Highlights
-
•
IRF3 deficiency increased Theiler's virus infection of RAW264.7 macrophages with decreased IL-33 expression and increased M2 macrophage markers, CD206 and ARG1.
-
•
IL-33 augments Theiler's virus infection of RAW264.7 macrophages.
-
•
IL-33 induction of anti-viral responses of RAW264.7 macrophages are IRF3-dependent.
-
•
Restoring IRF3 in IRF3-deficient RAW264.7 cells decreased Theiler's virus infection and increased IL-33 expression.
Keywords: Interleukin-33, Theiler's virus, Macrophages, RAW264.7 cells, IRF3
Abstract
Interleukin-33 (IL-33), which promotes M2 macrophage development, may influence the control of viruses, such as Theiler's Murine Encephalomyelitis Virus (TMEV) that infect macrophages. Because Interferon Regulatory Factor-3 (IRF3) is also critical to control of TMEV infection in macrophages, information on the relationship between IL-33 and IRF3 is important. Thus, RAW264.7 Lucia murine macrophage lineage cells with an endogenous IRF3-ISRE promoter driving secreted luciferase and IRF3KO RAW Lucia, a subline deficient in IRF3, were challenged with TMEV. After the challenge, considerable TMEV RNA detected at 18 and 24 h in RAW cells was significantly elevated in IRF3KO RAW cells. TMEV induction of ISRE-IRF3 promoter activity, IFN-β and IL-33 gene expression, and IL-6 and IL-10 protein production, which was strong in RAW cells, was less in IRF3KO RAW cells. In contrast, expression of CD206 and ARG1, classical M2 macrophage markers, was significantly elevated in IRF3KO RAW cells. Moreover, RAW and IRF3KO RAW cells produced extracellular IL-33 prior to and after infection with TMEV and antibody blockade of the IL-33 receptor, ST2, reduced CD206 and ARG1 expression, but increased IL-6 gene expression. Pre-treating both RAW and IRF3KO RAW cells with IL-33 prior to challenge significantly increased TMEV infection, but also increased IL-33, IL-10, IL-6 mRNA expression, and NO production without increasing IFN-β. Notably, IL-33 induction of IL-33, IRF3-ISRE promoter activity, and IL-10 by TMEV or poly I:C/IFN-γ was significantly dependent upon IRF3. The results show that the expression of IL-33 and the repression of M2 macrophage phenotypic markers are dependent on IRF3 and that IL-33 decreases the ability of macrophages to control infection with macrophage-tropic viruses.
1. Introduction
IL-33 is a member of the IL-1 cytokine family, classified as an alarmin, that alerts the immune microenvironment of cell and tissue damage (Zhao and Hu, 2010). At first, it, distinctively, nuclear-localizes to chromatin but is released in the extracellular space by necrotic cells (Cayrol and Girard, 2022). Cytokine IL-33 is associated with the enhancement of Th2 CD4 T cell responses (Nechama et al., 2018) and as such is involved in Type I hypersensitivity immune responses (Schmitz et al., 2005). It also induces ROS (Caslin et al., 2018) and chemokine CCL22 from macrophages (Tjota et al., 2017), decreases TNF-alpha and CXCL10 chemokine from macrophages (Lin et al., 2022), and increases IL-13 release from mast cells (Schmitz et al., 2005). In the central nervous system (CNS), IL-33 and its receptor system are highly expressed, especially in glial cells (Hudson et al., 2008), and likely protect against traumatic or virus-induced brain injury by dampening inflammatory cytokine production from macrophages (Xie et al., 2022). However, neurotropic viruses, such as TMEV induce further IL-33 gene expression in the CNS (Hudson et al., 2008). Virus induction of IL-33 is likely through Pattern Recognition Receptor (PRR) pathways, such as the TLR3 pathway (Talabot-Ayer et al., 2012). However, TLR3 is a critical pathway for innate antiviral immune responses, mostly through immediate activation of Interferon Regulatory Factor 3 (IRF3) (Sarkar et al., 2004). Additionally, IL-33 expression appears to be dependent upon activation of IRF3, such that macrophages deficient in IRF3 fail to express IL-33 in response to poly I:C (Polumuri et al., 2012). Therefore, IL-33 dampens inflammation, which may be at the expense of viral clearance.
Recent evidence reveals that IL-33 has a significant role in macrophage plasticity, whereby pro-inflammatory M1 macrophage populations that are involved in microbial/viral destruction and clearance transition to pro-resolving M2 macrophage populations involved in healing after microbial infection (Wang et al., 2017). In this case, IL-33 supports the M2 phenotype. Moreover, it is likely that IL-33 actively reduces innate anti-viral immune responses by increasing the activity of Pin1 phospho-specific prolyl isomerase activity (Nechama et al., 2018), which regulates IRF3 activity (Saitoh et al., 2006), a transcription factor for many Interferon Stimulated Genes (ISGs) of anti-viral immune responses. This is an important consideration because certain viruses, such as TMEV, infect and persist in macrophages, which can migrate to other tissues (Clatch et al., 1990). The mechanism by which TMEV persists in macrophages is unresolved but one hypothesis is that TMEV persists in M2 macrophages, perhaps brought about by exposure of responding macrophages to IL-33. Therefore, the impact of IL-33 on the innate anti-viral immunity of macrophages needs clarification.
We set out here to test the hypothesis that IL-33 significantly reduces the innate anti-viral resistance of macrophages to myelotropic viruses, such as TMEV, by promoting certain M2 phenotypic markers. Our results show that IL-33 has a profound effect on M1/M2 macrophage factors and anti-viral immune responses that are IRF3 dependent. These effects of IL-33 on the anti-viral immunity result in elevated levels of TMEV infection suggesting a pathway that could play a role in TMEV persistence in macrophages.
2. Materials and methods
2.1. Cell lines, virus, and reagents
RAW264.7-Lucia-ISG and RAW264.7-Lucia-IRF3KO-ISG (Invivogen, San Diego, CA) were maintained in complete media consisting of DMEM with 10% FBS and 50 ug/ml gentamycin, as we have previously reported (Guinn and Petro, 2019). TMEV DA strain was originally obtained from Dr. Kristin Drescher (Creighton University) (Petro, 2005). TMEV for infections was obtained by passage onto BHK21 cells, harvesting supernatants after 72 h, and conducting plaque assays using BHK21 cells to quantify the virus. Poly I:C was obtained from Invivogen, recombinant mouse IFN-γ and mouse IL-33 were obtained from BioLegend (San Diego, CA). Purified anti-mouse IL-33Rα (ST2) blocking antibody was obtained from BioLegend.
2.2. Quantitative RT-PCR
To assess mRNA expression, 2 × 106 or 10 × 106 RAW cells were incubated overnight in complete cell culture media in either 24-well or 6-well plates, respectively. Cells were challenged with 1 × 107 or 5 × 107 TMEV, respectively, and/or stimulated with 20 ng/ml IFN-γ, 10 μg/ml poly I:C, or 20 ng/ml IL-33. After 18 or 24 h, RNA was isolated using PureLink RNA mini-kit (ThermoFisher, Waltham, MA) and quantified spectrophotometrically at 260/280 nm. Equal concentrations of RNA (500 ng) were used to synthesize cDNA with EasyScript Reverse Transcriptase (Midwest Scientific, Valley Park, MO). For qPCR, 2 ul of cDNA were incubated with EvaGreen qPCR MasterMix (Midwest Scientific) and with the following primer pairs: murine GAPDH sense 5′ TTG TCAGCAATGCATCCTGCAC 3′ and antisense 5′ ACAGCTTTCCAGAGGGGCCATC 3′; murine IFN-β sense 5’-ATGAACAACAG GTGGATCCTCC-3’, antisense 5’- AGGAGCTCCTGACATTTCCGAA-3’; murine IL-6 sense 5’- ATGAAGTTCCTCTCTGCAAGAGACT-3’, antisense 5’- CACTAGGTTTGCCGAGTAGATCTC-3’; murine IL-33 sense 5’ TCCAACTCCAAGATTTCCCCG 3’, antisense 5’- CATGCAGTAGACATGGCAGAA 3’; TMEV sense 5′ CTTCCCATTCTACTGCAATG 3′, antisense 5′ AGGGCAGCACGAATTATGAC 3′; murine IL-10 sense, 5’-ATGCAGGACTTT AAGGGTTACTTGGGTG-3’, antisense 5’-ATTTCGGAGAGAGGTACAAACGAGGTTT-3’; murine CD206 sense 5’ ATGTTGAAGGGAAGTGGCTTTG 3’, antisense 5’ CCTTTGTACG AAGAACAGTGGA 3’; murine ARG1 sense 5’ CTTCGGAACCTACTGGGACAAC 3’, antisense 5’ CAGAAGGCACTGTGGTTCCCAG 3’; murine NOS2 sense 5’ ACAAGCCT ACCCCTCTGGAGGA 3’, antisense 5’ GTGAGCTGGTAGGTTCCTGTTG 3’. Quantitative PCRs were run on an ABI Prism 7000 thermal cycler at 50°C for 2 min then 95 °C for 10 min followed by 40 cycles of 95°C/15s, 60°C/30s. Data were expressed as relative levels of mRNA using 2ΔΔCt.
2.3. ISRE promoter activity
To evaluate the activity of stably expressed ISRE-IRF3 promoter-reporter, RAW Lucia and IRF3KO RAW Lucia cells were challenged with TMEV, or stimulated with 10 μg/ml poly I:C with or without 20 ng/ml IFN-γ, (Guinn and Petro, 2019) or stimulated with 20 ng/ml IL-33 for 18-24 h. Ten μl of supernatant was mixed with 50 μl of QuantiLuc (Invivogen) in a 1.5 ml microcentrifuge tube and relative luminescence was immediately determined using a Turner Biosystems Luminometer TD20/20.
2.4. Cytokine ELISA
IL-33, IL-10 and IL-6 ELISA kits with antibody pairs were obtained from ThermoFisher and cytokine quantification in supernatants was made using the manufacturer's specifications. Briefly, rat monoclonal IgG anti-mouse IL-33, anti-mouse IL-10 or anti-mouse IL-6 capture antibody in coating buffer was applied to 96-well ELISA plates, sealed, and incubated at 4 °C overnight. After decanting the capture antibody and washing once with PBS/0.05% Tween 20, blocking buffer (PBS-10% FBS) was applied for 1 h at RT. Wells were washed 3X with PBS/Tween, after which supernatants or dilutions of cytokine standard were applied to respective wells and then incubated for 2 h at RT. Wells were then washed 3X and then incubated with the designated biotinylated-detection antibody that was rat monoclonal IgG anti-mouse IL-33, anti-mouse IL10 or anti-mouse IL-6 for 1 h at RT followed by avidin-peroxidase. The plates were washed five times with PBS/Tween 20, and incubated with 3’, 5, 5’ tetramethylbenzidine substrate, followed by the addition of a stopping reagent. ODs at 450 nm wavelength with reference ODs at 570 nm made using an ELISA spectrophotometric plate reader were used to calculate the concentration of cytokine per ml of supernatant using the standard curve readings.
2.5. Nitric oxide
Nitric oxide concentrations in supernatants were determined using the Griess Assay kit of ThermoFisher. Briefly, 150 μl of the supernatant sample was added to 130 μl of deionized water and then 20 μl of Griess reagent. After incubation for 30 min at RT, absorbance at 548 nm was used to determine molar concentrations of NO in each sample.
2.6. Transfections
To express IRF3 in IRF3KO RAW Lucia cells, 2 × 105 of these cells in complete culture media were incubated in 24-well plates overnight. After 12 h, cells were transfected with 0.5 ug pIRF3 (Genecopia Ex-Mm7218-M56 clone) or pEGFP empty vector using Lipofectamine 3000 (ThermoFisher). Twenty-four h after initiation of transfection, cells were stimulated with 10 ug/ml poly I:C with or without 20 ng/ml IFN- γ. After 24 h, IL-33, NO, IL-6, and IL-10 were assessed.
2.7. Statistical analysis
Cytokine expression measured by PCR and cytokine production measured by ELISA were analyzed using Student's t-test to determine the significance of differences; P-values of ≤ 0.05 were considered significant.
3. Results
3.1. IRF3 deficiency increases TMEV infection and decreases cytokine response to TMEV infection in RAW264.7 cells
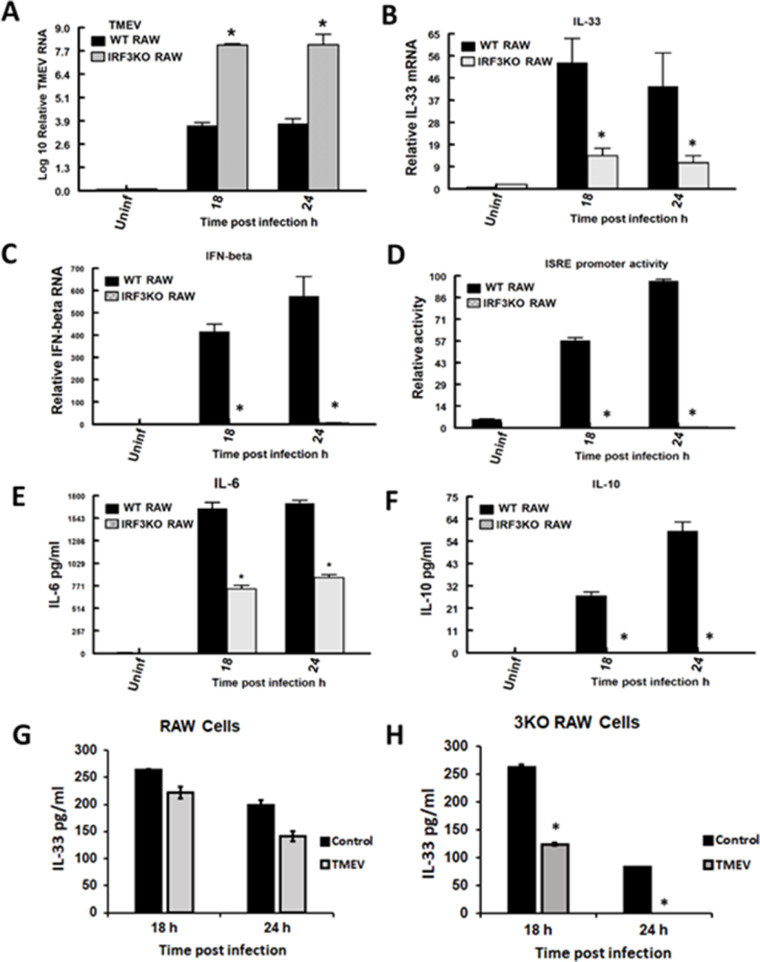
Previously, we provided evidence that IRF3 is critical to macrophage control of TMEV infection, through its role in the expression of IL-6, IFN-β, ISGs, and NO (Moore et al., 2012; Moore et al., 2013). While IL-6 and NO are factors produced by M1 macrophages, we did not examine M2 macrophage cytokines like IL-33 and IL-10, or the signature M2 markers CD206 and ARG1. Here we used the RAW-Lucia mouse macrophage cell line (RAW), which is a modified version of the original RAW264.7 cells with an ISG54/ISRE promoter containing numerous IRF3 binding elements driving secreted luciferase (Lucia) production. In addition, we used the IRF3KO-RAW Lucia cell line (IRF3KO RAW) that has a deleted IRF3 gene but contains the ISG54/ISRE promoter-reporter. RAW Lucia and IRF3KO RAW Lucia cells were challenged with TMEV and at 18 and 24h TMEV RNA, IFN-β and IL-33 gene expression, IL-6, IL-10, and IL-33 protein production, and ISRE promoter activity were assessed. RAW Lucia cells challenged with TMEV produced IL-6 and IL-10 protein, expressed IFN-β and IL-33 mRNA, and showed ISRE promoter activity (Fig. 1). As we have seen previously, IRF3 deficiency resulted in significantly elevated TMEV infection in RAW Lucia macrophages and significantly decreased IL-6 protein, IFN-β gene expression, and ISRE promoter activity (Guinn and Petro, 2019; Moore et al., 2013). However, we now provide evidence that IRF3 deficiency also significantly reduced the expression of IL-33 mRNA and IL-10 protein during TMEV infection. Because IL-33 is a unique cytokine retained intracellularly and released upon cellular lysis, whereby it brings about its effects, we also evaluated extracellular IL-33 protein during TMEV infection using ELISA. Unlike induction of IL-33 gene expression, extracellular IL-33 protein was found in supernatants of unstimulated cells and decreased with TMEV infection of RAW or IRF3KO RAW cells (Fig. 1 G, H). Supernatants of uninfected and TMEV-infected RAW cells had equivalent levels of IL-33 at 18 h post-infection. However, IL-33 protein levels at 18 and 24 h post-infection were less in supernatants of IRF3KO RAW cells compared with RAW cells. These data suggest that TMEV infection of macrophages induces both M1 and M2 macrophage factors, and IRF3 influences both sets of factors, including IL-33.
Fig. 1.
IRF3 deficiency impairs innate antiviral immune response to TMEV. 1 × 106 RAW Lucia or IRF3KO-RAW Lucia cells were incubated overnight in 6-well plates and then challenged with 5 × 106 TMEV. After 18 and 24 h, TMEV RNA (A), IL-33 (B) and IFN-beta (C) mRNA by qRT-PCR, secreted IL-6 (E), IL-10 (F) and IL-33 (G, H) by ELISA. For the reported experiment n=3 for each condition, which was repeated n=3. Data are means ± standard error and were analyzed by Student's t-test; * indicates significantly different than control, p≤ 0.05.
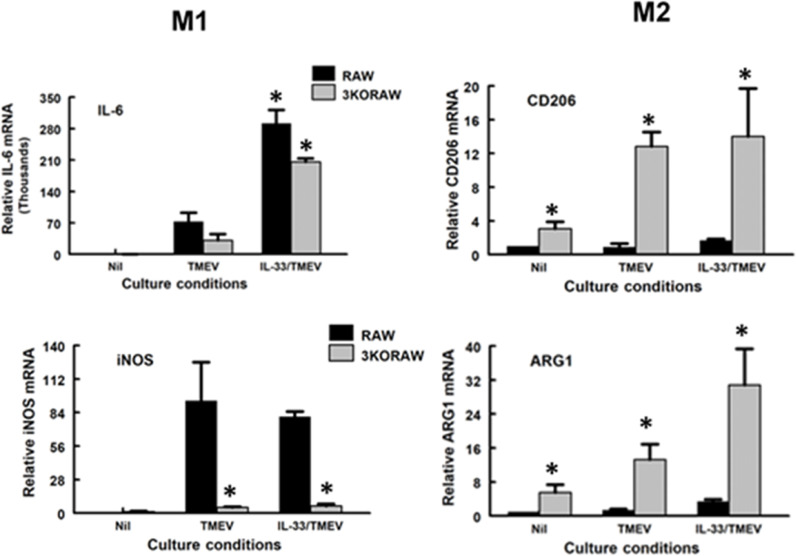
3.2. IRF3 deficiency increases expression of classical M2 macrophage markers
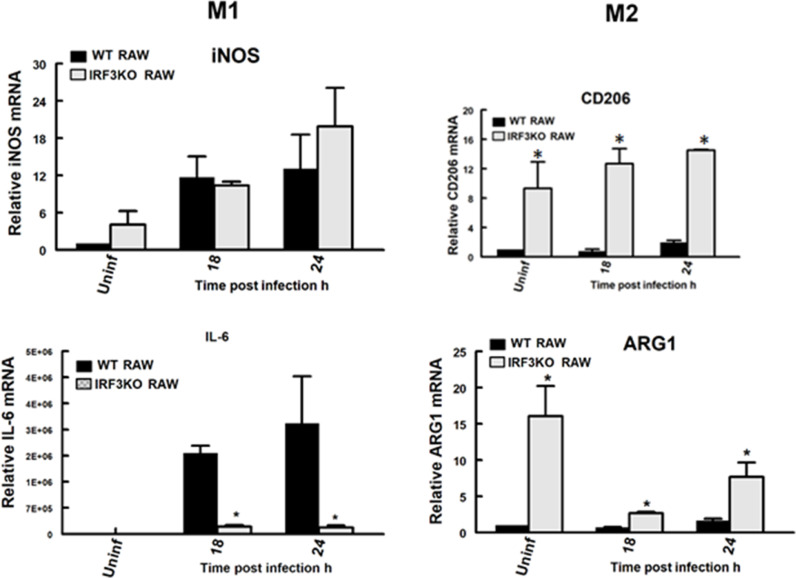
Arginase 1 (ARG1) and CD206 are recognized signature markers of M2, while iNOS (aka NOS2) and IL-6 are signature markers of M2. To evaluate the impact of IRF3 on M1 vs M2 classical phenotypic markers, we evaluated ARG1 and CD206, as well as iNOS and IL-6 gene expression in RAW264.7 Lucia and IRF3KO RAW Lucia cells with and without TMEV challenge at 18 and 24 h. Expression of both iNOS and IL-6 in RAW Lucia macrophages increased at 18 and 24 h post-TMEV infection (Fig. 2). As expected, expression of IL-6 was significantly impaired in IRF3KO RAW Lucia cells challenged with TMEV, while IRF3 deficiency did not significantly affect the expression of iNOS. In contrast, uninfected IRF3KO RAW cells expressed significantly elevated levels of CD206 and ARG1 mRNA compared with uninfected RAW cells. TMEV-infected IRF3KO RAW cells also expressed significantly higher levels of CD206 and ARG1 mRNA compared with TMEV-infected RAW cells. These data suggest that IRF3 not only promotes the expression of some M1 macrophage markers but also represses the expression of M2 markers.
Fig. 2.
IRF3 deficiency increases M2 phenotypic markers. 1 × 106 RAW Lucia or IRF3KO-RAW Lucia cells were incubated overnight in 6-well plates and then challenged with 5 × 106 TMEV. After 18 and 24 h, mRNA expression of iNOS and IL-6 M1 markers or CD206, and ARG1 M2 markers were assessed by qRT-PCR. For the reported experiment n=3 for each condition, which was repeated n=3. Data are means ± standard error and were analyzed by Student's t-test; * indicates significantly different than control, p≤ 0.05.
3.3. Blockade of IL-33 receptor decreases expression of M2 markers
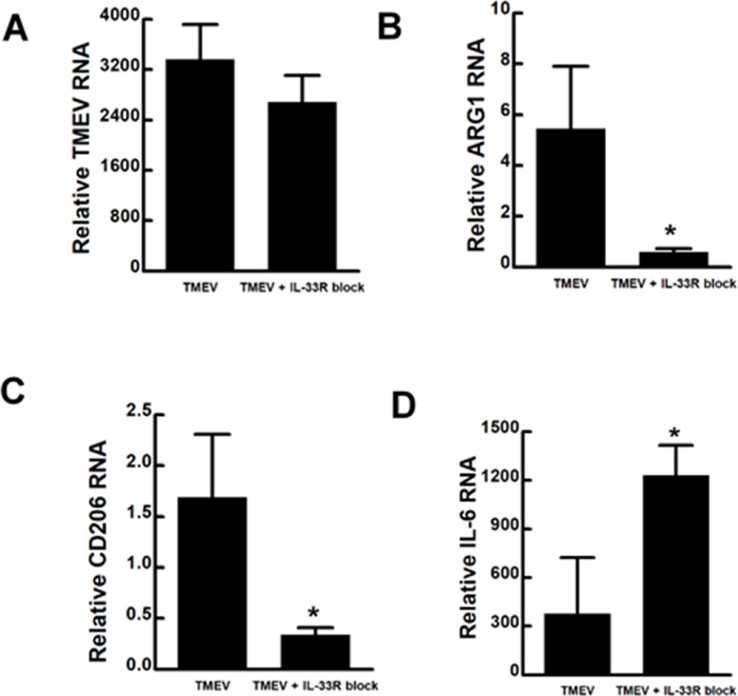
Since, IL-33 protein was constitutively released from RAW-Lucia cells with or without TMEV infection, we decided to reduce the effect of IL-33 with addition of a verified IL-33 receptor ST2 blocking antibody at the time of infection (Hashiguchi et al, 2014). Addition of IL-33R ST2 blocking antibody during the TMEV challenge of RAW Lucia cells only slightly reduced TMEV RNA, but significantly reduced CD206 and ARG1 expression at 24 h post-infection (Fig. 3). In contrast, IL-6 expression significantly increased upon the blockade of IL-33R ST2 during TMEV infection of RAW Lucia cells. These data suggest that in addition to IRF3 deficiency, IL-33 production from macrophages promotes the expression of M2 phenotypic markers.
Fig. 3.
Blockade of IL-33RA ST2 alters M1/M2 phenotypic markers during TMEV infection. 1 × 106 RAW Lucia cells were incubated overnight in 6-well plates and then challenged with 5 × 106 TMEV with and without 10 μg/ml monoclonal antibody to IL-33R ST2. After 24 h, mRNA expression of TMEV, IL-6 (M1 marker) or CD206 and ARG1 (M2 markers) was evaluated by qRT-PCR. For the reported experiment n=3 for each condition, which was repeated n=3. Data are means ± standard error and were analyzed by Student's t-test; * indicates significantly different than control, p≤ 0.05.
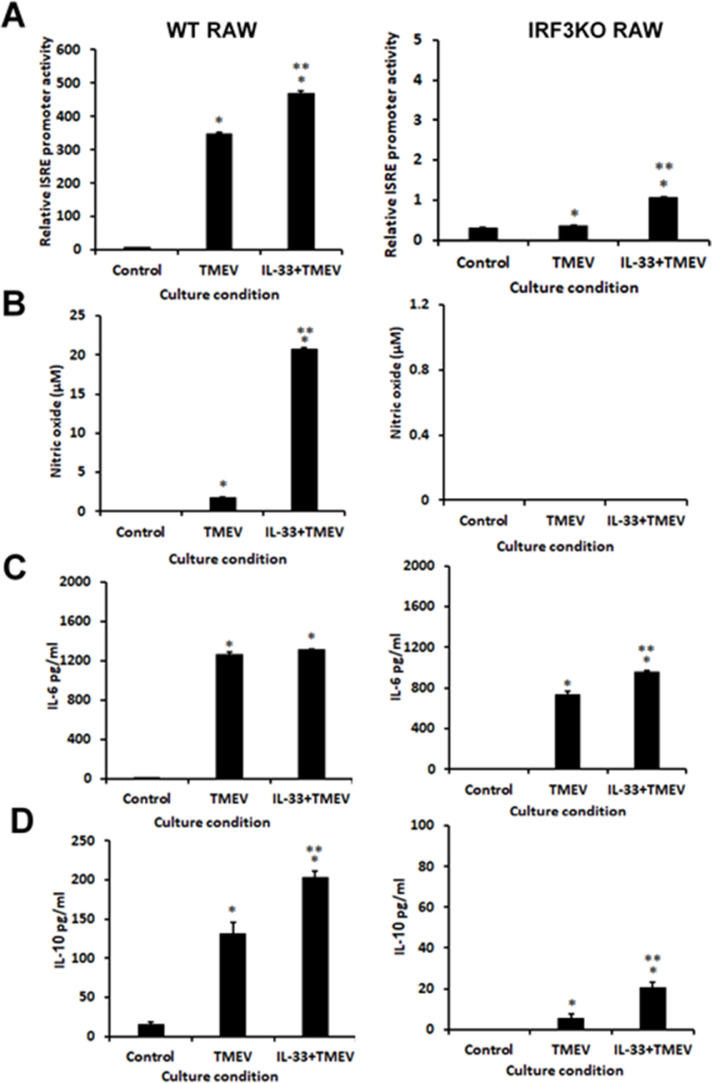
3.4. IL-33 pretreatment increases TMEV infection in RAW264.7 cells and increases cytokine response to infection in an IRF3-dependent fashion
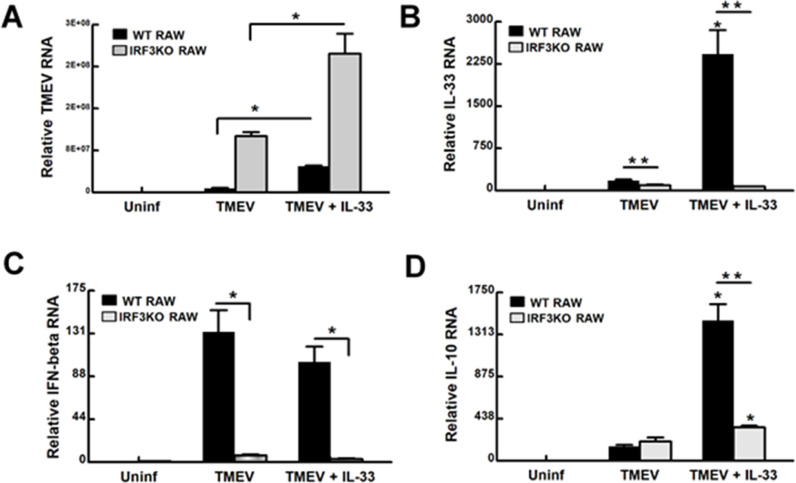
Challenging RAW264.7 cells with TMEV is known to result in persistent infection of these cells with this virus (Steurbaut et al., 2006). Since RAW cells express IL-33 (Hudson et al., 2008; Kiyomiya et al., 2015), we set out to determine if additional IL-33 protein could affect TMEV infection of macrophages or the response to the infection. RAW and IRF3KO-RAW cells were pretreated for 24 h with IL-33 protein and then challenged with TMEV. Pretreatment of RAW Lucia or IRF3KO RAW Lucia cells significantly increased TMEV infection at 24 h post-challenge (Fig 4A). At the same time, treatment with IL-33 increased the expression of IL-33 and IL-10 mRNA in an IRF3-dependent manner (Fig. 4B, D), but did not affect IFN-β induction in response to TMEV infection (Fig. 4C). Expression of the M1 marker, IL-6 (Fig. 5), but not iNOS, significantly increased with IL-33 pretreatment prior to infection with TMEV in both RAW Lucia and IRF3KO RAW Lucia cells. As with the previous experiment, M2 markers, CD206 and ARG1, were significantly elevated in IRF3KO RAW Lucia compared with RAW Lucia cells, which were elevated further with IL-33 pretreatment (Fig. 5). ISRE promoter activity, NO, and IL-10 protein production in response to TMEV infection in RAW Lucia increased with IL-33 pretreatment (Fig. 6). While the overall responses in IRF3KO RAW Lucia cells were substantially lower as expected, IL-33 increased the impaired TMEV-induced ISRE promoter activity, IL-6 protein, and IL-10 protein in IRF3KO RAW Lucia cells to a small degree (Fig. 6) and failed to restore NO production. Interestingly, IL-33 failed to increase IL-6 (Fig. 6C) but did increase IL-10 protein production (Fig. 6D) during TMEV infection of RAW Lucia or IRF3KO RAW Lucia cells. Therefore, IL-33 significantly increased susceptibility to TMEV replication in RAW Lucia cells while at the same time augmenting M2 markers, CD206 and ARG1.
Fig. 4.
IL-33 treatment increases TMEV infection of RAW cells. 1 × 106 RAW Lucia or IRF3KO RAW Lucia cells were incubated overnight in 6-well plates, pretreated with 20 ng/ml IL-33 for 24 h, and then challenged with 5 × 106 TMEV. After 24 h, cells were assessed for TMEV RNA (A), IL-33 (B), IFN-beta (C), and IL-10 (D) mRNA using qRT-PCR. For the reported data n=3 for each condition of an experiment, which was repeated n=3. Data are means ± standard error analyzed by Student's t-test; *; ** indicates significantly different from controls, p≤ 0.05.
Fig. 5.
IL-33 treatment increases M2 phenotypic markers during TMEV infection of RAW cells. 1 × 106 RAW Lucia or IRF3KO RAW Lucia cells were incubated overnight in 6-well plates, pretreated with 20 ng/ml IL-33 for 24 h, and then challenged with 5 × 106 TMEV. After 24 h, gene expression of cellular IL-6 and iNOS (M1 markers) or CD206 and ARG1 (M2 markers) was assessed using qRT-PCR. For the reported data n=3 for each condition of an experiment, which was repeated n=3. Data are means ± standard error analyzed by Student's t-test; *; ** indicates significantly different from controls, p≤ 0.05.
Fig. 6.
IL-33 treatment increased innate immune responses during TMEV infection. 1 × 106 RAW Lucia (Left) or IRF3KO RAW Lucia (Right) cells were incubated overnight in 6-well plates, pretreated with 20 ng/ml IL-33 for 24 h, and then challenged with 5 × 106 TMEV. After 24 h, (A) IRF3 activity was assessed by secreted Luciferase directed by ISRE-IRF3 promoter activity, (B) supernatant Nitric Oxide (NO) assessed using Griess assay, (C) secreted IL-6 and (D) IL-10 assessed using ELISA. For the reported data n=3 for each condition of an experiment, which was repeated n=3. Data are means ± standard error and were analyzed by Student's t-test; * indicates significantly different from controls; ** indicates significantly different from TMEV only infected cells, p≤ 0.05.
To determine the impact of IL-33 on innate antiviral responses, RAW and IRF3KO-RAW cells were pretreated with IL-33 and then stimulated with poly I:C, a TLR3 agonist (Alexopoulou et al., 2001), with or without IFN-γ (Guinn and Petro, 2019). IL-33 pretreatment alone stimulated NO, IL-6, and IL-10 production from RAW cells (Supplemental Fig. 1). Poly I:C in combination with IL-33 pretreatment increased IL-10 and IL-6, but not NO from RAW cells. IL-33 inhibited ISRE promoter activity in response to poly I:C/IFN-γ-stimulated IRF3KO-RAW Lucia cells. While IRF3 deficiency increased the expression of ARG1 and CD206 (Supplemental Fig. 2), IL-33 did not increase these expressions in unstimulated or stimulated RAW or IRF3KO RAW cells. Therefore, IL-33 modulates key innate antiviral immune responses, but its effect in some conditions is dependent on IRF3.
3.5. IRF3 overexpression restores IL-33 induced IL-33, IL-10 and ISRE promoter activity in IRF3KO RAW Lucia cells
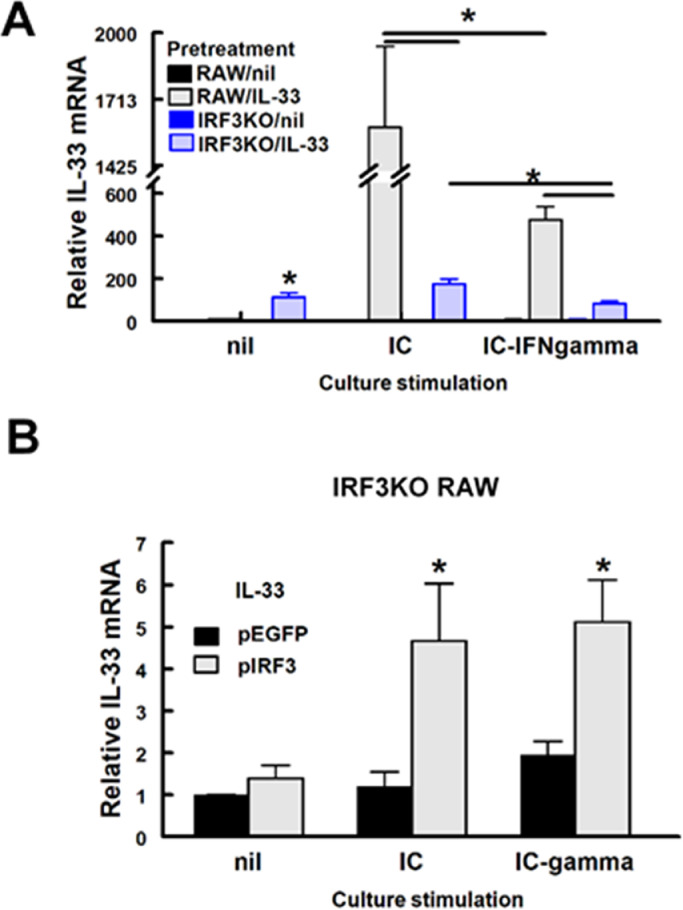
IRF3 deficiency decreased IL-33 mRNA expression in cells stimulated with TMEV infection or poly I:C with or without IFN-γ (Fig. 7A). Interestingly, IL-33 pretreatment induced some IL-33 mRNA expression in unstimulated IRF3KO-RAW Lucia cells, but not in unstimulated RAW Lucia cells. Stimulation with poly IC or poly IC/IFN-γ did not increase IL-33 expression further in IRF3KO-RAW Lucia cells. IL-33 expression in RAW Lucia cells was induced to a great extent by poly I:C, but poly I:C-induced IL-33 was significantly diminished with IFN-γ costimulation (Fig. 7A). Therefore, these results suggest that the expression of IL-33 is dependent on IRF3 activity.
Fig. 7.
Induced IL-33 gene expression in IL-33 pretreated RAW cells is IRF3 dependent. (A) 1 × 106 RAW Lucia or IRF3KO RAW Lucia cells were incubated overnight in 6-well plates, pretreated with 20 ng/ml IL-33 for 24 h, and then stimulated with 10 μg/ml poly I:C (IC) with or without 20 ng/ml IFN-gamma (IFN-γ). (B) 2 × 105 IRF3KO RAW Lucia cells were incubated overnight in 24-well plates and then cells were transfected with 0.5 μg per well pEGFP Empty vector or pIRF3 expression vector. After 24 h of transfection, cells were stimulated with 10 ug/ml poly I:C (IC) with or without 20 ng/ml IFN-gamma (IFN-g). After 24 h of stimulation, qRT-PCR assessed IL-33 expression (A, B). For A, n=3 for each condition of an experiment, which was repeated n=3 and for B, n=4. Data are means ± standard error and were analyzed by Student t test; * indicates significantly different from controls (nil), p≤ 0.05.
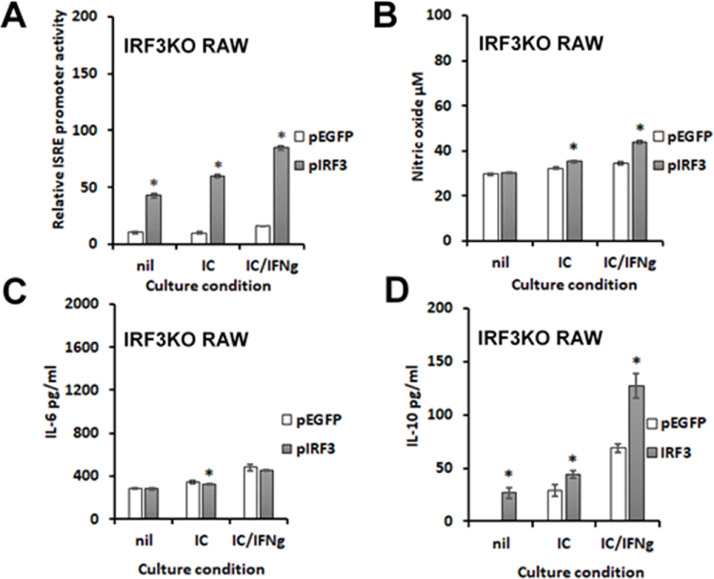
To confirm that IRF3 contributes significantly to IL-33-induced gene expressions seen here, IRF3KO-RAW Lucia cells were transiently transfected with pIRF3, a plasmid expression vector encoding wild-type mouse IRF3. After transfection, cells were stimulated with poly I:C with or without IFN-γ. The results confirm that IRF3KO RAW Lucia cells, which do not express IL-33 in response to poly I:C or poly I:C/IFN-γ, express significantly increased IL-33 with pIRF3 plasmid transfection (Fig. 7B). As expected pIRF3 restored the ISRE response of IRF3KO RAW Lucia cells to poly I:C and poly I:C/IFN-γ (Fig. 8A), while overexpression of IRF3 alone stimulated ISRE promoter activity. In contrast, poly I:C- and poly I:C/IFN-γ-induced production of NO and IL-6 from IRF3KO RAW cells was only slightly affected by IRF3 overexpression (Fig. 8B, C). In contrast, IL-33 induced IL-10 production from IRF3KO-RAW cells was detected with IRF3 overexpression alone and significantly enhanced in IL-33 pretreated IRF3KO-RAW cells stimulated with poly I:C or poly I:C plus IFN-γ (Fig. 8D). These data confirm that IRF3 activity is a critical component in IL-33 gene expression.
Fig. 8.
Induced IL-10 expression and IRF3-ISRE promoter activity in RAW cells is IRF3 dependent. 2 × 105 IRF3KO RAW Lucia cells were incubated overnight in 24-well plates and then cells were transfected with 0.5 μg per well pEGFP Empty vector or pIRF3 expression vector. After 24 h cells were stimulated with 10 μg/ml poly I:C (IC) with or without 20 ng/ml IFN-gamma (IFN-g). After 24 h of stimulation, IRF3-ISRE promoter activity was assessed with secreted luciferase (A), NO assessed with Griess assay (B), and IL-6 (C) or IL-10 (D) assessed by ELISA. For A, n=3 for each condition of an experiment, which was repeated n=3 and for B-F, n=4. Data are means ± standard error and were analyzed by Student t test; * indicates significantly different from pEGFP controls, p≤ 0.05.
4. Discussion
The present study provides evidence that IL-33, a unique cytokine of the IL-1 family, can increase macrophage susceptibility to replication of myelotropic viruses, like TMEV (Lipton et al., 1995; Takata et al., 1998). We show here that RAW264.7 mouse macrophage cells treated with IL-33 exhibited significantly greater intracellular TMEV genome 24 h after infection compared with untreated cells, while blockade of IL-33R ST2 prevented this enhancement in TMEV infection. Moreover, RAW264.7 cells deficient in IRF3, a key antiviral transcription factor of the innate antiviral immune response, exhibited greater levels of TMEV infection after treatment with IL-33. Macrophage populations are phenotypically diverse and are known reservoirs for persistent viral infection, such as the Influenza virus (Sprenger et al., 1994), HIV (Ushijima et al., 1992), and Zika virus (Quicke et al., 2016). However, the mechanism for persistent viral infection of macrophages is unclear. M1 macrophages are destructive to microbes, mostly because they produce ROS, NO, ISGs, and inflammatory cytokines, such as IL-6 (Murray, 2017). None of these anti-viral factors declined following IL-33 pretreatment prior to TMEV infection. However, macrophages undergo phenotypic differentiation upon exposure to cytokines, such as IL-4 and IL-33 (Murray, 2017). These alternatively activated macrophage subsets, termed M2, contribute to the healing of damaged tissue through the production of anti-inflammatory cytokines, such as IL-10, growth factors, such as fibroblast growth factors, and expression of M2 signature proteins CD206 and ARG1. Here we show that IL-10 increases following IL-33 pretreatment. More importantly, expression of CD206 and ARG1 were elevated in RAW 264.7 cells deficient in IRF3, suggesting that IRF3 activity downregulates M2 phenotypic markers. SJL/J mice, which exhibit persistent infection with TMEV in the CNS, express heightened IL-10 production in macrophages during the infection (Uhde et al., 2018). Many cell types, including macrophages, produce IL-33 in response to microbial infection, such as with viruses. Initially, IL-33 protein is localized to the nucleus and associated with chromatin (Cayrol and Girard, 2022). Upon cellular necrosis, which can occur due to viral infection of a cell, IL-33 exits the cell. The extracellular IL-33 alerts the immune system of cell and tissue damage, wherein macrophage populations initiate an M2 resolving phenotype and less of an M1 microbial destructive phenotype. The results here show that TMEV infection of RAW Lucia macrophage cells induces IL-33 mRNA. However, TMEV infection did not stimulate greater release of IL-33 from infected cells. A steady state release of IL-33 occurs from RAW Lucia cells even without viral challenge. Moreover, addition of exogenous recombinant IL-33 increased TMEV infection of RAW Lucia cells, while antibody blockade of IL-33R ST2 at these cells only slightly decreased TMEV replication. Thus, the pro-M2 cytokine, IL-33, likely contributes significantly to persistence of myelotropic viruses but the mechanism by which it produces this effect is unclear and may be related to the induction of the signature M2 phenotypic markers, CD206 and ARG1. It is interesting to speculate that the elevated expression of ARG1, which is an enzyme that catalyzes the conversion of arginine to ornithine, depletes intracellular arginine. This would prevent the enzyme iNOS, an M1 phenotypic marker, from producing sufficient NO for antiviral activity from its arginine substrate.
Another key finding here is that the optimum expression of IL-33 depends upon IRF3, an essential transcription factor for immediate anti-viral innate immunity. RAW cells deficient in IRF3 expressed greatly diminished IL-33 protein production and IL-33 mRNA expression either in response to TMEV infection or in response to poly I:C with or without IFN-γ. These data are in agreement with a previous study that suggested the expression of IL-33 is dependent on IRF3 in macrophages (Polumuri et al., 2012). Moreover, IRF3 deficiency impaired the response of RAW264.7 cells to IL-33, wherein they express diminished IL-10, which is an M2 macrophage cytokine. Restoration of IRF3 expression in IRF3KO-RAW cells restored IL-33-induced IL-10 and IL-33 expression in response to poly I:C/IFN-γ. Previously, we showed that IRF3 is crucial to IL-6 responses to TMEV infection of macrophages and that IL-6 induces the production of NO (Moore et al., 2012; Moore et al., 2013). In landmark experiments, many others have shown that activation of IRF3 controls early IFN-β responses of cells to viral infection (Wathelet et al., 1998). Secreted IFN-β then stimulates global expression of a wide range of additional ISGs, therein securing robust innate antiviral immunity (Grandvaux et al., 2002). The IRF3/IFN-β/ISG axis is so crucial to anti-viral immunity, it is not difficult to appreciate why most viruses, including TMEV through its L-protein (Ricour et al., 2009), target some part of the IRF3 activation pathway to promote viral replication in host cells (Kuo et al., 2010). It is not clear if failure to express IL-33 in IRF3 deficient macrophages is a direct effect or the result of deficient production of an IRF3-dependent cytokine that then stimulates IL-33 expression. However, our results here show that IRF3 is a key transcriptional repressor of M2 phenotypic markers, CD206 and ARG1.
Since its discovery, the evidence points to a key role of IL-33 in both innate and adaptive immune responses. In contrast to its effect on innate anti-viral immune responses, IL-33 is beneficial in the development of cytolytic CD8 T cell adaptive immune responses during viral infection (Bonilla et al., 2012) and it amplifies Treg responses (Schiering et al., 2014). Moreover, IL-33 decreases the expression of Blimp-1 and increases the expression of IRF-8 in RAW264.7 macrophages (Kiyomiya et al., 2015). Therefore, IL-33 influences many components of antiviral immunity.
The mechanism by which IL-33 increases susceptibility to macrophage infection with myelotropic viruses is unclear. IL-33 pretreatment augmented IL-10 production, which is a M2 macrophage cytokine. IL-33 pretreatment did not augment IL-6 production in response to TMEV infection, but it did stimulate IL-6 from RAW264.7 cells without TMEV infection. Despite its limited impact on IL-6 here, there is evidence that IL-6 has diverse effects on target cells, which affect the response to IL-33. In that regard, JAK1, JAK2, and Tyk2 of the IL-6 receptor system can activate cytoplasmic STAT1 and STAT3 (Akira, 1997), and depending on whether STAT1 or STAT3 is activated by IL-6 could control macrophage responses to viruses. It is reasonable to speculate that if IL-6 activates pSTAT1 (Lv et al., 2017) in the presence of IL-33 this could lead to M1 phenotype with ISG expression, including ISG54 (IFIT2) and control of virus infection. But if IL-6 activates pSTAT3 (Wei et al., 2022) in the presence of IL-33 then M2 phenotype with survivin protein expression could occur, ultimately leading to virus persistence(Zhang et al., 2021). In support of this assertion is evidence that IL-33 induces STAT3 (Liang et al., 2018) and downregulates STAT1 (Stier et al., 2017). Thus, while IL-33 does not augment IL-6 production substantially during virus infection, it could change the intracellular response to IL-6 in such a manner as to contribute to virus persistence. In conclusion, IL-33, a unique cytokine termed an alarmin and known to be involved in M2 and Th2 differentiation, could play a role in the increased susceptibility of macrophages to persistent viral infection. However, the role of IRF3 in preventing persistent infection of macrophages by viruses now includes the ability of IRF3 to downregulate macrophage M2 phenotypic markers. It remains to be seen if IL-33 augments infection in macrophages with other viruses shown to be myelotropic, such as ATCV-1 chlorovirus (Petro et al., 2015).
Author statement
The authors declare that research described was conducted in an ethical manner.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests that influenced the work reported in this paper.
Acknowledgements
This study was funded by Stuart Nichols Research Foundation and research support from the UNMC College of Dentistry.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2022.199007.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Akira S. IL-6-regulated transcription factors. Int. J. Biochem. Cell Biol. 1997;29(12):1401–1418. doi: 10.1016/s1357-2725(97)00063-0. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-[kappa]B by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Bonilla W.V., Fröhlich A., Senn K., Kallert S., Fernandez M., Johnson S., Kreutzfeldt M., Hegazy A.N., Schrick C., Fallon P.G., Klemenz R., Nakae S., Adler H., Merkler D., Löhning M., Pinschewer D.D. The alarmin interleukin-33 drives protective antiviral CD8⁺ T cell responses. Science. 2012;335(6071):984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- Caslin H.L., Taruselli M.T., Haque T., Pondicherry N., Baldwin E.A., Barnstein B.O., Ryan J.J. Inhibiting glycolysis and ATP production attenuates IL-33-mediated mast cell function and peritonitis. Front. Immunol. 2018;9:3026. doi: 10.3389/fimmu.2018.03026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C., Girard J.P. Interleukin-33 (IL-33): a critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine. 2022;156 doi: 10.1016/j.cyto.2022.155891. [DOI] [PubMed] [Google Scholar]
- Clatch R.J., Miller S.D., Metzner R., Dal Canto M.C., Lipton H.L. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler's murine encephalomyelitis virus (TMEV) Virology. 1990;176(1):244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- Grandvaux N., Servant M.J., tenOever B., Sen G.C., Balachandran S., Barber G.N., Lin R., Hiscott J. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 2002;76(11):5532–5539. doi: 10.1128/JVI.76.11.5532-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn Z.P., Petro T.M. Interferon regulatory factor 3 plays a role in macrophage responses to interferon-γ. Immunobiology. 2019;224(4):565–574. doi: 10.1016/j.imbio.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C.A., Christophi G.P., Gruber R.C., Wilmore J.R., Lawrence D.A., Massa P.T. Induction of IL-33 expression and activity in central nervous system glia. J. Leukoc. Biol. 2008;84(3):631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomiya H., Ariyoshi W., Okinaga T., Kaneuji T., Mitsugi S., Sakurai T., Habu M., Yoshioka I., Tominaga K., Nishihara T. IL-33 inhibits RANKL-induced osteoclast formation through the regulation of Blimp-1 and IRF-8 expression. Biochem. Biophys. Res. Commun. 2015;460(2):320–326. doi: 10.1016/j.bbrc.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Kuo R.L., Zhao C., Malur M., Krug R.M. Influenza A virus strains that circulate in humans differ in the ability of their NS1 proteins to block the activation of IRF3 and interferon-beta transcription. Virology. 2010;408(2):146–158. doi: 10.1016/j.virol.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Yang N., Pan G., Jin B., Wang S., Ji W. Elevated IL-33 promotes expression of MMP2 and MMP9 via activating STAT3 in alveolar macrophages during LPS-induced acute lung injury. Cell. Mol. Biol. Lett. 2018;23:52. doi: 10.1186/s11658-018-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.C., Lin Y.C., Tsai M.L., Tsai Y.G., Kuo C.H., Hung C.H. IL-33 regulates M1/M2 chemokine expression via mitochondrial redox-related mitophagy in human monocytes. Chem. Biol. Interact. 2022;359 doi: 10.1016/j.cbi.2022.109915. [DOI] [PubMed] [Google Scholar]
- Lipton H.L., Twaddle G., Jelachich M.L. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Virol. 1995;69(4):2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv R., Bao Q., Li Y. Regulation of M1‑type and M2‑type macrophage polarization in RAW264.7 cells by Galectin‑9. Mol. Med. Rep. 2017;16(6):9111–9119. doi: 10.3892/mmr.2017.7719. [DOI] [PubMed] [Google Scholar]
- Moore T.C., Bush K.L., Cody L., Brown D.M., Petro T.M. Interleukin-6 control of early Theiler's Murine Encephalomyelitis Virus replication in macrophages occurs in conjunction with STAT1 activation and nitric oxide production. J. Virol. 2012;86:10841–10851. doi: 10.1128/JVI.01402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T.C., Cody L., Kumm P.M., Brown D.M., Petro T.M. IRF3 helps control acute TMEV infection through IL-6 expression but contributes to acute hippocampus damage following TMEV infection. Virus Res. 2013;178(2):226–233. doi: 10.1016/j.virusres.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- Nechama M., Kwon J., Wei S., Kyi A.T., Welner R.S., Ben-Dov I.Z., Arredouani M.S., Asara J.M., Chen C.H., Tsai C.Y., Nelson K.F., Kobayashi K.S., Israel E., Zhou X.Z., Nicholson L.K., Lu K.P. The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in IL-33-induced allergic airway inflammation. Nat. Commun. 2018;9(1):1603. doi: 10.1038/s41467-018-03886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro T.M. ERK-MAP-kinases differentially regulate expression of IL-23 p19 compared with p40 and IFN-beta in Theiler's virus-infected RAW264.7 cells. Immunol. Lett. 2005;97(1):47–53. doi: 10.1016/j.imlet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Petro T.M., Agarkova I.V., Zhou Y., Yolken R.H., Van Etten J.L., Dunigan D.D. Response of Mammalian macrophages to challenge with the chlorovirus Acanthocystis turfacea chlorella virus 1. J. Virol. 2015;89(23):12096–12107. doi: 10.1128/JVI.01254-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polumuri S.K., Jayakar G.G., Shirey K.A., Roberts Z.J., Perkins D.J., Pitha P.M., Vogel S.N. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J. Immunol. 2012;189(1):50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke K.M., Bowen J.R., Johnson E.L., McDonald C.E., Ma H., O'Neal J.T., Rajakumar A., Wrammert J., Rimawi B.H., Pulendran B., Schinazi R.F., Chakraborty R., Suthar M.S. Zika virus infects human placental macrophages. Cell Host Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricour C., Delhaye S., Hato S.V., Olenyik T.D., Michel B., van Kuppeveld F.J.M., Gustin K.E., Michiels T. Inhibition of mRNA export and dimerization of interferon regulatory factor 3 by Theiler's virus leader protein. J. Gen. Virol. 2009;90(1):177–186. doi: 10.1099/vir.0.005678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Tun-Kyi A., Ryo A., Yamamoto M., Finn G., Fujita T., Akira S., Yamamoto N., Lu K.P., Yamaoka S. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat. Immunol. 2006;7(6):598–605. doi: 10.1038/ni1347. [DOI] [PubMed] [Google Scholar]
- Sarkar S.N., Peters K.L., Elco C.P., Sakamoto S., Pal S., Sen G.C. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 2004;11(11):1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- Schiering C., Krausgruber T., Chomka A., Fröhlich A., Adelmann K., Wohlfert E.A., Pott J., Griseri T., Bollrath J., Hegazy A.N., Harrison O.J., Owens B.M.J., Löhning M., Belkaid Y., Fallon P.G., Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513(7519):564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D.M., Bazan J.F., Kastelein R.A. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Sprenger H., Bacher M., Rischkowsky E., Bender A., Nain M., Gemsa D. Characterization of a high molecular weight tumor necrosis factor-alpha mRNA in influenza A virus-infected macrophages. J. Immunol. 1994;152(1):280–289. [PubMed] [Google Scholar]
- Steurbaut S., Rombaut B., Vrijsen R. Persistent infection of RAW264.7 macrophages with the DA strain of Theiler's murine encephalomyelitis virus: an in vitro model to study viral persistence. J. Neurovirol. 2006;12(2):108–115. doi: 10.1080/13550280600714120. [DOI] [PubMed] [Google Scholar]
- Stier M.T., Goleniewska K., Cephus J.Y., Newcomb D.C., Sherrill T.P., Boyd K.L., Bloodworth M.H., Moore M.L., Chen K., Kolls J.K., Peebles R.S., Jr STAT1 represses cytokine-producing group 2 and group 3 innate lymphoid cells during viral infection. J. Immunol. 2017;199(2):510–519. doi: 10.4049/jimmunol.1601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata H., Obuchi M., Yamamoto J., Odagiri T., Roos R.P., Iizuka H., Ohara Y. L* protein of the DA strain of Theiler's murine encephalomyelitis virus is important for virus growth in a murine macrophage-like cell line. J. Virol. 1998;72(6):4950–4955. doi: 10.1128/jvi.72.6.4950-4955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talabot-Ayer D., McKee T., Gindre P., Bas S., Baeten D.L., Gabay C., Palmer G. Distinct serum and synovial fluid interleukin (IL)-33 levels in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Joint Bone Spine. 2012;79(1):32–37. doi: 10.1016/j.jbspin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Tjota M.Y., Camacho D.F., Turnquist H.R., Sperling A.I. IL-33 drives monocyte recruitment to lung interstitium through chemokine upregulation. Immunohorizons. 2017;1(6):101–108. doi: 10.4049/immunohorizons.1700024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde A.K., Ciurkiewicz M., Herder V., Khan M.A., Hensel N., Claus P., Beckstette M., Teich R., Floess S., Baumgärtner W., Jung K., Huehn J., Beineke A. Intact interleukin-10 receptor signaling protects from hippocampal damage elicited by experimental neurotropic virus infection of SJL mice. Sci. Rep. 2018;8(1):6106. doi: 10.1038/s41598-018-24378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima H., Kunisada T., Ami Y., Tsuchie H., Takahashi I., Klocking H.P., Muller W.E. Characterization of human immunodeficiency virus-1-infected cells of myeloid-monocytic lineage (ML-1, HL-60, THP-1, U-937) J. Acquir. Immune Defic. Syndr. 1992;5(10):1001–1004. [PubMed] [Google Scholar]
- Wang C., Dong C., Xiong S. IL-33 enhances macrophage M2 polarization and protects mice from CVB3-induced viral myocarditis. J. Mol. Cell Cardiol. 2017;103:22–30. doi: 10.1016/j.yjmcc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Wathelet M.G., Lin C.H., Parekh B.S., Ronco L.V., Howley P.M., Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell. 1998;1(4):507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- Wei L.Y., Lin H.C., Tsai F.C., Ko J.Y., Kok S.H., Cheng S.J., Lee J.J., Chia J.S. Effects of Interleukin-6 on STAT3-regulated signaling in oral cancer and as a prognosticator of patient survival. Oral Oncol. 2022;124 doi: 10.1016/j.oraloncology.2021.105665. [DOI] [PubMed] [Google Scholar]
- Xie D., Miao W., Xu F., Yuan C., Li S., Wang C., Junagade A., Hu X. IL-33/ST2 axis protects against traumatic brain injury through enhancing the function of regulatory T cells. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.860772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.M., Wei C.Y., Wang Q., Wang L., Lu L., Qi F.Z. M2-polarized macrophages mediate wound healing by regulating connective tissue growth factor via AKT, ERK1/2, and STAT3 signaling pathways. Mol. Biol. Rep. 2021;48(9):6443–6456. doi: 10.1007/s11033-021-06646-w. [DOI] [PubMed] [Google Scholar]
- Zhao W., Hu Z. The enigmatic processing and secretion of interleukin-33. Cell. Mol. Immunol. 2010;7(4):260–262. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.