Abstract
BACKGROUND
For people with HIV and CD4+ counts >500 cells/mm3, early initiation of antiretroviral therapy (ART) reduces serious AIDS and serious non-AIDS (SNA) risk compared with deferral of treatment until CD4+ counts are <350 cells/mm3. Whether excess risk of AIDS and SNA persists once ART is initiated for those who defer treatment is uncertain.
METHODS
The Strategic Timing of AntiRetroviral Treatment (START) trial, as previously reported, randomly assigned 4684 ART-naive HIV-positive adults with CD4+ counts .500 cells/mm3 to immediate treatment initiation after random assignment (n = 2325) or deferred treatment (n= 2359). In 2015, a 57% lower risk of the primary end point (AIDS, SNA, or death) for the immediate group was reported, and the deferred group was offered ART. This article reports the follow-up that continued to December 31, 2021. Cox proportional-hazards models were used to compare hazard ratios for the primary end point from randomization through December 31, 2015, versus January 1, 2016, through December 31, 2021.
RESULTS
Through December 31, 2015, approximately 7 months after the cutoff date from the previous report, the median CD4+ count was 648 and 460 cells/mm3 in the immediate and deferred groups, respectively, at treatment initiation. The percentage of follow-up time spent taking ART was 95% and 36% for the immediate and deferred groups, respectively, and the time-averaged CD4+ difference was 199 cells/mm3. After January 1, 2016, the percentage of follow-up time on treatment was 97.2% and 94.1% for the immediate and deferred groups, respectively, and the CD4+ count difference was 155 cells/mm3. After January 1, 2016, a total of 89 immediate and 113 deferred group participants experienced a primary end point (hazard ratio of 0.79 [95% confidence interval, 0.60 to 1.04] versus hazard ratio of 0.47 [95% confidence interval, 0.34 to 0.65; P<0.001]) before 2016 (P=0.02 for hazard ratio difference).
CONCLUSIONS
Among adults with CD4+ counts >500 cells/mm3, excess risk of AIDS and SNA associated with delaying treatment initiation was diminished after ART initiation, but persistent excess risk remained. (Funded by the National Institute of Allergy and Infectious Diseases and others.)
Introduction
In 2015, a recommendation to initiate antiretroviral therapy (ART) in people as soon as possible after HIV diagnosis, regardless of CD4+ count, became globally accepted.1,2 This followed the reporting of interim findings of the Strategic Timing of AntiRetroviral Treatment (START)3,4 and Early Antiretroviral Treatment and/or Early Isoniazid Prophylaxis Against Tuberculosis in HIV-infected Adults (TEMPRANO) studies in 2015.5 The planning of START began after the interim report of the Strategies for Management of Antiretroviral Therapy (SMART) study in 2006.6 SMART established that interrupting ART increased the risk of both AIDS and serious non-AIDS (SNA) outcomes. The results of SMART in 2006 provided the necessary impetus to initiate a study of whether to begin lifelong ART in people living with HIV who had a CD4 count of more than 500 cells/mm3, a question about which there was substantial uncertainty.7
START was designed to determine whether immediate initiation of ART after randomization among adults with HIV and CD4+ counts of more than 500cells/mm3 reduced major morbidity and mortality compared with deferred ART until the CD4+ count declined to less than 350cells/mm3 or AIDS developed.8 After an average of 3years of followup, the risk of the primary composite end point of serious AIDS or SNA was reduced by 57% among those randomly assigned to immediate initiation of ART compared with those randomly assigned to deferred ART initiation.4
Globally, delays in the diagnosis of HIV and initiation of ART remain an important public health challenge.9–11 Experimental evidence that quantifies the long-term excess risk from delayed diagnosis and delayed treatment initiation is lacking.
After the interim findings from the START trial were reported, participants in the deferred group were advised to initiate ART if they had not already done so, and both treatment groups were observed until the end of 2021. The primary objective of the 6-year extended follow-up reported herein was to determine whether excess risk as a result of deferring ART was maintained, increased, or reduced. The randomized design of START was ideally suited to inform this question.
Methods
The International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) (Supplementary Appendix, Section 1) designed and conducted START.
PARTICIPANTS
As described previously, people with HIV who were 18 years of age or older and had not yet initiated ART, had no history of AIDS, and were in general good health were eligible for the study if they had two CD4+ cell counts of more than 500 cells/mm3.4,8 Full eligibility details are provided in the Supplementary Appendix (Section 3). The study was approved by the ethics committees at participating sites. Written informed consent was obtained from all participants.
STUDY DESIGN
The START study evaluated two strategies for initiating ART: (1) immediate initiation of ART after random assignment; and (2) deferral of ART until the CD4+ count declined to less than 350 cells/mm3 or when AIDS or other conditions that dictated the use of ART (e.g., pregnancy) occurred.
Participants were randomly assigned to the immediate and deferred treatment groups with equal probability.4,8 ART that was consistent with U.S. Department of Health and Human Services guidelines was donated through 2017 and provided to sites through a central drug repository. The 2.5-year period between 2015 (when the START study results were originally reported) and 2017 provided the sites the time needed to transition participants in both treatment groups from the centrally provided ART to locally obtained ART. As noted below, the sites were successful in this goal.
The primary end point was a composite outcome that included: (1) serious AIDS or death from AIDS (“serious AIDS”): opportunistic events consistent with the 1993U.S. Centers for Disease Control and Prevention expanded surveillance definition12 plus Hodgkin’s lymphoma (nonfatal esophageal candidiasis and Herpes simplex virus infection were not counted as primary events because of their lesser severity); and (2) SNA13 or death not related to AIDS (“serious non-AIDS”): cardiovascular disease (CVD), end-stage renal disease, liver disease, non–AIDS-defining cancer (except basal or squamous cell skin cancer), and death not attributable to AIDS or one of the non-AIDS conditions (Supplementary Appendix, Section 3).
An end point review committee that was blinded to the treatment group reviewed all reported serious AIDS and SNA events and deaths using preestablished criteria. Events considered confirmed or probable by the end point review committee were counted as end points.
Secondary end points included serious AIDS, SNA, and death from any cause. Section 3 of the Supplementary Appendix provides additional details on the primary end point and its components.
FOLLOW-UP
Participants were seen every 4 months through 2015. During 2016 and 2017, follow-up visits were conducted every 6 months; for 2018 through 2021, data collection was performed annually. For the last year of data collection, sites were asked to confirm the primary and secondary end point status of each participant as of December 31, 2021.
The authors vouch for the accuracy and completeness of the analyses and data reported. The primary funding organization participated in the preparation of the protocol and the manuscript.
STATISTICAL ANALYSIS
In version 4.0 of the protocol for long-term follow-up (as discussed in the protocol and Section 3 of the Supplementary Appendix), two overarching scientific hypotheses were formulated for the period after 2015: (1) the HIV-RNA hypothesis — the primary end point event rate in the immediate and deferred groups will be similar between 2016 and 2021, because ART use and HIV RNA levels after 2015 would be similar for the two treatment groups; and (2) the nadir CD4+ count hypothesis — the primary end point rate in the deferred group would remain substantially higher than in the immediate group between 2016 and 2021 as a consequence of initiating ART at a lower CD4+ count an estimated 2.5 years after the immediate ART group. (Section 3 of the Supplementary Appendix includes the rationale for the two hypotheses, and Section 5 discusses power considerations.) No corresponding statistical hypotheses were formulated in terms of predetermined boundaries for the treatment difference in the primary end point event rate (hazard ratio for the immediate vs. deferred ART group); therefore, results are not reported as distinguishing between the two hypotheses from the data gathered. The extent to which excess risk as a result of deferring ART was reduced is described by the hazard ratios and their 95% confidence intervals (CIs) for the periods before and post–January 1, 2016, as detailed below.
Time-to-event methods, including Kaplan–Meier survival curves and Cox proportional-hazards regression models, stratified according to geographic region, were used to estimate the immediate and deferred randomized treatment groups for three time periods: (1) from randomization through 2015 (pre-2016); (2) from 2016 through 2021 (post–January 1, 2016); and (3) from randomization through 2021.
Hazard ratios for the immediate versus deferred group for the pre-2016 and post–January 1, 2016, periods were estimated by using a single Cox proportional-hazards model that included data from randomization through 2021 for all participants, an indicator variable for the treatment group, a time-updated indicator variable for calendar time (0 for the time from randomization to December 31, 2015, and then 1 thereafter), and their interaction. The interaction term assesses the extent to which the hazard ratios differ for the pre-2016 and post–January 1, 2016, periods.
P values and CIs were not adjusted for multiplicity, and results should not be considered clinically directive. Additional analysis details are given in the Supplementary Appendix (Sections 5 and 6). Statistical analyses were performed by using SAS version 9.4 (SAS Institute, Inc.) or R version 4.0 (R Foundation for Statistical Computing).
Results
As reported previously, from April 2009 through December 2013, a total of 4684 participants were randomly assigned to receive immediate (n=2325) or deferred (n=2359) ART (Fig. S1, in the Supplementary Appendix). Participants were enrolled at 215 sites in 35 countries. Selected baseline characteristics are provided in Table S1A. The participants enrolled were globally representative of adults with HIV who had not initiated ART (Supplementary Appendix, Section 2).
The following two sections summarize treatment comparisons for ART use, HIV RNA level, and CD4+ cell count during the pre-2016 and post–January 1, 2016, periods. These sections are followed by summaries of primary and secondary end point comparisons for this report of extended follow-up.
For the pre-2016 period, analyses are based on all randomly assigned participants (2325 in the immediate group and 2359 in the deferred group). At the beginning of the post–January 1, 2016, period, 4436 randomly assigned participants (2210 in the immediate group and 2226 in the deferred group) were alive, had not withdrawn consent, and had vital status determined in the post–January 1, 2016, period. Of these, 4322 participants (2177 in the immediate group and 2145 in the deferred group) had not experienced a primary event (Fig. S1).
PRE-2016: IMMEDIATE COMPARED WITH DEFERRED ART
Median (25th, 75th percentiles) follow-up was 3.6 years (2.9, 4.8 years) during the pre-2016 follow-up period. The distribution of CD4+ cell counts at the time of treatment initiation is shown in Figure S2. As expected, the median CD4+ count in the immediate group was comparable to the baseline count (648 and 651 cells/mm3, respectively) but lower in the deferred group (460 cells/mm3).
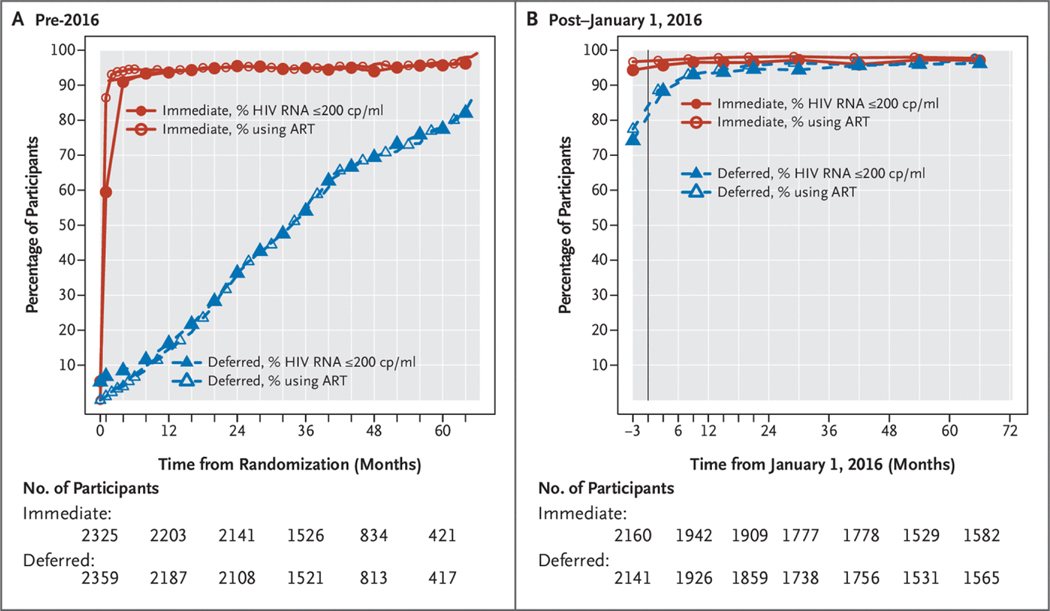
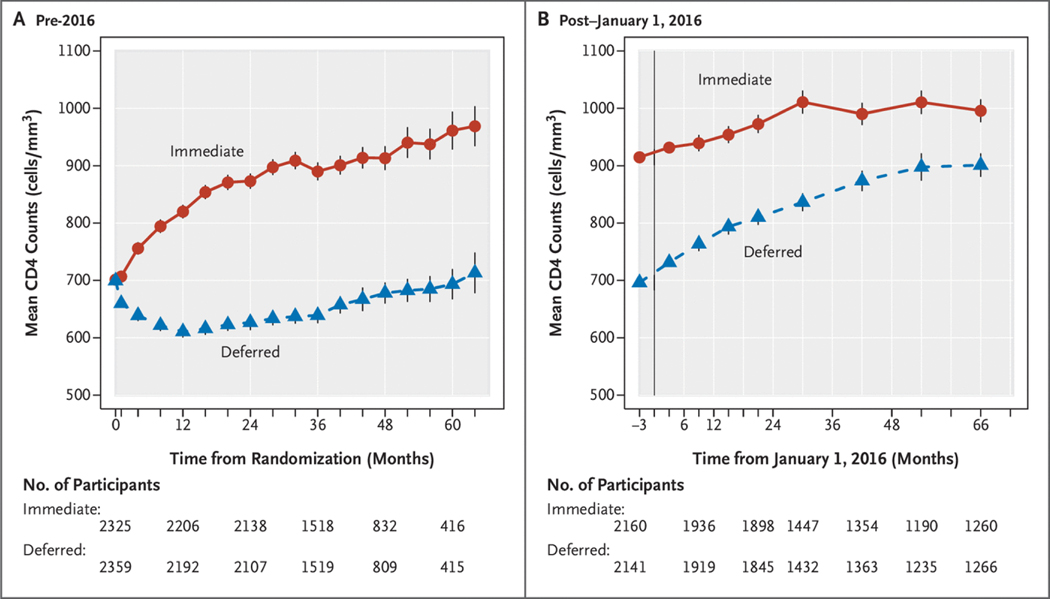
Figures 1A and 2A summarize ART use and HIV RNA levels and CD4+ counts, respectively, from randomization through the end of 2015. The percentage of follow-up time spent on therapy was 95% for the immediate group and 36% for the deferred group. Almost all participants on ART achieved an HIV RNA level ≤200 copies/ml. ART was initiated in the deferred arm a median of 2.5years (interquartile range, 1.6 to 3.5years) after randomization. Large differences in the CD4+ cell counts were observed soon after randomization, and the time-averaged difference between treatment groups during this period was 199cells/mm3 (95% CI, 190 to 208) (Fig. 2A).
Figure 1.
Percentage of Participants Receiving ART and Percentage with HIV RNA Levels 200 copies/ml.
(Panel A) Summary of percentage of participants receiving antiretroviral therapy (ART) and percentage with HIV RNA levels ≤200 copies (cp)/ml according to month of follow-up after randomization in the pre-2016 period. (Panel B) Summary of percentage of participants receiving ART and percentage with HIV RNA levels ≤200 cp/ml according to calendar year of follow-up in the post–January 1, 2016, period.
Figure 2.
Summary of Average CD4+ Counts.
(Panel A) Summary of average CD4+ counts according to month of follow-up after randomization in the pre-2016 period. (Panel B) Summary of average CD4+ counts according to calendar year of follow-up in the post–January 1, 2016, period.
AFTER JANUARY 1, 2016: ART FOR ALL PARTICIPANTS
By the end of 2015, for the cohort of 4436 participants followed from 2016 through 2021 (Fig. S1), 99% of the immediate group and 85% of the deferred group had initiated ART (Table S1B). Figures 1B and 2B summarize ART and HIV RNA levels and CD4+ counts, respectively, from the beginning of 2016 through 2021. In the deferred group, lower CD4+ count and higher HIV RNA level were strongly associated with initiating therapy by 2016 (Table S2). During the post–January 1, 2016, period, the percentage of follow-up time during which participants were receiving ART was 97.2% for the immediate group and 94.1% for the deferred group. As in the pre-2016 period, initiation of ART led to rapid declines in HIV RNA levels to ≤200 copies/ml. The time-averaged CD4+ count difference during this period was 155 cells/mm3 (95% CI, 148 to 168) (Fig. 2B).
Figures S3 and S4 provide similar summaries for ART/HIV RNA and CD4+ counts, respectively, over the full follow-up period from randomization through 2021 for all 4684 randomly assigned participants. At the end of follow-up for all randomly assigned participants, primary end point status and vital status on December 31, 2021, were verified for 82.5% and 81.8% (Figs. S1 and S5).
PRIMARY END POINT
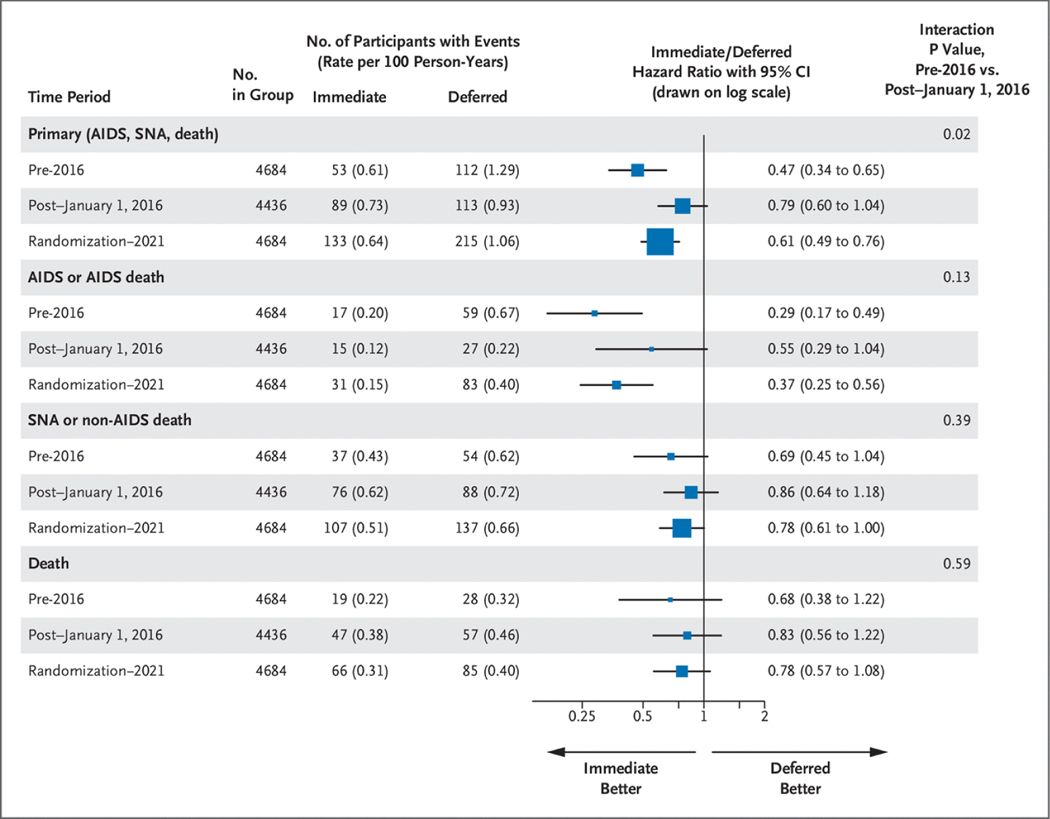
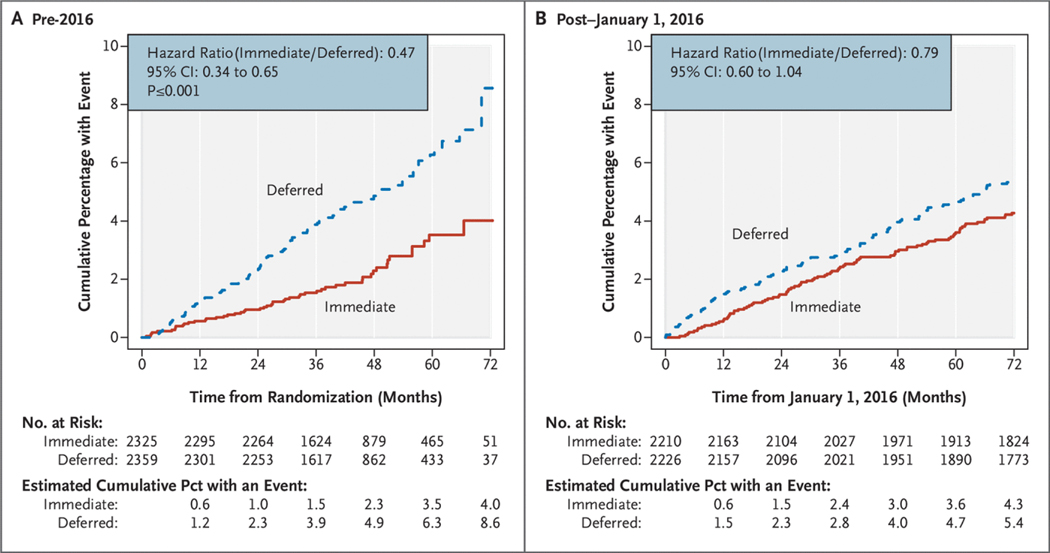
The rate of the composite primary end point declined in the deferred group from 1.29 per 100 person-years during the pre-2016 period to 0.93 per 100 person-years during the post–January 1, 2016, period. Rates for these two time periods for the immediate group were 0.61 and 0.73 per 100 person-years, respectively. The hazard ratio (immediate/deferred) increased from 0.47 (95% CI, 0.34 to 0.65) in pre-2016 to 0.79 (95% CI, 0.60 to 1.04) in the post–January 1, 2016, period (P50.02 for difference; Figs. 3 and 4). The increase in the hazard ratio denotes a decrease in the treatment difference between the immediate and deferred groups post–January 1, 2016, compared with the pre-2016 time period. The increase in the hazard ratio, expressed as the ratio between hazard ratios for the post–January 1, 2016, and pre-2016 periods, was 1.67 (95% CI, 1.09 to 2.96); a ratio more than 1.0 indicates a higher hazard ratio (and smaller treatment difference) in the post–January 1, 2016, period compared with the pre-2016 period. Hazard ratios increased from 0.65 (95% CI, 0.41 to 1.01) for 2016 through 2017, to 0.88 (95% CI, 0.54 to 1.42) for 2018 through 2019, and to 0.91 (95% CI, 0.54 to 1.56) for 2020 through 2021 (P50.04 for the test of proportional hazards) (Table S3).
Figure 3.
Primary and Secondary End Point Event Rates (per 100 Person-Years) According to Treatment Group and Hazard Ratios, Pre-2016 and Post–January 1, 2016.
CI denotes confidence interval; and SNA, serious non-AIDS.
Figure 4.
Kaplan–Meier Plots of the Cumulative Percentage of Participants with the Primary End Point According to Treatment Group.
(Panel A) Curves for the pre-2016 period. (Panel B) Curves for the post–January 1, 2016 period. CI denotes confidence interval.
For the above analyses, participants who developed the primary end point before 2016 and survived were included in the post–January 1, 2016, cohort and observed for a new event. When participants who experienced the primary event pre-2016 are excluded in the post–January 1, 2016, period — that is, a time-to-first-event analysis — results are similar (Table S4).
During the median 9.3years (interquartile range, 8.5 to 10.4years; average, 9.0years) of follow-up from randomization through the end of follow-up in 2021, the hazard ratio for the primary end point was 0.61 (95% CI, 0.49 to 0.76) (Fig. S6).
The impact of missing data on the primary end point results was assessed by using data from 162 sites with the most complete follow-up (Supplementary Appendix, Section 6). This accounted for 3581 of 4684 enrolled participants.
Hazard ratios were similar to those for all participants (Table S5). Findings were also consistent with the inclusion of additional end points that were reported to the end point review committee but on adjudication were not considered to fulfill the definition of the primary end point (Table S6).
COMPONENTS OF THE PRIMARY END POINT
Hazard ratios for the pre-2016 and post–January 1, 2016, periods and for randomization through 2021 for serious AIDS and SNA, and for all-cause mortality are summarized in Figure 3. Kaplan–Meier curves for the three time periods are given in Figures S7, S8, and S9. The difference in hazard ratios between the post–January 1, 2016, and pre-2016 periods expressed as the ratio between the two hazard ratios was 1.91 (95% CI, 0.83 to 4.29) for serious AIDS, 1.26 (95% CI, 0.75 to 2.11) for SNA, and 1.21 (95% CI, 0.60 to 2.44) for death.
Causes of death are summarized in Table S7. Cause of death could not be ascertained for 33 (32%) of 104 deaths (16 in the immediate group and 17 in the deferred group) during the post–January 1, 2016, period. Among participants for whom the cause was determined, non-AIDS cancer was the most common cause of death (8 immediate and 11 deferred), followed by suicide (4 immediate and 7 deferred).
Tuberculosis and AIDS-defining cancers were the most common AIDS events (Table S8). The rate of tuberculosis in the deferred group declined from 0.30 per 100 person-years pre-2016 to 0.08 per 100 person-years in the post–January 1, 2016, period and treatment hazard ratios for the pre-2016 and post–January 1, 2016, periods were 0.31 (95% CI, 0.14 to 0.68) and 0.89 (95% CI, 0.36 to 2.20), respectively. AIDS-defining cancer rates were 0.07 per 100 person-years for the immediate group and 0.28 per 100 person-years for the deferred group in the pre-2016 period and 0.04 for the immediate group and 0.11 for the deferred group in the post–January 1, 2016, period. Corresponding hazard ratios (immediate/deferred) for the pre-2016 and post–January 1, 2016, periods were 0.24 (95% CI, 0.10 to 0.59) and 0.38 (95% CI, 0.14 to 1.07), respectively (Table S10).
The most common SNA events were cancer and CVD (Table S9). For non-AIDS cancer, hazard ratios for the pre-2016 and post–January 1, 2016, periods were 0.45 (95% CI, 0.21 to 0.99) and 0.85 (95% CI, 0.48 to 1.50), respectively (Table S10). Findings for all cancers (AIDS and SNA) are also summarized in Table S10. For CVD, hazard ratios for the pre-2016 and post–January 1, 2016, periods were 1.07 (95% CI, 0.53 to 2.17) and 0.87 (95% CI, 0.48 to 1.59).
Kaplan–Meier curves from randomization through 2021 are shown in Figures S7, S8, and S9. Hazard ratios for serious AIDS, SNA, and all-cause mortality through 2021 were 0.37 (95% CI, 0.25 to 0.56), 0.78 (95% CI, 0.61 to 1.00), and 0.78 (95% CI, 0.57 to 1.08), respectively.
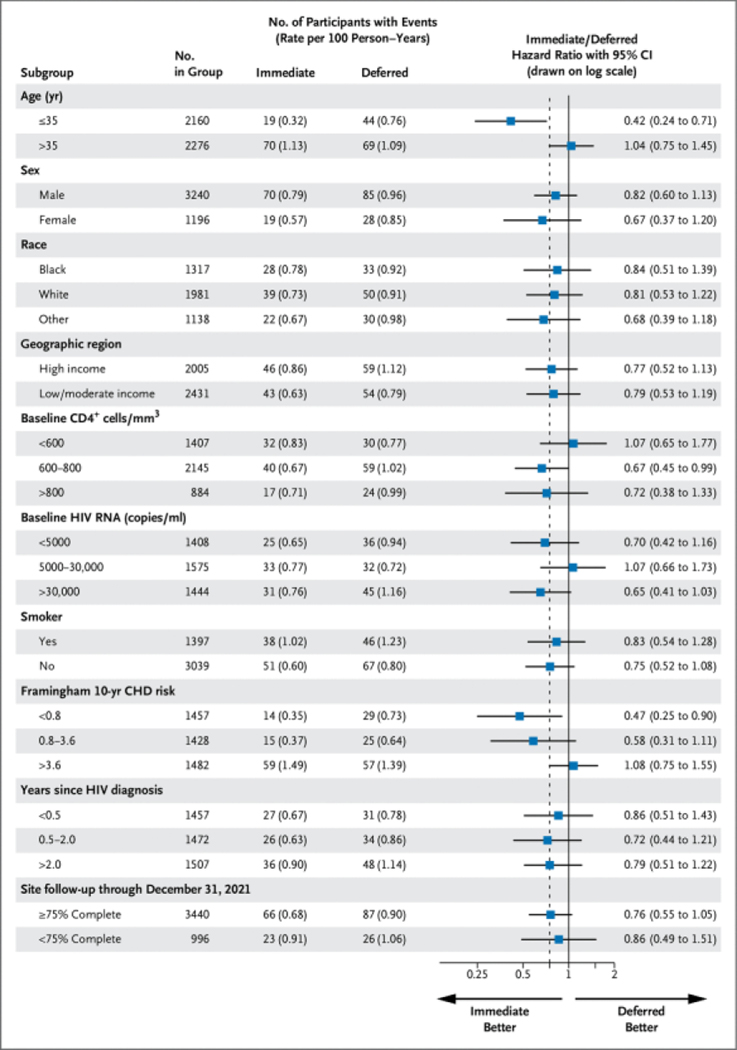
PRIMARY END POINT ACCORDING TO SUBGROUPS
Outcomes of subgroups based on characteristics at baseline and follow-up experience for the primary end point over the post–January 1, 2016, follow-up period are summarized in Figure 5 for the post–January 1, 2016, period and in Figure S10 for the full follow-up period. In the post–January 1, 2016, period the hazard ratio (immediate/deferred) among those older than 35 years of age (median age at entry) was 1.04 compared with 0.42 for those 35 years of age or younger. The absolute excess risk from deferring ART expressed as the difference in rates between the immediate and deferred groups was −0.45 per 100 person-years (95% CI, −0.71 to −0.18) among younger participants compared with 0.04 (95% CI, −0.33 to 0.41) among older participants. When age was considered as a continuous variable, the hazard ratio (immediate/deferred) increased by 47% (95% CI, 14 to 88) per 10 years’ older age.
Figure 5.
Primary End Point Event Rate (per 100 Person-Years) and Hazard Ratios during the Post–January 1, 2016, Period According to Treatment Group by Subgroups.
CHD denotes coronary heart disease; and CI, confidence interval. The dashed line denotes the overall hazard ratio of 0.79.
Apart from age, there was no evidence for heterogeneity in hazard ratios for the subgroups, including sex, self-declared race, and geographic region, for the primary end point in the post–January 1, 2016, or follow-up period from randomization through 2021. Of note, hazard ratios for sites categorized according to their completeness of follow-up were similar.
Discussion
In 2015, we reported from this large, international, randomized study involving previously untreated adults with HIV and a CD4+ count of more than 500 cells/mm3 that immediate initiation of ART after randomization was superior to deferring therapy until the CD4+ count declined to less than 350 cells/mm3 or AIDS developed. During 6 years of additional follow-up after recommending initiation of ART in all participants, we report on the diminished, yet persistent, excess risk of serious clinical disease and lower immune recovery from deferring treatment once therapy was initiated in this group of participants.
Although the excess risk of developing AIDS, non-AIDS, or death was substantially reduced in the deferred group after 2015, there remained a 1.27-fold (1/0.79) excess risk from 2016 through 2021 of this composite end point among those allocated initially to deferring ART compared with those initiating therapy immediately after randomization (albeit that the 95% CI just included zero excess risk). When comparing this with a 2.13-fold (1/0.47) excess risk during the follow-up period before 2016, the decline in excess risk was statistically significant (P=0.02). Of note, the reduction in risk, as reflected by the difference in hazard ratios between the two time periods, may vary according to the type of disease; this is the subject of additional study.
A potential explanation for the enduring excess risk is the fact that the CD4+ count remained clearly lower in participants in the deferred arm. Part of the explanation for this could be that the percentage of follow-up time from 2016 through 2021 not spent on ART was higher in the deferred group compared with the immediate group, albeit only slightly, and especially in the first 2years post–January 1, 2016. This incomplete initial uptake of ART occurred despite a strong recommendation by trial leadership in 2015 to initiate ART for all study participants and similar recommendations of treatment guidelines.1,2 It is possible that at least part of the 27% excess risk in the deferred group post–January 1, 2016, was explained by this continued deferral of ART. This hypothesis is consistent with the observed gradual decline of excess risk from 2016 through 2021; however, alternate explanations include effects from slower recovery of immune function or that intrinsic factors linked to deferred therapy may take some time to reverse.
The present findings experimentally inform the long-term effects of deferral of ART initiation over several years after ART was initiated. Numerous observational studies have attempted to address this question but with inconsistent conclusions.7,14–18 The validity of the findings from these cohort studies relies on unverifiable assumptions, including the absence of unmeasured confounders — prognostic factors that are causally related to when ART was initiated and hence to the nadir CD4+ count. In our study, the random allocation of START participants to immediate versus deferred initiation of ART provides better protection against the presence of such confounding factors compared with these observational studies.
The degree to which excess risk remained seemed to differ according to the participant’s age. Among younger participants, excess risk persisted overall and after January 1, 2016, whereas this was not observed in older participants. We considered multiple subgroups and had no prior hypothesis of a difference according to age; thus, this finding may well be largely a chance effect, or age may be marker for something else. One other possibility, for which we can only speculate, is that it could reflect the more robust T-cell receptor repertoire in the younger group and the inability to reconstitute elements of the repertoire once lost. Uptake of ART did not vary according to age group and is therefore not a plausible explanation, per se.
The strengths of the present findings include randomized comparisons of how immediate versus deferred initiation of ART affect serious clinical events in a large international cohort of HIV-positive people observed for more than 9years.
Weaknesses of the present study include an inability to provide complete follow-up for the entire cohort. Of note, restricting the analysis to sites with more comprehensive follow-up did not materially affect the key reported findings. An underlying cause could not be established in 32% of the deaths after 2016; therefore, the two most common underlying causes after 2016 — non-AIDS cancer and suicide — and other causes are likely underestimated. The severe acute respiratory syndrome coronavirus 2 pandemic affected the ability to see participants in 2020 and 2021, but was rarely reported as the underlying cause of death.
Here, we further document the extent of the detrimental effects for the individual’s health from deferring the initiation of therapy. This is in addition to risks to sexual partners from failure to diagnose HIV and start treatment.19,20 Hence, our findings underline and provide quantifiable risk estimates that can be used as arguments to support the Joint United Nations Programme on HIV/AIDS (UNAIDS) 95:95:95 targets and further intensify efforts to diagnose and have all HIV-positive persons initiate ART as quickly as possible after the time of infection.21–24
In summary, among adults with HIV, delays in ART initiation result in excess risk of AIDS and SNA conditions, even among those with CD4+ counts of more than 500cells/mm3. This ongoing risk persists after ART is initiated. Overall, the data reinforce the benefit of early diagnosis of HIV and prompt initiation of ART.
Supplementary Material
Acknowledgments
We thank the START participants without whom this work would not have been possible. We also recognize the contributions of Drs. David Cooper (Sydney, NSW, Australia) and Fred Gordin (Washington, D.C.) to the design and implementation of START, including this long-term follow-up work. Both colleagues led INSIGHT International Coordinating Centers before their untimely deaths on the same day, March 18, 2018.
Primary funding was from the National Institute of Allergy and Infectious Diseases, Division of AIDS, National Institutes of Health (grant no. 5U01AI136780; START ClinicalTrials.gov number, NCT00867048). Additional funding was received from the NIH Clinical Center, National Cancer Institute, National Heart, Lung and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (France), National Health and Medical Research Council (Australia), National Research Foundation (Denmark), Bundesministerium für Bildung und Forschung (Germany), European AIDS Treatment Network, Medical Research Council (United Kingdom), National Institute for Health and Care Research, and the National Health Service (United Kingdom). Antiretroviral drugs were donated through 2017 to the central drug repository by AbbVie, Bristol Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck & Co.
Footnotes
Disclosures
Author disclosures and other supplementary materials are available at evidence.nejm.org.
A complete list of members of the Strategic Timing of AntiRetroviral Treatment (START) study group is provided in the Supplementary Appendix, available at evidence.nejm.org.
References
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed. June 1, 2016. (https://www.who.int/publications/i/item/9789241549684). [PubMed] [Google Scholar]
- 2.Ryom L, Boesecke C, Gisler V, et al. Essentials from the 2015 European AIDS Clinical Society (EACS) guidelines for the treatment of adult HIV-positive persons. HIV Med 2016;17:83–88. DOI: 10.1111/hiv.12322. [DOI] [PubMed] [Google Scholar]
- 3.INSIGHT Network. Strategic Timing of AntiRetroviral Treatment(START). July 20, 2015. (http://insight.ccbr.umn.edu/start/).
- 4.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373: 795–807. DOI: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808–822. DOI: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 6.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–2296. DOI: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren JD, Babiker AG, Gordin FM, Borges ÁH, Neaton JD. When to start antiretroviral therapy: the need for an evidence base during early HIV infection. BMC Med 2013;11:148. DOI: 10.1186/1741-7015-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials 2013;10:S5–S36. DOI: 10.1177/1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brazier E, Tymejczyk O, Zaniewski E, et al. Effects of national adoption of treat-all guidelines on pre-antiretroviral therapy (ART) CD4 testing and viral load monitoring after ART initiation: a regression discontinuity analysis. Clin Infect Dis 2021;73:e1273–e1281. DOI: 10.1093/cid/ciab222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Justice AC, Goetz MB, Stewart CN, et al. Delayed presentation ofHIV among older individuals: a growing problem. Lancet HIV 2022; 9:e269–e280. DOI: 10.1016/S2352-3018(22)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connell S, Enkelmann J, Sadlier C, Bergin C. Late HIV presentation — missed opportunities and factors associated with a changing pattern over time. Int J STD AIDS 2017;28:814–821. DOI: 10.1177/0956462416674093. [DOI] [PubMed] [Google Scholar]
- 12.Ward M, Buehler MJW, Jaffe MHW, Berkelman RL. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992;41:1–19. [PubMed] [Google Scholar]
- 13.Lifson AR, Belloso WH, Davey RT, et al. Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV Clin Trials 2010;11:205–219. DOI: 10.1310/hct1104-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caniglia EC, Cain LE, Sabin CA, et al. Comparison of dynamic monitoring strategies based on CD4 cell counts in virally suppressed, HIV-positive individuals on combination antiretroviral therapy in high-income countries: a prospective, observational study. Lancet HIV 2017;4:e251–e259. DOI: 10.1016/S2352-3018(17)30043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keruly JC, Moore RD. Immune status at presentation to care didnot improve among antiretroviral-naive persons from 1990 to 2006. Clin Infect Dis 2007;45:1369–1374. DOI: 10.1086/522759. [DOI] [PubMed] [Google Scholar]
- 16.Lodi S, Phillips A, Logan R, et al. Comparative effectiveness ofimmediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV 2015;2:e335–e343. DOI: 10.1016/S2352-3018(15)00108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocroft A, Phillips AN, Gatell J, et al. Normalisation of CD4 countsin patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet 2007;370:407–413. DOI: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 18.Trickey A, Zhang L, Gill MJ, et al. Associations of modern initial antiretroviral drug regimens with all-cause mortality in adults with HIV in Europe and North America: a cohort study. Lancet HIV 2022;9:e404–e413. DOI: 10.1016/S2352-3018(22)00046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. DOI: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019;393:2428–2438. DOI: 10.1016/S0140-6736(19)30418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2021 (2020 data). November 30, 2021. (https://www.ecdc.europa.eu/en/publications-data/hiv-aids-surveillance-europe-2021-2020-data).
- 22.UNAIDS. Performance report demonstrates how the UN Joint Programme on HIV and AIDS has helped save lives. June 18, 2022. (https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2022/june/20220618_Performance_Monitoring_PPF).
- 23.ClinicalInfo. HIV/AIDS Treatment Guidelines. Office of AIDS Research. September 21, 2022. (https://clinicalinfo.hiv.gov/en/guidelines).
- 24.European AIDS Clinical Society. EACS Guidelines. December 7, 2022. (https://www.eacsociety.org/guidelines/eacs-guidelines/).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.