To the Editor
Tuberculosis (Tbc) is a granulomatous disease caused by Mycobacterium tuberculosis bacillus (M. tuberculosis). Although it is primarily seen in the lungs, other organs may also be affected with a lower prevalence.1 The second most common prevalence of extrapulmonary Tbc cases, nearly 27% of extrapulmonary Tbc cases, are seen in the urogenital system. The most important mechanism introduced in urogenital Tbc pathophysiology is the hematogenous spreading after primary lung infection.2,3 After the initial settlement of bacillary in kidney parenchyma, the bacillary may not cause an infection if the host defense is satisfactory or the virulence of bacillary is low. However, the presence of these factors (low host defense or increased bacillary virulence) may cause parenchymal infiltration and granulomatous infection resulting in fibrosis. In addition, caseous necrosis in papilla and calyxes and chronic abscesses in renal parenchyma may also occur as the disease progresses. In addition to stricture in the ureteropelvic junction and ureter, it may spread to the bladder and cause bladder fibrosis and caseous necrosis. Apart from these, urogenital Tbc may be presented with epididymis and prostate involvement in male patients and the involvement of the fallopian tube, cervix, endometrium, and ovaries in female patients.4,5
Urogenital Tbc formation and development may take long periods after primary infection. The diagnosis is hard due to no specific findings. Thus, it may cause severe outcomes. The aim of this work was to present an urinary Tbc case with nonspecific symptoms for a long time and caused organ loss.
Our case was a 32-year-old female patient who was admitted to the urology outpatient clinic with severe lower urinary system symptoms such as dysuria, frequency, and urgency present for 1 year. She had no additional diseases and surgery history. No pathological findings were observed in the physical examination. Because there was leukocyte positivity in urine analysis, urinary culture was taken and antibiotic treatment was started. Kidney function tests were normal in the blood biochemistry of the patient who had no bacteria growth in the urinary culture. The HbsAg, anti-HIV, and anti-HCV were negative. Since the symptoms recurred, urinary system ultrasonography was taken. Bladder irregularity, bladder wall thickening, hydronephrosis, and parenchymal thinning in the left kidney were observed in the urinary system ultrasonography. Contrasted abdominopelvic computed tomography (CT) was performed. There was left renal hydronephrosis and no ureteral dilatation in abdominopelvic CT. Asymmetrical wall thickening was observed in right posterior and left lateral wall in the bladder (Fig. 1). Afterward, intravenous pyelography showed no function in the left kidney (Fig. 2).
Figure 1.
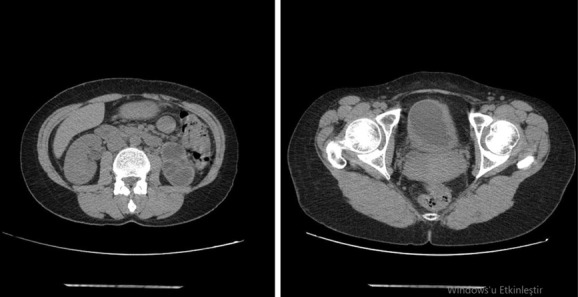
Abdomen CT: The left kidney is hydronephrotic, with no significant dilatation of the left ureter. Asymmetric wall thickening was observed in the right posterior and left lateral bladder.
Figure 2.
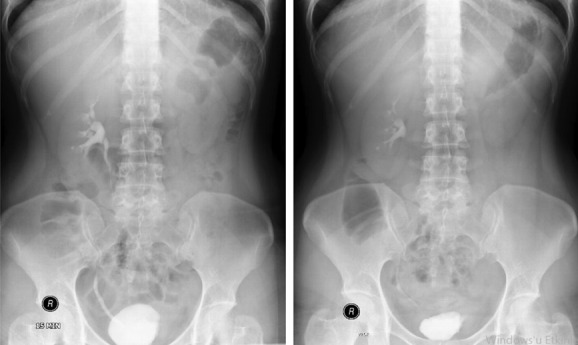
IVP: The left kidney does not enter the nephrogram and the pyelogram phase. Bladder wall thickness was normal, and no significant luminal pathology was detected.
In the Tc-99m DTPA, radioactivity involvement was not observed in the left kidney. It was considered that this appearance could correspond to a nonfunctional left kidney. The right kidney had normal size and morphological structure. Blood supply, concentration, and excretion functions were within normal limits in the right kidney. Right kidney GFR was calculated as 60.1 ml/min.
Because there was a left nonfunctional kidney and an irregularity in the bladder wall, a cystoscopy was planned for the patient. In cystoscopy, a nearly four cm lesion with a caseous necrotic image was observed around the left ureteral orifice. The lesion was completely resected through a 26-fr resectoscope, and specimens were sent to the pathology department. Pathological examination showed “a caseous granulomatous inflammation” (Fig. 3). Therefore, a urogenital Tbc was investigated. Tbc PCR and urinary Tbc culture were performed to investigate the Tbc. In addition, thorax CT was performed. Tbc culture and PCR results were compatible with urogenital Tbc. In thorax CT, there were multiple lymphadenopathies, a maximum size of 17 mm with spot microcalcifications, were observed in the mediastinum and there was a soft tissue appearance at the left kidney.
Figure 3.
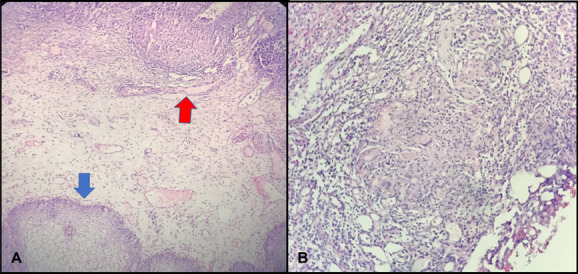
Tur-B pathology specimen image: H&E, ×40 bladder epithelium (blue arrow) and granuloma structure (red arrow) (A); H&E, ×100, granuloma structure in the bladder and Langhans giant cell (B).
Afterward, the patient was consulted with the pulmonary diseases department. Isoniazid, rifampicin, ethambutol, and pyrazinamide treatment was started. After 6 months of treatment, a nephrectomy was planned for the left kidney which did not function in scintigraphy. Left open transperitoneal nephroureterectomy and lymphadenectomy were performed. The pathology result was reported as “granulomatous pyelonephritis, granulomatous inflammation, and granulomatous lymphadenitis” (Fig. 4).
Figure 4.
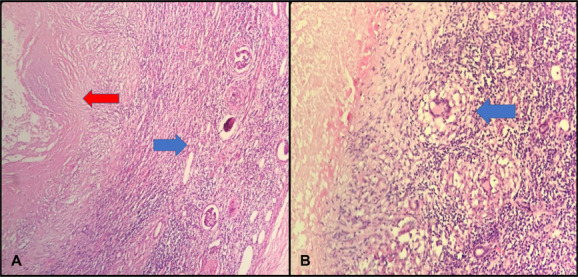
Nephroureterectomy and lymphadenectomy pathology specimen image: H&E, ×40 renal parenchyma, glomerular (blue arrow) and caseeification necrosis (red arrow) (A); H&E, ×100, granuloma Langhans giant cells (blue arrow) and caseeification necrosis (left arrow) (B).
The patient has been followed up in correlation with urology and pulmonary diseases for the last 30 months. Additional systemic treatment was not required in the postoperative period. In the follow-up period, it was observed that the pulmonary lesions completely declined. In addition, it was observed that the symptoms of the patient had completely recovered.
Urinary Tbc is a severe problem that can cause pathologies such as renal failure and chronic kidney disease. The diagnosis is difficult because there is no specific symptom, and a long time passes between the diagnosis and treatment. Some patients can be asymptomatic, but nonspecific symptoms such as dysuria, frequency, renal colic, hematuria, acidic urine pH, and sterile pyuria can be observed. If there is presence of these symptoms, this disease may imitate pyelonephritis and kidney stone.6 Chaker et al reported a renal tuberculosis case seeming like a renal mass.7 Different findings can be observed in imaging methods, depending on the affected localization and organ.8
The most important laboratory finding which may raise doubts about urinary Tbc is sterile pyuria. While sterile pyuria is concealed due to secondary infections in nearly 20% of the patients, microscopic hematuria can be detected in nearly 50%.9 The presence of sterile pyuria and chronic lower urinary tract symptoms may be important findings for urinary Tbc. Therefore, Tbc culture should be performed at the first evaluation of the patient if there is presence of these symptoms. The diagnosis depends on the presence of bacillus in urine. After the cultivation of minimum three urine analysis in Löwenstein–Jensen medium, it was observed that M. tuberculosis bacillus provided a positive result in 90% of the patients after waiting for 6–8 weeks.10 In recent years, PCR and nucleic acid amplification tests are also used in Tbc diagnosis, and PCR was found as positive in 94% of genitourinary Tbc cases. In our present case, the Tbc culture and Tbc-PCR were positive. Another diagnosis tool is the granulomatous inflammation in the bladder biopsy.9,11 Granulomatous inflammation was observed in the bladder biopsy samples in our case (Figs. 3 and 4).
There are no specific findings in imaging methods for diagnosis. Perirenal abscess and hydronephrosis can be observed. Findings such as bladder wall thickening and asymmetrical appearance are among the detectable findings in bladder involvement cases. In case of renal failure, a nonfunctional kidney image may be present in intravenous pyelography. In our case, the left kidney had a nonfunctional appearance, and there was an asymmetrical wall thickening in the bladder (Fig. 2).
Thorax imaging must be performed if urinary Tbc is suspected in the patient. Most of the patients have primary pulmonary Tbc, and urinary Tbc formation follows this.4 Our patient was evaluated with thorax CT after urinary Tbc was detected. In thorax CT, multiple lymph nodes in line with Tbc were observed in the mediastinum.
Systemic treatment should primarily be planned in urinary Tbc treatment. A combination of isoniazid, rifampicin, ethambutol, pyrazinamide, and streptomycin is used in primary care. The minimum treatment is at 6 months. In case of resistance against first-line drugs, second-line antituberculosis drugs are among the options to be used for treatment. In severe fibrosis, corticosteroid treatment is among the options which can be added to antituberculosis treatment.12,13 Percutaneous nephrostomy opening, JJ catheter insertion, pyeloplasty, ureterostomy, ureteroneocystostomy, partial nephrectomy, total nephrectomy, bladder augmentation, and urethral reconstruction are among the applicable surgical treatments based on the affected organ and the severity.13 In our case, nephroureterectomy and partial cystectomy were performed due to a nonfunctional kidney, and the pathology was reported as granulomatous inflammation.
Urinary Tbc is a rare but important disease that may cause severe problems. Diagnosis takes time because there is no specific finding. Severe complications may occur because of late diagnosis. Recurrent urinary tract infection should be considered for this distinctive diagnosis in presence of persistent dysuria.
Conflicts of interest
The authors report no conflicts of interest.
Contributor Information
Mehmet Balasar, Email: drbalasar@gmail.com.
Mehmet Giray Sönmez, Email: drgiraysonmez@gmail.com.
Muzaffer Tansel Kilinç, Email: m.tanselkilinc@gmail.com.
Pembe Oltulu, Email: drpembe@yahoo.com.
Eren Erol, Email: drerenerl@gmail.com.
Yunus Emre Göger, Email: dr_yegoger@yahoo.com.
References
- [1].Global Tuberculosis Report 2017. World Health Organization. https://www.who.int/tb/publi cations/global_report/gtbr 2017_main_text.pdf. Accessed April 16, 2019; 2017. [Google Scholar]
- [2].Toccaceli S, Persico Stella L, Diana M, et al. Renal tuberculosis: a case report. G Chir. 2015;36(2):76–78. [PMC free article] [PubMed] [Google Scholar]
- [3].Figueiredo AA, Lucon AM, Srougi M. Urogenital tuberculosis. Microbiol Spectr. 2017;5(1):TNMI7-0015-2016. [DOI] [PubMed] [Google Scholar]
- [4].Büyükalpelli R. Tuberculosis of the genitourinary system. Tuberculosis Symposium in the 21st Century and II. Samsun: Tuberculosis Laboratory Diagnostic Methods Course, 1996;13:327. [Google Scholar]
- [5].Dülek H, Gönenç I, Yazıcı M, et al. Tuberculosis of the urinary tract in pediatric patient. Turkish J Fam Pract. 2017;21(2):90–93. [Google Scholar]
- [6].Daher EDF, Barros EJG, da Silva Junior GB. Renal tuberculosis in the modern era. Am J Trop Med Hyg. 2013;88(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chaker K, Chakroun M, Gharbi M, Chebil M. Renal tuberculosis mimicking renal cell carcinoma: a case report. J Med Case Rep. 2019;13(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sallami S, Ghariani R, Hichri A, Zrayer O. Imaging findings of urinary tuberculosis on computerized tomography versus excretory urography: through 46 confirmed cases. Tunis Med. 2014;92(12):743–747. [PubMed] [Google Scholar]
- [9].Hemal AK, Gupta NP, Rajeev TP, Kumar R, Dar L, Seth P. Polymerase chain reaction in clinically suspected genitourinary tuberculosis: comparison with intravenous urography, bladder biopsy, and urine acid fast bacilli culture. Urology. 2000;56(4):570–574. [DOI] [PubMed] [Google Scholar]
- [10].Wise GJ, Marella VK. Genitourinary manifestations of tuberculosis. Urol Clin North Am. 2003;30(1):111–121. [DOI] [PubMed] [Google Scholar]
- [11].Buchholz NP, Salahuddin S, Haque R. Genitourinary tuberculosis: a profile of 55 in patients. J Pak Med Assoc. 2000;50(8):265–269. [PubMed] [Google Scholar]
- [12].Hanno P. Genitourinary tuberculosis. AUA News. 2001;6:15. [Google Scholar]
- [13].Carl P, Stark L. Indications for surgical management of genitourinary tuberculosis. World J Surg. 1997;21(5):505–510. [DOI] [PubMed] [Google Scholar]


