Abstract
The Escherichia coli rhaBAD operon encodes the enzymes for catabolism of the sugar l-rhamnose. Full rhaBAD activation requires the AraC family activator RhaS (bound to a site that overlaps the −35 region of the promoter) and the cyclic AMP receptor protein (CRP; bound immediately upstream of RhaS at −92.5). We tested alanine substitutions in activating regions (AR) 1 and 2 of CRP for their effect on rhaBAD activation. Some, but not all, of the substitutions in both AR1 and AR2 resulted in approximately twofold defects in expression from rhaBAD promoter fusions. We also expressed a derivative of the α subunit of RNA polymerase deleted for the entire C-terminal domain (α-Δ235) and assayed expression from rhaBAD promoter fusions. The greatest defect (54-fold) occurred at a truncated promoter where RhaS was the only activator, while the defect at the full-length promoter (RhaS plus CRP) was smaller (13-fold). Analysis of a plasmid library expressing alanine substitutions at every residue in the carboxyl-terminal domain of the α subunit (α-CTD) identified 15 residues (mostly in the DNA-binding determinant) that were important at both the full-length and truncated promoters. Only one substitution was defective at the full-length but not the truncated promoter, and this residue was located in the DNA-binding determinant. Six substitutions were defective only at the promoter activated by RhaS alone, and these may define a protein-contacting determinant on α-CTD. Overall, our results suggest that CRP interaction with α-CTD may not be required for rhaBAD activation; however, α-CTD does contribute to full activation, probably through interactions with DNA and possibly RhaS.
Regulation of the Escherichia coli rhaBAD operon responds to both availability of l-rhamnose and catabolite repression. In the presence of l-rhamnose, the AraC family activator RhaS (reviewed in reference 12) binds to a site that spans from position −32 to position −81 relative to the rhaBAD transcription start site (9, 10). This RhaS-binding site consists of two 17-bp inverted repeat half-sites that are separated by 16 bp of DNA not contacted by RhaS (9). RhaS alone can activate rhaBAD expression approximately 1,000-fold above the extremely low basal level (10). The cyclic AMP receptor protein (CRP) mediates catabolite repression at rhaBAD by binding to a site immediately upstream of RhaS that is centered at position −92.5 relative to the rhaBAD transcription start site (10). CRP alone does not activate rhaBAD expression, but in the presence of RhaS CRP can contribute 30- to 50-fold additional activation (10).
CRP is a global regulator of catabolite repression in E. coli (reviewed in reference 6). Interactions between CRP and RNA polymerase (RNAP) that are required for transcription activation have been well defined for promoters where CRP is the only activator. These simple CRP-dependent promoters are categorized according to the location of the CRP-binding site. At class I CRP-dependent promoters CRP binds upstream but not adjacent to RNAP, with sites for CRP usually centered at positions −62.5, −72.5, or −92.5 relative to the transcription start site. CRP activation at class I promoters involves protein-protein contacts between a surface-exposed loop on CRP activating region 1 (AR1), and the carboxyl-terminal domain of the α subunit (α-CTD) of RNAP (31, 35, 36; reviewed in reference 6). At class II CRP-dependent promoters CRP binds to a site that is centered at position −42.5 and overlaps the −35 region. In this situation, contacts are made between a second activating region on CRP, AR2, and the N-terminal domain of α (α-NTD) (21, 27, 29; reviewed in references 5 and 6), as well as between CRP AR1 and α-CTD (32, 36).
Activation by CRP at promoters where CRP acts in conjunction with a regulon-specific activator, called class III promoters, has been less thoroughly studied. In contrast to class I and class II promoters, a pattern or patterns for the role of CRP at class III promoters has not yet emerged. For example, at the uhpT promoter, CRP binds at position −103.5 and acts in conjunction with the uhp-specific activator, UhpA, bound at position −64. Merkel et al. (17) found that CRP AR1 substitutions were not defective for uhpT activation, suggesting that CRP activation of uhpT does not depend on the previously defined α-CTD–AR1 interactions. More recent work has shown that α-CTD is required for uhpT activation (23). CRP is also involved in activation with regulon-specific proteins at several pairs of divergent promoters. At some divergent promoters, such as mal, the only role of CRP appears to be to reposition other activators (28), while at others there is evidence that CRP plays a role in both DNA structure and in protein-protein interactions (4, 25, 26, 34).
α-CTD is dispensable at many activator-independent promoters; however, it is required for interaction with DNA (especially UP elements) or activator proteins and DNA at a large number of other promoters. Three determinants on the surface of α-CTD have been identified based on their functions at class I CRP-dependent promoters and UP elements (reviewed in reference 6). The 265 determinant is important for both UP element activation and CRP-dependent activation and is proposed to identify residues involved in DNA binding. The 261 determinant of α-CTD is also important for both UP element and CRP-dependent activation; however, these residues are not required for α-CTD interactions with DNA, and their function is not clear. The 287 determinant is required for activation by CRP but is not required for DNA binding or UP element-dependent activation. Mutations in the 287 determinant disrupt cooperativity between CRP and α; hence, it is proposed that these residues are required for interactions between CRP and α-CTD.
The goal of this work was to characterize rhaBAD transcription activation by CRP and α-CTD. We found that alanine substitution of some residues within both AR1 and AR2 of CRP resulted in small defects in rhaBAD activation. To determine whether α-CTD was required for rhaBAD activation, we expressed a derivative of α deleted for the entire C-terminal domain, α-Δ235. Expression of α-Δ235 resulted in a 54-fold defect at a promoter with only a RhaS-binding site and a 13-fold defect at a promoter with binding sites for both CRP and RhaS. Deletion of rhaS from the cell eliminated the α-CTD deletion defects at all promoters. Using a library of alanine substitutions in α-CTD, we found strong evidence for an α-CTD interaction with DNA, suggestive evidence for a possible interaction between α-CTD and RhaS, and no evidence for an α-CTD–CRP interaction. Overall, our results are most consistent with a model for rhaBAD activation in which CRP activates by a mechanism other than interaction with α-CTD and in which α-CTD activates by interacting with DNA and possibly RhaS.
MATERIALS AND METHODS
General methods.
Standard methods were used for restriction endonuclease digestion, ligation, and transformation of DNA. Most DNA sequences were verified using automated dideoxy sequencing on a LI-COR 4000L sequencer. Primers for the LI-COR 4000L were labeled with IRD-41 and were custom made by LI-COR, Inc. (Lincoln, Nebr.). The Thermo Sequenase fluorescent-labeled primer cycle sequencing kit from Amersham Life Science (Piscataway, N.J.) was used for sequencing reactions. Additional DNA sequence verification was performed on an ABI Prism 310. Primers for ABI Prism sequencing were synthesized by Oligos, Etc. (Wilsonville, Oreg.), and sequencing reactions were carried out using a Thermo Sequenase dye terminator sequencing kit from Amersham Life Science.
Culture media.
Morpholinepropanesulfonic acid (MOPS; 1×) buffered medium (20) was used to grow cultures for β-galactosidase assay and consisted of 40 mM MOPS, 4 mM Tricine, 0.01 mM FeSO4, 9.5 mM NH4Cl, 0.276 mM K2SO4, 0.5 μM CaCl2, 0.528 mM MgCl2, 50 mM NaCl, 3 × 10−9 M Na2MoO4, 4 × 10−7 M H3BO3, 3 × 10−8 M CoCl2, 10−8 M CuSO4, 8 × 10−8 M MnCl2, 10−8 M ZnSO4, 1.32 mM K2HPO4, 10 mM NaHCO3, 0.2% Casamino Acids, and 0.002% thiamine. Overnight medium consisted of 1× MOPS medium containing 0.04% glycerol. Growth medium consisted of 1× MOPS medium containing 0.4% glycerol, 125 mg of ampicillin per ml, and 0.2% l-rhamnose. For other experiments (cloning, strain construction, etc.) cells were grown in tryptone-yeast (TY) medium (16) with or without antibiotic.
Plasmids, phages, and strains.
The E. coli strains, λ phages, and plasmids used in this study are listed in Table 2. Wild-type crp was cloned by PCR amplification using primers 2003 and 2004 (Table 1) with whole cells of strain ECL116 (Table 2) serving as template. The PCR product was digested at the EcoRI site in 2003 and the BamHI site in 2004 and cloned between the EcoRI and BamHI sites of pHG165, resulting in pSE186. The DNA sequence of the entire cloned crp gene was verified by automated sequencing on both strands.
TABLE 2.
Strains used in this study
| Strain, phage, or plasmid | Genotype | Source or referencea |
|---|---|---|
| E. coli | ||
| ECL116 | F− ΔlacU169 endA hsdR thi | 1 |
| LS854 | trpA9605 his-85 rpsL136 Δcrp-3 metE70 trpR55 λ− IN(rrnD-rrnE)1 | CGSC |
| JW375 | supE42 λ− zhc-511::Tn10 | CGSC |
| L8854 | trpA9605 his-85 rpsL136 Δcrp-3 zhc-511::Tn10 metE70 trpR55 λ− IN(rrnD-rrnE)1 | E. Elsinghorst |
| SME1035 | ECL116 λSME103 recA::cat | 10 |
| SME1036 | ECL116 λSME104 recA::cat | 10 |
| SME1037 | ECL116 λSME105 recA::cat | 10 |
| SME1088 | ECL116 λSME104 ΔrhaS recA::cat | 10 |
| SME1089 | ECL116 λSME105 ΔrhaS recA::cat | 10 |
| SME1222 | ECL116 λSME103 ΔrhaS | 2 |
| SME1853 | ECL116 λSME101 Δcrp-3 zhc-511::Tn10 | This study |
| SME1854 | ECL116 λSME103 Δcrp-3 zhc-511::Tn10 | This study |
| SME1855 | ECL116 λSME104 Δcrp-3 zhc-511::Tn10 | This study |
| Phages | ||
| λSME101 | λ RS45 Φ(rhaB-lacZ)Δ226 | 10 |
| λSME103 | λ RS45 Φ(rhaB-lacZ)Δ110 | 10 |
| λSME104 | λ RS45Φ(rhaB-lacZ)Δ84 | 10 |
| λSME105 | λ RS45Φ(rhaB-lacZ)Δ70 | 10 |
| Plasmids | ||
| pREIIα | Ampr pREIIα rpoA | 3 |
| pHTf1α | Ampr pHTf1α rpoA | 31 |
| pSE186 | Ampr pHG165crp+ | This study |
| pSE187 | pSE186 (Thr158Ala) | This study |
| pSE188 | pSE186 (Gly162Ala) | This study |
| pSE189 | pSE186 (His19Ala) | This study |
| pSE190 | pSE186 (His21Ala) | This study |
| pSE191 | pSE186 (Lys101Ala) | This study |
| pSE192 | Ampr pREIIα rpoA (α-Δ235) | This study |
CGSC, E. coli Genetic Stock Center, Yale University, New Haven, Conn.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide no. | Oligonucleotide sequence, 5′–3′a | Use |
|---|---|---|
| 2003 | GACGAATTCGAGTACGCGTACTAACCA | Upstream crp amplification |
| 2004 | GACGGATCCAATCAGTCTGCGCCACAT | Downstream crp amplification |
| 2006 | CCAGACGCTATGGCTCACCCGGACG | Thr158Ala |
| 2007 | CTCACCCGGACGCTATGCAAATCAA | Gly162Ala |
| 2061 | TTGTCTCATTGCGCCATTCATAAGTACC | His19Ala |
| 2062 | CATTGCCACATTGCTAAGTACCCATC | His21Ala |
| 2063 | AAATTTCGTACAAAGCATTTCGCCAATTGA | Lys101Ala |
| 2078 | CCGTGCAGTACAGTTGATAGb | crp sequencing |
| 2080 | ATCAGTCTGCGCCACATCGGb | crp sequencing |
| 2094 | AGCTTTCGTTGACTTACGTGTTAAACTGACCTAAG | α-CTD deletion linking fragment |
| 2095 | GATCCTTAGGTCAGTTTAACACGTAAGTCAACGAA | α-CTD deletion linking fragment |
| 2126 | GTTTGAAGAGGGCCAGGAb | crp sequencing |
| 2127 | TCCGGGTTTACCTGAATCb | crp sequencing |
Regions of oligonucleotides not complementary to crp or rpoA (for cloning or site-directed mutagenesis purposes) are underlined.
These oligonucleotides were IRD41 dye labeled for use in a LI-COR automated sequencer.
ECL116 cells were infected with λSME101, λSME103, and λSME104 to generate strains carrying promoter fusions with lacZ. Lysogens were identified as blue colonies on plates with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plus l-rhamnose, and single lysogens were identified by β-galactosidase assay and the Ter test (13). Δcrp was moved into the resultant fusion strains by phage P1-mediated generalized transduction (18) using tetracycline plates (20 μg/ml) to select for the linked zhc-511::Tn10. Strains that recombined Δcrp along with zhc-511::Tn10 were identified by screening on MacConkey agar plates containing 1% l-rhamnose or maltose.
The wild-type rpoA gene carried on pREIIα, as well as the library of alanine substitution derivatives in the α-CTD, were gifts from R. Gourse. Plasmid pSE192 encoding a carboxyl-terminal domain deletion derivative of α (α-Δ235; Table 2) was derived from pREIIα as follows. Oligonucleotides 2094 and 2095 (Table 1) were hybridized to generate a small linking DNA fragment. pREIIα was cut at the unique HindIII site within rpoA and a BamHI site beyond rpoA. The linking fragment was ligated between the two sites to generate a plasmid encoding α that is wild-type through position 235, followed by the amino acids VKLT encoded by the linker and a stop codon. The amino acids encoded in the linker were identical to those fused to the C-terminal end of the original α-235 constructed by Igarishi and Ishihama (14). The sequence of the rpoA deletion derivative was verified by automated sequencing on both strands.
β-Galactosidase assay.
Strains for β-galactosidase assay were grown as described by Bhende and Egan (2). Briefly, starter cultures were grown in tryptone-yeast extract broth (with 125 μg of ampicillin per ml added for strains containing plasmid) for approximately 7 h at 37°C. Then, 40 μl of starter culture was used to inoculate 2.5 ml of 1× MOPS overnight medium (see recipe above), and this was grown for approximately 17 h. An approximately 200-μl volume of the overnight culture, or the appropriate volume to reach a starting optical density at 600 nm of 0.01 to 0.02 in the growth flask, was then used to inoculate 10 ml of 1× MOPS growth medium (see recipe above) in 125-ml baffled flasks. Cultures were grown at 37°C with vigorous shaking (∼280 rpm) to an A600 of approximately 0.4. After resuspension of the cell pellets in Z buffer (18), β-galactosidase activity was determined as described by Miller (18) except that incubation with substrate (o-nitrophenyl-β-d-thiogalactopyranoside) was done at room temperature. Specific activities were averaged from at least three independent assays, with two replicates in each assay.
Mutagenesis of crp.
Oligonucleotide primers for site-directed mutagenesis (Table 1) were synthesized by NBI (Beverly, Mass.) and Oligos, Etc. Alanine substitutions were introduced using the Gene Editor In Vitro Mutagenesis System (Promega Corp., Madison, Wis.) with plasmid pSE186 as template. In all cases, the entire crp gene was sequenced on both strands to confirm the mutation and to confirm that there were no additional mutations.
RESULTS
CRP AR1 and AR2 substitution derivatives at rhaBAD.
To begin to understand the mechanism of CRP activation of rhaBAD expression, we wished to determine whether AR1 and/or AR2 of CRP are necessary for activation at rhaBAD. We tested two alanine substitutions in AR1 (T158A and G162A) and three in AR2 (H19A, H21A, and K101A) which cause approximately 5- to 40-fold activation defects at standard class I and II promoters (21, 22, 35). Each of the CRP substitution derivatives was tested at two different fusions of the rhaBAD promoter region with lacZ. In the first fusion [Φ(rhaB-lacZ)Δ226; Fig. 1], rhaBAD promoter DNA extended upstream to position −226 and included the RhaS and CRP-binding sites at rhaBAD as well as the RhaR and CRP-binding sites at rhaSR. At Φ(rhaB-lacZ)Δ226, G162A and H21A both resulted in approximately twofold defects, and K101A was also slightly defective (Table 3). In the second fusion [Φ(rhaB-lacZ)Δ110], rhaBAD promoter DNA extended upstream to position −110 and included only the RhaS and CRP-binding sites at rhaBAD. At Φ(rhaB-lacZ)Δ110, G162A and H21A again resulted in approximately twofold defects, K101A was again slightly defective, and H19A also resulted in nearly a twofold defect.
FIG. 1.
rhaSR-rhaBAD intergenic region. The top line shows a schematic representation of the regulatory region between rhaBAD and rhaSR. The relative positions of the two RNA polymerases and the activator proteins RhaS, CRP, and RhaR are shown. The bottom lines show the DNA sequence upstream of rhaBAD extending back to position −239 (the rhaSR transcription start site). The positions of the RhaS and RhaR-binding sites are shown by everted arrows, and the position of the CRP-binding site is shown by inverted arrows. The −10 and −35 regions of the two promoters are marked. The upstream endpoints of rhaBAD promoter fusions are identified.
TABLE 3.
Effects of alanine substitutions in CRP AR1 and AR2 at Φ(rhaB-lacZ)
| CRP derivative | Φ(rhaB-lacZ)Δ226
|
Φ(rhaB-lacZ)Δ110
|
||
|---|---|---|---|---|
| β-Gal sp acta | % wt | β-Gal sp act | % wt | |
| wt | 1,018 | 100 | 812 | 100 |
| T158A | 1,051 | 103 | 684 | 84 |
| G162A | 532 | 52 | 466 | 57 |
| H19A | 912 | 90 | 382 | 47 |
| H21A | 418 | 41 | 356 | 44 |
| K101A | 733 | 72 | 631 | 77 |
β-Galactosidase (β-Gal) specific activity was measured from single-copy rhaB-lacZ fusions in crp deletion strains transformed with plasmids encoding either a wild-type (wt) or substitution derivative of CRP. Cultures were grown in MOPS growth media containing glycerol, l-rhamnose, and 125 μg of ampicillin per ml. Standard errors were less than 27% of the average units.
As expression of rhaS is also dependent on CRP activation (C. C. Holcroft and S. M. Egan, submitted for publication), it was possible that the small effects of the CRP AR1 and AR2 substitutions were indirect, due to decreased RhaS protein. However, assays of the same CRP substitutions at rhaS-lacZ fusions (Holcroft and Egan, submitted) suggest that the defects of the AR1 and AR2 substitutions at rhaBAD were not due to indirect effects on rhaS expression. To further support this conclusion, the AR1 and AR2 CRP substitution derivatives were also tested for activation at Φ(rhaB-lacZ)Δ84 (Table 4). This fusion does not include the CRP-binding site at rhaBAD; thus, any observed defect in activation would be due to an indirect effect of decreased rhaS expression. None of the AR1 or AR2 CRP substitution derivatives was defective for activation of Φ(rhaB-lacZ)Δ84; in fact, they were all at least 120% of the wild-type level.
TABLE 4.
Effects of alanine substitutions in CRP AR1 and AR2 at Φ(rhaB-lacZ)Δ84
| CRP derivative | Φ(rhaB-lacZ)Δ84
|
|
|---|---|---|
| β-Gal sp acta | % wt | |
| wt | 19 | 100 |
| T158A | 59 | 313 |
| G162A | 33 | 174 |
| H19A | 31 | 168 |
| H21A | 25 | 134 |
| K101A | 23 | 122 |
β-Galactosidase (β-Gal) specific activity was measured from a single-copy rhaB-lacZ fusion in a crp deletion strain transformed with plasmids encoding either a wild-type (wt) or substitution derivative of CRP. Cultures were grown in MOPS growth media containing glycerol, l-rhamnose, and 125 μg of ampicillin per ml. Standard errors were less than 10% of the average units.
Effect of deleting α-CTD at rhaBAD.
To determine whether α-CTD is important for activation of rhaBAD, we constructed a plasmid that expresses a derivative of α deleted for the entire α-CTD, α-Δ235. We assayed Φ(rhaB-lacZ) expression in a strain expressing wild-type α or α-Δ235 from a plasmid as well as wild-type α from the chromosome (Table 5). At the shortest promoter, Φ(rhaB-lacZ)Δ70, there was a sixfold defect upon expression of α-Δ235. There was no difference between expression of wild-type α and α-Δ235 at the same promoter in a ΔrhaS strain background, indicating that activation by α-CTD requires RhaS. It is likely that the higher level of expression (0.056U) from Φ(rhaB-lacZ)Δ70 in the rhaS+ strain expressing wild-type α reflects some ability of RhaS to bind to the partial RhaS-binding site that remains in this fusion, at least in the presence of α-CTD.
TABLE 5.
Effect of expressing an α-CTD deletion derivative at rhaBAD
| Promoter fusion | β-Gal sp acta
|
|||
|---|---|---|---|---|
|
rhaS+
|
ΔrhaS
|
|||
| wt α | α-Δ235 | wt α | α-Δ235 | |
| Φ(rhaB-lacZ)Δ110 | 330 | 26 | 0.018 | <0.01 |
| Φ(rhaB-lacZ)Δ84 | 8.7 | 0.16 | <0.01 | <0.01 |
| Φ(rhaB-lacZ)Δ70 | 0.056 | <0.01 | <0.01 | 0.010 |
β-Galactosidase (β-Gal) specific activity was measured from single copy rhaB-lacZ fusions in rhaS+ or ΔrhaS strains expressing either wild-type (wt) α or an α-CTD deletion derivative, α-Δ235. Cultures were grown in MOPS growth media containing glycerol, l-rhamnose, and 125 μg of ampicillin per ml. Standard errors were less than 44% of the average units.
Notice that the level of expression in the ΔrhaS strain background was extremely low for all of the fusions tested. We estimate that 0.01 Miller units is equivalent to >1 β-galactosidase monomer per cell. Any cell that transcribed rhaB-lacZ would be expected to synthesize more than one β-galactosidase monomer, suggesting that the majority of cells had no β-galactosidase enzyme at all. Our estimate suggests, therefore, that the level of expression in the ΔrhaS strain backgrounds is very close to zero.
Interestingly, expression of α-Δ235 resulted in a 54-fold defect at the RhaS-dependent Φ(rhaB-lacZ)Δ84 fusion compared to wild-type α. This suggests that a significant α-CTD interaction had been lost, possibly with DNA and/or RhaS. Again, in the ΔrhaS strain there was no difference between expression of wild-type α and α-Δ235, indicating that α-CTD could not contribute to activation in the absence of RhaS. Finally, the effect of expressing α-Δ235 at Φ(rhaB-lacZ)Δ110, where both the CRP and RhaS-binding sites are present, fell to 13-fold. This smaller defect in the presence of CRP activation was not expected given the hypothesis that the primary role of α-CTD would be interaction with CRP. Since there is no detectable rhaBAD activation by CRP in the absence of RhaS, it was not possible to test the effect of α-Δ235 expression on CRP activation independent of RhaS activation. Overall, these results indicate that α-CTD is required for full activation of both full-length and truncated rhaBAD promoters; however, they are not strongly supportive of a model in which the primary role of α-CTD is interaction with CRP.
RNAP α-CTD alanine scan.
Based upon the results of our analysis with α-Δ235, it appears that α-CTD is required for full rhaBAD activation. We wished to identify residues in α-CTD involved in this activation and to investigate whether α-CTD activation depends on interactions with DNA and/or CRP and/or RhaS. To do this, we tested a plasmid-borne library of α-CTD alanine substitution derivatives for activation at Φ(rhaB-lacZ)Δ110 and Φ(rhaB-lacZ)Δ84 in strain backgrounds that expressed wild-type α from the chromosome. The results of our analysis are shown in Fig. 2 and are summarized in Table 6. We have divided the important residues in α-CTD identified at rhaBAD into “DNA-binding” and “Other” categories. Assignment of residues into the DNA-binding category was based on the residues identified as part of the DNA-binding “265 determinant” based on studies with CRP and UP elements (6, 11, 19), on the fluorescence characterization of α-CTD binding to a factor-independent promoter and CRP and UP element-dependent promoters (24), and on the predicted position of the residues on the structure of α-CTD (accession no. 1COO.PDB).
FIG. 2.
Effect of single alanine substitutions within α-CTD on activation from Φ(rhaB-lacZ)Δ110 (SME1035) and Φ(rhaB-lacZ)Δ84 (SME1036). Activities are expressed as a percentage of the average activity measured from cells transformed with plasmids encoding the wild-type α subunit. Values shown are the averages of at least three independent experiments. Each α-CTD alanine substitution that significantly lowered expression compared to wild-type α-CTD as determined by analysis of variance statistical analysis is indicated by an asterisk above the bar.
TABLE 6.
Summary of important residues in α-CTD
| Proposed determinant | Residues defective at:
|
|
|---|---|---|
| (rhaB-lacZ)Δ110 | (rhaB-lacZ)Δ84 | |
| DNA binding | 263 | 263 |
| 265 | 265 | |
| 266 | 266 | |
| 268 | 268 | |
| 269 | 269 | |
| 273 | 273 | |
| 291 | 291 | |
| 296 | 296 | |
| 297 | 297 | |
| 298 | 298 | |
| 299 | 299 | |
| 300 | ||
| 301 | ||
| 302 | 302 | |
| 303 | ||
| 305 | 305 | |
| Other | 255 | 255 |
| 278 | ||
| 279 | ||
| 315 | ||
| 321 | ||
| 322 | ||
| 323 | ||
Given that α-CTD and CRP can interact to activate transcription at a variety of promoters (29; reviewed in reference 6), we looked for evidence of such an interaction at rhaBAD. We reasoned that alanine substitution of α-CTD residues that were involved in specific interactions with CRP would be defective compared to wild-type at Φ(rhaB-lacZ)Δ110 but would probably not be defective at Φ(rhaB-lacZ)Δ84. Interestingly, only one of the α-CTD substitutions (at residue 301) fits this pattern (Fig. 2; summarized in Table 6). α-CTD residue 301 appears to be located within the DNA-binding determinant of the α-CTD and therefore is not likely to define an α-CTD–CRP interaction site. We identified 14 α-CTD substitutions defective for activation at both Φ(rhaB-lacZ)Δ110 and Φ(rhaB-lacZ)Δ84 (Fig. 2; summarized in Table 6). Of these, 13 map in the DNA-binding category, suggesting that α-CTD does make important contacts with DNA at both promoters. The other residue that was defective at both Φ(rhaB-lacZ)Δ110 and Φ(rhaB-lacZ)Δ84 was at position 255. Arg255 is quite surface exposed on the structure of α-CTD and is immediately adjacent to the 261 determinant on α-CTD (8, 31).
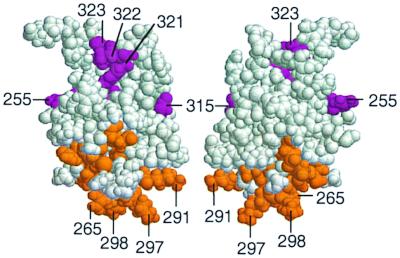
Eight substitutions in α-CTD were defective at Φ(rhaB-lacZ)Δ84 but not defective at Φ(rhaB-lacZ)Δ110 (Fig. 2; summarized in Table 6). Two of these were substitutions that map in the likely DNA-binding region of α-CTD. Of the other six residues, two (residues 278 and 279) are located within helix 2, and the other four (residues 315, 321, 322, and 323) are located in the C-terminal loop of the α-CTD structure (15). Residues 278 and 279 are not significantly surface exposed and likely function by altering the structure of α-CTD. Residues 321, 322, and 323 form a patch on α-CTD that is opposite the α-CTD DNA-binding determinant (Fig. 3). Since this region is located far from residues shown to be involved in DNA binding, it is tempting to speculate that it might define a region of interaction between α-CTD and RhaS; however, other explanations are also possible.
FIG. 3.
Space-filling model of predicted α-CTD structure. The model was based on the atomic coordinates of Jeon et al. (15). Colored residues are those identified as important at the Φ(rhaB-lacZ)Δ84 promoter fusion. Orange residues are those that may be involved in interaction with DNA (DNA-binding category), while violet residues are the residues that are unlikely to be involved in interaction with DNA (Other category). Residue numbers for some of the important residues are shown. The two models are related to one another by a 90° rotation around the vertical axis.
DISCUSSION
Model for activation of rhaBAD expression.
Our original hypothesis for the mechanism of transcription activation at the rhaBAD promoter was that RhaS activation would not require α-CTD but would occur by another mechanism such as interaction with ς70. We expected that CRP activation would involve contacts with α-CTD; hence, α-CTD would be required for full activation. Overall, our results indicate that α-CTD is required for full activation of rhaBAD and that at least some of the α-CTD activation involves interaction with DNA. We have some evidence that α-CTD activation, at least in the absence of CRP, may involve contacts with RhaS. CRP activation of rhaBAD expression appears to occur by a mechanism other than interaction with α-CTD.
Role of CRP AR1 and AR2 in rhaBAD expression.
While substitution of some residues in CRP AR1 and AR2 resulted in small defects in rhaBAD expression, we feel that these small AR1 and AR2 defects may not indicate loss of the same interactions found at simple class I and class II CRP-dependent promoters. In fact, the defects were so small that they may simply be the result of small effects on the stability or structure of the CRP proteins. While alanine substitution of CRP T158 resulted in a 40-fold at a synthetic class I promoter (35), this substitution had no defect at rhaBAD, suggesting that AR1 may not be important at rhaBAD. The small defect upon substitution of CRP G162 leaves open the possibility that CRP activation of rhaBAD could involve AR1 contacts with α-CTD; however, the evidence for such an interaction is not strong.
CRP AR2 is proposed to interact with the α subunit N-terminal domain at class II CRP-dependent promoters (21). CRP is not bound adjacent to RNAP at rhaBAD; hence, it is difficult to imagine an identical interaction between CRP AR2 and α-NTD. We do not believe that an overlapping class II CRP-dependent promoter contributes in any significant manner to rhaBAD activation since expression from this promoter is extremely low (0.01 U) in the absence of RhaS (under conditions where CRP would be expected to activate if it could). However, it is possible that amino acids in or near AR2 interact with other proteins involved in rhaBAD activation or, as mentioned above, that these substitutions have a small effect on the stability or overall structure of CRP.
Is α-CTD important for rhaBAD activation?
The overall conclusion from our analysis of the importance of α-CTD in rhaBAD activation is that some activation can occur in the absence of α-CTD, but maximal activation requires the contribution of α-CTD (Table 5). Two alternative mechanisms could explain this partial dependence on α-CTD. First, the only contribution of α-CTD to activation could be by interaction with DNA, and all of the activation signals that CRP and RhaS transmit to RNAP could be transmitted by α-CTD-independent mechanisms. Alternatively, α-CTD could be one of two or more sites for transmission of information from RhaS and/or CRP to RNAP.
Does activation by α-CTD involve DNA contacts?
Our assays identified five (positions 265, 268, 296, 298, and 299) of the seven residues within the α-CTD 265 determinant which has been shown to be required for DNA binding (11, 19; reviewed in reference 6). We also found a variety of other residues that were defective at both of the tested promoters (Table 6), most of which lie very near the 265 determinant and are likely to be required for full DNA binding (Fig. 3). Hence, we conclude that α-CTD contacts with DNA are important for full activation at both the full-length [Φ(rhaB-lacZ)Δ110] and truncated [Φ(rhaB-lacZ)Δ84] promoters (Table 6 and Fig. 2 and 3).
Does activation by α-CTD involve contacts with RhaS?
We were surprised to find that activation at a promoter including only the RhaS-binding site [Φ(rhaB-lacZ)Δ84] was greatly dependent on α-CTD (54-fold; Table 5). This result indicates that α-CTD makes productive interactions, at least with DNA, at the Φ(rhaB-lacZ)Δ84 promoter. However, since the defect upon expression of α-Δ235 required rhaS+, it is possible that α-CTD also interacts with RhaS. This dependence of α-CTD activation on RhaS could also be explained by other mechanisms. For example, it is possible that RhaS binding shifts α-CTD to a stronger DNA site or that RNAP is capable of little to no rhaBAD transcription initiation in the absence of RhaS (recall the low rhaBAD expression in the absence of RhaS) (Table 6). In this second model, RhaS would overcome the rate-limiting step in initiation of rhaBAD transcription, and only then could α-CTD contribute to activation. While Φ(rhaB-lacZ)Δ84 has an unnatural promoter, assays of this fusion are relevant because they may provide clues to the activation mechanism at the full-length promoter and/or insight into the mechanism of activation by AraC family proteins that function without the aid of a second activator such as CRP.
Finally, we identified a region of α-CTD that has not been identified as important for interaction with either CRP or UP elements (reviewed in reference 5). These residues (positions 278, 279, 315, 321, 322, and 323) were only important at the promoter which was activated by RhaS in the absence of CRP [Φ(rhaB-lacZ)Δ84] (Fig. 2 and Table 6) and are located within helix 2 and the C-terminal loop of α-CTD. All of the residues we identified within the C-terminal loop of α-CTD except residue 321 have been suggested to define α-CTD contacts with other activators. Residue 315 is a part of the “287 determinant” of α-CTD that is believed to contact CRP (6, 29) and is also important for activation by FNR (33). Activation by MerR requires residue 323 (7), and both residues 322 and 323 have been identified as important for OmpR activation (30). Since residues 315, 321, 322, and 323 were only important at Φ(rhaB-lacZ)Δ84, where the only activator protein is RhaS, it is possible that at least some of them define an interaction between α-CTD and RhaS.
Does activation by α-CTD involve contacts with CRP?
As mentioned above, it was not possible to test the contribution of α-CTD to CRP activation in the absence of RhaS since CRP does not activate under such conditions. Instead, we must draw conclusions about CRP activation by comparing results at the Φ(rhaB-lacZ)Δ84 and Φ(rhaB-lacZ)Δ110 fusions. Expression of α-Δ235 in a strain carrying a Φ(rhaB-lacZ)Δ110 fusion resulted in a smaller defect than that found at Φ(rhaB-lacZ)Δ84. This smaller defect may indicate that CRP displaces α-CTD to a weaker DNA site, thereby reducing the ability of α-CTD to contribute to activation. Alternatively, the smaller defect could indicate that the roles of α-CTD and CRP in rhaBAD activation are partially redundant. Several of our results are consistent with this. First, the fold activation by α-CTD increased in the absence of CRP [compare the defects upon α-CTD deletion at Φ(rhaB-lacZ)Δ110, 13-fold, and Φ(rhaB-lacZ)Δ84, 54-fold]. Second, the importance of CRP increased in the absence of α-CTD (compare the defects upon deleting the CRP-binding site with wild-type α, 38-fold, and with expression of α-Δ235, 162-fold). This increased activation by CRP and α-CTD in the absence of the other is the opposite of what would be expected if CRP activation required contacts with α-CTD.
Our assays utilizing the α-CTD alanine library also provided no evidence to support an interaction between α-CTD and CRP. We identified only one (residue 315) of the eight residues that make up the α-CTD 287 determinant (6), which is proposed to be the site of interaction with CRP AR1. At rhaBAD this residue was only important at the truncated (RhaS-binding site only) promoter. Although our results suggested that the 287 determinant was not important at rhaBAD, it was also possible that different residues were required for this α-CTD–CRP interaction. However, only one residue in α-CTD (residue 301) was defective at the full-length promoter but not the truncated promoter, and this residue is located near the α-CTD DNA-binding determinant. So, while we cannot rule out an interaction between α-CTD and DNA, none of our results support such an interaction.
ACKNOWLEDGMENTS
We thank the members of our laboratory for critical discussions and Prasanna Bhende for comments on the manuscript. We thank Richard Gourse for the generous gift of the α-CTD alanine substitution library. We thank the University of Kansas Biochemical Research Service Laboratory for help with automated DNA sequencing.
This work was supported by Public Health Service grant GM55099 from the National Institute of General Medical Sciences, the National Science Foundation under grant no. EPS-9550487, and matching support from the state of Kansas, a General Research Fund award from the University of Kansas, and the Franklin Murphy Molecular Biology Endowment (all to S.M.E.).
REFERENCES
- 1.Backman K, Chen Y-M, Magasanik B. Physical and genetic characterization of the gln A-glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci USA. 1981;78:3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhende P M, Egan S M. Amino acid-DNA contacts by RhaS: an AraC family transcription activator. J Bacteriol. 1999;181:5185–5192. doi: 10.1128/jb.181.17.5185-5192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatter E E, Ross W, Tang H, Gourse R L, Ebright R H. Domain organization of RNA polymerase α subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell. 1994;78:889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- 4.Buchet A, Eichler K, Mandrand-Berthelot M. Regulation of the carnitine pathway in Escherichia coli: investigation of the cai-fix divergent promoter region. J Bacteriol. 1998;180:2599–2608. doi: 10.1128/jb.180.10.2599-2608.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busby S, Ebright R H. Transcription activation at class II CAP-dependent promoters. Mol Microbiol. 1997;23:853–859. doi: 10.1046/j.1365-2958.1997.2771641.x. [DOI] [PubMed] [Google Scholar]
- 6.Busby S, Ebright R H. Transcription activation by catabolite activator protein (CAP) J Mol Biol. 1999;293:199–213. doi: 10.1006/jmbi.1999.3161. [DOI] [PubMed] [Google Scholar]
- 7.Caslake L F, Ashraf S I, Summers A O. Mutations in the alpha and sigma-70 subunits of RNA polymerase affect expression of the mer operon. J Bacteriol. 1997;179:1787–1795. doi: 10.1128/jb.179.5.1787-1795.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebright R H. Transcription activation at class I CAP-dependent promoters. Mol Microbiol. 1993;8:797–802. doi: 10.1111/j.1365-2958.1993.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 9.Egan S M, Schleif R F. DNA-dependent renaturation of an insoluble DNA binding protein. Identification of the RhaS binding site at rhaBAD. J Mol Biol. 1994;243:821–829. doi: 10.1006/jmbi.1994.1684. [DOI] [PubMed] [Google Scholar]
- 10.Egan S M, Schleif R F. A regulatory cascade in the induction of rhaBAD. J Mol Biol. 1993;234:87–98. doi: 10.1006/jmbi.1993.1565. [DOI] [PubMed] [Google Scholar]
- 11.Gaal T, Ross W, Blatter E E, Tang H, Jia X, Krishnan V V, Assa-Munt N, Ebright R H, Gourse R L. DNA-binding determinants of the α subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 1996;10:16–26. doi: 10.1101/gad.10.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Gallegos M-T, Schleif R, Bairoch A, Hofmann K, Ramos J L. AraC/XylS family of transcriptional regulators. Microbiol Mol Biol Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottesman M E, Yarmolinsky M B. The integration and excision of the bacteriophage lambda genome. Cold Spring Harbor Symp Quant Biol. 1968;33:735–747. doi: 10.1101/sqb.1968.033.01.084. [DOI] [PubMed] [Google Scholar]
- 14.Igarishi K, Ishihama A. Bipartite functional map of the E. coli RNA polymerase α subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell. 1991;65:1015–1022. doi: 10.1016/0092-8674(91)90553-b. [DOI] [PubMed] [Google Scholar]
- 15.Jeon Y H, Negishi T, Shirakawa M, Yamazaki T, Fujita N, Ishihama A, Kyogoku Y. Solution structure of the activator contact domain of the RNA polymerase α subunit. Science. 1995;270:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- 16.Maloy S R, Stewart V J, Taylor R K. Genetic analysis of pathogenic bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 17.Merkel T J, Dahl J L, Ebright R E, Kadner R J. Transcription activation at the Escherichia coli uhpT promoter by the catabolite gene activator protein. J Bacteriol. 1995;177:1712–1718. doi: 10.1128/jb.177.7.1712-1718.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 19.Murakami K, Fujita N, Ishihama A. Transcription factor recognition surface on the RNA polymerase α subunit is involved in contact with the DNA enhancer element. EMBO J. 1996;15:4358–4367. [PMC free article] [PubMed] [Google Scholar]
- 20.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu W, Kim Y, Tau G, Heyduk T, Ebright R H. Transcription activation at class II CAP-dependent promoters: Two interactions between CAP and RNA polymerase. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu W, Zhou Y, Dong Q, Ebright Y W, Ebright R H. Characterization of the activation region of Escherichia coli catabolite gene activator protein (CAP) I. Saturation and alanine-scanning mutagenesis. J Mol Biol. 1994;243:595–602. doi: 10.1016/0022-2836(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 23.Olekhnovic I N, Kadner R J. RNA polymerase α and ς70 subunits participate in transcription of the Escherichia coli uhpT promoter. J Bacteriol. 1999;181:7266–7273. doi: 10.1128/jb.181.23.7266-7273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozoline O N, Fujita N, Ishihama A. Transcription activation mediated by the carboxyl-terminal domain of the RNA polymerase α-subunit. J Biol Chem. 2000;275:1119–1127. doi: 10.1074/jbc.275.2.1119. [DOI] [PubMed] [Google Scholar]
- 25.Plumbridge J. Induction of the nag regulon of Escherichia coli by N-acetylglucosamine and glucosamine: role of the cyclic AMP-catabolite activator protein complex in expression of the regulon. J Bacteriol. 1990;172:2728–2735. doi: 10.1128/jb.172.5.2728-2735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plumbridge J, Kolb A. DNA bending and expression of the divergent nagE-B operons. Nucleic Acids Res. 1998;26:1254–1260. doi: 10.1093/nar/26.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodius V A, West D M, Webster C L, Busby S J W, Savery N J. Transcription activation at class II CRP-dependent promoters: the role of different activating regions. Nucleic Acids Res. 1997;25:326–332. doi: 10.1093/nar/25.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richet E, Sogaard-Andersen L. CRP induces the repositioning of MalT at the Escherichia coli malKp promoter primarily through DNA bending. EMBO J. 1994;13:4558–4567. doi: 10.1002/j.1460-2075.1994.tb06777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savery N J, Lloyd G S, Kainz M, Gaal T, Ross W, Ebright R H, Gourse R L, Busby S J W. Transcription activation at class II CRP-dependent promoters: identification of determinants in the C-terminal domain of the RNA polymerase α-subunit. EMBO J. 1998;17:3439–3447. doi: 10.1093/emboj/17.12.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slauch J M, Russo F D, Silhavy T J. Suppressor mutations in rpoA suggest that OmpR controls transcription by direct interaction with the α subunit of RNA polymerase. J Bacteriol. 1991;173:7501–7510. doi: 10.1128/jb.173.23.7501-7510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang H, Severinov K, Goldfarb A, Fenyo D, Chait B, Ebright R H. Location, structure, and function of the target of a transcription activator protein. Genes Dev. 1994;8:3058–3067. doi: 10.1101/gad.8.24.3058. [DOI] [PubMed] [Google Scholar]
- 32.West D, Williams R, Rhodius V, Bell A, Sharma N, Zou C, Fujita N, Ishihama A, Busby S. Interactions between the Escherichia coli cyclic AMP receptor protein and RNA polymerase at class II promoters. Mol Microbiol. 1993;10:789–797. doi: 10.1111/j.1365-2958.1993.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 33.Williams S M, Savery N J, Busby S J, Wing H J. Transcription activation at class I FNR-dependent promoters: identification of the activating surface of FNR and the corresponding contact site in the C-terminal domain of the RNA polymerase α subunit. Nucleic Acids Res. 1997;25:4028–4034. doi: 10.1093/nar/25.20.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Schleif R. Catabolite gene activator protein mutations affecting activity of the araBAD promoter. J Bacteriol. 1998;180:195–200. doi: 10.1128/jb.180.2.195-200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Merkel T J, Ebright R H. Characterization of the activation region of Escherichia coli catabolite gene activator protein (CAP). II. Role at class I and class II CAP-dependent promoters. J Mol Biol. 1994;243:603–610. doi: 10.1016/0022-2836(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Pendergrast P S, Bell A, Williams R, Busby S, Ebright R H. The functional subunit of a dimeric transcription activator protein depends on promoter architecture. EMBO J. 1994;13:4549–4557. doi: 10.1002/j.1460-2075.1994.tb06776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]