Abstract
Immune checkpoint inhibitors have revolutionized the treatment paradigm of several cancers. However, not all patients respond to treatment. Tumor cells reprogram metabolic pathways to facilitate growth and proliferation. This shift in metabolic pathways creates fierce competition with immune cells for nutrients in the tumor microenvironment and generates by-products harmful for immune cell differentiation and growth. In this review, we discuss these metabolic alterations and the current therapeutic strategies to mitigate these alterations to metabolic pathways that can be used in combination with checkpoint blockade to offer a new path forward in cancer management.
Keywords: immune checkpoint inhibitors, glucose metabolism, amino acid metabolism, lipid metabolism, adenosine pathway
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have significantly changed the treatment landscape of various cancers.[1] However, only 20–40% of patients respond to immunotherapy due to complex resistance mechanisms.[2] This is because malignant cells continue to evolve and use different strategies to evade the immune system and inhibit the immune response. Metabolic reprogramming is one such strategy adopted by tumor cells to provide for the increased metabolic demand of the rapidly proliferating tumor cells.[3] These changes in tumor metabolism not only promote tumor cell growth but also induce immune suppression by competing with cytotoxic T cells and NK cells for metabolic requirements and nutrients in the tumor microenvironment (TME).[4] This competition for resources limits the immune protective capabilities of the immune cells. In addition, accumulation of metabolic by-products such as lactate produces an immunosuppressive TME, which facilitates tumor progression and metastasis. As such, metabolic reprogramming is considered to be a hallmark of malignancy.[3] An understanding of how these alterations to metabolic pathways in the TME affects tumor and immune cells will shed light on the mechanisms of resistance to ICIs. In this review, we discuss the metabolic changes in tumor cells that have led to identification of therapeutic targets and development of therapeutic strategies for treatment of cancer that are currently under investigation.
METABOLIC REPROGRAMMING IN TUMOR CELLS
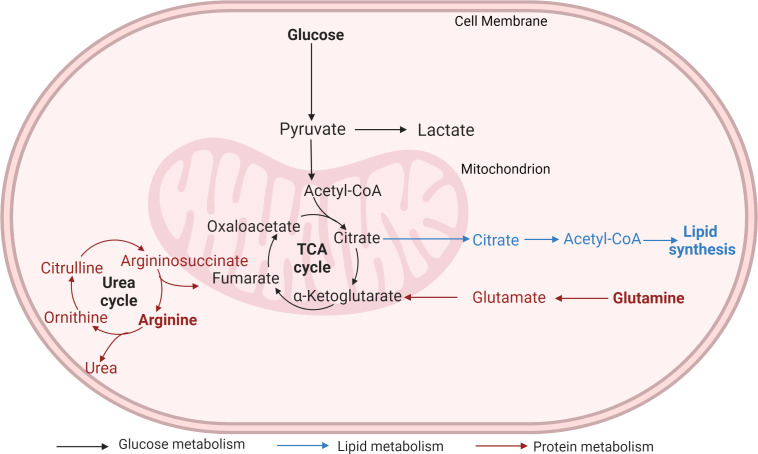
Tumor cells hijack metabolic pathways (Fig. 1) to meet the energy and nutrient requirements of the rapidly proliferating cells.
Figure 1.
Metabolic pathways hijacked by cancer cells.
acetyl-CoA, acetyl coenzyme A; TCA, tricarboxylic acid. Created with BioRender.com.
Glucose Metabolism
Glucose is the primary nutrient required for cellular growth and proliferation. In a normal resting state, quiescent cells derive energy through oxidative phosphorylation of glucose (OXPHOS).[5] Briefly, glucose undergoes glycolysis in the cytoplasm and is metabolized to pyruvate. Under aerobic conditions, pyruvate is transported into the mitochondria, where it is transformed to acetyl-CoA by pyruvate dehydrogenase complex. Oxidation of acetyl-CoA via the tricarboxylic acid (TCA) cycle releases 36 molecules of adenosine triphosphate (ATP). However, under anaerobic conditions, oxidation of glucose yields only two molecules of ATP as pyruvate is reduced to lactate by lactate dehydrogenase in the cytosol.
In rapidly proliferating cancer cells, energy is primarily derived through aerobic glycolysis.[6] Paradoxically, even in the presence of oxygen, only 5% of pyruvate enters the TCA cycle due to inactivation of the pyruvate dehydrogenase complex by pyruvate dehydrogenase kinase (PDK) to produce four molecules of ATP, while most of the pyruvate is converted into large amounts of lactate.[7] Although aerobic glycolysis is not as energy efficient as OXPHOS, most cancer cells prefer to metabolize glucose by aerobic glycolysis rather than OXPHOS. This is because aerobic glycolysis not only provides energy but also provides important precursors for the synthesis of amino acids, fatty acids, and nucleotides required for cellular proliferation.[8] On the contrary, although OXPHOS provides more energy through complete oxidation of glucose into carbon dioxide and water, it does not provide the building blocks for nucleic acid synthesis required for formation of new cells. Furthermore, glycolytic phenotype, which is believed to be an initial adaptation to hypoxia in the TME, results in emergence of tumor cells with a powerful growth advantage to overcome environmental constraints.[9] Additionally, acidity of the TME due to increased lactate production causes degradation of the extracellular matrix and promotes angiogenesis, facilitating tumor invasion and metastasis. Due to these advantages conferred by aerobic glycolysis, cancer cells prefer to switch from mitochondrial respiration to aerobic glycolysis. This phenomenon is termed the Warburg effect, named after Otto Warburg, who first reported it in the 1920s.[7] Thus, glycolysis provides the energy and the carbon required for new cell production.
Amino Acid Metabolism
Glutamine is the most abundant amino acid in the body. Cancer cells depend on glutamine for several metabolic processes, such as synthesis of nucleotides, lipids and other nonessential amino acids, and maintenance of redox balance.[10] As glutamine is indispensable, cancer cells have increased glutamine uptake and are its greatest consumers,[11] to the extent that some cancer cells cannot survive without glutamine and are in a sense “addicted” to it. Oxidation of glutamine via glutaminolysis fuels the TCA cycle to generate cellular energy.[12] Influx of glutamine into the cell is mediated via the SLC1A5 transporter and is converted to glutamate by glutaminase.[13] Glutamate is then converted to α-ketoglutarate by either glutamate dehydrogenase or aminotransferases. Action of glutamate dehydrogenase on glutamate releases NADH (reduced form of nicotinamide adenine dinucleotide [NAD]) or NADPH (reduced form of nicotinamide adenine dinucleotide phosphate [NADP]), while action of aminotransferases facilitates synthesis of nonessential amino acids. Thus, glutamine serves as the nitrogen source for amino acid and nucleotide biosynthesis. α-Ketoglutarate then enters the TCA cycle. The metabolic intermediaries of glutaminolysis, such as malate, aspartate, and citrate are exported to cytoplasm to promote amino acid, nucleotide, and lipid synthesis. Thus, a growing body of evidence suggests that upregulated SLC1A5 expression in many cancers favors glutamine uptake and use by tumor cells,[14] and increased expression of glutaminase in hepatocellular carcinoma and colorectal and breast cancer is associated with high-grade tumors.[10]
Arginine is another nonessential amino acid, which is primarily synthesized endogenously in healthy adults and is also supplemented in the diet.[15] It regulates several immunological processes, including proliferation of effector T cells and natural killer cells,[16] and modulates signaling pathways.[17] Arginase and isoforms of nitric oxide synthase (NOS) are the main enzymes involved in the metabolism of arginine. However, their effect on normal cells is different from that on tumor cells. Under normal conditions, hydrolysis of arginine by arginase produces urea and ornithine, which is a precursor to several compounds, such as proline and polyamines, that are required for collagen synthesis and cellular proliferation.[15] Nitric oxide and citrulline are metabolites derived by the action of NOS. Nitric oxide exhibits a wide range of tumoricidal effects by suppression of cellular respiration and DNA synthesis, upregulation of p53, and expression of apoptosis-associated proteins.[18] However, unregulated synthesis of NO is associated with tumor-promoting events as evidenced by expression of NOS in cervical, breast, central nervous system, laryngeal, and head and neck cancers. Similarly, arginase-expressing tumor myeloid cells were found in abundance in lung, gastrointestinal tract, and bladder cancer, causing arginine depletion and inhibition of T-cell function.[15,16] Furthermore, 70% of tumors lose their ability to synthesize arginine, making them dependent on exogenous arginine.[19] Thus, arginine deprivation is being investigated as a therapeutic strategy.
Lipid Metabolism
Fatty acid synthesis is heavily dependent on glucose and glutamine metabolism for its precursors.[8] The carbon required for fatty acid synthesis is provided by intermediaries released during glucose and glutamine metabolism in the mitochondria. Citrate formed by the condensation of acetyl-CoA and oxaloacetate in the TCA cycle is exported from the mitochondria to the cytosol by mitochondrial citrate transporter SLC25A1.[20] In the cytosol, the enzyme ATP citrate lyase breaks down citrate to oxaloacetate and acetyl-CoA, which is then used as the carbon source for the synthesis of palmitate and other fatty acids required for synthesis of new cell membranes of the proliferating cells. Thus, there is increased consumption of citrate by the rapidly proliferating cancer cells.[21] At the same time, as cancer cells use aerobic glycolysis for generation of energy due to the Warburg effect,[7] mitochondrial production of citrate is reduced. Thus, the reduced citrate production and increased consumption results in low citrate levels in the cytosol, favoring glycolysis and tumor growth.[21,22]
Adenosine Pathway
Adenosine is an immunosuppressive metabolite found in small quantities in extracellular fluid. Large amount of ATP is released into the extracellular fluid in response to inflammation, hypoxia, ischemia, and mechanical stress[23] through transport channels, exocytosis, or direct release through cell damage.[24] Extracellular ATP triggers an immune response and is subsequently catabolized by ectoenzymes CD39 and CD73. CD39 hydrolyses ATP to adenosine monophosphate (AMP), and AMP is dephosphorylated to adenosine by CD73.[25] Extracellular adenosine initially protects cells from excessive inflammatory damage, but a sustained increase in adenosine promotes immune suppression and tissue fibrosis, allowing tumor cells to proliferate.[26] Both CD39 and CD73 are upregulated in tumor cells and can be further increased in the hypoxic condition in the TME.[24]
Extracellular adenosine can bind to four G-protein–coupled receptors, A1, A2A, A2B, and A3.[24] Adenosine receptor signaling via A1 and A3 receptors inhibits adenylyl cyclase, while signaling via A2A and A2B receptors stimulates adenylyl cyclase, which decreases or increases intracellular cyclic AMP levels, respectively.[23] Stimulation of A2A and A2B receptors blunts the immune response through several mechanisms,[25] such as impaired maturation of dendritic cells and suppression of CD8+ T-cell and NK-cell cytotoxic activity, while promoting the proliferation of other immunosuppressive cells and emergence of immune checkpoints.[27] Stimulation of A2AR can result in the development of Treg cells with high levels of CD39 and CD73 expression. CD73 inhibits tumor apoptosis and aids tumor cell invasion by degrading the extracellular matrix. Similarly, A2BR activation results in differentiation of macrophages to M2 phenotype[28] and enhanced angiogenesis through vascular endothelial growth factor (VEGF) production by myeloid-derived suppressor cells recruited to the tumor.[29] Thus, adenosine pathway inhibitors may be a viable treatment option for the management of cancer.
CROSS TALK BETWEEN MOLECULAR SIGNALING AND METABOLIC PATHWAYS
Several signaling pathways have been implicated in carcinogenesis. These aberrant signaling pathways induce metabolic reprogramming in cancer cells such that the nutrient uptake and metabolism favor cellular proliferation and confer survival advantage.[8] Moreover, this process results in buildup of waste materials, such as lactate, that will dampen the effector functions of tumor-infiltrating lymphocytes and support the differentiation of macrophages to the M2 phenotype that promotes tumor growth and metastasis.[30] As factors affecting cellular proliferation and cellular metabolism are interrelated, an understanding of these factors is critical for identification of therapeutic targets.
Hypoxia-Inducible Factors
Hypoxia-inducible factors (HIFs), HIF-1α and HIF-2α, are transcription factors that regulate the cellular responses to hypoxic conditions. Due to rapid proliferation of cancer cells, the tumor outgrows its blood supply. The resultant hypoxia causes stabilization of HIF-1α, which induces expression of glucose transporters and glycolytic enzymes that orchestrates the switching from mitochondrial metabolism to glycolysis.[30] HIF-1 also directly activates the genes encoding PDKs, which inhibits the entry of pyruvate into the TCA cycle.[31] Thus, HIF-1 initiates the Warburg effect.[32] HIF-1 favors neovascularization through upregulation of VEGF. HIF-1 proteins are expressed in several tumors, such as bladder, brain, breast, colon, ovarian, pancreatic, prostate, and renal cell carcinoma (RCC).[33] HIF-1 expression can be upregulated by oncogenes such as Myc,[34] activation of the phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR)[35] and RAS pathways,[36] loss of tumor suppressers such as Von Hippel-Lindau (VHL),[37] or accumulation of lactate. HIF-2α, which shares the functional characteristics of HIF-1α, is overexpressed in patients with Von Hippel-Lindau (VHL) disease. In response to chronic hypoxia in cancer cells, HIF-2α promotes angiogenesis through upregulation of VEGF in vascular endothelial cells.[38] It further promotes metastasis and invasion contributing to the aggressive nature of the malignant cells.[39] Because the HIF pathway plays an important role in metabolic reprogramming in cancer cells and promotes angiogenesis, several therapeutic strategies targeting this pathway are under investigation.
Pyruvate Dehydrogenase Kinase
PDK is a mitochondrial enzyme that regulates the switch between OXPHOS and aerobic glycolysis. The PDKs limit the action of pyruvate dehydrogenase complexes required for complete oxidation of pyruvate in the mitochondria via the TCA cycle to release 36 molecules of ATP.[7] Thus, PDKs prevent pyruvate from entering into the TCA cycle (mitochondrial respiration) but instead promote fermentation of pyruvate to lactate in the cytosol (aerobic glycolysis).[5] PDKs are overexpressed in several cancers, such as breast, colon, gastric, head and neck, liver, lung, and renal cancer, and melanoma, myeloma, and glioblastoma. Overexpression of PDK is associated with aggressive tumors and poor prognosis; therefore, targeting the PDKs is a potential therapeutic strategy.
Oncogenes
Oncogenes are known to regulate certain enzymes or metabolic processes that promote glucose and amino acid uptake and metabolism to provide proteins, lipids, and nucleotides for the proliferating new cells.
MYC
The MYC oncogene, which encodes for c-Myc, a transcription factor, is considered to be the master regulator as metabolic reprogramming and cellular proliferation is mostly driven by c-Myc–regulated genes.[40,41] Myc induces expression of glucose transporters and glycolytic enzymes, favoring increased glucose uptake and glycolysis.[41] Like HIF-1, Myc promotes enhanced conversion of pyruvate to lactate by inducing expression of PDK1, which prevents pyruvate from entering the TCA cycle.[42] Taken together with HIF-1, Myc also induces VEGF, promoting angiogenesis. MYC also upregulates SLC1A5 expression, promoting glutamine entry into the cell.[41] Furthermore, Myc upregulates glutaminase expression leading to increased glutaminolysis, eventually leading to glutamine addiction. Myc also promotes lipid synthesis by its direct action on the enzymes driving the TCA cycle, thereby promoting citrate production required for fatty acid synthesis. Myc also upregulates expression of ATP citrate lyase and fatty acid synthase to facilitate breakdown of cytosolic citrate to the acetyl-CoA required for fatty acid synthesis.[43] MYC is upregulated in several cancers, such as colon, breast, prostate, and bladder cancer, and is associated with aggressive tumors.[44] Growth factor–responsive signal transduction pathways, such as PI3K/AKT/mTOR signaling, enhances MYC activity.[34] As Myc upregulates all pathways contributing to synthesis of substrates required for formation of new cells, it serves as a promising target for development of novel therapeutics.
PI3K/AKT/mTOR Pathway
The PI3K pathway is the most dysregulated in human cancers.[45] On aberrant activation, downstream AKT signaling augments glucose uptake by increasing the expression of glucose transporters and promotes aerobic glycolysis by upregulating glycolytic enzymes such as hexokinase 2 and PFKB3.[46–48] Furthermore, AKT inhibits pyruvate dehydrogenase complex and promotes fermentation of pyruvate to lactate. Additionally, AKT, by its action on downstream transcription factors such as HIF-1, MYC, sterol regulatory element-binding protein (SREBP), and Forkhead box O (FOXO), regulates the expression of metabolic enzymes that contribute to fatty acid and nucleotide synthesis.[48] For example, AKT activates SREBP-1c, which in turn upregulates ATP citrate lyase and fatty acid synthase, promoting break down of citrate in the cytosol to oxaloacetate and acetyl-CoA, the substrate required for fatty acid synthesis.[43,49] AKT, through activation of the mTORC1 pathway, induces glycolysis and promotes lipid and nucleotide synthesis, enabling proliferation of cancer cells.[41] Downstream of PI3K, mTOR signaling promotes transcription of HIF-1, which upregulates the expression of glucose transporter GLUT-1 promoting glycolysis[50] and expression of SLC1A5, increasing the use of glutamine.[41] Thus, activation of the PI3K/AKT/mTOR pathway promotes cellular proliferation and metastasis.[51] The PI3K pathway is inhibited by phosphatase and tensin homologue (PTEN), a tumor suppression gene. Therefore, PTEN loss promotes glycolysis.[52] Dysregulation of the PI3K pathway induces metabolic reprogramming of cancer cells at several levels, which could be therapeutically targeted.
RAS Pathway
As molecular alterations in the RAS pathway signal through PI3K pathway, most of the metabolic effects such as upregulation of glucose transporters are mediated through the PI3K/AKT/mTOR pathway,[53] while others are mediated through Myc and MAPK pathways.[54]
Tumor Suppressor Genes
Tumor suppressor proteins control metabolic pathways by mechanisms similar to oncogenes.
TP53
p53, a commonly mutated gene in cancer, is a key regulatory factor that maintains the balance between use of mitochondrial respiration and aerobic glycolysis for supply of energy.[55] p53 promotes OXPHOS through upregulation of synthesis of cytochrome c oxidase 2 (SCO2) and downregulates expression of glucose transporters.[56] p53 inhibits glycolysis through upregulation of hexokinase and TP53-induced glycolysis and apoptosis regulator (TIGAR), both of which negatively regulate glycolysis.[57] Furthermore, p53 inhibits glycolysis by upregulation of PTEN, a negative regulator of the PI3K pathway.[58]
Other Suppressor Genes
LKB1, PTEN, and VHL are other tumor suppressor genes that modulate metabolic pathways by promoting glycolysis, inhibiting OXPHOS, and inhibiting lipid synthesis, along with many other metabolic alterations with the goal to suppress tumor growth.[37,58–60]
An understanding of the metabolic pathways and their interactions with oncogenes and tumor suppressor genes sheds light on the ability of tumor cells to propagate proliferation and growth by evading and even suppressing immune cells in the TME. These metabolic pathways are potential targets for therapeutic interventions to overcome resistance to immune checkpoint blockade. Currently, drugs targeting metabolic pathways are being evaluated in combination with ICIs (Table 1). However, their activity may be at the cost of increased and compounded toxicities associated with combination therapies.
Table 1.
Therapeutic agents used in combination with immune checkpoint inhibitors (ICIs) to target metabolic pathways
|
Target and Drug
|
Combination with ICI
|
Indication
|
ClinicalTrials.gov Identifier(s)
|
Clinical Trial Status*
|
| mTOR inhibitor | ||||
| Metformin | Nivolumab | Non–small cell lung cancer | NCT03048500 | Active, not recruiting |
| Metformin | Nivolumab | Refractory microsatellite stable colorectal cancer | NCT03800602 | Active, not recruiting |
| Metformin | Pembrolizumab | Head and neck squamous cell cancer | NCT04414540 | Recruiting |
| Metformin | Pembrolizumab | Melanoma | NCT03311308 | Recruiting |
| Metformin | Durvalumab | Head and neck squamous cell cancer | NCT03618654 | Active, not recruiting |
| HIF-2α inhibitor | ||||
| Belzutifan | Pembrolizumab | Postnephrectomy clear cell renal cell carcinoma | NCT05239728 | Recruiting |
| Glutaminase inhibitor | ||||
| CB-839 (telaglenastat) | Nivolumab | Melanoma | NCT02771626 | Completed |
| Clear cell renal cell carcinoma | ||||
| Non–small cell lung cancer | ||||
| Glutamine antagonist | ||||
| DRP-104 (sirpiglenastat) | Atezolizumab | Advanced solid tumors | NCT04471415 | Recruiting |
| Arginase inhibitor | ||||
| CB-1158 (INCB001158; numidargistat) | Pembrolizumab | Solid tumors | NCT02903914 | Completed |
| Arginine depletion | ||||
| AEB1102 (pegzilarginase) | Pembrolizumab | Small cell lung cancer | NCT03371979 | Completed |
| A2AR antagonist | ||||
| CPI-444 (ciforadenant) | Atezolizumab | Renal cell cancer | NCT02655822 | Completed |
| Metastatic castration-resistant prostate cancer | ||||
| CPI-444 (ciforadenant) | Ipilimumab, nivolumab | Renal cell cancer | NCT05501054 | Not yet recruiting |
| AZD4635 (imaradenant) | Durvalumab | Solid tumors | NCT02740985 | Active, not recruiting |
| Non–small cell lung cancer | ||||
| Metastatic castration-resistant prostate cancer | ||||
| AZD4635 (imaradenant) | Durvalumab, cabazitaxel | Metastatic castration-resistant prostate cancer | NCT04495179 | Completed |
| NIR178 (taminadenant) | Spartalizumab | Solid tumors | NCT03207867 | Active, not recruiting |
| NIR178 (taminadenant) | Spartalizumab | Non–small cell lung cancer | NCT02403193 | Completed |
| EOS100850 (inupadenant) | EOS-448, dostarlimab, Pembrolizumab | Solid tumors | NCT05060432 | Recruiting |
| NCT05054348 | ||||
| A2A and A2B antagonist | ||||
| AB928 (etrumadenant) | Atezolizumab, chemotherapy | Pancreatic ductal adenocarcinoma | NCT03193190 | Recruiting |
| AB928 (etrumadenant) | AB122 (zimberelimab), radiotherapy, consolidation chemotherapy | Rectal cancer | NCT05024097 | Recruiting |
| AB928 (etrumadenant) | AB122 (zimberelimab), AB154 (domvanalimab) | Non–small cell lung cancer | NCT04791839 | Recruiting |
| AB928 (etrumadenant) | AB122 (zimberelimab), pemetrexed | Methylthioadenosine phosphorylase-deficient urothelial carcinoma | NCT05335941 | Not yet recruiting |
| AB928 (etrumadenant) | AB122 (zimberelimab), SRF617 | Metastatic castration-resistant prostate cancer | NCT05177770 | Recruiting |
| AB928 (etrumadenant) | Pembrolizumab, carboplatin/pemetrexed | Non–small cell lung cancer | NCT03846310 | Active, not recruiting |
| CD73 inhibitor | ||||
| CPI-006 | Pembrolizumab | Solid tumors | NCT03454451 | Active, not recruiting |
| MEDI9447 (oleculmab) | Durvalumab | Solid tumors | NCT02503774 | Active, not recruiting |
| MEDI9447 (oleculmab) | Durvalumab | Sarcoma | NCT04668300 | Recruiting |
| MEDI9447 (oleculmab) | Durvalumab | Bladder cancer | NCT03773666 | Completed |
| MEDI9447 (oleculmab) | Durvalumab | Non–small cell lung cancer | NCT05221840 | Recruiting |
| MEDI9447 (oleculmab) | Durvalumab, paclitaxel, carboplatin | Triple-negative breast cancer | NCT03616886 | Active, not recruiting |
| MEDI9447 (oleculmab) | Durvalumab, gemcitabine, nab-paclitaxel | Pancreatic cancer | NCT04940286 | Recruiting |
| BMS-986179 | Nivolumab | Solid tumors | NCT02754141 | Completed |
| TJ004309 (uliledlimab) | Atezolizumab | Solid tumors | NCT03835949 | Active, not recruiting |
| TJ004309 (uliledlimab) | Atezolizumab | Ovarian cancer, head and neck cancer, non–small cell lung cancer, gastrointestinal cancer, triple-negative breast cancer | NCT05001347 | Recruiting |
| TJ004309 (uliledlimab) | Toripalimab | Solid tumors | NCT04322006 | Recruiting |
| NZV930 | Spartalizumab | Solid tumors | NCT03549000 | Active, not recruiting |
| NZV930 | Spartalizumab, NIR178 | Solid tumors | NCT03549000 | Active, not recruiting |
| LY3475070 | Pembrolizumab | Solid tumors | NCT04148937 | Active, not recruiting |
Status of the clinical trial obtained from ClinicalTrials.gov as of December 6, 2022.
THERAPEUTIC AGENTS USED IN COMBINATION WITH ICIs TO TARGET TUMOR METABOLISM
Drugs Targeting Glucose Metabolism
Metformin, a safe and inexpensive medication used in the treatment for type II diabetes, has been recognized to have antitumor activity through several mechanisms.[61] Increasing evidence suggests that metformin decreases incidence and mortality in several cancer types.[62] The anticancer properties of metformin are attributed to its inhibitory action on mTOR, mediated through AMP-activated protein kinase (AMPK).[63] Metformin also inhibits mTOR signaling independent of AMPK through Rag GTPases[64] and induces cell cycle arrest through increased expression of REDD1 (a negative regulator of mTOR).[65,66] Through inhibition of the mTOR pathway, metformin inhibits both protein synthesis and gluconeogenesis, limiting the proliferation of cancer cells.[67] Additionally, metformin increases fatty acid oxidation and induces cell cycle arrest and apoptosis.[68] Hypoxia in the TME impairs T-cell metabolism and therefore is linked to immunotherapy resistance. Metformin was shown to decrease hypoxia in the TME by reducing the expression of HIF-1 and angiogenesis-associated factors and thus potentiate the anticancer effect of programmed cell death protein 1 (PD-1) inhibitors.[69] Immune exhaustion is a common phenomenon after treatment with ICI. Metformin enhances CD8 activity by restoring the functionality of exhausted cytotoxic T cells and protecting them from apoptosis.[70] Metformin also reduces the stability and membrane localization of programmed death ligand-1 (PD-L1) through an AMPK-dependent mechanism, resulting in reduced PD-L1 expression.[71] This reduction in PD-L1 signaling enhances the cytotoxic effector function of T cells. Due to the pleiotropic effects of metformin on the metabolic pathways and the immune system, metformin is currently being studied in combination with different ICIs in different types of cancers, with some showing promising results.[72–75] Some of these studies lacked statistical significance, likely due to small sample size, highlighting the need for larger prospective studies.
Drugs Targeting Amino Acid Metabolism
CB-839 (telaglenastat) is a first in class, oral glutaminase inhibitor that blocks glutamine consumption by the tumor. This blockade elevates glutamine levels in the TME and enhances immune cell activity. In preclinical studies, CB-839 reversed tumor cell–mediated immunosuppression, enhanced T-cell proliferation, and demonstrated antitumor activity in syngeneic mouse models.[76] Subsequently, CB-839 was evaluated alone and in combination with nivolumab in patients with advanced or metastatic cancer.[77] CB-839 was well tolerated. Among the 16 evaluable patients with melanoma who were progressing on a checkpoint inhibitor at study entry, an objective response rate (ORR) of 19% and a disease control rate (DCR) of 44% were reported. Stabilization of disease was achieved in 67% of patients with non–small cell lung cancer (NSCLC) and 75% of patients in RCC cohorts, despite prior progression on anti-PD-1/PD-L1 at study entry. These findings suggest that resistance to anti-PD-1/PD-L1 can be overcome by the addition of CB-839. However, mixed results were observed in a phase II randomized study (CANTATA)[78] of CB-839 in combination with cabozantinib vs placebo plus cabozantinib in 444 patients with advanced or metastatic clear cell RCC, who had progressed on antiangiogenic therapy or ICIs. CB-839 did not improve the efficacy of cabozantinib. The median progression-free survival (PFS) was 9.2 months vs 9.3 months, respectively (hazard ratio [HR] = 0.94; 95% CI: 0.74, 1.21). However, in a subgroup analysis in 128 patients who had failed prior ICI therapy, the median PFS was longer in patients treated with CB-839 in combination with cabozantinib vs those with placebo plus cabozantinib (11.1 vs 9.2 months, respectively, HR = 0.77; 95% CI: 0.56, 1.06), suggesting a need for biomarker-driven patient selection.
DRP-104 (sirpiglenastat) is an inactive prodrug of 6-Diazo-5-oxo-l-norleucine (DON), a glutamine antagonist. Although DON inhibited tumor growth markedly by its action on multiple enzymes regulating glutamine metabolism, clinical development of the drug was hampered due to gastrointestinal toxicities, such as intolerable nausea and vomiting.[79] DRP-104, a DON prodrug, a tumor-targeted glutamine antagonist, was developed to circumvent DON-related gastrointestinal toxicities.[80] In a first-in-human phase 1/2a, multicenter, multicohort study, the safety, tolerability, and antitumor activity of DRP-104 alone and in combination with atezolizumab, a PD-L1 inhibitor, is being evaluated.[81]
CB-1158 (numidargistat) is a small-molecule arginase inhibitor that inhibits the immunosuppressive enzyme arginase expressed by myeloid-derived suppressor cells in the TME.[82] This restores cellular proliferation and cytotoxic activity of activated T cells. In preclinical studies, CB-1158 reversed MDSC-mediated immunosuppression and inhibited tumor growth, particularly when combined with chemotherapy, ICIs and adoptive T-cell and NK-cell therapy.[16] Subsequently, CB-1158 was evaluated alone (n = 107) and in combination with pembrolizumab (n = 138) in patients with advanced or metastatic solid tumors.[82] Preliminary data from 245 patients treated in this study indicated that CB-1158 was well tolerated. An ORR of 3% and DCR of 27% was reported in patients with microsatellite stable colorectal cancer, who received monotherapy, and 7% ORR and 30% DCR in patients who received combination therapy with pembrolizumab, thus demonstrating preliminary antitumor activity in a tumor type resistant to PD-L1 treatment.
AEB1102 (pegzilarginase) is a bioengineered human PEGylated arginase 1 enzyme developed for degradation of arginine to ornithine and urea, thus lowering blood arginine levels.[83] In tumors dependent on extracellular arginine, due to reduced expression of argininosuccinate synthase required for intracellular synthesis of arginine, depletion of arginine inhibits proliferation and survival of cancer cells. Due to lack of stability of arginine-degrading enzymes, bioengineered human PEGylated arginase 1was developed. In preclinical studies, data suggested that small cell lung cancer, melanoma, and Merkel cell carcinoma may be responsive to arginine depletion. Furthermore, pegzilarginase increases CD8+ T-cell infiltration and enhances antitumor activity of PD-1 inhibitors. Based on these preclinical findings, pegzilarginase is being investigated in combination with pembrolizumab in patients with small cell carcinoma.[84]
Drugs Targeting Adenosine Pathway
Given the nonredundant signaling through the adenosine pathway, several cancer therapies that target A2AR or CD73 in combination with checkpoint inhibitors are being investigated.
CPI 444 (cifrodenant) is a small molecule that selectively binds to A2AR, inhibiting adenosine signaling. In animal models, it has been shown that treatment with PD-1 inhibitors upregulates A2AR and CD73 expression, conferring PD-1 resistance. Therefore, a first-in-human study was conducted with cifrodenant, either alone or in combination with atezolizumab (PDL-1 inhibitor) in patients with advanced refractory cancers.[85] In a study in 68 patients with RCC who were either resistant or refractory to prior PD-1/PD-L1 inhibitors, 3% of patients treated with single-agent and 11% of patients on combination therapy had an objective response. Disease control for at least 6 months was reported in 17% and 39% of those patients, respectively. AZD4635, EOS100850, and NIR178 are other A2AR antagonists under investigation in phase 1 trials.
AZD4635 (imaradenant) is an oral, small-molecule inhibitor of A2AR. In preclinical models, it was shown to increase antigen presentation by dendritic cells and cytotoxic T cells. Subsequently, antitumor activity of imaradenant is being evaluated in combination with durvalumab (Imfinzi) in patients with refractory solid tumors. In a cohort of 43 patients with heavily pretreated, metastatic castrate-resistant prostate cancer, objective response was seen in 16.2% of 37 evaluable patients.[86] Additionally, 48.6% of the patients achieved stable disease. The median PFS was 14.9 weeks. Enhanced adenosine gene expression signature[87] in peripheral blood was associated with improved PFS.
NIR178 (taminadenant), an oral, small-molecule, selective A2AR antagonist, inhibits A2AR that mediates immune suppression in the TME.[88] In murine models and in human ex vivo models, taminadenant significantly reduced tumor growth.[89] Based on the demonstrated synergistic activity of A2AR blockade in combination with ICIs,[90] a first-in-human phase I/Ib study of taminadenant in combination with spartalizumab, a PD-1 inhibitor, was conducted in patients with advanced cancer. In the study conducted in NSCLC patients,[88] 24/25 patients were evaluable for response. One patient had complete response (CR), 1 had partial response, and 14 additional patients had stable disease, providing an ORR of 8.3% and DCR of 66.7%. The median PFS and overall survival were 2.8 months and 5.4 months, respectively.
EOS100850 is an oral, potent, and highly selective A2AR antagonist that inhibits A2AR expressed on T-lymphocytes, resulting in proliferation and enhanced effector function.[91] A unique feature of this compound is the sustained A2AR inhibition even at the high adenosine concentrations found in tumors. In mouse models, EOS100850 combined with PD-L1 inhibitor exhibited enhanced antitumor activity compared to PD-L1 alone. Due to the distinct mechanisms of the two checkpoints, a phase I trial is evaluating the safety and efficacy of the combination in solid tumors.
AB928 (etrumadenant), a small-molecule dual antagonist of A2A and A2B receptors, is being evaluated in combination with chemotherapy and/or anti-PD-1 backbone in patients with advanced cancer.[92] Preliminary analyses in 26 patients indicates that the treatment was well tolerated. Four of the 12 evaluable patients had tumor reduction on first restaging. The combination is further evaluated in patients with triple-negative breast, ovarian, colorectal, gastroesophageal, non–small cell lung, renal cell, and castration-resistant prostate cancer.
CPI-006, an IgG1, produces complete inhibition of CD73 enzymatic activity. It inhibits the conversion of ATP to AMP by blocking CD73 on T and B cells, lowering extracellular adenosine levels. In preclinical studies, CPI-006 blocked adenosine production and inhibited tumor growth in syngeneic mouse models. Therefore, CPI-006 as monotherapy and in combination with cifrodenant is being investigated.[93] Additionally, CPI-006 in combination with PD-1 inhibitors, tyrosine kinase inhibitors, and chemotherapeutic agents is under evaluation.
MEDI9447 (oleculmab) is a human IgG1λ mAb that inhibits CD73, an enzyme required for synthesis of immunosuppressive adenosine. In preclinical studies, oleculmab reduced adenosine-mediated immune suppression and restricted tumor growth.[94] Furthermore, there was significant increase in effector T cells and activated macrophages. In murine models, oleculmab had an additive effect when combined with PD-1 inhibitors.[95] It inhibited the growth of epidermal growth factor receptor (EGFR)-mutated NSCLC in a xenograft mouse model when combined with durvalumab because CD73 is upregulated in EGFR-mutant NSCLC.[96] Based on these preclinical findings, the combination is being explored in solid tumors. In a phase I study of the combination, promising antitumor activity was reported in EGFR-mutant NSCLC.[97] Among 20 patients with microsatellite stable colorectal cancer, one patient had partial response (PR) and two had stable disease (SD).[98] Of the 21 patients with advanced pancreatic cancer, two patients had PR and three had SD. In a randomized, phase II study in NSCLC, ORR was significantly higher (30%) in 60 patients treated with oleculmab in combination with durvalumab compared to 17.9% in 67 patients treated with durvalumab alone.[99] The median PFS was not reached in patients treated with the combination vs 6.3 months in patients treated with durvalumab alone.
BMS-986179 is a high-affinity antibody that inhibits both the expression and the enzymatic activity of CD73. Based on findings from a preclinical study that combination with anti-mouse-CD73 antibody improved treatment outcomes more than with PD-1 blockade alone,[100] a first-in-human phase 1/2a study of BMS-986179 in combination with nivolumab was conducted in patients with solid tumors. Preliminary results reported confirmed PR in seven patients with head and neck, pancreatic, prostate, anal, and renal cancers, and disease stabilization in an additional 10 patients.[101]
LY3475070 is an oral, small-molecule, selective CD73 inhibitor that promotes internalization of CD73. It thereby reduces the amount of adenosine in the TME, preventing adenosine-mediated immune suppression. It also inhibits migration of cancer cells, thereby preventing metastasis.
TJ004309 (uliledlimab) is a humanized CD73 antibody that adopts a unique intradimer binding mode through the C-terminal domain of CD73.[102] This novel action enables complete inhibition of CD73 activity. Based on the antitumor activity of uliledlimab in combination with a PD-L1 inhibitor in several preclinical models, a phase I study of uliledlimab in combination with atezolizumab was conducted in solid tumors. Of the 20 patients enrolled in the study, 13 patients who received ≥10 mg/kg of uliledlimab were evaluable for response.[102] One patient had CR (ovarian clear cell carcinoma), two had PR (both NSCLC), and three additional patients had SD for an ORR of 23% and DCR of 46%. In a phase II study of uliledlimab in combination with toripalimab (PD-1 inhibitor), preliminary antitumor activity was observed in patients with NSCLC. Of the 48 patients evaluable for response, six patients had PR and 21 had SD for an ORR of 12.5% and DCR of 56.4%.[103] High CD73 expression was associated with response in both the studies.
NZV930 (SRF373) is a fully human anti-CD73 monoclonal antibody. It inhibits the enzymatic activity of CD73, thereby reducing availability of adenosine, and enhances T-cell proliferation. In preclinical studies, NZV930 in combination with a PD-1 inhibitor was shown to have antitumor activity. In a first-in-human phase I/Ib study of NZV930 alone and in combination with spartalizumab and/or taminadenant in patients with advanced cancer, 105 patients were enrolled in the dose-escalation phase. No objective response was reported. Twelve patients had SD. CD73 target engagement and modulation of adenosine pathway was observed posttreatment in biomarker studies.
Drugs Targeting Molecular Signaling Pathways
Another approach would be to target molecular signaling pathways that intersect with metabolic pathways. Although several pathway-specific targeted therapies, such as PI3K inhibitors, have undergone extensive clinical investigation, their use in combination with immunotherapeutic agents in treatment of solid tumors is limited. For example, everolimus has been U.S. Food and Drug Administration approved to treat patients with progressive neuroendocrine tumors of pancreatic, gastrointestinal, or lung origin; advanced RCC; subependymal giant cell astrocytoma; and renal angiomyolipomas associated with tuberous sclerosis, and is approved in combination with exemestane in postmenopausal women with advanced hormone receptor-positive (HR+) breast cancer. Alpelisib is another PI3K inhibitor, which has been approved for use in combination with fulvestrant for postmenopausal women and for men with HR+, human epidermal growth factor receptor 2 (HER2)–negative, PIK3CA-mutated, advanced or metastatic breast cancer. However, their use in combination with immunotherapeutic agents has been challenging due to off-target toxicities, lack of optimal bioavailability, drug resistance, and the target of PI3K inhibition (tumor vs T cell).[104] Based on the preclinical finding that resistance to ICIs is mediated by myeloid cells, which highly express PI3Kγ,[105] a phase I study of etrumadenant (dual antagonist of A2A and A2B receptors) and PEGylated liposomal doxorubicin with or without eganelisib (a PI3Kγ inhibitor), is being conducted in patients with triple-negative breast cancer and ovarian cancer. In the interim analysis, antitumor activity was observed in both the doublet and triplet combination without evidence of additional toxicity.[106] Currently, clinical studies are also evaluating etrumadenant in combination with zimberelimab (a PD-1 inhibitor).
Belzutifan is an HIF-2α inhibitor that was recently approved by FDA for early VHL disease–associated tumors such as RCC, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors that do not require surgery.[107] As inactivating mutations in the VHL gene lead to stabilization of HIFα and activation of downstream VEGF, HIF2α inhibitors such as belzutifan were developed for treatment of VHL disease. Antitumor activity (ORR of 25%) has been reported in a phase I study of belzutifan in patients with metastatic clear cell RCC.[108] Following the success of pembrolizumab in clear cell RCC in adjuvant setting,[109] belzutifan plus pembrolizumab is now being evaluated in a phase III LITESPARK-022 study.
SUMMARY
In summary, metabolic reprogramming contributes to therapeutic resistance of ICIs in the treatment of cancer. An understanding of these metabolic effects on the tumor and immune cells in the TME will guide development of therapeutic strategies to improve treatment outcomes with ICIs. Accumulating evidence suggests that combinatorial approaches that target metabolic pathways in addition to immune checkpoints may reactivate immune function and improve treatment response, justifying further evaluation. However, combination therapies are not without challenge. Enhanced response with combination therapies may be at the expense of increased toxicity, suggesting the need for patient selection. Correlative studies to identify biomarkers of response and target engagement (tumor versus T cell) are much needed.
Funding Statement
Source of Support: None.
Footnotes
Conflict of Interest: None.
References
- 1.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun . 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Osta B, Hu F, Sadek R, et al. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol . 2017;119:1–12. doi: 10.1016/j.critrevonc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell . 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Renner K, Singer K, Koehl GE, et al. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol . 2017;8:248. doi: 10.3389/fimmu.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Zhang SL, Hu XH, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target. Int J Biol Sci . 2015;11:1390–1400. doi: 10.7150/ijbs.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo M, Crochet RB, Lee Y-H. Targeting altered metabolism—emerging cancer therapeutic strategies. In: Neidle S, editor. Cancer Drug Design and Discovery 2nd ed. Academic Press;; 2014. 427–448. [Google Scholar]
- 7.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol . 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science . 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis. Nat Rev Cancer . 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 10.Pallett LJ, Dimeloe S, Sinclair LV, et al. A glutamine ‘tugofwar' targets to manipulate glutamine metabolism for cancer immunotherapy Immunotherapy Adv. 2021. 1:Itab010. [DOI] [PMC free article] [PubMed]
- 11.Reinfeld BI, Madden MZ, Wolf MM, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature . 2021;593:282–288. doi: 10.1038/s41586-021-03442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang CV. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells. Cell cycle . 2010;9:3884–3886. doi: 10.4161/cc.9.19.13302. [DOI] [PubMed] [Google Scholar]
- 13.Choi YK, Park KG. Targeting glutamine metabolism for cancer treatment. Biomol Ther (Seoul) . 2018;26:19–28. doi: 10.4062/biomolther.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witte D, Ali N, Carlson N, Younes M. Overexpression of the neutral amino acid transporter ASCT2 in human colorectal adenocarcinoma. Anticancer Res . 2002;22:2555–2557. [PubMed] [Google Scholar]
- 15.Rodriguez PC, Ochoa AC, Al-Khami AA. Arginine metabolism in myeloid cells shapes innate and adaptive immunity. Front Immunol . 2017;8:93. doi: 10.3389/fimmu.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steggerda SM, Bennett MK, Chen J, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer . 2017;5:101. doi: 10.1186/s40425-017-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patil MD, Bhaumik J, Babykutty S, et al. Arginine dependence of tumor cells: targeting a chink in cancer's armor. Oncogene . 2016;35:4957–4972. doi: 10.1038/onc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhari SK, Chaudhary M, Bagde S, et al. Nitric oxide and cancer: a review. World J Surg Oncol . 2013;11:118. doi: 10.1186/1477-7819-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CL, Hsu SC, Ann DK, et al. Arginine signaling and cancer metabolism. Cancers (Basel) . 2021;13:3541. doi: 10.3390/cancers13143541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eniafe J, Jiang S. The functional roles of TCA cycle metabolites in cancer. Oncogene . 2021;40:3351–6333. doi: 10.1038/s41388-020-01639-8. [DOI] [PubMed] [Google Scholar]
- 21.Icard P, Coquerel A, Wu Z, Gligorov J, et al. Understanding the central role of citrate in the metabolism of cancer cells and tumors: an update. Int J Mol Sci . 2021;22:6587. doi: 10.3390/ijms22126587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Icard P, Lincet H. The reduced concentration of citrate in cancer cells: an indicator of cancer aggressiveness and a possible therapeutic target. Drug Resist Updat . 2016;29:47–53. doi: 10.1016/j.drup.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer . 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 24.Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer . 2018;6:57. doi: 10.1186/s40425-018-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov . 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karmouty-Quintana H, Xia Y, Blackburn MR. Adenosine signaling during acute and chronic disease states. J Mol Med (Berl) . 2013;91:173–181. doi: 10.1007/s00109-013-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol . 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 28.Csoka B, Selmeczy Z, Koscso B, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J . 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorrentino C, Miele L, Porta A, et al. Myeloid-derived suppressor cells contribute to A2B adenosine receptor-induced VEGF production and angiogenesis in a mouse melanoma model. Oncotarget . 2015;6:27478–27489. doi: 10.18632/oncotarget.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell . 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab . 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Papandreou I, Cairns RA, Fontana L, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab . 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Talks KL, Turley H, Gatter KC, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol . 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell . 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brugarolas J, Kaelin WG. Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell . 2004;6:7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Gerald D, Berra E, Frapart YM, et al. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell . 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer . 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res . 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 39.Koh MY, Lemos R, Liu XP, Powis G. The hypoxia-associated factor switches cells from HIF-1 alpha- to HIF-2 alpha-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res . 2011;71:4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller DM, Thomas SD, Islam A. c-Myc and cancer metabolism. Clin Cancer Res . 2012;18:5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iurlaro R, Leon-Annicchiarico CL, Munoz-Pinedo C. Regulation of cancer metabolism by oncogenes and tumor suppressors. Methods Enzymol . 2014;542:59–80. doi: 10.1016/B978-0-12-416618-9.00003-0. [DOI] [PubMed] [Google Scholar]
- 42.Kim JW, Gao P, Liu YC, et al. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol . 2007;27:7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond) . 2018;38:27. doi: 10.1186/s40880-018-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dang CV. MYC on the path to cancer. Cell . 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janku F, Wheler JJ, Naing A, et al. PIK3CA mutations in advanced cancers: characteristics and outcomes. Oncotarget . 2012;3:1566–1575. doi: 10.18632/oncotarget.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res . 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 47.Plas DR, Talapatra S, Edinger AL. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem . 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 48.Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nature Rev Cancer . 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du XM, Kristiana I, Wong J, Brown AJ. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link between a key cell proliferative pathway and membrane synthesis. Mol Biol Cell . 2006;17:2735–2745. doi: 10.1091/mbc.E05-11-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1 alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science . 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell . 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tandon P, Gallo CA, Khatri S, et al. Requirement for ribosomal protein S6 kinase 1 to mediate glycolysis and apoptosis resistance induced by Pten deficiency. Proc Natl Acad Sci U S A . 2011;108:2361–2365. doi: 10.1073/pnas.1013629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen CH, Pore N, Behrooz A, et al. Regulation of glut1 mRNA by hypoxia-inducible factor-1—interaction between H-ras and hypoxia. J Biol Chem . 2001;276:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 54.Ying HQ, Kimmelman AC, Lyssiotis CA, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell . 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matoba S, Kang JG, Patino WD, et al. p53 regulates mitochondrial respiration. Science . 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 56.Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res . 2004;64:2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- 57.Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell . 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 58.Stambolic V, MacPherson D, Sas D, et al. Regulation of PTEN transcription by p53. Mol Cell . 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 59.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A . 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao D, Zheng S, Wang X, et al. iASPP is essential for HIF-1alpha stabilization to promote angiogenesis and glycolysis via attenuating VHL-mediated protein degradation. Oncogene . 2022;41:1944–1958. doi: 10.1038/s41388-022-02234-9. [DOI] [PubMed] [Google Scholar]
- 61.Hajjar J, Habra MA, Naing A. Metformin: an old drug with new potential. Expert Opin Investig Drugs . 2013;22:1511–1517. doi: 10.1517/13543784.2013.833604. [DOI] [PubMed] [Google Scholar]
- 62.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin . 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 63.Pierotti MA, Berrino F, Gariboldi M, et al. Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene . 2013;32:1475–1487. doi: 10.1038/onc.2012.181. [DOI] [PubMed] [Google Scholar]
- 64.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab . 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zakikhani M, Blouin MJ, Piura E, Pollak MN. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat . 2010;123:271–279. doi: 10.1007/s10549-010-0763-9. [DOI] [PubMed] [Google Scholar]
- 66.Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res . 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 67.Zhou GC, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest . 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug. Mol Cancer Ther . 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 69.Scharping NE, Menk AV, Whetstone RD, et al. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res . 2017;5:9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eikawa S, Nishida M, Mizukami S, et al. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A . 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cha JH, Yang WH, Xia W, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell . 2018;71:606–620.e7. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Svaton M, Zemanova M, Zemanova P, et al. Impact of concomitant medication administered at the time of initiation of nivolumab therapy on outcome in non-small cell lung cancer. Anticancer Res . 2020;40:2209–2217. doi: 10.21873/anticanres.14182. [DOI] [PubMed] [Google Scholar]
- 73.Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer . 2018;6:64. doi: 10.1186/s40425-018-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verdura S, Cuyas E, Martin-Castillo B, Menendez JA. Metformin as an archetype immunometabolic adjuvant for cancer immunotherapy Oncoimmunology. 2019. 8. [DOI] [PMC free article] [PubMed]
- 75.Afzal MZ, Dragnev K, Sarwar T, Shirai K. Clinical outcomes in nonsmallcell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors Lung Cancer Manag. 2019. 8. [DOI] [PMC free article] [PubMed]
- 76.Meric-Bernstam F, Tannir NM, Mier JW, et al. Phase 1 study of CB839 a small molecule inhibitor of glutaminase (GLS) alone and in combination with everolimus (E) in patients (pts) with renal cell cancer (RCC) J Clin Oncol. 2016. 34:Abstract 4568.
- 77.Meric-Bernstam F, Gordon M, Tykodi S, et al. A phase 1/2 study of CB839 a firstinclass glutaminase inhibitor combined with nivolumab in patients with advanced melanoma (MEL) renal cell carcinoma (RCC) or nonsmall cell lung cancer (NSCLC) (abstract 16) 32nd Annual Meeting and PreConference Programs of the Society for Immunotherapy of Cancer (SITC. 2017). 2017.
- 78.Tannir NM, Agarwal N, Porta C, et al. Efficacy and safety of telaglenastat plus cabozantinib vs placebo plus cabozantinib in patients with advanced renal cell carcinoma: The CANTATA randomized clinical trial. JAMA Oncol . 2022;8:1411–1418. doi: 10.1001/jamaoncol.2022.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leone RD, Zhao L, Englert JM, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science . 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tenora L, Alt J, Dash RP, et al. Tumor-targeted delivery of 6-diazo-5-oxo-L-norleucine (DON) using substituted acetylated lysine prodrugs. J Medicinal Chem . 2019;62:3524–3538. doi: 10.1021/acs.jmedchem.8b02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson ML, Doroshow DB, Seiwert TY, et al. Phase 1 and phase 2a firstinhuman (FIH) study of DRP104 a broad glutamine antagonist in adult patients with advanced solid tumors J Clin Oncol. 2021. 39:TPS3149-TPS.
- 82.Naing A, Bauer T, Papadopoulos KP. et al Phase I study of the arginase inhibitor INCB001158(1158) alone and in combination with pembrolizumab (PEM) in patients (Pts) with advanced/metastatic (adv/met) solid tumours (abstract 1621) Ann Oncol. 30 (suppl_5)v159v193. 2019.
- 83.Agnello G, Alters SE, Rowlinson SW. Preclinical safety and antitumor activity of the arginine-degrading therapeutic enzyme pegzilarginase, a PEGylated, cobalt-substituted recombinant human arginase 1. Transl Res . 2020;217:11–22. doi: 10.1016/j.trsl.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Rasco DW, Eckhardt SG, Davar D, et al. Phase I dose escalation trial of pegzilarginase in patients with advanced solid tumors Cancer Res. 2018. 78.
- 85.Fong L, Hotson A, Powderly JD, et al. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discovery . 2020;10:40–53. doi: 10.1158/2159-8290.CD-19-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim EA, Bauer TM, Patel MR. A phase I openlabel multicenter study to assess the safety pharmacokinetics and preliminary antitumor activity of AZD4635 both as monotherapy and in combination in patients with advanced solid malignancies Results from prostate cancer patients ( NCT02740985) J Clin Oncol. 2020. 38.
- 87.Sidders B, Zhang P, Goodwin K, et al. Adenosine signaling is prognostic for cancer outcome and has predictive utility for immunotherapeutic response. Clin Cancer Res . 2020;26:2176–2187. doi: 10.1158/1078-0432.CCR-19-2183. [DOI] [PubMed] [Google Scholar]
- 88.Chiappori AA, Creelan B, Tanvetyanon T, et al. Phase I study of taminadenant (PBF509/NIR178), an adenosine 2a receptor antagonist, with or without spartalizumab (PDR001), in patients with advanced non-small cell lung cancer. Clin Cancer Res . 2022;28:2313–2320. doi: 10.1158/1078-0432.CCR-21-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mediavilla-Varela M, Castro J, Chiappori A, et al. A novel antagonist of the immune checkpoint protein adenosine A2a receptor restores tumor-infiltrating lymphocyte activity in the context of the tumor microenvironment. Neoplasia . 2017;19:530–536. doi: 10.1016/j.neo.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mittal D, Young A, Stannard K, et al. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res . 2014;74:3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 91.Buisseret L, Rottey S, de Bono J, et al. Abstract CT152 First in human study with EOS100850 a novel potent A2A antagonist shows excellent tolerance and clinical benefit in immune resistant advanced cancers Cancer Res. 2020. 80:CT152-CT.
- 92.Powderly J, Spira A, Gutierrez R, et al. 1206P– Phase I evaluation of AB928 a novel dual adenosine receptor antagonist combined with chemotherapy or AB122 (antiPD1) in patients (pts) with advanced malignancies Ann Oncol. 2019. 30:v493.
- 93.Mobasher M, Miller RA, Kwei L, et al. A phase I/Ib multicenter study to evaluate the humanized antiCD73 antibody CPI006 as a single agent in combination with CPI444 and in combination with pembrolizumab in adult patients with advanced cancers J Clin Oncol. 2019. 37:TPS2646-TPS.
- 94.Geoghegan JC, Diedrich G, Lu X, et al. Inhibition of CD73 AMP hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. MAbs . 2016;8:454–467. doi: 10.1080/19420862.2016.1143182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hay CM, Sult E, Huang Q, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology . 2016;5:e1208875. doi: 10.1080/2162402X.2016.1208875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tu E, McGlinchey K, Wang J. et al AntiPDL1 and antiCD73 combination therapy promotes T cell response to EGFRmutated NSCLC JCI Insight. 2022. 7. [DOI] [PMC free article] [PubMed]
- 97.Bendell JC, LoRusso P, Overman MJ, et al. EGFRm NSCLC, editor. Safety and efficacy of the antiCD73 monoclonal antibody (mAb) oleclumab +/ durvalumab in patients (pts) with advanced colorectal cancer (CRC) pancreatic ductal adenocarcinoma (PDAC) or EGFRmutant nonsmall cell lung cancer. J Clin Oncol . 2021. 39.
- 98.Overman MJ, LoRusso P, Strickler JH, et al. Safety efficacy and pharmacodynamics (PD) of MEDI9447 (oleclumab) alone or in combination with durvalumab in advanced colorectal cancer (CRC) or pancreatic cancer (panc) J Clin Oncol. 36Abstract 4123. 2018.
- 99.Herbst RS, Majem M, Barlesi F, et al. COAST An openlabel phase ii multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable stage III nonsmallcell lung cancer J Clin Oncol. 2022. JCO2200227. [DOI] [PubMed]
- 100.Barnhart BC, Sega E, Yamniuk A, et al. A therapeutic antibody that inhibits CD73 activity by dual mechanisms Cancer Res. 2016. 76:Abstract 1476.
- 101.Siu LL, Burris H, Le DT, et al. Abstract CT180 Preliminary phase 1 profile of BMS986179 an antiCD73 antibody in combination with nivolumab in patients with advanced solid tumors Cancer Res. 2018;78:CT180-CT [Google Scholar]
- 102.Robert F, Dumbrava EE, Xing Y, et al. Preliminary safety, pharmacokinetics (PK), pharmacodynamics (PD) and clinical efficacy of uliledlimab (TJ004309), a differentiated CD73 antibody, in combination with atezolizumab in patients with advanced cancer. J Clin Oncol . 2021;39:2511. [Google Scholar]
- 103.Zhou Q, Wu L, Cui J, et al. Safety, efficacy, pharmacokinetics of uliledlimab alone or combined with toripalimab in advanced solid tumor: initial results of a phase I/II study. J Clin Oncol . 2022;40:e21123–e. [Google Scholar]
- 104.Mishra R, Patel H, Alanazi S, et al. PI3K inhibitors in cancer clinical implications and adverse effects Int J Mol Sci. 2021. 22. [DOI] [PMC free article] [PubMed]
- 105.De Henau O, Rausch M, Winkler D, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature . 2016;539:443–447. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Franco R, Rivas-Santisteban R, Navarro G, Reyes-Resina I. Adenosine receptor antagonists to combat cancer and to boost anticancer chemotherapy and immunotherapy Cells. 2021. 10. [DOI] [PMC free article] [PubMed]
- 107.Fallah J, Brave MH, Weinstock C, et al. FDA Approval Summary: Belzutifan for von Hippel-Lindau Disease-Associated Tumors. Clin Cancer Res . 2022;28:4843–4848. doi: 10.1158/1078-0432.CCR-22-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jonasch E, Bauer TM, Papadopoulos KP, et al. Phase 1 LITESPARK001 (MK6482001) study of belzutifan in advanced solid tumors Update of the clear cell renal cell carcinoma (ccRCC) cohort with more than 3 years of total followup J Clin Oncol. 2022. 40.
- 109.Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. New England Journal of Medicine . 2021;385:683–694. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]