Highlights
-
•
Many coxsackievirus A10 isolates have low culture efficiency in vero cells.
-
•
HEK293A cell is a suitable cell line for coxsackievirus A10 propagation.
-
•
A serum-free HEK293A cell culture was developed for coxsackievirus A10 production.
-
•
Other enteroviruses could also be propagated in serum-free HEK293A cell culture.
Keywords: Coxsackievirus A10 (CVA10), Hand foot and mouth diseases, Inactivated whole virion vaccine, HEK293A, Serum-free cell culture
Abstract
Coxsackievirus A10 (CVA10) is one of enteroviral pathogens that cause the hand, foot, and mouth disease (HFMD). Since CVA10 was reported to be not easily propagated in the Vero cell culture, a feasible manufacture process for producing formalin-inactivated CVA10 vaccine is urgently needed. Several cell lines that commonly used for viral vaccine production was tested for CVA10 (M2014 strain) culture in this study, and our result showed that CVA10 could be easily propagated in the HEK293A cells. A serum-free HEK293A cell culture system was developed for CVA10 production and the yields have reached over 108 TCID50/mL. The biochemical and immunogenic properties of CVA10 particles obtained from this serum-free HEK293A culture were identical to our previous study. Two major particles of CVA10 were separated by ultracentrifugation, and only the infectious mature particles were capable of inducing CVA10 neutralizing antibody responses in the mouse immunogenicity studies. Additionally, we found that coxsackievirus A6 and enterovirus A71 could also be easily propagated using this serum-free HEK293A cell culture system. Our results provide a solution to overcome the obstacle in the propagation of CVA10 and facilitate the development of multivalent vaccines for prevention of HFMD.
1. Introduction
Coxsackievirus A10 (CVA10) is one of common pathogens that causes hand, foot, and mouth diseases (HFMD). CVA10 infection could lead to erythematous, onychomadesis and HFMD-related symptoms in children. Based on the pandemic survey in the past 20 years, several outbreaks of CVA10-related HFMD had been reported in China, Finland, France, India, Japan, Korea, Singapore, Spain, Taiwan, Thailand, and Vietnam (Lu et al., 2012; Liu et al., 2014; Klein and Chong, 2015; Yang et al., 2015; Gonzalez et al., 2019; Nhan et al., 2020; Jiang et al., 2021; Kim et al., 2021; Min et al., 2021; Zhang et al., 2022). With the successful development of the formalin-inactivated enterovirus A71 (FI-EV-A71) vaccine for preventing EV-A71-related HFMD, other types of HFMD-related viruses began to draw attention (Fang and Liu, 2018, 2022). However, the monovalent EV-A71 vaccines showed poor cross-neutralization against CVA10 in preclinical studies (Liu et al., 2016). Based on the concept of FI-EV-A71 whole-virion vaccine, the development of FI-CVA10 vaccine is considered essential to be included in the multivalent vaccine for preventing HFMD (Liu et al., 2016; Lim et al., 2018; Fang and Liu, 2022). Therefore, a feasible manufacturing process for CVA10 is urgently needed for the vaccine production.
CVA10 is a non-enveloped RNA virus of the family Picornaviridae, genus Enterovirus, which also includes poliovirus (PV1-PV3), coxsackieviruses (CVA group and CVB group), EV-A71, and echovirus. CVA10 is classified in the Enterovirus A specie, and its RNA genome size is approximately 7.4 Kbp (Solomon et al., 2010; Simmonds et al., 2020). Although more than 100 enteroviruses have been reported to have similar genome size, they all have their own unique characteristics. Traditionally, virions required for enterovirus vaccine production are harvested from Vero cell culture. However, most CVA10 clinical isolates were reported to have low culture efficiency in Vero cells, while only a few pre-screened, Vero-adapted CVA10 strains could propagate adequately in Vero cells (Liu et al., 2016; Lim et al., 2018; Wang et al., 2020; Zhang et al., 2022; Zhao et al., 2022). These results may indicate that CVA10 vaccine candidates deriving from the clinical-isolated pool may not be feasible, giving that they do not propagate well in the conventional Vero culture.
Cell lines, culture media and manufacturing processes are important elements for cell-based viral vaccine production. Poliovirus vaccines are developed from virions produced by Vero cells in microcarrier bioreactor (Montagnon et al., 1984). In the past years, Vero and KMB-17 cell cultures were used for manufacturing the FI-EV-A71 vaccines (Wu et al., 2004; Liu et al., 2007; Dong et al., 2011; Liu et al., 2018; 2011). Culturing systems including roller bottle, cell factory and microcarrier bioreactor were developed to produce the EV-A71 in serum-free condition (Liu et al., 2011; Chou et al., 2012; Chong et al., 2015). In order to produce virions for vaccine manufacturing, several potential host cell lines, including HEK293, MRC-5, CHO, MDCK, CAP, and AGE1.CR, were exploited and these cell lines have potential to be developed into applicable systems for vaccine production (Vlecken et al., 2013). In the past years, the development of cell-based influenza vaccines had revealed that by utilizing cell lines including Vero, MDCK, AGE1.CR, and HEK293, the vaccine yields could be significantly improved (Lohr et al., 2009; Le et al., 2010; Tapia et al., 2014; Milián et al., 2017; Pérez Rubio and Eiros 2018). Therefore, the availability of additional suitable cell lines for virion production may provide extra flexibility for manufacturing CVA10 and other enteroviral vaccines.
In this study, we have tested several cell lines for enterovirus production and found that HEK293A is a potent cell line for CVA10 production. We developed a serum-free HEK293A cell culture process using roller bottle and suspension culture system for CVA10 propagation. The produced virions were purified and tested to evaluate their immunological and biochemical characteristics. We also examined the propagation of CVA6 and EV-A71 using HEK293A cells in this serum-free system. We expect that these results will contribute to the development of a multivalent HFMD vaccine.
2. Materials and methods
2.1. Ethics statement
Animal experiments in this study were conducted in accordance with the guidelines of the Laboratory Animal Center of the National Health Research Institutes (NHRI), Taiwan. Protocols of animal study have been reviewed and approved by the NHRI Institutional Animal Care and Use Committee (Approved protocol No. NHRI-IACUC-107,095-A).
2.2. Cells, media and viruses
Vero (CCL-81) cells were obtained from the American Type Culture Collection (ATCC, USA). Rhabdomyosarcoma (RD) cells and Madin-Darby Canine Kidney (MDCK) cells were obtained from the Bioresource Collection and Research Center (BCRC), Hsinchu, Taiwan. Human embryonic kidney 293 adherent cells (HEK293A) were purchased from Invitrogen (Cat. R70507). Vero, RD and MDCK cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) with 10% fetal bovine serum (FBS). HEK293A were recovered in DMEM+10%FBS and adapted into serum-free medium using FreeStyle 293 medium (Gibco) with 1x cell culture media supplement (ITSE + A, InVitria) in roller bottle culture and BalanCD HEK293 medium (FUJIFILM Irvine Scientific) in suspension culture. The cell density was counted by a hemocytometer. The CVA10 (strain M2014) and CVA6 (strain M0746) were obtained from National Cheng Kung University Hospital, Taiwan. The EV-A71 (strain E59) was obtained from the Center of Disease Control, Taiwan. These virus stocks were prepared from the supernatants of infected RD cells at 3 days post-infection (DPI). The titers of virus were determined by the median tissue culture infectious dose (TCID50) assay.
2.3. Determination of viral titer
The virus titers were determined by TCID50 assay as previously described (Liu et al., 2016). Briefly, serially diluted virus samples (from 10−1 to 10−8) were added to RD cells cultured on 96-well plates, and 6 replicate samples were used for each dilution. The TCID50 values were obtained after counting the cytopathic effects (CPE) in the infected RD cells which grew in 96-well plates and incubated for six days at 37 °C. The 50% reduction of the viral infectivity was calculated using the Reed-Muench method.
2.4. CVA10 virus propagation in selected cell lines
RD, Vero, MDCK and HEK293A cells were grown in DMEM + 10% FBS for suitable cell line screening with CVA10. All tested cell lines were seeded at 1 × 106 cells with medium in T-25 flask. After 8 h of incubation, the medium was replaced with 10 mL fresh medium and the cells were infected with CVA10 at the multiplicity of infection (MOI) = 10−4. This MOI value was selected based on the result of CVA10 production in RD cells with three MOIs (supplementary Fig. S1). The CPE of tested cell line was documented after 6 DPI.
2.5. Propagation of CVA10 in roller bottle system
The propagation of CVA10 using serum-free FreeStyle 293 medium with supplement in the roller bottle was evaluated as reported in our previous study (Chou et al., 2012). The specific growth rate (µ) of the serum-free adapted HEK293A cells was determined (supplementary Fig. S2) as described in our previous study (Liu and Wu, 2004). The HEK293A cells (2 × 107 cells) were seeded into each CellBIND 850 cm2 roller bottle (Corning) containing 200 mL of culture medium in roller rack incubator at 37 °C for cell propagation. After 6 days of incubation, each roller bottle was replaced with 330 mL fresh medium containing CVA10 at MOI = 10−4. After 6 DPI, the culture supernatant was harvested for virus purification.
2.6. Propagation of CVA10 in suspension culture system
The cultivation of CVA10 using serum-free BalanCD HEK293 medium in suspension system was evaluated in both the spinner flask and bioreactor systems. The 500 mL spinner flasks (Corning) were placed on a magnetic stirrer at 120 rpm (RPM) in a 5% CO2 incubator at 37 °C. The doubling time of suspension HEK293A cells was approximately 1.5 days of incubation (µ = 0.513 d−1). Suspension HEK293A cells were collected and centrifugated at 1200 RPM for 5 min from high-cell density spinner flask. Cells (1 × 106 cells/mL) were transferred into spinner flask with fresh medium, and a working volume of 250 mL culture were then infected with CVA10 at MOI = 10−4. One mL sample of spinner culture was taken daily to assess the condition of the cells and to collect sample for virus titer determination. The duration of propagation and determination of virus harvest were based on the condition of cell viability after CVA10 infection. The cell viability was evaluated by 0.4% trypan blue staining (Gibco). When cell viability decreased to lower than 50%, the suspension culture was stopped and proceeded with virus harvest (Kwang et al., 2016). For bioreactor system, the Biostat-B bioreactor (Sartorius AG) was inoculated with 5 × 105 cells per mL at 120 RPM, and the cell density reached 1.5–2 × 106 cells per mL (working volume = 1.5 L) after 3 days of cultivation. The suspension HEK293A cells were fed with medium containing glucose (2 g/L) and glutamine (20 mM/L), and then infected with CVA10 at MOI = 10−4. Ten mL samples of bioreactor culture were taken daily to assess the condition of the cells and to collect sample for virus titer determination. The supernatant and pellet of five mL sample were separated by centrifugation at 1200 RPM centrifugation. The pellet samples were then wash twice with PBS and resuspended in PBS, and samples were placed at -80 °C to freeze and then thaw to break the cells for virus titer determination. When cell viability decreased to lower than 50%, the bioreactor culture process was stopped and proceeded to virus harvest.
2.7. Preparation of concentrated CVA10 solution for purification
The virus liquids of CVA10 were harvested from the bioreactor culture and then placed at -80 °C to freeze and then thaw to break the cells (Shao et al., 2016). Cell debris in the viral harvest was eliminated by passing through 0.65 µm (Sartorius AG), 0.45 µm and 0.22 µm filter sequentially (Thermo Fisher Scientific). The filtrate was concentrated to near 20-fold with a 100 K tangential flow filtration (TFF) capsule (Pall). The concentrated CVA10 solution was used for purification experiment.
2.8. Purification of CVA10 particles using liquid chromatography
To purify CVA10 particles, concentrated CVA10 solution was loaded in size-exclusion column containing Sephocryl S-500 gel (GE Healthcare), as described in our previous study (Chou et al., 2012). Phosphate buffered saline (PBS) was used as the elution buffer and the flow-rate was set to 20 mL/min. Fractions (10 mL per fraction) were collected sequentially and analyzed by Western blotting assay, and viral infectivity was determined by TCID50 assay. The CVA10 particles were then further concentrated by centrifuging in a 100 k molecular weight cutoff (MWCO) Amicon ultra centrifugal filter tube (Millipore), and recovered in 1x PBS.
2.9. Purification of CVA10 particles by continuous sucrose gradient ultracentrifugation
The CVA10 empty (E)-particle and full (F)-particle were isolated by continuous sucrose gradient ultracentrifugation, as described in our previous study (Liu et al., 2016). In this study, the concentrated CVA10 solution was applied for purification of the E- and F- particles using a 10 to 60% continuous sucrose gradient and centrifuged in a Hitachi CP80 ultracentrifuge (32,000 rpm for three hours) (Liu et al., 2016). The CVA10 particles were further concentrated by centrifuging in a 100 k MWCO Amicon ultra centrifugal filter tube (Millipore), and recovered in 1x PBS.
2.10. SDS-PAGE and western blotting analysis of viral proteins
Western blotting analyses of CVA10 were performed by 4–12% Bis-Tris SDS-PAGE (Invitrogen). The CVA10 proteins were separated by SDS-PAGE and transferred onto a PVDF membrane (Invitrogen) using a Mini Trans-Blot Cell (Bio-Rad) according to the manufacturer's instructions. The anti-CVA6 VP1 rabbit polyclonal antibody GTX132346 (GeneTex) that could detect not only the CVA6 but also cross-react with CVA10 and CVA16, was used (supplementary Fig. S3). The secondary, goat anti-rabbit IgG (AP132P, Millipore) conjugated with horseradish peroxidase (HRP), was then applied. Immobilon crescendo western HRP substrate WBLUR0500 (Millipore) was used for chemiluminescence development and detected by the Amersham Imager 600 system (GE Healthcare).
2.11. Transmission electron microscopy
Transmission electron microscopy (TEM) was used to examine the purified viral particle samples. The purified CVA10 samples were inactivated by formalin solution (v/v 1:4000 dilution) at 37 °C for 3 days. The sample (4 µL) was loaded on carbon-vaporized copper grid (200-mesh) for 15 min at room temperature. The excess sample was removed with paper, and washing twice with water. The grid was stained with 2% uranyl acetate solution. The stained sample was air-dried and examined using the Joel JEM-1400 transmission electron microscope (Liu et al., 2016).
2.12. Immunogenicity studies of CVA10 in animal
The purified formalin-inactivated (v/v 1:4000 dilution) samples of CVA10 particles were adsorbed with aluminum phosphate (Alum, InvivoGen). A group of 6 female BALB/c mice (6–8 weeks old) were immunized intramuscularly with 0.2 mL antigen (0.5 µg viral protein + 60 µg Alum). Mice were boosted twice with identical dose at two-week intervals after priming. Blood from the immunized mice were collected one week after the final boost, and the serum was used for virus neutralization study.
2.13. Virus neutralizing test
The virus neutralization test (Nt) was performed as described previously (Liu et al., 2011). Serum samples collected from immunized mice were inactivated at 56 °C for 30 min. Each serum sample was then serially diluted (2-fold) with culture medium. Two hundred µL of virus solution with titer equal to 200 TCID50 were added to tubes containing 200 µL of the diluted sera. After incubation at 4 °C for 18–24 h, these samples (100 µL/well) were added to 96-well plates containing RD cells. The cultures were incubated for 6 days at 37 °C, and TCID50 were measured after quantifying the CPE in the infected RD cells. The Nt value is the geometric reciprocal of the serum dilution yielding a 50% reduction in the viral titer, was obtained using the Reed-Muench methods.
2.14. ELISA analysis
Mouse anti sera (1,000x dilution), and mAb979 (anti-EV-A71; 1,000x dilution), anti-CVA6 VP1 (GTX132346; 1,000x dilution), were used in ELISA to recognize the formalin-inactivated viral particles (CVA6, CVA10, and EV-A71). Viral particles were coated on a 96-well ELISA plate (Corning) at 0.5 µg/well in 50 µL coating buffer (1 M NaHCO3, pH9.5) (Liu et al., 2016). After antigen coating, 250 µL of 5% skim milk in PBS was added for blocking. One hundred µL of testing sera and antibodies were added to testing wells and incubated for 2 h at room temperature. The wells were washed four times with 250 µL wash buffer (PBS+0.05%Tween20), and 100 µL of an HRP-conjugated secondary antibody (1:30,000 dilution; Jackson ImmunoResearch) was added to each well for 30 min incubation at room temperature. The plate was washed six times with wash buffer and dried with filter paper. 50 µL of TMB peroxidase substrate (SureBlueTM, KPL) was added for 30 min reaction and the reaction was stopped by adding 50 µL of 2 N H2SO4. The absorbance at 450 nm was measured by an ELISA reader (Spectra Max M2 model, USA).
3. Results
3.1. Propagation of CVA10 in selected cell lines
To boost CVA10 production, several cell lines, including RD, Vero, MDCK, and HEK293A cells were tested for CVA10 replication. After CVA10 infection at MOI = 10−4, CPE could be observed in RD and HEK293A cells and most of these cells were lyzed. On the contrary, Vero and MDCK cells did not show obvious CPE and most of these cells remained fully attached to the flask surface after 6 DPI (Fig. 1A). The kinetics of virus replication showed that the titers of CVA10 reached 1 × 1010 TCID50/mL in RD cell culture at 3 DPI (Fig. 1B). The virus titer of CVA10 reached 1 × 109 TCID50/mL in HEK293A cells at 6 DPI, whereas the virus titers were nearly undetectable in Vero and MDCK cell cultures. Given that RD is a tumor cell line and not a suitable host for conventional vaccine manufacturing, our result shows that HEK293A cell line could be a satisfactory host for propagating CVA10 and was therefore used to develop CVA10 culture process in the following experiments.
Fig. 1.
Propagation of CVA10 in RD, Vero, MDCK, and HEK293A cells. All tested cell lines were seeded with 1 × 106 cells in T-25 flask. After 8 h of incubation, the medium was replaced with 10 mL fresh medium and the cells were infected with CVA10 at MOI = 10−4. (A) Cell morphologies of CVA10-infected and control cells. (B) The replication kinetics of CVA10 in different cell lines. The bar represents 100 µm.
3.2. Adaptation of HEK293A cell to serum-free culture condition for CVA10 production in a roller bottle culture system
In order to develop serum-free culture process for CVA10 vaccine production, HEK293A cell was adapted to serum-free condition. HEK293A cells were cultured with gradually reduced FBS ratio (10, 5, 2.5 and 1%) in each passage. When HEK293A cells were adapted in DMEM + 1% FBS medium, FreeStyle 293 medium with supplement was used to replace the serum-containing medium for cell culture. After 3 passages, some HEK293A cells were stably adapted to the FreeStyle 293 medium by showing cell division and growth. The specific growth rate of this adapted HEK293A cells is 0.415 d−1 in FreeStyle 293 medium with supplement (supplementary Fig. S2). The adapted HEK293A cells were seeded in roller bottles, and the cells were attached and grew on the inner surface of the roller bottles (Fig. 2A). Based on the specific growth rate, the doubling time of the adapted HEK293A cells was approximately two days. After 6 days of culture, the cell density reached 9 × 107 cells in a roller bottle (data not shown). After CVA10 infection, CPE was presented in HEK293A cells and the cells dissociated from the culture surface (Fig. 2B). The kinetics of virus replication revealed that the virus titers of CVA10 reached 2–5 × 108 TCID50/mL after 4 DPI (Fig. 2C). Our result shows that HEK293A cells cultured in serum free condition could propagated CVA10 properly as Vero cells do in EV-A71 viral production.
Fig. 2.
Propagation profiles of CVA10 in serum-free roller bottle culture. HEK293A cells were infected with CVA10 at an MOI of 10−4. (A) Morphology of serum-free HEK293A cells in roller bottle. (B) Morphology of HEK293A cells after CVA10 infection. (C) TCID50 values of three roller bottles after counting CPE in infected RD cells. The TCID50 values were calculated using the Reed-Muench method. The bar represents 100 µm.
3.3. Adaptation of HEK293A cell to suspension culture system for CVA10 production
Suspension culture system can be easily scaled up for manufacturing, and HEK293 cells were reported to be able to grow in suspension condition (Dekevic et al., 2022; Jang et al., 2022). The HEK293A cells adapted in FreeStyle 293 medium were seeded in spinner flasks, and several candidate serum-free media, including FreeStyle 293, VP-SFM (Gibco), Pro293s-CDM (Gibco), BalanCD 293, and CDM4HEK293 (HyClone), were tested in suspension culture (supplementary Fig. S4). During the first three passages of adaptation, the cells were retained by centrifugation and resuspended with fresh medium. We found that HEK293A cells cultured in BalanCD HEK293 medium revealed a better growth condition, and the adapted HEK293A cells can proliferate properly in suspension condition with 120 RPM blending in spinner flasks (Fig. 3A). Suspended HEK293A cells appeared spherical with slightly aggregate multicell clumps, and cells grew a bit faster in BalanCD HEK293 medium than in other media (supplementary Fig. S4). After CVA10 infection, these cells were shriveled and most cells revealed abnormal appearance (Fig. 3B). The kinetics of virus replication showed that the virus titers of CVA10 reached 1.2 - 15 × 109 TCID50/mL after 3 DPI (Fig. 3C) in spinner culture. Based on these results, a suspension bioreactor system was developed to propagate CVA10 (as described in Methods). The growth kinetic data showed that the virus titers of CVA10 could reach 9 - 12 × 109 TCID50/mL after 4 DPI (Fig. 3D). The extracellular and intracellular virus titers were evaluated individually by separating the supernatants and pellets of samples. Higher CVA10s were present in the cells than in the culture supernatants. These results show that serum-free suspension HEK293A culture could produce high yield of CVA10 and may facilitate large-scale viral production for vaccine development.
Fig. 3.
Propagation profiles of CVA10 in serum-free suspension culture. HEK293A cells were infected with CVA10 at an MOI of 10−4. (A) Suspension HEK293A cells in a spinner. (B) Suspension HEK293A cells after CVA10 infection. (C) TCID50 values of four spinners after counting CPE in the infected RD cells. (D) Extracellular and intracellular TCID50 values of serum-free bioreactor culture after counting CPE in infected RD cells. The TCID50 values were calculated using the Reed-Muench method. The bar represents 100 µm.
3.4. Protein and viral particle profiles of CVA10 purified by size exclusion liquid chromatography and sucrose gradient zonal ultracentrifugation
CVA10 virus was harvested and collected from serum-free suspension HEK293A culture. Two purification methods were used to characterize CVA10 particles as our previous study (Liu et al., 2016). Given that no specific anti-CVA10 antibody was commercially available at this moment, a polyclonal anti-CVA6 VP1 antibody (GTX132346) was used to detect the CVA10 antigens. This antibody has been confirmed in our lab by western blot to show that it recognizes CVA6, CVA10, and CVA16 but not EV-A71 proteins (supplementary Fig. S3). The CVA10 solution was loaded into a S500 size exclusion liquid chromatography for viral particle purification, and CVA10 antigens were found to be concentrated among fractions 18 to 22 (Fig. 4A). The same CVA10 solution was also loaded onto a zonal rotor containing a 10–60% continuous sucrose gradient. The sucrose gradient fractions were collected and analyzed (Fig. 4B). There were two regions of factions that viral antigens could be identified. The first region was fractions 6 to 7, and second region was fractions 12 to 16, which represents the E-particle fractions and F-particle fractions of CVA10 (Liu et al., 2016; Lien et al., 2022).
Fig. 4.
The purification profiles of CVA10 particles by two methods. (A) Purification of CVA10 by S-500 size exclusion chromatography. Total viral particles were eluted among fractions 18 to 22. (B) Purification of CVA10 by 10–60% sucrose gradient zonal ultracentrifugation. E-particles were eluted at fractions 6–7 and F-particles were eluted at fractions 12 to 16. M: protein ladder; C: concentrated CVA10 solution before purification. The numbers on the top of panels indicate the fraction collected during elution. The VP1 viral antigen was detected by western blot with a polyclonal antibody GTX132346.
To determine the protein profiles of CVA10 derived from the serum-free HEK293A culture, samples purified by size exclusion liquid chromatography and sucrose gradient zonal ultracentrifugation were analyzed by SDS-PAGE (Fig. 5A). The concentrated virus solution contained a lot of impurities, and most of these impurities were removed after size exclusion chromatography and sucrose gradient ultracentrifuge. These major protein bands that correspond to the VP0, VP1, VP2, and VP3 based on their predicted protein sizes are shown in Fig. 4A. The purified CVA10 mixture-particles from liquid chromatography (LC) presented similar protein composition to that of the E-particle (E) from sucrose-gradient ultracentrifuge. The F-particle (F) fraction from sucrose gradient ultracentrifugation showed thicker VP2-VP3 protein on gel. CVA10 VP1 antigen was confirmed in these purified samples by western blotting assay (Fig. 5B). TEM revealed the irregular icosahedral morphologies of the purify CVA10 antigens from two purification methods (Fig. 6). These CVA10 viral particles were found to have a diameter around 30–35 nm, and the F-particles of CVA10 were smaller than the E-particles. These results demonstrate that the viral protein compositions and particle morphologies of CVA10 from serum-free suspension HEK293A culture were similar to our previous study (Liu et al., 2016).
Fig. 5.
Viral antigen profile of CVA10 particles analyzed by SDS-PAGE and western blotting. (A) Samples of CVA10 particles analyzed on a NuPAGE 4–12% gel. (B) CVA10 VP1 protein detected by the GTX132346 antibody. Twenty µL of each sample was loaded for western blot analyses. M: Protein ladder; Conc.: concentrated CVA10 solution before purification; LC: viral fractions from size exclusion chromatography; E: E-particle fractions from sucrose gradient ultracentrifugation; and F: F-particle fractions from sucrose gradient ultracentrifugation.
Fig. 6.
CVA10 particles analyzed by TEM. (A) CVA10 viral particles purified by size exclusion chromatography. (B) E-particle fractions of CVA10 purified from sucrose gradient ultracentrifugation. (C) F-particle fractions of CVA10 purified from sucrose gradient ultracentrifugation. Four µL of sample was loaded on carbon-vaporized copper grid (200-mesh) and stained with 2% uranyl acetate solution. The stained sample was examined by the Joel JEM-1400 TEM. The bar represents 100 nm.
3.5. Immunogenicity studies of CVA10 viral particles from different purification methods
Our previous study has shown that the E- and F- particles of EV-A71 and CVA16 had profound difference in infectivity and immunogenicity (Liu et al., 2011; Chong et al., 2012). To investigate whether the E- and F-particles of CVA10 also reveal this kind of variance, mice were immunized with formalin-inactivated, E- and F- CVA10 viral particles from ultracentrifugation and mixed viral particles from LC. Antisera from the mice immunized with either the formalin-inactivated mixture-particles from LC or the F-particles of CVA10 generated good neutralizing antibody responses, while those immunized with the E-particle of CVA10 had not (Nt < 8) (Table 1). The F-particle of CVA10 generated higher neutralizing titers (Nt = 361.3) than the mixture-particles in mice (Nt = 124.5). Our result indicated that the F-particle of CVA10 should be used as the immunogens for the induction of a good and specific neutralization response in CVA10 vaccine development.
Table 1.
The neutralization titers of BALB/c mice immunized with FI-CVA10 antigens.
| Antigen groups | Control (PBS only) |
The mixture-particles of CVA10 from liquid chromatography | The E-particle of CVA10 from sucrose gradient ultracentrifugation | The F-particle of CVA10 from sucrose gradient ultracentrifugation |
|---|---|---|---|---|
| Average virus neutralization titer (N = 6; Mean±SD) |
< 8 | 124.5 ± 248.2 | < 8 | 361.3 ± 192.3 |
3.6. Reactivity and specificity of mouse anti-CVA10 sera
In order to confirm the reactivity and specificity of mouse sera generated from CVA10 vaccinations, three enteroviral particles were tested in this ELISA analysis. Four groups of mouse anti-sera were used to detect the CVA6, CVA10, and EV-A71 viral particles. The mAb979 (specific against VP2 of EV-A71) and the anti-CVA6 VP1 antibodies were included as controls, and the results are shown in Fig. 7. Interestingly, mice immunized with the mixed CVA10 particles (from size-exclusion chromatography purification, containing both E- and F- particles) were found to recognize not only the CVA10 but also the CVA6, albeit to a much lesser extent. Mice immunized with the CVA10 E-particles reacted strongly with both the CVA6 and CVA10, while mice immunized with the CVA10 F-particle recognized the CVA10 viral particles specifically. The mAb979 antibody reacted with the EV-A71 viral particles only, and the anti-CVA6 antibody recognized both the CVA6 and CVA10. These results show that the reactivity and specificity for CVA10 could be elicited in mice immunized with the CVA10 F-particle, while the specificity could be varied in mice immunized with mixed or CVA10 E-particles.
Fig. 7.
Antibody specificity assay of immunized mice to three enteroviruses (CVA6, CVA10 and EV-A71). Sera of mice immunized with CVA10 mixture particles (CVA10-LC), E-particles (CVA10-E), F-particles (CVA10-F), and mock group (PBS) were tested by ELISA. Anti-EV-A71 (mAb979) and anti-CVA6 VP1 (GTX132346) antibodies were included as controls.
3.7. Serum-free HEK293A culture process for CVA6 and EV-A71
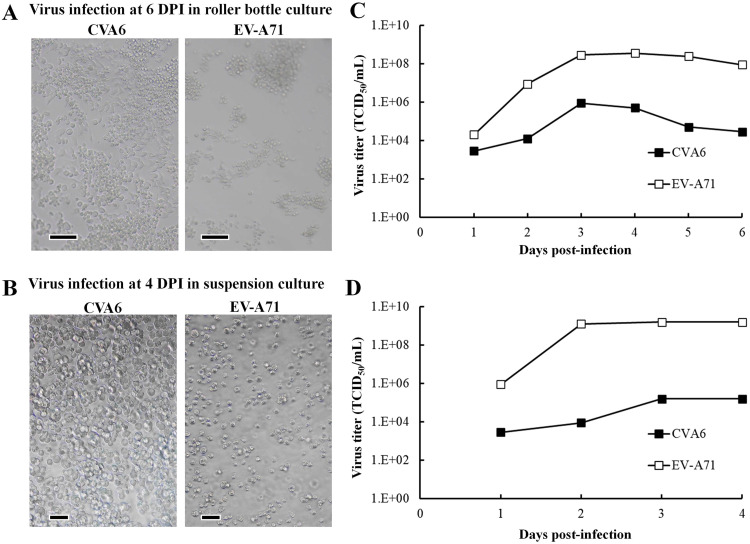
Given the success of serum-free HEK293A culture process developed for CVA10 production, we investigated whether this can be applied to other HFMD-related enteroviruses. The CVA6 and EV-A71 infected serum-free HEK293A cells were processed using the same roller bottle and spinner system as aforementioned. The cells were infected with viruses at MOI = 10−4, and the results are shown in Fig. 8. Serum-free HEK293A cells presented CPE after infection in roller bottle (Fig. 8A) and spinner culture (Fig. 8B). In roller bottle culture, the virus titers of CVA6 reached 8.9 × 105 TCID50/mL after 3 DPI, while the virus titers of EV-A71 reached 3.5 × 108 TCID50/mL after 3 DPI (Fig 8C). In suspension spinner culture, the virus titers of CVA6 reached 1.6 × 105 TCID50/mL after 3 DPI, while the virus titers of EV-A71 reached 1.6 × 109 TCID50/mL after 3 DPI (Fig 8D). Interestingly, CVA6 is also a virus that showed low propagation in Vero cells but could be propagated properly in HEK293A cells in our study. Our results show that CVA6 and EV-A71 could also be propagated using HEK293A cells in this serum-free HEK293A process, which proves that this system is a suitable production process for other enterovirus types.
Fig. 8.
Propagation profiles of other HFMD-related viruses in serum-free HEK293A cell culture. HEK293A cells were infected with each virus (CVA6 and EV-A71) at MOI = 10−4. The culture and harvest conditions were identical to that described in the Method for CVA10 propagation. (A) Morphology of HEK293A cells after virus infection in roller bottle at 6 DPI. (B) Morphology of suspension HEK293A cells after virus infection in spinner at 4 DPI. (C) TCID50 values of serum-free roller bottle cultures after counting CPE in infected RD cells. (D) TCID50 values of serum-free spinner cultures after counting CPE in infected RD cells. The TCID50 values were calculated using the Reed-Muench method. The bar represents 100 µm.
4. Discussion
The susceptibility of cell lines to enteroviruses is widely different. In previous studies, many cell lines were evaluated for their susceptibility, but not a single cell line is found susceptible to infection by all serotypes of enterovirus (Prim et al., 2013). RD cell was found to be a highly susceptible cell for culturing coxsackievirus A group (Tsao et al., 2010). In CVA10 propagation study, major CPE was observed in RD cells at 3 DPI and the cells were mostly lysed at 6 DPI. However in the HEK293A cells, only a small number of CPE was noted at 3DPI and the major CPE was occurred at 6 DPI (Fig. 1). Vero and MDCK cells were unaffected and continued to grow during the study. Compared to RD, the HEK293A cells required three more days to achieve a major CPE when infected at MOI = 10−4. This result shows the difference in susceptibility of cell lines to CVA10 infection. Although being a highly susceptible cell, RD is a cancer cell line and is unsuitable for vaccine application. Therefore, the availability of suitable cell lines for CVA10 production may provide important advances for HFMD vaccine development. In addition, the serum-free culture systems and the low MOI condition could facilitate large-scale production of enteroviruses (Liu et al., 2011; Wu et al., 2015). In this study, infection at MOI = 10−4 is found to be an optimal condition for virus production (supplementary Fig. S1).
Many traditional vaccines were manufactured via Vero cell culture, and cell-based flu vaccines were manufactured using MDCK cells. Our results showed that HEK293A cell line is a suitable cell line to propagate CVA10 (Fig. 1) compared to Vero and MDCK cells. Currently, the HEK293 cell lines have been widely used in research and biotechnology fields for over 30 years, with roles mainly focus on the production of viral vectors for gene and cell therapy (Tan et al., 2021). HEK293 cell line was established from an embryonic human kidney and transformed with sheared adenovirus 5 DNA, and the cell line currently had many subclones (Yuan et al., 2018). HEK293 cells also have been used to produce recombinant proteins and viral vaccines. Several HEK293 cell-produced therapeutic agents have been approved by the FDA or the European Medicines Agency (EMA) for clinical use (Dumont et al., 2016). In 2020, the Oxford-AstraZeneca chimpanzee adenovirus vectored COVID-19 vaccine (Vaxzevria), which was generated by HEK293 cells, is authorized by the EMA for protection against SARS-COV-2 infection (van Doremalen et al., 2020; Falsey et al., 2021; European Medicines Agency, 2022).
The HEK293A cell line is a subclone of HEK293 cells with attaching morphology that is commonly used for recombinant adenovirus production. Previous studies have shown that HEK293 cells can grow in serum-free medium for adherent or suspension cultures (Le et al., 2010; Chahal et al., 2014; Shao et al., 2016; Milián et al., 2017; Gélinas et al., 2019; Dekevic et al., 2022; Jang et al., 2022). In this study, serum-free HEK293A cells were infected with CVA10 at a low MOI for virus production, and the virus titers have reached over 108 TCID50/mL in both adherent and suspension cultures. Serum-free HEK293A cell suspension culture is an easier way to scale-up the production of CVA10, and we demonstrated the production of CVA10 by spinner culture and bioreactor culture system in this study. However, under such conditions, we did not observe apparent burst of cells after virus infection and the produced CVA10 particles seemed to be retained within cells (Fig. 3B and D). In order to harvest all the CVA10 yields, the cell pellets should be bursted by methods such as freezing and thawing at -80 °C, as similar to the recombinant adenovirus vector production (Shao et al., 2016).
Immunogenicity studies in mouse showed that the formalin-inactivated CVA10 F-particles elicited far stronger CVA10-specific neutralizing antibody responses than those obtained from CVA10 E-particle. This also explains that mice immunized with the CVA10 mixture particles (from liquid chromatography purification, containing both E- and F- particles) elicited weaker neutralizing antibody responses than the mice of the CVA10 F-particle group (Table 1). These results showed that the biochemical and immunogenic properties of CVA10 viral particles obtained from serum-free HEK293A culture were similar to our previous study in which the CVA10 was produced in serum-containing culture (Liu et al., 2016). The ELISA analysis has confirmed the specific recognition of CVA10 by sera of mice immunized with the F-particles of CVA10 (Fig. 7). Mice immunized with the E-particle of CVA10 were found to react with both the CVA6 and CVA10 viral particles. Although mice immunized with the E-particle showed antibody responses to CVA10 (Fig. 7), they failed to reveal any protection capability in the neutralizing assay (Table 1). These results certainly indicate that E-particle and F-particle of CVA10 have different structural conformation for eliciting specific antibody responses, and reinforce the concept that the F-particle of CVA10 should be used as the immunogens for the induction of a good and specific neutralization response in CVA10 vaccine development (Liu et al., 2016). In addition, our study showed that CVA6 and EV-A71 viruses could also be propagated using HEK293A cells in this serum-free culture, which proves that this system is a suitable production process for other enterovirus types.
In conclusion, we demonstrated that HEK293A cell is a suitable cell line for the production of CVA10 and other HFMD-related enteroviruses. Serum-free HEK293A cell suspension culture is an easier way to scale-up the production of CVA10 using spinner culture and bioreactor culture system. Serum-free HEK293A cell system can greatly improve the CVA10 yields and maintains the biochemical and immunogenic properties as those produced from Vero cells. CVA6 and EV-A71 could also be propagated using the serum-free HEK293A system. We expect that serum-free HEK293A cell culture process could provide an important solution for the manufacture of multivalent HFMD vaccines.
CRediT authorship contribution statement
Sheng-Chieh Lien: Conceptualization, Methodology, Investigation, Writing – original draft. Yu-Sheng Shen: Conceptualization, Methodology, Investigation, Writing – original draft. Hsiao-Yu Lin: Conceptualization, Investigation. Shang-Rung Wu: Investigation. Chih-Yeu Fang: Formal analysis, Writing – original draft. Chi-Hsun Chen: Investigation. Yi-An Chen: Investigation. Pele Choi-Sing Chong: Conceptualization, Resources. Ming-Hsi Huang: Resources. Yen-Hung Chow: Conceptualization, Resources. Jen-Ren Wang: Resources. Suh-Chin Wu: Resources. Chia-Chyi Liu: Conceptualization, Methodology, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Chia-Chyi Liu has patent #TWI688652B; CN106661102B; JP6774149B2; WO2015179979A1 licensed to National Health Research Institutes, Taiwan. Hsiao-Yu Lin has patent #TWI688652B; CN106661102B; JP6774149B2; WO2015179979A1 licensed to National Health Research Institutes, Taiwan. Pele Choi-Sing Chong has patent #TWI688652B; CN106661102B; JP6774149B2; WO2015179979A1 licensed to National Health Research Institutes, Taiwan. Yen-Hung Chow has patent #TWI688652B; CN106661102B; JP6774149B2; WO2015179979A1 licensed to National Health Research Institutes, Taiwan.
Acknowledgments
Funding
This work was supported by the National Health Research Institutes, Taiwan [Grant Nos. 06A1-IVPP13–014, 09A1-IVPP13–014, 11A1-IVPP14–014, 12A1-IVPP18–014].
Acknowledgments
This work was supported by the Ministry of Health and Welfare, the Taiwan CDC and the National Health Research Institutes, Taiwan. We also thank the technical services provided by “the i-MANI center of the National Core Facility for Biopharmaceuticals, Ministry of Science and Technology, Taiwan” as well as the technical service from the Instrument Development Center of the National Cheng Kung University.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199101.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Chahal P.S., Schulze E., Tran R., Montes J., Kamen A.A. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods. 2014;196:163–173. doi: 10.1016/j.jviromet.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P., Guo M.S., Lin F.H., Hsiao K.N., Weng S.Y., Chou A.H., Wang J.R., Hsieh S.Y., Su I.J., Liu C.C. Immunological and biochemical characterization of coxsackie virus A16 viral particles. PLOS One. 2012;7:e49973. doi: 10.1371/journal.pone.0049973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P., Liu C.C., Chow Y.H., Chou A.H., Klein M. Review of enterovirus 71 vaccines. Clin. Infect. Dis. 2015;60:797–803. doi: 10.1093/cid/ciu852. [DOI] [PubMed] [Google Scholar]
- Chou A.H., Liu C.C., Chang C.P., Guo M.S., Hsieh S.Y., Yang W.H., Chao H.J., Wu C.L., Huang J.L., Lee M.S., Hu A.Y., Lin S.C., Huang Y.Y., Hu M.H., Chow Y.H., Chiang J.R., Chang J.Y., Chong P. Pilot scale production of highly efficacious and stable enterovirus 71 vaccine candidates. PLOS One. 2012;7:e34834. doi: 10.1371/journal.pone.0034834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekevic G., Tasto L., Czermak P., Salzig D. Statistical experimental designs to optimize the transient transfection of HEK 293T cells and determine a transfer criterion from adherent cells to larger-scale cell suspension cultures. J. Biotechnol. 2022;346:23–34. doi: 10.1016/j.jbiotec.2022.01.004. [DOI] [PubMed] [Google Scholar]
- Dong C., Liu L., Zhao H., Wang J., Liao Y., Zhang X., Na R., Liang Y., Wang L., Li Q. Immunoprotection elicited by an enterovirus type 71 experimental inactivated vaccine in mice and rhesus monkeys. Vaccine. 2011;29:6269–6275. doi: 10.1016/j.vaccine.2011.06.044. [DOI] [PubMed] [Google Scholar]
- Dumont J., Euwart D., Mei B., Estes S., Kshirsagar R. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit. Rev. Biotechnol. 2016;36:1110–1122. doi: 10.3109/07388551.2015.1084266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., Neuzil K.M., Hahn W., Hunt J., Mulligan M.J., McEvoy C., DeJesus E., Hassman M., Little S.J., Pahud B.A., Durbin A., Pickrell P., Daar E.S., Bush L., Solis J., Carr Q.O., Oyedele T., Buchbinder S., Cowden J., Vargas S.L., Guerreros B.A., Call R., Keefer M.C., Kirkpatrick B.D., Pullman J., Tong T., Brewinski I.M., Benkeser D., Janes H.E., Nason M.C., Green J.A., Kelly E.J., Maaske J., Mueller N., Shoemaker K., Takas T., Marshall R.P., Pangalos M.N., Villafana T., Gonzalez-Lopez A., AstraZeneca AZD1222 Clinical Study Group Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N. Engl. J. Med. 2021;385:2348–2360. doi: 10.1056/nejmoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency, 2022. Vaxzevria (previously COVID-19 vaccine AstraZeneca): COVID-19 Vaccine (ChAdOx1-S [recombinant]), https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria (accessed 18 March 2023).
- Fang C.Y., Liu C.C. Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease. Expert Rev. Vaccines. 2018;17:819–831. doi: 10.1080/14760584.2018.1510326. [DOI] [PubMed] [Google Scholar]
- Fang C.Y., Liu C.C. Novel strategies for the development of hand, foot, and mouth disease vaccines and antiviral therapies. Expert Opin. Drug. Discov. 2022;17:27–39. doi: 10.1080/17460441.2021.1965987. [DOI] [PubMed] [Google Scholar]
- Gélinas J.F., Azizi H., Kiesslich S., Lanthier S., Perdersen J., Chahal P.S., Ansorge S., Kobinger G., Gilbert R., Kamen A.A. Production of rVSV-ZEBOV in serum-free suspension culture of HEK 293SF cells. Vaccine. 2019;37:6624–6632. doi: 10.1016/j.vaccine.2019.09.044. [DOI] [PubMed] [Google Scholar]
- Gonzalez G., Carr M.J., Kobayashi M., Hanaoka N., Fujimoto T. Enterovirus-associated hand-foot and mouth disease and neurological complications in Japan and the rest of the World. Int. J. Mol. Sci. 2019;20:5201. doi: 10.3390/ijms20205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Pete E.S., Bruheim P. The impact of serum-free culture on HEK293 cells: from the establishment of suspension and adherent serum-free adaptation cultures to the investigation of growth and metabolic profiles. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.964397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Zhang Z., Rao Q., Wang X., Wang M., Du T., Tang J., Long S., Zhang J., Luo J., Pan Y., Chen J., Ma J., Liu X., Fan M., Zhang T., Sun Q. The epidemiological characteristics of enterovirus infection before and after the use of enterovirus 71 inactivated vaccine in Kunming, China. Emerg. Microbes Infect. 2021;10:619–628. doi: 10.1080/22221751.2021.1899772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Bae K.S., Kim J.H., Kang J.H., Choi U.Y. Seroprevalence of neutralizing antibodies against candidate serotypes of enterovirus vaccines among Korean Children. Viral Immunol. 2021;34:62–67. doi: 10.1089/vim.2020.0073. [DOI] [PubMed] [Google Scholar]
- Klein M., Chong P. Is a multivalent hand, foot, and mouth disease vaccine feasible? Hum. Vaccin. Immunother. 2015;11:2688–2704. doi: 10.1080/21645515.2015.1049780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwang T.W., Zeng X., Wang S. Manufacturing of AcMNPV baculovirus vectors to enable gene therapy trials. Mol. Ther. Methods Clin. Dev. 2016;3:15050. doi: 10.1038/mtm.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le R.A., Jacob D., Transfiguracion J., Ansorge S., Henry O., Kamen A.A. Scalable production of influenza virus in HEK-293 cells for efficient vaccine manufacturing. Vaccine. 2010;28:3661–3671. doi: 10.1016/j.vaccine.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Lien S.C., Lu C.C., Shen Y.S., Yang Y.T., Wu S.R., Fang C.Y., Chow Y.H., Liao C.L., Chiang J.R., Liu C.C. Separation and purification of highly infectious enterovirus A71 particles using a strong anion-exchange column. J. Chromatogr. A. 2022;1680 doi: 10.1016/j.chroma.2022.463427. [DOI] [PubMed] [Google Scholar]
- Lim H., In H.J., Lee J.A., Sik Y.J., Lee S.W., Chung G.T., Choi Y.K., Chung J.K., Cho S.J., Lee J.W. The immunogenicity and protection effect of an inactivated coxsackievirus A6, A10, and A16 vaccine against hand, foot, and mouth disease. Vaccine. 2018;36:3445–3452. doi: 10.1016/j.vaccine.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Wu S.C. Mosquito and mammalian cells grown on microcarriers for four-serotype dengue virus production: variations in virus titer, plaque morphology, and replication rate. Biotechnol. Bioeng. 2004;85:482–488. doi: 10.1002/bit.10918. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Lian W.C., Butler M., Wu S.C. High immunogenic enterovirus 71 strain and its production using serum-free microcarrier Vero cell culture. Vaccine. 2007;25:19–24. doi: 10.1016/j.vaccine.2006.06.083. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Guo M.S., Lin F.H., Hsiao K.N., Chang K.H., Chou A.H., Wang Y.C., Chen Y.C., Yang C.S., Chong P.C. Purification and characterization of enterovirus 71 viral particles produced from vero cells grown in a serum-free microcarrier bioreactor system. PLOS One. 2011;6:e20005. doi: 10.1371/journal.pone.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.C., Chow Y.H., Chong P., Klein M. Prospect and challenges for the development of multivalent vaccines against hand, foot and mouth diseases. Vaccine. 2014;32:6177–6182. doi: 10.1016/j.vaccine.2014.08.064. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Guo M.S., Wu S.R., Lin H.Y., Yang Y.T., Liu W.C., Chow Y.H., Shieh D.B., Wang J.R., Chong P. Immunological and biochemical characterizations of coxsackievirus A6 and A10 viral particles. Antiviral Res. 2016;129:58–66. doi: 10.1016/j.antiviral.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Wu S.C., Wu S.R., Lin H.Y., Guo M.S., Hu A.Y.C., Chow Y.H., Chiang J.R., Shieh D.B., Chong P. Enhancing enterovirus A71 vaccine production yield by microcarrier profusion bioreactor culture. Vaccine. 2018;36:3134–3139. doi: 10.1016/j.vaccine.2017.02.042. [DOI] [PubMed] [Google Scholar]
- Lohr V., Rath A., Genzel Y., Jordan I., Sandig V., Reichl U. New avian suspension cell lines provide production of influenza virus and MVA in serum-free media: studies on growth, metabolism and virus propagation. Vaccine. 2009;27:4975–4982. doi: 10.1016/j.vaccine.2009.05.083. [DOI] [PubMed] [Google Scholar]
- Lu Q.B., Zhang X.A., Wo Y., Xu H.M., Li X.J., Wang X.J., Ding S.J., Chen X.D., He C., Liu L.J., Li H., Yang H., Li T.Y., Liu W., Cao W.C. Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLOS One. 2012;7:e52073. doi: 10.1371/journal.pone.0052073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milián E., Julien T., Biaggio R., Venereo-Sanchez A., Montes J., Manceur A.P., Ansorge S., Petiot E., Rosa-Calatrava M., Kamen A. Accelerated mass production of influenza virus seed stocks in HEK-293 suspension cell cultures by reverse genetics. Vaccine. 2017;35:3423–3430. doi: 10.1016/j.vaccine.2017.04.065. [DOI] [PubMed] [Google Scholar]
- Min N., Ong Y.H.B., Han A.X., Ho S.X., Yen E.W.P., Ban K.H.K., Maurer-Stroh S., Chong C.Y., Chu J.J.H. An epidemiological surveillance of hand foot and mouth disease in paediatric patients and in community: a Singapore retrospective cohort study, 2013-2018. PLOS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0008885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnon B.J., Fanget B., Vincent-Falquet J.C. Industrial-scale production of inactivated poliovirus vaccine prepared by culture of Vero cells on microcarrier. Rev. Infect. Dis. 1984;6:S341–S344. doi: 10.1093/clinids/6.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- Nhan L.N.T., Khanh T.H., Hong N.T.T., Van H.M.T., Nhu L.N.T., Ny N.T.H., Nguyet L.A., Thanh T.T., Anh N.T., Hang V.T.T., Qui P.T., Viet H.L., Tung T.H., Ha D.Q., Tuan H.M., Thwaites G., Chau N.V.V., Thwaites L., Hung N.T., van Doorn H.R., Tan L.V. Clinical, etiological and epidemiological investigations of hand, foot and mouth disease in southern Vietnam during 2015-2018. PLOS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Rubio A., Eiros J.M. Cell culture-derived flu vaccine: present and future. Hum. Vaccin. Immunother. 2018;14:1874–1882. doi: 10.1080/21645515.2018.1460297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prim N., Rodríguez G., Margall N., Del Cuerpo M., Trallero G., Rabella N. Combining cell lines to optimize isolation of human enterovirus from clinical specimens: report of 25 years of experience. J. Med. Virol. 2013;85:116–120. doi: 10.1002/jmv.23426. [DOI] [PubMed] [Google Scholar]
- Shao H.Y., Hsu H.S., Yu S.L., Wu S.R., Hu K.C., Chang C.K., Liu C.C., Chow Y.H. Immunogenicity of an adeno-vector vaccine expressing the F protein of a respiratory syncytial virus manufactured from serum-free suspension culture. Antiviral Res. 2016;130:27–35. doi: 10.1016/j.antiviral.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Gorbalenya A.E., Harvala H., Hovi T., Knowles N.J., Lindberg A.M., Oberste M.S., Palmenberg A.C., Reuter G., Skern T., Tapparel C., Wolthers K.C., Woo P.C.Y., Zell R. Recommendations for the nomenclature of enteroviruses and rhinoviruses. Arch. Virol. 2020;165:793–797. doi: 10.1007/s00705-019-04520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T., Lewthwarte P., Perera D., Cardosa M.J., McMinn P., Ooi M.H. Virology, epidemiology, pathogenesis and control of enterovirus 71. Lancet Infect. Dis. 2010;10:778–790. doi: 10.1016/s1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- Tan E., Chin C.S.H., Lim Z.F.S., Ng S.K. HEK293 cell line as a platform to produce recombinant proteins and viral vectors. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.796991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia F., Vogel T., Genzel Y., Behrendt I., Hirschel M., Gangemi J.D., Reichl U. Production of high-titer human influenza A virus with adherent and suspension MDCK cells cultured in a single-use hollow fiber bioreactor. Vaccine. 2014;32:1003–1011. doi: 10.1016/j.vaccine.2013.11.044. [DOI] [PubMed] [Google Scholar]
- Tsao K.C., Huang C.G., Huang Y.L., Chen F.C., Huang P.N., Huang Y.C., Lin T.Y., Shih S.R., Chang S.C. Epidemiologic features and virus isolation of enteroviruses in Northern Taiwan during 2000-2008. J. Virol. Methods. 2010;165:330–332. doi: 10.1016/j.jviromet.2010.03.001. [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V.A., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E.R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Pérez-Pérez L., Edwards N.J., Wright D., Bissett C., Gilbride C., Williamson B.N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P.W., Scott D., Saturday G., de Wit E., Gilbert S.C., Munster V.J. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlecken D.H., Pelgrim R.P., Ruminski S., Bakker W.A., van der Pol L.A. Comparison of initial feasibility of host cell lines for viral vaccine production. J. Virol. Methods. 2013;193:28–41. doi: 10.1016/j.jviromet.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Wang M., Yan J., Zhu L., Wang M., Liu L., Yu R., Chen M., Xun J., Zhang Y., Yi Z., Zhang S. The establishment of infectious clone and single round infectious particles for Coxsackievirus A10. Virol. Sin. 2020;35:426–435. doi: 10.1007/s12250-020-00198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.Y., Lin Y.W., Kuo C.H., Liu W.H., Tai H.F., Pan C.H., Chen Y.T., Hsiao P.W., Chan C.H., Chang C.C., Liu C.C., Chow Y.H., Chen J.R. Inactivated enterovirus 71 vaccine produced by 200-l scale serum-free microcarrier bioreactor system provides cross-protective efficacy in human scarb2 transgenic mouse. PLOS One. 2015;10 doi: 10.1371/journal.pone.0136420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.C., Liu C.C., Lian W.C. Optimization of microcarrier cell culture process for the inactivated enterovirus type 71 vaccine development. Vaccine. 2004;22:3858–3864. doi: 10.1016/j.vaccine.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Yang Q., Ding J., Cao J., Huang Q., Hong C., Yang B. Epidemiological and etiological characteristics of hand, foot, and mouth disease in Wuhan, China from 2012 to 2013: outbreaks of coxsackieviruses A10. J. Med. Virol. 2015;87:954–960. doi: 10.1002/jmv.24151. [DOI] [PubMed] [Google Scholar]
- Yuan J., Xu W.W., Jiang S., Yu H., Poon H.F. The scattered twelve tribes of HEK293. Biomed. Pharmacol. J. 2018;11:621–623. http://biomedpharmajournal.org/?p=20696 (accessed 18 March 2023) [Google Scholar]
- Zhang J., Xu D., Liu H., Zhang M., Feng C., Cong S., Sun H., Yang Z., Ma S. Characterization of coxsackievirus A10 strains isolated from children with hand, foot, and mouth disease. J. Med. Virol. 2022;94:601–609. doi: 10.1002/jmv.27268. [DOI] [PubMed] [Google Scholar]
- Zhao H., Yang T., Yue L., Li H., Xie T., Xiang H., Wang J., Wei X., Zhang Y., Xie Z. Comparative analysis of the biological characteristics of three CV-A10 clones adaptively cultured on Vero cells. J. Med. Virol. 2022;94:3820–3828. doi: 10.1002/jmv.27796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.