Abstract
Objective
Informal caregivers (ICs) are vital to supportive cancer care and assisting cancer patients, but this caregiving burden is associated with significant distress. While addressing caregiving, it is important to explore if the caregivers are receiving care they need. Evaluating interventions that address burden and distress is integral to targeting ICs needs. This study evaluated interventions addressing IC burden and distress.
Methods
Randomized control trials (RCT) assessing interventions for IC burden and distress and exploring supportive care as an adjunct to the intervention were included. Six electronic databases were searched in accordance with the Preferred Reporting Items for Systematic reviews and Meta-analyses guidelines through October 2021. Effect sizes were estimated, and risk of bias was assessed.
Results
Of 678 studies, 11 were included. Most ICs were spouses, females, and white. Interventions included educational programs, cognitive behavioral treatment, and a telephone support program. Five studies utilized behavioral theories and seven included supportive care. Pooled results showed no significant effect on reducing caregiver distress (ES, -0.26, p<0.001).
Conclusions
Caring for the caregiver with interventions for reducing burden and distress are not efficacious. Innovative, well-designed, more pragmatic RCTs are needed.
Innovation
This study exclusively focused on interventions and supportive care needs for reducing distress and burden among cancer ICs.
Keywords: Cancer, Caregiver distress, Caregiver burden, Interventions, Supportive care, Systematic reviews
Highlights
-
•
Current interventions had no significant effect on reducing caregiver distress.
-
•
Five out of 11 studies used a behavioral theory or model.
-
•
Interventions included cognitive behavioral therapy, educational programs and supportive care.
-
•
Supportive care providers were mostly oncology nurses and family physicians.
-
•
Seven out of the 11 studies included supportive care as part of the caregiver intervention.
1. Introduction
Cancer, one of the leading causes of death in the US, has debilitating effects on people [1]. In 2022, 1.9 million individuals will receive a new cancer diagnosis [2]. With technological advancement and therapeutic innovation, the cancer death rate has decreased, resulting in a growing number of cancer survivors. In most cases, survivorship demands long-term assistance and medical care, making supportive care integral to cancer care. For example, along with surgical, radiation, and chemotherapy treatments, patients often experience treatment side effects and need additional supportive care services such as nutritional support, pain management, self-care plans, physical rehabilitation, and psychotherapy to improve their emotional well-being and quality of life. The Multinational Association of Supportive Care has been a proponent of a host of individuals providing care in ‘the prevention and management of the adverse effects of cancer and its treatment’ [3]. One vital source of such supportive care is provided by informal caregivers (ICs). An IC shares a personal relationship (family member, partner, friends, or neighbors), whilst providing unpaid care to a patient with life-threatening illnesses [4]. There are multiple models for informal caregiving, although not mutually exclusive, such as family caregiving, rotating caregiving, long distance caregiving, and caregiving by friends or neighbors [5]. In rotating caregiving, multiple ICs often take turns in providing care to the patient, especially in situations where a primary IC is unavailable. Long distance caregiving is common when families are scattered geographically. Friends or neighbors may volunteer for caregiving if the patient lives alone or without a proximal (or willing) family. Irrespective of the relation with the patient, ICs are integral in providing long-term assistance to cancer patients/survivors [6].
Prevalence estimates of ICs vary in the US; the 2020 National Alliance of Caregiving and American Association of Retired Persons (AARP) annual report estimated 53 million US adults serving as unpaid ICs to an adult; 10% provided care to a cancer patient [7]. Caregiving is labor intensive; average of 32.9 hours/week is spent on unpaid cancer caregiving [8]. Providing care for individuals with cancer can be demanding and stressful. The responsibility of ICs often includes caring for and emotionally supporting the individual as well as handling the financial burden of treatment. The demanding role of caregiving and associated burden has been linked to elevated levels of both physical (fatigue, loss of appetite, sleep deprivation) and psychological distress [9,10]. This burden experienced by ICs can lead to distress. This distress is conceptualized as strain or existential distress and can include feelings of hopelessness, loss of personal meaning and dignity, and the desire for death or the decreased will to live [11]. In addition to distress, ICs may experience anxiety and depressive symptoms resulting in poor health outcomes [10]. Such outcomes are often prevalent in rotating and long-distance caregiving; the IC may experience guilt and stress for their own lack of availability because of work or personal needs [12,13]. ICs can also face psychosocial problems such as inability to work and/or participate in their usual social activities, work activities, their own leisure activities, or maintain physical and mental health. This dynamic is concerning in cancer care because an IC’s physical and psychological status may influence the quality of care provided to the cancer patient.[14] It can affect communication between individuals leading to a negative effect on marital and family relationships [15,16]. The impact of relationship quality on caregivers’ psychological health outcomes has also been noted in studies by Reblin et al, wherein the discord and conflicts in relationships may contribute to IC burden and distress [17]. Langer et al., focused on a couple-based communication intervention and has noted reduction in distress due to self-disclosure and partner support [18].
ICs who support patients with cancer, especially those with minimal or no formal training, can experience unmet supportive care needs. Meeting IC’s unmet needs is integral in cancer care due to the inter-relation of the patient-IC dyad experience and often the dependence of the patient [19]. Using supportive care in caregiver interventions related to domains like comprehensive cancer care, information on emotional and psychological impact and daily activities, relationship support, access to health services, etc., may improve caregiver’s wellbeing as well as patient’s distress and overall quality of life [20]. Thus, an emergent question from the often-intense caregiving burden of ICs is: who is providing supportive care for the IC as supportive carer?
Although interventions aimed at reducing caregiver distress and burden are readily available for a variety of chronic and neurological conditions [21,22], a growing number of interventions are now focusing on cancer ICs [10,[23], [24], [25], [26]], Although many have included Cognitive Behavioral Therapies [27,28], and psychoeducational techniques to reduce distress [29], evidence regarding efficacy is still unclear with little to no progress made in addressing the caregiver outcomes. Therefore, we need additional information on interventions that have been developed and tested through a randomized control trial (RCT) and included a caregiver-based supportive care component.
To date, we identified one systematic review with a meta-analysis that investigated caregiver-based interventions in cancer [23]. Although an excellent review, the paper was published over ten years ago, and many recent intervention studies have emerged to address distress and burden in ICs. The paper focused on family caregivers and their psychosocial needs, they examined general characteristics such as informational needs of ICs, ability to cope, and their perceived self-efficacy. We identified no study to date that has exclusively focused on distress and caregiver burden interventions and supportive care needs for reducing distress among cancer ICs.
Thus, the purpose of the current study was to conduct a systematic review with meta-analysis of RCTs to evaluate interventions aimed at reducing IC distress and burden among those caring for patients with cancer. Specifically, we sought to understand the type and efficacy of the interventions, to identify the potential benefits, and to examine mode of supportive care received.
2. Methods
2.1. Overview
The objective was to conduct an aggregate data meta-analysis with individual studies as the unit of analysis. The review followed guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [30]. The study protocol is registered in PROSPERO (ID #CRD42020171011).
2.2. Study eligibility
The eligibility measures were designed in accordance with Population, Intervention, Comparator, Outcome, Study setting (PICOS) criteria [31]. Studies that met the following inclusion-exclusion criteria were deemed eligible. RCTs assessing interventions, strategies, or programs aimed at reducing caregiver distress and caregiver burden at the participant level were included. Selected studies were restricted to only RCTs because they are the gold standard to determine the true effect of an intervention [32]. Interventions for adult ICs engaged in provision of adult cancer care, including spouses, partners, parents, other family members, friends, and neighbors were considered. The inclusion of a comparator/control group (no intervention, usual care, wait-list control, routine care, education) was necessary. Exclusion criteria included interventions focusing on addressing distress and burden only in patients. In addition, non-English articles, other systematic reviews or meta-analysis, editorials, commentaries, gray literature, and non-randomized trials were excluded. While the current study was limited to English-language articles, recent research has suggested that excluding non-English articles does not change the direction of results [33].
2.3. Data sources
Six electronic databases (PubMed, Medline, Scopus, CINAHL, Web of Science, and PsycINFO) were searched from their inception dates forward through October 2021 for English language articles.
2.4. Search strategy
A list of terms relevant with the study objectives were developed and adapted to create the search strategy, first on PubMed using the Medical Subjects Headings (MeSH) terms, title and abstracts (tiab), and other controlled vocabularies. The search strategies were modified accordingly for each database.
2.5. Study selection
Individual searches were conducted, and all the studies were imported into EndNote X9 (Clarivate Analytics; Philadelphia, PA) for screening by the first reviewer (TD). Potential duplicates were removed. The second reviewer (ZS) utilized a copy of the databases for duplicate screening. Both reviewers (TD and ZS) independently undertook title/abstract screening using the pre-defined eligibility criteria. Any study deviating from the eligibility criteria or the PICOS components was excluded. An excel sheet record was maintained for the excluded studies along with the reason for exclusion, The two screening files were compared intermittently. Any discrepancies were resolved. Using Cohen’s kappa statistic (κ), the overall agreement rate prior to correcting disagreements was 0.70.
2.6. Data abstraction
A codebook was developed by the two reviewers (TD and ZS) a priori. Major categories of variables to be coded included study characteristics (author, study year, journal, study setting, objectives); participant characteristics(age, race, relationship); intervention characteristics (type, length, mode, outcome assessed, supportive care, behavioral theory/model). Upon completion, the coding results were compared, and any discrepancies were resolved between reviewers. The two codebooks were then merged into one primary codebook for data analysis.
2.7. Outcome measures
The primary outcome for this study was changes in caregiver distress and caregiver burden. Secondary outcomes were changes in anxiety and depression.
2.8. Risk of bias (ROB) assessment (for individual studies)
Two reviewers (TD and ZS) independently assessed the ROB in individual studies using the Cochrane's ROB instrument (v2)[34] for RCTs with a focus on the primary outcome. This instrument evaluated bias across five domains: 1) randomization process, 2) deviations from intended interventions, 3) missing outcome data, 4) measurement of the outcome, and 5) selection of the reported result. Within each domain, signaling questions lead to judgments of either low, high, or some concerns for bias. Using the judgments within each domain, the instrument further calculates the overall risk of bias for the entire study. After individually assessing risk of bias, any potential discrepancies during the assessment were discussed and resolved by consensus between the reviewers. Using Cohen’s kappa statistic (κ), the overall agreement rate for ROB assessment prior to correcting disagreements was 0.54
2.9. Data synthesis
2.9.1. Summary measures
The principal summary measure utilized in this study was the standardized mean difference (SMD) effect size. The a priori rationale for using this metric was based on the different scales expected for the instruments used to assess caregiver distress/caregiver burden, anxiety, and depression.
2.9.2. Calculation of effect sizes
The SMD effect size used for the primary outcome and secondary outcomes was Hedge’s g. This estimate corrects for any potential small sample bias compared to Cohen’s traditional d statistic [35], a limitation of the previous study [23]. Change scores and their standard deviations [36] were utilized for calculation of effect sizes for both the intervention and control groups. For studies reporting change outcomes at multiple time-points, the mean value of the assessment that took place immediately after intervention administration (T1-post intervention) was used. The following cut-points were used to interpret the magnitude of effect size results: 0.2 = small effect, 0.5= medium effect, and 0.8= large effect [37].
2.9.3. Pooled estimates for changes in outcomes
Effect sizes for outcomes were pooled and assessed using the inverse variance heterogeneity (IVhet) model [38]. The IVhet model is a quasi-likelihood model assessing both within and between study variances [38]. It is considered more robust compared to the traditional random-effects model [38], which was used in the previous study [23].
Heterogeneity and inconsistency for each pooled outcome were estimated using the Q and I2 statistics. An alpha level of <0.10 for Q represented statistically significant heterogeneity while inconsistency was categorized as very low (<25%), low (25% to <50%), moderate (50% to <75%) or large (>75%) [39]. Influence analysis with each study removed from the analysis once was conducted. Cumulative meta-analysis (CMA), ranked by year, was conducted to examine the accumulation of results over time. All analyses were conducted using MetaXL (version 5.3). For results that were statistically significant, an a-priori decision was made to calculate the Number-needed to treat (NNT) [31].
2.10. Risk of bias across studies- Meta-biases (small study effects)
Small-study effects (publication bias, etc.) for primary and secondary outcomes were examined qualitatively using Doi plots and quantitatively using the LFK index [40]. Values for the LFK index were considered to represent no, minor, and major asymmetry, respectively [40]. Doi plot and LFK analyses were conducted using both Meta XL (version 5.3) and the user-written lfk and admetan routines in Stata (version 16.1).
3. Results
3.1. Overview
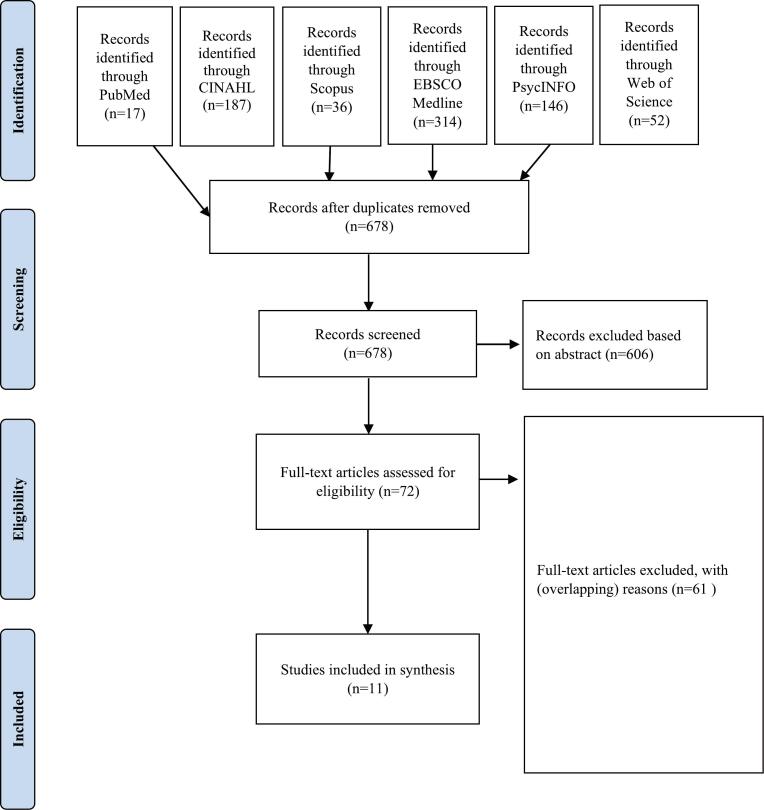
The study selection process is depicted in the PRISMA flowchart (Fig. 1). The search yielded 752 studies of which 11 met the final criteria and was subjected to analysis [19,[41], [42], [43], [44], [45], [46], [47], [48], [49]]. Full text articles were excluded if the study population, outcome, intervention type, or study design did not meet the inclusion criteria Detailed reasons for exclusion were recorded in an excel sheet.
Fig. 1.
PRISMA chart.
3.2. Study characteristics
Study characteristics for the included studies are shown in Table 1. Of the 11 studies, five (45%) were published in the US [41,[43], [44], [45],50], two (18%) in Iran [46,49], and one (9%) each in France [47], Australia (9%) [42], China (9%) [51], and Canada (9%). All studies were published in peer-reviewed journals, with 5-year impact factors for the journals ranging from 0.64 to 3.9 (mean = 2.25). Supportive care to IC was included in seven studies (67%) as part of the intervention plan. The studies (n=10) reported their estimates using the intention to treat (ITT) approach [[42], [43], [44], [45], [46], [47], [48], [49],51] while one reported results using the per-protocol approach [41].
Table 1.
Study characteristics.
| Author (year) | Objective | Country | Participants | Cancer type and stage | Baseline distress/burden levels Mean (SD) |
Assessment used | Supportive care and personnel | Theory/model |
|---|---|---|---|---|---|---|---|---|
| Abdullahzadeh et al[49] (2021) | To illustrate the efficacy of a designed family-need-based program on relieving stress, anxiety, and depression of family caregivers of leukemia patients | Iran | Female= 71.9% Treatment: (n= 32, mean age= 39 years) Control: (n= 32, mean age= 40.3 years) Race: N/A Relationship with the patient: Spouse/partner (50%), Children (19%) |
Leukemia- All stages | Treatment= 31.16 (4.14) Control= 31.09 (4.48) |
DASS-42 | Peer support, support group Personnel: Nurse researchers |
N/A |
| Applebaum et al[41] (2017) | Evaluate feasibility, acceptability, and preliminary efficacy of the CCC workshop among ICs across the US through a pilot RCT | United States of America (USA) | Female- 86% Treatment: (n= 42, mean age= 48.3 years) Control: (n= 42, mean age= 51.9 years) Race: White (86%) Relationship with the patient: Spouse/partner (61%), Parent (13%), Children (15%), Sibling (4%) |
Blood cancer and breast cancer- All stages | Treatment= 137.2 (19.8) Control= 140.6 (22.1) |
24-item Caregiver reaction assessment | Webcasts Personnel: N/A |
N/A |
| Aubin et al[48] (2021) | To assess the feasibility and preliminary effects of an intervention to improve FC supportive care | Canada | Female- 72.5% Treatment: (n=54) Control: (n=55) Mean age= 61.8 years Race: N/A Relationship with the patient: Spouse/partner (78%), Parent (19%), Other-children, siblings (11%) |
Lung cancer | Treatment= 12.3 (7.8) Control 10.4 (7.1) |
Hospital Anxiety and Depression Scale | Informational aids Personnel: Family physicians And Oncology nurses |
N/A |
| Belgacem et al[47] (2013) | Assess the efficacy of a caregiver educational program by measuring two outcomes: patients and caregivers’ quality of life and caregivers’ burden | France | Female- 58.5% Treatment: (n= 33, mean age= 56.6 years) Control: (n= 34, mean age= 62.5 years) Race: N/A Relationship with the patient: Spouse/partner (61%), Parent (9%), Children (17%), Sibling (9%), Friend (3%) |
Hematological | Treatment= 23.5 (14.4) Control= 28.5 (14.6) |
22-item ZBI | Nursing care, welfare care Personnel: Oncology nurses, | N/A |
| Chen et al[53] (2019) | To examine the effect of a reminiscence therapy (RT) intervention on the spousal caregivers of elderly patients with advanced cancer | China | Male- 51.9% Treatment: (n= 27, mean age= 69.1 years) Control: (n= 29, mean age= 66.3 years) Race: Asian Relationship with the patient: Spouse/partner (50%) |
Advanced solid- Stage IV | Treatment= 39.22 (15.01) Control= 41.31 (8.69) |
22-item ZBI | N/A | Positive psychology theory |
| Ferrell et al[67] (2020) | To test a palliative care support intervention for oncology family caregivers | USA | Female- 80% Treatment: (n=117) Control (n=123), Mean age=65 years Race: White (49%) Relationship with the patient: Spouse/partner (64%), Children (23%) |
Solid tumors- Stage IV | Treatment= 48.9 (8.26) Control= 50.3 (8.07) |
Caregiver burden scale | Selfcare plan and informational aids Personnel: Registered nurses |
Family caregiver quality of life |
| Heckal et al[42] (2018) | Test the efficacy of a telephone outcall program to reduce caregiver burden and unmet needs, and improve psychological well-being among cancer caregivers, evaluate the potential impact on patient outcomes | Australia | Female- 63% Treatment: (n= 108, mean age= 56.3 years) Control: (n= 108, mean age= 57.2 years) Race: N/A Relationship with the patient: Spouse/partner (77.3%) Other- parent, children, friend (21%) |
Solid tumors- Stage I to III | Treatment= 18.99 (12.15) Control= 18.05 (12.10) |
22-item ZBI | Education and counseling Personnel: Oncology nurses |
N/A |
| Mosher et al[44] (2019) | Examine the feasibility and preliminary effects of telephone based ACT for symptomatic, advance lung cancer patients and their distressed family caregivers | USA | Female-76% Treatment: (n= 25, mean age= 61.64 years) Control: (n= 25, mean age= 52.40 years) Race: White (84%) Relationship with the patient: Spouse/partner (72%) Other family member (28%) |
Advanced lung cancer Stage III or IV |
Treatment= 4.12 (0.46) Control= 4.12 (0.46 |
One-item Distress Thermometer | N/A | Acceptance and commitment therapy model |
| Mosher et al[45] (2018) | Examine whether adding a peer helping component to a coping skills intervention leads to improved meaning in life and peace for advanced gastrointestinal cancer patients and their caregivers | USA | Female- 64% Treatment: (n= 25, mean age= 55.32 years) Control: (n= 25, mean age= 52.40 years) Race: White (88%) Relationship with the patient: Spouse/partner (76%) Other family member (24%) |
Advanced gastrointestinal cancer Stage IV |
Treatment= 14.36 (7.44) Control= 14.64 (8.40) |
12-item short form of the ZBI | N/A | Social Cognitive Theory |
| Nejad et al[46] (2016) | Determine and compare the caregiver strain index scores of breast cancer informal caregivers, before and after a patient-caregiver educational and telephone follow-up program | Iran |
Treatment: (n= 30, mean age= 40 years) Control: (n= 30, mean age= NA) Race: Relationship with the patient: Spouse/partner (41%), Children (31%), Siblings (15%), Parents (5%), Other (5%) |
Breast cancer Stage: N/A |
Treatment= 4.8 (2.3) Control= 7.8 (2.8) |
12-item caregiver strain index questionnaire | N/A | N/A |
| Pensak et al[54] (2021) | To help caregivers of oncology patients manage distress |
USA | Female- 73% Treatment: (n= 26, mean age= 53.3 years) Control: (n= 30 mean age= 55 years) Race: White (92%) Relationship with the patient: Spouse/partner (76%) |
Solid tumors- Stage I | Treatment= 13.60 (4.52) Control= 23.46 (5.51) |
Perceived stress scale | Support groups Personnel: social workers |
Cognitive Behavioral Stress Management |
CCC= Care for the Cancer Caregiver, DASS= Scale for stress, anxiety, and depression, IC= Informal caregiver, RCT= Randomized clinical trial, ACT= Acceptance and Commitment Therapy, ZBI= Zarit Burden Interview, BSI= Brief Symptom Inventory.
3.3. Participant characteristics
A greater proportion of patients were diagnosed with breast cancer (in adults) [41,42,46] and hematological cancer [43,47]. Most IC were white (86%), most of the IC were spouses (62%) of the patient and most were females (70%). For the four studies that provided information on number of hours per day spent on providing care, the mean was estimated to be 6.7 hours per day for both the intervention and control groups [19,41,48,50]. Additional details can be found in Table 1.
3.4. Intervention characteristics
Intervention characteristics for each study are shown in Table 2. We categorized the interventions based on intervention format and intervention components.
Table 2.
Intervention characteristics.
| Author (year) | Intervention/Type | Length | Sessions/Frequency | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|---|
| Abdullahzadeh et al[49] (2021) | Family-need-based program Type: Behavioral Component: Educational Format: In-person |
4-weeks | 5 sessions of 90-minute each | Stress, anxiety, depression | N/A |
| Applebaum et al[41] (2017) | Care for the Cancer Caregiver (CCC) Workshop Type: Behavioral Component: Self-administered Format: Web-based |
14 weeks | 5 sessions | Meaning in caregiving, Sense of meaning and purpose, Caregiver burden, Depression and anxiety, Spiritual wellbeing, Benefit-finding scale |
N/A |
| Aubin et al[48] (2021) | Family caregiver supportive care Type: Behavioral Component: Counseling Format: In-person |
8 weeks | N/A | Family caregiver distress | Quality of life, psychological burden, caregiving preparedness |
| Belgacem et al[47] (2013) | Type: Welfare care Component- Educational program Format: In-person |
N/A | N/A | Patient and caregivers’ quality of life, Caregiver burden | Patient satisfaction |
| Chen et al[53] (2019) | Reminiscence therapy (RT) Type: Behavioral Component: Psychosocial Format: In-person |
4-weeks | 8 sessions of 60 minute each | Caregiver burden | Positive feelings, level of hope |
| Ferrell et al[67] (2020) | Family Caregiver Palliative Care Intervention Type: Behavioral Component: Educational Format: In-person and telephonic |
4-weeks | 4 sessions | Caregiver burden, psychological distress, caregiving skill preparedness, and Quality of life | N/A |
| Heckal et al[42] (2018) | PROTECT study Type: Supportive Component: Information and support Format: Telephonic |
16 weeks | 3 sessions | Caregiver burden | Depression, Perceived needs of caregivers and cancer patients, Health literacy |
| Mosher et al[44] (2019) | Acceptance and Commitment Therapy Type: Behavioral Component: CBT Format: Virtual |
6 weeks | 6 sessions of 60-minute each | Patient symptom interference, Patient and caregiver distress |
Patient physical symptoms, Patient breathlessness, Patient and caregiver acceptance of illness |
| Mosher et al[45] (2018) | Coping skill intervention + Peer help Type: Behavioral Component: Peer help Format: Telephonic |
NA | 5 sessions of 50 to 60 minute | Sense of meaning in life and peace in patients and caregivers | Patient and caregiver fatigue, Pain intensity Depression/anxiety, Patient and caregiver distress, Confidence, Perceived available of emotional support, Personal and role strain |
| Nejad et al[46] (2016) | Type: Behavioral Component: Educational program Format: In-person and telephonic |
6 weeks | 2 face-face, and 4 telephonic sessions | Caregiver strain | N/A |
| Pensak et al[54] (2021) | Pep-Pal Type: Behavioral Component: Cognitive Behavioral Stress Management Format: Web based |
12-weeks | 10 sessions of 20-minute each | Anxiety and depression | Perceived stress, current status, sexual dysfunction |
CCC= Care for the Cancer Caregiver, CBT= Cognitive behavioral therapy.
The types of the interventions based on intervention components included educational programs [46,47,49], cognitive behavioral treatment [43,44,50], a peer helping intervention [45], supportive care [48], and reminiscence therapy [51], whereas, based on intervention format included a web based program [41], a telephone support service outcall program [42]. Outcomes assessed by these interventions varied across the studies and included a range of primary and secondary measures. Nine studies [19,[41], [42], [43], [44], [45], [46], [47], [48]] analyzed caregiver distress and caregiver burden as one of their primary outcomes followed by anxiety [41,[43], [44], [45],49,50], depression [[41], [42], [43], [44], [45]], and sense of meaning and purpose in life [41,45], skill preparedness and quality of life [52]. Two studies assessed caregiver distress as a secondary outcome measure [45,50]. Only five studies (45%) used a behavioral theory or model. These theories/models included: Social Cognitive Theory, Cognitive Behavioral Stress Management theory, Positive Psychology theory, Acceptance and Commitment Therapy model, and family caregiver quality of life theory. Supportive care for ICs included informational aids, welfare care, problem solving plans, and self-care plans. Supportive care providers were mostly oncology nurses (71%), followed by family physicians.
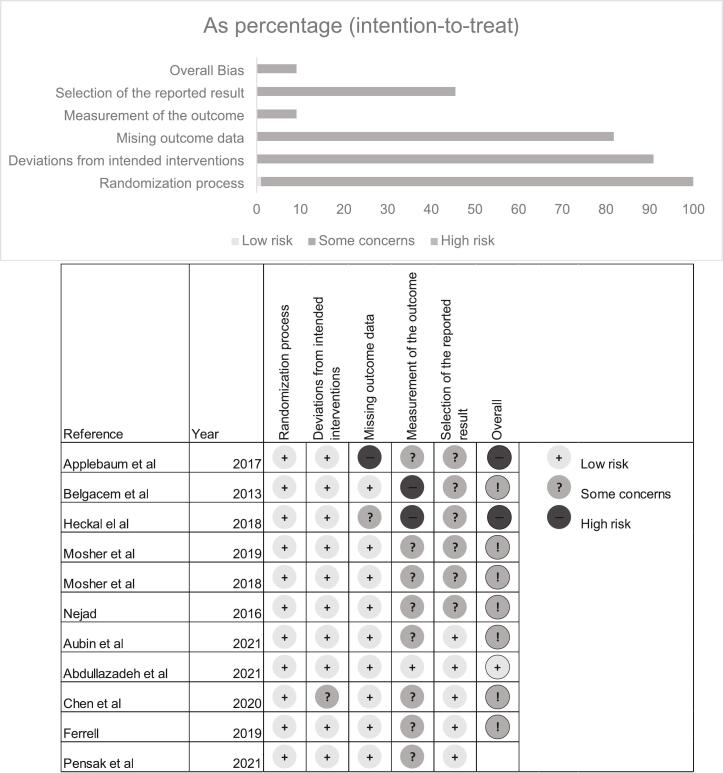
3.5. Risk of Bias assessment within studies
Overall results using the Cochran ROB (v2) are shown in Fig. 2. Approximately 18% of the studies were considered to have a high risk of bias. Due to the inability to truly blind participants in these types of psychological and educational interventions, all studies were considered to have deviated from intervention blinding of participants to their assigned interventions.
Fig. 2.
Summary risk of bias results.
3.6. Synthesis of results
3.6.1. Overall results for primary outcome (caregiver distress/burden)
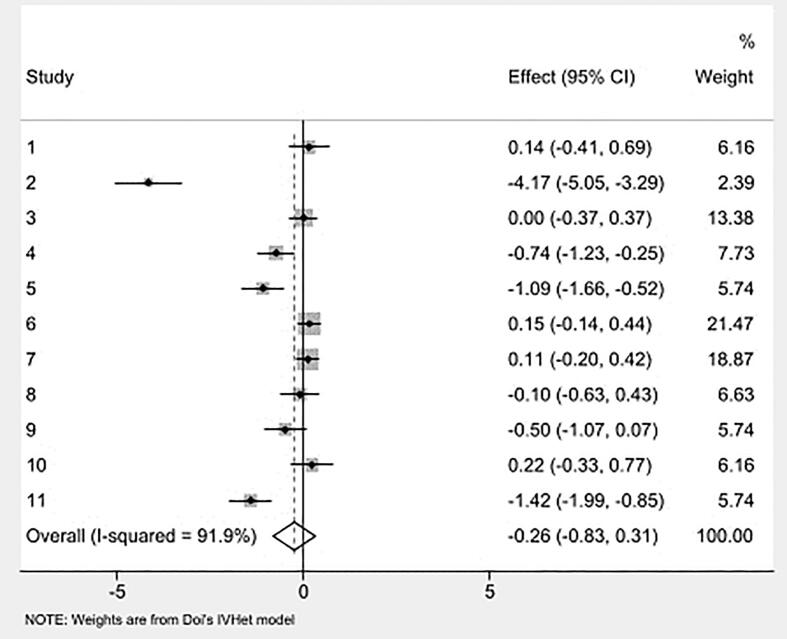
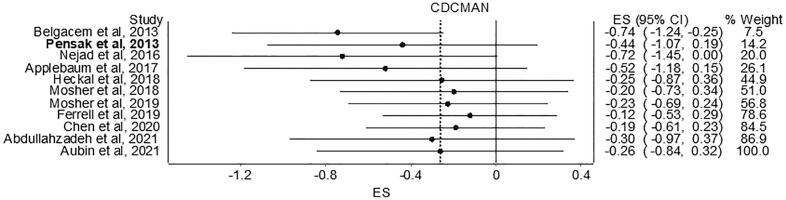
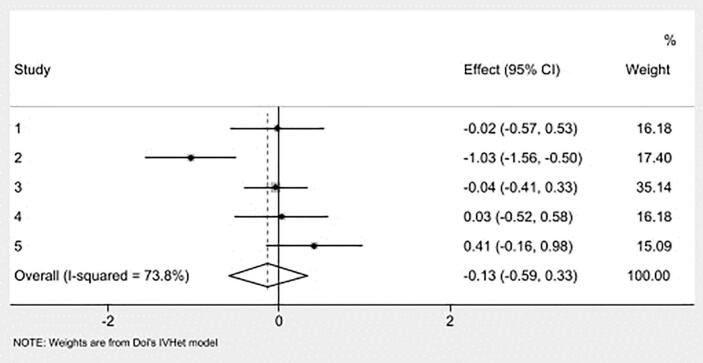
Overall pooled estimates for changes in caregiver distress and burden is shown in Table 3 and study level results are displayed in Fig. 3. Caregiver distress and burden was measured using instruments such as 24-item caregiver reaction assessment, 22-item Zarit Burden Interview (ZBI), burden scale [42,47,53], one-item distress thermometer [44], 12-item ZBI short form [45], perceived stress scale [54], 12-item caregiver strain index [46]. While in the direction of benefit, the overall magnitude of change was small and not statistically significant. Statistically significant heterogeneity was observed, and inconsistency was high. Major asymmetry suggested small-study effects (Fig. 4). CMA demonstrated an improvement in effect size over the years with results in the direction of benefit but continuously non-significant (Fig. 5). Deletion of three outliers from the model also resulted in non-significant overall estimates that were marginally close to individual estimates. Heterogeneity was still significant, and overall inconsistency was 51% (ES= -0.02, 95% CI -0.24 to 0.21, Q= 14.17, p=0.05, I2= 51%, 95% CI 0.00 to 77.9). Results for influence analysis are shown in Table 4. Because results were not statistically significant, NNT estimates were not calculated.
Table 3.
Results for primary and secondary outcomes (data reported as standardized mean difference effect size).
| Variable | ES (n) |
Participants (n) |
g (95% CI) |
Q (p) | I2 (95% CI) |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Caregiver distress Caregiver burden |
11 | 905 | -0.26 (-0.84, 0.32) | 126.19(<0.001)* | 92.08 (87.81, 94.85) |
| Secondary outcomes | |||||
| Anxiety | 5 | 328 | -0.29 (-1.26, 0.67) | 15.85 (<0.001)* | 74.7 (37.7, 89.8) |
| Depression | 6 | 485 | -0.04 (-0.22, 0.13) | 0.91 (0.97)* | 0(0.00, 0.00) |
Notes: ES (n) represent number of included studies for each outcome; Participants (n) represent number of total participants from the studies, and includes both treatment and comparator groups; g, pooled effect size represented by Hedges g; Q, Cochran Q statistics with p as alpha value for Q; *Statistically significant (alpha value ≤0.05 and non-overlapping confidence intervals
Fig. 3.
Forest plot for changes in caregiver distress. g, Hedges’ effect size.
Fig. 4.
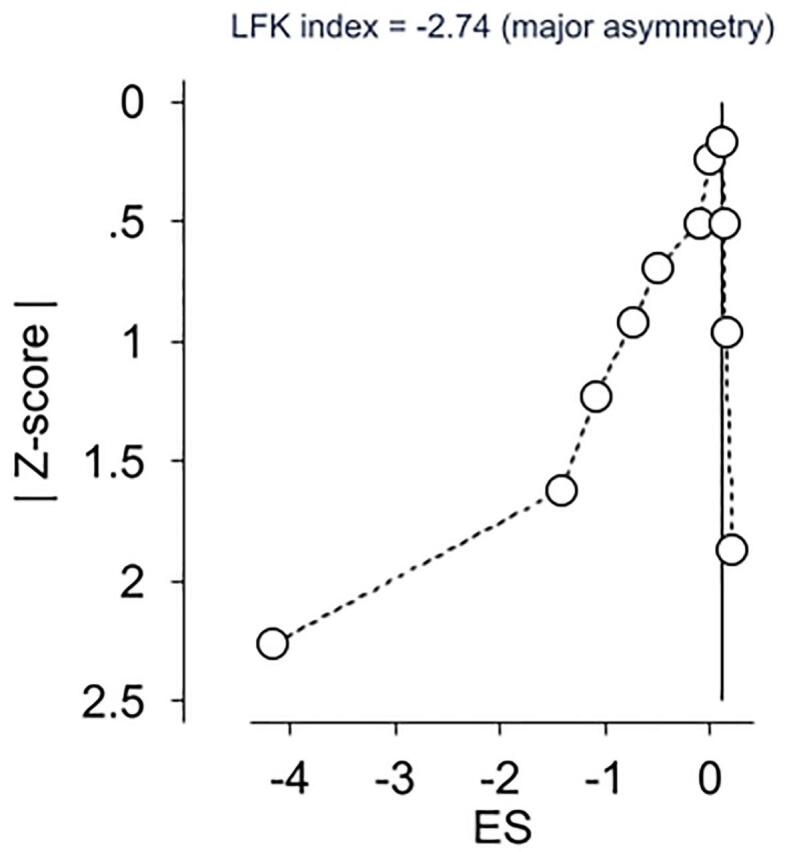
Doi plot and LFK index for changes in caregiver distress. g, effect size (Hedges’g).
Fig. 5.
Cumulative meta-analysis for changes in caregiver distress. g, effect size (Hedges’g).
Table 4.
Results for influence analysis for primary and secondary outcomes (data reported as standardized effect size).
| Excluded study | Pooled g | LCI 95% | HCI 95% | Q | p | I2 | I2 LCI 95% | I2 HCI 95% |
|---|---|---|---|---|---|---|---|---|
| Primary outcome (Caregiver distress) | ||||||||
| Applebaum et al, 2017 | -0.29 | -0.92 | 0.34 | 123.91 | 0.00 | 92.74 | 88.70 | 95.33 |
| Abdullahzadeh et al, 2021 | -0.16 | -0.53 | 0.20 | 47.23 | 0.00 | 80.94 | 65.94 | 89.34 |
| Aubin et al, 2021 | -0.30 | -0.97 | 0.36 | 124.04 | 0.00 | 92.74 | 88.71 | 95.34 |
| Belgacem et al, 2013 | -0.22 | -0.85 | 0.41 | 122.25 | 0.00 | 92.64 | 88.52 | 95.28 |
| Chen et al, 2020 | -0.21 | -0.82 | 0.40 | 117.37 | 0.00 | 92.33 | 87.98 | 95.11 |
| Ferrell et al, 2019 | -0.38 | -1.02 | 0.26 | 116.27 | 0.00 | 92.26 | 87.85 | 95.07 |
| Heckal et al, 2018 | -0.35 | -1.01 | 0.31 | 119.49 | 0.00 | 92.47 | 88.22 | 95.18 |
| Pensak et al, 2013 | -0.27 | -0.91 | 0.36 | 125.79 | 0.00 | 92.84 | 88.89 | 95.39 |
| Mosher et al, 2019 | -0.25 | -0.87 | 0.38 | 125.48 | 0.00 | 92.83 | 88.86 | 95.38 |
| Mosher et al, 2018 | -0.29 | -0.92 | 0.33 | 123.09 | 0.00 | 92.69 | 88.61 | 95.31 |
| Nejad et al, 2016 | -0.19 | -0.77 | 0.39 | 109.20 | 0.00 | 91.76 | 86.96 | 94.79 |
| Secondary outcome (Anxiety) | ||||||||
| Applebaum et al, 2017 | -0.15 | -0.75 | 0.44 | 15.66 | 0.00 | 80.85 | 49.79 | 92.69 |
| Abdullahzadeh et al, 2021 | 0.06 | -0.18 | 0.31 | 1.86 | 0.60 | 0.00 | 0.00 | 75.25 |
| Aubin et al, 2021 | -0.18 | -0.80 | 0.44 | 15.47 | 0.00 | 80.60 | 49.00 | 92.62 |
| Mosher et al, 2019 | -0.16 | -0.75 | 0.42 | 15.45 | 0.00 | 80.58 | 48.95 | 92.62 |
| Mosher et al, 2018 | -0.23 | -0.73 | 0.27 | 11.59 | 0.01 | 74.11 | 27.67 | 90.73 |
| Secondary outcome (Depression) | ||||||||
| Applebaum et al, 2017 | -0.03 | -0.22 | 0.16 | 0.80 | 0.94 | 0 | 0 | 0.00 |
| Heckal et al, 2018 | -0.04 | -0.26 | 0.18 | 0.91 | 0.92 | 0 | 0 | 8.55 |
| Abdullahzadeh et al, 2021 | -0.02 | -0.21 | 0.17 | 0.43 | 0.98 | 0 | 0 | 0.00 |
| Mosher et al, 2019 | -0.06 | -0.24 | 0.13 | 0.78 | 0.94 | 0 | 0 | 0.00 |
| Mosher et al, 2018 | -0.04 | -0.23 | 0.14 | 0.91 | 0.92 | 0 | 0 | 8.80 |
| Aubin et al, 2021 | -0.07 | -0.28 | 0.13 | 0.56 | 0.97 | 0 | 0 | 0.00 |
Notes: g, Hedges g; * Statistically significant (alpha value ≤0.05 and non-overlapping confidence interval.
3.6.2. Results for secondary outcomes (anxiety and depression)
Pooled estimates for anxiety can be seen in Table 3 while study-level results are shown in Fig. 6. Anxiety was assessed in six studies [41,[43], [44], [45],48,49] using various instruments including the Hospital Anxiety and Depression Scale [19,41,48], State-Trait Anxiety Inventory [43], DASS-42 scale [49], and PROMIS short-form anxiety measure [45]. The pooled results showed negligible and non-significant change in anxiety. Heterogeneity was significant, and inconsistency was categorized as high. Small-study effects (LFK index= -2.41) showed major asymmetry. CMA demonstrated a decrease in improvements over the years. Deletion of one outlier resulted in non-significant estimates, with non-significant heterogeneity and overall inconsistency as moderate (ES -0.02, 95% CI -0.29 to 0.25, Q= 0.04, p=0.980, I2= 0%, 95% CI 0.000 to 0.000). Results remained non-significant when each study was deleted from the model once (Table 4).
Fig. 6.
Forest plot for changes in Anxiety. g, Hedges’ effect size.
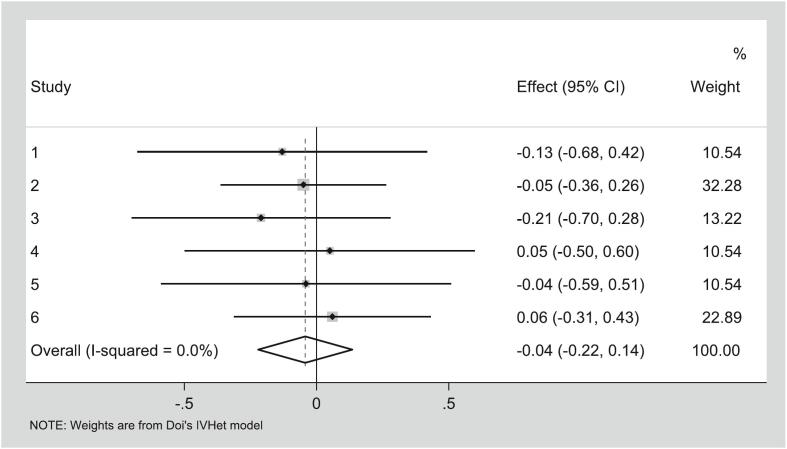
Pooled estimates for the seven studies [[41], [42], [43], [44], [45],48,49] assessing depression are shown in Table 4 while study-level results are shown in Fig. 7. Depression was assessed using the Hospital Anxiety and Depression Scale [41], Beck Depression Inventory [43], PROMIS short-form depression measure [44], DASS-42 [49], CES-D [42], and PROMIS anxiety and depression measure [45]. No significant changes in depression scores were observed. Heterogeneity was significant, and inconsistency was categorized as high. Small-study effects (LFK index= 0.48) showed no asymmetry. CMA demonstrated a decrease in improvements over the years. Deletion of two outliers resulted in non-significance, with non-significant heterogeneity and overall inconsistency as moderate (ES 0.00, 95% CI -0.21 to 0.22, Q= 0.24, p=0.97, I2= 0%, 95% CI 0.000 to 0.000). Results were still non-significant when each study was deleted from the model once (Table 4).
Fig. 7.
Forest plot for changes in Depression. g, Hedges’ effect size.
4. Discussion and conclusion
The current study examined the effects of interventions addressing caregiver distress or burden among cancer ICs. While caregiving is associated with increased physical and psychological stress, the overall result of the study suggests that current interventions are not efficacious in reducing distress or burden among cancer ICs. These findings are supported primarily by the pooled results as well as outlier and other sensitivity analyses. The CMA of articles ranging from the years 2013 to 2021 showed some improvement in distress reduction in the effect size over time (-0.74 in 2013 to -0.26 in 2021); however, the improvement remained non-significant. Similar non-significant results were observed for the secondary outcomes (anxiety and depression). The small effect size in each study or non-significant findings may be understood in terms of the wide variability in the intervention type, overall design, small sample size, mode of administration, intervention dose (sessions), and methods used to assess distress. The effect of the interventions may be moderated by other factors such as characteristics of the IC, pre-existing mental health issues, characteristics of the caregiving situation, their relationship, and quality of relationship with the patient. It is important to acknowledge that, despite the development of multiple interventions and the rise in the cancer caregiver population, little progress has been made to support caregiver outcomes.
One area for future research is to explore the nature of dyadic relations when designing IC distress interventions: considering both patient and IC concurrently and the complex interplay of the relationship. A caregiver’s negative state of mind can affect patients physical and mental outcomes and overall quality of life [55]. Northouse et al., highlighted the benefit of a dyadic intervention, which showed significant improvements in coping and self-efficacy in both cancer patient and the caregiver [56]. Relationship quality may also influence health outcomes, which may be particularly salient given ICs involvement in providing homecare and symptom management for cancer patients [57,58]. Other studies, including those by Ellington, Reblin and colleagues, have explored the reciprocal nature of patient-caregiver relationships and the extent to which they impact outcomes in the other partner [[59], [60], [61]]. The Me in We dyadic intervention and another couples-based communication intervention further demonstrated that a dyadic design led to improved communication and coping among cancer patients and ICs, thereby further reducing distress and burden in ICs [18,62]. These studies demonstrating the value of dyadic designs in improving outcomes for ICs, and future interventions are likely to benefit from a dyadic approach in seeking to improve outcomes for both patients and ICs.
Despite limitations in findings from the individual focused intervention studies, our analysis suggests a path forward for future interventions for ICs. One consideration might be to create easier access to services. Applebaum et al. [41], highlighted caregiver’s interest in online/web-based support tool, and Heckal et al [42], presented a feasible approach to address the unmet needs of IC using telephone outreach. The flexibility of using web-based or telephone interventions may be particularly beneficial for ICs unable to leave the patient alone. Our results also identified the importance of using supportive care such as welfare care as part of their caregiving management [47]. Researchers are encouraged to explore more psychoeducational techniques in future studies. A study comparing mindfulness based techniques and educational interventions supported the idea of implementing integrative educational approaches in the reduction of caregiver burden [29]. Similar findings were observed in the study by Northouse et al. and Belgacem et al [23,47]. Our review found five studies using a behavioral model or theory. Use of behavioral models or developing theory informed interventions fosters a systematic approach to designing interventions.
Timely integration of supportive care in caregiver interventions to address unmet needs can help IC feel better and improve their quality of life by reducing distress and treatment delays. A study by Ullrich et al., reported that a higher number of IC unmet needs (such as information related to patient’s treatment, interactions with patient’s physician) are associated with increased psychological distress and overall IC burden [7]. Our study results highlighted that caregiver interventions need to take into consideration a holistic understanding of different sources of distress and burden. There are several triggers that can contribute to an increase in distress such as relationship concerns during cancer, accessibility to treatment location, role adaptation or managing daily activities [20]. Instances of rotating caregiving and caregiving by friends or neighbors may also create inconsistencies in care provision (either due to changes in caregiving style by each person or in situations wherein health information is not exchanged or communicated well, thus adding to the unmet needs) [63]. One can further consider the role of relationship quality and its impact on the patient-IC relationship as well as IC distress and burden [17]. It is crucial for interventions to address and assess these core issues to ensure seamless provision of care. Our results identified that assessment of needs and using supportive care resources like tailored self-care plans, caregiving preparedness, problem-solving strategies, and communication training may promote care satisfaction among the IC. We suggest that studies need to also acknowledge the different models of caregiving (rotating caregiving, long distance caregiving) and different type of ICs (family caregiver, friends, neighbors) when designing an intervention to reduce IC distress and burden.
The results observed in this study have important implications for research. For example, variability among the interventions suggests a need for further refinement in the way we are designing and examining caregiver interventions, supportive care, and their impact. The cumulative meta-analysis did not show any significant improvement over the years, suggesting the need for further exploration given the common-sense notion that interventions should be tailored to the right people, at the right time, and for the right purposes. Currently, a limited number of interventions are available focused specifically on IC’s distress and burden; thus, it is important for researchers to continue to innovate interventions beyond those that are currently existing. Since many of the interventions were psychological and educational in nature, high/unclear risk of bias was inherently present as blinding is difficult in these types of interventions. The challenges of blinding notwithstanding, effective measures are needed to try and better address these experimental design issues. Therefore, the present findings should encourage researchers to evaluate interventions and promote studies beyond the traditional methods employed.
The evidence from this study has important implications for practice. While there is a wide range of caregiver distress interventions available for other chronic conditions, only a limited number are currently available for cancer caregivers [24,64]. Although much attention is given to the efficacy of an intervention, only a few interventions are assessed for their implementation into practice and utilization by clinicians [65]. While the results of our analysis were non-significant, our evidence highlighted important characteristics of the developed interventions in improving health literacy, identifying unmet needs, enhancing quality of life, and confidence among ICs. Clinicians (e.g., oncologists, nurses) who wish to decrease caregiver distress should be encouraged to utilize this information. Cancer centers that have implemented a routine distress screening for cancer patients can offer a similar initiative for ICs. Aubin et al., suggested improving health communication between the clinician and the IC considering the IC’s role as a communicator on behalf of the patient [19]. Additionally, nurses and social workers may offer counseling or mindfulness referrals to the distressed ICs and connect them with social support groups at their institution. Incorporating IC initiative in oncology practice can be challenging considering the workload on healthcare force, thus innovative strategies to implement distress management and supportive care is needed to acknowledge evolving oncology practice, changing disease patterns and the changing patient demographic. Our results also suggest a need for policy makers to support funding for novel research aimed at developing interventions that specifically target caregiver distress and burden.
There are several potential limitations to the current study. Since this was an aggregate data meta-analysis, group level results may not apply at the individual level; a potential for ecological fallacy exists [66]. Due to a broad definition of distress, inclusion of different instruments for outcome assessment and interventions could have affected our findings, resulting in decreased sensitivity to find change. Distress or burden is multidimensional, consisting of both subjective and objective dimensions with minimal clarity of what differentiates the subjective from the objective [21]. The current study was not able to disaggregate the dimensions of distress. All assessments in the individual studies were conducted using self-report instruments, the possibility of self-report bias exists. Given the small number of studies, we were unable to conduct a detailed subgroup and sensitivity analyses. We have not assessed the varying models of caregiving in our analysis.
4.1. Innovation
Upon extensive examination, this is the first systematic review with meta-analysis to investigate RCT-based interventions addressing caregiver distress among cancer ICs. Including only RCTs offers the most valid type of study design by controlling for unknown and unmeasured confounders. A novel approach, the IVhet model, was used to pool findings resulting in more robust estimates compared to the traditional random-effects model [38]. We also explored utilization of behavioral theories and models for intervention development. Our focus on supportive care needs for reducing distress and exploring supportive care providers offers novel insights that have not been previously explored for cancer ICs. The study suggests exploring dyadic relations in intervention design, assessment of unmet needs, and enhanced communication between multiple domains including oncologists, nurses, policy makers, family members, and other informal caregivers. To summarize, in comparison to the previously published study, our study includes 12 additional years of research, uses more recent meta-analysis methods that give higher quality studies more weight in the analysis, and also explicitly considers modality of supportive care as part of the caregiver intervention plan.
4.2. Conclusions
Despite the unpromising findings, we see many opportunities for future investigation. The involvement of IC in cancer care for supporting patients will continue to increase. We must acknowledge that distress and burden among ICs is problematic; the study results offer information and provide best ways on how we can refine future strategies to improve IC’s overall quality of life and psychological outcomes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical consideration
Any ethical review was not sought for this study as it met the criteria for exemption according to the institution policy.
Disclaimer
Preliminary data associated with this research was submitted for presentation at the annual meeting of The Society of Behavioral Medicine.
Category
Systematic review and meta-analysis
Contributions
Trupti Dhumal: Conceptualization, data curation, formal analysis, investigation, methodology, writing-original draft, and writing- review and editing.
Zasim Siddiqui: Data curation and formal analysis.
Dr. George Kelley: Methodology, software, supervision, validation, and writing-original draft.
Dr. Felicity Harper: Writing- review and editing.
Dr. Kimberly M Kelly: Investigation, supervision, validation, writing-original draft, and writing- review and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data sharing not applicable – no new data generated: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Centers for Disease Control and Prevention Leading Causes of Death. 2017. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm accessed September 11 2022.
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Berman R., Davies A., Cooksley T., Gralla R., Carter L., Darlington E., et al. Supportive Care: An Indispensable Component of Modern Oncology. Clin Oncol (R Coll Radiol) 2020;32(11):781–788. doi: 10.1016/j.clon.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Research Council . The Role of Human Factors in Home Health Care: Workshop Summary. National Academies Press (US); 2010. Informal caregivers in the United States: prevalence, caregiver characteristics, and ability to provide care.https://www.ncbi.nlm.nih.gov/books/NBK210048/ [Google Scholar]
- 5.Cagle J.G., Munn J.C. Long-distance caregiving: a systematic review of the literature. J Gerontol Soc Work. 2012;55(8):682–707. doi: 10.1080/01634372.2012.703763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer caregiving in the US: an intense, episodic, and challenging care experience. National Alliance for Caregiving; Bethesda, MD: 2016. https://www.caregiving.org/wp-content/uploads/2020/05/CancerCaregivingReport_FINAL_June-17-2016.pdf (accessed September 11 2022) [Google Scholar]
- 7.Ullrich A., Marx G., Bergelt C., Benze G., Zhang Y., Wowretzko F., et al. Supportive care needs and service use during palliative care in family caregivers of patients with advanced cancer: a prospective longitudinal study. Support Care Cancer. 2021;29(3):1303–1315. doi: 10.1007/s00520-020-05565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yabroff K.R., Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115(S18):4362–4373. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 9.Northouse L.L., Katapodi M.C., Schafenacker A.M., Weiss D. The Impact of Caregiving on the Psychological Well-Being of Family Caregivers and Cancer Patients. Semin Oncol Nurs. 2012;28(4):236–245. doi: 10.1016/j.soncn.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Dumont S., Turgeon J., Allard P., Gagnon P., Charbonneau C., Vézina L. Caring for a loved one with advanced cancer: determinants of psychological distress in family caregivers. J Palliat Med. 2006;9(4):912–921. doi: 10.1089/jpm.2006.9.912. [DOI] [PubMed] [Google Scholar]
- 11.Miller B., Townsend A., Carpenter E., Montgomery R.V., Stull D., Young R.F. Social support and caregiver distress: A replication analysis. J Gerontol B Psychol Sci Soc Sci. 2001;56(4):S249–S256. doi: 10.1093/geronb/56.4.s249. [DOI] [PubMed] [Google Scholar]
- 12.Mazanec P. Distance caregiving a parent with cancer. Semin Oncol Nurs. 2012;28(4):271–278. doi: 10.1016/j.soncn.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanberg J.E. Making it work: informal caregiving, cancer, and employment. J Psychosoc Oncol. 2006;24(3):1–18. doi: 10.1300/J077v24n03_01. [DOI] [PubMed] [Google Scholar]
- 14.Litzelman K. Caregiver Well-being and the Quality of Cancer Care. Semin Oncol Nurs. 2019;35(4):348–353. doi: 10.1016/j.soncn.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manne S.L., Ostroff J.S., Norton T.R., Fox K., Goldstein L., Grana G. Cancer-related relationship communication in couples coping with early stage breast cancer. Psychooncology. 2006;15(3):234–247. doi: 10.1002/pon.941. [DOI] [PubMed] [Google Scholar]
- 16.Badr H., Taylor C.L. Sexual dysfunction and spousal communication in couples coping with prostate cancer. Psychooncology. 2009;18(7):735–746. doi: 10.1002/pon.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reblin M., Donaldson G., Ellington L., Mooney K., Caserta M., Lund D. Spouse cancer caregivers’ burden and distress at entry to home hospice: The role of relationship quality. J Soc Pers Relat. 2016;33(5):666–686. doi: 10.1177/0265407515588220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langer S.L., Porter L.S., Romano J.M., Todd M.W., Lee S.J. A Couple-Based Communication Intervention for Hematopoietic Cell Transplantation Survivors and Their Caregiving Partners: Feasibility, Acceptability, and Change in Process Measures. Biol Blood Marrow Transplant. 2018;24(9):1888–1895. doi: 10.1016/j.bbmt.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Jang Y., Jeong Y. Unmet Needs and Quality of Life of Cancer Patients and Their Families: Actor-Partner Interdependence Modeling. Healthcare (Basel, Switzerland) 2021;9(7):874. doi: 10.3390/healthcare9070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert S.D., Girgis A. Unmet Supportive Care Needs Among Informal Caregivers of Patients with Cancer: Opportunities and Challenges in Informing the Development of Interventions. Asia Pac J Oncol Nurs. 2017;4(2):136–139. doi: 10.4103/2347-5625.204485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acton G.J., Kang J. Interventions to reduce the burden of caregiving for an adult with dementia: A meta-analysis. Res Nurs Health. 2001;24(5):349–360. doi: 10.1002/nur.1036. [DOI] [PubMed] [Google Scholar]
- 22.Spalding-Wilson K.N., Guzmán-Vélez E., Angelica J., Wiggs K., Savransky A., Tranel D. A novel two-day intervention reduces stress in caregivers of persons with dementia. Alzheim & Dement (N Y) 2018;4:450–460. doi: 10.1016/j.trci.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Northouse L.L., Katapodi M.C., Song L., Zhang L., Mood D.W. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin. 2010;60(5):317–339. doi: 10.1016/j.trci.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Applebaum A.J., Breitbart W. Care for the cancer caregiver: a systematic review. Palliat Support Care. 2013;11(3):231–252. doi: 10.1017/S1478951512000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaltenbaugh D.J., Klem M.L., Hu L., Turi E., Haines A.J., Hagerty Lingler J. Using Web-based interventions to support caregivers of patients with cancer: a systematic review. Oncol Nurs Forum. 2015;42(2):156–164. doi: 10.1188/15.ONF.156-164. [DOI] [PubMed] [Google Scholar]
- 26.Shin J.Y., Kang T.I., Noll R.B., Choi S.W. Supporting Caregivers of Patients With Cancer: A Summary of Technology-Mediated Interventions and Future Directions. Am Soc Clin Oncol Educ Book. 2018;38:838–849. doi: 10.1200/EDBK_201397. [DOI] [PubMed] [Google Scholar]
- 27.O’Toole M.S., Zachariae R., Renna M.E., Mennin D.S., Applebaum A. Cognitive behavioral therapies for informal caregivers of patients with cancer and cancer survivors: a systematic review and meta-analysis. Psychooncology. 2017;26(4):428–437. doi: 10.1002/pon.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Applebaum A.J., Panjwani A.A., Buda K., O’Toole M.S., Hoyt M.A., Garcia A., et al. Emotion regulation therapy for cancer caregivers-an open trial of a mechanism-targeted approach to addressing caregiver distress. Transl Behav Med. 2020;10:413–422. doi: 10.1093/tbm/iby104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Daken L.I., Ahmad M.M. The implementation of mindfulness-based interventions and educational interventions to support family caregivers of patients with cancer: A systematic review. Perspect Psychiatr Care. 2018;54(3):441–452. doi: 10.1111/ppc.12286. [DOI] [PubMed] [Google Scholar]
- 30.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins J., Thomas J., Chandler J. 2019. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0 (Updated July 2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn E., Kang H. Introduction to systematic review and meta-analysis. Korean J Anesthesiol. 2018;71(2):103–112. doi: 10.4097/kjae.2018.71.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nussbaumer-Streit B., Klerings I., Dobrescu A., Persad E., Stevens A., Garritty C., et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42–54. doi: 10.1016/j.jclinepi.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Sterne J.A., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 35.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. 2009. Effect sizes Based on Means. Introduction to Meta-Analysis; pp. 21–32. [DOI] [Google Scholar]
- 36.Follmann D., Elliott P., Suh I., Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. 2013. Statistical power analysis for the behavioral sciences: Academic press. [DOI] [Google Scholar]
- 38.Doi S.A., Barendregt J.J., Khan S., Thalib L., Williams G.M. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp Clin Trials. 2015;45:130–138. doi: 10.1016/j.cct.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuya-Kanamori L., Barendregt J.J., Doi S.A. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16(4):195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 41.Applebaum A., Buda K., Farberov M., Teitelbaum N., Evans K., Cowens-Alvarado R., et al. Care for the cancer caregiver: A Web-based intervention to address caregiver burden. Psychooncology. 2017;26:54. doi: 10.1002/pon.4583. [DOI] [Google Scholar]
- 42.Heckel L., Fennell K.M., Reynolds J., Boltong A., Botti M., Osborne R.H., et al. Efficacy of a telephone outcall program to reduce caregiver burden among caregivers of cancer patients [PROTECT]: a randomised controlled trial. BMC Cancer. 2018;18:59. doi: 10.1186/s12885-017-3961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marsland A.L., Long K.A., Howe C., Thompson A.L., Tersak J., Ewing L.J. A Pilot Trial of a Stress Management Intervention for Primary Caregivers of Children Newly Diagnosed With Cancer: Preliminary Evidence That Perceived Social Support Moderates the Psychosocial Benefit of Intervention. J Pediatr Psychol. 2013;38(4):449–461. doi: 10.1093/jpepsy/jss173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosher C.E., Secinti E., Hirsh A.T., Hanna N., Einhorn L.H., Jalal S.I., et al. Acceptance and Commitment Therapy for Symptom Interference in Advanced Lung Cancer and Caregiver Distress: A Pilot Randomized Trial. J Pain Symptom Manage. 2019;58(4):632–644. doi: 10.1016/j.jpainsymman.2019.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosher C.E., Secinti E., Hirsh A.T., Hanna N., Einhorn L.H., Jalal S.I., et al. Acceptance and Commitment Therapy for Symptom Interference in Advanced Lung Cancer and Caregiver Distress: A Pilot Randomized Trial. J Pain Symptom Manage. 2019;58(4):632–644. doi: 10.1016/j.jpainsymman.2019.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nejad Z.K., Aghdam A.M., Hassankhani H., Sanaat Z. The effects of a patient-caregiver education and follow-up program on the breast cancer caregiver strain index. Iran Red Crescent Med J. 2016;18(3) doi: 10.5812/ircmj.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belgacem B., Auclair C., Fedor M.C., Brugnon D., Blanquet M., Tournilhac O., et al. A caregiver educational program improves quality of life and burden for cancer patients and their caregivers: A randomised clinical trial. Eur J Oncol Nurs. 2013;17(6):870–876. doi: 10.1016/j.ejon.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Aubin M., Vézina L., Verreault R., Simard S., Desbiens J.-F., Tremblay L., et al. A randomized clinical trial assessing a pragmatic intervention to improve supportive care for family caregivers of patients with lung cancer. Palliat Support Care. 2021;19(2):146–153. doi: 10.1017/S1478951520000711. [DOI] [PubMed] [Google Scholar]
- 49.Abdullahzadeh M., Khosravi N. Designing a need-based program for relieving psychological distress of family caregivers of leukemia patients: a randomized controlled trial. Support Care Cancer. 2021;29(12):7601–7610. doi: 10.1007/s00520-021-06353-z. [DOI] [PubMed] [Google Scholar]
- 50.Pensak N.A., Carr A.L., Jones J., Mikulich-Gilbertson S.K., Kutner J.S., Kilbourn K., et al. A pilot study of mobilized intervention to help caregivers of oncology patients manage distress. Psycho-Oncology. 2021;30(4):520–528. doi: 10.1002/pon.5597. [DOI] [PubMed] [Google Scholar]
- 51.Chen J., Xiao H., Chen Y., Sun H., Chen S., Zheng J. Effect of reminiscence therapy based on positive psychology theory (RTBPPT) on the positive feelings of the spousal caregivers of elderly patients with advanced cancer in China. Eur J Cancer Care (Engl) 2020;29(6):1–10. doi: 10.1111/ecc.13324. [DOI] [PubMed] [Google Scholar]
- 52.Ferrell B., Kravits K., Borneman T., Pal S.K., Lee J. A Support Intervention for Family Caregivers of Advanced Cancer Patients. J Adv Pract Oncol. 2019;10(5):444–455. doi: 10.6004/jadpro.2019.10.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J., Xiao H., Chen Y., Sun H., Chen S., Zheng J. Effect of reminiscence therapy based on positive psychology theory (RTBPPT) on the positive feelings of the spousal caregivers of elderly patients with advanced cancer in China. Eur J Cancer Care (Engl) 2020;29(6):1–10. doi: 10.1111/ecc.13324. [DOI] [PubMed] [Google Scholar]
- 54.Pensak N.A., Carr A.L., Jones J., Mikulich-Gilbertson S.K., Kutner J.S., Kilbourn K., et al. A pilot study of mobilized intervention to help caregivers of oncology patients manage distress. Psychooncology. 2021;30(4):520–528. doi: 10.1002/pon.5597. [DOI] [PubMed] [Google Scholar]
- 55.Best A.L., Shukla R., Adamu A.M., Martinez Tyson D., Stein K.D., Alcaraz K.I. Impact of caregivers’ negative response to cancer on long-term survivors’ quality of life. Support Care Cancer. 2021;29(2):679–686. doi: 10.1007/s00520-020-05509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Northouse L.L., Mood D.W., Schafenacker A., Kalemkerian G., Zalupski M., LoRusso P., et al. Randomized clinical trial of a brief and extensive dyadic intervention for advanced cancer patients and their family caregivers. Psychooncology. 2013;22(3):555–563. doi: 10.1002/pon.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berli C., Lüscher J., Luszczynska A., Schwarzer R., Scholz U. Couples' daily self-regulation: The Health Action Process Approach at the dyadic level. PloS One. 2018;13(10) doi: 10.1371/journal.pone.0205887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adashek J.J., Subbiah I.M. Caring for the caregiver: a systematic review characterising the experience of caregivers of older adults with advanced cancers. ESMO Open. 2020;5(5) doi: 10.1136/esmoopen-2020-000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reblin M., Iacob E., Tay D.L., Li H., Thomas Hebdon M.C., Beck A., et al. Family Caregiver Reports of Their Own and Patient Symptoms in Cancer Home Hospice Approaching End-of-Life. Am J Hosp Palliat Care. 2022 doi: 10.1177/10499091221108119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otto A.K., Ketcher D., Heyman R.E., Vadaparampil S.T., Ellington L., Reblin M. Communication between advanced cancer patients and their family caregivers: relationship with caregiver burden and preparedness for caregiving. Health Commun. 2021;36(6):714–721. doi: 10.1080/10410236.2020.1712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otto A.K., Gonzalez B.D., Heyman R.E., Vadaparampil S.T., Ellington L., Reblin M. Dyadic effects of distress on sleep duration in advanced cancer patients and spouse caregivers. Psychooncology. 2019;28(12):2358–2364. doi: 10.1002/pon.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ketcher D., Thompson C., Otto A.K., Reblin M., Cloyes K.G., Clayton M.F., et al. The Me in We dyadic communication intervention is feasible and acceptable among advanced cancer patients and their family caregivers. Palliat Med. 2021;35(2):389–396. doi: 10.1177/0269216320972043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen S.C., Tsai M.C., Liu C.L., Yu W.P., Liao C.T., Chang J.T. Support needs of patients with oral cancer and burden to their family caregivers. Cancer Nurs. 2009;32(6):473–481. doi: 10.1097/NCC.0b013e3181b14e94. [DOI] [PubMed] [Google Scholar]
- 64.Ferrell B., Wittenberg E. A review of family caregiving intervention trials in oncology. CA Cancer J Clin. 2017;67(4):318–325. doi: 10.3322/caac.21396. [DOI] [PubMed] [Google Scholar]
- 65.O'Cathain A., Croot L., Duncan E., Rousseau N., Sworn K., Turner K.M., et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2019-029954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rücker G., Schumacher M. Simpson's paradox visualized: the example of the rosiglitazone meta-analysis. BMC Med Res Methodol. 2008;8:34. doi: 10.1186/1471-2288-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrell B., Kravits K., Borneman T., Pal S.K., Lee J. A support intervention for family caregivers of Advanced cancer patients. J Adv Pract Oncol. 2019;10(5):444–455. doi: 10.6004/jadpro.2019.10.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.