsorption of MSRV particles, which suggested that UA may affect viral adsorption by influencing membrane receptors rather than by damaging viral particles. In addition, some studies have shown that agents affect viral binding, suggesting that agents may affect early stages of viral replication. Meanwhile, our study showed that UA could prevent MSRV infection and inhibit the MSRV release. We hypothesized that UA may affect the early and late stages of MSRV replication. Several studies have demonstrated which stages of viral replication are affected by time of additional assay. (Hu et al., 2019b; Shen et al., 2020). Experiments to corroborate these hypotheses are currently underway.
Furthermore, we investigated whether UA was also effective against MSRV infection in vivo. Our results indicated that largemouth bass massive died after infection and MSRV replication rapidly. Reassuringly, UA was effective to MSRV infection in largemouth bass within 3 and 5 d post-infection, and the survival rate of infected largemouth bass increased after UA treatment. The RT-qPCR assay indicated that UA had reduced antiviral activity against MSRV in 7 d post-infection, especially in the heart and brain. It is worth noting that similar results have emerged in several studies, in which drugs only play a role in the early phases (Hu et al., 2019a; Li et al., 2019; Liu et al., 2019). Therefore, we speculate that the therapeutic effect is limited by the disappearance of UA in largemouth bass. Structure optimization based on metabolism is important to improve the therapeutic effect of UA, which needs further study. Given the above, UA has been identified with antiviral activity against MSRV in vivo and could be an alternative anti-MSRV agent in aquaculture.
5. Conclusions
In summary, our study demonstrated that UA inhibited MSRV replication in vitro. Mechanically, UA does not damage the virion, but UA can affect the adsorption and release of MSRV. Simultaneously, UA plays a preventive and therapeutic role. More importantly, UA injection could suppress MSRV infection in largemouth bass and increase the survival time. Overall, UA is expected to be used as a therapeutic agent in aquaculture.
CRediT authorship contribution statement
Bo-Yang Li: Methodology, Writing – original draft. Jia-Cheng Qin: Writing – review & editing, Methodology. Yu-Feng Shen: Formal analysis, Writing – review & editing. Fei Yang: Writing – review & editing, Methodology. Tao Wang: Writing – review & editing. Fei Ling: Writing – review & editing, Visualization. Gao-Xue Wang: Writing – review & editing, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2022.198965.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Bell T.M., Espina V., Senina S., Woodson C., Brahms A., Carey B., Lin S.C., Lundberg L., Pinkham C., Baer A. Rapamycin modulation of p70 S6 kinase signaling inhibits Rift Valley fever virus pathogenesis. J. Antivir. Res. 2017;143:162–175. doi: 10.1016/j.antiviral.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinbaiar A.S., Leehuang S. Potentiation of anti-HIV activity of anti-inflammatory drugs, dexamethasone and indomethacin, by MAP30, the antiviral agent from bitter melon. J. Biochem. Biophys. Res. Commun. 1995;208:779–785. doi: 10.1006/bbrc.1995.1405. [DOI] [PubMed] [Google Scholar]
- Chen Q.Y., Shi H., Ho C.T. Effects of rosemary extracts and major constituents on lipid oxidation and soybean lipoxygenase activity. J. Am. Oil Chem. Soc. 1992;69:999–1002. [Google Scholar]
- Fontanay S., Grare M., Mayer J., Finance C., Duval R.E. Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008;120:272–276. doi: 10.1016/j.jep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Gao E.B., Chen G. Micropterus salmoides rhabdovirus (MSRV) infection induced apoptosis and activated interferon signaling pathway in largemouth bass skin cells. Fish Shellfish Immunol. 2018;76:161–166. doi: 10.1016/j.fsi.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Guo Z.R., Zhao Z., Zhang C., Jia Y.J., Qiu D.K., Zhu B., Wang G.X. Carbon nanotubes-loaded subunit vaccine can increase protective immunity against rhabdovirus infections of largemouth bass (micropterus salmoides) Fish Shellfish Immunol. 2020;99:548–554. doi: 10.1016/j.fsi.2020.02.055. [DOI] [PubMed] [Google Scholar]
- Hu Y., Chen W.C., Shen Y.F., Zhu B., Wang G.X. Synthesis and antiviral activity of a new arctigenin derivative against IHNV in vitro and in vivo. Fish Shellfish Immunol. 2019;92:736–745. doi: 10.1016/j.fsi.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Hu Y., Shen Y., Li B., Wang G.X., Zhu B. Evaluation on the antiviral activity of ribavirin against infectious hematopoietic necrosis virus in epithelioma papulosum cyprini cells. Virus Res. 2019;263:73–79. doi: 10.1016/j.virusres.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Murakami A., Ohigashi H. Ursolic acid: an anti- and pro-inflammatory, triterpenoid. Mol. Nutr. Food Res. 2008;52:26–42. doi: 10.1002/mnfr.200700389. [DOI] [PubMed] [Google Scholar]
- Ko S., Gu M.J., Kim C.G., Kye Y.C., Lim Y., Lee J.E., Park B.C., Chu H., Han S.H., Yun C.H. Rapamycin-induced autophagy restricts porcine epidemic diarrhea virus infectivity in porcine intestinal epithelial cells. J. Antivir. Res. 2017;146:86–95. doi: 10.1016/j.antiviral.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Li S., Liao Q., Zhang Y., Sun R., Zhu X., Zhang Q., Wang J., Wu X., Fang X., Zhu Y. Oleanolic acid and ursolic acid: novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013;98:44–53. doi: 10.1016/j.antiviral.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Li B.Y., Hu Y., Li J., Shi K., Shen Y.F., Zhu B., Wang G.X. Ursolic acid from prunella vulgaris L. efficiently inhibits IHNV infection in vitro and in vivo. Virus Res. 2019;273 doi: 10.1016/j.virusres.2019.197741. [DOI] [PubMed] [Google Scholar]
- Li B.Y., Yang F., Zhang Z.Y., Shen Y.F., Wang T., Zhao L., Qin J.C., Ling F., Wang G.X. Quinoline, with the active site of 8-hydroxyl, efficiently inhibits Micropterus salmoides rhabdovirus (MSRV) infection in vitro and in vivo. J. Fish Dis. 2022;45:895–905. doi: 10.1111/jfd.13615. [DOI] [PubMed] [Google Scholar]
- Liu L., Hu Y., Lu J., Wang G. An imidazole coumarin derivative enhances the antiviral response to spring viremia of carp virus infection in zebrafish. Virus Res. 2019;263:112–118. doi: 10.1016/j.virusres.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Liu M., Yu Q., Yi Y., Xiao H., Putra D.F., Ke K., Zhang Q., Li P. Antiviral activities of lonicera japonica thunb. Components against grouper iridovirus in vitro and in vivo. Aquaculture. 2020;519 [Google Scholar]
- Lyu S.J., Yuan X.M., Zhang H.Q., Shi W.D., Hang X.Y., Liu L., Wu Y.L. Isolation and characterization of a novel strain (YH01) of micropterus salmoides rhabdovirus and expression of its glycoprotein by the baculovirus expression system. J. Zhejiang Univ. Sci. B. 2019;20:728–739. doi: 10.1631/jzus.B1900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Deng G., Bai J., Li S., Yu L., Quan Y., Yang X., Jiang X., Zhu Z., Ye X. A strain of siniperca chuatsi rhabdovirus causes high mortality among cultured largemouth bass in South China. J. Aquat. Anim. Health. 2013;25:197–204. doi: 10.1080/08997659.2013.799613. [DOI] [PubMed] [Google Scholar]
- Moreno L., Jacoby D.B., Fryer A.D. Dexamethasone prevents virus-induced hyperresponsiveness via multiple mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L451. doi: 10.1152/ajplung.00046.2003. [DOI] [PubMed] [Google Scholar]
- Plant K.P., Lapatra S.E. Advances in fish vaccine delivery. J. Dev. Comp. Immunol. 2011;35:1256–1262. doi: 10.1016/j.dci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Rahul C., Sandur S.K., Deepak S., Patwardhan R.S., Jayakumar S., Vineet K., Gautam S., Aggarwal B.B., Sainis K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS One. 2012;7:e31318. doi: 10.1371/journal.pone.0031318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Shen Y.F., Liu Y.H., Li B.Y., Liu T.Q., Wang G.X. Evaluation on antiviral activity of a novel arctigenin derivative against multiple rhabdoviruses in aquaculture. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198019. [DOI] [PubMed] [Google Scholar]
- Takada K., Nakane T., Masuda K., Ishii H. Ursolic acid and oleanolic acid, members of pentacyclic triterpenoid acids, suppress TNF-α-induced E-selectin expression by cultured umbilical vein endothelial cells. J. Phytomed. 2010;17:1114–1119. doi: 10.1016/j.phymed.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Tan W., Lu J., Huang M., Li Y., Chen M., Wu G., Gong J., Zhong Z., Xu Z., Dang Y., Guo J., Chen X., Wang Y. Anti-cancer natural products isolated from chinese medicinal herbs. Chin. Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme M.J., Gimenez M.C., Peralta A., Colombo M.I., Delgui L.R. Ursolic acid: a novel antiviral compound inhibiting rotavirus infection in vitro. Int. J. Antimicrob. Agents. 2019;54:601–609. doi: 10.1016/j.ijantimicag.2019.07.015. [DOI] [PubMed] [Google Scholar]
- Vagi E., Rapavi E., Hadolin M., Peredi K.V., Balazs A., Blazovics A., Simandi B. Phenolic and triterpenoid antioxidants from origanum majorana L. herb and extracts obtained with different solvents. J. Agric. Food Chem. 2005;53:17–21. doi: 10.1021/jf048777p. [DOI] [PubMed] [Google Scholar]
- Xu H.X., Zeng F.Q., Wan M., Sim K.Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996;59:643–645. doi: 10.1021/np960165e. [DOI] [PubMed] [Google Scholar]
- Yang F., Song K., Zhang Z., Chen C., Wang G., Yao J., Ling F. Evaluation on the antiviral activity of ribavirin against Micropterus salmoides rhabdovirus (MSRV) in vitro and in vivo. Aquaculture. 2021;543 [Google Scholar]
- Zhang L., Li N., Lin Q., Liu L., Liang H., Huang Z., Fu X. An avirulent micropterus salmoides rhabdovirus vaccine candidate protects Chinese perch against rhabdovirus infection. Fish Shellfish Immunol. 2018;77:474–480. doi: 10.1016/j.fsi.2018.03.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.