Abstract
Exposure to ubiquitous plastic-associated endocrine disrupting chemicals (EDCs) is associated with the increased risk of many chronic diseases. For example, phthalate exposure is associated with cardiometabolic mortality in humans, with societal costs ~ $39 billion/year or more. We recently demonstrated that several widely used plastic-associated EDCs increase cardiometabolic disease in appropriate mouse models. In addition to affecting adult health, parental exposure to EDCs has also been shown to cause metabolic disorders, including obesity and diabetes, in the offspring. While most studies have focused on the impact of maternal EDC exposure on the offspring’s health, little is known about the effects of paternal EDC exposure. In the current study, we investigated the adverse impact of paternal exposure to a ubiquitous but understudied phthalate, dicyclohexyl phthalate (DCHP) on the metabolic health of F1 and F2 offspring in mice. Paternal DCHP exposure led to exacerbated insulin resistance and impaired insulin signaling in F1 offspring without affecting diet-induced obesity. We previously showed that sperm small non-coding RNAs including tRNA-derived small RNAs (tsRNAs) and rRNA-derived small RNAs (rsRNAs) contribute to the intergenerational transmission of paternally acquired metabolic disorders. Using a novel PANDORA-seq, we revealed that DCHP exposure can lead to sperm tsRNA/rsRNA landscape changes that were undetected by traditional RNA-seq, which may contribute to DCHP-elicited adverse effects. Lastly, we found that paternal DCHP can also cause sex-specific transgenerational adverse effects in F2 offspring and elicited glucose intolerance in female F2 descendants. Our results suggest that exposure to endocrine disrupting phthalates may have intergenerational and transgenerational adverse effects on the metabolic health of their offspring. These findings increase our understanding of the etiology of chronic human diseases originating from chemical-elicited intergenerational and transgenerational effects.
Keywords: Phthalate, Paternal exposure, Transgenerational inheritance, Metabolic disease, Sperm small non-coding RNA, PANDORA-seq
1. Introduction
Numerous endocrine disrupting chemicals (EDCs) that are used in plastic production have become a significant health concern (Eriksen et al., 2014; Seltenrich, 2015). Plastic base chemical bisphenol A (BPA), and many phthalate plasticizers, are associated with increased metabolic disease risk in humans (Helsley and Zhou, 2017; Lang et al., 2008; Lind and Lind, 2012; Lind and Lind, 2011; Melzer et al., 2012; Melzer et al., 2010; Olsen et al., 2012; Trasande et al., 2022; Vom Saal and Myers, 2008). The adverse health effects of BPA have been extensively studied, and phthalate exposure has also been shown to be associated with increased cardiometabolic disease risk and mortality in humans, with societal costs ~$39 billion/year or more (Casals-Casas and Desvergne, 2011; Halden, 2010; Lind et al., 2012; Trasande et al., 2022; Wu et al., 2021). However, the mechanisms by which exposure to those endocrine disrupting phthalates influences cardiometabolic disease are not well understood.
In addition to affecting adult health, parental exposure to a wide range of environmental toxicants including plastic-associated EDCs can cause metabolic disorders, including obesity and diabetes, and these metabolic disease risks can be transmitted to their offspring (Heindel and Blumberg, 2019; Lee and Blumberg, 2019; Sales et al., 2017). Studies from worm to mammal suggest environmental stress-induced phenotypes can be “memorized” in the germline and transmitted to future generations (Daxinger and Whitelaw, 2012; Heard and Martienssen, 2014; Lane et al., 2014; Ost et al., 2014). Most data are obtained from the impact of maternal exposure on the offspring health. For example, we previously provided the first evidence that maternal murine exposure to BPA exacerbates atherosclerosis in their adult offspring (Sui et al., 2018). However, emerging evidence suggests epigenetic inheritance can also occur between father and offspring. A subsequent wave of research searches for sperm regulatory factors that could relay this transmission of phenotype in a non-DNA sequence-based manner (Chen et al., 2016). We and others have recently demonstrated that sperm small non-coding RNAs (sncRNAs) including rRNA-derived small RNAs (rsRNAs) and tRNA-derived small RNAs (tsRNAs) form the ‘sperm RNA code’ to carry important epigenetic information that can induce intergenerational transmission of paternally acquired metabolic disorders (Chen et al., 2016; Sarker et al., 2019; Zhang et al., 2019; Zhang et al., 2018).
Compared to other well-studied sncRNAs (e.g., microRNAs or miR-NAs), many tsRNAs and rsRNAs have RNA modifications from their precursors and may show different RNA termini due to cleavage by different RNase (Phizicky and Hopper, 2010; Schimmel, 2018; Sergiev et al., 2018; Shi et al., 2021; Shi et al., 2022). When performing RNA-seq analysis for highly modified sperm sncRNAs, certain RNA modifications (including RNA methylations and terminal RNA modifications) can interfere with the cDNA library construction process (Cozen et al., 2015; Honda et al., 2015; Zheng et al., 2015), which prevents discovery of highly modified tsRNAs and rsRNAs that may be responsible for transmitting paternal phenotypes (Shi et al., 2022). To address this problem, we have successfully developed an innovative RNA-seq protocol, PANDORA-seq (Panoramic RNA Display by Overcoming RNA Modification Aborted Sequencing) to overcome RNA modification-elicited sequence interferences (Shi et al., 2021).
In the current study, we investigated the effects of paternal exposure to a ubiquitous phthalate, dicyclohexyl phthalate (DCHP) on the metabolic health of F1 and F2 offspring in mice. We also performed PANDORA-seq to reveal the paternal DCHP exposure-elicited sperm tsRNA and rsRNA changes.
2. Materials and methods
2.1. Animals
Eight-week-old male wild-type (WT) mice (C57BL/6J strain, purchased from the Jackson Laboratory) were fed a normal chow diet (ND; PicoLab Rodent Diet 20, Lab Supply) containing 20 % protein and 4.5 % fat by weight (Calories: 25 % protein, 13 % Fat, 62 % Carbohydrate) and received daily oral gavage of corn oil vehicle control or DCHP (10 mg/kg body weight, Sigma-Aldrich) for 4 weeks. At 12 weeks old, the mice were mated with 12-week-old unexposed female WT mice (C57BL/6J strain, purchased from the Jackson Laboratory). After copulation was confirmed by vaginal plug detection, the male mice were removed from the mating cage and humanely euthanized. These initial mouse pairs were designated F0. The F0 dams were kept on the ND. The F1 offspring were weaned at 3 weeks and provided with either a ND or Western-type high-fat diet (HFD, TD.88137, Envigo) containing 17.3 % protein and 21 % fat by weight (Calories: 15 % protein, 42 % fat, and 42 % carbohydrate) (Helsley et al., 2016; Lu et al., 2019) until euthanasia at 12 weeks old. Before euthanasia, the F1 male mice on ND were mated with 12-week-old unexposed WT females (C57BL/6J strain, purchased from the Jackson Laboratory) to generate F2 generation. The F2 mice were also weaned at 3 weeks old and fed with the same HFD for 9 weeks before euthanasia (Fig. 1A). Mice were restricted to food for 6 h prior to euthanasia, and tissue collection was performed as described in previous publication (Sui et al., 2021; Wang et al., 2018). All animal studies followed protocols approved by the University of California, Riverside Institutional Animal Care and Use Committee.
Fig. 1. Scheme of paternal DCHP exposure study and the impact of DCHP exposure on F0 sires and F1 litters.
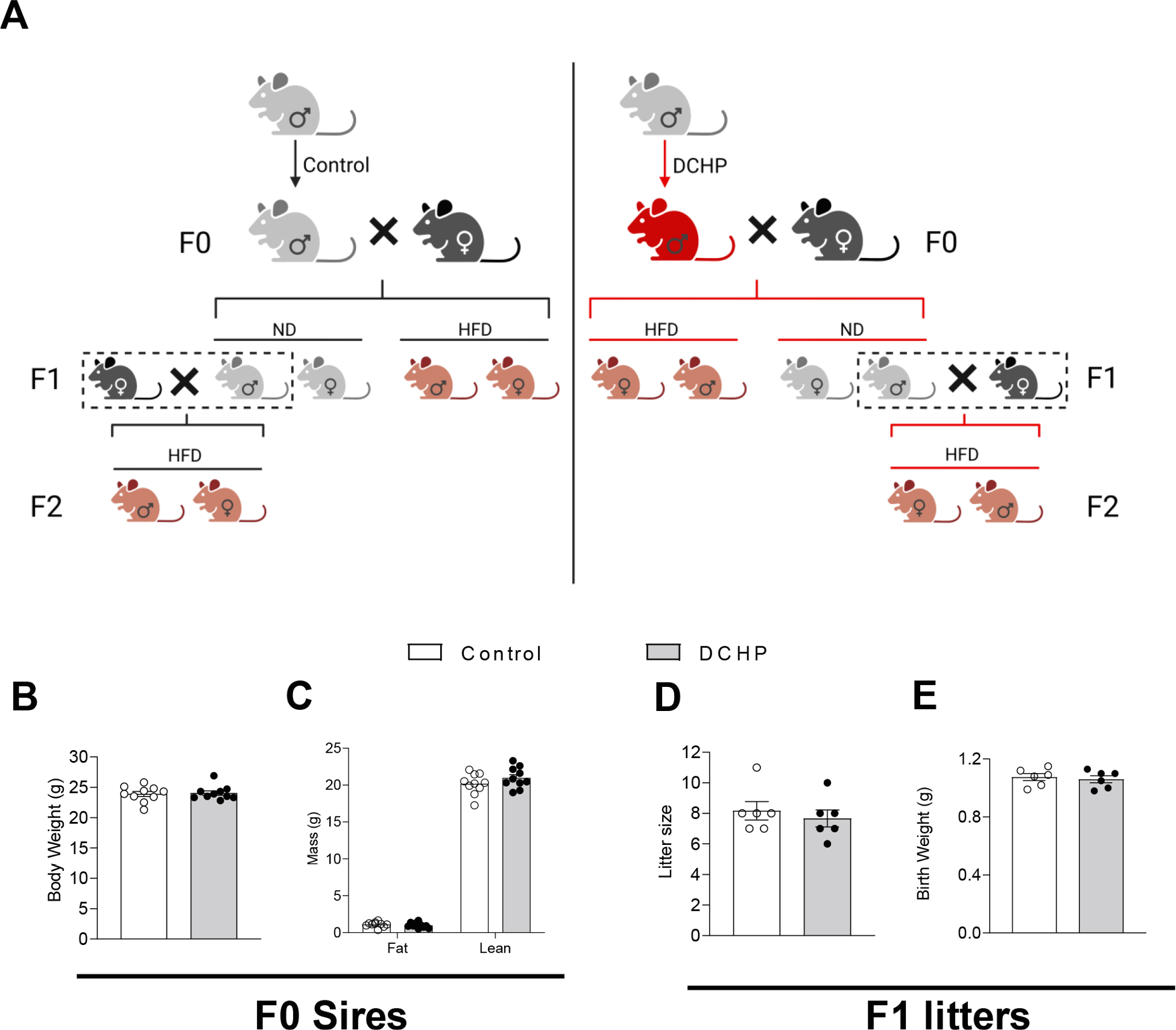
(A) Eight-week-old male C57BL/6 wild-type (WT) mice were treated with vehicle control or 10 mg/kg/day of DCHP by oral gavage for 4 weeks. Control or DCHP-exposed male mice were mated with 12-week-old unexposed female WT mice. The F1 offspring were weaned at 3-week-old and were fed a normal chow diet (ND) or a high-fat diet (HFD) for 9 weeks. ND-fed male F1 mice then mated with 12-week-old unexposed female WT mice to generate F2 offspring that were also fed HFD for 9 weeks after weaning. (B and C) The impact of DCHP exposure on body weight (B) and lean and fat mass (C) of F0 sires (n = 9–10, two-sample, two tailed Student’s t-test). (D and E) The impact of paternal DCHP exposure on the litter size (D) and average birth weight (E) of F1 offspring (n = 6, two-sample, two tailed Student’s t-test).
2.2. Metabolic phenotypic analyses
Body weight was recorded weekly. NMR spectroscopy (EchoMRI) was performed before euthanasia to measure the lean and fat mass of the mice (Helsley et al., 2016; Lu et al., 2019). We performed glucose tolerance test (GTT) and insulin tolerance test (ITT) as described in previous studies (Helsley et al., 2016; Sui et al., 2014). For insulin stimulation studies, mice were injected with 0.35 U/kg body weight Humulin R U-100 insulin (Lilly USA. LLC) into the inferior vena cava (Helsley et al., 2016). Mice were euthanized after 5 min and major tissues (skeletal muscle, liver, white adipose fat) were collected for further analysis (Helsley et al., 2016).
2.3. Sperm isolation
We collected mouse sperm from the F0 sires at the end of treatment using a previously described procedure (Shi et al., 2021). After release from the cauda epididymis, sperm were incubated for 15 min (37 °C) in 1 × phosphate-buffered saline (PBS). Tissue debris was removed by filtering the sperm through a cell strainer (pore size 40-μm, Corning, CLS431750). Somatic cell residues were eliminated by incubating the sperm with somatic cell lysis buffer for 40 min on ice. The precipitation of sperm was achieved through centrifugation (600 × g, 5 min). Afterwards, sperm was washed twice with 1 × PBS solution and precipitated again at 600 × g, 5 min. The sperm was finally resuspended in 1 mL TRIzol Reagent (Thermo Fisher Scientific 15596026) and stored at −80 °C until further use.
2.4. Western blotting
We used previously described methods for the extraction of protein and immunoblotting analyses (Helsley et al., 2016; Sui et al., 2021). The denatured protein lysates were resolved using SDS-PAGE. The separated proteins were transferred onto a nitrocellulose membrane (Bio-Rad, 1620115). After blocking with 5 % bovine serum albumin (BSA, Sigma-Aldrich, A9647) for 1 h at room temperature, the membranes were incubated with primary antibodies overnight at 4 °C: anti-Actin (1:5000 dilution, Millipore Sigma A2066), anti-Akt (1:1000 dilution, Cell Signaling 9272), and anti-Phospho-Akt (Ser473) antibodies (1:1000 dilution, Cell Signaling 9271). After 4 × 5 min washes with 1 × PBS with 0.1 % Tween20 (PBST), the membranes were incubated for 1 h with an anti-rabbit secondary antibodies (1:5000 dilution, Millipore Sigma 12–348) at room temperature. After 4 × 5 min washes, the membranes were developed by Pierce ECL Western Blotting Substrate kit (Thermo Fisher Scientific, 32209), and exposed with Bio-Rad Chemidoc imaging machine. Uncropped immunobloting images are included in Supplemental Figure 1.
2.5. RNA extraction and quantitative Real-Time PCR assay
TRIzol Reagent (Thermo Fisher Scientific 15596026) was used to perform total RNA extraction from mouse tissues following manufacturer instructions (Satta et al., 2022; Shi et al., 2021; Sui et al., 2021). We measured the relative mRNA expression levels by Quantitative Real-Time PCR with the SYBR Green (Bio-Rad 170–8886) kit using a Bio-Rad CFX Real-Time-PCR Machine (184–5096) (Satta et al., 2022; Sui et al., 2021). The primer sequences are included in Supplemental Table 1.
2.6. RNA-seq and transcriptomic data analysis
After isolation RNA integrity was checked using a Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA). The Illumina standard operation pipeline was used for cDNA library construction and sequencing as described previously (Meng et al., 2022; Shi et al., 2021; Sui et al., 2020), as were the detailed RNA-seq data analysis methods (Meng et al., 2022; Shi et al., 2021; Sui et al., 2020). The cut-off threshold for differential expression was set as fold change (FC) > 1.5 and a false discovery rate (FDR) < 0.1. Further, Gene Ontology (GO) Biological process analysis proceeded with the differentially expressed genes as previously described (Meng et al., 2022; Sui et al., 2020). The raw transcriptome datasets have been uploaded and can be accessed in the Gene Expression Omnibus (GSE215807).
2.7. PANDORA-seq of sperm small RNAs
We recently developed the PANDORA-seq protocol (Shi et al., 2021). Detailed information for PANDORA-seq of sperm small RNAs is also described as following.
Small RNA extraction and cDNA library creation were done using our recently published protocol (Shi et al., 2021). Briefly, RNA loading dye from New England Biolabs (B0363S) was added to the sperm RNA sample, mixed well, and incubated for 5 min at 75 °C. The RNA mixture was electrophoresed through a urea polyacrylamide gel (15 %). SYBR Gold solution (Invitrogen; S11494) was used to stain and visualize the small RNA. The excised small RNA (15–50 nucleotides) was eluted overnight with sodium acetate (0.3 M, Invitrogen, AM9740) at 4 °C. After 10 min centrifugation (12,000 × g, 4 °C), the aqueous phase was collected. Subsequently, linear acrylamide (Invitrogen, AM9520), sodium acetate (3 M) and ethanol (100 %) were added into the collected supernatant and incubated for 2 h at −20 °C. The RNA was precipitated and resuspended in Nuclease-free H2O.
200 ng sperm RNA was added into 50 μL reaction buffer, which consists of α-ketoglutaric acid (1 mM, Sigma–Aldrich; K1128), AlkB (4 μg mL−1), sodium ascorbate (2 mM), HEPES (50 mM, Fisher Scientific, 15630080), BSA (50 mg L−1, Sigma-Aldrich, A9647), ferrous ammonium sulfate (75 μM), and RNase inhibitor (2,000 U mL−1). The mixture was incubated for 20 min (37 °C), after which 500 μL TRIzol reagent was added into the mixture to extract the RNA. The isolated RNA was transferred into another reaction buffer (50 μL) containing 10 × PNK buffer (5 μL, New England Biolabs, B0201S), ATP (1 mM, New England Biolabs, P0756S), T4PNK (10 U, New England Biolabs, M0201L) and incubated for 20 min (37 °C). 500 μL TRIzol was added for RNA extraction.
NEBNext Small RNA Library Prep Set for Illumina kit (New England Biolabs; E7330S) was used to conduct small RNA library construction. Detailed PCR parameters were published previously (Shi et al., 2021). PAGE gel was run for purification of the PCR product. Qualified libraries were amplified and sent for sequencing at the Genomics Center of University of California, San Diego (Illumina system, SE75 strategy). The software SPORTS1.1 (parameter setting: -M 1) was used to analyze and annotate the raw sequencing outputs (Shi et al., 2018; Shi et al., 2021). To investigate the relative abundance of sncRNAs, we normalized the expression of the individual sncRNA species by total miRNA expression (Shi et al., 2021). The 20,000 sncRNA species with the highest normalized mean expression were retained. The samr package was then applied to identify the differentially expressed sncRNAs (based on the normalized expression) between DCHP-exposed and control groups. The sncRNA species with q-value < 10 % and FC > 2 were deemed differentially expressed. The sperm RNA sequence datasets have been uploaded and can be accessed in the Gene Expression Omnibus (GSE215807).
2.8. Statistical analysis
All data are displayed in the format of the mean ± standard error (SE). Two-sample, two-tailed Student’s t-test was used for individual pairwise comparisons. Two-way ANOVA was applied when multiple comparisons were performed (with Bonferroni correction method). Data analyses were performed using GraphPad Prism software with the statistically significant level set at p < 0.05.
3. Results
3.1. Paternal exposure to phthalate DCHP had no effect on litter size or birth weight of F1 offspring
To investigate the potential effects of paternal DCHP exposure on offspring health, 8-week-old male WT mice were fed a normal chow diet (ND) and treated with vehicle control or 10 mg/kg body weight/day of DCHP by oral gavage for 4 weeks before mating with 12-week-old unexposed female WT mice (Fig. 1A). After the vaginal plug was detected (embryonic day 0.5), pregnant females were housed separately and fed the ND. The sires were euthanized for tissue and sperm collection. Exposure to DCHP did not affect the body weight of sires (Fig. 1B). We also measured their body composition by Echo MRI (NMR spectroscopy) and found that DCHP exposure affected neither lean nor fat mass of these mice (Fig. 1C). In addition, paternal DCHP treatment affected neither litter size nor birth weight of F1 offspring (Fig. 1D and 1E).
3.2. Paternal DCHP exposure leads to exacerbated insulin resistance and impaired insulin signaling in F1 offspring without affecting diet-induced obesity
F1 pups were weaned on postnatal day 21 and fed a ND or high-fat diet (HFD) (Helsley et al., 2016; Lu et al., 2019; Sui et al., 2014; Wang et al., 2018) for 9 weeks before euthanasia at 12 weeks of age. To determine whether paternal DCHP exposure affected diet-induced obesity in the offspring, the body weight of male and female F1 offspring was measured weekly. In addition, body composition was analyzed by EchoMRI. Body weight and fat mass were significantly increased following HFD feeding in both male and female F1 mice. However, paternal DCHP exposure had no effect on the growth curve or body composition (e.g., lean and fat mass) of ND or HFD-fed male and female F1 offspring (Fig. 2). Therefore, paternal DCHP exposure did not affect diet-induced obesity in the F1 offspring.
Fig. 2. Paternal DCHP exposure does not affect diet-induced obesity in the F1 offspring.
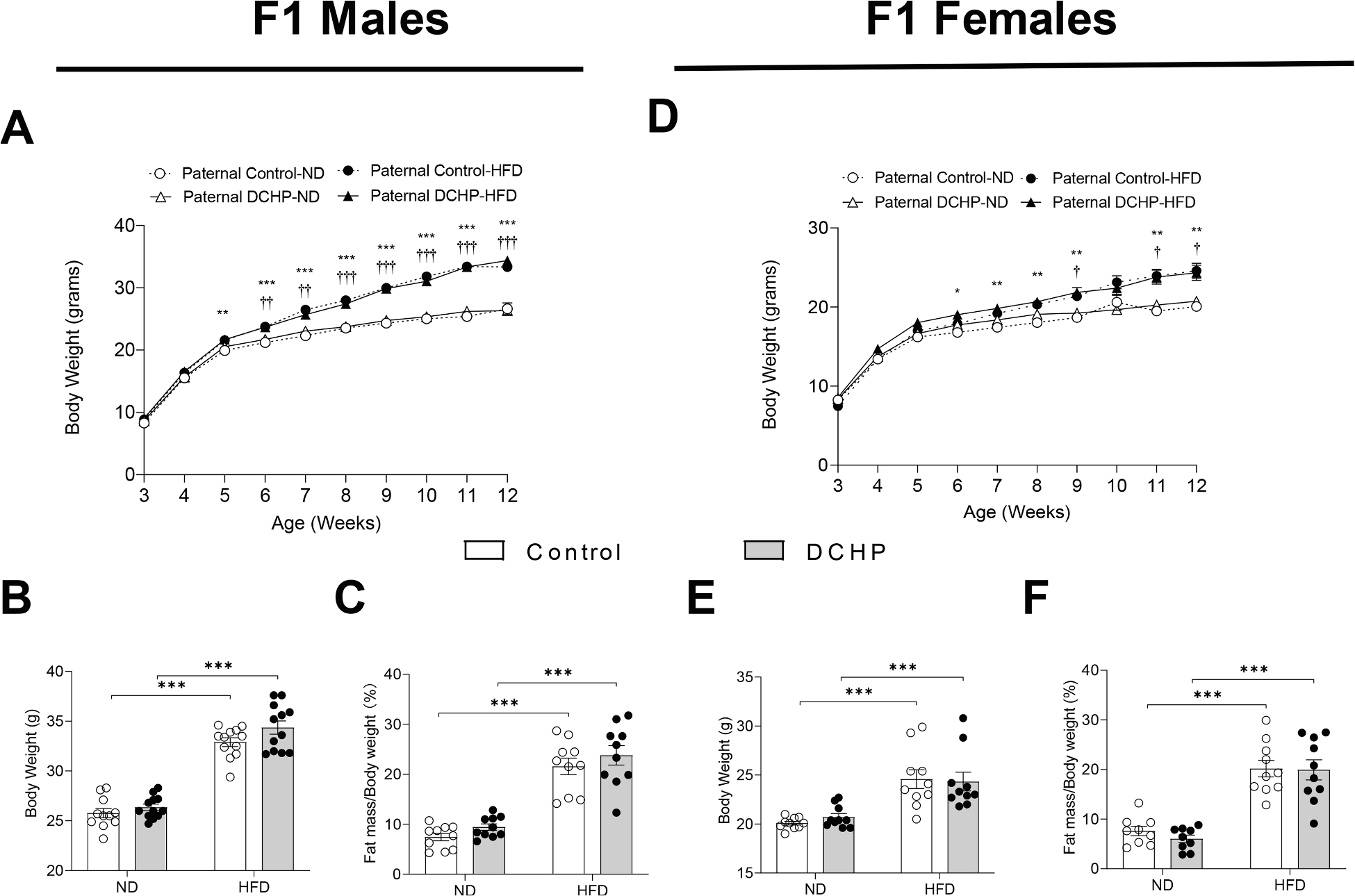
Eight-week-old male WT mice were treated with vehicle control or 10 mg/kg/day of DCHP by oral gavage for 4 weeks. F1 descendants of control or DCHP-exposed sires were fed a ND or HFD for 9 weeks. Growth curve (A and D), final body weight (B and E), and fat mass (percentage of body weight) (C and F) of male and female F1 mice were measured (n = 9–12, two-way ANOVA followed by Bonferroni’s multiple comparison test). Statistically significant differences between ND and HFD fed F1 mice from control sires were indicated with * (*P < 0.05, **P < 0.01, and ***P < 0.001). Statistically significant differences between ND and HFD fed F1 mice from DCHP sires were indicated with † (†P < 0.05, ††P < 0.01, and †††P < 0.001).
Despite similar body weight and adiposity, paternal DCHP exposure led to increased diabetic phenotypes in F1 offspring. We performed glucose and insulin tolerance tests (GTT & ITT) in those mice and found that both male and female offspring from paternal DCHP-exposed sires had worse glucose tolerance (Fig. 3A and 3C) and exhibited reduced hypoglycemic response to administered insulin (Fig. 3B and 3D). To understand the impact of paternal DCHP exposure on insulin signaling in obese F1 offspring, HFD-fed F1 male mice were treated with insulin prior to euthanasia, and phosphorylated Akt levels were evaluated in multiple tissues. We found that paternal DCHP exposure impaired Akt phosphorylation in response to insulin in liver, skeletal muscle, and white adipose tissue (WAT) (Fig. 3E), which indicates impaired insulin signaling in multiple tissues of the offspring.
Fig. 3. Paternal DCHP exposure leads to exacerbated diabetic phenotypes and impaired insulin signaling in HFD-fed F1 offspring.
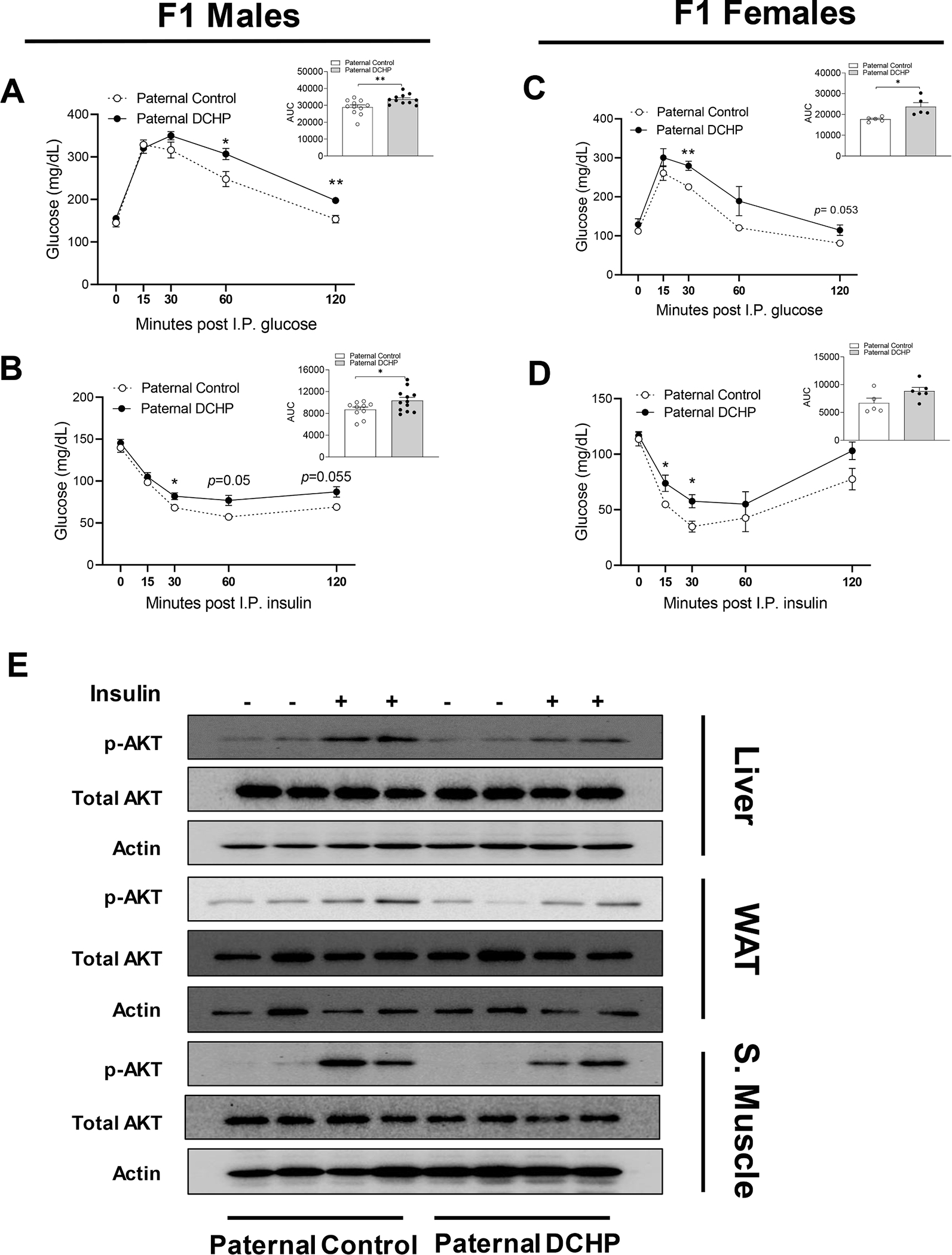
Eight-week-old male WT mice were treated with vehicle control or 10 mg/kg/day of DCHP by oral gavage for 4 weeks. F1 descendants of control or DCHP-exposed sires were fed a HFD for 9 weeks. (A-D) Glucose tolerance test (GTT) and the area under the curve (AUC) of GTT (A and C), and insulin tolerance test (ITT) and AUC of ITT (B and D) in HFD-fed F1 male and female offspring (n = 5–12, two-sample, two tailed Student’s t-test, *P < 0.05 and **P < 0.01). (E) Immunoblotting of phosphorylated Akt and total Akt levels in the liver, white adipose tissue (WAT), and skeletal muscle of F1 male mice injected with saline or 0.35 units/kg body weight insulin (n = 3).
3.3. Paternal exposure to DCHP induces hepatic transcriptomic changes in F1 offspring
To further understand transcriptomic changes in key organs (e.g., liver) from paternal DCHP exposure that may have contributed to the metabolic disorders of F1 offspring, we isolated total RNA from the liver of F1 males and performed RNA sequencing (RNA-seq) analysis. Liver plays a central role in whole-body glucose homeostasis (Lin and Accili, 2011; Nordlie et al., 1999) and previous studies also reported that paternal EDC exposure can affect key hepatic signaling in the offspring to increase insulin resistance (Gong et al., 2021). RNA-seq results showed that paternal DCHP exposure led to 289 differentially expressed genes (DEGs, 227 upregulated and 62 downregulated) in the liver of F1 offspring with a cut-off threshold of fold change (FC) > 1.5 and false discovery rate (FDR) < 0.1 (Fig. 4A and Supplemental Table 2). Gene Oncology (GO) Biological Process analysis was then performed and the results revealed DEGs were enriched in biological processes related to metabolic disease risk including “inflammatory response”, “lipid metabolism process”, and “cellular response to leptin stimulus”. (Fig. 4B and C) (Hernandez and Zhou, 2021; Hotamisligil and Erbay, 2008; Saengnipanthkul et al., 2021). The hepatic DEGs associated with these pathways were either upregulated or downregulated in F1 offspring of DCHP-exposed sires (Fig. 4C and Supplemental Table 2).
Fig. 4. Paternal DCHP exposure alters hepatic transcriptome in F1 male offspring.
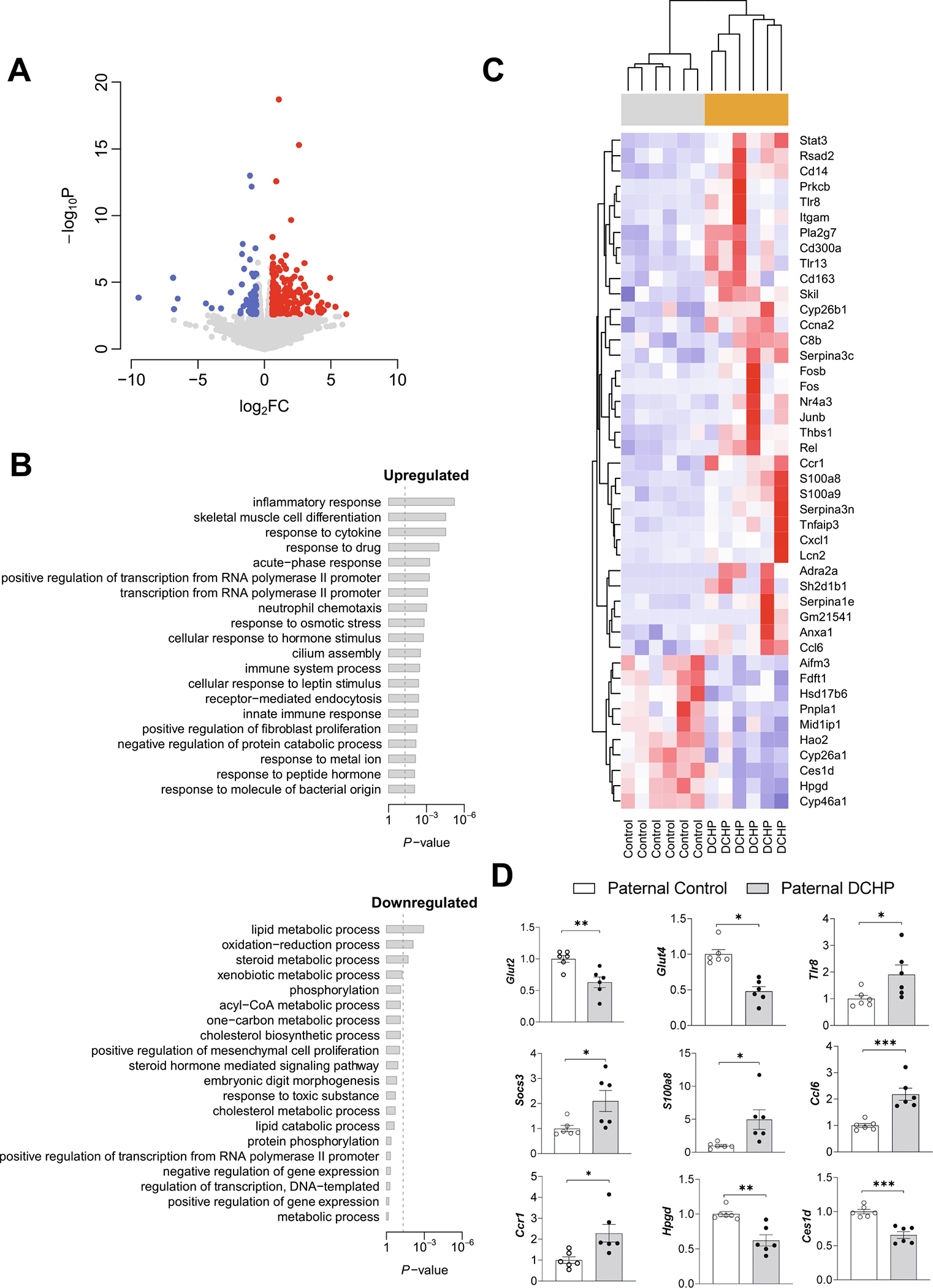
Eight-week-old male WT mice were treated with vehicle control or 10 mg/kg/day of DCHP by oral gavage for 4 weeks. F1 descendants of control or DCHP-exposed sires were fed a HFD for 9 weeks. Total RNAs were extracted from the liver of F1 male mice for RNA-seq analysis. (A) Volcano plot of differentially expressed genes (DEGs) between F1 descendants of DCHP-exposed sires and F1 descendants of control sires. Colored dots represent the up-regulated (red) and down-regulated (blue) hepatic DEGs of F1 offspring with a false discovery rate (FDR) < 0.1 and a fold change (FC) > 1.5 as a cut-off threshold. (B) Gene Ontology (GO) Biological Process terms associated with the hepatic DEGs of F1 offspring. The P-values were computed by Fisher’s exact test. The vertical dash line indicates the significance level of α = 0.05. The y-axis displays the GO terms while the x-axis displays the adjusted P-values. (C) Heatmap representation of DEGs involved in the GO Biological Processes of “inflammatory response”, “lipid metabolism process”, “cellular response to leptin stimulus”, “immune system process”, “oxidation–reduction process’, “neutrophil chemotaxis”, “response to cytokine’, and “ cellular response to hormone stimulus” shown in panel B. Each row shows one individual genes and each column shows a biological replicate of mouse. Red represents relatively increased gene expression while blue denotes downregulation. (D) QPCR analyses of representative hepatic genes of F1 offspring (n = 6, two-sample, two tailed Student’s t-test, *P < 0.05, **P < 0.01, and ***P < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We next performed QPCR analysis to verify the expression changes of several DEGs discovered by RNA-seq analysis. Paternal DCHP exposure consistently caused significantly increased hepatic expression of genes known to be associated with inflammation or metabolic disorders (e.g., Tlr8, Ccl6, Ccr1, and S100a8) (Fig. 4D) (Ahmad et al., 2016; Catalán et al., 2011; Jiao et al., 2009; Lylloff et al., 2017; Zeyda and Stulnig, 2009). By contrast, several genes known to promote insulin sensitivity (e.g., Hpgd and Ces1d) (Fig. 4D) (Li et al., 2022; Quiroga et al., 2012; Schmidleithner et al., 2019) were downregulated in the liver of F1 offspring of DCHP-exposed sires. Further, QPCR analyses showed paternal DCHP exposure led to decreased hepatic expression of Glut2 and Glut4, two key genes involved in hepatic glucose homeostasis (Fig. 4D). Collectively, these results suggest paternal DCHP exposure leads to altered hepatic genes and pathways associated with insulin resistance or metabolic disorders.
3.4. PANDORA-seq unveils paternal DCHP exposure-elicited sperm tsRNA and rsRNA changes
We and others have demonstrated that sperm sncRNAs, including tsRNAs and rsRNAs, can sensitively respond to environmental exposures and act as causative agents in mediating offspring’s metabolic phenotypes (Chen et al., 2016; Frye et al., 2018; Zhang et al., 2016; Zhang et al., 2019; Zhang et al., 2018). However, many of these sncRNAs bear various RNA modifications, preventing the discovery of highly modified sperm tsRNAs and rsRNAs in widely used traditional RNA-seq methods (Shi et al., 2021; Shi et al., 2022). To overcome this obstacle, we developed a novel small RNA sequencing method, PANDORA-seq, to eliminate RNA modification-elicited sequence interferences (Shi et al., 2021).
To determine whether PANDORA-seq can detect novel sperm sncRNAs that may confer metabolic dysfunction in the offspring of DCHP-exposed sires, total RNAs isolated from sperms of control and DCHP-exposed sires were subjected to both traditional RNA-seq and PANDORA-seq, and the sequencing data were analyzed by SPORTS1.1 bioinformatics analysis software (Shi et al., 2018; Shi et al., 2021). Consistent with our previous studies (Shi et al., 2021), PANDORA-seq revealed an overall rsRNA- and tsRNA-enriched sperm sncRNA landscape that was not detected by traditional RNA-seq (Fig. 5A). The origins of sperm tsRNAs in regards to their loci from tRNA precursors including 3′tsRNAs, 5′tsRNAs, 3′tsRNAs with a CCA end, and internal tsRNAs, were also analyzed. PANDORA-seq revealed an increased relative expression (normalized to miRNAs) of specific tsRNA origins as compared to traditional RNA-seq (Fig. 5B). Further, exposure to DCHP induced differentially expressed tsRNAs and rsRNAs was detected only by PANDORA-seq but not by traditional RNA-seq, and the significantly changed tsRNA and rsRNA sequences are shown in the heatmap (Fig. 5C and Supplemental Table 3). Consistently, mapping of tsRNA expression patterns on individual tRNA length scales (e.g., tRNA-Glu-CTC, tRNA-Arg-CCT) revealed that the tsRNAs also contain distinct dynamic responses to DCHP treatment (Fig. 5D). These results showed that PANDORA-seq can detect more sperm tsRNAs and rsRNAs that were otherwise undetectable using the traditional RNA-seq method, consistent with previous results (Shi et al., 2021). The functions of individual tsRNAs or rsRNAs are mostly unknown, but their overall changed signature after DCHP treatment may have functional consequences that contribute to DCHP-induced intergenerational metabolic disorders.
Fig. 5. PANDORA-seq identifies significantly changed tsRNAs and rsRNAs in the sperm of DCHP treated sires.
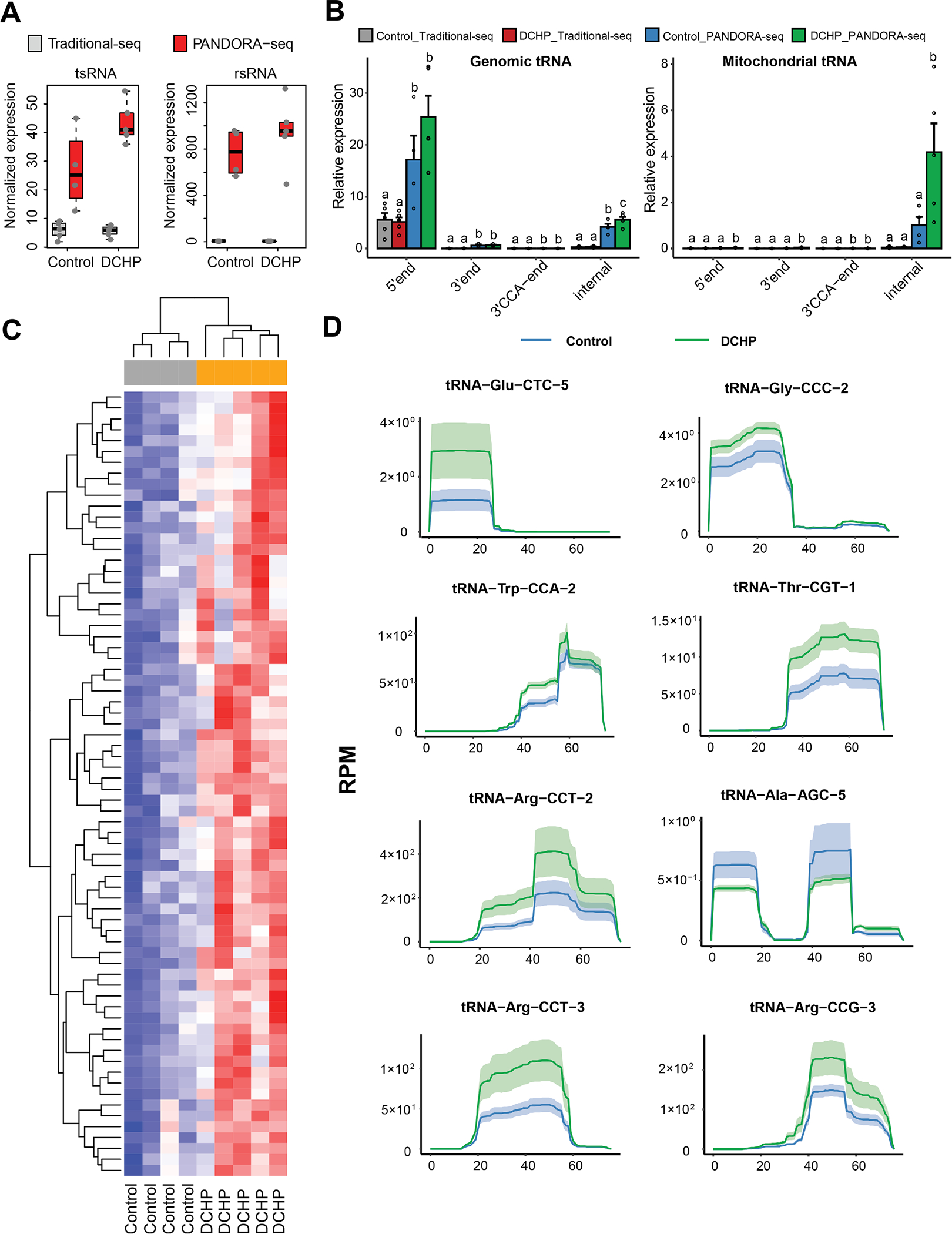
Eight-week-old male WT mice were treated with vehicle control or 10 mg/kg/day of DCHP by oral gavage for 4 weeks. Total RNAs were isolated from the sperms of F0 male mice and used for PANDORA-seq or traditional small RNA sequencing. (A) Normalized tsRNA and rsRNA (normalized to miRNAs) under traditional-seq and PANDORA-seq protocols. (B) tsRNA responses to traditional-seq and PANDORA-seq corresponding to different origins (5′ tsRNA, 3′ tsRNA, 3′ tsRNA-CCA end, and internal tsRNAs). The y axes represent the relative expression levels compared with total reads of miRNAs. Statistical significance was determined by two-sided one-way ANOVA with uncorrected Fisher’s LSD test. Bars bearing different letters above were significantly different from each other (P < 0.05). All data are plotted as means ± SEM (n = 4–5 in each group). (C) Relative expression heatmap of the differentially expressed tsRNAs and rsRNAs in the sperm of control or DCHP-exposed mice detected by PANDORA-seq (n = 4–5). Each row shows one individual tsRNA or rsRNA and each column shows a biological replicate of mouse. Red represents higher relative expression while blue denotes lower relative expression. (D) Dynamic responses to vehicle control or DCHP of representative individual tsRNAs detected by PANDORA-seq. The solid curves indicate the mean reads per million (RPM) values for the control and DCHP-exposed groups. The colored bands represent the 95 % confidence interval (n = 4–5). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Paternal exposure to DCHP leads to impaired glucose tolerance in F2 female mice
In addition to F1 offspring, we also mated ND-fed 12-week-old F1 males to age-matched unexposed female WT mice to generate F2 offspring. After weaning, F2 litters were fed the same HFD as the F1 descendants for 9 weeks. Similar to F1 offspring, paternal DCHP exposure had no effect on litter size or birth weight of F1 offspring (Fig. 6A and B). In addition, paternal DCHP exposure affected neither diet-induced obesity nor body composition, including lean and fat mass, in HFD-fed male and female F2 offspring (Fig. 6A–H).
Fig. 6. Paternal DCHP exposure does not affect the litter size, birth weight, and growth curves of F2 offspring.
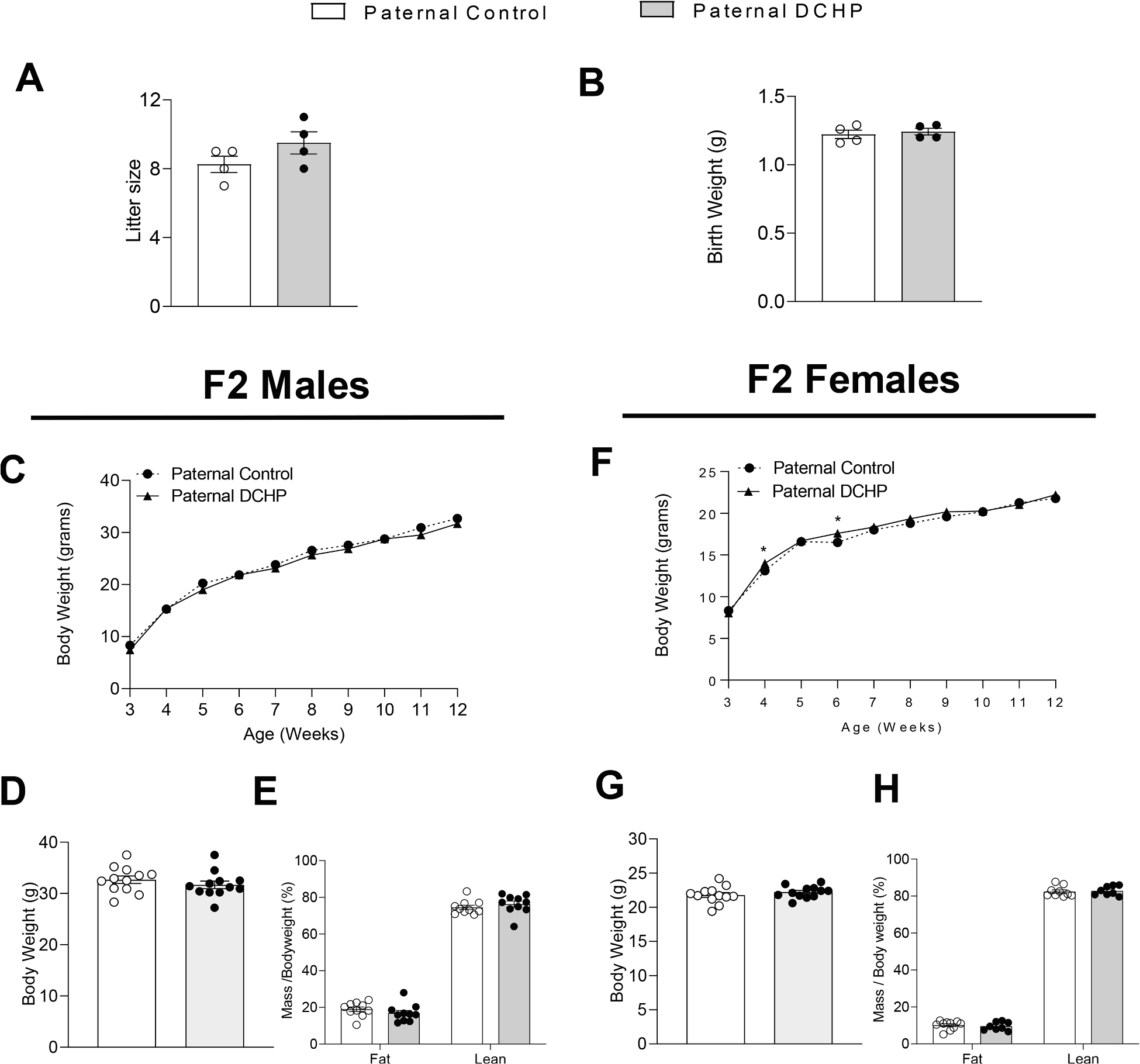
F2 descendants of control or DCHP-exposed sires were fed a HFD for 9 weeks. Litter size (A) and average birth weights (B) of the F2 mice (n = 4). The growth curves, final body weight, and lean and fat mass (percentage of body weight) of the F2 male (C-E) and female (F-H) offspring (n = 8–12, two-sample, two tailed Student’s t-test, *P < 0.05).
Interestingly, female but not male F2 mice from DCHP exposed F0 sires had impaired glucose tolerance, but comparable insulin tolerance results, as compared to F2 mice from unexposed sires (Fig. 7A–D), suggesting paternal DCHP exposure has sex-specific transgenerational effects on offspring metabolic health. We analyzed hepatic gene expression to understand the potential mechanisms underlying the glucose intolerant phenotype in F2 female mice. Several genes, including Glut 2 and Tlr8, that were altered by paternal DCHP exposure in F1 mice were unchanged in F2 mice (Fig. 7E). However, we found that another glucose transporter family gene, Glut9, was significantly downregulated by paternal DCHP exposure in the liver of F2 female mice (Fig. 7E). Further, paternal DCHP exposure significantly increased the hepatic expression of Socs3 in F2 females, consistent with the expression patten in F1 offspring (Fig. 7E). These results suggest that paternal DCHP exposure can have transgenerational impact on hepatic gene expression that may contribute to the observed metabolic disorders.
Fig. 7. Paternal DCHP exposure elicits sex-specific transgenerational effects in F2 offspring.
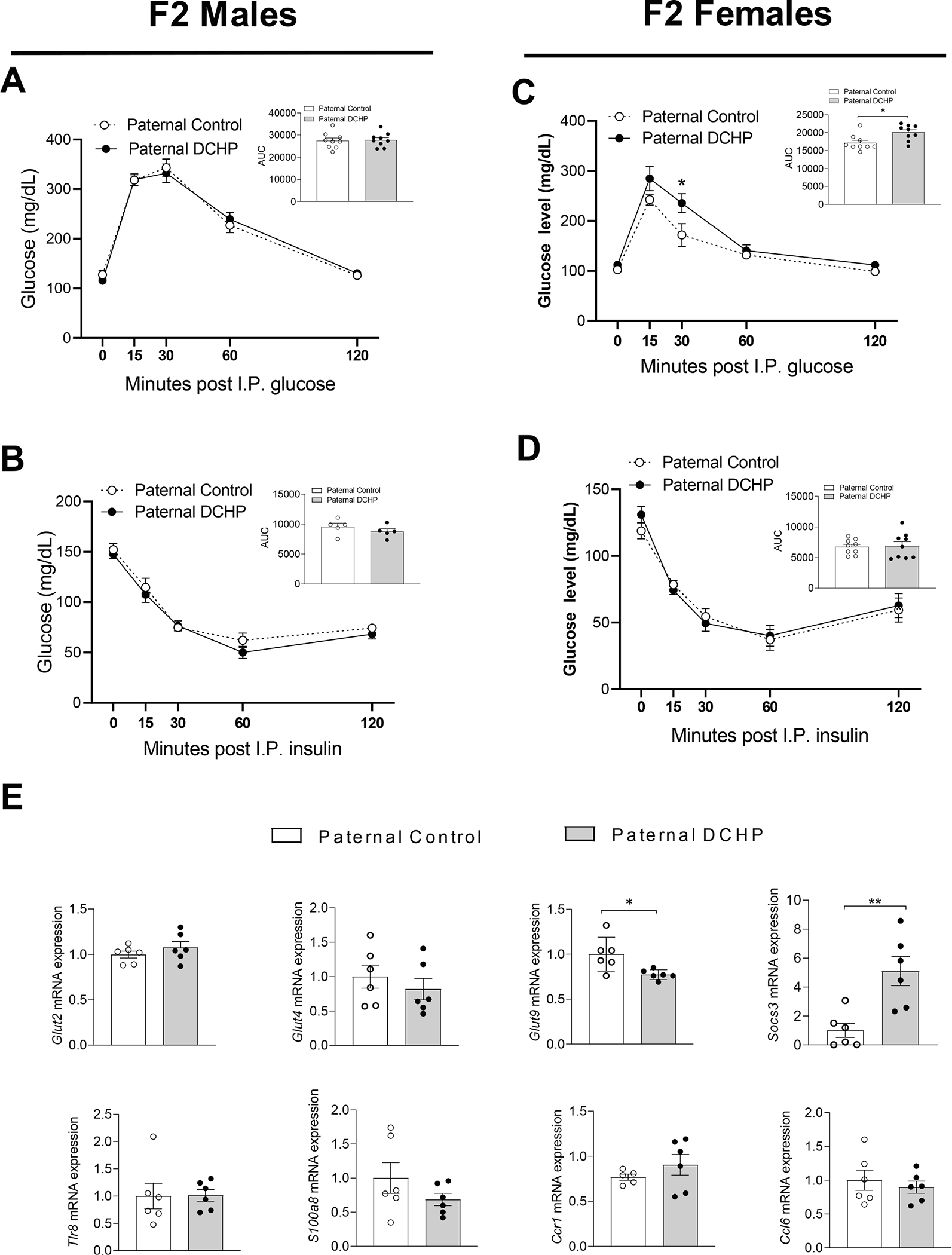
F2 descendants of control or DCHP-exposed sires were fed a HFD for 9 weeks. (A-D) Glucose tolerance test (GTT) and the area under the curve (AUC) of GTT (A,C), and insulin tolerance test (ITT) and AUC of ITT (B,D) in HFD-fed F2 offspring (n = 5–9, two-sample, two tailed Student’s t-test, *P < 0.05). (E) QPCR analyses of hepatic gene expression of F2 female mice (n = 6, two-sample, two tailed Student’s t-test, *P < 0.05 and ** P < 0.01).
4. Discussion
Mounting evidence demonstrates exposure to EDCs can cause numerous adverse health effects in humans (Casals-Casas and Desvergne, 2011; Colborn et al., 1996; Diamanti-Kandarakis et al., 2009; Gore et al., 2015; Gross, 2007; Heindel et al., 2017; Helsley and Zhou, 2017; Kavlock et al., 1996). One challenge in risk assessment for EDC exposure is that individuals may or may not display health issues from the direct EDC exposure, but the adverse effects of these ancestral exposures may manifest in their non-exposed offspring. In our current study, we investigated the adverse effects of paternal exposure to DCHP, a widely used phthalate, on offspring metabolic health. We found that paternal DCHP exposure can induce intergenerational metabolic disorders in the F1 offspring. Using an innovative PANDORA-seq method, we revealed that DCHP exposure can lead to sperm tsRNA and rsRNA landscape changes undetected by traditional RNA-seq. Paternal DCHP exposure-elicited metabolic phenotype in the F1 offspring can be transmitted to F2 offspring, as F2 female descendants from DCHP-exposed sires also developed glucose intolerance. To the best of our knowledge, the current study is the first one demonstrating that paternal exposure to endocrine disrupting phthalates can induce intergenerational and transgenerational metabolic disorders in the offspring.
Previous studies have demonstrated the detrimental effects of parental exposure to plastic-associated EDCs, including BPA and several phthalates, on the long-term health outcome of progeny (Bansal et al., 2017; Brehm and Flaws, 2019; Li et al., 2014; Meltzer et al., 2015; Rattan et al., 2018; Zhou et al., 2017). These adverse effects include altered anogenital distance (Mammadov et al., 2018; Miao et al., 2011; Sun et al., 2018), impaired glucose/insulin tolerance (Li et al., 2014; Mao et al., 2015), altered neonatal behavior (Engel et al., 2008), and cardiometabolic disorders (Liu et al., 2022). However, most evidence has been obtained from maternal exposure studies, and little is known about the impact of paternal EDC exposure on offspring health. Further, BPA and several other phthalates (e.g., DEHP) have been extensively studied, and their adverse health effects have attracted considerable attention (Casals-Casas and Desvergne, 2011; Clara et al., 2010; Fierens et al., 2012; Halden, 2010). However, the impact of exposure to DCHP on human health are poorly understood. DCHP is used in numerous products and can also be detected in food, water, and indoor particulate matter (Cao et al., 2015; Cheng et al., 2016; EPA, 2019; Sakhi et al., 2014; Schecter et al., 2013). DCHP and its metabolites can also be found in human urinary and blood samples, and DCHP exposure levels can be high in certain populations (Blount et al., 2000; Hartmann et al., 2018; Huang et al., 2014; Saravanabhavan et al., 2013; Wang et al., 2013). Therefore, EPA has recently designated DCHP as one of twenty high-priority substances for risk evaluation (EPA, 2019). Our study revealed the intergenerational and transgenerational effects of paternal DCHP exposure on the F1 and F2 offspring. Therefore, future studies should be conducted on the adverse health effects of these understudied phthalates.
While paternal DCHP exposure induced metabolic disorders in both male and female F1 offspring, it is intriguing that female but not male F2 offspring from DCHP-exposed sires showed impaired glucose tolerance phenotype. These results indicated that paternal DCHP exposure can lead to sex-specific transgenerational effects on the metabolic health of their progenies. Previous human epidemiological studies and animal studies have reported sexual dimorphic responses to early life perturbations, from either ancestral side (Aiken and Ozanne, 2013; Sandovici et al., 2022; Vallaster et al., 2017; Vickers et al., 2011). Most of the existing paradigms regarding sex-specific inheritance traits are intergenerational, whereas evidence regarding transgenerational sex-different transmission is very limited. For example, Gong et al. found that paternal inorganic arsenic exposure elicited hepatic insulin resistance and glucose intolerance in F1 female but not male mice (Gong et al., 2021). Similarly, another study showed only female offspring of HFD-fed mice displayed glucose intolerance and resistance to diet-induced obesity (de Castro Barbosa et al., 2016). By contrast, Maloney et al., reported that maternal methyl-deficient diet feeding during the peri-conception period led to impaired glucose homeostasis only in male offspring (Maloney et al., 2011). Another study reported that paternal chronic social defeat preferentially affects the locomotor activity and sucrose preference in male offspring but the altered depression- and anxiety-related behavior was observed in both genders (Dietz et al., 2011). Our current study only used F1 males to breed with unexposed female mice to generate F2 offspring. It would be interesting to investigate whether F2 offspring generated by mating F1 females with unexposed male mice have similar sex-specific metabolic phenotypes in the future. While the underlying mechanisms for sex-specific inheritance are largely unknown, some hypotheses have been proposed, including epigenetic modifications on sex chromosomes (Pembrey et al., 2006), sex differences related to mitochondiral DNA and sncRNAs (Godschalk et al., 2017; Wang et al., 2022a; Wang et al., 2022b), and sex hormone-induced differential gene expression (Carone et al., 2010; Tramunt et al., 2020; Waxman and O’Connor, 2006). Therefore, future studies are required to test those hypotheses and understand how parental exposure affects sex-specific effects on the offspring’s health.
To understand how paternal DCHP exposure affects genes or pathways in key organs that may contribute to the observed phenotypes in the offspring, we performed RNA-seq and QPCR analyses of hepatic genes in F1 mice. Our results revealed the altered expression of key genes or pathways by paternal DCHP exposure. In addition to reduced glucose transporters (e.g., Glut2 and Glut4), we also found higher expression of inflammatory genes or inflammatory pathway genescores in the liver of F1 mice from paternal DCHP-exposed sires. It has been well-established that chronic inflammation contributes to the pathogenesis of metabolic disorders or insulin resistance (Gregor and Hotamisligil, 2011; Hernandez and Zhou, 2021; Kahn et al., 2006; Shoelson et al., 2006). Similar to our findings, Bansal et al., previously reported that maternal exposure to BPA impaired insulin secretion and increased proinflammatory cytokines in F1 and F2 male mice (Bansal et al., 2017). Paternal DCHP exposure led to increased expression of hepatic Socs3 in both F1 and F2 offspring. Socs3 can be induced by certain inflammatory cytokines (e.g., TNFα and IL6) to contribute to inflammation-mediated insulin resistance in the liver and adipose tissue (Cao et al., 2018; Jorgensen et al., 2013; Torisu et al., 2007). It is plausible that Socs3 and other inflammatory genes contribute to paternal DCHP exposure-elicited metabolic phenotypes in the offspring.
While accumulating evidence shows that paternal environmental input (e.g., dietary manipulation, exercise, toxicant exposure) may confer lifetime metabolic phenotype alterations in offspring (Gong et al., 2021; Oluwayiose et al., 2021; Ost et al., 2014; Stanford et al., 2018; Wang et al., 2022a; Watkins et al., 2018; Zhang et al., 2021), the underlying mechanisms for paternally acquired phenotypes remain elusive. We and others have recently demonstrated that paternally acquired phenotypic traits are achieved at least partially through sperm sncRNAs (Chen et al., 2016; Gapp et al., 2014; Grandjean et al., 2015; Klastrup et al., 2019; Ord et al., 2020; Rodgers et al., 2015; Sarker et al., 2019; Wang et al., 2021; Zhang et al., 2021; Zhang et al., 2018). For example, we previously discovered that mature mouse sperms carry a dominant form of small RNAs including tsRNAs and rsRNAs (Chen et al., 2016; Peng et al., 2012; Zhang et al., 2018). We demonstrated that the injection of sperm tsRNA/rsRNA-enriched RNA fractions (30–40nt) from HFD-exposed male mice into control zygotes can lead to the development of metabolic disorders in F1 mice (Chen et al., 2016; Zhang et al., 2018). We further showed that RNA modifications can alter secondary sncRNA structures and biological properties and are required for sperm sncRNAs’ biological impact in epigenetic inheritance (Zhang et al., 2018). These studies support the concept of ‘sperm RNA code’ in programming offspring metabolic phenotypes (Chen, 2022; Zhang et al., 2019). However, many RNA modifications prevent the detection of important sncRNAs in widely used RNA-seq methods (Shi et al., 2022). To overcome this obstacle, we developed a novel RNA sequencing method, PANDORA-seq (Shi et al., 2021). In the current study, DCHP exposure led to changes in sperm tsRNAs and rsRNAs that were detectable by PANDORA-seq but not traditional RNA sequencing.
It remains unclear how DCHP causes sperm tsRNA and rsRNA changes. We recently demonstrated that DCHP is a ligand of a nuclear hormonal receptor, pregnane X receptor (PXR) and DCHP may induce adverse effects in adult mice through PXR signaling (Sui et al., 2021). It is plausible that DCHP-mediated activation of PXR or other signaling pathways may regulate biogenesis of sperm tsRNAs and rsRNA. In addition, it is also possible that DCHP altered sperm sncRNAs through indirect mechanisms. For example, previous studies demonstrated that exposure to various environmental factors including stress, malnutrition, and chemicals can alter the sperm RNA profiles (Chen et al., 2016; Gapp et al., 2014; Rodgers et al., 2013; Rodgers et al., 2015; Rompala et al., 2018). These paternal preconceptual life experiences can also lead to the dysregulation of hypothalamic–pituitaryadrenal (HPA) axis and altered physical activities in the offspring (Rodgers et al., 2013; Short et al., 2016; Short et al., 2017), which may contribute to the development of metabolic disorders (Broadney et al., 2018; Stull, 2016; Wareham et al., 2000). It would be interesting to investigate whether DCHP exposure can also affect physiological and behavioral phenotypes of the sires and their offspring in the future by characterizing HPA stress axis responsivity, prepulse inhibition, performance on the tail suspension test, or performance in the light–dark box (Rodgers et al., 2013). In addition, detailed measurements of food and water consumption, physical activities, and indirect calorimetry using metabolic cages as we previous described (Park et al., 2016; Sui et al., 2014) would help us to understand the impact of DCHP exposure on energy balance and physical activities of the sires and their offspring. DCHP-elicited unfavorable changes of those factors may lead to altered sperm sncRNAs including tsRNAs and rsRNAs. Lastly, the functions of the altered tsRNAs and rsRNAs by DCHP are also unknown. Further studies, including the isolation and zygotic injection of individual tsRNAs/rsRNAs, are needed to explore the functions of these tsRNAs/rsRNAs in mediating DCHP and other EDC-induced intergenerational and transgenerational metabolic disorders.
In summary, paternal exposure to DCHP, an EPA-designated high-priority substance for risk evaluation, can cause intergenerational and transgenerational adverse metabolic effects in F1 and F2 offspring without affecting diet-induced obesity. Paternal DCHP exposure led to impaired insulin signaling and altered hepatic gene expression in their offspring. Using a novel PANDORA-seq, we revealed that DCHP exposure can lead to sperm tsRNA/rsRNA landscape changes undetected by traditional RNA-seq, which may contribute to DCHP-elicited adverse effects. Our study suggests that exposure to ubiquitous phthalates may not only have adverse effects on adults but also increase the risk of metabolic diseases in their progeny. These findings are intended to increase our understanding of the etiology of chronic human diseases originating from chemically-elicited intergenerational effects.
Supplementary Material
Acknowledgements
We thank all lab members for their technical assistance and Dr. Linlin Zhao lab for preparing recombinant AlkB. We also thank Ms. Jo Gerrad for proofreading of our manuscript and providing suggestions. This work was partially supported by National Institutes of Health grants (R01ES023470, R01ES032024, R01HL167206, and R01HL131925) and American Heart Association grant (19TPA34890065). R.H. was supported by a National Institutes of Health T32 training grant (T32ES018827) and an American Heart Association predoctoral fellowship (23PRE1018751).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Jingwei Liu: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Visualization, Writing – original draft. Junchao Shi: Methodology, Formal analysis, Investigation, Data curation, Visualization, Writing – original draft. Rebecca Hernandez: Methodology, Validation, Formal analysis, Investigation. Xiuchun Li: Methodology, Validation, Formal analysis, Investigation. Pranav Konchadi: Formal analysis, Investigation. Yuma Miyake: Formal analysis, Investigation. Qi Chen: Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Funding acquisition. Tong Zhou: Software, Formal analysis, Data curation, Visualization, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Changcheng Zhou: Conceptualization, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2023.107769.
Data availability
Data will be made available on request.
References
- Ahmad R, Kochumon S, Thomas R, Atizado V, Sindhu S, 2016. Increased adipose tissue expression of TLR8 in obese individuals with or without type-2 diabetes: significance in metabolic inflammation. J. Inflamm. 13, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken CE, Ozanne SE, 2013. Sex differences in developmental programming models. Reproduction 145, R1–R13. [DOI] [PubMed] [Google Scholar]
- Bansal A, Rashid C, Xin F, Li C, Polyak E, Duemler A, van der Meer T, Stefaniak M, Wajid S, Doliba N, 2017. Sex-and dose-specific effects of maternal bisphenol A exposure on pancreatic islets of first-and second-generation adult mice offspring. Environ. Health Perspect. 125, 097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW, 2000. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect 108, 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm E, Flaws JA, 2019. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 160, 1421–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadney MM, Belcher BR, Berrigan DA, Brychta RJ, Tigner IL Jr., Shareef F, Papachristopoulou A, Hattenbach JD, Davis EK, Brady SM, Bernstein SB, Courville AB, Drinkard BE, Smith KP, Rosing DR, Wolters PL, Chen KY, Yanovski JA, 2018. Effects of Interrupting Sedentary Behavior With Short Bouts of Moderate Physical Activity on Glucose Tolerance in Children With Overweight and Obesity: A Randomized Crossover Trial. Diabetes Care 41, 2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wang Z, Wan W, 2018. Suppressor of cytokine signaling 3: emerging role linking central insulin resistance and Alzheimer’s disease. Front. Neurosci. 12, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XL, Zhao W, Dabeka R, 2015. Di-(2-ethylhexyl) adipate and 20 phthalates in composite food samples from the 2013 Canadian Total Diet Study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32, 1893–1901. [DOI] [PubMed] [Google Scholar]
- Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, Meissner A, Weng Z, Hofmann HA, Friedman N, Rando OJ, 2010. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B, 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73, 135–162. [DOI] [PubMed] [Google Scholar]
- Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Fernandez-Real JM, Salvador J, Frühbeck G, 2011. Increased´ Levels of Calprotectin in Obesity Are Related to Macrophage Content: Impact on Inflammation and Effect of Weight Loss. Mol. Med. 17, 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, 2022. Sperm RNA-mediated epigenetic inheritance in mammals: challenges and opportunities. Reprod Fert Develop 35, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng GH, Peng H, Zhang X, Zhang Y, Qian J, Duan E, Zhai Q, Zhou Q, 2016. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Li HH, Wang HS, Zhu XM, Sthiannopkao S, Kim KW, Yasin MSM, Hashim JH, Wong MH, 2016. Dietary exposure and human risk assessment of phthalate esters based on total diet study in Cambodia. Environ. Res. 150, 423–430. [DOI] [PubMed] [Google Scholar]
- Clara M, Windhofer G, Hartl W, Braun K, Simon M, Gans O, Scheffknecht C, Chovanec A, 2010. Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere 78, 1078–1084. [DOI] [PubMed] [Google Scholar]
- Colborn T, Dumanoski D, Meyers JP, 1996. Our Stolen Future. Dutton, New York, p. 306. [Google Scholar]
- Cozen AE, Quartley E, Holmes AD, Hrabeta-Robinson E, Phizicky EM, Lowe TM, 2015. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat. Methods 12, 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxinger L, Whitelaw E, 2012. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet 13, 153–162. [DOI] [PubMed] [Google Scholar]
- de Castro Barbosa T, Ingerslev LR, Alm PS, Versteyhe S, Massart J, Rasmussen M, Donkin I, Sjögren R, Mudry JM, Vetterli L, Gupta S, Krook A, Zierath JR, Barrès R, 2016. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Molecular Metabolism 5, 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC, 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ, 2011. Paternal transmission of stress-induced pathologies. Biol Psychiatry 70, 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Calafat AM, Zhu C, Liao L, Silva MJ, Wolff MS, 2008. Prenatal Phthalate Exposure is Associated with Altered Neonatal Behavior in a Multiethnic Pregnancy Cohort. Epidemiology 19, S181–S182. [Google Scholar]
- EPA. 2019. Proposed Designation of Dicyclohexyl Phthalate (CASRN 84–61-7) as a High-priority Substance for Risk Evaluation. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100XPOZ.txt.
- Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J, 2014. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS One 9, e111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierens T, Servaes K, Van Holderbeke M, Geerts L, De Henauw S, Sioen I, Vanermen G, 2012. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem. Toxicol. 50, 2575–2583. [DOI] [PubMed] [Google Scholar]
- Frye M, Harada BT, Behm M, He C, 2018. RNA modifications modulate gene expression during development. Science 361, 1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM, 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godschalk R, Remels A, Hoogendoorn C, van Benthem J, Luijten M, Duale N, Brunborg G, Olsen A-K, Bouwman FG, Munnia A, Peluso M, Mariman E, van Schooten FJ, 2017. Paternal Exposure to Environmental Chemical Stress Affects Male Offspring’s Hepatic Mitochondria. Toxicol. Sci. 162, 241–250. [DOI] [PubMed] [Google Scholar]
- Gong Y, Xue Y, Li X, Zhang Z, Zhou W, Marcolongo P, Benedetti A, Mao S, Han L, Ding G, 2021. Inter-and Transgenerational Effects of Paternal Exposure to Inorganic Arsenic. Adv. Sci. 8, 2002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36, E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean V, Fourre S, De Abreu DA, Derieppe MA, Remy JJ, Rassoulzadegan M, 2015. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep 5, 18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS, 2011. Inflammatory mechanisms in obesity. Annu Rev Immunol 29, 415–445. [DOI] [PubMed] [Google Scholar]
- Gross L, 2007. The Toxic Origins of Disease. PLoS Biol 5, e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halden RU, 2010. Plastics and health risks. Annu. Rev. Public Health 31, 179–194. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Uhl M, Weiss S, Scharf S, Konig J, 2018. Austrian reference values for phthalate metabolite exposure in children/adolescents and adults. Int. J. Hyg. Environ. Health 221, 985–989. [DOI] [PubMed] [Google Scholar]
- Heard E, Martienssen RA, 2014. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, 2019. Environmental Obesogens: Mechanisms and Controversies. Annu Rev Pharmacol Toxicol 59, 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F, 2017. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 68, 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsley RN, Sui Y, Park SH, Liu Z, Lee RG, Zhu B, Kern PA, Zhou C, 2016. Targeting IkappaB kinase beta in Adipocyte Lineage Cells for Treatment of Obesity and Metabolic Dysfunctions. Stem Cells 34, 1883–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsley RN, Zhou C, 2017. Epigenetic impact of endocrine disrupting chemicals on lipid homeostasis and atherosclerosis: a pregnane X receptor-centric view. Environ Epigenet 3, dvx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez R, Zhou C, 2021. Recent Advances in Understanding the Role of IKKbeta in Cardiometabolic Diseases. Front Cardiovasc Med 8, 752337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Loher P, Shigematsu M, Palazzo JP, Suzuki R, Imoto I, Rigoutsos I, and Kirino Y. 2015. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc Natl Acad Sci U S A 112:E3816–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Erbay E, 2008. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 8, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, Wang L, Tan Y, Luo J, Qiu Z, Chen JA, Shu W, 2014. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One 9, e87430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H, 2009. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes 58, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SB, O’Neill HM, Sylow L, Honeyman J, Hewitt KA, Palanivel R, Fullerton MD, Öberg L, Balendran A, Galic S, van der Poel C, Trounce IA, Lynch GS, Schertzer JD, Steinberg GR, 2013. Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes 62, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM, 2006. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846. [DOI] [PubMed] [Google Scholar]
- Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA, 1996. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect 104 Suppl 4, 715–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klastrup LK, Bak ST, Nielsen AL, 2019. The influence of paternal diet on sncRNA-mediated epigenetic inheritance. Mol. Genet. Genomics 294, 1–11. [DOI] [PubMed] [Google Scholar]
- Lane M, Robker RL, Robertson SA, 2014. Parenting from before conception. Science 345, 756–760. [DOI] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D, 2008. Association of urinary bisphenol a concentration with medical disorders and laboratory abnormalities in adults. JAMA 300, 1303–1310. [DOI] [PubMed] [Google Scholar]
- Lee MK, Blumberg B, 2019. Transgenerational effects of obesogens. Basic Clin Pharmacol Toxicol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chang H, Xia W, Mao Z, Li Y, Xu S, 2014. F0 maternal BPA exposure induced glucose intolerance of F2 generation through DNA methylation change in Gck. Toxicol Lett 228, 192–199. [DOI] [PubMed] [Google Scholar]
- Li G, Li X, Yang L, Wang S, Dai Y, Fekry B, Veillon L, Tan L, Berdeaux R, Eckel-Mahan K, Lorenzi PL, Zhao Z, Lehner R, Sun K, 2022. Adipose tissue-specific ablation of Ces1d causes metabolic dysregulation in mice. Life Sci Alliance 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Accili D, 2011. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab 14, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Lind L, 2011. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis 218, 207–213. [DOI] [PubMed] [Google Scholar]
- Lind L, Lind PM, 2012. Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease? J. Intern. Med. 271, 537–553. [DOI] [PubMed] [Google Scholar]
- Lind PM, Zethelius B, Lind L, 2012. Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care 35, 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liao M, Huang R, You Y, Lin X, Yang H, Fan L, Zhong Y, Li X, Li J, Xiao X, 2022. Perinatal Combinational Exposure to Bisphenol A and a High-Fat Diet Contributes to Transgenerational Dysregulation of Cardiovascular and Metabolic Systems in Mice. Front Cell Dev Biol 10, 834346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Park SH, Meng Z, Wang F, Zhou C, 2019. Deficiency of Adipocyte IKKbeta Affects Atherosclerotic Plaque Vulnerability in Obese LDLR Deficient Mice. J. Am. Heart Assoc. 8, e012009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lylloff L, Bathum L, Madsbad S, Grundtvig JLG, Nordgaard-Lassen I, Fenger M, 2017. S100A8/A9 (Calprotectin), Interleukin-6, and C-Reactive Protein in Obesity and Diabetes before and after Roux-en-Y Gastric Bypass Surgery. Obes Facts 10, 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney CA, Hay SM, Young LE, Sinclair KD, Rees WD, 2011. A methyl-deficient diet fed to rat dams during the peri-conception period programs glucose homeostasis in adult male but not female offspring. J Nutr 141, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammadov E, Uncu M, Dalkan C, 2018. High Prenatal Exposure to Bisphenol A Reduces Anogenital Distance in Healthy Male Newborns. J Clin Res Pediatr Endocrinol 10, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Xia W, Chang H, Huo W, Li Y, Xu S, 2015. Paternal BPA exposure in early life alters Igf2 epigenetic status in sperm and induces pancreatic impairment in rat offspring. Toxicol Lett 238, 30–38. [DOI] [PubMed] [Google Scholar]
- Meltzer D, Martinez-Arguelles DB, Campioli E, Lee S, Papadopoulos V, 2015. In utero exposure to the endocrine disruptor di (2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod. Toxicol 51, 47–56. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS, 2010. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One 5, e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Luben R, Khaw KT, Wareham NJ, Galloway TS, 2012. Urinary bisphenol a concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125, 1482–1490. [DOI] [PubMed] [Google Scholar]
- Meng Z, Hernandez R, Liu J, Gwag T, Lu W, Hsiai TK, Kaul M, Zhou T, Zhou C, 2022. HIV Protein Tat Induces Macrophage Dysfunction and Atherosclerosis Development in Low-Density Lipoprotein Receptor-Deficient Mice. Cardiovasc Drugs Ther 36, 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M, Yuan W, He Y, Zhou Z, Wang J, Gao E, Li G, Li DK, 2011. In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res. A 91, 867–872. [DOI] [PubMed] [Google Scholar]
- Nordlie RC, Foster JD, Lange AJ, 1999. Regulation of glucose production by the liver. Annu. Rev. Nutr. 19, 379–406. [DOI] [PubMed] [Google Scholar]
- Olsen L, Lampa E, Birkholz DA, Lind L, Lind PM, 2012. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Ecotoxicol. Environ. Saf. 75, 242–248. [DOI] [PubMed] [Google Scholar]
- Oluwayiose OA, Marcho C, Wu H, Houle E, Krawetz SA, Suvorov A, Mager J, Richard Pilsner J, 2021. Paternal preconception phthalate exposure alters sperm methylome and embryonic programming. Environ Int 155, 106693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord J, Heath PR, Fazeli A, Watt PJ, 2020. Paternal effects in a wild-type zebrafish implicate a role of sperm-derived small RNAs. Mol. Ecol. 29, 2722–2735. [DOI] [PubMed] [Google Scholar]
- Ost A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, Ruf M, Rajewsky N, Reuter G, Iovino N, Ribeiro C, Alenius M, Heyne S, Vavouri T, Pospisilik JA, 2014. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 159, 1352–1364. [DOI] [PubMed] [Google Scholar]
- Park SH, Liu Z, Sui Y, Helsley RN, Zhu B, Powell DK, Kern PA, Zhou C, 2016. IKKbeta Is Essential for Adipocyte Survival and Adaptive Adipose Remodeling in Obesity. Diabetes 65, 1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M, Golding J, The AST, 2006. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 14, 159–166. [DOI] [PubMed] [Google Scholar]
- Peng H, Shi J, Zhang Y, Zhang H, Liao S, Li W, Lei L, Han C, Ning L, Cao Y, Zhou Q, Chen Q, Duan E, 2012. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 22, 1609–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky EM, Hopper AK, 2010. tRNA biology charges to the front. Genes Dev 24, 1832–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga AD, Li L, Trötzmüller M, Nelson R, Proctor SD, Köfeler H, Lehner R, 2012. Deficiency of carboxylesterase 1/esterase-x results in obesity, hepatic steatosis, and hyperlipidemia. Hepatology 56, 2188–2198. [DOI] [PubMed] [Google Scholar]
- Rattan S, Brehm E, Gao L, Flaws JA, 2018. Di (2-ethylhexyl) phthalate exposure during prenatal development causes adverse transgenerational effects on female fertility in mice. Toxicol. Sci. 163, 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, and Bale TL. 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proceedings of the National Academy of Sciences 112:13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL, 2013. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 33, 9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, Homanics GE, 2018. Heavy Chronic Intermittent Ethanol Exposure Alters Small Noncoding RNAs in Mouse Sperm and Epididymosomes. Front Genet 9, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengnipanthkul S, Noh HL, Friedline RH, Suk S, Choi S, Acosta NK, Tran DA, Hu X, Inashima K, Kim AM, Lee KW, Kim JK, 2021. Maternal exposure to high-fat diet during pregnancy and lactation predisposes normal weight offspring mice to develop hepatic inflammation and insulin resistance. Physiol. Rep. 9, e14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi AK, Lillegaard IT, Voorspoels S, Carlsen MH, Loken EB, Brantsaeter AL, Haugen M, Meltzer HM, Thomsen C, 2014. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int 73, 259–269. [DOI] [PubMed] [Google Scholar]
- Sales VM, Ferguson-Smith AC, Patti ME, 2017. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab 25, 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandovici I, Fernandez-Twinn DS, Hufnagel A, Constância M, Ozanne SE, 2022. Sex differences in the intergenerational inheritance of metabolic traits. Nature Metabolism 4, 507–523. [DOI] [PubMed] [Google Scholar]
- Saravanabhavan G, Guay M, Langlois E, Giroux S, Murray J, Haines D, 2013. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian Health Measures Survey (2007–2009). Int. J. Hyg. Environ. Health 216, 652–661. [DOI] [PubMed] [Google Scholar]
- Sarker G, Sun W, Rosenkranz D, Pelczar P, Opitz L, Efthymiou V, Wolfrum C, Peleg-Raibstein D, 2019. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc Natl Acad Sci U S A 116, 10547–10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta S, Meng Z, Hernandez R, Cavallero S, Zhou T, Hsiai TK, Zhou C, 2022. An engineered nano-liposome-human ACE2 decoy neutralizes SARS-CoV-2 Spike protein-induced inflammation in both murine and human macrophages. Theranostics 12, 2639–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D, Colacino JA, Birnbaum LS, 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect 121, 473–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P, 2018. The emerging complexity of the tRNA world: mammalian tRNAs beyond protein synthesis. Nat. Rev. Mol. Cell Biol. 19, 45–58. [DOI] [PubMed] [Google Scholar]
- Schmidleithner L, Thabet Y, Schonfeld E, Köhne M, Sommer D, Abdullah Z, Sadlon T, Osei-Sarpong C, Subbaramaiah K, Copperi F, Haendler K, Varga T, Schanz O, Bourry S, Bassler K, Krebs W, Peters AE, Baumgart AK, Schneeweiss M, Klee K, Schmidt SV, Nüssing S, Sander J, Ohkura N, Waha A, Sparwasser T, Wunderlich FT, Förster I, Ulas T, Weighardt H, Sakaguchi S, Pfeifer A, Blüher M, Dannenberg AJ, Ferreirós N, Muglia LJ, Wickenhauser C, Barry SC, Schultze JL, Beyer M, 2019. Enzymatic Activity of HPGD in Treg Cells Suppresses Tconv Cells to Maintain Adipose Tissue Homeostasis and Prevent Metabolic Dysfunction. Immunity 50, 1232–1248.e1214. [DOI] [PubMed] [Google Scholar]
- Seltenrich N, 2015. New link in the food chain? Marine plastic pollution and seafood safety. Environ Health Perspect 123, A34–A41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergiev PV, Aleksashin NA, Chugunova AA, Polikanov YS, Dontsova OA, 2018. Structural and evolutionary insights into ribosomal RNA methylation. Nat Chem Biol 14, 226–235. [DOI] [PubMed] [Google Scholar]
- Shi J, Ko EA, Sanders KM, Chen Q, Zhou T, 2018. SPORTS1.0: A Tool for Annotating and Profiling Non-coding RNAs Optimized for rRNA-and tRNA-derived Small RNAs. Genomics Proteomics Bioinformatics 16, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhang Y, Tan D, Zhang X, Yan M, Zhang Y, Franklin R, Shahbazi M, Mackinlay K, Liu S, Kuhle B, James ER, Zhang L, Qu Y, Zhai Q, Zhao W, Zhao L, Zhou C, Gu W, Murn J, Guo J, Carrell DT, Wang Y, Chen X, Cairns BR, Yang XL, Schimmel P, Zernicka-Goetz M, Cheloufi S, Zhang Y, Zhou T, Chen Q, 2021. PANDORA-seq expands the repertoire of regulatory small RNAs by overcoming RNA modifications. Nat Cell Biol 23, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhou T, Chen Q, 2022. Exploring the expanding universe of small RNAs. Nat Cell Biol 24, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB, 2006. Inflammation and insulin resistance. J Clin Invest 116, 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short AK, Fennell KA, Perreau VM, Fox A, O’Bryan MK, Kim JH, Bredy TW, Pang TY, Hannan AJ, 2016. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry 6, e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short AK, Yeshurun S, Powell R, Perreau VM, Fox A, Kim JH, Pang TY, Hannan AJ, 2017. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl Psychiatry 7, e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Rasmussen M, Baer LA, Lehnig AC, Rowland LA, White JD, So K, De Sousa-Coelho AL, Hirshman MF, Patti M-E, 2018. Paternal exercise improves glucose metabolism in adult offspring. Diabetes 67, 2530–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull AJ, 2016. Lifestyle Approaches and Glucose Intolerance. Am J Lifestyle Med 10, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Park SH, Xu J, Monette S, Helsley RN, Han SS, Zhou C, 2014. IKKbeta links vascular inflammation to obesity and atherosclerosis. J Exp Med 211, 869–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Park SH, Wang F, Zhou C, 2018. Perinatal Bisphenol A Exposure Increases Atherosclerosis in Adult Male PXR-Humanized Mice. Endocrinology 159, 1595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Meng Z, Park SH, Lu W, Livelo C, Chen Q, Zhou T, Zhou C, 2020. Myeloid-specific deficiency of pregnane X receptor decreases atherosclerosis in LDL receptor-deficient mice. J Lipid Res 61, 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Meng Z, Chen J, Liu J, Hernandez R, Gonzales MB, Gwag T, Morris AJ, Zhou C, 2021. Effects of Dicyclohexyl Phthalate Exposure on PXR Activation and Lipid Homeostasis in Mice. Environ Health Perspect 129, 127001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Li D, Liang H, Miao M, Song X, Wang Z, Zhou Z, Yuan W, 2018. Maternal exposure to bisphenol A and anogenital distance throughout infancy: A longitudinal study from Shanghai, China. Environ Int 121, 269–275. [DOI] [PubMed] [Google Scholar]
- Torisu T, Sato N, Yoshiga D, Kobayashi T, Yoshioka T, Mori H, Iida M, Yoshimura A, 2007. The dual function of hepatic SOCS3 in insulin resistance in vivo. Genes Cells 12, 143–154. [DOI] [PubMed] [Google Scholar]
- Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, Gourdy P, 2020. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Liu B, Bao W, 2022. Phthalates and attributable mortality: A population-based longitudinal cohort study and cost analysis. Environ Pollut 292, 118021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallaster MP, Kukreja S, Bing XY, Ngolab J, Zhao-Shea R, Gardner PD, Tapper AR, Rando OJ, 2017. Paternal nicotine exposure alters hepatic xenobiotic metabolism in offspring. Elife 6, e24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MH, Clayton ZE, Yap C, Sloboda DM, 2011. Maternal Fructose Intake during Pregnancy and Lactation Alters Placental Growth and Leads to Sex-Specific Changes in Fetal and Neonatal Endocrine Function. Endocrinology 152, 1378–1387. [DOI] [PubMed] [Google Scholar]
- Vom Saal FS, Myers JP, 2008. Bisphenol a and risk of metabolic disorders. JAMA 300, 1353–1355. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen Z-P, Hu H, Lei J, Zhou Z, Yao B, Chen L, Liang G, Zhan S, Zhu X, 2021. Sperm microRNAs confer depression susceptibility to offspring. Science. Advances 7, eabd7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo X, Hong X, Wang G, Pearson C, Zuckerman B, Clark AG, O’Brien KO, Wang X, Gu Z, 2022b. Association of mitochondrial DNA content, heteroplasmies and inter-generational transmission with autism. Nat. Commun. 13, 3790. 10.1038/s41467-022-30805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Liu Z, Park SH, Gwag T, Lu W, Ma M, Sui Y, Zhou C, 2018. Myeloid beta-Catenin Deficiency Exacerbates Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol 38, 1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Xia L, Zhu D, Zeng H, Wei B, Lu L, Li W, Shi Y, Liu J, Zhang Y, 2022a. Paternal high-fat diet altered sperm 5’tsRNA-Gly-GCC is associated with enhanced gluconeogenesis in the offspring. Front. Mol. Biosci. 9, 857875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou Y, Tang C, He Y, Wu J, Chen Y, Jiang Q, 2013. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PLoS One 8, e56800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareham NJ, Wong MY, Day NE, 2000. Glucose intolerance and physical inactivity: the relative importance of low habitual energy expenditure and cardiorespiratory fitness. Am J Epidemiol 152, 132–139. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Dias I, Tsuro H, Allen D, Emes RD, Moreton J, Wilson R, Ingram RJ, 2018. Paternal diet programs offspring health through sperm-and seminal plasma-specific pathways in mice. Proc Natl Acad Sci U S A 115, 10064–10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, O’Connor C, 2006. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20, 2613–2629. [DOI] [PubMed] [Google Scholar]
- Wu H, Kupsco A, Just A, Calafat AM, Oken E, Braun JM, Sanders AP, MercadoGarcia A, Cantoral A, Pantic I, Tellez-Rojo MM, Wright RO, Baccarelli AA, Deierlein AL, 2021. Maternal Phthalates Exposure and Blood Pressure during and after Pregnancy in the PROGRESS Study. Environ Health Perspect 129, 127007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM, 2009. Obesity, inflammation, and insulin resistance–a mini-review. Gerontology 55, 379–386. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cozen AE, Liu Y, Chen Q, Lowe TM, 2016. Small RNA Modifications: Integral to Function and Disease. Trends Mol Med 22, 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Shi J, Tuorto F, Li X, Liu Y, Liebers R, Zhang L, Qu Y, Qian J, Pahima M, Liu Y, Yan M, Cao Z, Lei X, Cao Y, Peng H, Liu S, Wang Y, Zheng H, Woolsey R, Quilici D, Zhai Q, Li L, Zhou T, Yan W, Lyko F, Zhang Y, Zhou Q, Duan E, Chen Q, 2018. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol 20, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shi J, Rassoulzadegan M, Tuorto F, Chen Q, 2019. Sperm RNA code programmes the metabolic health of offspring. Nat Rev Endocrinol 15, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ren L, Sun X, Zhang Z, Liu J, Xin Y, Yu J, Jia Y, Sheng J, Hu G-F, 2021. Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsRNAs. Nat. Commun. 12, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Qin Y, Clark WC, Dai Q, Yi C, He C, Lambowitz AM, Pan T, 2015. Efficient and quantitative high-throughput tRNA sequencing. Nat. Methods 12, 835–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Gao L, Flaws JA, 2017. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology 158, 1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


