Highlights
-
•
FMDVs have brought great challenges to virus molecular analysis and caused several outbreaks with continuously emerging viruses in Egypt.
-
•
Samples were collected from the most recent FMDV outbreak in the Egyptian bovine in 2022.
-
•
First report on newly emerging FMDV type A of EURO-SA lineage isolated from Egypt.
-
•
Results showed that the Egyptian EURO-SA strain was related to strains detected previously in Venezuela, Brazil, and Colombia.
Abstract
A newly emerging and exotic foot-and-mouth disease virus (FMDV) caused a recent outbreak of serotype A in Egypt in 2022, which affected cattle and water buffalo. Previous phylogenetic studies on FMDV circulating in Egypt have mainly focused on genomic regions encoding the structural proteins which determine FMDV serotype. No study has yet determined structural proteins sequences of the newly emerging Europe-South America (EURO-SA) lineage which was recently isolated from Egypt during a routine surveillance in 2022. The objective of the current study was to analyze the structural proteins of the Venezuelan type which belongs to EURO-SA. The new isolate was related to serotype A lineage Euro-South America. Phylogentic analyses have reveled that the newly isolated lineage samples were closely related to reported sequences that have been identified in Venzuela and Colombia. Analysis of structural protein sequences revealed the recent isolates belong to prototype strain A24 Cruzeiro. Notably, nucleotide sequences of the Egyptian isolate was related to Venezuelan, Brazilian, and Colombian strains with identity not exceeding 90%. The divergence which appears in the genetic identity of the Egyptian A/EURO-SA lineage from other related strains may be attributed to the absence of Euro-SA lineage sequence from Egypt. The present study is the first report on the detection of EURO-SA lineage in Egypt. The recent detection of the EURO-SA lineage samples may be explained due to imported animals from Colombia or Brazil which share geographical borders with Venezuela. The findings of the present study highlight the significance of continuous monitoring of FMDV in Egypt for newly emerging FMDVs.
Background
Foot-and-mouth disease virus (FMDV) caused a highly devastating vesicular disease. It affected both the domestic and wild cloven-hoofed animals causing an infectious and contagious disease. The disease caused enormous global economic losses in the livestock industry (Hussein et al., 2019; Mahajan et al., 2021). FMD is a transboundary animal disease (TAD), affected by international trade movements, especially between endemic countries in Africa, Asia, South America, and the Middle East (Marqués et al., 2019).
FMDV is known for seven well-distinguished serotypes known as serotypes A, O, C, Southern African territories, SAT1, SAT2, SAT3, and Asia-1 (Brown et al., 2022; Liu et al., 2021; Mahajan et al., 2021; Singanallur et al., 2022). The virus conquered Egypt in the 1950s through serotype O. Since then, serotype O is the predominant circulating among the governorates in the country, but other serotypes have stricken the country especially serotype A (Hussein et al., 2019) and serotype SAT2 which caused massive outbreaks in 2012 (Ahmed et al., 2012; Soltan et al., 2019). Serotype A was first recorded in 1967 and 1972 (Vosloo et al., 2002). It's worthy to mention that the serotype-related viruses have the most genetic and antigenic variations among the seven FMDVs and have 3 known topotypes including Africa, Asia, and Europe-South America (Euro-SA) (Audarya, 2020; Jamal & Belsham, 2013). Furthermore, devastating outbreaks attacked many governorates by serotype A of Egyptian and East African origin in 2006 due to the dilemma of live animals trading among the countries (Knowles et al., 2007). Until 2010, serotype A Iran-05, the Asian topotype was circulating among the districts extending to 2015 (Singanallur et al., 2022; Sobhy et al., 2018). Recently, the African topotype emerged in early 2012, and since that time, the A- Africa topotype G-IV was dated in the country and re-emerged in 2016 and 2018 (Sobhy et al., 2018) besides its dominance in early 2020 till now (Hassan, et al., 2022a).
FMDV belongs to genus Aphthovirus of the Picornaviridae family. The virus is a naked, single-stranded, positive-strand polarity RNA virus with approximately 8.5 Kb in length (Zell et al., 2021). The viral genome has four major regions. For instance, the open reading frame region is flanked by untranslated regions (UTR) at the 3̀ and 5̀ terminus and the poly-A tail (Longjam et al., 2011). The ORF encodes viral polyproteins (Leader Protein (Lpro), P1, P2, and P3) that their translation process intitated at two AUG codons spaced by 84 nucleotides (Longjam et al., 2011; Ryan et al., 1989). ORF proteins are co-/post-translation processed by virus-encoded proteinases (Liu et al., 2021; Zell et al., 2021). The P1 region encodes viral structural proteins including VP1, VP2, VP3, and VP4 encoded by 1D, 1B, 1C, and 1A, respectively (Seago et al., 2012). The genomic material is enclosed in an icosahedral capsid, where the building blocks (i.e. pentamers) are comprised of the five copies of heteropolymeric viral proteins (VPs, VP1-4) (Longjam et al., 2011; Seago et al., 2012).
Furthermore, structural proteins exhibit the highest rates of nucleotide and amino acid variability among all viral proteins (likely a response to intense selective pressures) except VP4 since 73% to 84% of its nucleotide sequence is conserved among all FMDV isolates. VP4 carries a swine-specific immunodominant and heterotypic T-cell antigenic site that is capable of providing help to a B-cell epitope when in tandem. (Grubman & Baxt, 2004).
Regarding VP1, the protein consists of 213 residues and is responsible for virus attachment and entry, protective immunity, and serotype specificity (Acharya et al., 1989; Grubman and Baxt, 2004), as it contains two main immunogenic sites in the G-H loop (residues 141–160) and the C-terminus (residues 200–213) (Fry et al., 2005; Lawrence et al., 2013). Whereas the viral protein 2 (VP2) plays a critical role in capsid stability and particle maturation, supported by the observation that 47% of its amino acids are invariant between and among different FMDV serotypes (Grubman and Baxt, 2004). Furthermore, the N-terminus of the VP3 protein of various protomers stimulates the packing of protomers into pentamers (Acharya et al., 1989). Besides, it has also established several important conformational neutralizing epitopes and one T-cell epitope (Bari et al., 2015; Dong et al., 2022).
Moreover, the diagnostic testing is based on the virus isolation using a cell-based method conducted in baby-hamster kidney cells (BHK-21 cell line), and the molecular characterization of VP1 using PCR and amino acids sequence differentiation as the protein has serotype-distinguishable domains (Carrillo et al., 2005; Freimanis et al., 2016). The full genome sequence of FMDV provides full virus imaging regarding the inter-serotypes variability, amino acid difference affecting the antigenic and immunogenic proteins, and the virus virulence, infection rate, and severity (Carrillo et al., 2005; Knowles et al., 2016). Basically, molecular analysis of the whole virus genome especially for the ORF region is required to determine the amino acid sequences different changes which are reflecting the nature of immunogencity, antigenicity, disease prognosis, and spreading of FMDV certain serotypes (Hassan, et al., 2022b).
Likewise, the molecular inspection of the viral capsid proteins of FMDV is considered a full data gathering about the antigenic and genomic determinants of the tested virus serotype. Therefore, the current study is based on the genomic analysis of the capsid proteins (i.e. VP1, VP2, VP3, and VP4) using the single Sanger sequence for the determination of the antigenic determinants of the virus.
Material and methods
Sample processing and Virus isolation
A total of 24 specimens were collected from clinically infected animals (2 vesicular fluids and 22 epithelial tissues) in March 2022. The specimens were prepared according to the guidelines of the World Organization for Animal Health (Bonbon, 2020). Briefly, the epithelial tissue was ground using sterile sand, the sample suspension was clarified at 3000 rpm for 10 minutes at 4°C. The supernatant was aspirated and incubated with 1% of antibiotic solution (i.e. 100 IU/ml penicillin, and 100 mg/ml streptomycin) for 3 hours at room temperature. Afterward, the prepared samples were cultured in virus adapted BHK-21 cell line and the remaining part of the sample suspension was kept at -80°C for further analysis. The prepared samples were inoculated into the virus-adapted cell line (i.e. BHK-21) under restricted biosafety level-2 conditions. The inoculated cells were maintained in 1-2% fetal calf serum-provided minimum essential medium (MEM). The flasks were incubated in a 5% CO2-supplied incubator. The flasks were observed for the viral cytopathogenic effects on the BHK-21 formed monolayer for 18-48 hours. The three successive passages were carried out.
Quantitative Real-time RT-PCR (qRT-PCR) and One-Step Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
The viral genome extraction was carried out using EasyPure viral-RNA kit (TransGen Biotech, Beijing, China). Based on the amplification of the most conserved segment of the FMDV-RNA polymerase gene (3D gene), Quantitative Real-time Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) was carried out for FMDV detection using the pan-FMDV primers/probe set as shown in (Callahan et al., 2002). The partial genomic analysis with the serotype-distinguishable protein, VP1, was performed for FMDV serotyping using three-selective primers for serotypes A, O, and SAT2 as given in (Hassan, et al., 2022a). The procedures were performed using the EasyScript One-Step RT-PCR SuperMix (TransGen, Beijing, China).
Sequencing of capsid coding region
RNA extraction, RT–PCR using six primers sets (Table 1), and DNA Sanger sequencing were performed for the purified PCR product using BigDye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher, USA) according to the manufacturer's instructions. A 3.2 pmol of forward and reverse primers were used in two different reactions. The sequencing product was purified by spinning in Centri-Sep™ Spin Columns (Thermo Fisher, USA) and the electro-kinetic injection on capillary electrophoresis systems 3500 Genetic analyzer (Applied Biosystems, USA).
Table 1.
Primers used for FMDV capsid coding region Sequencing
| Primer set | Primer Designation | Primer sequence (5′-3′) | Annealing Temperature | Amplicon size | Reference |
|---|---|---|---|---|---|
| 1 | FMD-1842-F | GAG GAC TTY TAC CCY TGG AC | 60°C | 1362 bp | (Dill et al., 2017) |
| FMD-3223-R | TGG GHC CWG TGA ACA TGA ART G | ||||
| 2 | FMD-3161-F | TCG CVC AGT ACT ACR CAC AGT A | 60°C | 1143 bp | (Dill et al., 2017) |
| FMD-4303-R | TGA CGT CRG AGA AGA AGA ARG G | ||||
| 3 | FMD-3161-F | TCG CVC AGT ACT ACR CAC AGT A | 52°C | 281 bp | (Dill et al., 2017) |
| A-VP3 3232 R | TTC CCG TGT GTA ATT TGG T | This study | |||
| 4 | A1F | CCG ATC TGG AGA TTG TGG TGC G | 59°C | 524 bp | (Muhammad, 2014) |
| NK72 | GAA GGG CCC AGG GTT GGA CTC | (Knowles, N. J., 1994) | |||
| 5 | A-1C562F | TAC CAA ATT ACA CAC GGG AA | 56.5°C | 789 bp | (Knowles et al., 2007) |
| FMD-4303-R | TGA CGT CRG AGA AGA AGA ARG G | (Dill et al., 2017) | |||
| 6 | FMD-4249-F | GCA GGR GAC GTB GAG TCC AA | 60°C | 943 bp | (Dill et al., 2017) |
| FMD-5191-R | CGT CRA AGT GGT CRG GGT C |
Sequence analysis
Sequences obtained from both directions were assembled and checked for accuracy with SeqMan (Lasergene, version 16; DNASTAR, Inc., WI, USA). Multiple nucleotide and deduced amino acid sequence alignment was performed using Clustal W (Chenna et al., 2003) algorithm in the BioEdit software-version 7.1 (Hall, 1999). The sequences were also independently aligned in MEGA11 software (Tamura et al., 2021) and the rate of nucleotide substitution per site (genetic distance) was estimated in the Megalign module of DNASTAR package. The similarity percentages of the investigated sequences were estimated using the GenBank using the alignment tool of Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Phylogenetic trees were constructed for VP1, VP2, VP3, and VP4 sequences. Phylogenetic trees were inferred using the neighbor-joining approach implemented within MEGA11 software, the topology was estimated by bootstrapping over 1000 replicates (Saitou & Nei, 1987; Tamura et al., 2004, 2021).
Results
Virus isolation
A confluent proliferative monolayer of the Baby-hamster kidney cell line (BHK-21 cell line) was used for virus propagation. Thus, the suspected sample suspension was inoculated (approximately 250µL in 25 mL volume tissue culture flask). Under normal conditions of isolation, cytopathogenic effects (CPE) in the cells were observed promptly 18 hours postinoculation. CPEs were observed in these samples. The flasks were removed directly after the second day of the inoculation. The three successive blind passages were obtained with a mighty CPE. Astonishingly, the BHK-21 cells showed a high sensitive permeability with a prompt propagation rate contrary to the other isolated strains.
FMDV molecular identification and Serotype-specific assay
RNA was extracted from suspected FMDV cases and RNA extracts were tested using the well-established diagnostic qRT-PCR . Results showed that all the tested samples were positive for FMDV targeting part of the 3D coding region within the FMDV genome with Ct values ranging from 18 to 28. The serotype of these positive samples was determined using the serotype-specific RT-PCR targeting the variable region in the (1D) gene as previously described (Hassan et al., 2022b). Results showed that all the samples belonged to serotype A. Five samples were sequenced for molecular characterization of the partial VP1 region and nucleotide sequences were submitted to GenBank under accession numbers (OP131709, OP131710, OP131711, and OP131712). One sample (OP093730) was selected for the complete capsid sequencing and characterization.
The P1 region sequence and encoded capsid protein sequences of FMDV/A
The capsid region of the selected A-serotyped sample was sequenced, and nucleotide sequence was submitted to GenBank (accession number OP093730). The entire P1 sequence of the recent Egyptian FMDV/A isolate was determined in the current study and the corresponding amino acid sequence was deduced. This amino acid sequence was then compared to the corresponding virus sequences, to identify amino acid substitutions and to track the genetic diversification and associated antigenic evolution of the virus. The P1 region contains 2205 nucleotides encoding 735 amino acids . The P1 polyprotein was processed to contain VP1 (212 amino acids), VP2 (218 amino acids), VP3 (221 amino acids), and VP4 (86 amino acids). The pairwise alignment of the complete P1 region showed 80.74% nucleotide identity to the local Egyptian vaccinal strain (accession number KC440882), and revealed various nucleotide and amino acid identities among the different available FMDV/A isolates in the NCBI database. The studied isolate showed the highest pairwise identity at the nucleotide level with the Brazilian strain (accession number MT412356, 86.23%), the Venezuelan strain (accession number MH426566, 86.20%), and the Argentinian strain (accession number AY593786, 82.61%). At the amino acid level, the recent Egyptian isolate showed a higher pairwise identity to the Brazilian strain (accession number MT412356, 90.8), Venezuelan strain (accession number AWU46704, 90.75%), and the Argentinian strain (accession number AAT01729, 90.34%).
Capsid region selection pressure
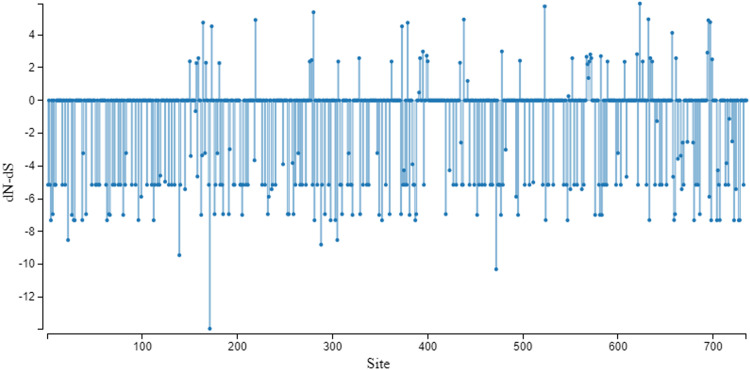
The site selection pressure for the recent FMDV/A capsid region to the prototype strain (accession number AY593768) was measured as nonsynonymous (dN) –synonymous (dS) nucleotide substitutions per site as previously reported (Nei & Gojobori, 1986). The substitution differences were estimated using the approximate method which revealed the number of synonymous substitutions per synonymous site (ds; 0.63), and the number of nonsynonymous substitutions per nonsynonymous site (dn; 0.055). Thus, the number of synonymous substitutions was about 85.3%, nonsynonymous substitutions about 14.7%, and the dn/ds value was 0.087 which reflects positive selection. The selection pressure assessment on the capsid region among the Egyptian isolate (accession number OP093730, the prototype sequence (AY593768), and the Egyptian vaccinal strain (KC440882) using the Single-Likelihood Ancestor Counting (SLAC) method (Sergei et al., 2005) identified 20 codons with 0.0814 nonsynonymous /synonymous rate ratio (Fig. 1).
Fig. 1.
SLAC site graph method in the capsid coding region (P1 region) was used to identify positively and negatively codons/sites.The calculation was averaged over the FMDV-A-Egy-AHRI-RL385-1-Ven-2022 isolate (OP093730), the prototype sequence (AY593768), and the Brazilian strain (MT412356).
Comparison of VP1 amino acid sequences and phylogenetic analysis
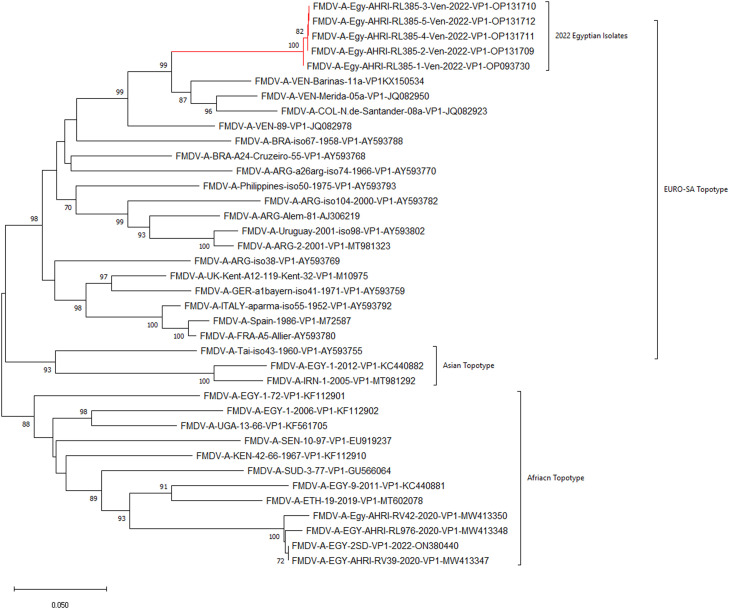
The topology of the VP1 constructed tree (Fig. 2), and with considering a bootstrap value of 1000 replicates, the three different genetic groups of FMDV/A topotypes could be distinguished (African, Asian, and EURO-SA). The tested Egyptian isolates belonged to topotype (EURO-SA) and clustered separately from the latter two topotypes strains that have been circulating in Egypt.
Fig. 2.
VP1 Phylogenic analysis of the recently isolated FMDV strains in Egypt. FMDV serotype A/VP1 coding region-based phylogenetic tree. The tree was reconstructed with the neighbor-joining method with 1,000 bootstrap replicates (shown next to the branches) in MEGA11. Phylogeny showed the relationships between the FMDV serotype A isolates from Egypt (lined with red color lines) and other contemporary viruses.
The nucleotide composition of the Egyptian isolates was 24. 7% (A), 18 % (T), 30.30% (C), and 27% (G) . Comparing the prototype strain of EURO-SA nucleotide sequences to recent Egyptian isolates isolated in 2022 showed that the transitional substitution rate was 66.14 %, and the transversional substitutions rate was 33.86%. The number of amino acid substitutions per site using the Poisson correction model from averaging over the prototype sequence and the Egyptian isolates sequences pairs was 0.06 (Tamura et al., 2021; Zuckerkandl & Pauling, 1965). Divergence average among them and the recent 2020 and 2022 Egyptian isolates (accession number ON380440 and MW413347) which belonged to African topotype/G-IV was 23.2%.
The structural proteins sequences (VP2, VP3, and VP4)
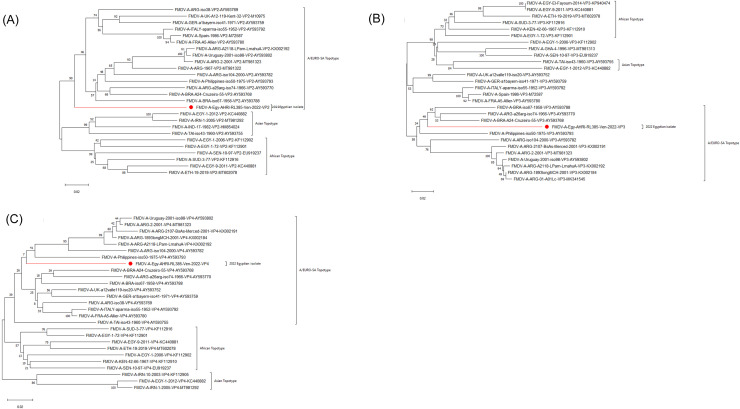
The phylogenetic analyses (Fig. 3) and the pairwise distances (data not shown) showed that the investigated VP2, VP3, and VP4 regions of the recent FMDV type A virus isolates from Egypt belonged to the EURO-SA topotype. The amino acid difference values for VP2 and VP3 regions ranged between 9% and 8.3, the VP4 region showed complete identity with respect to EURO-SA topotype strain.
Fig. 3.
VP2, VP3 and VP4 Phylogenic analysis of the recently isolated FMDV isolate in Egypt. FMDV serotypecoding region-based phylogenetic tree A) VP2, B) VP3 and C) VP4. The tree was reconstructed with the neighbor-joining method with 1,000 bootstrap replicates (shown next to the branches) in MEGA11. Phylogeny showed the relationships between the FMDV serotype A recent isolate from Egypt (accession number OP093730) (lined with red color lines) and other contemporary viruses.
Discussion
FMD is a highly contagious viral disease which has devastating effects on livestock production in Egypt. Multiple epidemic outbreaks occurred in Egypt since the 1950s. Consequently, the Egyptian government has listed FMD as a sovereign and endemic disease. Despite the efforts and measures by the government to eradicate the virus, different FMDV serotypes are circulating and causing outbreaks yearly. During the last decade, the emergence of different lineages of SAT2, O and A serotypes were reported from Egypt. Although these lineages are included in the locally-produced vaccines, FMDV have been evading the vaccine-induced immunity in the Egyptian livestock due to its quasi-species nature.
Animal movements and international trade are risk factors for the emergence of various exotic FMDVs including serotypes and lineages which are not included in vaccination plans. The Egyptian trade relationships were extended during the last decade especially in livestock imports. The trade routes included imports from Sudan, India and South America specifically from Brazil and Colombia, according to a report issued by the USDA in 2021 (Omar et al., 2021). South American countries are FMD-free zones that either with or without the inclusion of vaccination except for Venezuela where there is no official FMD status (OIE, 2021). According to recent reports, Venezuela is still suffering from endemic FMDV infections with no reported notification from Venezuela (Sierra, 2021).
Brazil and Colombia share geographical borders with Venezuela where it endangers the free-status of both countries. Recently, Colombia reported the occurrence of FMD outbreaks in areas near the Venezuelan geographical borders in 2017 and 2018 (COSALFA, 2019). Colombia has regained its FMD-Free status after the successful eradication of previous FMDV infections. However, there is a continued risk that may expose other countries which are in trade relationships with Colombia and Brazil, such as Egypt.
The tested samples in the current study revealed the presence of a new serotype A linage that is closely related to the reference isolate A24 Cruzeiro (accession number AY593768) and more specifically to samples circulating in Venezuela. The structural protein sequence of the tested samples showed close relatedness with samples isolated from the Venezuelan field in 2005 and 2011. To the best of our knowledge, the available genetic data of strains from Venezuela were for the VP1 region in the period between the 1990s and early 2010s. The unknown FMD status of Venezuela and the lack of sufficient FMDV genetic data about Venezuelan strains, is a challenge for the appropriate determination and identification of the original source of the newly emerging Egyptian FMDV isolates reported in the present study.
The virus strains reported in the present study were only detected in a single Egyptian farm. Continuous surveillance and molecular identification of samples collected from nearby locations revealed no infection incidence with the same viral strain identified. A recent study reported on the detection of serotype O, Europe-South America topotype in Egypt (Soltan et al., 2022), however, we haven't detected any FMD virus isolate with the same genetic make-up in the present study. This may be explained due to virus introduction through imported animals. However, we recommend continuous surveillance for the Egyptian field to avoid viral outbreaks and to monitor other newly emerging FMDVs. Inclusion of the newly emerging EURO-SA lineage in locally produced vaccines is highly recommended and will help prevent and contain the spread of FMDV infection to other Egyptian farms and governarates.
Funding
The study was supported by funds from the Animal Health Research Institute, Agriculture Research Center, Egypt.
Ethics statement
Not applicable.
CRediT authorship contribution statement
Naglaa M. Hagag: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition, Supervision. Ayah M. Hassan: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, critical proof revisions. Mostafa R. Zaher: Validation, Writing – original draft, Writing – review & editing. Sara M. Elnomrosy: Investigation, Methodology. Omayma A. Shemies: Investigation, Methodology. Heba A. Hussein: Investigation, Methodology, Writing – review & editing. Eman S. Ahmed: Investigation, Methodology. Mohamed H. Ali: Investigation, Methodology. Mohamed Ateay: Investigation, Methodology. Mahmoud A. Abdel-Hakim: Investigation, Methodology. Ahmed R. Habashi: Investigation, Methodology. Samah Eid: Investigation, Methodology, Writing – review & editing. Mohamed E. El Zowalaty: Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing, critical proof revisions, Supervision. Momtaz A. Shahein: Supervision, Methodology, Project administration.
Declaration of Competing Interest
None to declare.
Acknowledgment
Authors would like to thank the veterinarians and farm owners for their help and cooperation during the collection of the samples used in the present study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2022.198960.
Contributor Information
Naglaa M. Hagag, Email: naglaahagagahri@gmail.com.
Mohamed E. El Zowalaty, Email: elzow005@gmail.com.
Appendix. Supplementary materials
Data availability
References
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 Å resolution. Nature. 1989;337(6209):709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- Ahmed H.A., Salem S.A.H., Habashi A.R., Arafa A.A., Aggour M.G.A., Salem G.H., Gaber A.S., Selem O., Abdelkader S.H., Knowles N.J. Emergence of foot-and-mouth disease virus SAT 2 in Egypt During 2012. Transbound. Emerg. Dis. 2012;59(6):476–481. doi: 10.1111/tbed.12015. [DOI] [PubMed] [Google Scholar]
- Audarya S.D. In: Some RNA Viruses. Shah Y., Abuelzein E., editors. IntechOpen.; 2020. Foot-and-mouth disease in India: past, present and future outlook-a review. [DOI] [Google Scholar]
- Bari F.D., Parida S., Asfor A.S., Haydon D.T., Reeve R., Paton D.J., Mahapatra M. Prediction and characterization of novel epitopes of serotype A foot-and-mouth disease viruses circulating in East Africa using site-directed mutagenesis. J. General Virol. 2015;96(5):1033–1041. doi: 10.1099/vir.0.000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonbon E. A framework for national official assurance systems with reference to World Organisation for Animal Health standards. Revue Scientifique et Technique (International Office of Epizootics) 2020;39(1):193–200. doi: 10.20506/rst.39.1.3072. [DOI] [PubMed] [Google Scholar]
- Brown E., Nelson N., Gubbins S., Colenutt C. Airborne transmission of foot-and-mouth disease virus: a review of past and present perspectives. Viruses. 2022;14(5):1009. doi: 10.3390/v14051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan J.D., Brown F., Osorio F.A., Sur J.H., Kramer E., Long G.W., Lubroth J., Ellis S.J., Shoulars K.S., Gaffney K.L. Use of a portable real-time reverse transcriptasepolymerase chain reaction assay for rapid detection of foot-and-mouth disease virus. J. Am. Vet. Med. Assoc. 2002;220(11):1636–1642. doi: 10.2460/javma.2002.220.1636. [DOI] [PubMed] [Google Scholar]
- Carrillo C., Tulman E.R., Delhon G., Lu Z., Carreno A., Vagnozzi A., Kutish G.F., Rock D.L. Comparative genomics of foot-and-mouth disease virus. J. Virol. 2005;79(10):6487–6504. doi: 10.1128/JVI.79.10.6487-6504.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna R., Sugawara H., Koike T., Lopez R., Gibson T.J., Higgins D.G., Thompson J.D. Multiple sequence alignment with the Clustal series of programs. Nucl. Acids Res. 2003;31(13):3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill V., Beer M., Hoffmann B. Simple, quick and cost-efficient: A universal RT-PCR and sequencing strategy for genomic characterisation of foot-and-mouth disease viruses. J. Virol. Methods. 2017;246:58–64. doi: 10.1016/j.jviromet.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Dong H., Liu P., Bai M., Wang K., Feng R., Zhu D., Sun Y., Mu S., Li H., Harmsen M., Sun S., Wang X., Guo H. Vol. 13. Higher Education Press Limited Company; 2022. Structural and molecular basis for foot-and-mouth disease virus neutralization by two potent protective antibodies; pp. 446–453. (Protein and Cell). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimanis G.L., Di Nardo A., Bankowska K., King D.J., Wadsworth J., Knowles N.J., King D.P. Genomics and outbreaks: foot-and-mouth disease. Rev. Sci. Tech. 2016;35(1):175–189. doi: 10.20506/rst.35.1.2426. [DOI] [PubMed] [Google Scholar]
- Fry E.E., Stuart D.I., Rowlands D.J. Vol. 288. Springer Verlag; 2005. The structure of foot-and-mouth disease virus; pp. 71–101. (Current Topics in Microbiology and Immunology). [DOI] [PubMed] [Google Scholar]
- Grubman M.J., Baxt B. Vol. 17. American Society for Microbiology (ASM); 2004. Foot-and-Mouth Disease; pp. 465–493. (Clinical Microbiology Reviews). [DOI] [Google Scholar]
- Hall, T.A. (1999). BIOEDIT: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser., 41 (1999), pp. 95-98. Available at: https://scholar.google.com/scholar?q=BioEdit:%20a%20user-friendly%20biological%20sequence%20alignment%20editor%20and%20analysis%20program%20for%20Windows%209598NT.
- Hassan A.M., El-Mayet F.S., El-Habbaa A.S., Shahein M.A., El Zowalaty M.E., Hagag N.M., Sharawi S.S.A. Molecular characterization of newly emerging foot-and-mouth disease virus serotype SAT 2 of Lib-12 Lineage Isolated from Egypt. Virus Res. 2022;311 doi: 10.1016/j.virusres.2021.198651. [DOI] [PubMed] [Google Scholar]
- Hassan A.M., Zaher M.R., Hassanien R.T., Abd-El-Moniem M.I., Habashi A.R., Ibraheem E.M., Shahein M.A., El Zowalaty M.E., Hagag N.M. Molecular detection, phylogenetic analysis and genetic diversity of recently isolated foot-and-mouth disease virus serotype A African topotype, Genotype IV. Virol. J. 2022;19(1) doi: 10.1186/S12985-021-01693-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein H.A., Hassan R.Y.A., El Nashar R.M., Khalil S.A., Salem S.A., El-Sherbiny I.M. Designing and fabrication of new VIP biosensor for the rapid and selective detection of foot-and-mouth disease virus (FMDV) Biosens. Bioelectr. 2019;141 doi: 10.1016/j.bios.2019.111467. [DOI] [PubMed] [Google Scholar]
- Jamal S.M., Belsham G.J. Foot-and-mouth disease: past, present and future. Vet. Res. 2013;44(1):1–14. doi: 10.1186/1297-9716-44-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N.J., Samuel A.R. Report of the Session of the Research Group of the Standing Technical Committee of the European Commission for the control of foot-and-mouth disease. Vienna, Austria. Food and Agriculture Organization of the United Nation; Rome, Italy, Appendix 8: 1994. Polymerase chain reaction amplification and cycle sequencing of 1D (VP1) gene of foot-and-mouth disease viruses; pp. 45–53. [Google Scholar]; https://www.fao.org/3/ca9413en/ca9413en.pdf.
- Knowles N.J., Wadsworth J., Bachanek-Bankowska K., King D.P. VP1 sequencing protocol for foot-and-mouth disease virus molecular epidemiology. Rev. Sci. Tech. 2016;35(3):741–755. doi: 10.20506/rst.35.3.2565. [DOI] [PubMed] [Google Scholar]
- Knowles, N. J., Wadsworth, J., Reid, S. M., Swabey, K. G., El-Kholy, A. A., Abd El-Rahman, A. O., Soliman, H. M., Ebert, K., Ferris, N. P., Hutchings, G. H., Statham, R. J., King, D. P., Paton, D. J., El-Rahman, A. O. A., Soliman, H. M., Ebert, K., Ferris, N. P., Hutchings, G. H., Statham, R. J., … Paton, D. J. (2007). Foot-and-mouth disease virus serotype A in Egypt. 13(10). doi: 10.3201/eid1310.070252. [DOI] [PMC free article] [PubMed]
- Lawrence P., Larocco M., Baxt B., Rieder E. Examination of soluble integrin resistant mutants of foot-and-mouth disease virus. Virol. J. 2013;10 doi: 10.1186/1743-422X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Xue Q., Zhu Z., Yang F., Cao W., Liu X., Zheng H. Foot-and-mouth disease virus inhibits RIP2 protein expression to promote viral replication. Virol. Sin. 2021;36(4):608–622. doi: 10.1007/s12250-020-00322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longjam N., Deb R., Sarmah A.K., Tayo T., Awachat V.B., Saxena V.K. A brief review on diagnosis of foot-and-mouth disease of livestock: conventional to molecular tools. Vet. Med. Int. 2011;2011 doi: 10.4061/2011/905768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S., Sharma G.K., Subramaniam S., Biswal J.K., Pattnaik B. Selective isolation of foot-and-mouth disease virus from coinfected samples containing more than one serotype. Braz. J. Microbiol. 2021;52(4):2447–2454. doi: 10.1007/s42770-021-00604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués F.J., Battistessa E.I., Peek S.F., Raabis S.M., Darien B.J. The effect of foot-and-mouth disease vaccination on early pregnancy loss in beef heifers in Argentina. Prevent. Vet. Med. 2019;170 doi: 10.1016/j.prevetmed.2019.104716. [DOI] [PubMed] [Google Scholar]
- Muhammad I. Development and optimization of multiplex PCR for the identification of A, O and Asia-1 serotypes of FMDV in Pakistan. J. Inf. Mol. Biol. 2014;2(1):11–15. doi: 10.14737/JIMB.2307-5465/2.1.11.15. [DOI] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- OIE . World Organization for Animal Health; Paris, France: 2021. Recognition of the Foot-and-mouth disease Status of Members. [Google Scholar]; Available at: https://www.woah.org/app/uploads/2021/05/a-r13-2021-fmd.pdf
- Omar S., Mello E., Seifarth K. 2021. Livestock and Products Annual. [Google Scholar]; Available at: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Livestock%20and%20Products%20Annual_Cairo_Egypt_09-01-2021.pdf
- Ryan M.D., Belsham G.J., King A.M.Q. Specificity of enzyme-substrate interactions in foot-and-mouth disease virus polyprotein processing. Virology. 1989;173(1):35–45. doi: 10.1016/0042-6822(89)90219-5. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4(4):406–425. doi: 10.1093/OXFORDJOURNALS.MOLBEV.A040454. [DOI] [PubMed] [Google Scholar]
- Seago J., Jackson T., Doel C., Fry E., Stuart D., Harmsen M.M., Charleston B., Juleff N. Characterization of epitope-tagged foot-and-mouth disease virus. J. General Virol. 2012;93(Pt 11):2371. doi: 10.1099/vir.0.043521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergei L. Kosakovsky Pond, Simon D.W. Frost. 2005, Not so different after all: a comparison of methods for detecting amino acid sites under selection, Mol. Biol. Evol. 22(5), 1208–1222, doi: 10.1093/molbev/msi105. [DOI] [PubMed]
- Sierra D.L. 2021. 45 th ORDINARY MEETING OF THE SOUTH AMERICAN COMMISSION FOR THE FIGHT AGAINST FOOT-AND-MOUTH DISEASE 45th COSALFA RESOLUTIONS. [Google Scholar]; https://iris.paho.org/bitstream/handle/10665.2/50443/resolutionsCOSALFA45_eng.pdf#:~:text=45thORDINARY%20MEETING%20OF%20THE%20SOUTH%20AMERICAN%20COMMISSION%20FORT,la%20Sierra%20-%20Bolivia%20%7C%2020%20April%202018.
- Singanallur N.B., Eblé P.L., Ludi A.B., Statham B., Bin-Tarif A., King D.P., Dekker A., Vosloo W. A vaccine based on the A/ASIA/G-VII lineage of foot-and-mouth disease virus offers low levels of protection against circulating viruses from the A/ASIA/Iran-05 lineage. Viruses. 2022;14(1):97. doi: 10.3390/v14010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhy N.M., Bayoumi Y.H., Mor S.K., El-Zahar H.I., Goyal S.M. Outbreaks of foot-and-mouth disease in Egypt: Molecular epidemiology, evolution and cardiac biomarkers prognostic significance. Int. J. Vet. Sci. Med. 2018;6(1):22–30. doi: 10.1016/j.ijvsm.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltan M.A., Dohreig R.M.A., Abbas H., Ellawa M., Yousif I., Aly A.E., Wasfy M., El-Sayed M.M. Emergence of Foot-and-mouth disease virus, Lib 12 lineage of topotype VII, serotype SAT 2 in Egypt, 2018. Transbound. Emerg. Dis. 2019 doi: 10.1111/tbed.13152. [DOI] [PubMed] [Google Scholar]
- Soltan M.A., Mahmoud M.M., Hegazy Y., Abd-Eldiam M.M. Emergence of foot and mouth disease virus, serotype O, Europe-South America topotype in Egypt, 2022. Transbound. Emerg. Dis. 2022 doi: 10.1111/TBED.14612. [DOI] [PubMed] [Google Scholar]
- South American Commission for the fight Against Foot-and-Mouth Disease (COSALFA), 2019. Cartagena, Colombia. The Pan American Center for Foot-and-Mouth Disease and Veterinary Public Health, Pan American Health Organization, Washington, DC, USA. https://iris.paho.org/handle/10665.2/51363. Accessed (5 May, 2022).
- Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. 2004;101(30):11030–11035. doi: 10.1073/PNAS.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38(7):3022–3027. doi: 10.1093/MOLBEV/MSAB120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosloo W., Bastos A.D.S., Sangare O., Hargreaves S.K., Thomson G.R. Review of the status and control of foot-and-mouth disease in sub-Saharan Africa. Revue Scientifique et Technique-Office International Des Épizooties. 2002;21(3):437–445. doi: 10.20506/rst.21.3.1349. [DOI] [PubMed] [Google Scholar]
- Zell R., Knowles N.J., Simmonds P. A proposed division of the family Picornaviridae into subfamilies based on phylogenetic relationships and functional genomic organization. Arch. Virol. 2021;166(10):2927–2935. doi: 10.1007/s00705-021-05178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerkandl E., Pauling L. Evolutionary Divergence and Convergence in Proteins. Evol. Genes Proteins. 1965:97–166. doi: 10.1016/B978-1-4832-2734-4.50017-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.