Abstract
The soil bacterium Sinorhizobium meliloti is capable of entering into a nitrogen-fixing symbiosis with Medicago sativa (alfalfa). Particular low-molecular-weight forms of certain polysaccharides produced by S. meliloti are crucial for establishing this symbiosis. Alfalfa nodule invasion by S. meliloti can be mediated by any one of three symbiotically important polysaccharides: succinoglycan, EPS II, or K antigen (also referred to as KPS). Using green fluorescent protein-labeled S. meliloti cells, we have shown that there are significant differences in the details and efficiencies of nodule invasion mediated by these polysaccharides. Succinoglycan is highly efficient in mediating both infection thread initiation and extension. However, EPS II is significantly less efficient than succinoglycan at mediating both invasion steps, and K antigen is significantly less efficient than succinoglycan at mediating infection thread extension. In the case of EPS II-mediated symbioses, the reduction in invasion efficiency results in stunted host plant growth relative to plants inoculated with succinoglycan or K-antigen-producing strains. Additionally, EPS II- and K-antigen-mediated infection threads are 8 to 10 times more likely to have aberrant morphologies than those mediated by succinoglycan. These data have important implications for understanding how S. meliloti polysaccharides are functioning in the plant-bacterium interaction, and models are discussed.
The establishment of the symbiotic relationship between the soil bacterium Sinorhizobium meliloti (also referred to as Rhizobium meliloti) and its host plant, Medicago sativa (alfalfa), is a complex process involving the exchange of a series of signals between the plant and bacteria (7, 9, 27, 40). Flavonoid compounds released into the soil by alfalfa attract rhizobia and stimulate bacterial production of Nod factors, lipochitooligosaccharides that trigger root hair curling and the formation of root nodules on the host plant. S. meliloti cells trapped in curled root hairs invade the developing root nodule via tubes known as infection threads and are ultimately released into the nodule, where they differentiate into nitrogen-fixing bacteroids.
Polysaccharides produced by S. meliloti are critical for the establishment of a productive plant-bacterium symbiosis. Bacterial mutants that fail to produce certain polysaccharides are substantially impaired in their ability to invade developing root nodules and thus primarily yield root nodules devoid of bacteria and bacteroids (14, 24, 32, 37). The wild-type S. meliloti laboratory strain Rm1021 is capable of producing two symbiotically important exopolysaccharides, termed succinoglycan and EPS II. Both succinoglycan and EPS II can be produced in symbiotically active forms (i.e., forms sufficient to mediate nodule invasion). In culture, Rm1021 produces succinoglycan alone; EPS II is not produced at detectable levels. EPS II production by Rm1021 can occur in the presence of the expR101 mutation (14), a mucR::Tn5 mutation (19), or very low phosphate conditions (47). However, symbiotically active EPS II is produced only in an expR101 derivative of strain Rm1021 (15, 29). Rm41, an independently isolated wild-type S. meliloti strain, is also able to produce succinoglycan. However, it also produces a symbiotically active form of a capsular polysaccharide, termed K antigen (also known as KPS), which can substitute for succinoglycan and EPS II in mediating the nodule invasion step of symbiosis (35, 37, 39). Rm1021 lacks the ability to produce symbiotically active K antigen (39) and is therefore dependent on EPS production for root nodule invasion.
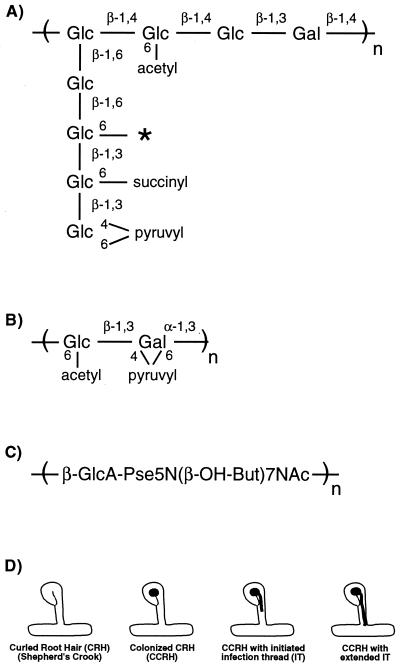
Intriguingly, succinoglycan, EPS II, and K antigen are structurally diverse polysaccharides. Succinoglycan is a polymer of an octasaccharide repeating unit composed of one galactose and seven glucose residues with acetyl, succinyl, and pyruvyl modifications (Fig. 1A) (1, 36), whereas EPS II is a polymer of a glucose-galactose disaccharide repeating unit modified with acetyl and pyruvyl substituents (Fig. 1B) (14, 16). K antigen has a disaccharide repeating unit containing glucuronic acid and a modified pseudaminic acid residue (Fig. 1C) (38). It is currently unclear how these three structurally distinct polysaccharides are each able to mediate root nodule invasion by S. meliloti, though it appears that low-molecular-weight forms of all three polysaccharides are the symbiotically active forms (4, 15, 39, 45). In the case of succinoglycan, the symbiotically active form is the trimer of the octasaccharide repeating unit (45), and in the case of EPS II, it is the class of oligosaccharides consisting of 15 to 20 disaccharide repeating units (15).
FIG. 1.
(A) Structure of the succinoglycan repeating unit. The asterisk indicates the position of a second succinyl modification (45) in some repeating units. (B) The structure of the EPS II repeating unit. (C) The structure of the K-antigen repeating unit. Pse, pseudaminic acid. (D) Schematic diagram of a curled root hair (CRH) and the three indices scored in GFP-based plant assays: a colonized curled root hair (CCRH), a colonized curled root hair with initiated infection thread (IT), and a colonized curled root hair with extended infection thread (adapted from reference 8).
Accumulating evidence suggests that low-molecular-weight polysaccharides are acting as signaling molecules that trigger developmental responses in the host plant or regulate host defense responses. First, small quantities of purified, low-molecular-weight succinoglycan or EPS II can partially rescue the nodule invasion defect of a strain producing no symbiotically active polysaccharide (4, 15, 44, 45). Second, polysaccharide function can be provided in trans to a strain producing no symbiotically active polysaccharide by a second, polysaccharide-proficient strain (22, 30). Finally, strains carrying mutations that perturb the molecular weight distribution of succinoglycan or EPS II have a reduced nodule invasion ability (8, 23).
Recently, our laboratory (8) has refined a previously described system (13) for examining the kinetics and efficiency of nodule invasion events in the alfalfa-S. meliloti symbiosis. Briefly, alfalfa plants growing on glass microscope slides coated with nitrogen-free media are inoculated with S. meliloti constitutively expressing the green fluorescent protein (GFP) from a stably maintained plasmid vector. Early events in symbiotic nodulation, including formation of colonized, curled root hairs, initiation of infection threads, and extension of infection threads, are visualized (Fig. 1D) and quantified using fluorescence microscopy. Using this technique, members of our group previously (8) examined the role of succinoglycan in symbiosis and determined that succinoglycan production by strain Rm1021 is important for infection thread initiation and absolutely required for infection thread extension. Additionally, we determined that the presence of the acetyl and succinyl modifications of succinoglycan are important for infection thread extension and that overproduction of succinoglycan profoundly reduces the efficiency of colonized, curled root hair formation.
To gain further insight into the mechanisms by which three S. meliloti polysaccharides of such strikingly different structures can promote the common biological outcome of nodule invasion, we used our GFP-based approach to analyze nodule invasion by S. meliloti derivatives that produced only EPS II or K antigen. Our work has revealed that succinoglycan, EPS II, and K antigen do not function equally well in mediating root nodule invasion. Succinoglycan is more efficient than K antigen and much more efficient than EPS II in mediating the growth of infection threads on alfalfa. In addition, we have observed distinct morphologies for infection threads promoted by different polysaccharides. This suggests that succinoglycan, EPS II, and K antigen are acting by related but not identical mechanisms to promote nodule invasion.
MATERIALS AND METHODS
Bacterial strains and growth media.
The strains used in this study are listed in Table 1. Strain Rm9011 was constructed by generalized transduction (12) of the expA3::lacZ (Gmr) allele from strain RmAR1011 (5) into Rm9000. Strains were grown in Luria-Bertani liquid medium supplemented with 2.5 mM CaCl2 and 2.5 mM MgSO4. Antibiotics were used at the following concentrations: streptomycin, 500 μg/ml; neomycin, 200 μg/ml; gentamicin, 50 μg/ml; spectinomycin, 100 μg/ml; and tetracycline, 10 μg/ml. All strains used for fluorescence microscopy analyses contained pHC60 (8), a stably maintained plasmid that constitutively expresses GFP.
TABLE 1.
S. meliloti strains used in this studya
| Designation | Genotype | SG | EPS II | KPSb | Reference |
|---|---|---|---|---|---|
| Rm1021 | SU47 Smr | + | − | − | 28 |
| Rm7210 | Rm1021 exoY210::Tn5 | − | − | − | 24 |
| Rm9000 | Rm1021 expR101 exoY210::Tn5 | − | + | − | 15 |
| Rm8530 | Rm1021 expR101 | + | + | − | 14 |
| Rm9011 | Rm1021 expR101 exoY210::Tn5 expA3::lacZ (Gmr) | − | − | − | This study |
| Rm41 | Spr | + | ? c | + | 34 |
| AK631 | Rm41 exoB631 | − | − | + | 3 |
| PP674 | AK631 rkpA::Tn5 | − | − | − | 35 |
+ indicates production of a symbiotically active form of the polysaccharide. − indicates that the strain does not produce a symbiotically active form of the polysaccharide.
KPS, K antigen.
It is not known whether Rm41 produces EPS II.
Nodulation assays.
Standard plant assays were performed as previously described (24), and each plant was inoculated with 1 ml of a cell suspension with an optical density at 600 nm of 0.05. Plant height means and nodule percentage means were calculated from data from several separate inoculations. At least 60 total plants inoculated with each strain were scored. The error ranges represent the standard errors of the means computed using mean values from groups of 5 to 10 plants.
Nodulation of alfalfa (M. sativa cv. Iroquois) by S. meliloti for GFP analyses was analyzed on microscope slides as described by Cheng and Walker (8). To determine the kinetics of nodule invasion, at least three separate sets of 12 plants were inoculated with each strain and examined by fluorescence microscopy for 12 days. The overall efficiencies of nodule invasion for each strain were calculated from data collected on day 12 of the experiments. Infection threads that had a wide, irregular region in excess of double the width of another section of the thread were scored as aberrant. Additionally, any infection thread with more than one densely packed pocket of bacteria along its length was scored as aberrant.
Fluorescence microscopy.
Alfalfa seedlings inoculated with GFP-expressing S. meliloti strains were examined at ×100 magnification (10× eyepiece; 10× objective) using a Zeiss fluorescence microscope, model Axioskop (Carl Zeiss, Inc., Thornwood, N.Y.) equipped with a fluorescein isothiocyanate filter set. Image acquisition was performed at ×400 magnification (10× eyepiece; 40× objective) using a Zeiss fluorescence microscope, model Axioplan2, equipped with an integrated 3-chip cooled charge-coupled device color camera (model DEI-750T; Optronics Engineering). Images of GFP-expressing S. meliloti cells were obtained by using a filter set consisting of a 460- to 500-nm bandpass exciter, a 505-nm longpass dichroic filter, and a 510-nm longpass emitter (model 41012; Chroma, Brattleboro, Vt.). Images of root hair cells were obtained by using a filter set consisting of a 510- to 560-nm bandpass exciter, a 565-nm longpass dichroic filter, and a 582- to 647-nm bandpass emitter (model 41002; Chroma). Images were captured using Scion Image 1.62 (public domain) installed on a Power Macintosh 8600/200 computer. Composite images were generated using Adobe Photoshop 4.0 software.
Acetylene reduction assays.
Acetylene reduction assays were performed as follows: 1.5 ml of acetylene gas (generated by the reaction of water and calcium carbide) was injected into stoppered 15-ml tubes containing alfalfa root systems from 4-week-old plants inoculated with various S. meliloti strains. The tubes were incubated for 10 h before analysis. A 100-μl sample from each tube was analyzed for the presence of acetylene and ethylene using a Shimadzu GC-9A gas chromatograph equipped with a Porapak N (Supelco, Bellafonte, Pa.) molecular sieve column (6 feet by 1/8 inch; mesh size, 80/100) and a flame ionization detector. The flow rate of the nitrogen carrier gas was set at 40 ml/min. The injector temperature was 100°C, and the column temperature was 65°C. Under these conditions, ethylene typically eluted after 60 s, while acetylene eluted after 90 s. Following the incubation and gas analyses, all pink nodules were removed and weighed. The amount of ethylene produced per pink nodule (fresh weight) was calculated for Rm1021, Rm9000, Rm8530, Rm41, and AK631. No acetylene reduction was observed for root systems from plants that had been inoculated with water alone, Rm7210, Rm9011, or PP674.
RESULTS
Alfalfa plants inoculated with succinoglycan-producing S. meliloti strains grow better than those inoculated with a strain producing EPS II alone.
Over the course of many separate inoculations in standard plant assays scored 4 weeks after inoculation, it became clear that the mean plant height of alfalfa (M. sativa cv. Iroquois) inoculated with a succinoglycan-producing S. meliloti strain (Rm1021) was noticeably greater (8.0 cm [±1.4 cm]) than the average height of plants inoculated with Rm9000 (3.7 cm [±0.8 cm]), an Rm1021 derivative producing only EPS II. Additionally, 93.0% (±2.7%) of the nodules on plants inoculated with the strain producing succinoglycan were pink, nitrogen-fixing nodules, whereas only 55.3% (±3.8%) of the nodules induced by a strain producing EPS II alone were pink 4 weeks after inoculation. Since pink nodules are symbiotically successful, this suggested that the strain producing EPS II alone was forming nitrogen-fixing nodules at a reduced rate. We did not observe a significant difference in either plant height (8.8 cm [±1.6 cm] for Rm41 versus 8.37 cm [±1.9 cm] for AK631) or the percentage of nodules that were pink (90.4% [±3.3%] for Rm41 versus 89.1% [±3.9%] for AK631) on plants inoculated with AK631, a strain that produces K antigen alone, or its wild-type parent, Rm41, which produces both succinoglycan and K antigen. Very similar standard plant assay results were obtained regardless of whether alfalfa plants were inoculated with strains carrying the GFP plasmid (pHC60 [8]), carrying the pHC60 parent plasmid (pHC41 [8]), or containing no plasmid (data not shown).
To determine whether the stunted growth of the EPS II-inoculated plants relative to those inoculated with succinoglycan or K-antigen-producing strains was due to a deficiency in the ability of the EPS II-producing strain to fix nitrogen, we used acetylene reduction assays to compare the nitrogen-fixing capacities of pink nodules taken from 4-week-old alfalfa plants inoculated with S. meliloti strains that differed with respect to the polysaccharides they produced. Pink nodules from plants inoculated with strains producing various polysaccharides (Rm1021, Rm9000, Rm8530, Rm41, or AK631) reduced acetylene to ethylene with efficiencies that were similar in terms of the amount of ethylene generated per pink nodule (fresh weight) (data not shown). This suggested that the above-described differences in plant condition at 4 weeks were not due to an inherent decrease in the nitrogen-fixing capacity and potential of the EPS II-producing strain relative to those of the other strains examined. Additionally, the total number (both pink and white) of nodules induced by the EPS II-producing strain was comparable to the number of nodules induced by the succinoglycan- or K-antigen-producing strains (data not shown), and the kinetics of nodule appearance were similar in all strains examined (data not shown). This suggested that the stunted growth of the EPS II-producing strain was not due to a defect in the strain's ability to induce nodule formation. Taken together with the differences in the percentages of pink nodules, these results suggested that there might be a difference between nodule invasion mediated by EPS II and nodule invasion mediated by succinoglycan or K antigen.
EPS II-mediated nodule invasion is less efficient than succinoglycan-mediated nodule invasion.
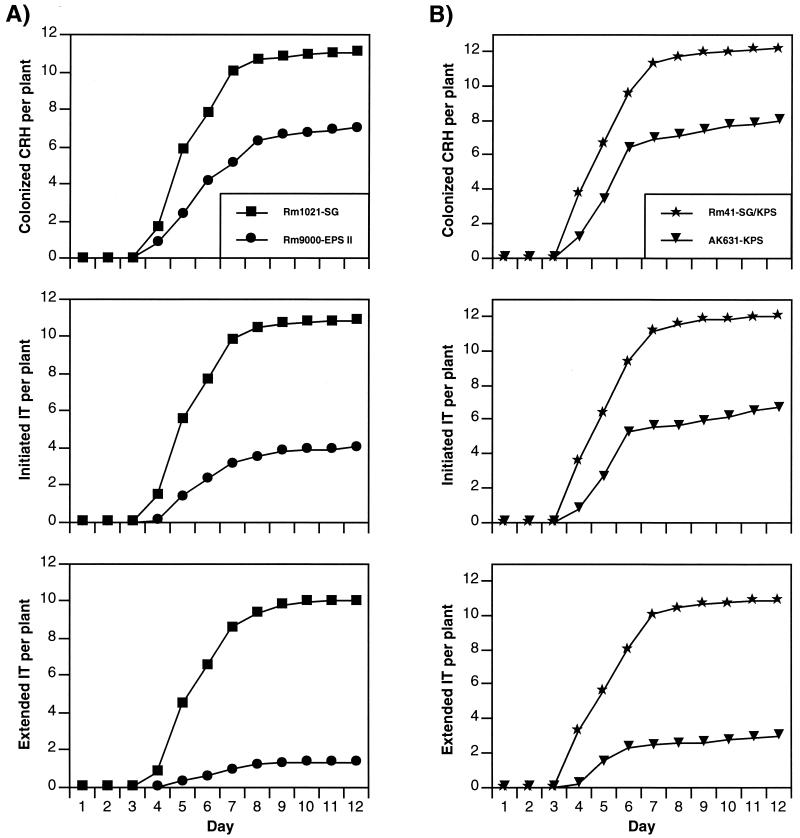
To determine whether the differences observed in the standard plant assays were the result of an invasion defect in the EPS II-producing strain, we used GFP-labeled S. meliloti strains to examine the kinetics (Fig. 2) and efficiency (Fig. 3) of nodule invasion in the symbioses mediated by succinoglycan, EPS II, and K antigen. At least 36 plants inoculated with each strain were scored daily for the appearance of colonized curled root hairs, initiated infection threads, and extended infection threads (Fig. 2). Plants inoculated with the succinoglycan-producing strain, Rm1021, developed colonized curled root hairs, initiated infection threads, and extended infection threads beginning on day 4, and the numbers of all three events increased rapidly until day 8 or 9, at which time they reached a plateau (Fig. 2A). A high percentage (>98%) of colonized curled root hairs initiated infection threads, and most of the infection threads (>92%) were extended to the base of the root hair (Fig. 3). As noted previously (8), nearly all (>95%) of the nodules induced by GFP-expressing Rm1021 fluoresced very brightly throughout when viewed under the fluorescence microscope.
FIG. 2.
Kinetics of colonized curled root hair (CRH) formation, infection thread (IT) initiation, and infection thread extension on alfalfa plants inoculated with various S. meliloti strains. Sets of at least 36 plants were inoculated with Rm1021 (succinoglycan [SG]-producing strain) or Rm9000 (EPS II-producing strain) (A) or with Rm41 (succinoglycan- and K-antigen [KPS]-producing strain) or AK631 (K-antigen-producing strain) (B). The numbers of colonized curled root hairs, initiated infection threads, and extended infection threads per plant were recorded for 12 days. The colonized curled root hair count includes those with initiated and extended infection threads, and the initiated infection thread count includes extended infection threads. Standard error of the mean calculations were performed with the mean daily values from at least 3 groups of 12 plants. The standard errors for the time points were nonoverlapping.
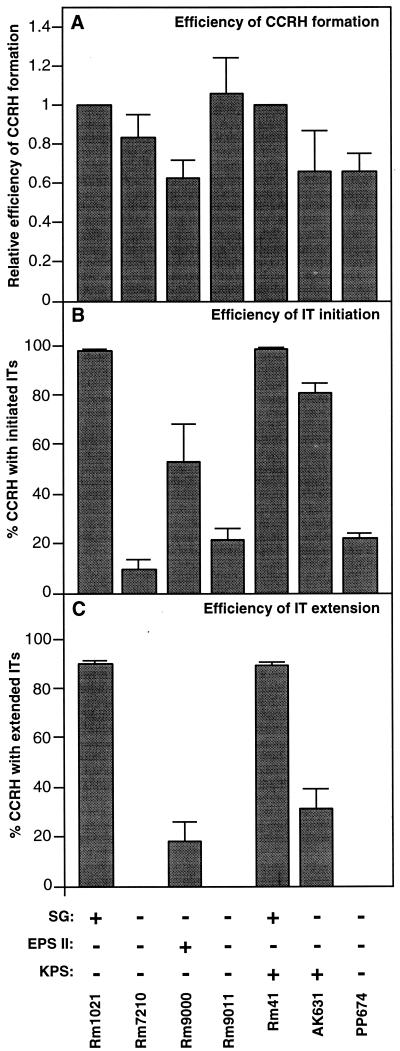
FIG. 3.
Efficiencies of nodule invasion by various S. meliloti strains. Sets of at least 36 plants were inoculated with different strains and scored on day 12 for numbers of colonized curled root hairs (CCRHs), numbers of initiated infection threads (ITs), and numbers of extended infection threads. (A) The relative efficiencies of colonized curled root hair formation were computed by normalizing the numbers of colonized curled root hairs to that of Rm1021 (for Rm7210, Rm9000, and Rm9011) or Rm41 (for AK631 and PP674). (B) The efficiency of infection thread initiation is the percentage of curled root hairs colonized by a particular strain that initiate an infection thread. (C) The efficiency of infection thread extension is the percentage of curled root hairs colonized by a particular strain that have infection threads that are extended (reach the base of the root hair cell). The error bars represent the standard errors of the means computed using the mean values from at least three groups of 12 plants.
The EPS II-producing strain (Rm9000) also developed colonized curled root hairs on day 4, but in contrast to results for Rm1021, substantial numbers of initiated infection threads and extended infection threads did not appear until day five (Fig. 2A). Furthermore, relative to the results for the succinoglycan-producing strain, the EPS II-producing strain exhibited a 40 to 50% reduction in both the total number of colonized curled root hairs and the percentage of colonized curled root hairs that initiated infection threads (Fig. 3A and B). Strikingly, less than 20% of colonized curled root hairs on plants inoculated with the EPS II-producing strain developed extended infection threads compared to the >90% seen with succinoglycan (Fig. 3C). Also, in contrast to the high percentage (>95%) of bright nodules induced by succinoglycan-producing strains, a significant proportion (∼50%) of the nodules induced by Rm9000 were dark on day 12 when viewed under the fluorescence microscope, suggesting that these nodules had not been filled with bacteria. These analyses clearly indicate that nodule invasion mediated by EPS II proceeds less efficiently than nodule invasion mediated by succinoglycan.
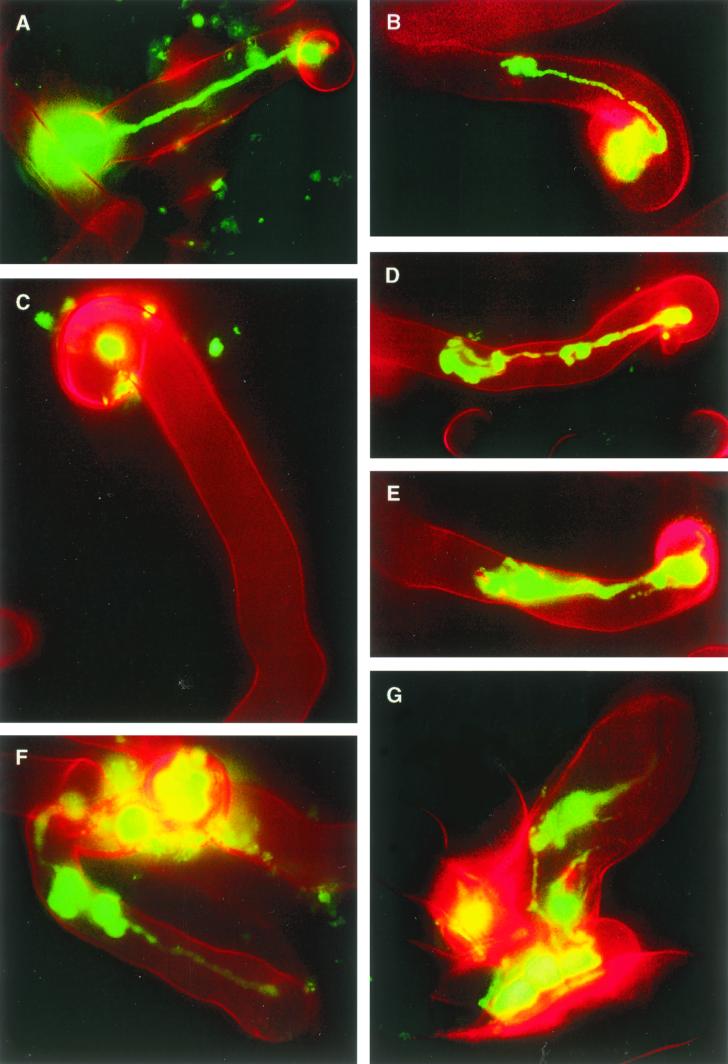
In the course of our GFP analyses of succinoglycan- and EPS II-mediated symbioses, we observed that infection threads formed on plants inoculated with the S. meliloti strain producing EPS II alone frequently had morphologies that were unusual relative to those induced by succinoglycan-producing strains. Infection threads induced by succinoglycan-producing strains almost always maintained a constant width and were located in the centers of the root hairs (Fig. 4A). This regular infection thread phenotype was observed in 96.4% (±1.2%) of infection threads observed on plants inoculated with succinoglycan-producing strains. In the rare instances that succinoglycan-mediated infection threads aborted prior to reaching the base of the root hair, they usually had, at most, one densely populated pocket of bacteria located at the terminus of the aborted infection thread (Fig. 4B). In contrast, we observed that 39.5% (±3.5%) of EPS II-mediated infection threads had morphologies that were aberrant compared to those of typical succinoglycan-mediated infection threads. Infection threads that had a wide, irregular region in excess of double the width of another section of the thread and threads that had more than one densely packed pocket of bacteria along their length were classified as aberrant. A majority (66%) of aberrant EPS II-mediated infection threads exhibited irregular width variation (Fig. 4E), though some (34%) had constant widths and had two or more densely packed pockets of bacteria along their lengths (Fig. 4D). Just over half (53%) of aberrant EPS II-mediated infection threads aborted before reaching the base of the root hair cell.
FIG. 4.
Fluorescence microscopy analyses of infection thread formation mediated by various S. meliloti polysaccharides. All images are composite images of GFP-expressing S. meliloti cells (green) and root hair cells (red). (A) Typical succinoglycan-mediated extended infection thread formed by Rm1021. The infection thread extends from the colonized, curled root hair to the base of the root hair cell. (B) Aborted succinoglycan-mediated infection thread with a densely packed pocket of bacteria near the terminus. (C) Colonized, curled root hair formed by Rm7210, an exoY210::Tn5 mutant of Rm1021 that fails to produce a symbiotically active polysaccharide. (D and E) Aborted, aberrant EPS II-mediated infection threads present on plants inoculated with Rm9000. (F and G) Extended, aberrant, K-antigen-mediated infection threads on plants inoculated with AK631.
expR101-induced EPS II production does not compromise the efficiency of succinoglycan-mediated nodule invasion.
The fact that EPS II is less efficient than succinoglycan at mediating nodule invasion raised the question of whether EPS II production either by a succinoglycan-producing strain or by a coinoculated second strain could interfere with succinoglycan-mediated nodule invasion. However, strain Rm8530 (Rm1021 expR101 exo+), which produces both succinoglycan and EPS II, was able to invade nodules with virtually the same kinetics, efficiency, and infection thread morphology as those of strain Rm1021, which produces succinoglycan alone (data not shown). When alfalfa plants were coinoculated with a 1:1 ratio of GFP-expressing Rm1021 cells (which produce only succinoglycan) and GFP-expressing Rm9000 cells (which produce only EPS II), we also observed Rm1021-like nodule invasion kinetics, nodule invasion efficiency, and infection thread morphology. This indicates that neither expR101-induced production of EPS II in a succinoglycan-producing strain nor EPS II production by a coinoculated strain interferes with the efficiency of succinoglycan in mediating nodule invasion.
An S. meliloti strain producing K antigen alone is less proficient at mediating infection thread extension than a strain producing both succinoglycan and K antigen.
Rm41, an independently isolated S. meliloti strain, can produce symbiotically active forms of both succinoglycan and K antigen. In GFP-based plant assays, Rm41 performed similarly to Rm1021, the strain that makes succinoglycan alone, in terms of its ability to colonize curled root hairs, initiate infection threads, and extend infection threads (Fig. 2B and 3). Based on the results of our standard plant assays, we expected that K-antigen-mediated nodule invasion would proceed with approximately the same kinetics and efficiency as those observed for strain Rm41 (Fig. 2B and 3). We were therefore surprised to observe a slight delay relative to Rm41 in the appearance of extended infection threads on plants inoculated with AK631, an Rm41 derivative that produces only K antigen (Fig. 2B). Additionally, although AK631 was able to colonize curled root hairs and initiate infection threads with approximately the same efficiency as that of Rm41, it exhibited a sizable reduction in the efficiency of infection thread extension relative to that for Rm41; fewer than 35% of colonized curled root hairs on a plant inoculated with strain AK631 developed extended infection threads (Fig. 3). However, this reduced efficiency of infection thread extension did not appear to negatively impact the results of standard plant assays using the K-antigen-producing strain. Nearly all (>95%) of the nodules induced on plants inoculated with AK631 fluoresced brightly throughout when viewed under the fluorescence microscope on day 12. This suggested that despite the reduced occurrence of extended infection threads, most nodules were associated with a successful infection event (not all infection threads are nodule associated).
In results similar to those for EPS II-mediated infection threads, 40.7% (±3.4%) of the infection threads formed by an S. meliloti strain producing only K antigen were aberrant compared to only 6.4% (±3.35%) on plants inoculated with Rm41. Aberrant K-antigen-mediated infection threads almost always (96%) consisted of threads of constant widths having two or more densely packed pockets of bacteria along the length of the thread (Fig. 4F and G). Interestingly, almost half (45.5%) of aberrant K-antigen-mediated infection threads were extended to the base of the root hair cell (Fig. 4F and G), suggesting that the infection thread had aborted (or stalled) and then restarted several times during the extension process, resulting in the formation of several densely packed pockets of bacteria along the length of the thread.
As was the case with EPS II production in a succinoglycan-producing strain, the production of K antigen by Rm41 did not appear to interfere with the high efficiency of succinoglycan-mediated nodule invasion (Fig. 2B and 3). Additionally, the efficiencies of nodule invasion and the infection thread morphologies that we observed for Rm41-AK631 and Rm1021-AK631 coinoculations (data not shown) were similar to those seen for inoculations using Rm1021 alone (Fig. 2A and 3) or Rm41 alone (Fig. 2B and 3).
EPS II- and K-antigen-producing strains prevented from producing any symbiotically active polysaccharides are not defective in colonization of curled root hairs.
Previously we observed that Rm7210 (Rm1021 exoY210::Tn5), a succinoglycan-deficient mutant of Rm1021, was highly compromised in its ability to initiate and extend infection threads but retained its ability to colonize curled root hairs (8). To determine whether either the expR101 mutation in the EPS II-producing strain Rm9000 or the galE (exoB) mutation in the K-antigen-producing strain AK631 resulted in a reduced ability to colonize curled root hairs, we compared the kinetics and efficiency of nodule invasion of Rm9011 [Rm9000 expA3::lacZ (Gmr)], an EPS II-deficient derivative of Rm9000, and PP674 (AK631 rkpA::Tn5), a K-antigen-deficient derivative of AK631, to those of Rm7210 (Rm1021 exoY210::Tn5). All three strains performed similarly in terms of their infection kinetics (data not shown) and overall nodule invasion efficiencies (Fig. 3). Rm7210, Rm9011, and PP674 were able to colonize curled root hairs approximately as well as Rm1021 and Rm41 but were substantially compromised in their abilities to initiate infection threads and completely deficient in infection thread extension; most of the symbiotic events observed for plants inoculated with S. meliloti strains deficient in the production of succinoglycan, EPS II, and K antigen consisted of colonized, curled root hairs with no infection threads (Fig. 4C). These analyses demonstrated that neither the expR101 mutation nor the galE (exoB) mutation significantly reduced the colonized, curled-root-hair formation efficiencies of strains carrying them. As expected, these data indicated that, like succinoglycan in our previous investigation (8), EPS II and K antigen mediate infection thread initiation and extension in symbiosis.
DISCUSSION
Using S. meliloti strains constitutively expressing GFP from a stably maintained plasmid vector, we have shown that there are clearly defined quantitative and qualitative differences in the abilities of succinoglycan, EPS II, and K antigen to mediate M. sativa cv. Iroquois (alfalfa) root nodule invasion. K antigen is less efficient than succinoglycan at mediating infection thread extension, whereas EPS II is less efficient than succinoglycan at mediating both infection thread initiation and extension. Constitutive GFP expression did not appear to substantially impact the symbiotic efficiencies of the strains used in this study.
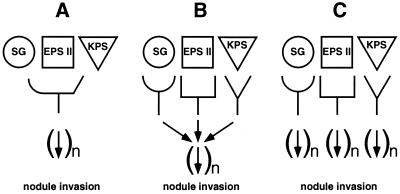
The differences in the efficiencies of nodule invasion mediated by succinoglycan, EPS II, and K antigen suggest that the three polysaccharides seem to act through related but different mechanisms. Specific low-molecular-weight forms of succinoglycan or EPS II provided at low levels are sufficient to promote nodule invasion (4, 15, 45), and it appears that low-molecular-weight forms of K antigen are similarly the symbiotically active forms (39). For this reason, it seems probable that succinoglycan, EPS II, and K antigen are acting as signals to the plant, likely by signaling via a plant receptor or receptors. In one model (Fig. 5A), one plant receptor is able to recognize all three polysaccharides. In this three-polysaccharide, one-receptor model, the differences in nodule-invasion-promoting activity among the three polysaccharides would most likely result from various interactions of three structurally diverse polysaccharides with the single receptor. In an alternate class of models (Fig. 5B and C), a distinct receptor is proposed to exist for each polysaccharide. Though it is formally possible that each of the three receptors could signal a completely distinct signal transduction pathway (Fig. 5C), it would seem that a more plausible version of this type of model would be one in which the signals from the three receptors feed into a common signal transduction pathway involved in nodule invasion (Fig. 5B). In three-polysaccharide, three-receptor models, the various efficiencies of nodule invasion mediated by succinoglycan, EPS II, and K antigen would most likely result from inherent differences in the signal transduction pathways that are specific to each receptor. The fact that both strains producing EPS II alone and strains producing K antigen alone induce distinct aberrant infection threads seems easiest to explain by a model in which there are three separate receptors that influence a common invasion pathway in somewhat different fashions (Fig. 5B).
FIG. 5.
Models for the perception of succinoglycan (SG), EPS II, and K antigen (KPS) by a host plant and subsequent signal transduction resulting in nodule invasion by S. meliloti. (A) A three-polysaccharide, one-receptor model. (B) A three-polysaccharide, three-receptor model in which the three signal transduction pathways feed into a common signal transduction pathway. (C) A three-polysaccharide, three-receptor model in which the three signal transduction pathways are independent of one another.
The model that we presently favor (Fig. 5B) is schematically simple, but if one considers what is known about the perception of Nod factor by host legumes, it is very likely that signal transduction pathways involved in the recognition of three structurally diverse signal polysaccharides are far more complicated than those represented in our figure. A core set of genes involved in Nod factor production is conserved among the rhizobia, and the various Nod factors produced by rhizobial strains adhere to common structural themes. Even so, isolation of candidate Nod factor receptors has proven very challenging (see reference 31 for a review), partially due to the identification of multiple candidates with various affinities for Nod factors, the possibility that lipochitooligosaccharides are a general class of developmental signaling molecules, and the fact that slightly different Nod factor molecules appear to control different aspects of the host's response to Nod factor. Because succinoglycan, EPS II, and K antigen have very different structures and each has at least one distinct cluster of genes involved in its synthesis (5, 14, 20, 21, 26), it seems very likely that the perception of each of these three polysaccharides by a host plant is at least as complex as the plant's perception of Nod factors.
Though succinoglycan, EPS II, and K antigen mediate infection thread initiation and extension, it is not clear which plant functions are being modulated. One attractive possibility is that rhizobial polysaccharides function as signals that direct cytoskeletal movements in the root hair cell which stimulate infection thread initiation and maintenance (41). Another intriguing possibility is that bacterial polysaccharides could function as signals that affect plant defense responses. The aberrant infection thread structures seen on plants inoculated with strains producing EPS II alone or K antigen alone could result from less efficient modulation of plant cytoskeletal movements or less efficient modulation of host defense responses. Many aberrant infection thread events consist of several densely packed pockets of bacteria along the length of the infection thread (Fig. 4F), and some aberrant threads are wider than typical succinoglycan-mediated infection threads (Fig. 4E). Because those few succinoglycan-mediated infection threads that abort prior to reaching the base of the root hair cell tend to have one such pocket of bacteria at the point of termination (Fig. 4B), those threads that have two or more of such pockets along their lengths may have arisen from multiple rounds of infection thread stalling (or abortion) and reinitiation. Both stalling (or abortion) or a widening of the infection thread could be a result either of inefficiently modulated plant defenses acting to halt or slow bacterial invasion or of inefficient modulation of plant cytoskeletal components.
The decrease in nodule invasion efficiency of a strain producing EPS II alone is manifested in reductions (relative to plants inoculated with succinoglycan-producing strains) in both the mean plant height and the percentage of root nodules that are pink after a 4-week growth period. However, plants inoculated with a strain producing K antigen alone do not manifest stunted phenotypes, despite the observed reduction in efficiency of nodule invasion. The stunting seen in plants inoculated with the strain producing EPS II alone appears to be a result of the strain's invasion deficiency rather than of a deficiency in its ability to induce root nodule formation or a defect in its innate ability to fix nitrogen. Our interpretation of this is that the reduced invasion efficiency of the strain producing EPS II alone (reduced infection thread initiation and extension) results in a delay in the appearance of symbiotically successful, nitrogen-fixing nodules. In contrast, the reduction in infection thread extension efficiency seen with the strain producing K antigen alone, though measurable, is not severe enough to result in a delay in the establishment of a productive symbiosis. Though pink nodules from plants inoculated with the EPS II-producing strain have the same nitrogen-fixing capacity per unit of fresh weight as pink nodules from plants inoculated with strains producing succinoglycan or K antigen, fewer symbiotically effective (pink) nodules are present on plants inoculated with strains producing EPS II alone.
Since a number of S. meliloti strains produce more than one symbiotically important polysaccharide (25, 34, 46), it seems possible that certain bacterial polysaccharides may be more efficient in mediating root nodule invasion on specific hosts or under specific environmental conditions. Thus, production of more than one symbiotically important polysaccharide by S. meliloti may provide a strain with a selective advantage, allowing the strain to interact as efficiently as possible under a variety of conditions with many cultivars or ecotypes of legumes that it can nodulate. Succinoglycan may be the most efficient polysaccharide of the three at mediating root nodule invasion on M. sativa cv. Iroquois under our assay conditions, but EPS II or K antigen may be more efficient than succinoglycan on another host plant or under different environmental conditions. It is also possible that some hosts of S. meliloti have receptors for only a subset of the three known symbiotically important polysaccharides produced by the bacterium. Either possibility could explain our lab's previous observation (14) that while succinoglycan is able to mediate nodule invasion on alfalfa, white clover, yellow clover, fenugreek, and the one ecotype of Medicago truncatula tested, EPS II is able to mediate nodule invasion on alfalfa but not the other hosts.
Given the general importance of rhizobial polysaccharides in the establishment of a successful symbiosis, the data presented in this work may be useful for thinking about the role(s) of rhizobial polysaccharides in determining host ranges and efficiencies of symbioses other than that between S. meliloti and alfalfa. For example, Bradyrhizobium japonicum strain 2143 and two derivative strains are capable of producing three exopolysaccharides that appear to be involved in the efficiency of their symbioses with Glycine max (18), and B. japonicum strain USDA 123 produces two structurally distinct polysaccharides, one when outside the nodule and the second when inside the nodule (2). Additionally, the symbiotic defects of EPS-deficient mutants of B. japonicum strain 110spc4 are host dependent, differing markedly on the hosts Glycine max and Glycine soja (33), and there are many other rhizobia for which production of specific polysaccharides is important for symbiosis, including Rhizobium sp. strain NGR234 (11), Rhizobium leguminosarum bv. viciae (6, 10), Rhizobium leguminosarum bv. trifolii (42, 43), and Rhizobium loti (17). Thus, the conclusions from this work are likely to be broadly applicable to analyses of bacterial polysaccharides that mediate interactions with host plants.
ACKNOWLEDGMENTS
We thank members of the Walker lab for helpful suggestions and discussions. We also thank C. A. Kaiser and S. L. Sanders for the use of their fluorescence microscopes and M. L. Guerinot for the use of her gas chromatograph.
This work was supported by Public Health Service grant GM31030 from the National Institutes of Health (NIH) to G.C.W. and NIH predoctoral training grant T32GM07287 (B.J.P.).
REFERENCES
- 1.Aman P, McNeil M, Franzen L-E, Darvill A G, Albersheim P. Structural elucidation, using HPLC-MS and GLC-MS, of the acidic exopolysaccharide secreted by Rhizobium meliloti strain Rm1021. Carbohydr Res. 1981;95:263–282. [Google Scholar]
- 2.An J, Carlson R W, Glushka J, Streeter J G. The structure of a novel polysaccharide produced by Bradyrhizobium species within soybean nodules. Carbohydr Res. 1995;269:303–317. doi: 10.1016/0008-6215(94)00361-i. [DOI] [PubMed] [Google Scholar]
- 3.Banfalvi Z, Sakanyan V, Koncz C, Kiss A, Dusha I, Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of Rhizobium meliloti. Mol Gen Genet. 1981;184:318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- 4.Battisti L, Lara J C, Leigh J A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci USA. 1992;89:5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker A, Rüberg S, Küster H, Roxlau A A, Keller M, Ivashina T, Cheng H, Walker G C, Pühler A. The 32-kilobase exp gene cluster of Rhizobium meliloti directing the biosynthesis of galactoglucan: genetic organization and properties of the encoded gene products. J Bacteriol. 1997;179:1375–1384. doi: 10.1128/jb.179.4.1375-1384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borthakur D, Barber C E, Lamb J W, Daniels M J, Downie J A, Johnston A W B. A mutation that blocks exopolysaccharide synthesis prevents nodulation of peas by Rhizobium leguminosarum but not of beans by R. phaseoli and is corrected by cloned DNA from Rhizobium or the phytopathogen Xanthomonas. Mol Gen Genet. 1986;203:320–323. [Google Scholar]
- 7.Brewin N J. Development of the legume root nodule. Annu Rev Cell Biol. 1991;7:191–226. doi: 10.1146/annurev.cb.07.110191.001203. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H P, Walker G C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J Bacteriol. 1998;180:5183–5191. doi: 10.1128/jb.180.19.5183-5191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denarie J, Cullimore J. Lipo-oligosaccharide nodulation factors: a new class of signalling molecules mediating recognition and morphogenesis. Cell. 1993;74:951–954. doi: 10.1016/0092-8674(93)90717-5. [DOI] [PubMed] [Google Scholar]
- 10.Diebold R, Noel K D. Rhizobium leguminosarum exopolysaccharide mutants: biochemical and genetic analyses and symbiotic behavior on three hosts. J Bacteriol. 1989;171:4821–4830. doi: 10.1128/jb.171.9.4821-4830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djordjevic M A, Redmond J W, Batley M, Rolfe B G. Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J. 1987;6:1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finan T M, Hartwieg E K, LeMieux K, Bergman K, Walker G C, Signer E R. General transduction in Rhizobium meliloti. J Bacteriol. 1984;159:120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gage D, Bobo T, Long S. Use of green fluorescent protein to visualize the early events of symbiosis between Rhizobium meliloti and alfalfa (Medicago sativa) J Bacteriol. 1996;178:7159–7166. doi: 10.1128/jb.178.24.7159-7166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glazebrook J, Walker G C. A novel exopolysaccharide can function in place of the Calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989;56:661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- 15.González J E, Reuhs B L, Walker G C. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc Natl Acad Sci USA. 1996;93:8636–8641. doi: 10.1073/pnas.93.16.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Her G-R, Glazebrook J, Walker G C, Reinhold V N. Structural studies of a novel exopolysaccharide produced by a mutant of Rhizobium meliloti strain Rm1021. Carbohydr Res. 1990;198:305–312. doi: 10.1016/0008-6215(90)84300-j. [DOI] [PubMed] [Google Scholar]
- 17.Hotter G S, Scott D B. Exopolysaccharide mutants of Rhizobium loti are fully effective on a determinate nodulating host but are ineffective on an indeterminate nodulating host. J Bacteriol. 1991;173:851–859. doi: 10.1128/jb.173.2.851-859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karr, D., R.-T. Liang, B. Reuhs, and D. Emerich. Altered exopolysaccharides of Bradyrhizobium japonicum mutants correlate with impaired soybean lectin binding, but not with effective nodule formation. Planta, in press. [DOI] [PubMed]
- 19.Keller M, Roxlau A, Weng W M, Schmidt M, Quandt J, Karsten N, Jording D, Arnold W, Pühler A. Molecular analysis of the Rhizobium meliloti mucR gene regulating the biosynthesis of the exopolysaccharides succinoglycan and galactoglucan. Mol Plant-Microbe Interact. 1995;8:267–277. doi: 10.1094/mpmi-8-0267. [DOI] [PubMed] [Google Scholar]
- 20.Kereszt A, Kiss E, Reuhs B L, Carlson R W, Kondorosi A, Putnoky P. Novel rkp gene clusters of Sinorhizobium meliloti involved in capsular polysaccharide production and invasion of the symbiotic nodule: the rkpK gene encodes a UDP-glucose dehydrogenase. J Bacteriol. 1998;180:5426–5431. doi: 10.1128/jb.180.20.5426-5431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiss E, Reuhs B, Kim J, Kereszt A, Petrovics G, Putnoky P, Dusha I, Carlson R, Kondorosi A. The rkpGHI and -J genes are involved in capsular polysaccharide production by Rhizobium meliloti. J Bacteriol. 1997;179:2132–2140. doi: 10.1128/jb.179.7.2132-2140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein S, Hirsch A M, Smith C A, Signer E R. Interaction of nod and exo Rhizobium meliloti in alfalfa nodulation. Mol Plant-Microbe Interact. 1988;1:94–100. doi: 10.1094/mpmi-1-094. [DOI] [PubMed] [Google Scholar]
- 23.Leigh J A, Reed J W, Hanks J F, Hirsch A M, Walker G C. Rhizobium meliloti mutants that fail to succinylate their Calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell. 1987;51:579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 24.Leigh J A, Signer E R, Walker G C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci USA. 1985;82:6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloret J, Bolaños L, Lucas M M, Peart J, Brewin N J, Bonilla I, Rivilla R. Ionic and osmotic pressure induce different alterations in the lipopolysaccharide of a Rhizobium meliloti strain. Appl Environ Microbiol. 1995;61:3701–3704. doi: 10.1128/aem.61.10.3701-3704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long S, Reed J W, Himawan J, Walker G C. Genetic analysis of a cluster of genes required for synthesis of the Calcofluor-binding exopolysaccharide of Rhizobium meliloti. J Bacteriol. 1988;170:4239–4248. doi: 10.1128/jb.170.9.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long S R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989;56:203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- 28.Meade H M, Long S R, Ruvkun G B, Brown S E, Ausubel F M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendrygal K E, González J E. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J Bacteriol. 2000;182:599–606. doi: 10.1128/jb.182.3.599-606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller P, Hynes M, Kapp D, Niehaus K, Pühler A. Two classes of Rhizobium meliloti infection mutants differ in exopolysaccharide production and in coinoculation properties with nodulation mutants. Mol Gen Genet. 1988;211:17–26. [Google Scholar]
- 31.Niebel A, Gressent F, Bono J J, Ranjeva R, Cullimore J. Recent advances in the study of nod factor perception and signal transduction. Biochimie. 1999;81:669–674. doi: 10.1016/s0300-9084(99)80124-2. [DOI] [PubMed] [Google Scholar]
- 32.Niehaus K, Becker A. The role of microbial surface polysaccharides in the Rhizobium-legume interaction. Subcell Biochem. 1998;29:73–116. doi: 10.1007/978-1-4899-1707-2_3. [DOI] [PubMed] [Google Scholar]
- 33.Parniske M, Schmidt P, Kosch K, Müller P. Plant defense responses of host plants with determinate nodules induced by EPS-defective exoB mutants of Bradyrhizobium japonicum. Mol Plant-Microbe Interact. 1994;5:631–638. [Google Scholar]
- 34.Putnoky P, Grosskopf E, Ha D T, Kiss G B, Kondorosi A. Rhizobium fix genes mediate at least two communication steps in symbiotic nodule development. J Cell Biol. 1988;106:597–607. doi: 10.1083/jcb.106.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putnoky P, Petrovics G, Kereszt A, Grosskopf E, Ha D T C, Banfalvi Z, Kondorosi A. Rhizobium meliloti lipopolysaccharide and exopolysaccharide can have the same function in the plant-bacterium interaction. J Bacteriol. 1990;172:5450–5458. doi: 10.1128/jb.172.9.5450-5458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinhold B B, Chan S Y, Reuber T L, Marra A, Walker G C, Reinhold V N. Detailed structural characterization of succinoglycan, the major symbiotically important exopolysaccharide of Rhizobium meliloti strain Rm1021. J Bacteriol. 1994;176:1997–2002. doi: 10.1128/jb.176.7.1997-2002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuhs B L, Carlson R W, Kim J S. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J Bacteriol. 1993;175:3570–3580. doi: 10.1128/jb.175.11.3570-3580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuhs B L, Geller D P, Kim J S, Fox J E, Kolli V S, Pueppke S G. Sinorhizobium fredii and Sinorhizobium meliloti produce structurally conserved lipopolysaccharides and strain-specific K antigens. Appl Environ Microbiol. 1998;64:4930–4938. doi: 10.1128/aem.64.12.4930-4938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reuhs B L, Williams M N V, Kim J S, Carlson R W, Cote F. Suppression of the Fix− phenotype of Rhizobium meliloti exoB mutants by lpsZ is correlated to a modified expression of the K-polysaccharide. J Bacteriol. 1995;177:4289–4296. doi: 10.1128/jb.177.15.4289-4296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhijn P V, Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol Rev. 1995;59:124–142. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridge R. A model of legume root hair growth and Rhizobium infection. Symbiosis. 1992;14:359–373. [Google Scholar]
- 42.Rolfe B, Carlson R, Ridge R, Dazzo F, Mateos P, Pankhurst C. Defective infection and nodulation of clovers by exopolysaccharide mutants of Rhizobium leguminosarum bv. trifolii. Aust J Plant Physiol. 1996;23:285–303. [Google Scholar]
- 43.Skorupska A, Bialek U, Urbanik-Sypniewska T, van Lammeren A. Two types of nodules induced on Trifolium pratense by mutants of Rhizobium leguminosarum bv. trifolii deficient in exopolysaccharide production. J Plant Physiol. 1995;147:93–100. [Google Scholar]
- 44.Urzainqui A, Walker G C. Exogenous suppression of the symbiotic deficiencies of Rhizobium meliloti exo mutants. J Bacteriol. 1992;174:3403–3406. doi: 10.1128/jb.174.10.3403-3406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L-X, Wang Y, Pellock B J, Walker G C. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J Bacteriol. 1999;181:6788–6796. doi: 10.1128/jb.181.21.6788-6796.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zevenhuizen L P T M, Faleschini P. Effect of the concentration of sodium chloride in the medium on the relative proportions of poly- and oligo-saccharides excreted by Rhizobium meliloti strain YE-2S1. Carbohydr Res. 1991;209:203–209. doi: 10.1016/0008-6215(91)80157-i. [DOI] [PubMed] [Google Scholar]
- 47.Zhan H J, Lee C C, Leigh J A. Induction of the second exopolysaccharide (EPSb) in Rhizobium meliloti SU47 by low phosphate concentrations. J Bacteriol. 1991;173:7391–7394. doi: 10.1128/jb.173.22.7391-7394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]