Abstract
Objective
To develop and evaluate a personalizable genomic results e-booklet that helps families understand their genomic testing results and navigate available resources.
Methods
The need for the Genomics Results e-Booklet was identified by families, after which this tool was developed by a team of clinical researchers and three parent-advisors. We customized the genomic results e-booklet for 50 families participating in a genomic sequencing research study. We conducted an assessment using a 19-question survey and semi-structured interviews to elicit feedback and iteratively improve the tool.
Results
25 users provided feedback via questionnaires and seven respondents were interviewed. Genomic Results e-Booklet recipients responded favorably: 96% of participants stated that it helped them remember information shared during their results appointment, 80% said it had or would help them communicate their results with other healthcare providers, 68% felt that it helped to identify and guide their next steps, and 72% anticipated that the e-booklet would have future utility.
Conclusion
The Genomic Results e-Booklet is a patient and family-oriented resource that complements post-test genetic counselling.
Innovation
Compared to traditional laboratory reports and clinical letters, the Genomics Results e-Booklet is patient-conceived and patient-centered, and allows clinicians to efficiently personalize content and prioritize patient understanding and support.
Key words: Genome sequencing, genomic results, genetic counselling, patient-oriented research, patient co-design
Highlights
-
•
The Genomic Results e-Booklet (GRB) is a patient-conceived, patient-centered tool.
-
•
The GRB aims to help families understand and navigate genomic testing results.
-
•
It is efficiently customizable by clinicians and complements genetic counselling.
-
•
96% of family users reported that the GRB helped them remember results information.
-
•
80% of family users felt the GRB helps them to communicate their results with others.
1. Introduction
Genome wide sequencing (GWS), which refers to diagnostic sequencing of the whole exome or genome, introduces complexities in communicating test results that may have significant impact on patients and their family members. GWS results are usually reported in technical medical language, and the pathogenicity of reported variants may be uncertain or subject to future reinterpretation. Non-genetics healthcare providers often express difficulty and may feel inadequately prepared to relay genomic outcomes [[1], [2], [3], [4], [5], [6], [7], [8], [9]], and geneticists are faced with a growing demand for consultation regarding testing results [[10], [11], [12]].
Despite the established value of providing patients with written information about genetic results [[13], [14], [15]], there have been few attempts to create GWS reporting tools appropriate for a patient audience [[16], [17], [18]]. Efforts have been primarily focused on developing laboratory reports that are clinician-friendly [[19], [20], [21], [22], [23], [24]], but these remain technical and do not address families’ needs for understanding and guidance. Consequently, families describe confusion and uncertainty after receiving genetic test results [[25], [26], [27], [28], [29]] and feel unequipped to participate in informed health management decisions [30].
The impetus for the Genomic Results e-Booklet (GRB) came from a parent-advisor to one of our GWS research studies: While involved in a social media group for families receiving genomic testing, they observed that many families felt “abandoned and lost” after receiving results. In collaboration with parent-advisors, genetic counsellors, and clinical geneticists, we co-developed the GRB to convey personalized results to families and empower them to identify a path forward. In pilot testing, we evaluated user perceived understanding, acceptability, and utility to inform improvement and future assessment.
2. Methods
2.1. Development
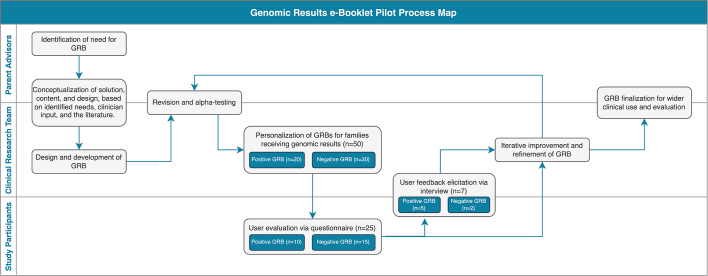
The GRB was developed and tested in the context of the IMAGINE study [31], a GWS research study of children with atypical cerebral palsy. The study’s parent-advisors (BL, KO, and IJ), whose families have experienced GWS and whose role is to represent and advocate for the research participants, were consulted about their needs and their vision for the content and design of the GRB (Supplementary Material - SM1). Content responsive to the identified needs was drafted by the research team in collaboration with the parent-advisors (SM1) and an iterative approach to development and pilot testing was used (Fig. 1).
Fig. 1.
Genomic Results e-Booklet Pilot Process Map.
The process map identifies steps in the iterative development and pilot testing of the GRB, and illustrates the parties involved at every step: the parent advisors, the clinical research team (including a clinical geneticist, genetic counsellors, a research nurse, and research assistants), and the study participants. The sample size at each stage is demonstrated in the teal boxes.
Content and design were informed by best practices for development and evaluation of patient resources [32,33]. Published guidelines recommend that patient-facing resources should facilitate “sufficient knowledge and appreciation of genetics to allow informed decision-making for personal wellbeing” [34] and offer breadth in resources, which can include avenues for support and information [5,18,23,35]. The GRB was developed under the framework of the Charter for Patient Information Resources [36] and generally targeted a Flesch-Kincaid reading level [37] of 6-7 but retained and explained essential medical terms, as per Canadian health resource guidelines [38] and the parent-advisors’ suggestions.
A printable e-booklet format was chosen to make the GRB accessible for both clinicians and patients while mitigating the privacy concerns of web-based applications. The static design was overlaid with an editable form in Adobe Acrobat Pro DCTM [39]. Patient-specific information can be generated efficiently through a set of form selections; explanatory sections throughout the booklet are auto-populated based on selections on the summary page. Results-related fields can be locked by clinicians to avoid inadvertent modification of information. Once drafted, the GRB was alpha tested and reviewed by three parent-advisors and five genetic clinicians, which resulted in refinements to language and design.
2.2. Pilot testing
A concise GRB pilot evaluation was completed as proof-of-concept and to identify areas of improvement prior to future extensive clinical implementation and assessment. We aimed to elicit feedback on perceived understandability, appropriateness of content, usability, actionability, and general helpfulness.
Families from the IMAGINE study all received genetic counselling for their results and were provided a results booklet that was personalized by a research assistant based on clinical reports and verified by a genetic counsellor. One week after booklet receipt, families who agreed to provide feedback were emailed a link to an online 19-question REDCap survey (SM2) [40]. Development of the questionnaire was based on the Patient Education Materials Assessment Tool [41] and previous evaluations of genomic reports (SM2) [16].
Questionnaire recipients were selected for semi-structured phone interviews (SM3) based on the depth and variety of their questionnaire responses. Feedback from both the questionnaires and interviews was used to iteratively improve the GRB, meaning that participants who received later GRB versions benefited from the input from recipients of earlier versions. Iterations were reviewed by the research team and the parent-advisors.
3. Results
3.1. The GRB
Using input from parent-advisors and clinicians (SM1), two versions of the GRB were developed: one for positive (or diagnostic) results (16 pages) and one for negative (non-informative or null) results (13 pages). Parent co-designers advocated for the inclusion of comprehensive information and details, noting that it was important to avoid a “more typical, brief, simplistic, medical document” in favour of creating a “family-centred and holistic” tool. The GRB has three customizable sections: genomic testing explanations; testing results; resources and support (Table 1). Information is stratified to provide additional detail, if the recipient desires. Key features of the GRB are exemplified in Fig. 2. Booklets can be viewed and modified using PDF-compatible software. The pilot-tested GRBs included some IMAGINE-specific information, however, generic booklets, as well as translated versions are available here: https://www.bcchr.ca/GenCOUNSEL/results-e-booklet
Table 1.
Content of the genomic results e-Booklet by section.
| Section and Purpose | Content |
|---|---|
|
Title Page Purpose: To provide organization and ease navigation. |
|
|
Summary Page Purpose: To allow for efficient customization (via auto-population of expanded educational sections) and to serve as an extractable shareable document. |
High-level summary of:
|
|
Background Genomic Information Purpose: To document testing and prime users for information about their results. |
|
|
Genomic Testing Results Purpose: To deliver the results stepwise and in plain language as to help decipher a laboratory-generated genomic testing report. |
For positive results only: the impacted gene and its known role; the variant and its associated medical condition; the laboratory classification, the clinical interpretation, and recommendations; inheritance and risk. For negative results only: The lack of genetic etiology versus the lack of ability to detect genetic etiology. For both: Limitations in analysis and interpretation, and the opportunity for re-testing or reanalysis in the future. |
|
Resources and Next Steps Purpose: To provide non-judgmental and non-prescriptive direction and instruction for users seeking further support and resources. |
|
Fig. 2.
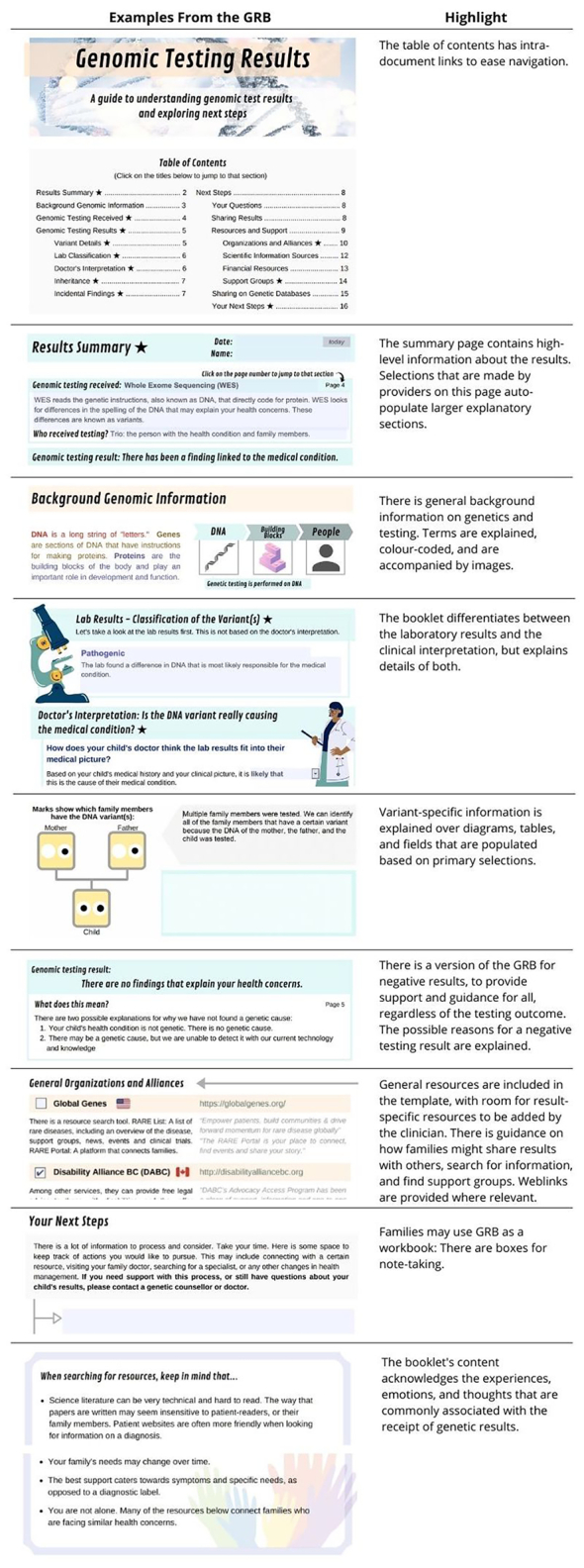
Examples of the GRB features requested by patients and providers.
Snapshots from various sections of the GRB exemplify key features that render this tool unique and patient-oriented.
3.2. Pilot testing
The GRB was distributed to 50 families: 20 received the positive GRB and 30 received the negative version. 26 families received the booklet proactively, immediately after their results appointment, and 24 families (who had enrolled earlier in IMAGINE, before the booklet was conceived) received the booklet retroactively, on average 11 months after results receipt. Families were emailed the e-booklet (n=40), were mailed a paper copy (n=7), or both (n=3), as requested.
Questionnaires were completed by 25 (10 positive, 15 negative) of 50 families (50%), seven of whom also provided additional feedback via interviews. Many minor iterative changes were made to wording (grammar, complexity) and design (images, organization). Feedback from the first 27 families resulted in two more major structural changes: These were the addition of a title page with a table of contents, and a single-page summary of the personalized results. The final 23 families received this more structured version.
3.3. User feedback
GRB recipients responded favorably to the booklet (Table 2, SM4, SM5). Of 25 questionnaire respondents, 24 (96%) stated that the GRB helped them to remember information shared during their results appointment; 20 (80%) said it had or would help them communicate their results with their other healthcare providers, 17 (68%) felt that it helped to identify and guide their next steps, and 18 (72%) anticipated future utility. Ratings of understanding (Fig. 3.A) and general helpfulness (Fig. 3.C) were high, and the level of detail was assessed as appropriate (Fig. 3.B). Results were similar between participants who received positive versus negative GRBs (SM5). Several recipients would have liked to receive more results-specific content, however, information is frequently limited for negative results and rare conditions.
Table 2.
Representative thoughts from family-user interviews and questionnaire data, and a interpretation of their implication.
| Finding | Sample of Supporting Quotes |
|---|---|
| General impressions are that the booklet is a family-oriented resource that aligns with the patient experience. |
“Not just giving a report expecting that we already know everything.” – Father, Family 29, Interview “It’s oriented for non-technical people for sure. Non-medical people, the parents. There’s great information. So this is a one-of-a-kind report that I’ve received, in my lifetime.” – Father, Family 72, Interview “I found the overall impression of the booklet is very friendly, and it’s a very different read from the [usual] small black and white print, … given that it’s an emotional subject anyways, it was easier to read.” – Mother, Family 46, Questionnaire |
| There was a range of perceived understandability and ease of reading. |
“It wasn’t frustrating at all because it was all presented for us, rather than us sifting through things trying to figure something out. So that was nice, and it was user friendly, easy to read.” – Mother, Family 66, Interview “I needed to read it three times before I was able to understand it enough to take notes. To get my brain working.” – Mother, Family 73, Interview “When I received the [GRB], it gave me a clearer picture of where we’re at. Even the drawings are really helpful.” – Mother, Family 49, Questionnaire |
| Some parents expressed wanting more detail on the genomic outcomes for their family. | “I found the explanation of the process that was taken to get the results helpful, however I hoped for more detailed medical information from our results.” – Family 61, Questionnaire |
| The GRB complements clinical encounters and reports. |
“Once you’re in those kinds of meetings, as a parent with all those overwhelming feelings, you normally forget things. So the [GRB] is really useful to remember some other details and notes.” – Father, Family 72, Interview “At the time [of the appointment] there was a lot of information. We sort of knew the gist of what was being tested, but [the GRB] helped to simplify the main things that were being looked at and the overall picture. It was also nice to just have very clearly written.” – Mother, Family 66, Interview “I think I probably looked at the booklet first […] and then I went back to the [lab report] […] and I was like, ‘okay yeah, I see that, that’s the variant.” – Mother, Family 73, Interview |
| The GRB aids communication with clinicians and family. |
“I sent a copy to my parents so that my parents […] And then when I want to explain something to them, I can refer back to [the GRB] because I have highlighted some parts for them.” – Mother, Family 73, Interview “ … it’s great to be able to share [the GRB] with her different specialists.” – Mother, Family 46, Interview |
| Families perceive the GRB as actionable, with future utility. |
“We got most of our information from the resources part, like the Facebook groups, and the research papers. I asked for [an EEG] referral because of [information in] the links that I got to the [disease] foundation page” – Mother, Family 73, Interview “When my kid would seize, I read through it again, you know, just to get a more in depth like what’s going on.” – Mother, Family 49, Interview |
| The GRB was still informative for families who received negative testing results. | “Now we know which stage we’re at. There’s still another stage of testing that might be done for him. So that’s still important to us if we might see other [health] changes.” – Mother, Family 49, Interview |
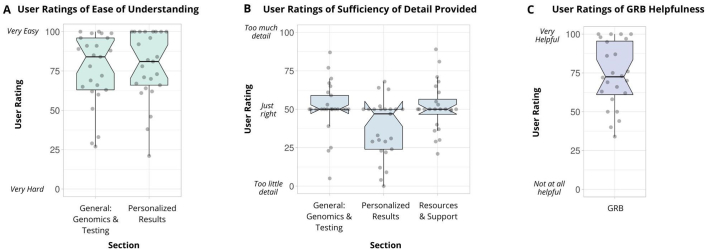
Fig. 3.
GRB user ratings.
(A) User ratings of ease of understating of general information for genomics and genomic testing and personalized information about genomic results. The graph shows the data points as jittered dots. The number of samples per condition is: General=25, Personalized=25. The summary of the data is shown as a boxplot, with the box indicating the IQR, the whiskers showing the range of values that are within 1.5*IQR and a horizontal line indicating the median. The notches represent for each median the 95% confidence interval (approximated by 1.58*IQR/sqrt(n)). No statistical difference was found for any measures when comparing negative and positive versions of the booklet using the 2-sided Wilcoxon Rank Sum test for non-parametric data (p=0.637, p=0.143).
(B) User ratings of sufficiency of detail provided in sections about general genomics and testing, personalized results, and resources and support. The graph shows the data points as jittered dots. The number of samples per condition is: General=25, Personalized=25, Resources = 24. The summary of the data is shown as a boxplot, with the box indicating the IQR, the whiskers showing the range of values that are within 1.5*IQR and a horizontal line indicating the median. The notches represent for each median the 95% confidence interval (approximated by 1.58*IQR/sqrt(n)). No statistical difference was found for any measures when comparing negative and positive versions of the booklet using the 2-sided Wilcoxon Rank Sum test for non-parametric data (p=0.363, p=0.429, p=1.00).
(C) User ratings of GRB helpfulness. The graph shows the data points as jittered dots. The number of samples per condition is: 25. The summary of the data is shown as a boxplot, with the box indicating the IQR, the whiskers showing the range of values that are within 1.5*IQR and a horizontal line indicating the median. The notches represent for each median the 95% confidence interval (approximated by 1.58*IQR/sqrt(n)). No statistical difference was found when comparing negative and positive versions of the booklet using the 2-sided Wilcoxon Rank Sum test for non-parametric data (p=0.206).
4. Discussion and conclusion
4.1. Discussion
The GRB was developed to address a critical gap in care by equipping families with lay-friendly, personalized information, and guidance in navigating genomic results. This pilot project shows that the GRB appears to meet patient and family needs.
The GRB pilot was limited in several ways. First, the GRB was altered iteratively in parallel to ongoing evaluation, meaning that our results may not fully reflect users’ perceptions of the GRB in its current state. The sample size was too small to evaluate differences between iterations. Only 50% of study participants returned a questionnaire, introducing the possibility of response bias. Furthermore, all participants in this study had a child with atypical cerebral palsy, and all were fluent in English. To avoid overburdening participants in the pilot stage of implementation, we did not collect demographic information. Therefore, our results may not be generalizable to all families who receive GWS.
Next steps include testing the final versions of the GRB for effectiveness in clinical practice and evaluating translations (French, Arabic, Punjabi, Simplified Chinese). Future research will further evaluate impact on patient utility, long-term understanding and management of results, as well as clinician utility, time spent, implementation and usage costs, and broader acceptability from the genetics professionals’ standpoint.
4.2. Innovation
Traditional laboratory reports and clinical letters are too technical and online sources are too general to meet family needs [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]]. The GRB is novel in that it 1) is patient-conceived and patient-centered, 2) contains elements that are efficiently customizable by clinicians to cater to patient-specific results and needs, and 3) aims to both help families understand their genomic testing results and navigate their next steps. The generalized GRB may be used by providers to communicate results efficiently and comprehensively in diverse clinical and research settings.
4.3. Conclusion
The GRB meets a patient-identified gap in emerging genomic healthcare by presenting personalized, comprehensive information to supplement genetic counselling and support families receiving GWS results. The framework of the GRB shows great potential to enhance patient education and support in a variety of settings. We welcome correspondence with potential GRB users, and comments on its utility.
Author contributions
All authors contributed to the design and development of the GRB. BL, KO, and IJ were the parent-advisors that provided vision and guidance in the development of the GRB, and were consulted throughout the development, the pilot evaluation, and the iterative improvement of the GRB. JH prepared and CG verified distributed GRBs for this study. JH, PB, and CG designed the evaluation framework. JH conducted interviews and analyzed survey responses, with oversight from PB and CG. JH and PB drafted the initial manuscript, and all authors reviewed the submitted manuscript. The IMAGINE Study was the source of all research participants for the current study.
Compliance with ethical standards
This study protocol was approved by the University of British Columbia Children’s and Women’s Hospital Research Ethics Board (H16-02126) as human subjects’ research.
Data availability statement
Evaluation data are available in the supplementary materials.
Declaration of Competing Interest
There are no conflicts of interest.
Acknowledgements
We would like to thank all parent-advisors and family participants involved in this study. We are grateful to genetic counsellors Alison Elliott and Nicole Si Yan Liang for their revisions of the GRB and recommendations. The IMAGINE study was funded by the Canadian Institutes of Health Research (CIHR – SCA-145104) through CHILD-BRIGHT (Child Health Initiative Limiting Disability – Brain Research Improving Growth and Health Trajectories), with additional support provided by BC Children’s Hospital Foundation and the Michael Smith Foundation for Health Research. This project was also supported by the University of British Columbia and by GenCOUNSEL (funded through Genome Canada’s Large Scale Applied Research Project Competition).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pecinn.2022.100039.
Appendix A. Supplementary data
Supplementary material includes (1) the GRB elements requested by families and solutions implemented, (2) the GRB evaluation and feedback e-questionnaire, (3) the prompts for the semi-structured interviews, (4) family user insight and feedback shared through questionnaire and interviews, and (5) GRB questionnaire data.
References
- 1.Baars M.J.H., Henneman L., ten Kate L.P. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: A global problem. Genet Med. 2005;7:605–610. doi: 10.1097/01.gim.0000182895.28432.c7. [DOI] [PubMed] [Google Scholar]
- 2.Baars M.J.H., Scherpbier A.J.J.A., Schuwirth L.W., Henneman L., Beemer F.A., Cobben J.M., et al. Deficient knowledge of genetics relevant for daily practice among medical students nearing graduation. Genet Med. 2005;7:295–301. doi: 10.1097/01.GIM.0000162877.87333.9A. [DOI] [PubMed] [Google Scholar]
- 3.Guttmacher A.E., Porteous M.E., McInerney J.D. Educating health-care professionals about genetics and genomics. Nat Rev Genet. 2007;8:151–157. doi: 10.1038/nrg2007. [DOI] [PubMed] [Google Scholar]
- 4.Acheson L.S., Stange K.C., Zyzanski S. Clinical genetics issues encountered by family physicians. Genet Med. 2005;7:501–508. doi: 10.1097/01.gim.0000177418.24176.9b. [DOI] [PubMed] [Google Scholar]
- 5.Haga S.B., Kim E., Myers R.A., Ginsburg G.S. Primary care physicians’ knowledge, attitudes, and experience with personal genetic testing. J Pers Med. 2019;9:29. doi: 10.3390/jpm9020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn J., Lewis K., Amendola L.M., Bernhardt B.A., Biswas S., Joshi M., et al. Clinical providers’ experiences with returning results from genomic sequencing: an interview study. BMC Med Genomics. 2018;11:45. doi: 10.1186/s12920-018-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora N.S., Davis J.K., Kirby C., McGuire A.L., Green R.C., Blumenthal-Barby J., et al. Communication challenges for nongeneticist physicians relaying clinical genomic results. Pers Med. 2017;14:423–431. doi: 10.2217/pme-2017-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubanovich C.K., Cheung C., Torkamani A., Bloss C.S. Physician communication of genomic results in a diagnostic odyssey case series. Pediatrics. 2019;143:S44–S53. doi: 10.1542/peds.2018-1099I. [DOI] [PubMed] [Google Scholar]
- 9.Carroll J.C., Allanson J., Morrison S., Miller F.A., Wilson B.J., Permaul J.A., et al. Informing integration of genomic medicine into primary care: an assessment of current practice, attitudes, and desired resources. Front Genet. 2019;10 doi: 10.3389/fgene.2019.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukenik-Halevy R., Ludman M.D., Ben-Shachar S., Raas-Rothschild A. The time-consuming demands of the practice of medical genetics in the era of advanced genomic testing. Genet Med. 2016;18:372–377. doi: 10.1038/gim.2015.96. [DOI] [PubMed] [Google Scholar]
- 11.Berg J.S., Khoury M.J., Evans J.P. Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time. Genet Med. 2011;13:499–504. doi: 10.1097/GIM.0b013e318220aaba. [DOI] [PubMed] [Google Scholar]
- 12.Dragojlovic N., Borle K., Kopac N., Ellis U., Birch P., Adam S., et al. The composition and capacity of the clinical genetics workforce in high-income countries: a scoping review. Genet Med. 2020;22:1437–1449. doi: 10.1038/s41436-020-0825-2. [DOI] [PubMed] [Google Scholar]
- 13.Cassini C., Thauvin-Robinet C., Vinault S., Binquet C., Coron F., Masurel-Paulet A., et al. Written information to patients in clinical genetics: What’s the impact? Eur J Med Genet. 2011;54:277–280. doi: 10.1016/j.ejmg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Collins V., Halliday J., Warren R., Williamson R. Assessment of education and counselling offered by a familial colorectal cancer clinic. Clin Genet. 2001;57:48–55. doi: 10.1034/j.1399-0004.2000.570107.x. [DOI] [PubMed] [Google Scholar]
- 15.Kausmeyer D.T., Lengerich E.J., Kluhsman B.C., Morrone D., Harper G.R., Baker M.J. A survey of patients’ experiences with the cancer genetic counselling process: recommendations for cancer genetics programs. J Genet Couns. 2006;15:409–431. doi: 10.1007/s10897-006-9039-2. [DOI] [PubMed] [Google Scholar]
- 16.Williams J.L., Rahm A.K., Zallen D.T., Stuckey H., Fultz K., Fan A.L., et al. Impact of a patient-facing enhanced genomic results report to improve understanding, engagement, and communication. J Genet Couns. 2018;27:358–369. doi: 10.1007/s10897-017-0176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams M.S., Kern M.S., Lerch V.R., Billet J., Williams J.L., Moore G.J. Implementation of a patient-facing genomic test report in the electronic health record using a web-application interface. BMC Med Inform Decis Mak. 2018;18:32. doi: 10.1186/s12911-018-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haga S.B., Mills R., Pollak K.I., Rehder C., Buchanan A.H., Lipkus I.M., et al. Developing patient-friendly genetic and genomic test reports: formats to promote patient engagement and understanding. Genome Med. 2014;6:58. doi: 10.1186/s13073-014-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheuner M.T., Hilborne L., Brown J., Lubin I.M. for the members of the RAND, a report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genet Test Mol Biomarkers. 2012;16:761–769. doi: 10.1089/gtmb.2011.0328. [DOI] [PubMed] [Google Scholar]
- 20.Scheuner M.T., Edelen M.O., Hilborne L.H., Lubin I.M. Effective communication of molecular genetic test results to primary care providers. Genet Med. 2013;15:444–449. doi: 10.1038/gim.2012.151. [DOI] [PubMed] [Google Scholar]
- 21.Vassy J.L., McLaughlin H.L., MacRae C.A., Seidman C.E., Lautenbach D., Krier J.B., et al. A one-page summary report of genome sequencing for the healthy adult. Public Health Genomics. 2015;18:123–129. doi: 10.1159/000370102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorschner M.O., Amendola L.M., Shirts B.H., Kiedrowski L., Salama J., Gordon A.S., et al. Refining the structure and content of clinical genomic reports. Am J Med Genet Part C Semin Med Genet. 2014;166:85–92. doi: 10.1002/ajmg.c.31395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubin I.M., McGovern M.M., Gibson Z., Gross S.J., Lyon E., Pagon R.A., et al. Clinician perspectives about molecular genetic testing for heritable conditions and development of a clinician-friendly laboratory report. J Mol Diagn. 2009;11:162–171. doi: 10.2353/jmoldx.2009.080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin H.M., Ceyhan-Birsoy O., Christensen K.D., Kohane I.S., Krier J., Lane W.J., et al. A systematic approach to the reporting of medically relevant findings from whole genome sequencing. BMC Med Genet. 2014;15:134. doi: 10.1186/s12881-014-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hylind R., Smith M., Rasmussen-Torvik L., Aufox S. Great expectations: patient perspectives and anticipated utility of non-diagnostic genomic-sequencing results. J Community Genet. 2018;9:19–26. doi: 10.1007/s12687-017-0314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeldt D.L., Cheung C., Ariniello L., Darst B.F., Topol S., Schork N.J., et al. Patient perspectives on whole-genome sequencing for undiagnosed diseases. Pers Med. 2017;14:17–25. doi: 10.2217/pme-2016-0050. [DOI] [PubMed] [Google Scholar]
- 27.McGowan M.L., Glinka A., Highland J., Asaad G., Sharp R.R. Genetics patients’ perspectives on clinical genomic testing. Pers Med. 2013;10:339–347. doi: 10.2217/pme.13.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanderson S.C., Linderman M.D., Suckiel S.A., Zinberg R., Wasserstein M., Kasarskis A., et al. Psychological and behavioural impact of returning personal results from whole-genome sequencing: the HealthSeq project. Eur J Hum Genet. 2017;25:280–292. doi: 10.1038/ejhg.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang N.S.Y., Adam S., Elliott A.M., Siemens A., Souich C., Friedman J.M., et al. After genomic testing results: Parents’ long-term views. J Genet Couns. 2021:jgc4.1454. doi: 10.1002/jgc4.1454. [DOI] [PubMed] [Google Scholar]
- 30.Morren M., Rijken M., Baanders A.N., Bensing J. Perceived genetic knowledge, attitudes towards genetic testing, and the relationship between these among patients with a chronic disease. Patient Educ Couns. 2007;65:197–204. doi: 10.1016/j.pec.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 31.The IMAGINe Study, (n.d.)https://www.child-bright.ca/imagine.
- 32.Hung Y.-L., Stones C. In: Des. User Exp. Usability. User Exp. Des. Everyday Life Appl. Serv. Marcus A., editor. Springer International Publishing; Cham: 2014. Visual Design in healthcare for low-literate users -- a case study of healthcare leaflets for new immigrants in Taiwan; pp. 44–55. [Google Scholar]
- 33.Frascara J. Allworth Press; 2004. Communication Design: Principles, Methods, and Practice. [Google Scholar]
- 34.Bowling B.V., Acra E.E., Wang L., Myers M.F., Dean G.E., Markle G.C., et al. Development and evaluation of a genetics literacy assessment instrument for undergraduates. Genetics. 2008;178:15–22. doi: 10.1534/genetics.107.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farmer G.D., Gray H., Chandratillake G., Raymond F.L., Freeman A.L.J. Recommendations for designing genetic test reports to be understood by patients and non-specialists. Eur J Hum Genet. 2020;28:885–895. doi: 10.1038/s41431-020-0579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patients Included Charter for Patient Information Resources. 2016. https://patientsincluded.org/patient-information-resources/
- 37.Kincaid B.S., Peter J., Fishburne Robert P., Jr., Rogers Richard L., Chissom Derivation of new readability formulas (automated readability index, fog count and flesch reading ease formula) for navy enlisted personnel. Inst Simul Train. 1975;56 https://stars.library.ucf.edu/istlibrary/56/ [Google Scholar]
- 38.Wizowski L., Harper T., Hutchings T. 4th ed. Hamilton Health Sciences; 2014. Writing health information for patients and families: A guide to developing patient education materials that promote health literacy.http://www.hamiltonhealthsciences.ca/workfiles/PATIENT_ED/Writing_HI_Edition4.pdf [Google Scholar]
- 39.Adobe Inc Adobe Acrobat Pro DC. 1993. acrobat.adobe.com
- 40.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoemaker S.J., Wolf M.S., Brach C. Development of the Patient Education Materials Assessment Tool (PEMAT): A new measure of understandability and actionability for print and audiovisual patient information. Patient Educ Couns. 2014;96:395–403. doi: 10.1016/j.pec.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material includes (1) the GRB elements requested by families and solutions implemented, (2) the GRB evaluation and feedback e-questionnaire, (3) the prompts for the semi-structured interviews, (4) family user insight and feedback shared through questionnaire and interviews, and (5) GRB questionnaire data.
Data Availability Statement
Evaluation data are available in the supplementary materials.