Abstract
Introduction
Complement activation is highly involved in membranous nephropathy. Identifying the mechanism of the complement activation pathway carries crucial therapeutic implications yet remains controversial. This study explored lectin complement pathway activation in PLA2R-associated membranous nephropathy (MN).
Methods
One hundred and seventy-six patients with biopsy-proven PLA2R-associated MN were enrolled in the retrospective study and divided into the remission group (24-hour urine protein <0.75g and serum albumin >35 g/L) and the nephrotic syndrome group. The clinical manifestation and C3, C4d, C1q, MBL, and B factor in renal biopsy tissues and C3, C4, and immunoglobulins in serum were evaluated.
Results
Deposition of glomerular C3, C4d, and mannose-binding lectin (MBL) was significantly higher in the activated state than in the remission state in PLA2R-associated MN. MBL deposition was the risk factor for no remission. During follow-up, the persistent non-remission patients have significantly lower serum C3 levels.
Conclusion
Activation of the lectin complement pathway in PLA2R-associated MN may contribute to proteinuria progression and disease activity.
Keywords: membranous nephropathy, anti-PLA2R antibody, complement, lectin pathway
Background
Membranous nephropathy (MN) is an autoimmune disease caused by the deposition of immune complexes on the subepithelial surface of the glomerular capillary wall.1 It is the most common cause of nephrotic syndrome (NS) in adults.2 In 2009, it was discovered that the phospholipase A2 receptor (PLA2R) on podocytes acts as the primary antigen triggering autoimmune reactions.3 Currently, PLA2R antibodies are used as markers for the diagnosis and monitoring of MN. Persistently high serum PLA2R antibody levels predict a high risk of disease progression.4 Conversely, the disappearance of serum PLA2R antibody concentration is termed immunological remission and predicts clinical remission.2,5 The majority of PLA2R-associated MN was primary MN, and most likely develops governed by factors such as genetic susceptibility, loss of tolerance, and alterations in antigen expression with a role for environmental factors like air pollution, smoking, and infections.6
PLA2R-associated MN is believed to be developed when immune complexes activate the complement system and form C5b-9, leading to podocyte damage and proteinuria.3,7 The complement system consists of three pathways: the classical pathway, lectin pathway, and alternative pathway, forming C5b-9 and triggering cellular injury.8–10 In addition to C5b-9, C3a and C5a anaphylatoxins are generated by the activity of the C3 and C5 convertase, and have been also shown to be important effectors of complement-mediated damage in primary MN11,12 (Figure 1).
Figure 1.
Three pathways of complement activation in PLA2R-associated MN. The classical pathway is often initiated by IgG1, IgG2, and IgG3. The lectin pathway is normally initiated by bindings of LP-recognition molecules (ie MBL, ficolins) to various carbohydrates or acetylated residues on the surface of pathogens (most commonly mannose residues) and aberrant glycosylated IgG4 of anti-PLA2R antibodies particularly in PLA2R-associated MN. The alternative pathway is spontaneously activated by the hydrolysis of the C3. After factor B (FB) has been activated to Bb by factor D (FD), Bb interacts with C3 (H2O) to form a C3 convertase. C3 convertases cleave C3 into the anaphylatoxin C3a and C3b. C3b deposited on surfaces can form additional C3 convertases (C3bBb), and this drives the C3 amplification loop. Then the downstream complements are activated and lead to the assembly of the membrane attack complex (MAC).
Most PLA2R antibodies are IgG4 subclasses and do not activate the classical pathway.9,13 The classical pathway is usually activated by IgG1, IgG2, and IgG3.14 Positive staining for C4d and mannose-binding lectin (MBL) in the absence of C1q suggest that the lectin pathway plays a role in MN complement activation and is associated with disease progression.15 Seifert found glomerular IgG4 deposition closely correlated with MBL signals, suggesting glomerular lectin pathway activation by bound autoantibodies.16 Lhotta found MBL is present in the glomeruli of patients with glomerulonephritis in 1999.17 Hayashi found that intrinsic antigen-related MN was more strongly associated with complement activation by the lectin pathway, which may contribute to a less favorable clinical outcome.12 Segawa found global MN patients showing complement activation of both the alternative and lectin pathways associated with IgG2 and IgG4.18 Zhao found no correlation between serum MBL levels and clinical manifestations or prognosis. Meanwhile, Zhang observed that patients with MBL deposition reached ICR faster than those without.12 The inconsistency of the above studies indicated further research necessary. The mechanism of activation of the complement pathway also remains controversial. This study explored lectin complement pathway activation in PLA2R-associated membranous nephropathy (MN), and, therefore, contributes to a better understanding of the role of the lectin complement pathways in PLA2R-associated MN progression.
About one-third MN patients undergo spontaneous remission, especially those with absent or low anti-PLA2R levels, one-third progress to ESRD over 10 years, and the remainder develop nonprogressive CKD.19 Because of the poor prognosis of these people, this study is needed to find remission predictors and provide a research base for future drug targets.
Methods
Study Population
Two hundred and fifty-six patients with biopsy-proven PLA2R-associated MN were enrolled at the China-Japan Friendship Hospital from 2014 to 2021 in the retrospective study. The inclusion criteria were as follows: (1) positive glomerular PLA2R or positive serum PLA2R antibodies, (2) ≥5 glomeruli on each renal biopsy tissue, and (3) signed informed consent. The exclusion criteria were as follows: (1) already receiving glucocorticoid and/or immunosuppressive therapy before biopsy, (2) secondary MN, (3) incomplete clinical data, (4) infectious disease with hs-CRP ≥20 mg/L, and (5) patients not in remission state or nephrotic syndrome state. As a result, 176 patients were enrolled in the study, with 30 patients in partial remission state (proteinuria <0.75g/d and serum albumin >35 g/L) and 146 in NS state (proteinuria >3.5g/d and serum albumin <30 g/L). Eighty patients were excluded from our study, and among them, 57 patients with proteinuria between 0.75 and 3.5g/d, 20 with proteinuria >3.5 g/d but serum albumin >30g/l, 3 with proteinuria <1 g/d but serum albumin <30g/l. Clinical data, including serum albumin, proteinuria, serum creatine, serum C3, C4, and immunoglobulins, and pathological data, were collected at the time of renal biopsy and during follow-up every two months until the patients reach complete remission (proteinuria <0.3g/d and serum ALB >35g/l). This study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the China-Japan Friendship Hospital, approval number 2021–113-K71. Informed consent was obtained for the medical use of data and patient samples.
Kidney Biopsies
MN was confirmed by kidney biopsy. Light microscopy, electron microscopy, immunofluorescence staining, and immunohistochemical staining were performed on the specimens. The deposition of C3, C1q, IgG, IgG1, and IgG4 in the glomeruli was analyzed using immunofluorescence staining, and the deposition of PLA2R, C4d, MBL, and factor B in the glomeruli was analyzed using immunohistochemical staining. At least 5 glomeruli per patient were analyzed for immunofluorescence and immunohistochemistry.
Hematoxylin and eosin, periodic Schiff stain (PAS), and Masson and Jones silver staining were routinely examined by light microscopy. The stage of MN progression was assessed by light and electron microscopy according to the classification of Ehrenreich and Churg.
Immunofluorescence Staining
Staining for IgG, IgG1, IgG4, IgM, IgA, C3, and C1q were performed by indirect immunofluorescence. The frozen sections were blocked with goat serum working solution for 30 minutes. Primary antibodies to human IgG1 (9052–02, Southern Biotech, Birmingham, AL), IgG4 (9190–09, Southern Biotech, Birmingham, AL), IgG (Dako A0423), IgM (Dako A0426), IgA (Dako A0262), C3 (Dako A0062), and C1q (Dako F0254) were incubated overnight at 4°C. In immunofluorescence staining, the intensity of IgG, IgM, IgA, C3, C1q, IgG1, and IgG4 staining in the glomerular capillary wall was scored from 0 to 3 (0, negative; 1, weak; 2, moderate; 3, strong) by indirect immunofluorescence. Scores above 1 were considered positive. Five glomeruli from each biopsy tissue were randomly selected and scored, using the average score as the final score for each patient. All sections were evaluated by two blinded pathologists.
Immunohistochemistry and Quantitative Analysis
Kidney tissues were fixed, embedded in paraffin, and sectioned at a thickness of 3 µm. After dewaxing through hydration, the kidney tissue was repaired using an antigen repair method. Antigen repair of PLA2R and C4d was performed using high-pressure thermal repair, and repair of MBL and B factors using proteinase K repair. The repair was followed by inactivation of endogenous peroxidase using hydrogen peroxide. Sections were then incubated in goat serum working solution for 30 min before being incubated overnight at 4°C with the following antibodies (all from Abcam, Cambridge, UK): (1) PLA2R antibody (ab211573) and (2) C4d antibody (ab167093) diluted 1:100. (3) MBL antibody (ab23457) diluted 1:100, and (4) B factor antibody (ab192577) diluted 1:500. After visualized with horseradish peroxidase and diaminobenzidine, sections were viewed under a microscope (Nikon, Tokyo, Japan) at a magnification of 400 × using a Moticam 2506 instrument (Motic, Fujian, China). Images were taken with a digital camera system (Nikon) for qualitative and semi-quantitative assessment in a blinded manner. Semi-quantitative assessments were performed using Image Pro-plus computer image analysis software (Media Cybernetics, Bethesda, MD, USA) to analyze the average optical density (AOD) and quantify protein levels. Five glomeruli from each biopsy tissue were randomly captured and analyzed, using the average AOD as the final result for each patient.
Detection of Serum PLA2R Antibody Levels
Serum PLA2R antibodies were measured using an ELISA kit from Euroimmun AG (Lubeck, Germany), according to the manufacturer’s instructions. Serum was collected from all patients 1–3 days before renal biopsy and analyzed directly after collection. PLA2R antibodies >20Ru/mL were defined as positive and PLA2R antibodies <14Ru/mL as negative.
Statistical Analysis
Mean ± standard deviation, median (25–75% interquartile range [IQR]), and counts (percentages) were used to summarize normally distributed variables, non-normally distributed variables, and categorical variables, respectively. The Student’s t-test (for normally distributed variables), Mann–Whitney U-test (for non-normally distributed variables), and Fisher’s exact test (for categorical variables) were used to assess differences between groups. Pearson correlation tests (for normally distributed variables) or Spearman correlation tests (between two non-normally distributed variables) were used to analyze the correlation between the two variables. Univariable analysis followed by multivariable logistic regression analysis with a stepwise variable selection procedure was applied to identify risk factors for the no-remission of PLA2R-associated MN patients. All baseline variables entered the initial model and were maintained if p < 0.05. Statistical analyses were performed using SPSS 26.0, and a two-tailed p < 0.05 was considered significant.
Results
Demographic and Clinical Characteristics
Among the 176 patients with biopsy-proven PLA2R-associated MN, 30 were in the remission state, and 146 were in the NS state at the time of biopsy. The median urine protein levels between the remission state and NS state were 0.425 (0.295–0.525) and 6.54 (5.015–8.863) g/24 h, respectively. The median PLA2R antibody concentrations were 4.8 (4.3–5.0) and 61.2 (30.7–134.6) RU/mL, respectively. The median serum albumin levels were 38.7 (36.1–40.4) and 26.0 (22.0–27.1) g/L, respectively. Hematuria, proteinuria, serum albumin, anti-PLA2R, serum creatine, and eGFR were statistically different among groups. Although the median serum creatinine levels (65.4 vs 79.3) and glomerular filtration rates (101.3 vs 91.8) were significantly different between the 2 groups, they were all in the normal range, which indicated both groups have normal kidney function at baseline (Table 1).
Table 1.
Clinical Characteristics Between the Remission Group (n = 30) and Nephrotic Syndrome Group (n = 146)
| Parameters | Remission group (n=30) | Nephrotic Syndrome Group (n=146) | p |
|---|---|---|---|
| Gender (male/female) | 16/14 | 104/48 | 0.140 |
| Age (y) | 50.5 (39.3–62.8) | 53.0 (44.0–58.8) | 0.845 |
| Proteinuria (g/24 h) | 0.425 (0.295–0.525) | 6.54 (5.015–8.863) | 0.001 |
| Anti-PLA2R antibody concentrations (RU/mL) | 4.8 (4.3–5.0) | 61.2 (30.7–134.6) | 0.001 |
| Hematuria (HPF) | 4.9 (2.6–8.1) | 9.1 (4.5–16.3) | 0.005 |
| Albumin (g/l) | 38.7 (36.1–40.4) | 26.0 (22.0–27.1) | 0.001 |
| Hemoglobin (g/l) | 136 (130–146) | 136 (123–149) | 0.805 |
| eGFR (mL/(min*1.72m2)) | 101.3 (90.6–115.8) | 91.8 (73.0–106.9) | 0.006 |
| Serum creatinine (umol/L) | 65.4 (52.9–78.9) | 79.3 (65.3–100.0) | 0.001 |
Notes: Non-normally distributed variables were presented as median (25–75% interquartile range [IQR]).
Abbreviations: eGFR, estimated glomerular filtration rate; PLA2R, phospholipase A2 receptor.
Pathological Characteristics of Patients in 2 Groups, Including Deposition of C3, C1q, C4d, MBL, and Factor B in the Glomeruli
Glomerular C3 was observed in 25 (83.3%) patients in the remission group and 141 (96.6%) patients in the NS group. Glomerular C1q was detected in 20 (66.6%) and 110 (75.3%) patients in the remission and NS groups, respectively. IgG was detected in all the patients. IgG4 was observed in 26 (86.6%) and 145 (99.3%) patients in the remission and NS groups, respectively (Figure 2). The intensity of glomerular C3 staining was correlated with IgG1 (r = 0.219, p = 0.004).
Figure 2.
Positive examples of IgG (A), IgG1 (B), IgG4 (C) under immunofluorescence (original magnification×400) are present along the glomerular capillary wall in MN patients.
Glomerular C3 positivity and staining intensity were significantly lower in the remission group than in the NS group [83.3% vs 96.6%, p < 0.001; 3.0 (2.0–3.0) vs 3.0 (3.0–3.0), p = 0.003]. Glomerular IgG1 [3.0 (2.0–3.0) vs 3.0 (3.0–3.0), p = 0.004] and IgG4 [2.0 (2.0–3.0), 3.0 (3.0–3.0), p < 0.001] staining intensities were significantly lower in the remission group.
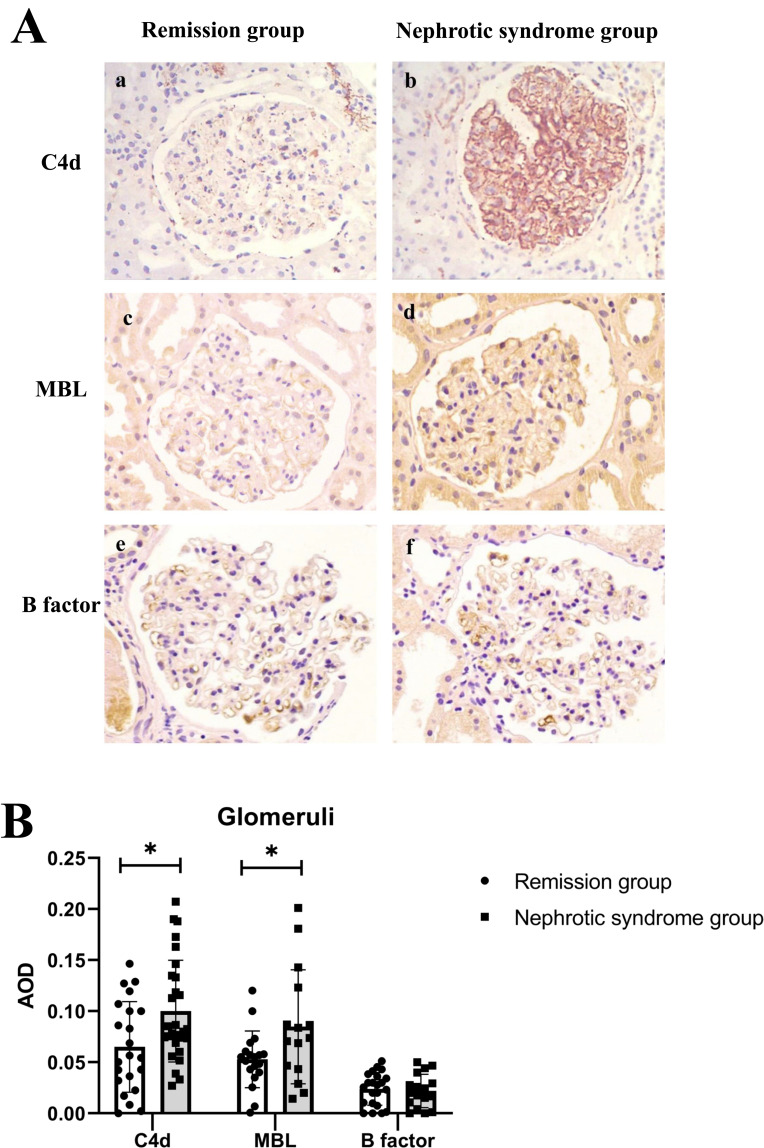
The average optical density (AOD) of C4d (0.065±0.044 vs 0.101±0.049, p = 0.013) and MBL (0.053±0.027 vs 0.084±0.055, p = 0.036) was significantly lower in the remission group than in the nephrotic group. Glomerular B-factor deposition did not differ between the two groups (Table 2) (Figure 3). The intensity of staining for glomerular MBL correlated with IgG4 (r = 0.481, p = 0.027). But the intensity of C1q was not found to be correlated with IgG1 (r = 0.216, p = 0.217).
Table 2.
Pathological Differences Between the 2 Groups
| Parameters | Remission Group (n=30) | Nephrotic Syndrome Group (n=146) | p |
|---|---|---|---|
| Immunofluorescence | |||
| IgG deposit, n (%) | 30 (100) | 146 (100) | NA |
| C3 deposit, n (%) | 25 (83.3) | 141 (96.6) | 0.003 |
| C1q deposit, n (%) | 20 (66.6) | 110 (75.3) | 0.390 |
| IgG staining intensity, median(quartile) | 3(3–3) | 3(3–4) | 0.144 |
| C3 staining intensity, median(quartile) | 3(2–3) | 3(3–3) | 0.003 |
| C1q staining intensity, median(quartile) | 2(1–2) | 2(1–2) | 0.929 |
| Immunohistological chemistry staining | |||
| C4d staining intensity, mean±SD | 0.065±0.044 | 0.101±0.049 | 0.013 |
| MBL staining intensity, mean±SD | 0.053±0.027 | 0.084±0.055 | 0.036 |
| Factor B staining intensity, mean±SD | 0.028±0.016 | 0.022±0.016 | 0.345 |
Note: Bold: having significant difference with p < 0.05.
Abbreviation: MBL, mannose-binding lectin.
Figure 3.
(A) Immunohistological chemistry staining for C4d (a and b), MBL (c and d), and Factor B (e and f) expression in the glomeruli of the remission group and the nephrotic syndrome group respectively (original magnification×400). (B) The expression of C4d, MBL, and Factor B in the two groups. *p < 0.05.
Abbreviation: AOD, average optical density.
Clinical Outcomes of PLA2R-Related MN Patients
Among 146 patients in the NS group, 109 were followed up at our institution and 35 had serum complement data (16 in persistent non-remission group and 19 in remission group). The median follow-up time was 14 months (5–19), and among them, 57 (52.3%) achieved partial remission state (24-hour urine protein <1.0 g) and 20 (18.3%) achieved complete remission (24-hour urine protein <0.3 g).
We performed Logistic regression analysis of the risk factors including age, gender, serum PLA2R-Ab, proteinuria, serum albumin, renal function, C3 deposition, IgG4 deposition, and MBL deposition, for no-remission of PLA2R-associated MN patients (Table 3). The result showed in univariable analysis, C3 deposition, MBL deposition, serum PLA2R-Ab, proteinuria, and serum albumin were risk factors that predicted no remission. After multivariable analysis, only proteinuria (OR = 1.997, 95% CI 1.028–3.879, p = 0.041) and MBL deposition (OR = 20.763, 95% CI 1.755–245.646, p = 0.016) were independent risk factors for no-remission (Table 3).
Table 3.
The Logistic Regression Analysis of the Risk Factors for No-Remission of PLA2R-Associated MN Patients
| Parameters | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Gender (male) | 1.032 (0.451–2.360) | 0.94 | ||
| Age (increased by 1 year) | 1.004 (0.974–1.035) | 0.801 | ||
| Serum PLA2R (increased by 1IU/mL) | 1.010 (1.004–1.017) | 0.005 | 0.990 (0.971–1.010) | 0.336 |
| Serum Creatine (increased by 1 umol/L) | 1.004 (0.993–1.015) | 0.483 | ||
| Serum Albumin (increased by 1 g/l) | 0.779 (0.702–0.865) | 0.001 | 0.952 (0.675–1.344) | 0.781 |
| 24h UP (increased by 1 g/d) | 1.579 (1.320–1.891) | 0.001 | 1.997 (1.028–3.879) | 0.041 |
| Serum C3 (increased by 1mg/l) | 1.003 (0.980–1.027) | 0.794 | ||
| Serum C4 (increased by 1mg/l) | 1.020 (0.960–1.083) | 0.530 | ||
| C3 deposition | 2.147 (1.068–4.314) | 0.032 | 0.880 (0.086–9) | 0.880 |
| MBL deposition | 9.625 (2.117–43.753) | 0.003 | 20.763 (1.755–245.646) | 0.016 |
| B factor deposition | 1.235 (0.256–5.970) | 0.793 | ||
| C1q deposition | 0.954 (0.567–1.606) | 0.859 | ||
| IgG deposition | 1.237 (0.517–2.958) | 0.632 | ||
| IgM deposition | 0.749 (0.478–1.173) | 0.206 | ||
| IgA deposition | 0.643 (0.371–1.114) | 0.115 | ||
| IgG1 deposition | 1.543 (0.634–3.753) | 0.339 | ||
| IgG4 deposition | 3.903 (1.590–9.583) | 0.003 | 0.485 (0.028–8.318) | 0.618 |
Note: Bold: having significant difference with p < 0.05.
Abbreviation: MBL, mannose-binding lectin.
The persistent non-remission group has significantly lower serum C3 levels compared with the remission group during follow-up (88.95 (77.75–97.3) vs 100.00 (95.6–103.5), p = 0.045). The serum IgM concentration is also lower in the non-remission group compared with the remission group (55.75 (32.45–68.60) vs 74.40 (60.55–190.05)). There were no significant differences in the serum concentrations of C4, C1q, IgG, and IgA (Table 4).
Table 4.
Follow-Up Serum Complement Concentrations Between the Non-Remission and Remission Groups
| Persistent Non-Remission Group (n=16) | Follow-Up Remission Group (n=19) | P | |
|---|---|---|---|
| Follow-up proteinuria (g/24 h) | 5.02 (2.68–7.29) | 0.31 (0.23–0.45) | 0.001 |
| Follow-up albumin (g/l) | 28.05 (21.60–35.03) | 42.10 (36.90–44.00) | 0.001 |
| Follow-up serum creatinine (umol/L) | 106.70 (74.50–177.30) | 73.95 (63.75–103.80) | 0.012 |
| Follow-up eGFR (mL/(min*1.72m2)) | 64.01 (26.15–91.80) | 91.24 (71.32–108.05) | 0.030 |
| Follow-up serum IgG (mg/dl) | 495.50 (359.25–615.50) | 701.00 (504.00–1231.50) | 0.089 |
| Follow-up serum IgA (mg/dl) | 151.00 (65.80–239.25) | 341.00 (131.00–381.50) | 0.128 |
| Follow-up serum IgM (mg/dl) | 55.75 (32.45–68.60) | 74.40 (60.55–190.05) | 0.002 |
| Follow-up serum C3 (mg/dl) | 88.30 (74.50–97.00) | 99.10 (84.20–111.25) | 0.044 |
| Follow-up serum C4 (mg/dl) | 24.20 (20.90–29.35) | 21.00 (15.85–37.05) | 0.731 |
| Follow-up serum C1q (mg/dl) | 156.00 (130.75–193.00) | 205.00 (147.50–275.50) | 0.088 |
Notes: Non-normally distributed variables were presented as median (25–75% interquartile range [IQR]). Bold: having significant difference with p < 0.05.
Abbreviation: eGFR, estimated glomerular filtration rate.
Discussion
We found that all three complement pathways were observed in glomeruli in the NS state of PLA2R-associated MN, and glomerular deposition of MBL was significantly higher in the activated state of PLA2R-associated MN than in the remission state. MBL deposition was an independent predictor for no-remission. Glomerular MBL expression is correlated with the intensity of IgG4, so IgG4 may activate complement via the lectin pathway. Moreover, during follow-up, the persistent non-remission patients have significantly lower serum C3 levels. We revealed that the activation of lectin pathways in PLA2R-associated MN is associated with proteinuria and disease activity.
Recently, various podocyte antigens have been identified, including M-type PLA2R,3 neutral endopeptidase (NEP),20 and thrombospondin type 1 domain-containing 7A (THSD7A).21 PLA2R and THSD7A are closely associated with primary MN.2,22 In this study, we used positive serological PLA2R antibodies or glomerular PLA2R as a diagnostic standard for PLA2R-associated MN. The decrease and disappearance of serum PLA2R antibody concentrations are known to indicate immunological remission and predict clinical remission.2 In contrast, glomerular PLA2R is more consistent with the development of PLA2R-associated MN.4
PLA2R in podocytes can act as the primary antigen that triggers an autoimmune response. Kerjaschk demonstrated in a model of Heymann nephritis9 that these antigens could form antigen-antibody complexes and could be released from the podocyte surface into the extracellular space, and bind to the glomerular basement membrane.23 Antigen-antibody complexes can activate complements and lead to cellular injury.24 Anti-podocyte antibodies can also directly mediate podocyte injury.25
The mechanism of complement activation in PLA2R-associated MN is not yet fully understood. C3, C4, and C5b-9 can be detected in glomerular basement membrane immune complexes, and the urinary excretion of C5b-9 is a marker of disease activity.26 The pathway activation is initiated by the antigen, subclass of IgG antibodies, and other antibodies bound to the cell surface.25 In adult MN, Ronco et al observed activation of C1q in a case of relapsed MN after renal transplantation without activation of the lectin pathway.25 Zhao et al also observed that glomerular C1q was expressed in some patients with primary MN, but probably not in close association with renal injury.27 C1q deposition is more commonly seen in secondary MN than in primary MN.27 However, C4d is commonly observed in primary MN, which supports the activation of the lectin pathway rather than the classical pathway activation in primary MN.8,28 In PLA2R-associated MN, IgG4 is the most commonly detected IgG subtype. However, it does not activate the classical pathway because it cannot combine with C1q.15 Studies have shown that abnormally glycosylated IgG4 can activate MBL and the lectin pathway in PLA2R-associated MN,12 which could be an unfavorable predictor of proteinuria and renal dysfunction.15,17,29 B factor is also often detected in the glomeruli in MN.18 This is consistent with our finding that the classical, lectin, and alternative pathway activations are all speculated in PLA2R-associated MN.
In previous literature, there have been conflicting findings regarding the correlation between glomerular MBL deposition and urinary protein, as well as the association between MBL and remission of membranous nephropathy. Lhotta found MBL is present in the glomeruli of patients with glomerulonephritis in 1999.16 Segawa found global MN patients showing complement activation of both the alternative and lectin pathways associated with IgG2 and IgG4.30 Zhao found no correlation between serum MBL levels and clinical manifestations or prognosis and did not focus on MBL deposition. Hayashi found that intrinsic antigen-related MN was more strongly associated with complement activation by the lectin pathway, which may contribute to a less favorable clinical outcome.15 Meanwhile, Zhang observed that patients with MBL deposition reached ICR faster than those without,17 which appears to contradict our study. Probable reason might be different sample size, different testing indicators, and different outcome events between our study and previous research, and our study included more PLA2R-associated MN patients. Our study supports the findings of Hayashi’s study and confirms that MBL deposition is more common in patients with renal syndrome and is a risk factor for non-remission of renal disease.
We found the deposition of lectin pathway complement, MBL, C4d, and C3, was significantly higher in glomeruli in nephrotic state of PLA2R-associated MN. We also found MBL deposition to be the risk factor for no remission. Therefore, we speculated that the activation of lectin complement pathways in PLA2R-associated MN glomeruli is associated with proteinuria and disease progression. Seifert found glomerular IgG4 deposition closely correlated with MBL signals, suggesting glomerular lectin pathway activation by bound autoantibodies.31 Our study showed that glomerular MBL expression correlated with the intensity of IgG4 expression, confirming that IgG4 may activate the complement system via the lectin pathway.
Tsai found low serum levels of C3 predicted poor renal outcomes.32 Our report is the first to show that patients who do not achieve remission have consistently lower levels of serum C3 during follow-up, suggesting that low C3 levels can be used as a monitoring tool for PLA2R-associated MN. In contrast, there was no significant difference in baseline C3 levels between the remission and renal syndrome groups. This may be because C3 levels continue to decrease during persistent unremitting states, but this depletion is not apparent at the onset.
This study provided increasing understanding for complement pathophysiology in MN and also potential prognostic value and therapeutic targets of lectin complement components. There are several complement inhibitors in MN investigated in clinical trials. Complement inhibitors OMS 721, which binds the lectin pathway protease mannan-binding lectin-associated serine protease-2 (MASP2), and APL2, which binds to C3 component, are in ongoing trials used for the treatment of MN.33
This study had some limitations. First, immunofluorescence and immunohistochemistry scoring was semi-quantitative. Second, this was a retrospective study using laboratory information derived from clinical records. In addition, there were 30 cases in the remission group, which was insufficient compared with 146 cases in the NS group. The smaller sample size may result in a larger variance, making it less likely to find true positive results in this study (smaller power), but will not affect the results of the statistical analysis of this study. We could increase the sample size and perform multiple corrections to find that there is a difference in MBL, and no difference in C1q and B factor, representing classical and alternative pathways, respectively, in the future. Finally, the results are highly dependent on the specificity of antibodies and on the antigen retrieval methods. These limitations may have some impact on the discussion of the results of this study. However, with the inclusion of 176 cases of PLA2R-associated MN, and a comprehensive study of the three complement pathways, we have gained a better understanding of the role of the lectin pathway in the progression of proteinuria and the mechanism of complement activation in MN pathogenesis.
In the future, we hope to explore complements in the lectin pathway in the sera of PMN. Recently, autoantigen-associated MN, such as PLA2R-associated MN and THSD7A-associated MN,21,34 have become a new classification criterion for MN.35,36 We will explore the characteristics of complement deposition and IgG isoforms in different autoantigen-associated MN, such as THSD7A-associated MN.
Conclusions
In conclusion, all three complement pathway activations were observed in PLA2R-associated MN, and glomerular deposition of MBL was significantly higher in the activated state of PLA2R-associated MN than in the remission state. MBL deposition was an independent predictor for no-remission. Glomerular MBL expression is correlated with the intensity of IgG4. Moreover, during follow-up, the persistent non-remission patients have significantly lower serum C3 levels. We revealed that the activation of lectin pathways in PLA2R-associated MN is associated with massive proteinuria and disease activity.
Acknowledgments
The authors gratefully acknowledge the support from China-Japan Friendship Hospital.
Funding Statement
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
MN, membranous nephropathy; MBL, mannose-binding lectin; NS, nephrotic syndrome; PLA2R, phospholipase A2 receptor; PAS, periodic Schiff stain; MASP2, mannan-binding lectin-associated serine protease-2.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the China-Japan Friendship Hospital, approval number 2021-113-K71. Informed consent included participation in this study/use of medical information and samples for this research study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
There are no conflicts of interest.
References
- 1.Debiec H, Ronco P. Immunopathogenesis of membranous nephropathy: an update. Semin Immunopathol. 2014;36(4):381–397. doi: 10.1007/s00281-014-0423-y [DOI] [PubMed] [Google Scholar]
- 2.Ronco P, Beck L, Debiec H, et al. Membranous nephropathy. Nat Rev Dis Primers. 2021;7(1):70. doi: 10.1038/s41572-021-00310-0 [DOI] [PubMed] [Google Scholar]
- 3.Beck LJ, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. New Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debiec H, Ronco P. Immune response against autoantigen PLA2R is not gambling: implications for pathophysiology, prognosis, and therapy. J Am Soc Nephrol. 2016;27(5):1275–1277. doi: 10.1681/ASN.2015101170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson A, Cattran DC, Blank M, Nachman PH. Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol. 2015;26(12):2930–2937. doi: 10.1681/ASN.2015010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Logt AE, Fresquet M, Wetzels JF, Brenchley P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96(6):1292–1302. doi: 10.1016/j.kint.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 7.Kerjaschki D. Pathomechanisms and molecular basis of membranous glomerulopathy. Lancet. 2004;364(9441):1194–1196. doi: 10.1016/S0140-6736(04)17154-7 [DOI] [PubMed] [Google Scholar]
- 8.Kerjaschki D, Neale TJ. Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J Am Soc Nephrol. 1996;7(12):2518–2526. doi: 10.1681/ASN.V7122518 [DOI] [PubMed] [Google Scholar]
- 9.Cybulsky AV, Rennke HG, Feintzeig ID, Salant DJ. Complement-induced glomerular epithelial cell injury. Role of the membrane attack complex in rat membranous nephropathy. J Clin Invest. 1986;77(4):1096–1107. doi: 10.1172/JCI112408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy YN, Siedlecki AM, Francis JM. Breaking down the complement system: a review and update on novel therapies. Curr Opin Nephrol Hy. 2017;26(2):123–128. [DOI] [PubMed] [Google Scholar]
- 11.Gao S, Cui Z, Zhao MH. Complement C3a and C3a receptor activation mediates podocyte injuries in the mechanism of primary membranous nephropathy. J Am Soc Nephrol. 2022;33(9):1742–1756. doi: 10.1681/ASN.2021101384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad G, Lorenzen JM, Ma H, et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J Clin Invest. 2021;131(5). doi: 10.1172/JCI140453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinico RA, Mezzina N, Trezzi B, Ghiggeri GM, Radice A. Immunology of membranous nephropathy: from animal models to humans. Clin Exp Immunol. 2016;183(2):157–165. doi: 10.1111/cei.12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CC, Lehman A, Albawardi A, et al. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Modern Pathol. 2013;26(6):799–805. doi: 10.1038/modpathol.2012.237 [DOI] [PubMed] [Google Scholar]
- 15.Hayashi N, Okada K, Matsui Y, et al. Glomerular mannose-binding lectin deposition in intrinsic antigen-related membranous nephropathy. Nephrol Dial Transpl. 2018;33(5):832–840. doi: 10.1093/ndt/gfx235 [DOI] [PubMed] [Google Scholar]
- 16.Lhotta K, Würzner R, König P. Glomerular deposition of mannose-binding lectin in human glomerulonephritis. NEPHROL DIAL TRANSPL. 1999;14(4):881–886. doi: 10.1093/ndt/14.4.881 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Liu Y, Liang L, et al. Effect of glomerular mannose-binding lectin deposition on the prognosis of idiopathic membranous nephropathy. Kidney Blood Press Res. 2020;45(5):713–726. doi: 10.1159/000508665 [DOI] [PubMed] [Google Scholar]
- 18.Bally S, Debiec H, Ponard D, et al. Phospholipase A2 receptor-related membranous nephropathy and mannan-binding lectin deficiency. J Am Soc Nephrol. 2016;27(12):3539–3544. doi: 10.1681/ASN.2015101155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couser WG. Primary Membranous Nephropathy. Clin J Am Soc Nephro. 2017;12(6):983–997. doi: 10.2215/CJN.11761116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debiec H, Guigonis V, Mougenot B, et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. New Engl J Med. 2002;346(26):2053–2060. doi: 10.1056/NEJMoa012895 [DOI] [PubMed] [Google Scholar]
- 21.Tomas NM, Beck LJ, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. New Engl J Med. 2014;371(24):2277–2287. doi: 10.1056/NEJMoa1409354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas-Rivera JE, Ortiz Arduán A. Primary membranous nephropathy in the era of autoantibodies and biological therapies. Med Clín. 2021;157(3):121–129. doi: 10.1016/j.medcle.2021.02.004 [DOI] [PubMed] [Google Scholar]
- 23.Kerjaschki D, Miettinen A, Farquhar MG. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987;166(1):109–128. doi: 10.1084/jem.166.1.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takano T, Elimam H, Cybulsky AV. Complement-mediated cellular injury. Semin Nephrol. 2013;33(6):586–601. doi: 10.1016/j.semnephrol.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 25.Ronco P, Debiec H. Molecular pathogenesis of membranous nephropathy. ANNU REV PATHOL. 2020;15:287–313. doi: 10.1146/annurev-pathol-020117-043811 [DOI] [PubMed] [Google Scholar]
- 26.Kon SP, Coupes B, Short CD, et al. Urinary C5b-9 excretion and clinical course in idiopathic human membranous nephropathy. Kidney Int. 1995;48(6):1953–1958. doi: 10.1038/ki.1995.496 [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Cui Z, Zhang Y, et al. Clinical and prognostic significance of glomerular C1q deposits in primary MN. Clin Chim Acta. 2018;485:152–157. doi: 10.1016/j.cca.2018.06.050 [DOI] [PubMed] [Google Scholar]
- 28.Debiec H, Hanoy M, Francois A, et al. Recurrent membranous nephropathy in an allograft caused by IgG3κ targeting the PLA2 receptor. J Am Soc Nephrol. 2012;23(12):1949–1954. doi: 10.1681/ASN.2012060577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo M, Ohi H, Ohsawa I, Fujita T, Matsushita M, Fujita T. Glomerular deposition of mannose-binding lectin (MBL) indicates a novel mechanism of complement activation in IgA nephropathy. Nephrol Dial Transpl. 1998;13(8):1984–1990. doi: 10.1093/ndt/13.8.1984 [DOI] [PubMed] [Google Scholar]
- 30.Segawa Y, Hisano S, Matsushita M, et al. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr Nephrol. 2010;25(6):1091–1099. doi: 10.1007/s00467-009-1439-8 [DOI] [PubMed] [Google Scholar]
- 31.Seifert L, Zahner G, Meyer-Schwesinger C, et al. The classical pathway triggers pathogenic complement activation in membranous nephropathy. Nat Commun. 2023;14(1):473. doi: 10.1038/s41467-023-36068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai SF, Wu MJ, Chen CH. Low serum C3 level, high neutrophil-lymphocyte-ratio, and high platelet-lymphocyte-ratio all predicted poor long-term renal survivals in biopsy-confirmed idiopathic membranous nephropathy. Sci Rep. 2019;9(1):6209. doi: 10.1038/s41598-019-42689-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zipfel PF, Wiech T, Rudnick R, Afonso S, Person F, Skerka C. Complement inhibitors in clinical trials for glomerular diseases. Front Immunol. 2019;10. doi: 10.3389/fimmu.2019.02166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen CP, Cossey LN, Beck LH. THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Modern Pathol. 2016;29(4):421–426. doi: 10.1038/modpathol.2016.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28(2):421–430. doi: 10.1681/ASN.2016070776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoxha E, Beck LJ, Wiech T, et al. An Indirect Immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol. 2017;28(2):520–531. doi: 10.1681/ASN.2016010050 [DOI] [PMC free article] [PubMed] [Google Scholar]