Abstract
Cardiovascular disease is the leading cause of non-noncommunicable disease mortality worldwide. Therefore, this study analyzes the mediating effect of dizziness and fatigue in the relationship between stress and sleep quality in patients with heart disease. This study was conducted on patients with heart disease diagnosed by a cardiologist from December 7, 2021 to August 30, 2022 at the Outpatient Department of Cardiology at Hanyang University Hospital in Guri-si, Gyeonggi-do. To verify the serial multiple mediation effect, serial multiple mediation analysis was performed using SPSS Macro Process Model 6 as the most appropriate verification method for this study. The analysis indicated that the more dizziness a participant experienced, the more severe their physical and psychological fatigue and the poorer their quality of sleep. Also, the more severe the physical fatigue, the worse the psychological fatigue and the worse the quality of sleep. In other words, the more severe the psychological fatigue, the poorer the quality of sleep. In summary, in the relationship in which stress in patients with heart disease affects sleep quality, stress is a variable that directly affects sleep quality, and this means that the stress of patients with heart disease can affect the quality of sleep through the parameters, dizziness and fatigue, sequentially; this research model can thus be considered a partial mediator model. Fatigue in patients with cardiovascular disease had a direct effect on sleep quality, and there was a mediating effect through dizziness and fatigue in the relationship between stress and sleep quality. Therefore, it is necessary to develop a sleep management program that can improve the quality of sleep in patients with cardiovascular disease as well as a nursing intervention plan that can alleviate fatigue and control stress in such patients.
Keywords: stressdizziness, dizzinessfatigue, fatiguesleep quality, sleep qualitystress
1. Introduction
Cardiovascular disease is the leading cause of non-noncommunicable disease mortality worldwide, with a reported mortality rate of about 32% in 2012.[1] Chronic mental health problems, such as stress and depression, were shown to be associated with the risk and prognosis of cardiovascular disease in an epidemiological study.[2] “Stress” is defined as a state that challenges homeostasis, “the property of maintaining a constant internal environment regardless of changes in the internal and external environment.”[3,4] When perceived stress is high, it negatively affects balanced health, mood, and cognitive functioning.[5,6] This stress can cause physical damage and disease. Stress is both a risk factor for the development of cardiovascular disease and a predictor of future cardiovascular disease morbidity.[7,8] Although stress contributes to cardiovascular events, it remains largely under-considered and underappreciated in clinical practice.
Sleep is a changeable lifestyle habit important for maintaining good mental and physical health.[9] Studies have shown that the higher one’s stress, the poorer the quality of sleep.[10,11] It has been found that even in people without illnesses, high levels of stress are associated with poor sleep quality. Therefore, it is even more important to investigate the relationship between stress and sleep quality in patients with cardiovascular disease.[11,12] In particular, although poor sleep is associated with a high risk of heart failure, coronary artery disease, and cardiovascular disease, this fact is not well known.[13–15] Stress was not correlated with sleep quality in 1 study.[16] Maintaining a habit of regular exercise or good sleep and a healthy lifestyle helps reduce the risk of cardiovascular disease.[17] In addition, metabolic reactions and unhealthy coping behaviors that occur during stress events increase cardiovascular risk.[18,19] Inadequate sleep and mental stress both raise physical stress markers such as cortisol, and low sleep quality aggravates cardiovascular disease, whereas high stress causes a response that increases blood pressure.[20] Prolonged and repeated exposure to stressful situations causes physical changes that eventually result in negative health outcomes.[21] Psychological stress is a major cause of sleep disturbance. Therefore, it is necessary to analyze the stress level of cardiovascular disease patients and the relationship between this stress and the quality of sleep.
Studies have also reported that dizziness in patients with cardiovascular disorder is frequent.[22] Fatigue in patients with cardiovascular disease is defined as an unpleasant feeling of an inability to perform physical or intellectual exertion.[23] Fatigue is a major symptom found in patients with cardiovascular disease.[24] This worsens disease prognosis and quality of life.[25] When considering symptoms related to fatigue, it is necessary to look at what causes changes in heart function in situations of high fatigue. However, studies examining the relationship between cardiovascular patients and fatigue are rare, and fatigue is a distinct symptom even in chronic heart failure. In addition, it is known to cause serious limitations in activities of daily living and a failure to meet the metabolic demands of muscles as well as be the main cause of cardiac output malfunction.[26]. Fatigue can cause inadequate or insufficient sleep. Indeed, fatigue can increase even after sleep restriction.[27,28] Previous studies have shown that improving the quality of sleep can have positive effects on mental health, such as reducing stress, depression, and anxiety.[29,30] Therefore, this study analyzes the mediating effect of dizziness and fatigue in the relationship between stress and sleep quality in patients with heart disease. Our hypotheses are as follows:
Hypothesis 1: Stress is positively associated with sleep quality among cardiovascular disease patients.
Hypothesis 2: Dizziness mediates the relationship between stress and sleep quality among cardiovascular disease patients.
Hypothesis 3: Fatigue mediates the relationship between stress and sleep quality among cardiovascular disease patients.
Hypothesis 4: The mediating effect of dizziness and fatigue forms a serial mediation pathway in the relationship between stress and sleep quality among cardiovascular disease patients.
2. Methods
2.1. Study design
This survey study was conducted from December 7, 2021 to August 30, 2022, for patients diagnosed by a cardiologist at Hanyang University Hospital in Gyeonggi-do to confirm the mediating effect of dizziness and fatigue in the relationship between stress and sleep quality.
2.2. Participants
In this study, cardiologists selected subjects with cardiovascular disease among patients treated by cardiologists, and the researchers conducted a face-to-face survey with subjects who could communicate, understood the purpose of the study, and agreed to participate.
The researchers collected a total of 220 questionnaires from Guri Hospital, which is affiliated with Hanyang University, and analyzed 207 of them (response rate, 94.09%), excluding 13 with insufficient responses or omissions. To confirm the appropriateness of this sample size, the G*Power 3.1 analysis software (G*Power, Düsseldorf, Germany) was used to calculate the minimum number of subjects at the significance level 0.05, median size effect 0.15, and power 0.95, which resulted in 199 useable responses.[31] In addition, considering a dropout rate of 10% and the number of responses that were insufficient for the study, the researcher distributed the questionnaire to 220 subjects. As the number of subjects ultimately included in the data analysis of this study is 207, it is sufficiently appropriate.
The inclusion criteria for subjects are as follows: Patients aged 20 years or older who have been diagnosed with cardiovascular disease by a doctor were selected for the study, as risk factors that contribute to the development of cardiovascular disease, such as smoking, hypertension, diabetes, and obesity, are typically absent or undiagnosed in individuals under the age of 20, making cardiovascular disease uncommon in this age group; New York Heart Association cardiac function classification II to IV patients; Participants who did not have a documented history of sleep disorders, depression, or anxiety, and who also underwent the Mini-Cog instrument screening[32] without showing any signs of cognitive impairment; Participants included in the study were selected from patients who did not have a documented history of psychiatric conditions such as depression, anxiety, or sleep disorders, as well as individuals who have not been diagnosed with mental health conditions by a psychiatrist. The patient’s psychiatric history was confirmed by research assistants through reviewing medical records in the patients’ chart, and the patients also reported their own history of mental health disorders through self-report measures.
According to the definition provided by the World Health Organization, cardiovascular diseases are a group of disorders that affect the heart and blood vessels, including coronary heart disease, cerebrovascular disease, peripheral arterial disease, congenital heart disease, rheumatic heart disease, deep vein thrombosis, and pulmonary embolism.[33]
The exclusion criteria for subjects included: Patients with vision or hearing impairment; Cognitive or language impairment due to stroke within the last 3 months, and; Cognitive impairment, mental illness (major depression and anxiety disorders), sleep disorders, and end-stage renal or hepatic failure. To ensure the validity of our sample, research assistants reviewed the medical charts of individuals who met the exclusion criteria and excluded them from the study population.
3. Measurement
3.1. Sleep quality
A self-enumeration questionnaire was completed using the Pittsburgh sleep quality index (PSQI) developed by Buysse et al.[34] The PSQI was administered in its Korean version[35] to evaluate the sleep quality and disturbances of the study participants. The PSQI measures the degree of subjective sleep disturbance for the past month, with “never in the past month” receiving 0 points, “once a week or less” receiving 1 point, “once or twice a week” receiving 2 points, and “3 or more times a week” receiving 3 points, and includes a total of 19 items. Seven items consist of subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction and are items used to evaluate functional impairment. The sum of each item is the global PSQI score, and the range is from 0 to 21 points. In all cases, a score of “0” means no sleeping difficulties, and a score of “3” indicates severe sleeping difficulties. The total score of the 7 items ranges from 0 to 21, and the higher the score, the worse the quality of sleep. Buysse et al[34] defined a score greater than >5 as a poor sleeper, and a score of 5 or less as a good sleeper. The Cronbach α of the Korean version-PSQI[35] was 0.94. The reliability of the tool was Cronbach α= = .86 at the time of development, and the reliability in this study was Cronbach α= = .88.
3.2. Stress
The perceived stress scale (PSS) analyzes the level of stress felt in daily life. It was developed by Cohen[36] and consists of 10 items. To measure the participants’ perceived stress levels, Lee et al[37] adapted the PSS and used its Korean version in their study. The PSS measures the subject’s perceived stressful experience over the past 4 weeks on a 5-point scale. In the case of the 10-item PSS, items 1, 2, 3, 6, 9, and 10 are positive questions (0 = never, 1 = rarely, 2 = sometimes, 3 = frequently, 4 = very often), and questions 4, 5, 7, and 8 are reverse-scored as negative questions. The total score ranges from 0 to 40, and the higher the total score, the greater the degree of perceived stress. In the study conducted by Lee et al[37], the tool used to measure perceived stress exhibited high reliability, as indicated by a Cronbach alpha coefficient of 0.88. The Cronbach alpha in this study was .90.
3.3. Fatigue
Fatigue was analyzed with Chalder fatigue scale (CFS),[38], which contains 7 items on physical fatigue and 4 items on mental fatigue, for a total of 11. The validation of the Korean version of the CFS was carried out in 2018 with a group of healthy participants.[39] Responses are composed of 0 to 3 points on a 4-point scale, with 0 points for “better than usual,” 1 point for “similar to usual,” 2 points for “worse than usual,” and 3 points for “much worse than usual.” Fatigue is divided into 2 subdomains consisting of 7 items for physical fatigue (measured by items 1–7) and 4 for mental fatigue (measured by items 8–11). The sum of the physical fatigue and mental fatigue items received was the degree of fatigue, and the total score consisted of 0 to 33 points. Respondents are asked to think about the past month, and higher scores indicate more severe fatigue. At the time of the development of this tool, the Cronbach alpha was.83 to.90. According to the study, the total scale of the Korean version of the CFS exhibited satisfactory internal consistency, as reflected by a Cronbach α value of 0.88. The Cronbach alpha in this study was.91.
3.4. Dizziness handicap inventory (DHI)
This questionnaire developed by Jacobson and Newman[40] is the first self-assessment inventory to investigate the dizziness-induced impairment of daily living in patients with dizziness. In this study, the assessment of participants was conducted using the Korean version of the 25-item DHI. It is divided into 3 steps and consists of a 7-item physical subscale, a 9-item emotional subscale, and a 9-item functional subscale. It consists of 25 questions in total, and each item is scored “0”,” “2” (sometimes), or “4”,” for a total score from 0 to 100 points. A score of 0 means no self-perceived disturbance of dizziness in daily life, and a score of 100 means severe self-perceived disturbance of dizziness in daily life. The DHI scores were categorized into mild (0–30), moderate (31–60), or severe (60–100) dizziness-related handicap.[41] Through its development, the DHI was found to have good internal consistency for the total score (α = = 0.89). At the time of tool development, the Cronbach alpha of satisfactory internal consistency for the subscales was .72 to .85,[40] and the Cronbach alpha in this study was .91. The reliability of the Korean version of the 25-item DHI was 0.96, and the subscales ranged from 0.83 to 0.90. The Cronbach alpha for each sub-tool was .83 to .89.
3.5. Data collection
This study was conducted on patients with heart disease diagnosed by a cardiologist from December 7, 2021, to August 30, 2022, at the outpatient department of cardiology at Hanyang University Hospital in Guri-si, Gyeonggi-do. It took about 20 minutes to fill out the questionnaire in this study, and data were collected through 1 questionnaire for patients. In the collected data, personal information such as the hospital consultation ticket number, resident registration number, and name of the subject was not used, and data that could identify the patient’s personal information through this study were not included. These data were distributed to patients with heart disease using a structured questionnaire and returned directly to the researchers upon completion by the subjects.
3.6. Ethical considerations
This study was approved by the Institutional Review Board (2021-10-006-004) of Hanyang University Hospital, Guri-si, Gyeonggi-do. All subjects were provided with detailed information about the purpose and process of the study, and written informed consent was obtained before filling out the questionnaire. The questionnaires were handed out only to those who directly agreed to participate in the study. Research subjects participated in the research voluntarily, and the data obtained through the survey were not used for any purpose other than research. The subjects had the option to withdraw from the survey at any time in the middle if desired, and the research they suffered no disadvantages due to the suspension of the study. The subjects took approximately 20 minutes to complete the questionnaire.
3.7. Statistical analyses
For data analysis in this study, SPSS statistical software (version 23; IBM Corp, Armonk, New York) and SPSS Macro Process v3.1 were used. Frequency analysis and descriptive statistical analysis were conducted to confirm the demographic characteristics as well as the characteristics of the main variables of the subjects of this study. To confirm the correlation between each variable, Pearson correlation coefficient was performed. Next, the serial multiple mediation effect of dizziness (M1) and fatigue (M2) in the relationship between stress (X) and sleep quality (Y) was verified. To verify the serial multiple mediation effect, serial multiple mediation analysis was performed using SPSS Macro Process v3.1 Model 6, developed by Hayes,[42] as the most appropriate verification method for this study. The bootstrapping method was used by measuring the 95% bias-corrected confidence interval (BC CI) for each proposed indirect path coefficient and 5000 bootstrap samples. The indirect path coefficient means that the mediating effect is significant when the 95% BC CI does not contain 0.
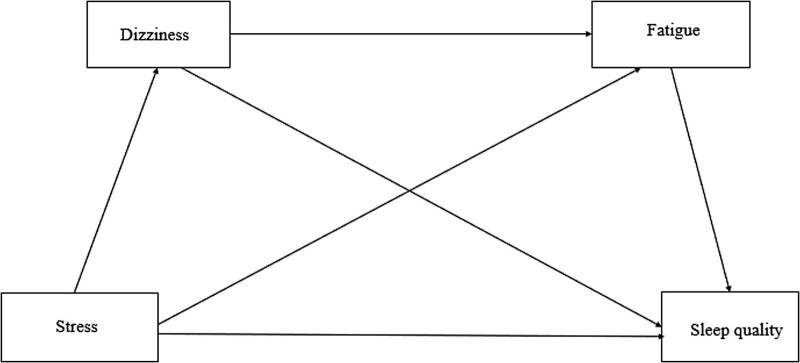
The model analyzed in this study is shown in Figure 1. The serial multiple mediator model was used to examine the structural relationship between dizziness, fatigue, stress, and sleep quality, which is the purpose of this study. Unlike the parallel multiple mediator model, which does not assume a causal relationship between the parameters in a parametric model with 2 or more parameters, the serial multiple mediator model recognizes the causal relationship among the parameters. It also has the advantage of being able to check direct and indirect effects while controlling for the effect of each variable by inputting the above parameters into 1 model.[43]
Figure 1.
A hypothetical model of the study.
The primary objective of a serial mediation model is to investigate the indirect relationship between X and Y, where the effect of X is transmitted through 1 or more intermediate variables (M1 and M2) in a specific order.[44]. This approach allows for a detailed examination of the direct and indirect effects of each mediator on the outcome variable. To run a serial multiple mediation model using the SPSS Macro Process software, it is necessary to specify Model 6, in which the order of the mediators is crucial, with the first mediator (M1) assumed to causally influence the second mediator (M2).
4. Results
4.1. General characteristics of subjects
Table 1 describes the general characteristics of the 207 subjects in this study. The average age of the subjects was 56.29 (11.48) years. Regarding sex, 63.3% of the subjects were male and 36.7% female; 67.6% were married, 54.1% were non-nondrinkers, 74.4% were non-nonsmokers, 56.5% did regular exercise. In the results of our study, 45.9% reported drinking alcohol. However, this does not mean that these patients have mental disorders such as a diagnosis of alcohol addiction from a physician. In terms of education level, high school graduates accounted for the highest proportion at 42.0%, and the majority of patients were taking cardiovascular drugs, accounting for 91.3%. The study participants self-reported their perceived level of economic status, which was categorized into upper class (wealthy), middle class, and lower class (poor). They were instructed to indicate their economic status by self-reporting and dividing it into 3 categories of high, middle, and low. As a result, 69.6% of the participants chose “middle” as their perceived economic status. The question, “Are you currently taking medication prescribed by a doctor for cardiovascular disease?” was answered by the respondents, of whom 91.3% were currently taking medication for cardiovascular disease. The duration of drug use was 37.4% for more than 9 years, followed by 31% for less than 3 years. Overall, 52.7% of subjects suffered from poor sleep quality, and 47.3% reported good sleep quality. The most commonly diagnosed disease among the subjects was coronary artery disease, accounting for 26.8% of cases, followed by hypertension (20.2%) and diabetes (11.7%). Other diagnosed diseases included hypothyroidism, hyperthyroidism, renal dysfunction, cancer, liver disease, and others. The prevalence of patients with a single diagnosed physical illness was the highest (51.7%) among all cases, followed by those with 3 diagnosed illnesses, which comprised 21.3% of cases.
Table 1.
Descriptive statistics of study population (N= = 207).
| Variable | N (%) |
|---|---|
| Mean age (years) (SD, range) | 56.29 [56.29 (11.48) (20 to 85)] |
| Sex | |
| Female | 76 (76 [36.7]) |
| Male | 131 (131 [63.3]) |
| Marital status | |
| Single | 22 (22 [10.6]) |
| Married | 140 (140 [67.6]) |
| Divorced or widowed | 45 (45 [21.7]) |
| Alcohol consumption | |
| Yes | 95 (95 [45.9]) |
| No | 112 (112 [54.1]) |
| Smoking | |
| Yes | 53 (53 [25.6]) |
| No | 154 (154 [74.4]) |
| Regular exercise | |
| Yes | 117 (117 [56.5]) |
| No | 90 (90 [43.5]) |
| Education level | |
| Elementary | 26 (26 [12.6]) |
| Middle school | 26 (26 [12.6]) |
| High school | 87 (87 [42.0]) |
| ≥College | 68 (68 [32.9]) |
| Economic status | |
| High | 12 (12 [5.8]) |
| Middle | 144 (144 [69.6]) |
| Low | 51 (51 [24.6]) |
| Medication | |
| Yes | 189 (189 [91.3]) |
| No | 18 (18 [18.7]) |
| Duration of drug | |
| <3 yr | 58 (58 [31.0]) |
| 3 yr to <6 yr | 18 (18 [15.0]) |
| 6 yr to <9 yr | 31 (31 [16.6]) |
| 9 yr or more | 70 (70 [37.4]) |
| Good sleep quality (PSQI < < 5) | 98 (98 [47.3]) |
| Poor sleep quality (PSQI ≥ ≥ 5) | 109 (109 [52.7]) |
| No. of physical disease | |
| 1 | 107 (107 [51.7]) |
| 2 | 36 (36 [17.4]) |
| 3 | 44 (44 [21.3]) |
| ≥4 | 20 (20 [9.7]) |
| †Types of Cardiovascular disease and physical disease | |
| Coronary artery disease | 105 (105 [26.8]) |
| Stroke | 8 (8 [2.0]) |
| Diabetes mellitus | 46 (46 [11.7]) |
| Hypertension | 79 (79 [20.2]) |
| Atrial fibrillation | 27 (27 [6.9]) |
| Heart failure | 26 (26 [6.6]) |
| Arrhythmias | 38 (38 [9.7]) |
| Hyperlipidemia | 20 (20 [5.1]) |
| Aneurysm | 3 (3 [0.8]) |
| Others | 40 (40 [11.0]) |
SD = standard deviation, PSQI = Pittsburgh sleep quality index.
† Multiple response.
4.2. Stress, dizziness, fatigue, and sleep quality
The overall average for sleep quality was 5.23 (2.86), >5, indicating poor sleep quality overall (Table 2). The average stress was 15.52 (4.48), which means that the total average stress level was moderate. The overall average of the subjects dizziness was 5.88 (11.95) out of 100 points, functional dizziness was 2.01 (4.57), emotional dizziness was 1.22 (3.59), and physical dizziness was 2.63 (4.51). The overall mean for fatigue was 8.69 (6.00). Among the 2 subscales of fatigue, the average for physical fatigue was 6.65 (4.48), and that for psychological fatigue was 2.03 (2.31). The average score for the overall PSQI is 5.23 (2.86) with a range between 0 and 16. The average scores for the subdomains of PSQI which include subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleeping medicine usage, and daytime dysfunction were 1.16, 1.30, 0.77, 0.28, 1.05, 0.16, and 0.48, respectively.
Table 2.
Level of stress, dizziness, fatigue, and sleep quality (N= = 207).
| Variable | Min-max | Mean (SD) |
|---|---|---|
| Total PSQI score | 0–16 | 5.23 (5.23 [2.86]) |
| Subjective sleep quality | 0–3 | 1.16 (1.16 [0.70]) |
| Sleep latency | 0–3 | 1.30 (1.30 [1.00]) |
| Sleep duration | 0–3 | 0.77 (0.77 [0.89]) |
| Habitual sleep efficiency | 0–3 | 0.28 (0.28 [0.66]) |
| Sleep disturbances | 0–3 | 1.05 (1.05 [0.50]) |
| Use of sleeping medicine | 0–3 | 0.16 (0.16 [0.70]) |
| Daytime dysfunction | 0–3 | 0.48 (0.48 [0.79]) |
| Perceived stress scale | 0–27 | 15.52 (15.52 [4.48]) |
| Dizziness handicap inventory | 0–94 | 5.88 (5.88 [11.95]) |
| Functional subscale | 0–34 | 2.01 (2.01 [4.57]) |
| Emotional subscale | 0–36 | 1.22 (1.22 [3.59]) |
| Physical subscale | 0–24 | 2.63 (2.63 [4.51]) |
| Fatigue | 1–31 | 8.69 (8.69 [6.00]) |
| Physical fatigue | 1–21 | 6.65 (6.65 [4.48]) |
| Psychological fatigue | 0–10 | 2.03 (2.03 [2.31]) |
SD = standard deviation, PSQI = Pittsburgh sleep quality index.
4.3. Correlation between variables
In Table 3, the correlation between stress, dizziness, physical fatigue, psychological fatigue, and sleep quality was analyzed. Stress was positively correlated with dizziness (r = .239, P < .001), physical fatigue (r = .301, P < .001), and sleep quality (r = .237, P < .001). It was found that the more severe the stress was, the more severe the dizziness, the more severe the physical fatigue and the poorer the quality of sleep.
Table 3.
Correlation among variables (N = 207).
| Variables | Stress | Dizziness | Physical fatigue | Psychological fatigue | Sleep quality |
|---|---|---|---|---|---|
| Stress | ― | ||||
| Dizziness | .239 (<.001) | ― | |||
| Physical fatigue | .301 (301 (<.001) | .944 (944 (<.001) | ― | ||
| Psychological fatigue | .038 (038 (.588) | .767 (767 (<.001) | .511 (511 [<.001]) | ― | |
| Sleep quality | .237 (237 (.001) | .429 (429 (<.001) | .420 (420 [<.001]) | .300 (300 (<.001) | ― |
Dizziness showed a positive correlation with physical fatigue (r = .944, P < .001), psychological fatigue (r = .767, P < .001), and sleep quality (r = .429, P < .001). The analysis indicated that the more the dizziness the participant experienced, the more severe their physical and psychological fatigue and the poorer their quality of sleep. Physical fatigue was positively correlated with psychological fatigue (r = .511, P < .001) and sleep quality (r = .420, P < .001). It was found that the more severe the physical fatigue was, the worse the psychological fatigue and the worse the quality of sleep. Psychological fatigue and sleep quality (r = .300, P < .001) were positively correlated. In other words, the more severe the psychological fatigue was, the poorer the quality of sleep.
5. Research model verification
5.1. Examination of the relationship among stress, dizziness, fatigue, and sleep quality: total effect, direct effect, and indirect effect
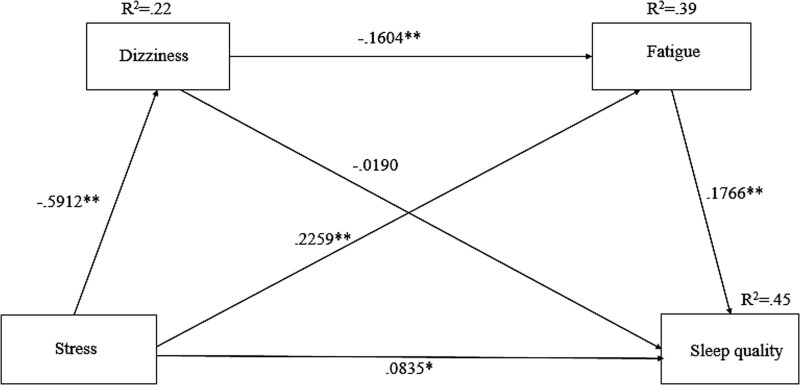
Table 4 shows the results of the analysis of the total, direct, and indirect effects of the evaluation of stress on sleep quality in patients with heart disease through the parameters, dizziness and fatigue, on the dependent variable, sleep quality. As a result of the verification, the path in which stress in patients with heart disease directly affects quality of sleep was statistically significant, and the total effect, meaning the sum of direct and indirect effects, was found to be significant (effect = 0.1514, P = .0006, 95% BC CI [0.0658, 0.2370]). In summary, in the relationship in which stress in patients with heart disease affects sleep quality, stress is a variable that directly affects sleep quality, and this means that the stress of patients with heart disease can affect quality of sleep through dizziness and fatigue parameters sequentially; this research model can thus be considered a partial mediator model.
Table 4.
Total, direct, and indirect effects for multiple mediator model (N= = 207).
| Model | Effect | SE | t | P value | 95% BC CI |
|---|---|---|---|---|---|
| Total effect of stress on sleep quality | 0.1514 | 0.0434 | 3.4872 | .0006 | [0.0658 0.2370] |
| Direct effect of stress on sleep quality | 0.0835 | 0.0416 | 2.0073 | .0460 | [0.0015, 0.1656] |
| Total indirect effect | 0.0679 | 0.0284 | [0.0196, 0.1301] | ||
| Indirect effect via | |||||
| Dizziness | 0.0112 | 0.0162 | [−0.0136, 0.0486]* | ||
| Fatigue | 0.0399 | 0.0173 | [0.0088, 0.0778] | ||
| Dizziness and fatigue | 0.0167 | 0.0084 | [0.0036, 0.0359] | ||
| Indirect 1 | Stress→ dizziness→ sleep quality | ||||
| Indirect 2 | Stress→fatigue→sleep quality | ||||
| Indirect 3 | Stress→dizziness→fatigue→ sleep quality | ||||
BC CI = bias-corrected confidence interval.
Not significant.
5.2. Significance of indirect effects through bootstrapping
Finally, the results of 5000 times of bootstrapping to verify the significance of the indirect effects are shown in Table 4. Verification of the mediating effect through bootstrapping is considered to have a statistically significant indirect effect if the resulting value representing the bootstrap confidence interval does not contain “0.” As a result of the verification, the total indirect effect is the sum of the effect coefficients of paths 1, 2, and 3, and the bootstrap confidence interval at the 95% confidence level is [0.0196, 0.1301], which is statistically significant because it does not contain “0.” Regarding the indirect effect by path, path 3 (stress → dizziness → fatigue → sleep quality) had a statistically significant indirect effect as the bootstrap confidence interval at the 95% confidence level did not include “0” at [0.0036, 0.0359]. Path 2 (stress → fatigue → sleep quality) did not include “0” in the bootstrap confidence interval [0.0088, 0.0778]; thus, the indirect effect was statistically significant. However, path 1 (stress → dizziness → sleep quality) showed a bootstrap confidence interval of [−0.0136, 0.0486], which includes “0,” so the indirect effect was not statistically significant. In other words, in this study, as shown in Figure 2, the dual mediating effect of experiencing both dizziness and fatigue, which are parameters presented in the research model, and the single mediating effect of experiencing fatigue (path 2) were found to be significant.
Figure 2.
The results of multiple mediation testing dizziness and fatigue as mediators of the effect of stress on sleep quality. *P < .05, **P < .01.
6. Discussion
Our study examined the mediating effects of dizziness and fatigue in the relationship between stress and sleep quality. Our results were similar to the stress score of diabetic patients (14.49–15.58), and the stress of hepatitis type c patients suffering from liver disease was 27.60 points, which was higher than that of the subjects in this study. It might be assumed that stress scores would have been high in patients who complained of physical symptoms such as edema or ascites even in normal times, and in the case of diseases for which symptoms can be prevented by drug therapy or lifestyle changes, the stress levels were lower than those of liver disease patients. In a previous study, the sleep quality score of coronary heart disease and heart failure patients was 5.94 points, showing poor sleep quality,[45] and the average sleep quality of subjects suffering from cardiovascular disease was low at 6.72 points.[46] These results showed that the quality of sleep was poor, similar to this study. Furthermore, in previous studies, it was found that the quality of sleep tended to be poorer in subjects with multiple illnesses compared to those with a single disease.[47,48] Our study also identified a considerable proportion of patients diagnosed with comorbidities in addition to cardiovascular disease. Since we did not investigate whether the medication these patients were taking had an impact on their quality of sleep or fatigue, we propose the need for further research as a follow-up study. It is important to investigate the impact of medication on the quality of sleep and fatigue in patients with comorbidities, including cardiovascular disease. A follow-up study can help to shed light on the relationship between medication use and sleep disturbances or fatigue, which may have implications for patient care and management. Possible areas for investigation in the follow-up study could include the type and dosage of medications used by patients, the timing and frequency of medication administration, and any reported side effects or interactions with other drugs. Additional factors such as age, sex, body mass index, and lifestyle behaviors could also be examined to better understand the complex interplay between medication use, comorbidities, and sleep-related outcomes.
In this study, the overall average of dizziness was as low as 5.88 points, and the sub-domains of functional dizziness, emotional dizziness, and physical dizziness all showed low scores. This differed from the results of a previous study, as dizziness in older adults was analyzed as moderate dizziness with emotional, functional, and physical dizziness over 30 points.[49] The overall dizziness of patients with acute unilateral peripheral vestibular disorders was 51 points for women and 47 points for men, which was significantly higher than the results in this study. The reason for this difference could be that the frequency of dizziness increases with age,[50] and the average age of the subjects in this study was 56.29 years old, with the age range evenly distributed from 20s to 60s or older. In a previous study, peripheral vestibular disorder commonly showed a high score for complaints of dizziness (51 points for females, 47 points for males), and dizziness in Meniere disease patients was also significantly high at 66.8 points.[51] The reason for this difference is that people with Meniere disease or peripheral vestibular disorder more commonly complain of severe dizziness than people with cardiovascular epilepsy, which is predicted to be more heavily influenced by anatomical and pathological factors.
In this study, the more severe the stress, the poorer was the quality of sleep; the more severe the dizziness, the more severe was the physical fatigue; and the more severe the emotional fatigue, the poorer was the quality of sleep. In previous studies, subjects with high levels of stress showed lower quality of sleep.[52] Previous studies also showed that the more stressed people with cardiovascular disease were, the poorer their sleep quality was, similar to the results of this study.[11,53] Sleep quality has been shown to be correlated with fatigue,[54] and fatigue is one of the most common symptoms in individuals with cardiovascular disease, who typically experience both physical and psychological fatigue.[55] However, very few studies have been conducted on the experience of fatigue, the contribution of fatigue to heart disease, and mental fatigue in Korea, despite their clinical importance. Therefore, this study is meaningful in that the relationship between fatigue and sleep quality in patients with heart disease was investigated.
As suggested by our findings, a high level of fatigue appeared to have a mediating effect on the relationship between stress and sleep quality. In preceding studies, the higher the stress, the lower the quality of sleep,[12] and it was reported that there is a significant correlation between cardiovascular disease and poor sleep.[56] In this study, a correlation was observed between stress and sleep quality in cardiovascular disease patients, and it was found that there was an indirect effect through fatigue. Previous studies also reported that fatigue is a factor that increases the severity of sleep disorders and diseases in cardiovascular patients and is a direct factor that affects sleep quality.[46,57] Studies have shown that the more severe the fatigue, the more difficult it is to take care of one’s body. Fatigue is one of the most commonly reported symptoms in heart failure patients, and it is persistent and not well relieved by normal recovery methods.[58–60] The results of this study revealed a causal relationship in that controlling stress in patients with cardiovascular disease can reduce poor sleep quality and fatigue. Nonetheless, because there are few studies applying stress relief therapy for patients with cardiovascular disease, intervention studies for such patients are needed. In addition, despite the fact that fatigue exacerbates the disease in patients with heart failure, patients have a low awareness of fatigue, consider it a part of aging, and fatigue is often underestimated and may be viewed as merely weakness or tiredness, which makes the life of patients with cardiovascular disease difficult. To improve the quality of life of patients with cardiovascular disease, we must also apply measures to manage and intervene regarding fatigue symptoms through long-term evaluation.
Our study showed no indirect effect through dizziness on the relationship between stress and sleep quality. However, in that relationship, it was found that there was an indirect effect through 2 parameters, dizziness and fatigue. Poor sleep quality, such as stress or lack of sleep, can contribute to dizziness. In addition, this dizziness is a symptom of heart disease, and as it is not a serious, life-threatening condition, it can be easily corrected. [61] Dizziness was shown to be a common symptom in patients with heart disease.[62] This dizziness is related to the condition of the heart and occurs when blood supply through the body or oxygen supply to the heart decreases and becomes insufficient. Therefore, an intervention plan is needed to monitor and manage the dizziness of patients with heart disease.
There are several limitations to consider in this study. First, although our proposal was based on a multiple mediator model, our study could not provide a clear inference of a causal relationship due to the nature of cross-sectional research. Second, there is a limitation in not examining the variables for this, although other relational variables in addition to dizziness and fatigue can affect the relationship between stress and sleep quality in cardiovascular disease patients. The fact that sleep quality was not measured by polysomnography, the lack of a complete psychiatric interview, and the exclusion of other comorbid psychiatric disorders are important limitations of the study. In addition, a limitation of this study is that it is not precisely known which medications the subjects are taking and how they may impact the variables used in the study. Third, our findings cannot be generalized because it only included patients from 1 hospital recruited by convenience sampling. Despite these limitations, this study presented new insights by analyzing the mediating effects of dizziness and fatigue in the relationship between stress and sleep quality. In addition, this study suggested that it is necessary to prepare measures to reduce the stress that can occur in daily life, prevent dizziness, and reduce fatigue to improve the quality of sleep for patients with cardiovascular disease in clinical practice. Therefore, it is most important that doctors and nurses prepare and provide priority interventions that can reduce stress for these patients.
7. Conclusion
In this study, stress, dizziness, and fatigue influenced the quality of sleep in patients with cardiovascular disease in Korea. Fatigue in patients with cardiovascular disease had a direct effect on sleep quality, and there was a mediating effect through dizziness and fatigue in the relationship between stress and sleep quality. Therefore, it is necessary to develop a sleep management program that can improve the quality of sleep in patients with cardiovascular disease as well as a nursing intervention plan that can alleviate fatigue and control stress in such patients. In addition, based on the results of this study, we propose a management plan according to the changing pattern of sleep quality in patients with heart disease, a longitudinal study that affects sleep quality, and a study of intervention measures considering the variables of various factors. Overall, a follow-up study could help to better understand the complex interplay between medication use, comorbidities, and sleep-related outcomes, which could ultimately inform more effective management and treatment strategies for patients with heart disease and sleep disturbances. Furthermore, it would be beneficial to prioritize the causal relationship between stress and sleep quality among cardiovascular disease patients and present various models as a follow-up study to this research.
Author contributions
Conceptualization: Hwan-Cheol Park.
Data curation: Hwan-Cheol Park, Jihyun Oh.
Formal analysis: Hwan-Cheol Park, Jihyun Oh.
Funding acquisition: Jihyun Oh.
Investigation: Hwan-Cheol Park.
Methodology: Jihyun Oh.
Project administration: Jihyun Oh.
Resources: Hwan-Cheol Park.
Software: Jihyun Oh.
Supervision: Hwan-Cheol Park.
Validation: Jihyun Oh.
Visualization: Jihyun Oh.
Writing – original draft: Jihyun Oh.
Writing – review & editing: Jihyun Oh.
Abbreviations:
- BC CI
- Bias-corrected confidence interval
- CFS
- Chalder Fatigue Scale
- DHI
- dizziness handicap inventory
- PSQI
- Pittsburgh sleep quality index
- PSS
- Perceived stress scale
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (Grant number 2022R1H1A2093192).
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Hanyang University Hospital Institutional Review Board (IRB No.1040968-A-2018–003).
Informed consent was obtained from all the subjects involved in the study.
The authors have no conflicts of interest to disclose.
How to cite this article: Park H-C, Oh J. The relationship between stress and sleep quality: The mediating effect of fatigue and dizziness among patients with cardiovascular disease. Medicine 2023;102:20(e33837).
References
- [1].Wadhera RK, Steen DL, Khan I, et al. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J Clin Lipidol. 2016;10:472–89. [DOI] [PubMed] [Google Scholar]
- [2].Levine GN, Cohen BE, Commodore-Mensah Y, et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation. 2021;143:e763–83. [DOI] [PubMed] [Google Scholar]
- [3].Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress. 2007;10:109–20. [DOI] [PubMed] [Google Scholar]
- [4].Lu S, Wei F, Li G. The evolution of the concept of stress and the framework of the stress system. Cell Stress. 2021;5:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boals A, Banks JB. Effects of traumatic stress and perceived stress on everyday cognitive functioning. Cogn Emot. 2012;26:1335–43. [DOI] [PubMed] [Google Scholar]
- [6].DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: psychological and social resources as mediators. J Pers Soc Psychol. 1988;54:486–95. [DOI] [PubMed] [Google Scholar]
- [7].Panaite V, Salomon K, Jin A, et al. Cardiovascular recovery from psychological and physiological challenge and risk for adverse cardiovascular outcomes and all-cause mortality. Psychosom Med. 2015;77:215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Steptoe A, Kivimäki M, Lowe G, et al. Blood pressure and fibrinogen responses to mental stress as predictors of incident hypertension over an 8-year period. Ann Behav Med. 2016;50:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Capezuti EA. The power and importance of sleep. Geriatr Nurs. 2016;37:487–8. [DOI] [PubMed] [Google Scholar]
- [10].Siddique RF, Ahmed O, Hossain KN. Relationship between the fear of COVID-19 disease and sleep quality: the mediating role of stress. Heliyon. 2021;7:e07033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao X, Lan M, Li H, et al. Perceived stress and sleep quality among the non-diseased general public in China during the 2019 coronavirus disease: a moderated mediation model. Sleep Med. 2021;77:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhai X, Wu N, Koriyama S, et al. Mediating effect of perceived stress on the association between physical activity and sleep quality among Chinese college students. Int J Environ Res Public Health. 2021;18:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].National Academies of Sciences Engineering and Medicine. The national academies collection: Reports funded by National Institutes of Health. 2020.
- [14].Redeker NS, Stein S. Characteristics of sleep in patients with stable heart failure versus a comparison group. Heart Lung. 2006;35:252–61. [DOI] [PubMed] [Google Scholar]
- [15].St-Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Demir B, Saritaş S. The relationship between anxiety and stress levels with quality of sleep in patients after living donor liver transplantation. Transpl Immunol. 2022;71:101561. [DOI] [PubMed] [Google Scholar]
- [17].Teo K, Lear S, Islam S, et al. Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle-and low-income countries: the prospective urban rural epidemiology (PURE) study. JAMA. 2013;309:1613–21. [DOI] [PubMed] [Google Scholar]
- [18].Gallo LC, Roesch SC, Fortmann AL, et al. Associations of chronic stress burden, perceived stress, and traumatic stress with cardiovascular disease prevalence and risk factors in the HCHS/SOL sociocultural ancillary study. Psychosom Med. 2014;76:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. [DOI] [PubMed] [Google Scholar]
- [20].Massar SA, Liu JC, Mohammad NB, et al. Poor habitual sleep efficiency is associated with increased cardiovascular and cortisol stress reactivity in men. Psychoneuroendocrinology. 2017;81:151–6. [DOI] [PubMed] [Google Scholar]
- [21].McEwen BS. Stressed or stressed out: what is the difference? J Psychiatry Neurosci. 2005;30:315–8. [PMC free article] [PubMed] [Google Scholar]
- [22].Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Casillas J, Damak S, Chauvet-Gelinier J, et al. Fatigue in patients with cardiovascular disease. Ann Readapt Med Phys. 2006;49:309–19, 392. [DOI] [PubMed] [Google Scholar]
- [24].Wilson JR, Martin J, Schwartz D, et al. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation. 1984;69:1079–87. [DOI] [PubMed] [Google Scholar]
- [25].Angius L, Crisafulli A. Exercise intolerance and fatigue in chronic heart failure: is there a role for group III/IV afferent feedback? Eur J Prev Cardiol. 2020;27:1862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1–20. [DOI] [PubMed] [Google Scholar]
- [27].Lekander M, Andreasson AN, Kecklund G, et al. Subjective health perception in healthy young men changes in response to experimentally restricted sleep and subsequent recovery sleep. Brain Behav Immun. 2013;34:43–6. [DOI] [PubMed] [Google Scholar]
- [28].Schwarz J, Axelsson J, Gerhardsson A, et al. Mood impairment is stronger in young than in older adults after sleep deprivation. J Sleep Res. 2019;28:e12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Scott AJ, Webb TL, Martyn-St James M, et al. Improving sleep quality leads to better mental health: a meta-analysis of randomised controlled trials. Sleep Med Rev. 2021;60:101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23:377–407. [DOI] [PubMed] [Google Scholar]
- [31].Faul F, Erdfelder E, Lang AG, et al. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- [32].Borson S, Scanlan JM, Chen P, et al. The Mini‐Cog as a screen for dementia: validation in a population‐based sample. J Am Geriatr Soc. 2003;51:1451–4. [DOI] [PubMed] [Google Scholar]
- [33].World Health Organization. Health Topics: Cardiovascular Diseases. 2013. Fact Sheet Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). [access date December 11, 2020].
- [34].Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [35].Sohn SI, Kim DH, Lee MY, et al. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16:803–12. [DOI] [PubMed] [Google Scholar]
- [36].. Cohen S. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, (eds). The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988:31–67. [Google Scholar]
- [37].Lee J, Shin C, Ko YH, et al. The reliability and validity studies of the Korean version of the perceived stress scale. Korean J Psychosom Med. 2012;20:127–34. [Google Scholar]
- [38].Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37:147–53. [DOI] [PubMed] [Google Scholar]
- [39].Ha H, Jeong D, Hahm B-J, et al. Cross-cultural validation of the Korean version of the chalder fatigue scale. Int J Behav Med. 2018;25:351–61. [DOI] [PubMed] [Google Scholar]
- [40].Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–7. [DOI] [PubMed] [Google Scholar]
- [41].Whitney SL, Wrisley DM, Brown KE, et al. Is perception of handicap related to functional performance in persons with vestibular dysfunction? Otol Neurotol. 2004;25:139–43. [DOI] [PubMed] [Google Scholar]
- [42].. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Publications; 2017. [Google Scholar]
- [43].Hayes AF. Mediation, moderation, and conditional process analysis. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach EDN. New York: Guilford publications; 2013;1:20. [Google Scholar]
- [44].Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. [DOI] [PubMed] [Google Scholar]
- [45].Qin Y, Liu R, Wang Y, et al. Self-reported sleep characteristics associated with cardiovascular disease among older adults living in rural eastern China: a population-based study. Clin Interv Aging. 2022;17:811–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hajj J, Mathelier H, Drachman B, et al. Sleep quality, fatigue, and quality of life in individuals with heart failure. J Nurse Pract. 2020;16:461–5. [Google Scholar]
- [47].Lee MK, Oh J. The relationship between sleep quality, neck pain, shoulder pain and disability, physical activity, and health perception among middle-aged women: a cross-sectional study. BMC Womens Health. 2022;22:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang J, Qin W, Pang M, et al. The effect of chronic disease and mental health on sleep quality among migrant elderly following children in Weifang City, China. Int J Environ Res Public Health. 2022;19:12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Takano NA, Cavalli SS, Ganança MM, et al. Quality of life in elderly with dizziness. Braz J Otorhinolaryngol. 2010;76:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Neuhauser HK. Epidemiology of vertigo. Curr Opin Neurol. 2007;20:40–6. [DOI] [PubMed] [Google Scholar]
- [51].Caruso S, Mauro D, Maiolino L, et al. Effects of combined oral contraception containing drospirenone on premenstrual exacerbation of Meniere’s disease: preliminary study. Eur J Obstet Gynecol Reprod Biol. 2018;224:102–7. [DOI] [PubMed] [Google Scholar]
- [52].Hwang Y, Oh J. The relationship between shoulder pain and shoulder disability in women: the mediating role of sleep quality and psychological disorders. Medicine (Baltimore). 2022;101:e31118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kashani M, Eliasson A, Vernalis M. Perceived stress correlates with disturbed sleep: a link connecting stress and cardiovascular disease. Stress. 2012;15:45–51. [DOI] [PubMed] [Google Scholar]
- [54].Kim JA, Kang SW. Relationship among sleep quality, heart rate variability, fatigue, depression, and anxiety in adults. Korean J Adult Nurs. 2017;29:87–97. [Google Scholar]
- [55].Falk K, Patel H, Swedberg K, et al. Fatigue in patients with chronic heart failure–a burden associated with emotional and symptom distress. Eur J Cardiovasc Nurs. 2009;8:91–6. [DOI] [PubMed] [Google Scholar]
- [56].Seixas AA, Vallon J, Barnes-Grant A, et al. Mediating effects of body mass index, physical activity, and emotional distress on the relationship between short sleep and cardiovascular disease. Medicine (Baltimore). 2018;97:e11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang TC, Huang JL, Ho WC, et al. Effects of a supportive educational nursing care programme on fatigue and quality of life in patients with heart failure: a randomised controlled trial. Eur J Cardiovasc Nurs. 2016;15:157–67. [DOI] [PubMed] [Google Scholar]
- [58].Jorgensen R. Chronic fatigue: an evolutionary concept analysis. J Adv Nurs. 2008;63:199–207. [DOI] [PubMed] [Google Scholar]
- [59].Kim HM, Kim J, Hwang SY. Health-related quality of life in symptomatic postmyocardial infarction patients with left ventricular dysfunction. Asian Nurs Res (Korean Soc Nurs Sci). 2015;9:47–52. [DOI] [PubMed] [Google Scholar]
- [60].Ream E, Richardson A. Fatigue: a concept analysis. Int J Nurs Stud. 1996;33:519–29. [DOI] [PubMed] [Google Scholar]
- [61].Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med. 2000;132:337–44. [DOI] [PubMed] [Google Scholar]
- [62].Čulić V, Mirić D, Eterović D. Correlation between symptomatology and site of acute myocardial infarction. Int J Cardiol. 2001;77:163–8. [DOI] [PubMed] [Google Scholar]