Abstract
Objective
To explore parents' self-reported experiences and information needs regarding recognition and management of pediatric anaphylaxis.
Methods
We searched Ovid Medline, Ovid PsychInfo, CINAHL Plus, the Cochrane Library, and grey literature to identify primary studies in English or French published since 2000. We used a mixed-method appraisal tool and convergent integrated approach to assess quality and synthesize data, respectively.
Results
43 studies were included (22 quantitative, 19 qualitative, and 2 mixed-method); 77% of studies had high methodological quality. Parents' experiences were categorized as: recognizing an anaphylactic reaction; managing and responding to a reaction; emotional impact of caring for a child at risk of anaphylaxis; and interaction with the health system and healthcare providers. Parents' information needs were categorized into themes relating to: gaps in knowledge and information; type of information desired; information sources; and information delivery format.
Conclusion
Negative emotional experiences and a general lack of information were commonly reported by parents of included studies. Provision of relevant and comprehensible information may help parents to make informed decisions and manage reactions promptly.
Innovation
The findings of this review are guiding the development of an innovative knowledge translation tool (KT) as part of a larger initiative of developing a suite of parent-focused KT tools for acute childhood conditions.
Keywords: Anaphylaxis, Child, Parent experience, Information need, Systematic review
1. Introduction
An anaphylactic reaction, or anaphylaxis, is a severe hypersensitivity reaction that is rapid in onset and can be potentially fatal [1,2]. Globally, the incidence of anaphylaxis and its related hospitalization rate has increased over recent years [3,4], with children and younger age groups being at a disproportionately increased risk of hospitalization and emergency department (ED) visits [5,6].
The etiology of anaphylaxis varies by region depending on different allergens present in the community, however, the reaction is most commonly caused by food, medication, and insect venom [7]. In some cases, the cause is un-identified and a combination of causes, including allergens, infection, strenuous physical activity, and psychological distress, is believed to trigger the reaction [2]. Food, including peanuts and tree nuts, has been identified as the most common trigger of allergic reaction in infants and children [8,9]. Consequently, recurrence and severe outcomes of anaphylaxis are potentially avoidable, and in this context, prompt recognition and management of anaphylaxis could be vital to prevent fatality [10]. Yet, management of anaphylaxis is compounded by the fact that the reactions can be severe and unexpected, and the symptoms can be non-specific [10,11]. This can place a heavy burden on those affected, their caregivers and families. The constant vigilance, continuous education of others (caregivers, teachers) and lifestyle restrictions required to keep the child safe can cause enormous emotional distress for families, and can negatively impacts the child and parents' quality of life [7,[12], [13], [14]].
Parents can play a crucial role in the prevention, early detection, and successful management of anaphylaxis. However, many factors including poor parental health literacy and conflicting information in practice guidelines may pose a challenge to acquisition of knowledge, adherence to healthcare recommendations, and management of acute reactions, leading to unnecessary healthcare utilization [10,15,16]. A comprehensive understanding of parents' experiences and information needs is therefore an essential step in establishing strategic decision-making pathways, improving quality of care, and meeting parents' care expectations [[17], [18], [19]]. Additionally, this information could be used to develop educational and knowledge translation (KT) resources to support parents. Such tools are becoming increasingly important for narrowing the gap between research and action in relation to parents managing their child's health [20]. Tailored KT tools and strategies developed with input from the target audience have been found to facilitate a higher uptake of knowledge to inform health-decision making, and showing large-to-moderate impact on care delivery [21,22].
With this in mind, we aim to develop KT tools (i.e., infographics, educational videos) to help parents of children at risk of anaphylaxis respond to acute events efficiently. To inform the development of these KT products, we conducted a systematic review to synthesize the available evidence on parents' self-reported experiences and information needs related to the management and prevention of their child's anaphylaxis.
2. Material and methods
We conducted a mixed studies systematic review [23] following an a priori protocol (https://osf.io/dpyns), and reported the review following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [24]. The PRISMA checklist and deviations from the protocol with justification are available in Appendix A.
2.1. Search strategy and study selection
A health research librarian developed and implemented the search, which was peer reviewed by a second librarian [25]. We combined subject headings and keywords for concepts related to anaphylaxis, hypersensitivity, children, parents, experiences, and information needs (complete search strategy in Appendix B). In May 2020, we conducted a comprehensive search of Ovid Medline, Ovid PsychInfo, CINAHL Plus via EBSCOhost, and the Cochrane Library for relevant studies. We limited our search to studies published in English or French and in January 2000 onwards. These limits were imposed to identify studies applicable to the Canadian/North American context and to ensure representativeness of current practice guidelines and information seeking behaviors of parents. We also searched ProQuest Dissertations and Theses Global to locate relevant grey literature. We manually scanned the reference lists of relevant reviews and overviews to identify any additional studies that were not captured by our search. After excluding duplicates, search results were exported into a structured Excel workbook for screening. Two reviewers independently screened titles and abstracts followed by full texts of studies marked as ‘include/unsure’. Any disagreements were resolved via consensus or deliberation with a third reviewer [26].
2.2. Eligibility criteria
We included primary research studies if they met the eligibility criteria outlined in Table 1. The term “parent” included familial caregivers, representing mother/father, grandparent, uncle/aunt and any other individual living in the same household as the child, who acted as their guardian and was responsible for looking after the child. To conceptualize experiences and information needs, we adopted the definitions used previously by our research team in similar published reviews [[27], [28], [29], [30]]. We defined experiences as how parents felt (e.g. anxious, frightened, confident) and acted (e.g. panicked, supportive, in-control) before, during, or after their child had an anaphylactic reaction; and information needs as the type, content or topic, quantity, frequency, and mode of delivery of information that parents required or desired to receive in order to understand, prevent, and manage anaphylaxis in their child.
Table 1.
Inclusion and exclusion criteria used for study selection.
| Characteristics | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study design | Primary studies of any design: quantitative, qualitative, mixed-method | |
| Population | Parents, familial caregivers, or legal guardians of children (0−18 years of age) who have experienced at least one anaphylaxis-related event |
|
| Outcome | Parents' self-reported experiencesc and/or information needsd related to the management of their child's acute anaphylaxis |
|
| Publication date | January 2000 and onwarde | Prior to January 2000 |
| Language | English or French | Any other language |
Reference lists were searched to identify relevant studies that were not captured in our literature search.
We included baseline data related to parents' experiences and/or information needs from intervention studies (e.g. educational interventions, behavioral modification interventions) that aimed to influence parents' management practices in relation to pediatric anaphylaxis.
Referred to how parents felt or acted before, during, and after an acute anaphylaxis event in their child, including their perspective, decision-making, compliance, seeking and accepting healthcare, and quality of life as a result of their child's anaphylaxis condition.
Referred to parents' opinions, beliefs, misconception, preferences, and information-seeking behavior.
To ensure the studies are relevant to current disease management and healthcare practices.
2.3. Data extraction and management
We extracted study characteristics (author, publication year, country, study design and objective, funding source); parents and children characteristics (sample size, recruitment setting, sociodemographic characteristics); severity and frequency of anaphylaxis; reported outcomes related to parents' experiences and information needs. Data was extracted by one reviewer and verified by a second reviewer. Any disagreements were resolved by discussion.
Relevant quantitative data (e.g., data-based outcomes of descriptive statistical test) were extracted directly into a structured data extraction Excel form. Relevant qualitative data (e.g. participant quotes or authors' reported data in ‘findings’ or ‘results’ section of the studies, excluding authors' interpretations) were extracted verbatim into NVivo data management software (v. 10, QSR International, Melbourne, Australia) by one reviewer and verified by a second reviewer.
2.4. Quality assessment
We used the Mixed-method Appraisal Tool (MMAT) version 2018 to assess the methodological quality of the studies [31,32]. Two reviewers assessed the quality of each study independently; any disagreement was resolved via discussion, until full agreement was reached. An overall quality score for each study was obtained by summing up the ‘yes’ responses for that study [33]. For mixed-method studies, the overall score was calculated based on the ‘yes’ responses in the lowest rated component. We did not exclude any studies based on their methodological quality, however, the quality of studies was taken into consideration when interpreting the findings.
2.5. Data synthesis
We used a convergent integrated approach to synthesize quantitative and qualitative data simultaneously [23]. This approach, recommended for the conduct of mixed-studies systematic reviews, requires transforming data into a “mutually compatible format” for integration [23]. Quantitative data were ‘qualitized’ i.e. transformed into texts or narrative descriptions. Qualitative data were analyzed thematically [34] using the NVivo software; one reviewer coded the qualitative data inductively by applying one or more codes to each line of text according to its meaning and content. The codes were then categorized into themes and sub-themes. This was repeated until no additional themes emerged from the data. A second reviewer verified all preliminary codes; any differences in interpretation were resolved via discussion or consultation with a third reviewer. Emergent themes and sub-themes were then finalized through iterative discussion with the review team.
To create a set of integrated findings, the ‘qualitized’ and qualitative data with similar meanings and contents were then pooled together, where possible. Where pooling was not possible or meaningful, the sub-themes were generated based on one data format, and the other format was used to complement the findings. Throughout the integration process, the ‘qualitized’ and qualitative data were constantly compared until no new themes emerged. An independent reviewer experienced in quantitative and qualitative methods reviewed and verified the final integrated themes and sub-themes.
3. Results
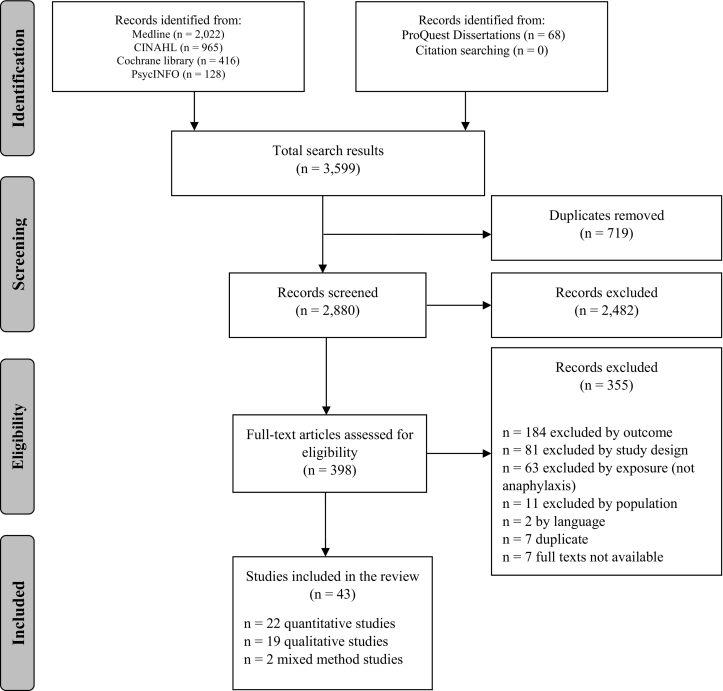
We identified 3599 unique records; 43 records met our inclusion criteria (Fig. 1). Table 2 and Appendix Table B.1 provide an overview and detailed characteristics of the included studies, respectively. In summary, 22 (51%) studies were quantitative, 19 (44%) qualitative, and two (5%) mixed-method. The median publication year was 2013, and more than half of the studies (n = 23, 53%) were conducted in USA and Canada. Overall, 77% (n = 33) of studies were of high methodological quality (i.e., MMAT score ≥ 80%) (Fig. 2 and Appendix Table B.2). Included studies represented a total parent population of 5235. Where reported, parents' mean age was 38 years (range: 23-65), and the majority (93%) of participants were mothers.
Fig. 1.
Study selection flow diagram. (From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. https://doi.org/10.1371/journal.pmed1000097.)
Table 2.
Overview of characteristics of the studies included in the review (n = 43).
| Study design, n (%) | Country, n (%) | Sample size across studies, mean (range) | Population a | Setting, n (%) | Data collection method, n (%) |
|---|---|---|---|---|---|
|
|
|
|
Numbers are based on studies that reported their sample size. One study included both parents, but did not report the number of mothers and fathers separately (Klinnert, 2015); another study involved female caregivers and fathers, but did not specify how the female caregivers were related to the child (Song, 2018).
One study included English-speaking participants from multiple countries (USA, UK, and Canada) using online recruitment (Broome, 2015).
Included Croatia, Germany, Iran, Japan, Netherlands, Sweden, and Turkey.
Including qualitative part of the mixed-method studies.
Number of children was not reported in thirteen studies.
E.g., allergy clinics, pediatric offices, hospitals and emergency departments.
E.g., participants home or workplace, community events, allergy campaigns, support groups, and schools.
Healthcare and community settings.
Fig. 2.
Summary of quality assessment of the studies included in the review using Mixed-method Appraisal tool (MMAT). Each bar represents the number of studies with corresponding MMAT percentage score (x-axis). Values on bars represent the number of studies in each design category with the corresponding MMAT percentage.
We grouped results based on our two main outcomes of interest. Table 3, Table 4 provide a summary of the integrated findings. The main themes are explored below:
Table 3.
Summary of integrated findings related to parents' experiences.
| Integrated findinga | Contributing studies n Sample size across the studies N |
Qualitative data example | Qualitized datab example |
|---|---|---|---|
| 1. Recognizing an anaphylactic reaction | Qualitative studies = 8 Quantitative studies = 1 N = 363 |
Hu 2005 (34): “…The other day he started to rub his eyes and sure enough we had just eaten a peanut butter sandwich, so for the next half hour I was constantly watching him…”➔ Able to recognize Vargas 2011 (23): “…And for the first 1 ½ year. I didn't know what my daughter [had], I didn't have Epi pens at home because I just didn't get it… I just didn't know the symptoms…”➔ Unable to recognize |
Teoh 2016 (14): Parents with greater perceived severity of child's food allergy were able to recognize anaphylaxis.➔Able to recognize |
| 2. Managing and responding to an anaphylactic reaction | |||
| 2.1. Ability to respond and take action quickly | Qualitative studies = 2 Quantitative studies = 3 N = 181 (sample size not reported in one study) |
Chooniedass 2018 (40): “…things are going to go into hyper overdrive and eventually shut down… You're in a race for time to make sure that things get fixed properly in a timely fashion. (M2).”➔ Acting quickly Munoz 2014 (33): “…So we rushed to the drugstore got him some Benadryl [and] took him in [to doctor's office].”➔ Acting quickly |
Herbert 2013 (8): Approximately two-thirds of the interviewed mothers carried an EpiPen with them and more than 10% had used one on their child at least once. ➔ Competency Warren 2015 (16): Mothers and fathers responded differently to questions related to how they would handle a problem arising with their allergic child, knowing what to do when problems arise, believing that they can solve the problem when it happens, they can calmly handle a crisis situation involving the child, and knowing what steps to take when the child is having a reaction. ➔ Competency |
| 2.2 Hesitation to act | Qualitative studies = 7 Quantitative studies = 2 N = 318 |
Chooniedass 2018 (40): “We should have given the EpiPen, but it was the fear of ‘I'm going to make this worse for my child.’ Like he's already dealing with the struggling… we just need to get him to the hospital because I don't want to hurt him more.” ➔ Hesitant Munoz 2014 (33): “Even though Jonathon assesses that Katy's allergic reaction was probably anaphylaxis, he still says that he would not give the Epipen if he were to be in the same situation again. He prioritizes her well-being and diffusing the stress of the allergic reaction over the medical concerns that Dr. Zawadzi raises.” ➔ Hesitant |
Kim 2005 (9): Parents reported of not having administered epinephrine injection although they were told by their physician they should have used it in a prior situation. ➔ Hesitant Topal 2013 (15): More than half of the parents who did not have a prescribed epinephrine injection believed the injection was no longer needed.➔ Hesitant |
| 2.3. Confidence using epinephrine injection | Qualitative studies = 7 Quantitative studies = 3 N = 1509 |
Graceffo 2008 (39): “The third participant that was comfortable administering an epinephrine shot was a mother that had at the time of the interview already administered several epinephrine shots to her son for what she felt were severe reactions.”➔ Comfortable administering the injection Chooniedass 2018 (40): “Doubt. Self-doubt; do I, am I doing the right thing? Am I over-reacting? Am I under-reacting?…I was questioning myself while I'm preparing to give it. (F2)”➔ Lack of confidence to administer the injection |
Herbert 2013 (8): Majority of mothers reported being ready to manage a reaction by carrying an epinephrine injection with them at all times.➔ Comfortable to administer the injection Chad 2013 (5): More than half of the parents reported being afraid/somewhat afraid to use epinephrine injection.➔ Lack of confidence to administer the injection |
| 2.4. Lessons learned | Qualitative studies = 4 N = 54 |
Dobbin 2019 (32): “I learned the hard way to call an ambulance to go to the hospital because one of his reactions when he was about 5, I gave him the epinephrine and ummm he was having a very severe reaction that time and I gave him the epinephrine and decided to get in the car and drive myself…”➔ Learn what to do Chooniedass 2018 (40): “Always think anaphylaxis even if there's been a long delay between a potential exposure, even if you haven't seen it. Because if you don't see it, your tendency is to figure out what's wrong and I've been caught in that trap so many times where you're trying to figure out was there an exposure? …Rather than just recognize the symptoms and respond appropriately. (F2)”➔ Learn to recognize |
None |
| 3. Emotional impact of caring for a child with anaphylaxis | |||
| 3.1. Anxiety and panic | Qualitative studies = 4 Quantitative studies = 11 Mixed method = 1 N = 1546 (sample size not reported in two quantitative studies) |
Gallagher 2011 (27): “I don't want the pen, I don't want the pen,’ she just kept screaming … there was no way that I could have safely gave her the EpiPen in the situation. There was too much panic, she was hysterical, I was kind of hysterical myself, not that I was screaming or anything, you know, but I thought If I give her this am I going to hurt her or am I going to put it in a vein, …”➔ Panic during a reaction Abdurrahman 2013 (42): “Nearly all participants (94%) experienced anxiety after their child's first reaction: “The frightening part was what could have been. And so that was a shock to the system, that I had nightmares for weeks on end after that…”➔ Anxiety after a reaction |
Song 2018 (12): Some parents reported being too nervous that they were not able to use an EAI properly.➔Panic during a reaction Ackerman 2008 (17): “Parents reported experiencing significantly higher levels of stress if they had a child with symptoms associated with anaphylaxis than parents of children without symptoms associated with anaphylaxis.”➔ Anxiety before a reaction |
| 3.2. Fear and trauma | Qualitative studies = 14 Quantitative studies = 2 Mixed method = 1 N = 1551 |
Rouf 2012 (35): “Horrible, horrible, thought she was going to die (…) I was just watching her to see if she was breathing (…) it was horrible. (Rebecca,5:74).”➔ Traumatic Munooz 2014 (33): “I was away out shopping or something, and [his dad] gave him the bottle, and he immediately passed out, stopped breathing… [His dad] called me; I met them at the hospital, but I didn't know if he was alive or dead sort of … So that was very traumatic.”➔ Traumatic |
Springston 2010 (13): Parents of children with a recent allergic event were frightened that the child would have a severe reaction.➔ Fear Fedele 2016 (7): Compared to Balanced Responders, Anxious High Responder mothers rated the likelihood of their child dying due to anaphylaxis significantly higher.➔ Fear |
| 3.3. Emotional burden, regret, and guilt | Qualitative studies = 3 Quantitative studies = 8 N = 1770 (sample size not reported in one quantitative study) |
Carstensen 2018 (37): “My husband and I are vigilant about everything that we give Nancy. We do read the back of everything but we made a mistake once that left us feeling embarrassed and disgusted in ourselves, and the reality is it will probably happen again in the future.”➔ Guilt Graceffo 2008 (39): “Several mothers mentioned during their interviews that they felt that they should have administered epinephrine for a reaction in the past and did not. They go on explain that they have really beat themselves up and blame themselves for not giving the medicine when they felt that their child probably needed it.”➔ Regret of failing to manage a reaction |
Fathi 2016 (6): Majority (~70%) of the parents in the survey were moderately to extremely troubled with the worry that they will not be able to help their child if they had an allergic reaction.➔ Emotional burden Allen 2015 (2): Parental burden was significantly greater in parents who reported administering an epinephrine injection to their child.➔ Burden of treatment |
| 4. Interaction with health system and HCPs | |||
| 4.1. Frustration | Qualitative studies = 5 Quantitative = 1 Mixed method = 1 N = 309 |
Akeson 2007 (25): “Nobody told us. We actually had to ask what happened to him … Nobody came around and said, ‘Well, we think he has had an anaphylactic reaction’. Anaphylaxis was never mentioned…I said ‘Is there anything we should avoid or anything we should do, does he need any medicine’. And they said ‘No, just carry on as before’.”➔ Frustrated with HCP's competency Chooniedass 2018 (40): “I know from that first time when we got to the hospital and they were like why didn't you give the EpiPen? And they were basically like yelling at us.”➔ Feeling berated |
Gore 2016 [41]: More than a quarter of parents (~39%) felt that the physician did not know enough about their child's allergic condition.➔ Frustrated with HCP's competency |
| 4.2. Negative experiences | Qualitative = 4 N = 52 |
Munoz 2014 [39]: “He [doctor] looks at me and is like, ‘You use the Epipen next time! Anytime you know that he's had exposure to peanuts you use that Epipen.’ (imitating a stern voice) And I was like, ‘Okay’ (in feigned frightened voice, nervous laugh). And so he kind of went in to explain. At first I kind of felt like chastised or whatever, like I'm such a bad parent (says in a low 144 voice).” Chooniedass 2018 [47]: “I know from that first time when we got to the hospital and they were like why didn't you give the EpiPen? And they were basically like yelling at us. (F4)” |
None |
EAI: Epinephrine auto-injector; HCP: Healthcare provider.
Where possible, qualitative and ‘qualitized’ data from included studies where integrated based on similarities in their meanings and contents.
Quantitative data extracted from quantitative and mixed-method studies were transformed into texts based on meaning and content.
Table 4.
Summary of integrated findings related to parents' information needs.
| Integrated findinga | Contributing studies n Sample size across the studies N |
Qualitative data example | Qualitized datab example |
|---|---|---|---|
| 1. Lack of information and instruction | Qualitative studies = 8 Quantitative studies = 1 Mixed method = 2 N = 762 |
McBride 2010 (36): “It was very frustrating, and even now, I find I struggle. There is not enough information…”➔ General lack of information Carstensen 2018 (37): “There was scant guidance about the purchasing of adrenaline autoinjectors for my daughter. We spent hundreds of dollars buying these because we thought they would save her life if she had a severe reaction, only to discover later that she was too young and underweight for them and we would have overdosed her had we used one.”➔ Lack of information related to auto-injectors |
Abdurrahman 2013 (42): More than half of the parents felt that they did not receive enough information about food allergy and anaphylaxis management, epinephrine autoinjectors, and coping strategies at each step of care received. ➔ General lack of information Gore 2016 (43): Only 19% of parents felt they have received enough information from emergency healthcare personnel.➔ Lack of information related to management |
| 2. Information topics and content needed | Qualitative studies = 5 Quantitative = 1 Mixed method = 2 N = 384 |
Hu 2007 (30): “Core information needs identified by parents were: what is anaphylaxis, recognizing a reaction, how to avoid and manage accidental exposures, when and how to give auto-injector.”➔ Topic preference around management Gallagher 2011 (27): “They showed us how to use it but I think that's how many years noo [now], three or four years? I mean I'd be struggling to remember exactly what tae dae [to do] … I would say maybe going back and getting trained maybe once a year or something because I honestly widnae ken [wouldn't know] what tae dae. (Father of Theodore, 13).”➔Information need related use of auto-injector |
Abdurrahman 2013 (42): Majority of parents were interested in educational program after a first reaction, and topics suggested were recognizing an allergic reaction, treating an allergic reaction, teaching others about food allergy, reading food labels, and coping with anxiety.➔ Topic preference around management |
| 3. Information seeking behavior and the sources of information sought | Qualitative studies = 8 Quantitative studies = 2 Mixed method = 2 N = 542 |
Hu 2007 (30): “Other information-seeking phases were those triggered by the anticipation, or occurrence, of new events and milestones, such as further acute reactions, starting childcare or school and travel.”➔ Information sought “as needed”. McBride 2010 (36): “A lot of it [information on allergies] seems to come from overseas, like the more helpful websites and they seem to be American or Canadian. There isn't any specific New Zealand information… Some of the overseas websites and books aren't that relevant to New Zealand, and what [products are] actually available here….”➔ Online Information source |
Alqurashi 2020 (21): Parents' most preferred sources of information about child's allergy were internet and doctors/nurses.➔ Online information source |
| 4. Preferences for mode and format of information | Qualitative studies = 3 Quantitative = 1 Mixed method = 1 N = 498 |
Broome 2015 (38): “I needed someone to teach me what I needed to do in the next few days/weeks specifically before I had time to pour over the Websites.”➔ Mode preference for instructions Vargas 2011 (23): “…almost 2 months have gone by since her bad reaction … I really needed like the one pager…”➔ Format preference for written, simple instructions |
Alqurashi 2020 (21): Parents' most common preferred method of receiving ED discharge instructions were paper handouts and less commonly Telephone line.➔ Format preference for written Abdurrahman 2013 (42): Preferred methods of receiving information were reading materials and online resources. Participants expressed the need for better education and its dissemination (i.e., reading material, referral to support groups or follow-up with a nurse).➔ Mode and format preference for written materials |
Where possible, qualitative and ‘qualitized’ data from included studies where integrated based on similarities in their meanings and contents.
Quantitative data extracted from quantitative and mixed-method studies were transformed into texts based on meaning and content.
3.1. Parents' experiences
Forty studies (93%) reported parent experiences relating to their child's anaphylaxis (captured via reflections or day-to-day living). Themes were categorized around: 1) recognizing an anaphylactic reaction; 2) managing and responding to an anaphylactic reaction; 3) emotional impact of caring for a child at risk of anaphylaxis; and 4) interactions with the health system and healthcare providers (HCPs).
3.1.1. (In)ability to recognize an anaphylactic reaction
Nine studies [[35], [36], [37], [38], [39], [40], [41], [42], [43]] (7 qualitative, 1 quantitative, 1 mixed-method) of variable quality (40%, 60%, 100% of MMAT criteria met) contributed data on parents' experiences around recognizing an anaphylactic reaction. Three qualitative studies reported on parents' experiences related to their ability to identify commonly occurring signs and symptoms of a reaction (e.g., breathing difficulty, swollen face, skin rash) [[35], [36], [37]]. Parents who were aware of the known allergens were vigilant and were able to identify a reaction right away, and associate it with an allergen intake [35,37].
In contrast, the majority of the qualitative data reflected on parent's inability to recognize a reaction because they didn't know the symptoms [35,[38], [39], [40], [41]]; they were uncertain and didn't know about the food or allergen causing the reaction [39,42]; or they didn't want to believe or accept their child had anaphylaxis [39,40]. Quantitative data complemented these findings by indicating that parent's ability to recognize anaphylaxis differed depending on perceived severity of their child's food allergy [43].
3.1.2. Ability to respond to and manage a reaction
Seventeen studies [35,36,[38], [39], [40],[43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]] (9 qualitative, 8 quantitative) of variable quality (40% to 100% MMAT criteria met) reported findings related to this theme. Findings reflected on parents' ability and level of preparedness (competency) to be able to respond quickly to a reaction, whether they acted accordingly or hesitated to act, whether they felt confident in their ability to administer an auto-injector (i.e., epinephrine/adrenaline auto-injector, EpiPen) when necessary, and lessons they learned after experiencing anaphylaxis in their child.
Hesitation to act was a common finding reported in several studies. This theme arose from parents' concern about the negative effects of auto-injector, or the idea that auto-injector was unnecessary. Parents hesitated because they were afraid of hurting the child and inflicting more pain [35,39,[47], [48], [49]]; worried about auto-injector's negative effects (including emotional and psychological impact on child) [39,[48], [49], [50], [51]]; did not think the reaction was serious enough or auto-injector was necessary [39,49,[51], [52], [53]]; or they preferred to go to a hospital instead of administering the injection themselves [47,54].
Parents' preparedness and confidence related to the use of auto-injectors was another sub-theme emerging from several studies. In one study, 94% of mothers reported having a prescribed auto-injector, while only 67% reported carrying it with them at all times, and 13% said they have used it on their child at least once [46]. One parent stated that carrying an auto-injector at all times was like a “safety net” and made her feel in control [35]. Two qualitative [38,51] and two quantitative [43,54] studies reported parents feeling comfortable/confident in administering an auto-injector. Any previous auto-injector training was associated with increased confidence in its administration [43]. Similarly, qualitative data indicated that parents who had previous experience of using an auto-injector on their child felt comfortable administering it [38,51,54]. In contrast, seven studies (5 qualitative, 2 quantitative) reported on parents' lack of confidence to administer auto-injector. In one study involving 1209 parents, 56% self-reported being afraid/somewhat afraid to use an auto-injector, while 44% were not afraid [48]. In another quantitative study, 75 parents (45%) felt outright uncomfortable administering auto-injector; the reason noted by 51% of them was the thought of not being able to recognize the symptoms of anaphylaxis, while 40% feared hurting their child and 36% thought they would forget how to use the auto-injector when under stress of an acute event [52]. Data from five qualitative studies complemented this finding with reported reasons being self-doubt [47]; feeling uncomfortable to administer auto-injector [51]; lack of confidence to use auto-injector or being afraid of doing it wrong [49]; and not knowing when to use an auto-injector [40].
Three qualitative [36,47,51] and one quantitative [54] studies reported on lessons learned and realizing the importance of taking action. Parents who witnessed anaphylaxis in their child reflected on their own responses and evaluated their coping skills to be better prepared in future [47]; other parents learned the importance of not delaying and accessing emergency services if they had to use them [36], and realized the importance of acting quickly and using auto-injector in a life-threatening scenario [51,54].
3.1.3. Emotional impact on parents of a child at risk of anaphylaxis
The majority (82%) of studies included in our review (17 qualitative, 17 quantitative, 1 mixed-method; 40% to 100% of MMAT criteria met) reported data related to parents' emotional experiences [35,36,[39], [40], [41],[45], [46], [47],[49], [50], [51],[54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72]]. Parents often experienced panic and anxiety, were living in constant fear, and were traumatized by seeing their child have a reaction. Parents reported of being greatly impacted by the burden of treatment, and coping with feelings of regret and guilt around managing their child's anaphylaxis.
Several quantitative studies (MMAT scores ranging 40% to 100%) reported on parents' constant feeling of anxiety and panic around anaphylaxis management and preventive practices [45,46,[55], [56], [57], [58]]. Three qualitative [47,49,61] and two quantitative [54,59] studies contributed data reporting parents' high levels of anxiety when responding to a reaction. The main reasons were related to auto-injector administration, with many parents reporting “being too nervous” [59], panicking [54] and feeling too anxious to administer an auto-injector safely and properly [39,49], or conversely, not wanting to use auto-injector or not being sure whether to use it led to panicking.
Parents' anxiety after a reaction in their child was also documented. Abdurrahman et al. found that 94% of parents experienced anxiety after their child's first anaphylactic reaction [61], and Scott et al. assessed parents' post-traumatic stress after their child's reaction and found that parents who were exposed to more allergic reactions in their child had higher stress and anxiety [62].
Emotional burden resulting from fear and trauma most commonly emerged from qualitative data (14 qualitative [[35], [36], [37],39,47,49,50,54,[64], [65], [66], [67], [68],73]; 1 mixed-method study [61]; 2 quantitative studies [57,69]). Referring to a reaction as a traumatic event often stemmed from seeing the child having severe symptoms of a reaction (e.g., covered in hives, swollen face, child gasping for air) [36,39,64,65]; the reaction being so severe that the parent had to rush to an ED [36,39]; the sudden-onset nature of the reaction [39,65]; or because the parents felt helpless and not able to control the situation [65,66]. Parents also reported having nightmares for weeks after seeing their child in a reaction, and felt the traumatic feeling had a lasting impact and would never leave them [50,61,65,66].
Fear also arose from parents' thoughts of not being able to manage a reaction or use an auto-injector [49]; or from the uncertainty of how severe a reaction could be [35,66,73]. Several studies reported on parents' fear of losing the child to a reaction [39,47,49,54,[65], [66], [67], [68],73]. Parents thought their fear of death would never go away and they had to live in constant fear of losing their child [54].
Eight quantitative studies [46,58,60,[69], [70], [71], [72], [73]] of high quality (MMAT scores ≥80%) contributed data related to burden of treatment and/or management of anaphylaxis. Studies reported on parental burden and intolerance of uncertainty due to treatment and management practices [46,58,60,70,71,73]. Psychological impact and parental burden was reported to be greater in parents whose child went to ED in the year leading to the study [69], or those who used an auto-injector [60].
Feelings of regret and guilt resulting from failing or hesitating to respond to a reaction was reported in three qualitative studies (100% MMAT score) [49,51,68]. Parents felt guilty thinking that they are the reason for their child's problem and they are struggling to manage it, or despite being vigilant they mistakenly exposed the child to an allergen [68]; other parents felt regret because of not acting quickly and not using an auto-injector when it was needed [49,51].
3.1.4. Experiences with the health system and health care providers
Eight studies [[39], [40], [41], [42],47,50,54,68] (6 qualitative, 80% to 100% MMAT; 1 quantitative, 40% MMAT; 1 mixed-method, 40% MMAT) contributed data to this theme. Many parents expressed frustration and detailed negative experiences when interacting with HCPs about their child's anaphylaxis. Studies indicated parents were concerned with HCP's level of competency [[40], [41], [42],47] related to their child's care; parents felt that primary care practitioners were not adequately competent to recognize the problem promptly [42]; did not take necessary steps, such as intubating the child when needed [47]; or lacked an understanding of anaphylaxis and did not know enough about allergens [40,41]. Studies also indicated parents' frustration with inconsistent advice or conflicting information they received from their HCPs[47,54,68], receiving inaccurate information regarding allergens and use of auto-injector [50], or because they felt the physician did not take the reaction seriously enough [47].
A negative experience captured by four qualitative studies [39,47,54,68] was feeling berated by HCPs when seeking care for their child. A parent, who was a nurse herself, felt she was criticized by the emergency healthcare staff because of not being vigilant enough to avoid the allergen causing her child's reaction [68]. Other studies indicated parents feeling embarrassed or felt they were being scolded by ED personnel for failing or hesitating to use an auto-injector [39,47,54].
3.2. Parents' information needs
Eighteen studies (42%) reported data related to parents' information needs and preferences for receiving anaphylaxis-related information. Themes that emerged from the data were categorized into themes relating to: 1) gaps in knowledge and information; 2)type of information desired; 3) information sources; and 4) information delivery format. Below we explore each theme.
3.2.1. Gaps in knowledge and information
Eleven studies [35,41,50,54,61,64,67,68,[74], [75], [76]] (10 qualitative, 1 quantitative, 2 mixed-methods) of relatively high quality (80% with 100% of MMAT criteria met) reported parents feeling that they lacked information and instructions to cope with anaphylaxis. Sixty-two percent of parents in one qualitative study felt they were poorly informed by their HCP about allergens, anaphylaxis management, and coping strategies [61]. In another study, 23% of parents felt important information was missing from their child's anaphylaxis action plan [76]. Similarly, Gore et al. found that only 19% of parents felt they had been given enough information by emergency HCPs [41]. Qualitative data from several studies also indicated that parents believed they lacked knowledge of anaphylaxis because HCPs did not give them enough information [41,50,61,64,67,74,75]. Some parents felt that they were expected to develop knowledge and expertise for managing anaphylaxis on their own [64]. Lack of sufficient information led to parents feeling “ill-prepared” and not knowing how to manage an emergency situation or how to use an auto-injector [35,41,50,54,61]. Parents were not only yearning for adequate information, they were also desperate to get more empathized guidance from HCPs to ensure survival of their child [41,50,54,64].
3.2.2. Type of information desired
Five qualitative [38,49,73,75,77] (MMAT ≥80%), one quantitative [54] (40% MMAT) and two mixed-method studies [41,61](40% MMAT) indicated parents' desire for more information around management of anaphylaxis and use of auto-injectors. Parents wanted to know how to avoid accidental exposures and how to manage an anaphylactic reaction [73,75,77]; when and how to use an auto-injector [75,77]; and information about available support groups[41,77]. Some parents felt there is a need for regular training related to auto-injector administration to update their skills [38,49]. In one qualitative study, parents reported their desire for a universal (provincial-level) policy or protocol for schools so that the school personnel know how to respond to an emergency arising from anaphylaxis [54]. In addition to management strategies, two studies [61,75] indicated parents' desire to receive information around recognizing the signs/symptoms of anaphylaxis.
3.2.3. Information sources
Findings related to this theme emerged from twelve studies [41,50,51,54,61,64,67,68,[74], [75], [76], [77]] (8 qualitative, MMAT ≥80%; 2 quantitative, MMAT ≥80%; 2 mixed-method, 40% MMAT). Parents were often triggered to search for information if there was an anticipation or occurrence of a new reaction, or if they had a planned activity coming up (i.e., travel, child starting the school, going to daycare)[75]. Across the studies, the internet and online resources (i.e., Google, Facebook, and blog sites) were the most commonly reported information sources sought by parents [51,54,61,64,67,68,74,75]. Other common sources were allergy organizations and campaigns (i.e., anaphylaxis campaign) [41,50,67,77], and other parents as a support group (online or in-person) [51,54,64,74]. Quantitative data also demonstrated that online sources of information were the most preferred source by parents [76].
3.2.4. Information delivery format
Five studies [38,61,64,75,76] (3 qualitative, MMAT ≥80%; 1 quantitative, MMAT ≥80%; 1 mixed-method, 40% MMAT) indicated parents' preferences for mode and format of information. At the point of contact with healthcare services or providers, parents in qualitative studies desired to receive some sort of written information (e.g., discharge instructions, pamphlet) that they could refer to when they forgot the instructions [38,64,75]. Quantitative data also showed that 45% of parents desired to receive ED discharge instructions in paper handouts, while 41% preferred it electronically, and only 4% preferred it via telephone line [76]. Further, some parents indicated that they did not like to search for information online, and would prefer to receive clear instructions in plain language provided to them by their HCP [38].
4. Discussion and conclusion
4.1. Discussion
Our objective was to synthesize the evidence around experiences and information needs, and use these findings to inform development of parent-focused KT tools for anaphylaxis. The concept behind this work is informed by findings from existing studies in the field of pediatric health, that support integrating experiences of target audience into designing effective KT tools [20,21]. Additionally, understanding information needs helps bridge the communication gap by providing parents the type of information they want to receive.
In this comprehensive mixed-studies systematic review we identified 43 studies that contributed to our understanding of parents' experiences and information needs. A common finding in our review was around parents' emotional experiences, reported in 35 studies. Many parents cited experiences associated with panic, anxiety, and fear when facing an acute reaction in their child, which largely could be due to a general lack of information and clear instructions on management strategies, which was also reported commonly in the included studies.
Anaphylaxis imposes an enormous psychosocial impact on the child and the family, caused by anxiety they may experience resulting from restrictions put in place to protect the allergic child [13,78,79], disruption in daily activities [80], and an overall poor quality of life [10,14]. The psychological impact on parents seems to be greater compared to having a child with other chronic conditions [81]. The uncertainty and un-predictability of anaphylaxis along with fear of a fatal reaction often causes stress and anxiety in parents. Several studies in our review reported an association between history of acute reactions with increased parental anxiety and greater perceived stress [[55], [56], [57], [58]]. While on-going anxiety and constant worrying are very common among parents of children with allergic conditions [82], our review found that parents whose child was diagnosed with anaphylaxis and had a prescribed auto-injector, became anxious and panicked when an acute reaction occurred, or when they had to access emergency services. Contrastingly, parents who were not aware of their child's condition also reported experiencing high levels of anxiety when they saw the first severe reaction in their child that led to the diagnosis of anaphylaxis. While the circumstances leading to anxiety in these two situations differ, the experience seems to be equally disturbing and upsetting for the parent. Fear of losing the child to a fatal reaction and feeling un-prepared to deal with a reaction appear to be the most common contributors to panic and anxiety experienced by parents. The experience of watching their child having a reaction was frightening and traumatizing to the parents and its impact lasted for a long time after the event. Day-to-day anxiety levels appear to be higher in mothers compared to fathers [56]; however, when it comes to managing an acute reaction, it has been reported that mothers are calmer and more responsive, while fathers may be more likely to panic and get nervous [39,54]. Nevertheless, caregivers' negative emotional experiences are commonly reported across experiences related to managing other child health conditions [29,82,83].
Knowledge of anaphylaxis and the resultant perceived severity of the condition may affect a parents' ability to recognize a reaction quickly and respond to it in a timely manner [43]. In line with an earlier review which identified gaps in parental knowledge related to anaphylaxis [10], data included in our review highlighted the fact that many parents felt they lacked general information on how to recognize and manage anaphylaxis. We found that many parents have difficulty distinguishing between serious and not so serious symptoms of a reaction, and by the time they realize a reaction is severe, they are caught off-guard and panic. This suggests that a lack of information on recognizing the signs and symptoms of anaphylaxis adds to the negative feelings experienced by parents. A similar phenomenon was also reported by studies assessing parent experiences related to childhood asthma [82], bronchiolitis [27], and fever [30], in that the anxiety experienced by many parents is largely due a lack of information and clear instructions for management.
While acknowledging the need for better education and training, it is worth noting that responding quickly to managing a reaction might be influenced to some extent by individual characteristics. It is believed that two common factors contributing to an anaphylactic reaction being fatal or non-fatal are “situational circumstances” and “individual management behaviors” [54]. One of the studies involving parents of 624 children found that mothers reported significantly greater empowerment compared to fathers in caring for their allergic child [44]. Similarly, findings from Warren et al. indicated that mothers and fathers rated their ability to deal with allergic reactions in their child differently [44]. Varying levels of emotional reactions were also evident from the study by Fedele and colleagues, where mothers of an allergic child were categorized into different types of responders (i.e., balanced/high/low/anxious high responders) according to their ratings on several food allergy anxiety and adaptation scales [57].
We found that parents felt HCPs and emergency personnel did not provide enough information about allergy triggers, anaphylaxis management, auto-injectors, and coping strategies [41,61,76]. Lack of information may mean that the seriousness of the reaction often goes under-appreciated by parents. For the same reason, although an auto-injector is often prescribed, families do not own one nor carry it with them at all times; or even if they did carry one, they were hesitant to use it on their child.
According to the existing recommendations, administering intra-muscular epinephrine is the first-line emergency management of an acute anaphylactic reaction, and delay in administering the injection can be fatal [84,85]. Yet, we found that many parents hesitate to use it and do not feel comfortable or confident in their ability to administer the injection. In one study, a mother stated that she was so uncomfortable to use the injection on her son that she decided to go to a hospital on multiple occasions [51]. Fear of hurting the child or thinking the injection was un-necessary were common reasons cited by parents. While owning an auto-injector made some parents feel confident and in-control [35], failing to use one was associated with feelings of regret or guilt [49,51]. Consistent with an earlier review [10], our review highlighted that lack of sufficient training and clear instructions on how to use the auto-injector was a common contributor to parents' lack of confidence and negative experiences with the device.
Although early recognition of signs and symptoms by parents is important, being able to act quickly and administer epinephrine within the first few minutes of a reaction is more life-saving [86]. Upon diagnosis of anaphylaxis, physicians often prescribe epinephrine injection, however, many parents felt they did not receive adequate training on how to use the device [87,88]. Parents expressed their desire to receive more information on when and how to use auto-injectors, particularly they wanted this information to be included in their action plans or post-discharge written instructions [38,77].
Physicians are known to be reliable sources of information by many parents [89]. Other parents found nurses as a valuable source of information because they were able to spend more time with them compared to a physician [64,67,75]. However, parents' experiences with inconsistent advice and management strategies by HCPs may cause them to turn to other sources (e.g., relatives, friends, online resources). Additionally, not receiving information from their preferred primary source caused some parents to worry and question the reliability of information on the internet and other sources [75].
Many parents referred to anaphylaxis campaigns and parental support groups (virtual and non-virtual) as useful sources of information that helped them cope. The internet was also identified as a very common source of information. However, although many parents accessed internet and online resources, they did not always believe that the information was trustworthy, an observation that is consistent with previous evidence [89]. Interestingly while some parents believed there is insufficient accessible information, others thought there is over-load of online information that is not always relevant to their situations or concerns [68]. Although we found limited data related to information mode and format preferences, it appears that parents generally wish to receive the information from their HCP in writing, and in plain language that is easy to understand and follow.
4.2. Limitations
Our review may be limited by the fact that most of the data relating to parents' experiences and information needs were collected retrospectively and therefore might be susceptible to recall bias [90,91]. This is particularly important with regard to emotional aspects of experiences, such as fear, panic and anxiety, which tend to fade away as more time passes from the occurrence of the event [92]. As most episodes of anaphylaxis tend to occur un-expectedly in a community setting, it was not reasonable to limit the studies to acute care settings (e.g., ED or hospital) where it is expected that experiences and information needs would be raw and more accurate.
Another potential limitation was that it was challenging to distinguish the experiences and information related to acute reactions from that related to long-term management of anaphylaxis. This was mitigated by having two reviewers extract and verify the data and its relevancy to the review question, and therefore minimize the possibility of errors in interpretation. Further, majority of participants in the included studies were mothers. Although this is anticipated, as generally mothers are more commonly involved in caring for a child, involving fathers and other caregivers in future studies could overcome this evidence gap. Similarly, almost all of the studies in our review were based on food-related anaphylaxis. While food has been identified as the most common allergy trigger in pediatric population, it is possible that parents would have different experiences and information needs with respect to other allergens.
4.3. Innovation
To our knowledge, this is the only systematic review that uses a mixed-studies design approach to synthesize evidence around experiences and information needs of parents of children at risk for anaphylaxis. While there is no theoretical framework underpinning this review, we specifically set out to collate this data in order to support the research practice gap, and inform the development of KT tools on this topic. In child health research, KT tools are often seen as a valuable resource to guide parents in informed decision-making [20,93].
It has been previously shown that parents receiving anaphylaxis educational modules have significantly improved knowledge and competency in emergency management, and reduced anxiety levels [45]. Providing comprehensible and accessible information to parents is one way to empower them in decision-making related to their child's health [94]. Innovative and tailored KT tools and interventions can ensure that the best available research evidence is accessible by parents in a user-friendly format to support informed decision-making. This is particularly important in relation to acute childhood conditions where proper management strategies and adherence to practice recommendation could be life-saving. Building on these theories, the findings of this review were combined with parent interviews to develop an innovative KT tool to help parents understand and manage anaphylaxis efficiently (https://www.echokt.ca/anaphylaxis/).
4.4. Conclusion
Parents' experiences and information needs seem to be inter-connected when it comes to managing their child's health conditions. This review highlighted that for many parents managing an acute anaphylactic reaction is frightening and stressful, leading to significant emotional burden. Coupled with the unpredictability and uncertainty of the reactions, these feelings often stemmed from gaps in crucial knowledge about anaphylaxis allergens, lack of information regarding management and HCP support. Furthermore, our review indicated that although parents lack knowledge and competency, they are interested to acquire more information and search for helpful resources in order to feel more confident in their ability when responding to a reaction. This highlights the importance of developing practical resources for parents while addressing contextual aspects and knowledge gaps.
Authors' contribution
SR contributed to all stages of this review including protocol development, project coordination, data collection and analysis and drafting the manuscript; SE provided overall guidance and contributed to review conceptualization and protocol development, data analysis, reviewing and editing the manuscript; SS and LH contributed to review conceptualization, drafting and editing the manuscript and secured funding for the project. The final version of this manuscript was reviewed and approved by all authors.
Funding
This work was supported by a Canadian Institutes of Health Research Foundation Grant [# 353462] awarded to Drs. Hartling and Scott. Dr. Hartling is a Canada Research Chair in Knowledge Synthesis and Translation and a Distinguished Researcher, Stollery Science Lab. Dr. Scott is a Canada Research Chair in Knowledge Translation in Children's Health and a Distinguished Researcher, Stollery Science Lab. The other authors received no external funding.
Trial registration
Not applicable.
Data availability
Data and forms associated with this manuscript are available upon request.
Declaration of Competing Interest
None.
Acknowledgements
We would like to thank Liza Bialy, Jocelyn Shulhan-Kilroy, and Lindsay Gaudet for their assistance with study selection, data extraction and data verification; and Diana Keto-Lambert for developing and implementing the search strategy.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pecinn.2022.100018.
Contributor Information
Sholeh Rahman, Email: sholeh1@ualberta.ca.
Sarah A. Elliott, Email: se2@ualberta.ca.
Shannon D. Scott, Email: shannon.scott@ualberta.ca.
Lisa Hartling, Email: hartling@ualberta.ca.
Appendices A & B. Supplementary data
Supplementary material
References
- 1.Johansson S., Haahtela T. World Allergy Organization guidelines for prevention of allergy and allergic asthma. Int Arch Allergy Immunol. 2004;135(1):83–92. doi: 10.1159/000080524. [DOI] [PubMed] [Google Scholar]
- 2.Brown S.G. Anaphylaxis: clinical concepts and research priorities. Emerg Med Australas. 2006;18(2):155–169. doi: 10.1111/j.1742-6723.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Allen K.J., Suaini N.H., McWilliam V., Peters R.L., Koplin J.J. The global incidence and prevalence of anaphylaxis in children in the general population: a systematic review. Allergy. 2019;74(6):1063–1080. doi: 10.1111/all.13732. [DOI] [PubMed] [Google Scholar]
- 4.Turner P.J., Campbell D.E., Motosue M.S., Campbell R.L. Global trends in anaphylaxis epidemiology and clinical implications. J Allergy Clin Immunol Pract. 2020;8(4):1169–1176. doi: 10.1016/j.jaip.2019.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee A.Y., Enarson P., Clarke A.E., La Vieille S., Eisman H., Chan E.S., et al. Anaphylaxis across two Canadian pediatric centers: evaluating management disparities. J Asthma Allergy. 2017;10:1. doi: 10.2147/JAA.S123053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson L.B., Arroyo A.C., Faridi M.K., Rudders S., Camargo C.A., Jr. Trends in US emergency department visits for anaphylaxis among infants and toddlers: 2006–2015. J Allergy Clin Immunol Pract. 2021;9(5):1931–1938. doi: 10.1016/j.jaip.2021.01.010. (e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baiu I., Melendez E. Anaphylaxis in children. JAMA. 2018;319(9):943. doi: 10.1001/jama.2018.0447. [DOI] [PubMed] [Google Scholar]
- 8.Turner P.J., Campbell D.E. Epidemiology of severe anaphylaxis: can we use population-based data to understand anaphylaxis? Curr Opin Allergy Clin Immunol. 2016;16(5):441. doi: 10.1097/ACI.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey N.B., Guffey D., Anagnostou K., Coleman N.E., Davis C.M. Epidemiology of anaphylaxis in critically ill children in the United States and Canada. J Allergy Clin Immunol Pract. 2019;7(7):2241–2249. doi: 10.1016/j.jaip.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastner M., Harada L., Waserman S. Gaps in anaphylaxis management at the level of physicians, patients, and the community: a systematic review of the literature. Allergy. 2010;65(4):435–444. doi: 10.1111/j.1398-9995.2009.02294.x. [DOI] [PubMed] [Google Scholar]
- 11.Fischer D., Vander Leek T.K., Ellis A.K., Kim H. Anaphylaxis. Allergy Asthma Clin Immunol. 2018;14(2):54. doi: 10.1186/s13223-018-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DunnGalvin A., Blumchen K., Timmermans F., Regent L., Schnadt S., Podestà M., et al. APPEAL-1: a multiple-country European survey assessing the psychosocial impact of peanut allergy. Allergy. 2020;75(11):2899–2908. doi: 10.1111/all.14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandell D., Curtis R., Gold M., Hardie S. Anaphylaxis: how do you live with it? Health Soc Work. 2005;30(4):325–335. doi: 10.1093/hsw/30.4.325. [DOI] [PubMed] [Google Scholar]
- 14.Cummings A.J., Knibb R.C., King R., Lucas J. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. 2010;65(8):933–945. doi: 10.1111/j.1398-9995.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 15.Morrison A.K., Glick A., Yin H.S. Health literacy: implications for child health. Pediatr Rev. 2019;40(6):263–277. doi: 10.1542/pir.2018-0027. [DOI] [PubMed] [Google Scholar]
- 16.Morrison A.K., Myrvik M.P., Brousseau D.C., Hoffmann R.G., Stanley R.M. The relationship between parent health literacy and pediatric emergency department utilization: a systematic review. Acad Pediatr. 2013;13(5):421–429. doi: 10.1016/j.acap.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byczkowski T.L., Gillespie G.L., Kennebeck S.S., Fitzgerald M.R., Downing K.A., Alessandrini E.A. Family-centered pediatric emergency care: a framework for measuring what parents want and value. Acad Pediatr. 2016;16(4):327–335. doi: 10.1016/j.acap.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Al-Abri R., Al-Balushi A. Patient satisfaction survey as a tool towards quality improvement. Oman Med J. 2014;29(1):3. doi: 10.5001/omj.2014.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjertnaes O.A., Sjetne I.S., Iversen H.H. Overall patient satisfaction with hospitals: effects of patient-reported experiences and fulfilment of expectations. BMJ Qual Saf. 2012;21(1):39–46. doi: 10.1136/bmjqs-2011-000137. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht L., Scott S.D., Hartling L. Knowledge translation tools for parents on child health topics: a scoping review. BMC Health Serv Res. 2017;17(1):686. doi: 10.1186/s12913-017-2632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kastner M., Makarski J., Hayden L., Lai Y., Chan J., Treister V., et al. Improving KT tools and products: development and evaluation of a framework for creating optimized, Knowledge-activated Tools (KaT) Implement Sci Commun. 2020;1:47. doi: 10.1186/s43058-020-00031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauchner H., Osganian S., Smith K., Triant R. Improving parent knowledge about antibiotics: a video intervention. Pediatrics. 2001;108(4):845–850. doi: 10.1542/peds.108.4.845. [DOI] [PubMed] [Google Scholar]
- 23.Stern C., Lizarondo L., Carrier J., Godfrey C., Rieger K., Salmond S., et al. Methodological guidance for the conduct of mixed methods systematic reviews. JBI Evid Synth. 2020;18(10):2108–2118. doi: 10.11124/JBISRIR-D-19-00169. [DOI] [PubMed] [Google Scholar]
- 24.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gates A., Shulhan J., Featherstone R., Scott S.D., Hartling L. A systematic review of parents’ experiences and information needs related to their child’s urinary tract infection. Patient Educ Couns. 2018;101(7):1207–1215. doi: 10.1016/j.pec.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Gates A., Shave K., Featherstone R., Buckreus K., Ali S., Scott S., et al. Parent experiences and information needs relating to procedural pain in children: a systematic review protocol. Syst Rev. 2017;6(1):109. doi: 10.1186/s13643-017-0499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gates M., Shulhan-Kilroy J., Featherstone R., MacGregor T., Scott S.D., Hartling L. Parent experiences and information needs related to bronchiolitis: a mixed studies systematic review. Patient Educ Couns. 2019;102(5):864–878. doi: 10.1016/j.pec.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Thompson A.P., Nesari M., Hartling L., Scott S.D. Parents’ experiences and information needs related to childhood fever: a systematic review. Patient Educ Couns. 2020;103(4):750–763. doi: 10.1016/j.pec.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Pace R., Pluye P., Bartlett G., Macaulay A.C., Salsberg J., Jagosh J., et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud. 2012;49(1):47–53. doi: 10.1016/j.ijnurstu.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Hong Q.N., Fàbregues S., Bartlett G., Boardman F., Cargo M., Dagenais P., et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inform. 2018;34(4):285–291. [Google Scholar]
- 33.Pluye P., Gagnon M.P., Griffiths F., Johnson-Lafleur J. A scoring system for appraising mixed methods research, and concomitantly appraising qualitative, quantitative and mixed methods primary studies in Mixed Studies Reviews. Int J Nurs Stud. 2009;46(4):529–546. doi: 10.1016/j.ijnurstu.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Thomas J., Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8(1):45. doi: 10.1186/1471-2288-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu W., Kerridge I., Kemp A. Risk, rationality, and regret: responding to the uncertainty of childhood food anaphylaxis. Med Humanit. 2005;31(1):12–16. doi: 10.1136/jmh.2004.000179. [DOI] [PubMed] [Google Scholar]
- 36.Dobbin-Williams K.A. University of Calgary (Canada); Ann Arbor: 2019. Understanding the experiences of mothers of teenaged children with life-threatening food allergies: A philosophical hermeneutic inquiry, nursing; p. 164. [Google Scholar]
- 37.Gunnarsson N., Hydén L.C. Organizing allergy and being a ‘good’ parent: parents’ narratives about their children’s emerging problems. Health (London) 2009;13(2):157–174. doi: 10.1177/1363459308099682. [DOI] [PubMed] [Google Scholar]
- 38.Vargas P.A., Sicherer S.H., Christie L., Keaveny M., Noone S., Watkins D., et al. Developing a food allergy curriculum for parents. Pediatr Allergy Immunol. 2011;22(6):575–582. doi: 10.1111/j.1399-3038.2011.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munoz V.L. Brandeis University; Ann Arbor: 2014. Managing food allergies, preserving childhoods; p. 222. [Google Scholar]
- 40.Anastos S.L. Massachusetts School of Professional Psychology; Ann Arbor: 2006. Application of the transtheoretical model to aid parents in managing pediatric peanut allergy; p. 140. [Google Scholar]
- 41.Gore C., Griffin R., Rothenberg T., Tallett A., Hopwood B., Sizmur S., et al. New patient-reported experience measure for children with allergic disease: development, validation and results from integrated care. Arch Dis Child. 2016;101(10):935–943. doi: 10.1136/archdischild-2015-309598. [DOI] [PubMed] [Google Scholar]
- 42.Burton C., Irshad T., Sheikh A. Understanding the experiences of allergy testing: a qualitative study of people with perceived serious allergic disorders. Postgrad Med J. 2010;86(1020):591–596. doi: 10.1136/pgmj.2009.092395. [DOI] [PubMed] [Google Scholar]
- 43.Teoh T., Mill C., Wong T., Baerg I., Alexander A., Hildebrand K.J., et al. Impact of supervised epinephrine autoinjector administration during food challenges on parent confidence. Ann Allergy Asthma Immunol. 2016;116(5):467–469. doi: 10.1016/j.anai.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Warren C.M., Gupta R.S., Sohn M.W., Oh E.H., Lal N., Garfield C.F., et al. Differences in empowerment and quality of life among parents of children with food allergy. Ann Allergy Asthma Immunol. 2015;114(2):117–125. doi: 10.1016/j.anai.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brockow K., Schallmayer S., Beyer K., Biedermann T., Fischer J., Gebert N., et al. Effects of a structured educational intervention on knowledge and emergency management in patients at risk for anaphylaxis. Allergy. 2015;70(2):227–235. doi: 10.1111/all.12548. [DOI] [PubMed] [Google Scholar]
- 46.Herbert L.J., Dahlquist L.M., Bollinger M.E. Maternal intolerance of uncertainty, anxiety, and adherence with food challenge referrals. J Health Psychol. 2013;18(9):1209–1219. doi: 10.1177/1359105312459895. [DOI] [PubMed] [Google Scholar]
- 47.Chooniedass R., Temple B., Martin D., Becker A. A qualitative study exploring parents' experiences with epinephrine use for their child's anaphylactic reaction. Clin Transl Allergy. 2018;8:43. doi: 10.1186/s13601-018-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chad L., Ben-Shoshan M., Asai Y., Cherkaoui S., Alizadehfar R., St-Pierre Y., et al. A majority of parents of children with peanut allergy fear using the epinephrine auto-injector. Allergy. 2013;68(12):1605–1609. doi: 10.1111/all.12262. [DOI] [PubMed] [Google Scholar]
- 49.Gallagher M., Worth A., Cunningham-Burley S., Sheikh A. Epinephrine auto-injector use in adolescents at risk of anaphylaxis: a qualitative study in Scotland, UK. Clin Exp Allergy. 2011;41(6):869–877. doi: 10.1111/j.1365-2222.2011.03743.x. [DOI] [PubMed] [Google Scholar]
- 50.Akeson N., Worth A., Sheikh A. The psychosocial impact of anaphylaxis on young people and their parents. Clin Exp Allergy. 2007;37(8):1213–1220. doi: 10.1111/j.1365-2222.2007.02758.x. [DOI] [PubMed] [Google Scholar]
- 51.Graceffo L.B. The University of Texas at Arlington; Arlington: 2008. Balancing safety and normalcy: a study of parents' management of young children's severe food allergies; p. 3098. [Google Scholar]
- 52.Kim J.S., Sinacore J.M., Pongracic J.A. Parental use of EpiPen for children with food allergies. J Allergy Clin Immunol. 2005;116(1):164–168. doi: 10.1016/j.jaci.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 53.Topal E., Bakirtas A., Yilmaz O., Ertoy I.H., Arga M., Demirsoy M.S., et al. A real-life study on acquired skills from using an adrenaline autoinjector. Int Arch Allergy Immunol. 2013;160(3):301–306. doi: 10.1159/000341367. [DOI] [PubMed] [Google Scholar]
- 54.Butler J.D. Memorial University of Newfoundland (Canada); Ann Arbor: 2005. Allergy management behaviours and sources of stress for schools and families living with life-threatening food allergies. (p. 218) [Google Scholar]
- 55.Baricic T.V., Catipovic M., Cetinic E.L., Krmek V., Horvat I. Parental perception, prevalence and primary care physicians’ knowledge on childhood food allergy in Croatia. Children (Basel) 2015;2(3):305–316. doi: 10.3390/children2030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klinnert M.D., McQuaid E.L., Fedele D.A., Faino A., Strand M., Robinson J., et al. Children’s food allergies: development of the food allergy management and adaptation scale. J Pediatr Psychol. 2015;40(6):572–580. doi: 10.1093/jpepsy/jsv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fedele D.A., McQuaid E.L., Faino A., Strand M., Cohen S., Robinson J., et al. Patterns of adaptation to children’s food allergies. Allergy. 2016;71(4):505–513. doi: 10.1111/all.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ackerman C.S. University of Maryland; Baltimore County, Ann Arbor: 2008. Parenting children with food allergy: stress, anxiety, and parenting style; p. 133. [Google Scholar]
- 59.Song T.T., Brown D., Karjalainen M., Lehnigk U., Lieberman P. Value of a second dose of epinephrine during anaphylaxis: a patient/caregiver survey. J Allergy Clin Immunol Pract. 2018;6(5):1559–1567. doi: 10.1016/j.jaip.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 60.Allen C.W., Bidarkar M.S., van Nunen S.A., Campbell D.E. Factors impacting parental burden in food-allergic children. J Paediatr Child Health. 2015;51(7):696–698. doi: 10.1111/jpc.12794. [DOI] [PubMed] [Google Scholar]
- 61.Abdurrahman Z.B., Kastner M., Wurman C., Harada L., Bantock L., Cruickshank H., et al. Experiencing a first food allergic reaction: a survey of parent and caregiver perspectives. Allergy Asthma Clin Immunol. 2013;9(1):18. doi: 10.1186/1710-1492-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott K.E. East Carolina University; Ann Arbor: 2016. Moderators of child anxiety due to food allergy; p. 99. [Google Scholar]
- 63.Knibb R.C., Cortes A., Barnes C., Stalker C. Validation of the English version of the scale for psychosocial factors in food allergy and the relationship with mental health, quality of life, and self-efficacy. J Allergy (Cairo) 2016;2016:4850940. doi: 10.1155/2016/4850940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Broome S.B., Lutz B.J., Cook C. Becoming the parent of a child with life-threatening food allergies. J Pediatr Nurs. 2015;30(4):532–542. doi: 10.1016/j.pedn.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Rouf K., White L., Evans K. A qualitative investigation into the maternal experience of having a young child with severe food allergy. Clin Child Psychol Psychiatry. 2012;17(1):49–64. doi: 10.1177/1359104511415636. [DOI] [PubMed] [Google Scholar]
- 66.Gupta R.S., Kim J.S., Barnathan J.A., Amsden L.B., Tummala L.S., Holl J.L. Food allergy knowledge, attitudes and beliefs: focus groups of parents, physicians and the general public. BMC Pediatr. 2008;8:36. doi: 10.1186/1471-2431-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillespie C.A., Woodgate R.L., Chalmers K.I., Watson W.T. "Living with risk": mothering a child with food-induced anaphylaxis. J Pediatr Nurs. 2007;22(1):30–42. doi: 10.1016/j.pedn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Carstensen C., Papps E., Thompson S. When a child is diagnosed with severe allergies: an autoethnographic account. Nurs Prax NZ. 2018;34(2):6–16. [Google Scholar]
- 69.Springston E.E., Smith B., Shulruff J., Pongracic J., Holl J., Gupta R.S. Variations in quality of life among caregivers of food allergic children. Ann Allergy Asthma Immunol. 2010;105(4):287–294. doi: 10.1016/j.anai.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Fathi S.M., Tavakol M., Rezaei N., Movahedi M., Aghamohammadi A., Shariat M., et al. Impact of IgE-mediated food allergy on parental quality of life in Iranian patients. Iran J Allergy Asthma Immunol. 2016;15(5):372–380. [PubMed] [Google Scholar]
- 71.Saleh-Langenberg J., Flokstra-de Blok B.M., Goossens N.J., Kemna J.C., van der Velde J.L., Dubois A.E. The compliance and burden of treatment with the epinephrine auto-injector in food-allergic adolescents. Pediatr Allergy Immunol. 2016;27(1):28–34. doi: 10.1111/pai.12458. [DOI] [PubMed] [Google Scholar]
- 72.Aika S., Ito M., Yamamoto Y. Food allergy response capabilities of mothers and related factors. Nurs Health Sci. 2017;19(3):340–350. doi: 10.1111/nhs.12351. [DOI] [PubMed] [Google Scholar]
- 73.Knibb R.C. Effectiveness of cognitive behaviour therapy for mothers of children with food allergy: a case series. Healthcare (Basel) 2015;3(4):1194–1211. doi: 10.3390/healthcare3041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McBride C., McBride-Henry K., van Wissen K. Parenting a child with medically diagnosed severe food allergies in New Zealand: the experience of being unsupported in keeping their children healthy and safe. Contemp Nurse. 2010;35(1):77–87. doi: 10.5172/conu.2010.35.1.077. [DOI] [PubMed] [Google Scholar]
- 75.Hu W., Grbich C., Kemp A. Parental food allergy information needs: a qualitative study. Arch Dis Child. 2007;92(9):771–775. doi: 10.1136/adc.2006.114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alqurashi W., Awadia A., Pouliot A., Cloutier M., Hotte S., Segal L., et al. The Canadian anaphylaxis action plan for kids: development and validation. Patient Educ Couns. 2020;103(1):227–233. doi: 10.1016/j.pec.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 77.Mandell D., Curtis R., Gold M., Hardie Susan. Families coping with a diagnosis of anaphylaxis in a child: a qualitative study of informational and support needs. ACI Int. 2002;14(96):101. [Google Scholar]
- 78.Bollinger M.E., Dahlquist L.M., Mudd K., Sonntag C., Dillinger L., McKenna K. The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol. 2006;96(3):415–421. doi: 10.1016/S1081-1206(10)60908-8. [DOI] [PubMed] [Google Scholar]
- 79.Dobbie A., Robertson C. Provision of self-injectable adrenaline for children at risk of anaphylaxis: its source, frequency and appropriateness of use, and effect. Ambulat Child Health. 1998;4:283–288. [Google Scholar]
- 80.Östblom E., Egmar A.C., Gardulf A., Lilja G., Wickman M. The impact of food hypersensitivity reported in 9-year-old children by their parents on health-related quality of life. Allergy. 2008;63(2):211–218. doi: 10.1111/j.1398-9995.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- 81.Primeau M.N., Kagan R., Joseph L., Lim H., Dufresne C., Duffy C., et al. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin Exp Allergy. 2000;30(8):1135–1143. doi: 10.1046/j.1365-2222.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 82.Shulhan-Kilroy J., Elliott S.A., Scott S.D., Hartling L. Parents' self-reported experiences and information needs related to acute pediatric asthma exacerbations: a mixed studies systematic review. PEC Innov. 2021;1 doi: 10.1016/j.pecinn.2021.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meherali S., Hartling L., Campbell A., Robin F., Scott S. Parent information needs and experience regarding acute otitis media in children: a systematic review. Patient Educ Couns. 2021;104(3):554–562. doi: 10.1016/j.pec.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 84.Sicherer S.H., Simons F.E.R. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017;139(3) doi: 10.1542/peds.2016-4006. [DOI] [PubMed] [Google Scholar]
- 85.Simons F., Chad Z., Gold M. Anaphylaxis in children: real-time reporting from a national network. Allergy Clin Immunol Int. 2004;1:242–244. [Google Scholar]
- 86.Sampson H.A., Mendelson L., Rosen J.P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327(6):380–384. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 87.Mehr S., Robinson M., Tang M. Doctor--how do I use my EpiPen? Pediatr Allergy Immunol. 2007;18(5):448–452. doi: 10.1111/j.1399-3038.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 88.Pouessel G., Deschildre A., Castelain C., Sardet A., Sagot-Bevenot S., de Sauve-Boeuf A., et al. Parental knowledge and use of epinephrine auto-injector for children with food allergy. Pediatr Allergy Immunol. 2006;17(3):221–226. doi: 10.1111/j.1399-3038.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 89.Khoo K., Bolt P., Babl F.E., Jury S., Goldman R.D. Health information seeking by parents in the Internet age. J Paediatr Child Health. 2008;44(7–8):419–423. doi: 10.1111/j.1440-1754.2008.01322.x. [DOI] [PubMed] [Google Scholar]
- 90.Colombo D., Suso-Ribera C., Fernández-Álvarez J., Cipresso P., Garcia-Palacios A., Riva B.C. Affect recall bias: being resilient by distorting reality. Cognit Ther Res. 2020;44(5):906–918. [Google Scholar]
- 91.Talari K., Goyal M. Retrospective studies - utility and caveats. J R Coll Physicians Edinb. 2020;50(4):398–402. doi: 10.4997/JRCPE.2020.409. [DOI] [PubMed] [Google Scholar]
- 92.Ritchie T.D., Batteson T.J., Bohn A., Crawford M.T., Ferguson G.V., Schrauf R.W., et al. A pancultural perspective on the fading affect bias in autobiographical memory. Memory. 2015;23(2):278–290. doi: 10.1080/09658211.2014.884138. [DOI] [PubMed] [Google Scholar]
- 93.Campbell A., Louie-Poon S., Slater L., Scott S.D. Knowledge translation strategies used by healthcare professionals in child health settings: an updated systematic review. J Pediatr Nurs. 2019;47:114–120. doi: 10.1016/j.pedn.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 94.Jackson C., Cheater F.M., Reid I. A systematic review of decision support needs of parents making child health decisions. Health Expect. 2008;11(3):232–251. doi: 10.1111/j.1369-7625.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data and forms associated with this manuscript are available upon request.