Abstract
Chronic kidney dysfunction is associated with increased mortality in multiple cancer types. Preliminary evidence suggests the same to be true for B-large cell lymphomas (B-LCL). To analyze the relationship of glomerular filtration rate (GFR) and outcome of B-LCL in detail we collected data on outcomes of 285 consecutive patients with newly diagnosed B-LCL treated at our institution with standard rituximab-containing regimens who did not have preexisting kidney disease or urinary tract obstruction at presentation. Median age was 59, range 18 to 87, 145 were male and 140 females. Forty-four had GFR < 60 mL/min, 123 had 60 to 90 mL/min, and 118 > 90 mL/min. Median follow-up of surviving patients was 49 months and estimated 3-year survival 76%. In univariate analysis age (P < .001), GFR (P = .014), stage (P < .001), performance status (P = .044), chemotherapy regimen (P < .01), and international prognostic index (IPI) (P < .001) were statistically significant prognostic factors. In multivariate analysis, age and GFR remained the only independent prognostic factors. Subtracting 1 from the IPI score of patients who had GFR > 90 mL/min and IPI > 1 resulted in a prognostic index that divides patients into 3 prognostic groups (low risk = 0–1, intermediate risk = 2–3 and high risk = 4–5) with an acceptable patient distribution frequency (38%, 39%, and 23%, respectively) and improved statistical significance and separation in comparison to IPI (5-year survival rates of 92%, 74%, and 42%, respectively). GFR is an important independent prognostic factor for B-LCL that should be taken into account in clinical decision making and data analysis and probably be incorporated in prognostic indices.
Keywords: B-large cell, diffuse, glomerular filtration rate, lymphoma, prognosis
1. Introduction
Chronic kidney disease (CKD) is strongly associated with increased cancer incidence[1–3]; patients with cancer often have a decreased glomerular filtration rate (GFR) at the time of diagnosis.[1,2,4,5] Decreased GFR is associated with increased risk of cancer, higher tumor grade and increased cancer-related mortality.[6–8] The exact pathogenetic mechanism is unknown; uremic toxins, chronic inflammation and oxidative stress are thought to be responsible for development of more aggressive cancers through accelerated cell differentiation, impairment of immune cells and their anti-tumor activity, angiogenesis, and transformation to higher histology grades.[9–11] It is currently unknown whether there is a threshold of GFR below which these effects take place. Survival of patients with various types of cancers is independently associated with CKD stage 3 or higher.[4,12,13] B-large cell lymphoma (B-LCL) is the most common non-Hodgkin type of lymphoma among adults.[14,15] The international prognostic index (IPI) is the most widely tested and used prognostic index for determining prognosis of this disease.[16] Patients can be divided in low-risk (IPI 0–1), low-intermediate risk (IPI 2), high-intermediate risk (IPI 3) and high-risk (IPI 4–5) based on their age, stage, ECOG performance status, LDH level and number of involved extranodal localizations.
Two studies, 1 from Japan and another from Korea reported inferior survival of B-LCL patients with GFR < 60 mL/min in comparison to those with normal renal function.[17,18] However, many questions regarding the effect of kidney function on survival of B-LCL patients remain unanswered. It is not clear whether this effect is limited to Asian patients, whether a decrease in GFR that is still within normal limits also influences survival, what is the relation with IPI and how does GFR influence outcome of different R-CHOP-like regimens. To try to answer some of these questions, we evaluated the prognostic value of baseline GFR in patients with B-LCL treated at our institution.
2. Methods
We retrospectively collected data on consecutive patients with B-LCL who started front-line treatment at our institution between 2011 and 2019. The study was performed in accordance to the Helsinki declaration, all relevant international, EU and Croatian laws and regulations and with the approval of the institutional Ethics Committee, and all participants gave written informed consent. Inclusion criteria were: age older than 18 years; newly diagnosed B-LCL according to the 2016 World Health Organization classification; no previous history of CKD; no obstructive nephropathy at diagnosis. Patients were treated with different R-CHOP-like regimens according to institutional policies.[19–21] Briefly, patients below 60 years of age with aaIPI ≥ 2 received R-CHOEP14; those above 60 with high-risk disease were treated with R-CHOP21 until beginning of 2016 and with DA-R-EPOCH after that; patients with cardiac disease and frail elderly received R-CEOP or R-miniCHOP; all other patients were treated with conventional R-CHOP21. Radiotherapy, 30 Gy in 15 fractions, was administered to sites of initial bulky disease, selected extranodal localizations and areas in PR after systemic therapy. Patients deemed high risk for central nervous system relapse received 2 cycles of high-dose methotrexate since beginning 2016. Final response assessment was performed by PET-CT. Patients were followed-up clinically at our outpatient department, every 3 to 4 months for the first 3 years, every 6 months in the 4th and 5th year and yearly thereafter. Baseline GFR (mL/min) was estimated using the CKD-EPI equation.[22]
Statistical analysis was performed using SPSS version 23.0 (IBM Corp.). Correlations were obtained using Spearman rank correlation. Survival analysis was performed using Kaplan–Meier curves; differences between them were tested with the log-rank test and hazard ratios estimated with Cox proportional hazards regression. Linear regression was used to explore the influence of multiple variables on survival. A P value <.05 (two-sided tests) was considered significant.
3. Results
We identified 285 patients with B-LCL who fulfilled the entry criteria. All were white (Caucasian). Median age was 59, range 18 to 87; 50.9% were male. Demographic, clinical and laboratory data are shown in Table 1. Fifty-one (17.9%) were smokers, 32 (11.2%) had diabetes and 105 (36.8%) arterial hypertension, 15.4% had a GFR < 60 mL/min. Baseline GFR negatively correlated with age (r = −0.567; P < .001) and positively with history of hypertension (R = 0.167; P < .03). In the linear regression model only age was a predictor of lower GFR.
Table 1.
Patient characteristics.
| Age (yr) (median [range]) | 59 (18–87) |
|---|---|
| Males (N(%)) | 145 (50.9) |
| Diabetes (N(%)) | 32 (11.2) |
| Hypertension (N(%)) | 105 (36.8) |
| Smoker (N(%)) | 51 (17.9) |
| Bulky disease (N(%)) | 112 (39.3) |
| Risk by IPI (N(%)) 0–1 2 3 4–5 |
79 (27.7) 59 (20.7) 61 (21.4) 86 (30.2) |
| Chemotherapeutic regimen (N(%)) R-CHOEP 14 DA-R-EPOCH R-CHOP R-CEOP/ R-miniCHOP |
81 (28.4) 36 (12.6) 137 (48.1) 31 (10.9) |
| Involved site radiotherapy Yes (N(%)) | 148 (51.9) |
| Response (N(%)) Complete remission Partial remission Stable disease Progressive disease Toxic death |
229 (80.4) 26 (9.1) 14 (4.9) 4 (1.4) 12 (4.2) |
| Creatinine (μmol/L) (mean ± SD) | 81.9 ± 1.3 |
| GFR (mL/min/1.73 m2) (mean ± SD) | 84.5 ± 1.5 |
| GFR < 60 mL/min/1.73 m2 (N(%)) | 44 (15.4) |
| GFR < 90 mL/min/1.73 m2 (N(%)) | 167 (58.6) |
DA-R-EPOCH = dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab, GFR = glomerular filtration rate, IPI = international prognostic index, R-CEOP = cyclophosphamide, vincristine, prednisone, etoposide and rituximab, R-CHOEP = cyclophosphamide, doxorubicin, vincristine, prednisone, etoposide and rituximab, R-CHOP = cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab.
Median follow-up of surviving patients was 49 months; estimated 3-year survival of the whole group was 76% and 5-year survival 74%.
3.1. Prognostic factors
Sex, smoking status and prevalence of diabetes mellitus and arterial hypertension did not significantly influence overall survival. In univariate analysis, age (P < .001), GFR (P = .014), stage (P < .001), performance status (P = .044), chemotherapy regimen (P < .01) and IPI (P < .001) were statistically significant prognostic factors. In multivariate analysis, age and GFR remained the only independent prognostic factors.
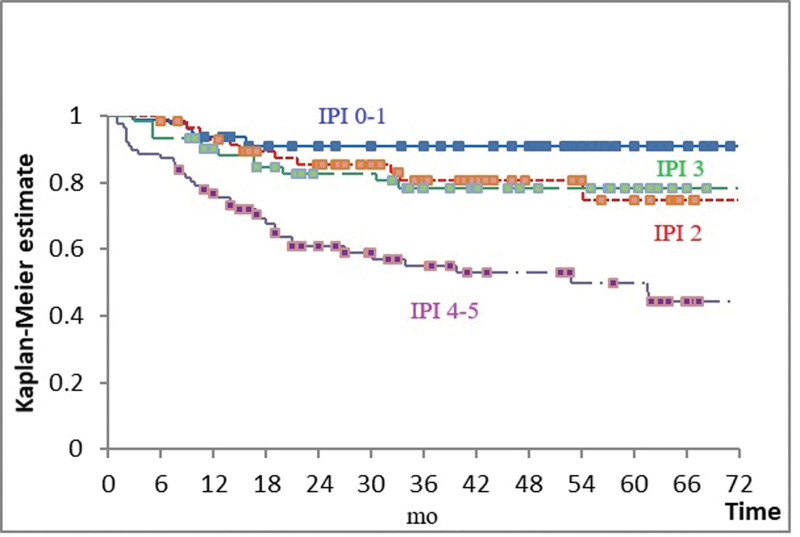
IPI had a very strong influence on survival (P < .001), but outcome of patients with low-intermediate and high-intermediate risk (i.e., IPI 2 and 3) was indistinguishable (Fig. 1 and Table 2). Even when those 2 groups were combined, the difference in OS between patients with IPI 0–1 and 2–3 failed to reach statistical significance (P = .061).
Figure 1.
Overall survival according to the international prognostic index (IPI). Blue = IPI 0–1; red = IPI 2; green = IPI = 3; purple = IPI 4–5. Boxes indicate censored observations. P < .001.
Table 2.
Overall survival (OS) according to the international prognostic index (IPI).
| IPI | N (%) | 5-yr OS (%) | Comparison | P value |
|---|---|---|---|---|
| 0–1 | 79 (27.7) | 91 | IPI 0–1 vs 2 | .086 |
| 2 | 59 (20.7) | 75 | IPI 2 vs 3 | .904 |
| 3 | 61 (21.4) | 78 | IPI 3 vs 4–5 | .001 |
| 4–5 | 86 (30.2) | 51 |
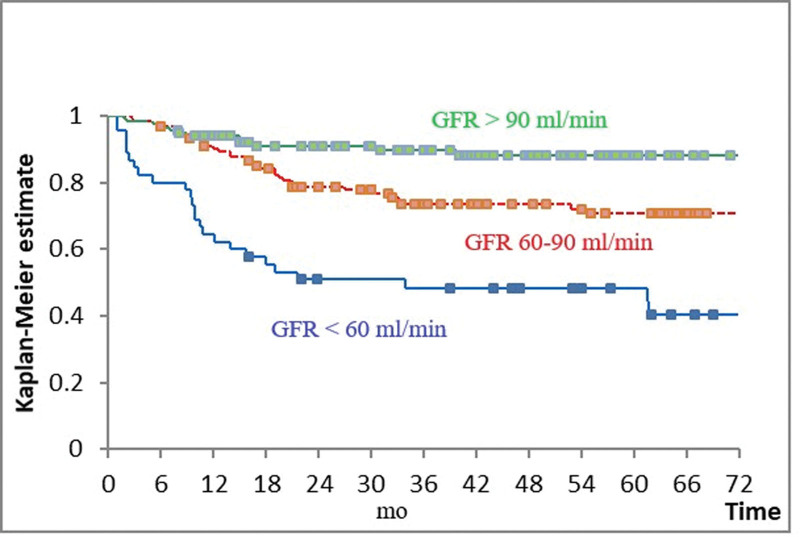
GFR had a very strong influence on survival (P < .001). Patients with baseline GFR < 60 mL/min fared worst, those with GFR 60 to 90 mL/min intermediate and those with GFR > 90 mL/min best (Fig. 2 and Table 3). These 3 prognostic subgroups defined by GFR were identifiable in patients treated with R-CHOEP14, R-CHOP, and DA-R-EPOCH and in those with IPI 2–3 and 4–5. There were only 3 patients with GFR > 90 mL/min in the group treated with R-CEOP/miniCHOP, while in the group with IPI 0–1 the survival curves of patients with GFR 60 to 90 mL/min and > 90 mL/min overlapped and were both superior to that of patients with GFR < 60 mL/min (data not shown).
Figure 2.
Overall survival according to the glomerular filtration rate (GFR). Blue = GFR < 60 mL/min; red = GFR 60–90 mL/min; green = GFR > 90 mL/min. Boxes indicate censored observations. P < .001.
Table 3.
Overall survival (OS) according to the glomerular filtration rate (GFR).
| GFR (mL/min/1.73 m2) | N (%) | 5-yr OS (%) | Comparison | P value |
|---|---|---|---|---|
| <60 | 44 (15.4) | 49 | <60 vs 60–90 | <.001 |
| 60–90 | 123 (43.2) | 71 | 60–90 vs > 90 | .004 |
| >90 | 118 (41.4) | 88 |
3.2. Combining IPI and GFR
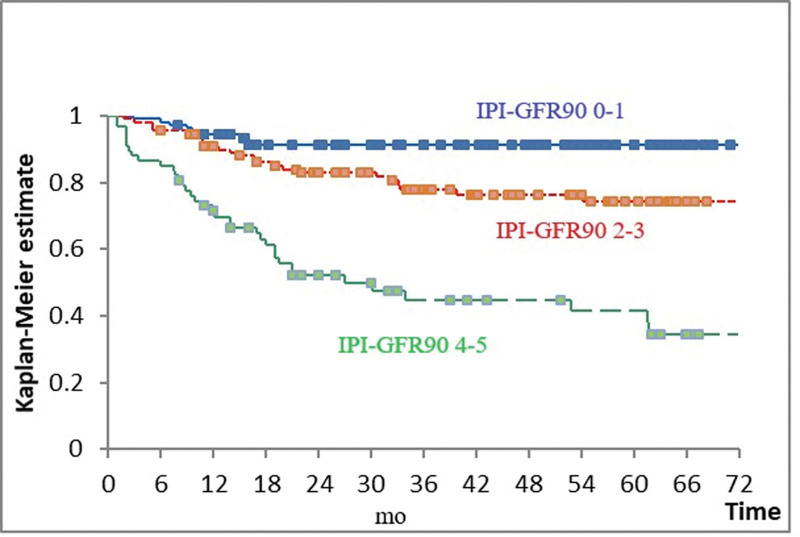
We tried to combine GFR with IPI to create an index with improved prognostic performance. The survival of patients with IPI 2 and GFR > 90 mL/min was equivalent to that of patients with IPI 0–1; survival of patients with IPI 4 and GFR > 90 mL/min was equivalent to that of patients with IPI 2 and GFR < 90 mL/min or IPI 3. Thus IPI-GFR90 was formed by subtracting 1 from the IPI score of patients who had GFR > 90 mL/min and IPI > 1. This index divides patients into 3 prognostic groups (low risk = IPI-GFR90 0–1, intermediate risk = IPI-GFR90 2–3, and high risk = IPI-GFR90 4–5) with an acceptable patient distribution frequency and improved statistical significance and separation in comparison to IPI (Fig. 3 and Table 4). Attempts to further improve the prognostication by adding 1 for those patients who have GFR < 60 mL/min and combining scores 0 to 1, 2 to 3, and 5 to 6 did not improve the performance of the index, except by identifying a small proportion of patients (approximately 10% of the total), who have IPI 4–5 and GFR < 60 mL/min. This group has a 5-year survival of only 29%.
Figure 3.
Overall survival according to IPI-GFR90. international prognostic index (IPI); glomerular filtration rate (GFR); Blue = IPI-GFR90 0–1; red = IPI-GFR 2–3; green 0 IPI-GFR 4–5. P < .001.
Table 4.
Overall survival (OS) according to the combination of the international prognostic index and glomerular filtration rate >90 mL/min (IPI-GFR90).
| IPI-GFR90 | N (%) | 5-yr OS (%) | Comparison | P value |
|---|---|---|---|---|
| 0–1 | 108 (37.9) | 92 | 0–1 vs 2–3 | .004 |
| 2–3 | 112 (39.3) | 74 | 2–3 vs 4–5 | <.001 |
| 4–5 | 65 (22.8) | 42 |
4. Discussion
Reductions in GFR below 60 mL/min and CKD have been associated with higher cancer incidence and higher mortality in cancers of the kidney, urinary tract, digestive tract and lung.[1,23] Only 2 published studies analyzed the impact of GFR reduction on survival of lymphoma patients, both were performed in east Asia.[17,18] The study of Ubukata et al analyzed patients with all types of lymphoma and compared the outcome of those with and without limited kidney function (defined as GFR below or above 60 mL/min).[18] In their series 34% of patients had limited renal function. The study of Hong et al focused on patients with B-LCL and explored differences in outcomes of patients who had GFR below 60 mL/min, between 60 and 90 mL/min and above 90 mL/min, as did our study.[17] In their study 10% of patients had GFR < 60 mL/min and an additional 16% 60 to 90 mL/min. This is different than in our series, in which, after exclusion of patients with history of CKD or renal obstruction, 15.7% had GFR < 60 mL/min and an additional 43% 60 to 90 mL/min. Similar to ours, these studies found that GFR < 60 mL/min is related to reduced overall survival and that this effect is independent of IPI. The exact causes of impaired survival of lymphoma patients, as well as those with other types of cancer, associated with reduced GFR are not fully understood. Reduction of GFR leads to development of CKD and its manifestations like uremic toxins, impaired immunity, anemia, induced cytokine production and pro-inflammatory condition; sarcopenia and malnutrition and oxidative stress lead to accelerated atherogenesis and development of subclinical vascular disease. These changes might be associated with development of more aggressive cancers, less tolerance to treatment, increased in cancer-unrelated mortality and shorter survival.[24–27] Irrespective of cause, the results of ours and the 2 previously published studies indicate that mild-to-moderate CKD is an important independent negative prognostic factor for overall survival in lymphoma patients, irrespective of race.
In contrast to the study of Hong et al, in which only patients with GFR < 60 mL/min had inferior prognosis, we were unable to identify a threshold value. Patients with GFR > 90 mL/min survived better than those with GFR 60 to 90 mL/min and patients from either of these groups did better than patients with GFR < 60 mL/min. The reason for this difference is not clear, but is probably related to differences in proportion of patients with intermediate GFR as stated above. Also, in contrast to their series, we did not routinely reduce the dose of cytotoxic agents in patients without previous history of CKD. Interestingly, in both studies was the effect of GFR less prominent in patients with favorable IPI.
We tried to combine data on GFR with IPI to improve the survival prognostication of the latter. Best results were obtained by moving patients who had GFR > 90 mL/min to a more favorable prognostic group. This IPI-GFR90 index distinguished 3 groups of patients with acceptable distribution of frequency and significantly different prognosis. Its survival prognostication was more sensitive than that of IPI. The negative prognostic impact of GFR < 60 mL/min seemed to be of exceeding importance only in patients with IPI 4–5, possibly identifying a small subgroup (approximately 10% in our series) with extremely bad prognosis. Similar percentage of all patients with Hodgkin´s lymphoma will not achieve complete remission and will relapse following standard therapy.[28] Randomized studies have shown improvement in both progression and event-free survival in relapsed or refractory Hodgkins´s and non-Hodgkins´s lymphoma after high-dose chemotherapy and autologous stem cell transplantation.[28–30] This therapeutic approach could be most suitable for our subgroup of patients with extremely bad prognosis. Nevertheless, we should still be careful with dosing of chemotherapeutic agents in patients with renal impairment.
Our work has several limitations. First, it is a retrospective study, we could not prove causality and various risk factors reported in previous studies like albuminuria were not recorded. Second, we have enrolled patients from only 1 center which might not be reflective of the general population, and our sample size is rather limited. Third, we have not analyzed the relative dose-intensity of chemotherapeutic agents, which might be decreased in patients with limited kidney function, even without a priori dose reduction, because of increased treatment-related toxicity. Also, we have not calculated CKD-EPI adjusted for BSA which is considered to be the most precise formula for kidney function assessment in cancer patients. However, despite these shortcomings, our study is the first to show that in non-Asian patients GFR could be a useful indicator for predicting survival in B-LCL patients. It is also the first study which suggests that even a mild reduction of GFR affects survival and that GFR could be combined with IPI in a new score with improved prognostic performance. These results should be validated in future large-scale multicentric studies.
Acknowledgments
All authors have read and approved the submission of the manuscript; the material is original research. Authors had no writing assistance. This paper was presented in part at the 2021 Virtual Annual Congress of the European Hematology Association.
Author contributions
Conceptualization: Vedran Premužić, Igor Aurer.
Data curation: Vedran Premužić, Dino Dujmović, Ivana Ilić, Neno Živković, Lucija Maleta, Margareta Dobrenić, Lea Galunić-Bilić, Pavle Rončević, Marijo Vodanović.
Formal analysis: Vedran Premužić, Ivo Radman, Ivana Ilić, Neno Živković, Lucija Maleta, Marko Kralik, Margareta Dobrenić, Lea Galunić-Bilić, Pavle Rončević.
Investigation: Sandra Bašić-Kinda, Dino Dujmović, Ivana Ilić, Neno Živković, Lucija Maleta, Marko Kralik, Margareta Dobrenić, Lea Galunić-Bilić.
Methodology: Vedran Premužić, Sandra Bašić-Kinda, Dino Dujmović, Ivana Ilić, Neno Živković, Marko Kralik, Margareta Dobrenić, Lea Galunić-Bilić, Pavle Rončević, Igor Aurer.
Project administration: Ivo Radman, Ivana Ilić, Igor Aurer.
Resources: Ivo Radman, Lea Galunić-Bilić.
Software: Vedran Premužić, Marko Kralik.
Supervision: Sandra Bašić-Kinda, Marijo Vodanović, Igor Aurer.
Validation: Ivo Radman, Lucija Maleta, Pavle Rončević, Marijo Vodanović, Igor Aurer.
Visualization: Sandra Bašić-Kinda, Dino Dujmović, Marijo Vodanović.
Writing – original draft: Vedran Premužić, Igor Aurer.
Writing – review & editing: Vedran Premužić, Sandra Bašić-Kinda, Pavle Rončević, Marijo Vodanović, Igor Aurer.
Abbreviations:
- B-LCL
- b-large cell lymphoma
- CKD
- chronic kidney disease
- GFR
- glomerular filtration rate
- IPI
- international prognostic index
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Premužić V, Bašić-Kinda S, Radman I, Dujmović D, Ilić I, Živković N, Maleta L, Kralik M, Dobrenić M, Galunić-Bilić L, Rončević P, Vodanović M, Aurer I. Glomerular filtration rate is an independent prognostic factor in patients with B-large cell lymphoma. Medicine 2023;102:20(e33675).
Contributor Information
Sandra Bašić-Kinda, Email: sandra.kinda@gmail.com.
Ivo Radman, Email: ivoradman5@gmail.com.
Dino Dujmović, Email: dujmovicdi@gmail.com.
Ivana Ilić, Email: ricilic@gmail.com.
Neno Živković, Email: nenozivkovic12@gmail.com.
Lucija Maleta, Email: maleta.lucija@yahoo.com.
Marko Kralik, Email: makralik@gmail.com.
Margareta Dobrenić, Email: margareta.dobrenic@gmail.com.
Lea Galunić-Bilić, Email: lgbilic@gmail.com.
Pavle Rončević, Email: kronac@gmail.com.
Marijo Vodanović, Email: marijo.vodanovic@gmail.com.
Igor Aurer, Email: igor.aurer@kbc-zagreb.hr.
References
- [1].Weng PH, Hung KY, Huang HL, et al. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol. 2011;6:1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–9. [DOI] [PubMed] [Google Scholar]
- [3].Cengiz K. Increased incidence of neoplasia in chronic renal failure (20-year experience). Int Urol Nephrol. 2002;33:121–6. [DOI] [PubMed] [Google Scholar]
- [4].Launay-Vacher V. Cancer and the kidney: individualizing dosage according to renal function. Ann Oncol. 2013;24:2713–4. [DOI] [PubMed] [Google Scholar]
- [5].Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110:1376–84. [DOI] [PubMed] [Google Scholar]
- [6].Dalrymple LS, Katz R, Kestenbaum B, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Iff S, Craig JC, Turner R, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis. 2014;63:23–30. [DOI] [PubMed] [Google Scholar]
- [8].Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–35. [DOI] [PubMed] [Google Scholar]
- [9].Betjes MG, Meijers RW, Litjens NH. Loss of renal function causes premature aging of the immune system. Blood Purif. 2013;36:173–8. [DOI] [PubMed] [Google Scholar]
- [10].Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Na SY, Sung JY, Chang JH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol. 2011;33:121–30. [DOI] [PubMed] [Google Scholar]
- [13].Yang Y, Li HY, Zhou Q, et al. Renal function and all-cause mortality risk among cancer patients. Medicine (Baltim). 2016;95:e3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the haematological malignancy research network. Br J Cancer. 2011;105:1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yi HG, Kim JS, Suh C, et al. Clinical features and survival outcomes of patients with diffuse large B-cell lymphoma: analysis of web-based data from the Korean Lymphoma Working Party Registry. Blood Res. 2013;48:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61. [DOI] [PubMed] [Google Scholar]
- [17].Hong J, Lee S, Chun G, et al. Baseline renal function as a prognostic indicator in patients with newly diagnosed diffuse large B-cell lymphoma. Blood Res. 2016;51:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ubukata M, Hara M, Nishizawa Y, et al. Prevalence and mortality of chronic kidney disease in lymphoma patients: a large retrospective cohort study. Medicine (Baltim). 2018;97:e9615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bašić-Kinda S, Radman I, Dujmović D, et al. R-CHOEP14 in younger high-risk patients with large B cell lymphoma: an effective front-line regimen with cardiac toxicity: a real-life, single-center experience. Ann Hematol. 2021;100:1517–24. [DOI] [PubMed] [Google Scholar]
- [20].Aurer I, Bašić-Kinda S, Dujmović D, et al. DA-R-EPOCH as front-line treatment of elderly patients with high-risk diffuse large B-cell lymphoma (DLBCL): a real-life single-center study. EHA Library 2020: abstract EP1283.
- [21].Hude I, Bašić-Kinda S, Radman I, et al. Substituting doxorubicin with etoposide in R-CHOP results in a regimen with similar efficacy for treatment of newly diagnosed elderly patients with B-large cell lymphoma (B-LCL). EHA Library 2017: abstract E971.
- [22].Rule AD. The CKD-EPI equation for estimating GFR from serum creatinine: real improvement or more of the same? Clin J Am Soc Nephrol. 2010;5:951–3. [DOI] [PubMed] [Google Scholar]
- [23].Lowrance WT, Ordoñez J, Udaltsova N, et al. CKD and the risk of incident cancer. J Am Soc Nephrol. 2014;25:2327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Witko-Sarsat V, Friedlander M, Nguyen Khoa T, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–32. [PubMed] [Google Scholar]
- [25].Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12:1549–57. [DOI] [PubMed] [Google Scholar]
- [26].Wilhelm-Leen ER, Hall YN, K Tamura M, et al. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vaziri ND, Pahl MV, Crum A, et al. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Linch D, Goldstone A, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin´s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4. [DOI] [PubMed] [Google Scholar]
- [29].Okay M, Büyükaşik Y, Demiroğlu H, et al. Mitoxantrone-melphalan conditioning regimen for autologous stem cell transplantation in relapsed/refractory lymphoma. Turk J Med Sci. 2019;49:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]