Abstract
Objective:
Sparse recent data are available on the epidemiology of surgical site infections (SSIs) in community hospitals. Our objective was to provide updated epidemiology data on complex SSIs in community hospitals and to characterize trends of SSI prevalence rates over time.
Design:
Retrospective cohort study.
Methods:
SSI data were collected from patients undergoing 26 commonly performed surgical procedures at 32 community hospitals in the southeastern United States from 2013 to 2018. SSI prevalence rates were calculated for each year and were stratified by procedure and causative pathogen.
Results:
Over the 6-year study period, 3,561 complex (deep incisional or organ-space) SSIs occurred following 669,467 total surgeries (prevalence rate, 0.53 infections per 100 procedures). The overall complex SSI prevalence rate did not change significantly during the study period: 0.58 of 100 procedures in 2013 versus 0.53 of 100 procedures in 2018 (prevalence rate ratio [PRR], 0.84; 95% CI, 0.66–1.08; P = .16). Methicillin-sensitive Staphylococcus aureus (MSSA) complex SSIs (n = 480, 13.5%) were more common than complex SSIs caused by methicillin-resistant S. aureus (MRSA; n = 363, 10.2%).
Conclusions:
The complex SSI rate did not decrease in our cohort of community hospitals from 2013 to 2018, which is a change from prior comparisons. The reason for this stagnation is unclear. Additional research is needed to determine the proportion of or remaining SSIs that are preventable and what measures would be effective to further reduce SSI rates.
Surgical site infections (SSIs) are among the most common and most costly hospital-acquired infections (HAIs) in the United States, accounting for almost one-quarter of all HAIs.1–3 Although the risk of SSI is generally low, SSIs are common due to the volume of surgical procedures performed across the United States. In 2018, an estimated 10 million surgical procedures occurred in the United States in nonfederal, inpatient surgical centers,4 and in 2016, another estimated 14 million surgeries occurred in nonfederal, ambulatory surgical centers.5 SSIs occur in 1%–2% of patients undergoing inpatient surgery.6–8
Overall, rates of SSI have been decreasing in the United States. Among 199 hospitals participating in serial point-prevalence surveys performed by the CDC, the rate of SSI decreased from 0.97 per 100 procedures in 2011 (n = 11,282 patients reviewed) to 0.56 per 100 procedures in 2015 (n = 12,299 patients reviewed; P = .001).9 Similarly, the publicly reported rates of SSI following abdominal hysterectomy and colon surgery decreased ~10% in 2017 compared to the national baseline reported in 2016, though the decreases were not statistically significant.10
Rates of SSI vary by type of procedure and by setting. The National Healthcare Safety Network no longer routinely reports national rates of SSI following commonly performed procedures; therefore, most nationwide estimates currently used are from data reported almost 10 years ago.
Data on the epidemiology of SSIs in community hospitals are also sparse. Most studies that have examined SSIs in community hospitals are limited by being single center and retrospective. We completed 2 prior studies that described epidemiology of SSIs in community hospitals from 2000 to 2005 and from 2008 to 2012.11,12 The objectives of this study were to provide an update on SSI epidemiology in a large network of community hospitals and to describe SSI trends stratified by pathogens that commonly cause SSIs.
Methods
Setting
These data were collected on patients undergoing 26 commonly performed surgical procedures at 32 community hospitals in the Duke Infection Control Outreach Network (DICON). DICON is a network of 60 community hospitals in 6 states in the southeastern United States.13 Community hospitals within our network have access to expert infection control consultation, educational services, benchmark data, and detailed data analysis. Trained and experienced infection preventionists prospectively enter data collected from patients undergoing 37 types of operative procedures into the DICON Surgical Surveillance database. The database contains the following information: type of surgical procedure; hospital; primary surgeon; patient age; procedure date and duration; NHSN risk index (calculated from the patient’s American Society of Anesthesiologists classification system score, wound class, and operative duration), and the presence or absence of postoperative SSI, including causative organism, if a postoperative culture was obtained and was positive.
DICON SSI surveillance methods have previously been described in detail.14 In brief, potential SSIs are identified through a combination of strategies that may include review of microbiology culture results, hospital readmissions following surgery, clinical rounds, and questionnaires sent to surgeons regarding postoperative patients at the discretion of each participating hospital. Infection preventionists used NHSN criteria to categorize SSIs into superficial (superficial incisional) and complex (deep incisional or organ-space) SSIs.15
Analysis
We limited our analysis to hospitals that were within the network from January 2013 through December 2018 (ie, the study period). We excluded procedures that were performed <4,000 times during the 6-year study period based on primary review of the data. Specifically, upon reviewing surgical volume, there was a clear inflection point between procedures performed more or less than 4,000 times during the study period.
We analyzed only complex SSIs given the surveillance bias and decreased sensitivity of surveillance for superficial incisional SSIs.16 We determined the overall prevalence rate of SSI during the 6-year study period and then stratified all collected data by procedure type and pathogen responsible for infection. We compared SSI prevalence rates in our network of hospitals to national data reported by NHSN. Next, we determined the prevalence rate of SSI for each year of the study from 2013 to 2018. Finally, we stratified annual prevalence rate of SSI by causative organism. We calculated annual crude prevalence rates and prevalence rate ratios (PRRs) using unadjusted log-binomial regression. We also constructed a log-binomial regression model controlling for clustering within hospitals to calculate adjusted annual SSI prevalence rates.
The Duke University Health System Institutional Review Board approved this research project. We analyzed all the data using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
In total, 3,561 complex SSIs occurred following 669,467 surgeries performed at 32 hospitals during the 6-year study period. The average age of the patients at the time of surgery was 54 years (SD, 17.9 years), and 432,187 patients (64.6%) in the cohort were female. Moreover, 40,222 patients (6.0%) had ASA scores of 1, 298,398 patients (44.6%) had ASA scores of 2, 264,555 patients (39.5%) had ASA scores of 3, and 66,292 patients (9.9%) had an ASA score of 4 or 5. Infection preventionists classified 262,601 (39.2%) wounds as clean–contaminated, 20,315 (3.0%) as contaminated, and 16,464 (2.5%) as dirty.
The overall prevalence rate was 0.53 complex SSIs per 100 procedures (Table 1). Among the 26 procedures that were performed >4,000 times during the study period, colon surgery had the highest prevalence rate of complex SSI (2.5 per 100 procedures), followed by small bowel surgery (1.8 of 100 procedures), craniotomy (1.0 per 100 procedures), and appendectomy (0.9 per 100 procedures).
Table 1.
Prevalence Rates of Complex Surgical Site Infections (SSIs) Stratified by Surgical Procedure in 32 DICON Hospitals Compared to NHSN 2018 Data
| NHSN Procedure Category | Procedures | Complex SSIs | Prevalence Rate, Complex SSI | NHSN Procedures, 2018 | NHSN Complex SSIs, 2018 | NHSN Complex SSI Prevalence Rate, 2018 |
|---|---|---|---|---|---|---|
| Colon surgery | 23,551 | 584 | 2.48 | 322,125 | 7,323 | 2.27 |
| Small bowel surgery | 13,131 | 237 | 1.80 | 37,455 | 603 | 1.61 |
| Craniotomy | 4,597 | 48 | 1.04 | 37,699 | 449 | 1.19 |
| Appendectomy | 20,998 | 185 | 0.88 | 38,641 | 128 | 0.33 |
| Hip arthroplasty | 40,126 | 341 | 0.85 | 403,624 | 2,630 | 0.65 |
| Coronary artery bypass graft | 12,914 | 89 | 0.69 | 125,865 | 860 | 0.68 |
| Exploratory laparotomy | 19,020 | 115 | 0.60 | 55,149 | 359 | 0.65 |
| Abdominal hysterectomy | 28,381 | 167 | 0.59 | 293,503 | 1,829 | 0.62 |
| Knee arthroplasty | 60,294 | 344 | 0.57 | 553,112 | 2,090 | 0.38 |
| Spinal fusion | 40,862 | 228 | 0.56 | 181,795 | 1,416 | 0.78 |
| Open reduction of fracture | 33,983 | 184 | 0.54 | 54,929 | 447 | 0.81 |
| Gastric surgery | 17,861 | 95 | 0.53 | 34,597 | 153 | 0.44 |
| Limb amputation | 15,665 | 77 | 0.49 | 10,495 | 76 | 0.72 |
| Vaginal hysterectomy | 10,660 | 51 | 0.48 | 23,710 | 120 | 0.51 |
| Breast surgery | 46,646 | 187 | 0.40 | 19,656 | 195 | 0.99 |
| Laminectomy | 35,232 | 132 | 0.37 | 70,031 | 281 | 0.40 |
| Cardiac surgery | 5,773 | 20 | 0.35 | 45,766 | 143 | 0.31 |
| Herniorrhaphy | 64,351 | 172 | 0.27 | 19,257 | 149 | 0.77 |
| Thoracic surgery | 9,023 | 23 | 0.25 | 27,440 | 77 | 0.28 |
| Gallbladder surgery | 68,249 | 150 | 0.22 | 66,062 | 261 | 0.40 |
| Prostate surgery | 4,274 | 8 | 0.19 | 4,199 | 24 | 0.57 |
| Ateriovenous shunt for hemodialysis | 5,592 | 10 | 0.18 | 1,745 | 3 | 0.17 |
| Caesarean section | 61,977 | 100 | 0.16 | 257,188 | 511 | 0.20 |
| Carotid endarterectomy | 5,634 | 8 | 0.14 | 9,909 | 17 | 0.17 |
| Ovarian surgery | 8,820 | 5 | 0.06 | 26,042 | 22 | 0.08 |
| Thyroid surgery | 11,853 | 1 | 0.01 | 4,253 | 4 | 0.09 |
| Total surgeries | 669,467 | 3,561 | 0.53 | 2,724,247 | 20,170 | 0.74 |
Note. DICON, Duke Infection Control Outreach Network; NHSN, National Health Safety Network.
For 14 procedure types, the prevalence rates of complex SSIs were similar to prevalence rates reported by the NHSN in 2018.17 DICON hospitals had higher complex SSI rates for colon surgery, appendectomy, hip arthroplasty, and knee arthroplasty compared to the complex SSI rates reported by NHSN for these procedures (Table 1). Conversely, DICON complex SSI rates were lower for spinal fusion, open reduction of fracture, limb amputation, breast surgery, herniorrhaphy, gall-bladder surgery, prostate surgery, and thyroid surgery.
Staphylococcus aureus was the most common organism (0.13 per 100 procedures), causing 843 complex SSIs (24%) in our cohort (Table 2). Methicillin-sensitive S. aureus (MSSA) complex SSIs (n = 480, 13%) occurred more frequently than methicillin-resistant S. aureus (MRSA) complex SSIs (n = 363, 10%). Escherichia coli was the most common gram-negative pathogen isolated (n = 337, 9%). In fact, E. coli SSI was more frequent than MRSA SSI in the last 2 years of the study period, and 783 complex SSIs (22%) were polymicrobial. Cultures were either negative or not obtained in 439 cases (12%).
Table 2.
Prevalence of Most Common Organisms Causing Complex Surgical Site Infections (SSIs)
| Organism | Complex SSIs No. (%) | Prevalence Rate, Complex SSI per 100 procedures |
|---|---|---|
| Staphylococcus aureus | 843 (23.7) | 0.13 |
| MSSA | 480 (13.5) | 0.07 |
| MRSA | 363 (10.2) | 0.05 |
| Escherichia coli | 337 (9.5) | 0.05 |
| Enterococcus spp | 180 (5.1) | 0.03 |
| Coagulase-negative staphylococci | 158 (4.4) | 0.02 |
| Klebsiella spp | 99 (2.8) | 0.01 |
| Streptococcus spp | 106 (3.0) | 0.02 |
| Pseudomonas aeruginosa | 49 (1.4) | 0.01 |
| Enterobacter spp | 69 (1.9) | 0.01 |
| Fungi | 102 (2.9) | 0.02 |
| Polymicrobiala | 783 (22.0) | 0.12 |
| No pathogen identifiedb | 439 (12.3) | 0.07 |
Note. MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resisitant Staphylococcus aureus.
Polymicrobial infections were also included in individual SSI counts for each organism isolated.
Negative cultures or no cultures taken.
We found no statistically significant change in rates of SSI during the study period: 0.58 infections per 100 procedures in 2013 to 0.53 infections per 100 procedures (PRR, 0.92; 95% confidence interval [CI], 0.82–1.03) (Table 3). From 2014 to 2017, the SSI prevalence rates declined each year; however, in 2018, the SSI prevalence rate increased.
Table 3.
Prevalence Rates of Complex Surgical Site Infection (SSI) from 2013 to 2018 at 32 Community Hospitals
| Year | Procedures, No. | Complex SSIs, No. | Prevalence Rate (95% CI) | PRR, Crude (95% CI) | PRR, Model (95% CI)a |
|---|---|---|---|---|---|
| 2013 | 100,643 | 579 | 0.58 (0.57–0.58) | 1 | 1 |
| 2014 | 104,516 | 606 | 0.58 (0.58–0.58) | 1.01 (0.90–1.13) | 1.01 (0.91–1.13) |
| 2015 | 106,913 | 593 | 0.56 (0.55–0.56) | 0.96 (0.86–1.08) | 0.97 (0.88–1.07) |
| 2016 | 114,389 | 578 | 0.51 (0.50–0.51) | 0.88 (0.78–0.99) | 0.88 (0.77–1.00) |
| 2017 | 121,354 | 563 | 0.46 (0.46–0.47) | 0.81 (0.72–0.91) | 0.79 (0.62–1.02) |
| 2018 | 121,652 | 642 | 0.53 (0.53–0.53) | 0.92 (0.82–1.03) | 0.90 (0.76–1.06) |
Note. CI, confidence interval; PRR, prevalence rate ratio.
Model calculation controls for clustering within individual hospitals.
The rate of complex S. aureus SSIs significantly decreased from 2013 to 2017 (PRR, 0.67; 95% CI, 0.49–0.90) (Table 4). This change was likely due to the significant decrease in MRSA complex SSIs in 2017 compared to 2013 (PRR, 0.61; 95% CI, 0.42–0.89). Notably, the S. aureus prevalence rate did increase in 2018. In general, the SSI prevalence rates for other pathogens appeared to be relatively stable over the study period (Fig. 1).
Table 4.
Prevalence Rates of Complex Surgical Site Infection (SSI) Due to Staphylococcus aureus From 2013 to 2018 at 32 Community Hospitals
| Year | S. aureus PRR (95% CI) | MSSA PRR (95% CI) | MRSA PRR (95% CI) |
|---|---|---|---|
| 2013 | 1 | 1 | 1 |
| 2014 | 0.97 (0.76–1.22) | 1.01 (0.76–1.35) | 0.91 (0.68–1.21) |
| 2015 | 0.96 (0.72–1.25) | 0.84 (0.61–1.16) | 1.10 (0.74–1.61) |
| 2016 | 0.88 (0.67–1.16) | 0.90 (0.59–1.38) | 0.86 (0.64–1.15) |
| 2017 | 0.67 (0.49–0.90) | 0.71 (0.47–1.08) | 0.61 (0.42–0.89) |
| 2018 | 0.84 (0.66–1.07) | 0.91 (0.66–1.25) | 0.76 (0.56–1.03) |
Note. CI, confidence interval; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resisitant Staphylococcus aureus; PRR, prevalence rate ratio.
Fig. 1.
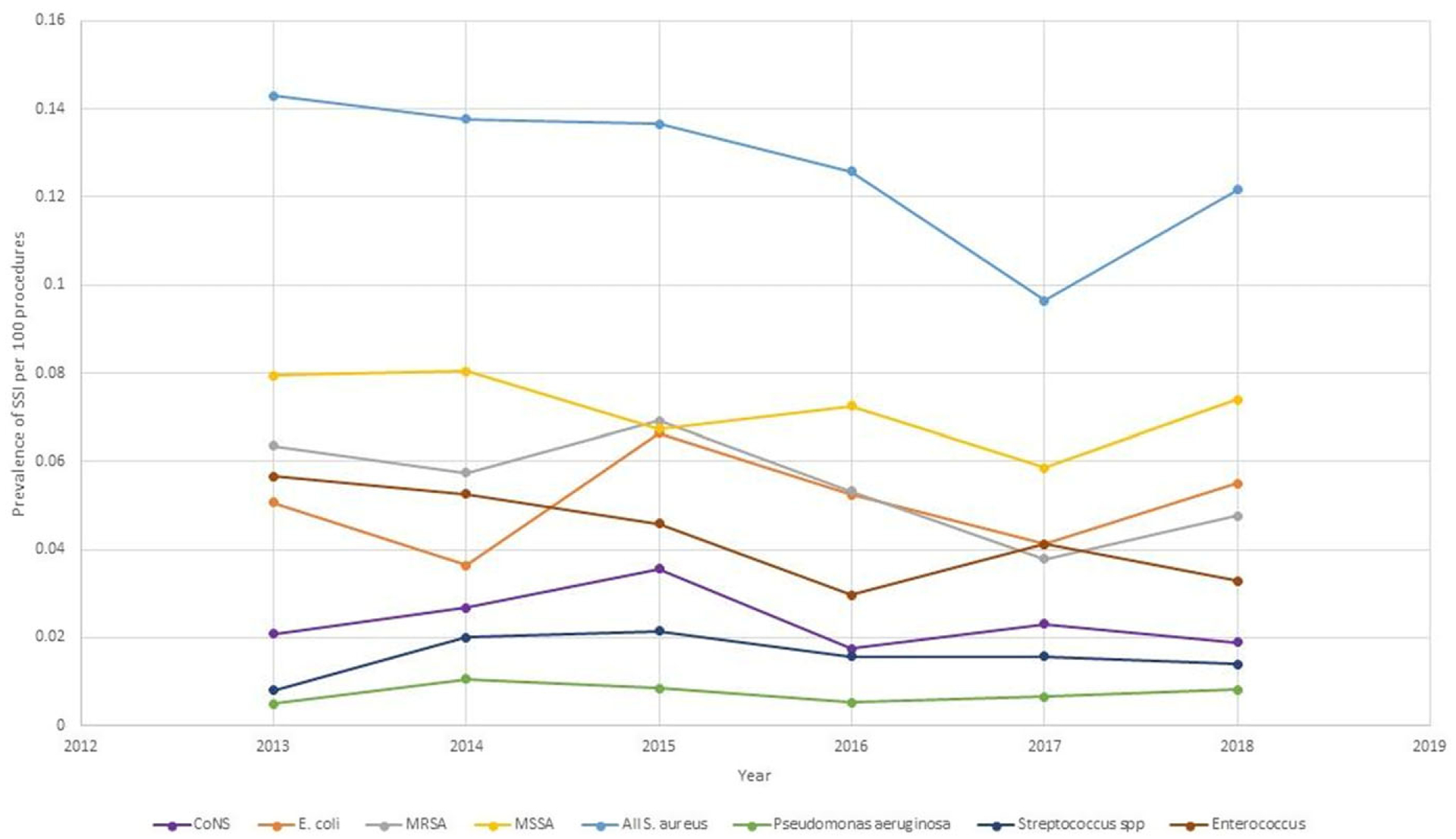
Prevalence rates of complex surgical site infections (SSI) by causative organism from 2013 to 2018
Discussion
In this large, multicenter, cohort study, we summarized the epidemiology of complex SSIs that occurred over a 6-year period in 32 community hospitals in the southeastern United States. Importantly, our results demonstrated that complex SSI prevalence rates did not significantly decrease, suggesting that hospitals may have reached the “glass floor” of SSI prevention.
The reason behind the increase in SSI prevalence rate from 2017 to 2018 compared to the consistent decrease SSI prevalence rates in earlier years is not immediately apparent. We are not aware of any major definitional changes made to SSI surveillance in 2018, nor do we know of any changes to NHSN procedures categories that would have significantly affected SSI prevalence rates between 2017 and 2018. Also, SSI prevalence rates remained stable throughout all 6 years of the study for the 5 most commonly performed procedures: hysterectomy, cesarean section, laparoscopic cholecystectomy, knee arthroplasty, and breast surgery. Finally, MSSA, MRSA, E. coli, and Enterococcus spp remained the most common pathogens that caused SSIs throughout the study period.
Although the rate of SSI was low in our cohort, SSIs are clinically substantial and warrant ongoing attention. We identified 3,561 complex SSIs over the 6-year period, causing significant morbidity, mortality, and cost. Badia et al18 demonstrated that the cost of providing care for patients with SSIs in Europe was consistently higher than costs accrued for patients without SSIs. The CDC estimates that SSIs cost the healthcare system an estimated $3.3 billion per year.19 Umscheid et al20 estimated that the cost of an SSI in the United States was between $5,600–$12,900 in 2011. Using these estimates, the complex SSIs identified in our cohort would cost between $20 million and $46 million. SSIs also prolong hospital stays, require readmission to hospitals and returns to the operating room, and have a negative impact on patients’ physical and mental health.18,21 Specifically, SSIs that occur in the United States add an estimated 1 million inpatient hospital days annually to length of stay.3,22 Lastly, SSIs area associated with a 3% mortality rate, and 75% of SSI-associated deaths are directly related to SSI.19
Consistent with prior studies, MSSA remained the most common cause of SSI,11,23 but the microbiological epidemiology of SSIs changed. MRSA SSIs continued to decrease. In fact, E. coli SSI PRs were higher than MRSA SSI PRs in 2017 and 2018, making MRSA only the third most prevalent pathogen causing SSIs. Although prior studies show that MRSA SSIs have been on the decline,23 ours is the first study of SSIs in community hospitals to show E. coli as the second most prominent SSI pathogen.
SSI reduction has recently stagnated. The Surgical Care Improvement Project (SCIP) started in 2006 as a core measure to reduce perioperative morbidity and mortality, with many measures addressing perioperative antibiotic usage and timing.24 High rates of compliance with SCIP measures as performance affected hospital payment under the Centers for Medicare & Medicaid Services Value-Based Purchasing Program were observed.25,26 After endorsement, compliance rates were high, and the differences between institutions were so small that these metrics were retired by the Joint Commission at the end of 2017.27,28 However, a recent study of SCIP compliance in our network of hospitals suggests that compliance with several core measures for SSI prevention was moderate to poor.29 Early on, there was a lot of enthusiasm for SCIP, but these measures have become routine and hospital priorities have shifted to other publicly reported quality metrics, there seems to be less energy directed toward SSI quality improvement.
If the goal is to decrease SSIs further, we should ensure that we are implementing evidence-based practices and we should consider adopting innovative strategies. The current dogma is that most SSIs arise from the microbiome of the patient’s skin or nares,30 and many evidence-based SSI prevention initiatives target nasal and/or skin antisepsis and decolonization. Our data demonstrate that the rate of SSIs caused by skin organisms like S. aureus has declined, whereas the rate of infectious caused by enteric pathogens such as E. coli has remained constant. Thus, considering various mechanisms of SSI pathogenesis will be important in development of future prevention initiatives. Thus, we may need to focus on prevention measures that target enteric flora given that E. coli SSIs were prevalent in our data set. In addition, traditional surgical attire and various types of headwear are under investigation for their benefit in preventing SSI.31 Also, novel surveillance methods using cell-phone applications and artificial intelligence may allow for earlier SSI detection and intervention.31,32
Our study had several limitations. This retrospective study was subject to the typical selection bias and misclassification bias inherent in this type of study. Not all patients with invasive SSIs were identified through our targeted surveillance if their infection diagnosis was not confirmed with a positive culture, if they presented to an outside facility for care of their infection, or if their infection was diagnosed >30 or 90 days postoperatively. As such, our study findings may represent the minimum rates of SSI in our cohort. Also, the generalizability of our findings to community hospitals outside the southeastern United States is uncertain. Moreover, outlier hospitals may have influenced prevalence rates for certain procedures, but this limitation was minimized by the large numbers of hospitals included and procedures performed. Finally, the prevalence rates were not risk adjusted based on patient-specific or procedure-specific risk factors.
With the institution of nationally mandated prevention practices, SSI rates are lower now than 20 years ago. However, the preventability of the remaining SSIs is unknown. The stagnation of SSI reduction rates over the past decade highlights the need to determine the proportion of remaining SSIs that are preventable and to develop prevention strategies geared toward these SSIs to break the current glass floor of SSI prevention.
Acknowledgments.
We acknowledge the individual group members of the group authorship Duke Infection Control Outreach Network Surveillance Team, which includes Linda Crane BSMT (ASCP) SM, CIC, Kathryn L. Crawford BSBA-HCM, RN, CIC, Andrea L. Cromer BSN, RN, MT, MPH, CIC, Polly Padgette RN, BSN, CIC, FAPIC, Linda Roach BSMT, CIC, CCHM, and Brittain Wood BSN, RN, CRCST, CIC.
Financial support.
A.W.B. was supported by NIH-NIAID (grant no. K08-AI163462).
Conflicts of interest.
S.A. reports grants from the CDC, the NIH-NIDDK (grant no. K12DK100024), the SHEA, and consulting fees from IPEC experts (co-owner) and IDSA (unrelated to this work). A.W.B. reports consulting fees from Medincell as an advisory board member.
Footnotes
PREVIOUS PRESENTATION. The data from this manuscript were presented as an abstract at ID Week 2020 on October 23, 2020, held virtually.
References
- 1.Burke JP. Infection control—a problem for patient safety. N Engl J Med 2003;348:651–656. [DOI] [PubMed] [Google Scholar]
- 2.Lewis SS, Moehring RW, Chen LF, Sexton DJ, Anderson DJ. Assessing the relative burden of hospital-acquired infections in a network of community hospitals. Infect Control Hosp Epidemiol 2013;34:1229–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimlichman E, Henderson D, Tamir O, et al. Healthcare-associated infections: a meta-analysis of costs and financial impact on the US healthcare system. JAMA Intern Med 2013;173:2039–2046. [DOI] [PubMed] [Google Scholar]
- 4.McDermott K, Liang Lan. Overview of operating room procedures during inpatient stays in US hospitals, 2018. Agency for Healthcare Research and Quality website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb281-Operating-Room-Procedures-During-Hospitalization-2018.pdf. Published August 2021. Accessed October 27, 2021. [PubMed] [Google Scholar]
- 5.Karaca Z, McDermott K. High-volume invasive, therapeuticambulatory surgeries performed in hospital-owned facilities, 2016. Agency for Healthcare Research and Quality website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb252-Invasive-Ambulatory-Surgeries-2016.pdf. Published 2019. Accessed October 27, 2021. [PubMed] [Google Scholar]
- 6.Scott RD. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Published 2009. Accessed May 31, 2022. [Google Scholar]
- 7.Healthcare cost and utilization project—statistics on hospital stays. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/. Published 2013. Accessed November 17, 2020. [Google Scholar]
- 8.Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 9.Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of healthcare-associated infections in US Hospitals. N Engl J Med 2018;379: 1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National data for acute-care hospitals, year 2017 HAI data. Centers for Disease Control and Prevention website. https://gis.cdc.gov/grasp/PSA/HAIreport.html. Accessed October 20, 2019. [Google Scholar]
- 11.Anderson DJ, Sexton DJ, Kanafani ZA, Auten G, Kaye KS. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2007;28:1047–1053. [DOI] [PubMed] [Google Scholar]
- 12.Baker AW, Dicks KV, Durkin MJ, et al. Epidemiology of surgical site infection in a community hospital network. Infect Control Hosp Epidemiol 2016;37:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagernes M, Lingaas E. Factors interfering with the microflora on hands: a regression analysis of samples from 465 healthcare workers. J Adv Nurs 2011;67:297–307. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DJ, Miller BA, Chen LF, et al. The network approach for prevention of healthcare-associated infections: long-term effect of participation in the Duke Infection Control Outreach Network. Infect Control Hosp Epidemiol 2011;32:315–322. [DOI] [PubMed] [Google Scholar]
- 15.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute-care setting. Am J Infect Control 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 16.Seidelman JL, Smith B, Shoff C, et al. Serious superficial incisional surgical site infections (SSISSIs): a proposed surveillance definition. Infect Control Hosp Epidemiol 2019;40:1258–1259. [DOI] [PubMed] [Google Scholar]
- 17.Harris JE. Smoke yields of tobacco-specific nitrosamines in relation to FTC tar level and cigarette manufacturer: analysis of the Massachusetts Benchmark Study. Public Health Rep 2001;116:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017;96:1–15. [DOI] [PubMed] [Google Scholar]
- 19.Surgical site infection (SSI) event. Centers for Disease Control and Prevention website. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. Updated January 2021. Accessed July 22, 2021. [Google Scholar]
- 20.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 2011;32:101–114. [DOI] [PubMed] [Google Scholar]
- 21.Andersson AE, Bergh I, Karlsson J, Nilsson K. Patients’ experiences of acquiring a deep surgical site infection: an interview study. Am J Infect Control 2010;38:711–717. [DOI] [PubMed] [Google Scholar]
- 22.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37:387–397. [DOI] [PubMed] [Google Scholar]
- 23.Baker AW, Dicks KV, Durkin MJ, et al. Epidemiology of surgical site infection in a community hospital network. Infect Control Hosp Epidemiol 2016;37:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Medicare and Medicaid Services. Hospital quality initiative overview. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/hospitalqualityinits/downloads/hospitaloverview.pdf. Published 2008. Accessed May 31, 2022. [Google Scholar]
- 25.Centers for Medicare and Medicaid Services. Medicare program; hospital inpatient value-based purchasing program. Final rule. Fed Regist 2011; 76:26490–26547. [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services. Medicare program; hospital inpatient prospective payment systems for acute-care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist 2012;77:53257–53750. [PubMed] [Google Scholar]
- 27.Tanner J, Dumville JC, Norman G, Fortnam M. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst Rev 2016: CD004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diab-Elschahawi M, Berger J, Blacky A, et al. Impact of different-sized laminar air flow versus no laminar air flow on bacterial counts in the operating room during orthopedic surgery. Am J Infect Control 2011;39:e25–e29. [DOI] [PubMed] [Google Scholar]
- 29.Baker A, Ilies I, Benneyan J, et al. Early recognition and response to increases in surgical site infections (SSI) using optimized statistical process control (SPC) charts— the early 2RIS trial: a multicenter stepped wedge cluster randomized controlled trial (RCT). Presented at IDWeek 2021, September 29–October 3, 2021, held virtually. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenzel RP. Surgical site infections and the microbiome: an updated perspective. Infect Control Hosp Epidemiol 2019;40:590–596. [DOI] [PubMed] [Google Scholar]
- 31.Fields AC, Pradarelli JC, Itani KM. Preventing surgical site infections: looking beyond the current guidelines. JAMA 2020;323:1087–1088. [DOI] [PubMed] [Google Scholar]
- 32.Anderson DJ, Ilieş I, Foy K, et al. Early recognition and response to increases in surgical site infections using optimized statistical process control charts—the early 2RIS trial: a multicenter cluster randomized controlled trial with stepped wedge design. Trials 2020;21:894. [DOI] [PMC free article] [PubMed] [Google Scholar]


