Abstract
Objective:
There is no licensed vaccine available to prevent the severe tick-borne disease Crimean-Congo hemorrhagic fever (CCHF), caused by the CCHF virus (CCHFV). This study sought to show that a combination of computational methods and data from published literature can inform the design of a multi-epitope antigen for CCHFV that has the potential to be immunogenic.
Methods:
Cytotoxic and helper T-cell epitopes were evaluated on the CCHFV GPC using bioinformatic servers, and this data was combined with work from previous studies to identify potentially immunodominant regions of the GPC. Regions of the GPC were selected for generation of a model multi-epitope antigen in silico, and the percent residue identity and similarity of each region was compared across sequences representing the widespread geographical and ecological distribution of CCHFV.
Results:
Eleven multi-epitope regions were joined together with flexible linkers in silico to generate a model multi-epitope antigen, termed EPIC, which included 812 (75.7%) of all predicted epitopes. EPIC was predicted to be antigenic by two independent bioinformatic servers, suggesting that multi-epitope antigens should be explored further for CCHFV vaccine development.
Conclusion:
The results presented within this manuscript provide information for potential targets within the CCHFV GPC for guiding future vaccine development.
Keywords: Bioinformatics, epitope prediction, Crimean-Congo hemorrhagic fever virus, CCHFV glycoprotein precursor, multi-epitope antigen
INTRODUCTION
Crimean-Congo hemorrhagic fever virus (CCHFV) has the most extensive geographic range of medically significant tick-borne viruses, being endemic to 30 countries in Western Asia, Southeast Europe, the Middle East, and Africa1. An estimated three billion people are at risk of CCHFV infection2. Human cases of Crimean-Congo hemorrhagic fever (CCHF) can result in severe disease, including hemorrhaging, multi-organ failure, shock, and death, with associated case fatality rates of up to 30%1,3,4. Consequently, CCHFV poses a high risk to public health and has been classified as a priority pathogen for research and development by the World Health Organization (WHO), and as a Biodefense Category A pathogen by the United States National Institutes of Health (NIH)5. Despite decades of vaccine development research for CCHFV, no approved vaccine is widely available for human use. The only vaccine available for human use is not licensed by the FDA and is only used in Bulgaria, as there are safety concerns associated with this inactivated mouse brain preparation1.
CCHFV contains a tri-segmented, negative sense, and single-stranded RNA genome, and is classified within the family Nairoviridae, genus Orthonairovirus6. The three segments, known as the small (S), medium (M), and large (L) segments, encode the nucleoprotein (NP), glycoprotein precursor (GPC), and RNA-dependent RNA polymerase (RdRp), respectively. Vaccine development for CCHFV has primarily focused on the use of the GPC or the NP in various platform technologies, with the majority of vaccine candidates demonstrating protection from challenge when encoding the glycoproteins. CCHFV is the most genetically diverse arbovirus, and the M segment demonstrates the largest nucleotide diversity of the three virus segments at 31%1. Despite the significant divergence of this segment, the M segment has been the most explored for antibody and T-cell epitope mapping, epitope predictions, and vaccine development for CCHFV since it encodes the structural glycoproteins present on the surface of the virion that induce both cellular and humoral immune responses. The CCHFV GPC undergoes the most extensive cleavage and processing of viruses in the order Bunyavirales to form the two structural glycoproteins GN and GC, and the three nonstructural glycoproteins, the mucin-like domain (MLD), GP38, and NSM7. The complex processing of the GPC includes N-linked glycosylation, many cysteine residues that may form disulfide bonds, and numerous O-linked glycosylations8. The complicated processing of the GPC, which requires the nonstructural proteins for the proper maturation of the structural glycoproteins, has led to the use of the whole GPC as a common vaccine antigen.
Correlates of protection for CCHFV have yet to be defined9. However, it is known that T cells play an important role in CCHFV immunity and are necessary for survival of CCHFV infection10,11. Depletion of CD4+ and/or CD8+ T-cells exacerbates morbidity and mortality during acute CCHFV infection, vaccination using the whole GPC can robustly activate T-cells, and human survivors of CCHF have long-lived CD8+ T-cell responses10–16. T-cell immunogenicity is not evenly distributed across the GPC, as certain regions generate stronger recall responses than others. These recall responses have also been shown to vary depending on the CCHFV strain of the stimulating peptide pool, where peptides from a strain heterologous to the immunizing strain show significantly reduced recall responses15. This pattern is also seen with antibody binding assays using GPC peptides17. This suggests that specific humoral or cellular responses could be induced from vaccination with specific GPC regions.
The size, complex processing, and uneven distribution of immunogenicity across the CCHFV GPC make it a strong candidate for vaccine development utilizing a multi-epitope antigen rather than the whole GPC18–21. Previous research has demonstrated the feasibility of a multi-epitope vaccine development strategy for other members of Bunyavirales with complex glycoprotein processing; a multi-epitope DNA vaccine generated with conserved epitopes selected from an alignment of the Hantaan virus (HTNV), Seoul virus (SEOV), and Puumala virus (PUUV) glycoproteins induced both humoral and cellular immunity against all three viruses in mice22. Ideally, this strategy for the development of a CCHFV multi-epitope antigen would allow for protection from diverse CCHF viruses, which has been a shortcoming of previous CCHFV vaccine candidates.
Previous studies attempting to develop multi-epitope vaccines for CCHFV have been limited to a “string of beads” approach, that links a few individual epitopes from the NP, structural glycoproteins, or RdRp, that are recognized only by specific major histocompatibility complex (MHC) alleles. In contrast, we sought to show that immunoinformatic analyses to predict cytotoxic T-lymphocyte (CTL) and helper T-lymphocyte (HTL) epitopes in the CCHFV GPC can be combined with regions that generate T-cell recall responses in the literature to generate a multi-epitope antigen that is composed of large GPC regions that include numerous predicted epitopes recognized by a variety of MHC alleles. It is hypothesized that generation of this type of multi-epitope antigen may generate robust T- and B-cell responses in future studies that are required for protection from lethal CCHFV challenge10,12,14–16,23. Regions of the GPC with multiple predicted epitopes were selected for generation of a model multi-epitope antigen, and the residue homology of each region was compared to the homology of whole GPC proteins from M segment sequences representing the widespread geographical and ecological distribution of the virus. Finally, a multi-epitope antigen was constructed in silico, and subcellular localization and antigenicity of the protein was predicted using bioinformatic servers. This work provides new information for potential targets during CCHFV vaccine development.
METHODS
Evaluation of CCHFV GPC Residue Diversity Across Clades
The amino acid sequence of the CCHFV GPC from strain Turkey200406546, designated throughout this work as Turkey2004 (Accession # KY362519) was used as a reference sequence for alignments, calculations of percent identity and percent similarity, and for prediction of CTL and HTL epitopes. This strain of CCHFV has not previously been used for in silico analysis of epitope prediction, despite the high annual incidence of CCHFV cases in Turkey1. Fifty GPC sequences were selected as representative sequences for the widespread geographical and ecological distribution of the virus, with sequences from each CCHFV clade, and from ticks, animals, and clinical cases. A phylogenetic tree was constructed using Geneious Tree Builder with default parameters, and clades were assigned based on previous publications1,24. Each full-length GPC sequence was aligned to the Turkey2004 sequence, and individual alignments were made for each GPC protein (MLD, GP38, GN, NSM, and GC) using Geneious Prime software (version 2021.1.1). The percent identity and percent similarity of each selected sequence to the Turkey2004 sequence was determined using William Pearson’s lalign program run through the Swiss Institute of Bioinformatics ExPASy Bioinformatics Resource Portal (now available through the European Molecular Biology Laboratory’s European Bioinformatics Institute (EMBL-EBI) https://www.ebi.ac.uk/Tools/psa/lalign/), where sequence identity considers only the residues that match strictly between two sequences, and sequence similarity considers residues that match exactly, and of the residues that differ, how similar the physicochemical properties of the residues are.
CTL Epitope Prediction
The translation of the GPC of CCHFV strain Turkey2004 was used for bioinformatic server predictions to identify epitopes likely to be presented by human MHC class I molecules to CD8+ cytotoxic T-lymphocytes. CTL epitopes were predicted using the NetCTL 1.2 Server (https://services.healthtech.dtu.dk/service.php?NetCTL-1.2) powered by the Department of Bio and Health Informatics at the Technical University of Denmark. The NetCTL 1.2 Server was used to predict binding of 9-mer CTL peptides to 12 MHC class I supertypes (A1, A2, A3, A24, A26, B7, B8, B27, B39, B44, B58, and B62) using neural networks. Predicted peptides were selected based on an inclusion criterion of a combined score of >1.0 given from prediction of MHC class I binding, proteasomal C-terminal cleavage, and TAP transport efficiency. A combined score of >1.0 yields 70% sensitivity and 98.5% specificity of CTL ligand prediction accuracy. Peptides were excluded if they fell across known cleavage sites on the GPC polyprotein.
Helper T-lymphocyte (HTL) Epitope Prediction
The translation of the GPC of CCHFV strain Turkey2004 was used for bioinformatic server predictions to identify epitopes likely to be presented by human MHC class II molecules to CD4+ helper T-lymphocytes. HTL epitopes were predicted using the NetMHCII 2.3 Server (https://services.healthtech.dtu.dk/service.php?NetMHCII-2.3) powered by the Department of Bio and Health Informatics at the Technical University of Denmark. The NetMHCII 2.3 Server was used to predict binding of 15-mer HTL peptides to 25 HLA-DR, 20 HLA-DQ, and 9 HLA-DP alleles using artificial neural networks. Predicted peptides were selected if they met both inclusion criteria of (1) a strong binder threshold of <2.00%-rank to a set of 1,000,000 random natural peptides and (2) a predicted IC50 value <50 nM. Peptides were excluded if they fell across known cleavage sites on the GPC polyprotein.
Alignment of Peptides to the CCHFV GPC and Selection of Multi-Epitope Regions
All predicted CTL and HTL peptides that met the above inclusion criteria were aligned to the Turkey2004 GPC sequence using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). Groups of peptides were assigned numerical values for the number of peptides that overlapped a given residue in the GPC alignment, and peptides were graphed in GraphPad Prism (Version 8) using these assigned values. The only two specific T-cell epitopes that have been experimentally demonstrated from convalescent CCHF cases13 were included in a different graphing panel for evaluation. Regions with the highest number of overlapping predicted peptides and regions including the two human T-cell epitopes were selected as multi-epitope regions.
Evaluation of Conservation of Selected Multi-Epitope Regions Across CCHFV Clades
Sequence conservation was assessed at the residue level for both percent identity and percent similarity. Each of the 11 selected Turkey2004 multi-epitope epitope sequences were compared individually to the same region of 50 representative CCHFV sequences, as mentioned previously, using William Pearson’s lalign program. The average percent identity and percent similarity of each multi-epitope region across the 50 sequences was compared to the average percent identity of the GPC protein where the multi-epitope region originated.
Construction of the Multi-Epitope Antigen and Antigenicity Prediction
The 11 multi-epitope regions were joined together in silico using a flexible linker of -glycine-glycine-glycine-serine- (-GGGS-). A start codon was placed at the N-terminus, and a six residue polyhistidine tag (6XHis) was added before a stop codon at the C-terminus. A signal peptide was not included at the N-terminus of the antigen to prevent localization for processing through the secretory pathway, as is the case for the GPC. The final model multi-epitope antigen, termed EPItope Construct (EPIC), with linkers and tag was 853 residues in length and will be referred to as EPIC throughout this manuscript. Subcellular localization and the residues important for localization of EPIC were predicted using the DeepLoc-1.0 server run through the Department of Bio and Health Informatics at the Technical University of Denmark (https://services.healthtech.dtu.dk/service.php?DeepLoc-1.0). Antigenicity of EPIC and individual GPC proteins was predicted with two independent servers, ANTIGENpro (http://scratch.proteomics.ics.uci.edu/) and VaxiJen (http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html).
RESULTS
Evaluation of CCHFV GPC Protein Diversity Across Clades
Fifty GPC sequences from ticks, animals, and clinical cases were selected for evaluation with representative sequences from each clade of CCHFV and spanning the widespread geographical distribution of the virus (Figure 1). The percent identity and similarity of each CCHFV GPC protein, MLD, GP38, GN, NSM, and GC, from each sequence to the Turkey2004 sequence was calculated using William Pearson’s lalign program. The Turkey2004 sequence aligns with clade V-Europe 1, and unsurprisingly, the representative sequences from this clade have the greatest homology to the Turkey2004 sequence. Sequences from clades I-IV and VI displayed greater differences in percent identity and similarity to the Turkey2004 sequence. There is a gradient in the homology of the GPC, with the N-terminus displaying a low level of homology, with increasing homology at the C-terminus of the GPC. The structural glycoproteins GN and GC display the greatest average percent identity and similarity across the six clades. The N-terminal nonstructural proteins MLD and GP38 display the lowest average percent identity and similarity across the six clades.
Figure 1: The percent identity and similarity of each GPC protein from 50 CCHFV GPC sequences compared to the Turkey2004 sequence.
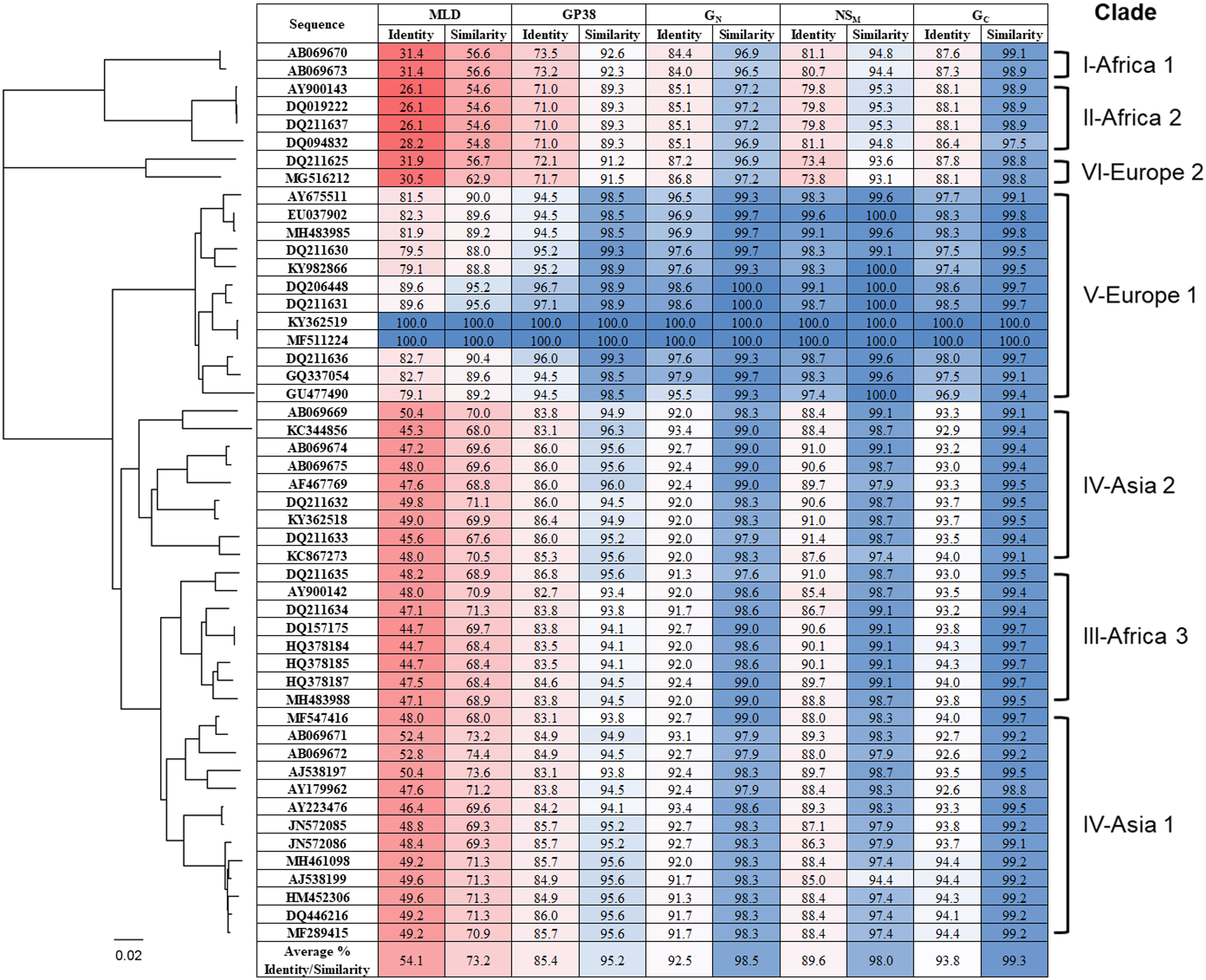
Fifty GPC sequences from ticks, animals, and clinical cases were selected with representative sequences from each clade of CCHFV and spanning the widespread geographical distribution of the virus. A phylogenic tree of the 50 sequences was generated using Geneious Tree Builder, and clades were assigned based on previous publications1,24. Percent identity and similarity was calculated using William Pearson’s lalign program run through the Swiss Institute of Bioinformatics ExPASy Bioinformatics Resource Portal (now available through the European Molecular Biology Laboratory’s European Bioinformatics Institute (EMBL-EBI) https://www.ebi.ac.uk/Tools/psa/lalign/). A color gradient was applied to the percent identity and similarity, with red highlighting areas with the lowest homology, and blue highlighting areas with the greatest homology to the Turkey2004 sequence.
CTL and HTL Epitope Prediction and Epitope Alignment to the GPC
The NetCTL 1.2 Server predicted 256 CTL peptides that met the initial inclusion criteria, and the NetMHCII 2.3 Server predicted 837 HTL peptides that met the initial inclusion criteria (Table 1). These 1093 predicted epitopes were aligned to the full-length GPC using Clustal Omega. After alignment, 4 CTL ligands and 17 HTL ligands were excluded for their location across known GPC cleavage sites, leaving 252 CTL ligands and 820 HTL ligands for consideration (Table 1). It was expected that the number of peptides that aligned to the individual proteins would be relative to the proportion of the GPC that the individual proteins comprise. The individual GPC proteins, MLD (249 residues), GP38 (272 residues), GN (288 residues), NSM (233 residues), and GC (647 residues), comprise 14.7%, 16.1%, 17.1%, 13.8%, and 38.3% of the polyprotein, respectively. The number of peptides that aligned to the individual proteins resulted in similar proportions of 19.1%, 16.2%, 13.1%, 14.9%, and 34.8% for MLD, GP38, GN, NSM, and GC, respectively (Table 1). Interestingly, the peptides predicted for the nonstructural proteins MLD, GP38, and NSM resulted in peptide prediction proportions higher than the proportion of the polyprotein the proteins comprise. At the same time, there were a lower proportion of peptides predicted for the structural glycoproteins GN and GC. The pattern of the predicted epitopes aligned to the GPC follows closely with graphs of antibody reactivity data and T-cell recall responses across the CCHFV GPC15,17, giving confidence in this prediction method for identifying potentially immunogenic regions of the GPC.
Table 1: Predicted epitopes by type per GPC region.
Total number of predicted CTL and HTL epitopes from using the NetCTL 1.2 and NetMHCII 2.3 servers. Epitopes were aligned to the Turkey2004 GPC sequence to determine their corresponding GPC region.
| Number of Predicted Epitopes | |||||
|---|---|---|---|---|---|
| GPC Region | Type of Epitope | Total | |||
| CTL | HLA-DR | HLA-DQ | HLA-DP | ||
| MLD | 19 | 39 | 140 | 11 | 209 (19.1%) |
| MLD-GP38 * | 0 | 0 | 0 | 0 | 0 (0%) |
| GP38 | 41 | 94 | 17 | 25 | 177 (16.2%) |
| GP38-G N * | 1 | 0 | 0 | 0 | 1 (0.1%) |
| G N | 54 | 42 | 10 | 37 | 143 (13.1%) |
| G N -NS M * | 2 | 5 | 5 | 0 | 12 (1.1%) |
| NS M | 40 | 43 | 66 | 14 | 163 (14.9%) |
| NS M -G C * | 1 | 3 | 4 | 0 | 8 (0.7%) |
| G C | 98 | 130 | 100 | 52 | 380 (34.8%) |
| Total | 256 | 356 | 342 | 139 | 1093 (100%) |
Epitopes located across known cleavage sites (indicated with *) were excluded from further consideration.
Identification of Multi-Epitope Regions
The 1072 CTL and HTL ligands that met the inclusion criteria were quantified and graphed by assigning numerical values for the number of predicted peptides that overlapped a given residue in the GPC sequence. This graphing method allowed for comparison to experimentally demonstrated CD8+ T-cell epitopes (Figure 2A)13, and for identification of regions with the most predicted epitopes (Figure 2B). There were 11 regions of the GPC, referred to as GPC-01 through GPC-11, chosen for further evaluation, which had the greatest number of overlapping predicted epitopes and included the two experimentally shown human T-cell epitopes (Figure 2C). The location of the 11 multi-epitope regions are as follows, using the residue numbers of the Turkey2004 GPC sequence: residues 96–172 (GPC-01), residues 205–244 (GPC-02), residues 253–283 (GPC-03), residues 303–399 (GPC-04), residues 643–742 (GPC-05), residues 923–1007 (GPC-06), residues 1043–1105 (GPC-07), residues 1270–1325 (GPC-08), residues 1335–1435 (GPC-09), residues 1472–1529 (GPC-10), and residues 1572–1663 (GPC-11). These 11 regions include 812 (75.7%) of all predicted CTL and HTL ligands and include 805 residues (47.7%) of the GPC sequence with regions selected from all five proteins of the GPC (Table 2).
Figure 2: Alignment of predicted epitopes to the GPC.
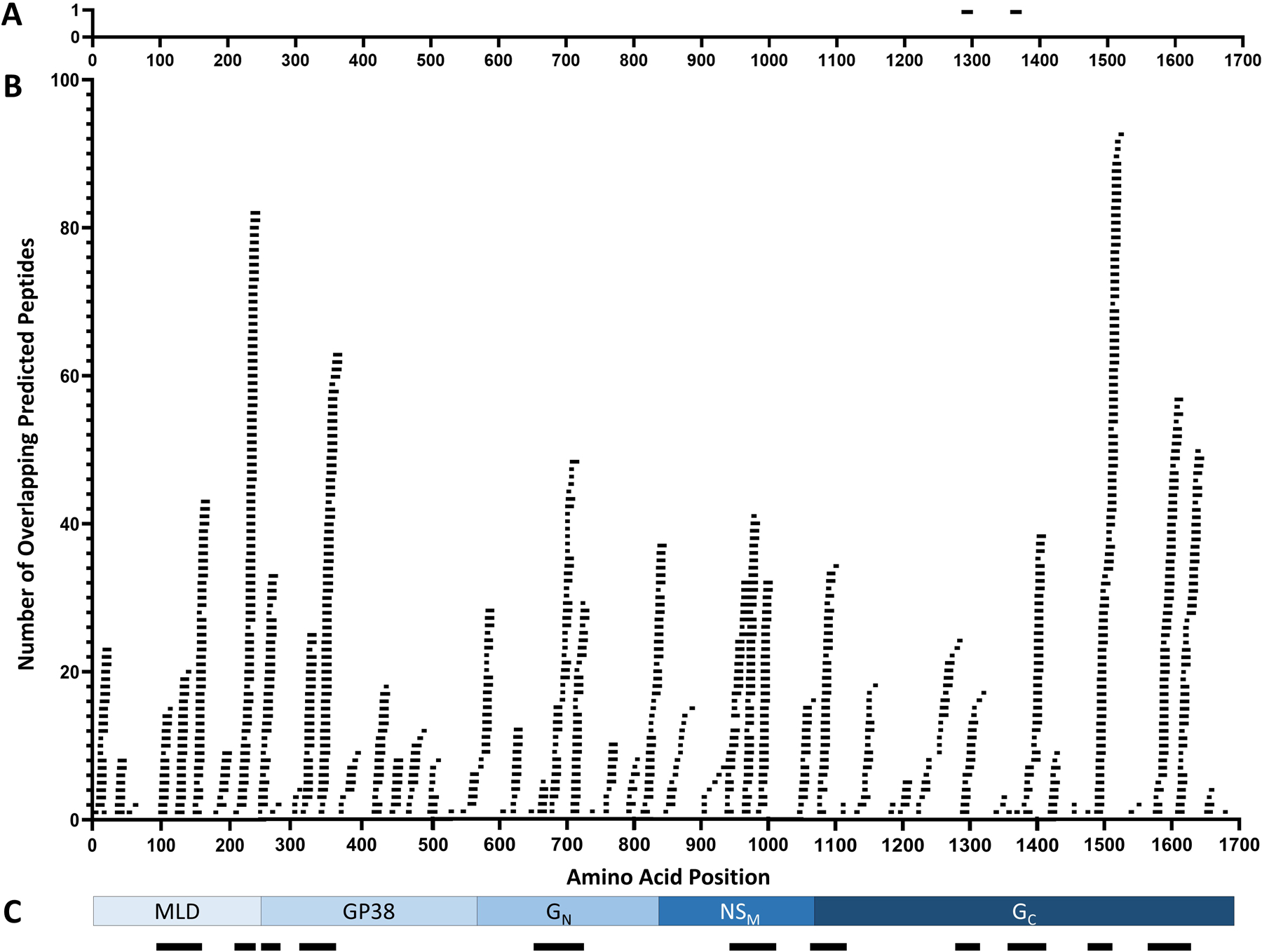
(A) Location of two experimentally demonstrated T cell epitopes aligned to the GPC13. (B) Alignment of all predicted epitopes to the GPC, excluding the 21 epitopes predicted across known GPC cleavage sites, for a total of 1072 aligned epitopes. (C) Schematic diagram of the five GPC proteins and 11 regions of the GPC chosen for generation of the multi-epitope antigen (black bars).
Table 2: Predicted epitopes by type per selected multi-epitope region.
Eleven multi-epitope regions were selected from alignment of the predicted epitopes to the GPC to generate the multi-epitope antigen. The length in residues of each region, GPC protein the region originated from, and the number and type of predicted epitopes included in each selected region are shown. A total of 812 epitopes (75.7% of total predicted epitopes) were included within the selected regions.
| Number of Predicted Epitopes | |||||||
|---|---|---|---|---|---|---|---|
| Epitope Region | Length (AA) | GPC Protein | Type of Epitope | Total | |||
| CTL | HLA-DR | HLA-DQ | HLA-DP | ||||
| GPC-01 | 77 | MLD | 6 | 11 | 62 | 0 | 79 |
| GPC-02 | 40 | MLD | 4 | 15 | 63 | 0 | 82 |
| GPC-03 | 36 | GP38 | 9 | 19 | 0 | 7 | 35 |
| GPC-04 | 97 | GP38 | 17 | 52 | 14 | 18 | 101 |
| GPC-05 | 100 | GN | 32 | 9 | 7 | 35 | 83 |
| GPC-06 | 85 | NSM | 15 | 36 | 45 | 10 | 106 |
| GPC-07 | 63 | GC | 11 | 16 | 23 | 0 | 50 |
| GPC-08 | 56 | GC | 8 | 2 | 10 | 0 | 20 |
| GPC-09 | 101 | GC | 14 | 22 | 16 | 0 | 52 |
| GPC-10 | 58 | GC | 11 | 39 | 44 | 0 | 94 |
| GPC-11 | 92 | GC | 21 | 43 | 0 | 46 | 110 |
| Total | 148 | 264 | 284 | 116 | 812 | ||
Evaluation of Conservation of Selected Multi-Epitope Regions Across CCHFV Clades
Multiple studies have demonstrated variability of immune responses to homologous or heterologous CCHFV strains15,17,25–30. Thus, it was essential to assess the residue conservation of the selected GPC regions between various CCHFV sequences. Each selected multi-epitope region was evaluated for residue identity across 50 different CCHFV sequences (Figure 3). As expected from the evaluation of identity across the whole CCHFV proteins, the multi-epitope regions originating from the MLD had the lowest average percent identity, while multi-epitope regions originating from the GC had the highest average percent identity. The average percent identity of each region was compared to its originating GPC protein, and 5/11 regions (GPC-04, GPC-05, GPC-06, GPC-10, and GPC-11) were less conserved than their respective whole proteins, and 6/11 regions (GPC-01, GPC-02, GPC-03, GPC-07, GPC-08, and GPC-09) had equal to, or greater, conservation than their respective whole proteins.
Figure 3: The percent identity of each multi-epitope region from 50 CCHFV GPC sequences compared to the Turkey2004 sequence.
Each of the 11 CCHFV Turkey2004 multi-epitope region sequences were compared individually to the same region in 50 selected sequences across all CCHFV clades using William Pearson’s align program run through the Swiss Institute of Bioinformatics ExPASy Bioinformatics Resource Portal (https://embnet.vital-it.ch/software/LALIGN_form.html). The average percent identity of each region was compared to the average percent identity of the GPC protein the region originated from. A color gradient was applied to the percent identity and similarity, with red highlighting areas with the lowest homology, and blue highlighting areas with the greatest homology to the Turkey2004 sequence.
Constuction of EPIC and Antigenicity Prediction
For the exercise of constructing a model multi-epitope antigen for CCHFV, EPIC was generated in silico using all 11 selected GPC regions. These regions included three transmembrane domains in GPC-05, GPC-06, and GPC-11, originating from GN, NSM, and GC, respectively. It was hypothesized that the lack of signal peptide in the protein, and the inclusion of the transmembrane domain from the structural glycoprotein GC, would localize EPIC to the cell membrane, rather than signaling EPIC to be processed through the secretory pathway in a manner similar to the full-length GPC. Localization prediction by the DeepLoc 1.0 server predicted that EPIC was likely to localize to the cell membrane, with a 78.7% likelihood (Figure 4A). The residues most important for the predicted subcellular localization of EPIC (Figure 4B) correspond to the three transmembrane domains included in the construct (Figure 4C). These results suggest that the inclusion of the transmembrane domains, but not signal peptide, from the GPC can result in a different predicted subcellular localization than the GPC, and the localization of future multi-epitope antigens can be modified by inclusion of different GPC regions.
Figure 4: Subcellular localization prediction and residues important for localization of EPIC.
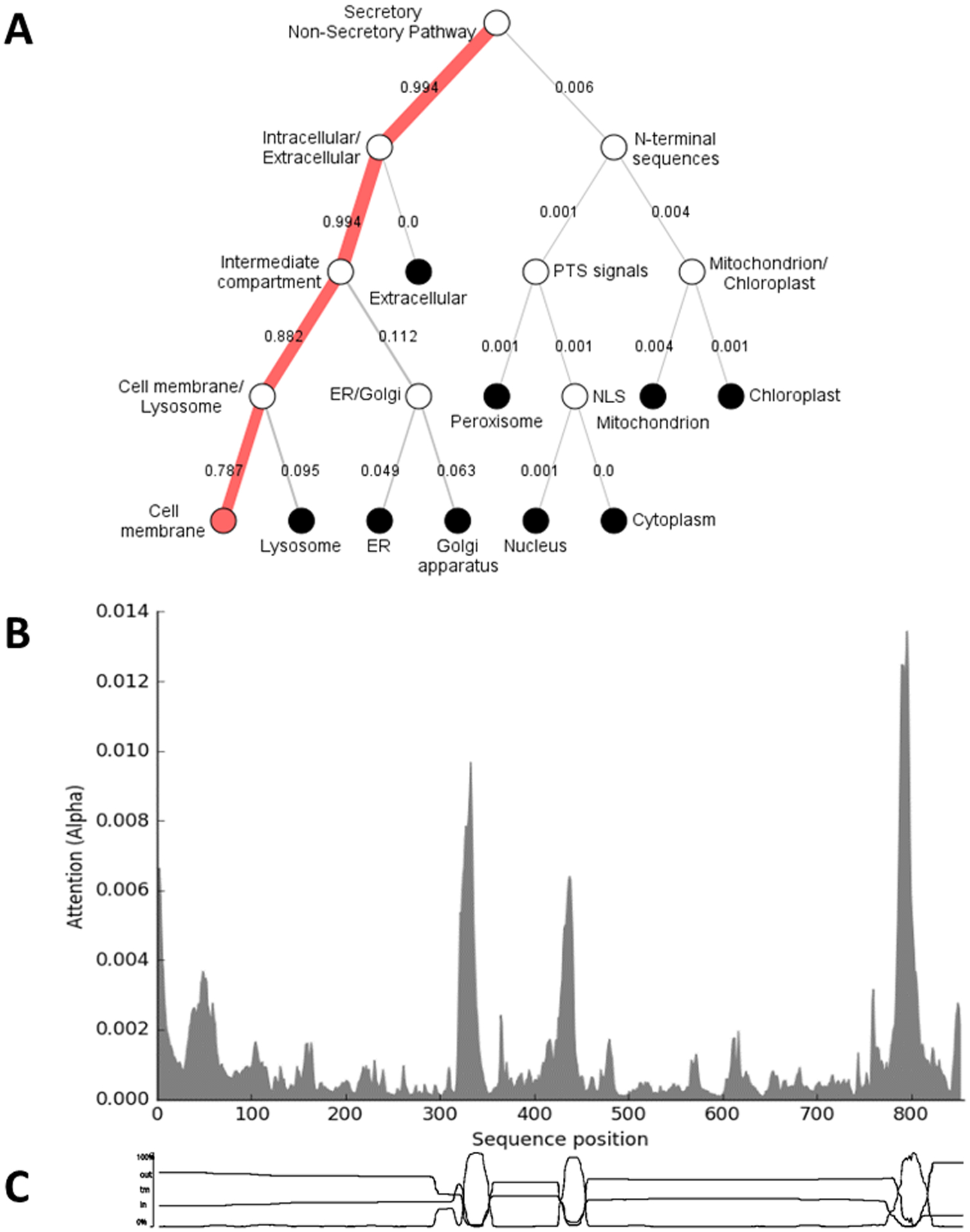
Results of the submission of the multi-epitope antigen sequence (EPIC) to the DeepLoc 1.0 Server (https://services.healthtech.dtu.dk/service.php?DeepLoc-1.0) for prediction of subcellular localization. (A) Hierarchical tree output showing the most likely localization of the antigen with numbers representing percent likelihood. (B) Graph of the importance of each residue for localization. (C) Location of the three transmembrane domains with EPIC, as shown by the transmembrane prediction function of Geneious Prime software.
The antigenicity of EPIC and the GPC proteins was predicted using two independent bioinformatic servers. ANTIGENpro predicted that EPIC was more likely to be antigenic than any of the GPC proteins alone with a predicted value of 0.94 out of 1 (MLD: 0.91; GP38: 0.56; GN: 0.73; NSM: 0.25; GC: 0.87). The VaxiJen server uses a threshold of 0.4 for indicating protein antigenicity. The VaxiJen server predicted that EPIC (0.49) would be less antigenic than the MLD (0.50), NSM (0.58) and GC (0.57) proteins, but more antigenic than GP38 (0.42) and GN (0.45). These results suggest that EPIC has antigenic potential, and further suggest that multi-epitope antigens such as EPIC should be evaluated in vitro and in vivo in future studies.
DISCUSSION
CCHF is a widespread and medically important tick-borne viral disease, yet, no vaccine is widely available for human use3. Vaccine development strategies for CCHFV have evaluated numerous platform and antigen combinations, and current CCHFV DNA vaccines being explored primarily encode the full-length GPC, or other whole protein-encoding sections from the GPC, including GP38, GN, and GC, due to the complex processing of the glycoproteins14–16,31,32. It has been suggested that the size and complex processing of the CCHFV GPC may hinder the GPC as a vaccine antigen and that the use of a multi-epitope antigen would be an improvement from current CCHFV DNA vaccine candidates18–21. Bioinformatic analyses towards generation of a CCHFV multi-epitope vaccine have previously evaluated the NP, GPC, or RdRp, for prediction of T- and B-cell epitopes, but these studies have been limited to evaluation of a single strain of CCHFV. The results presented herein are the first to provide bioinformatic epitope prediction using the Turkey2004 strain of the CCHFV GPC, evaluation of multi-epitope region homology between sequences from all CCHFV clades, and provide information for potential targets within the GPC for vaccine development.
Previous studies have discussed the diversity of the CCHFV M segment. However, this is the first study to compare the Turkey2004 GPC sequence to other CCHFV isolates spanning the widespread geographical distribution of CCHFV (Figure 1). Unsurprisingly, the Turkey2004 GPC displays high levels of similarity and identity to other isolates within Clade V-Europe 1, but greater diversity from other clades. The Turkey2004 GPC sequence displays greater homology to isolates from Asia and East Africa, than strains from West Africa, Central Africa, and the Mediterranean. This may be explained by migratory bird patterns, which play a role in the spread of Hyalomma ticks, the primary vector and reservoir of CCHFV1,33. These results highlight the importance of choosing specific regions of the GPC for vaccine development, with certain regions of the GPC demonstrating greater cross-clade homology than others.
The bioinformatic analyses in this manuscript predicted CTL and HTL epitopes using the amino acid sequence of the GPC from strain Turkey2004, followed by the evaluation of residue conservation of selected GPC regions across CCHFV clades. A total of 1093 T-cell epitopes were predicted across the Turkey2004 GPC sequence (Table 1). Alignment of these predicted epitopes to the GPC revealed 21 predicted epitopes that were located across experimentally demonstrated GPC cleavage sites, and were excluded from consideration (Table 1). All included epitopes were quantified and graphed to identify multi-epitope regions (Figure 2). Previous studies have shown that immunogenicity is not evenly distributed across the GPC, and certain regions of the GPC generate stronger antibody binding and T-cell recall responses than others12,15–17. Results of antibody epitope mapping of pooled Turkish or South African CCHFV convalescent sera to linear peptide pools generated from the Turkey-Kelkit06 strain of the CCHFV GPC demonstrated that the greatest reactivity was seen within the nonstructural glycoproteins, specifically, the C-terminus of MLD, N-terminus of GN, and middle of NSM17. Reactivity was also seen at the C-terminus of the structural glycoprotein GC17. Similarly, peptides generated from the MLD and NSM glycoproteins generate robust T-cell recall responses in GPC DNA vaccined mice and Cynomologous macaques15,16. Conversely, the N-terminus of GC generates stronger T-cell recall responses than the C-terminus in animals, however, the two specific human T-cell epitopes identified using PBMCs from convalescent patients are found in the middle of GC13. Herein, the in silico analyses of CTL and HTL epitopes within the Turkey2004 GPC demonstrate an uneven distribution across the GPC, with certain regions displaying more predicted epitopes than others. These results confirm the published results of splenocyte stimulation with peptide pools spanning the GPC, where splenocytes are not uniformly stimulated across the GPC. Additionally, the graphing pattern of the predicted T-cell epitopes was similar to the results of linear antibody epitope mapping of pooled Turkish sera to peptides generated against the CCHFV strain Turkey-Kelkit0617. Together, results from the reactivity of T-cells15,16, antibody binding17, and prediction of epitopes across the CCHFV GPC (this manuscript)18,20,21, provides information on the regions of the GPC which are predicted to be the most immunogenic, and can act as potential targets for vaccine development: the nonstructural glycoproteins and GC.
To generate a model multi-epitope antigen in silico, 11 regions of the GPC that contained the greatest number of overlapping epitopes, or included previously published T-cell epitopes13, were selected for further evaluation (Table 2). Multi-epitope regions were selected from each of the GPC proteins and contained 812 (75.7%) of all predicted CTL and HTL ligands and included 805 residues (47.7%) of the GPC sequence (Table 2). Interestingly, more epitopes were predicted in the nonstructural proteins than the structural glycoproteins, relative to protein size. This finding is similar to data showing that splenocyte stimulation with peptides homologous to the vaccine strain generates greater recall responses to nonstructural proteins than the structural glycoproteins, but not with heterologous peptides15. It was hypothesized that multi-epitope regions would demonstrate greater cross-clade homology than their whole originating GPC proteins, theoretically eliminating issues with reduced T-cell recall responses from heterologous strains. However, only 6/11 regions had equal to, or greater, conservation than their respective whole proteins (Figure 3). The MLD is the most diverse protein of the GPC (Figure 1), and this was the only GPC protein where both multi-epitope regions demonstrated greater than or equal levels of residue identity and similarity across 50 CCHFV sequences than the whole protein (Figure 3). These results suggest that a multi-epitope antigen derived strictly from regions of the most predicted epitopes may not be able to overcome the significant diversity of CCHFV between strains. In combination with published data showing variability in immune reactivity between strains15,17, these data suggest that given the widespread distribution of CCHFV, future work should focus on GPC regions with the most significant residue conservation to generate a vaccine candidate that produces heterologous protection across CCHFV clades. Alternatively, the development of regionally strain-specific vaccines is worth consideration.
The model multi-epitope antigen, EPIC, was constructed in silico by connecting each selected multi-epitope region with flexible linkers. The final multi-epitope antigen was predicted to localize to the cell membrane in mammalian cells, with the included transmembrane domains being important for localization prediction (Figure 4), and the protein was predicted to be antigenic by two different prediction servers. These results suggest that a multi-epitope antigen for CCHFV can be designed in silico, with favorable antigenic properties. This information can be used in downstream studies to modify the subcellular localization, and therefore processing and immunogenicity of multi-epitope antigens by inclusion of various domains of the GPC. Since a multi-epitope vaccine for HTNV, SEOV, and PUUV, was able to induce both cellular and humoral immunity to each virus in mice22, future in vitro and in vivo experiments utilizing EPIC or other CCHFV multi-epitope antigens should be undertaken to evaluate the immunogenicity and efficacy of this type of vaccine development strategy
The bioinformatic servers used within this manuscript predict epitopes based on linear amino acid sequences for protein cleavage and binding of peptides to MHC complexes. The in silico analysis is designed to provide information about epitopes that may bind to MHC complexes; however, the complex nature of this biological process is difficult to predict, so, likely, at least some or many of the predicted epitopes included within EPIC are not immunogenic. Sequence variability can affect the prediction of epitopes, leading to vastly different prediction results. These results from epitope prediction using the Turkey2004 strain of the CCHFV GPC can be combined with epitope prediction using other CCHFV strains to provide a broader understanding of how CCHFV sequence diversity may impact immunogenicity. Given the wide range of sequence variability within some of the GPC proteins, epitope predictions may be improved by completing the analyses with multiple CCHFV GPC sequences from diverse strains, then choosing regions based on homologously predicted peptides, rather than assessing conservation after regions were chosen from epitope predictions from a single sequence of GPC.
In conclusion, the results presented in this paper demonstrate that a model multi-epitope antigen using the Turkey2004 strain of the CCHFV GPC can be designed in silico by combining bioinformatic epitope predictions and T-cell immunogenicity data from the literature. The predicted antigenicity of EPIC suggests that a multi-epitope antigen based vaccine for CCHFV should be evaluated as a future vaccine development strategy for CCHFV.
ACKNOWLEDGEMENTS
MCM was supported by pre-doctoral fellowships from the UTMB Sealy Institute for Vaccine Sciences and NIAID Emerging and Tropical Infectious Diseases T32 (AI007526-20, PI: Dr. Lynn Soong).
Footnotes
Potential conflicts of interest. All authors: no reported conflicts.
REFERENCES
- 1.Bente DA et al. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 100, 159–189 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Control, E. C. for D. P. and. Factsheet about Crimean-Congo haemorrhagic fever. European Centre for Disease Prevention and Control Available at: https://www.ecdc.europa.eu/en/crimean-congo-haemorrhagic-fever/facts/factsheet. [Google Scholar]
- 3.Ergönül Ö Crimean-Congo haemorrhagic fever. Lancet Infect. Dis 6, 203–214 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehouse CA Crimean–Congo hemorrhagic fever. Antiviral Res. 64, 145–160 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Keshtkar-Jahromi M et al. Crimean-Congo hemorrhagic fever: Current and future prospects of vaccines and therapies. Antiviral Res. 90, 85–92 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Adams MJ et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol 162, (2017). [DOI] [PubMed] [Google Scholar]
- 7.Erickson BR, Deyde V, Sanchez AJ, Vincent MJ & Nichol ST N-linked glycosylation of Gn (but not Gc) is important for Crimean Congo hemorrhagic fever virus glycoprotein localization and transport. Virology 361, 348–355 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Sanchez AJ, Vincent MJ & Nichol ST Characterization of the Glycoproteins of Crimean-Congo Hemorrhagic Fever Virus. J. Virol 76, 7263 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez SE et al. Immunobiology of Crimean-Congo hemorrhagic fever. Antiviral Res. 199, 105244 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawman DW et al. T-Cells and Interferon Gamma Are Necessary for Survival Following Crimean-Congo Hemorrhagic Fever Virus Infection in Mice. Microorganisms 9, 1–17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowall SD et al. Protective effects of a Modified Vaccinia Ankara-based vaccine candidate against Crimean-Congo Haemorrhagic Fever virus require both cellular and humoral responses. PLoS One 11, e0156637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buttigieg KR et al. A Novel Vaccine against Crimean-Congo Haemorrhagic Fever Protects 100% of Animals against Lethal Challenge in a Mouse Model. PLoS One 9, e91516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedhals D, Paweska JT & Burt FJ Long-lived CD8+ T cell responses following Crimean-Congo haemorrhagic fever virus infection. PLoS Negl. Trop. Dis 11, e0006149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrison AR et al. A DNA vaccine for Crimean-Congo hemorrhagic fever protects against disease and death in two lethal mouse models. PLoS Negl. Trop. Dis 11, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suschak JJ et al. A CCHFV DNA vaccine protects against heterologous challenge and establishes GP38 as immunorelevant in mice. npj Vaccines 2021 61 6, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawman DW et al. A DNA-based vaccine protects against Crimean-Congo haemorrhagic fever virus disease in a Cynomolgus macaque model. Nat. Microbiol (2020). doi: 10.1038/s41564-020-00815-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritzen A et al. Epitope-mapping of the glycoprotein from Crimean-Congo hemorrhagic fever virus using a microarray approach. PLoS Negl. Trop. Dis 12, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosrati M, Behbahani M & Mohabatkar H Towards the first multi-epitope recombinant vaccine against Crimean-Congo hemorrhagic fever virus: A computer-aided vaccine design approach. J. Biomed. Inform 93, 103160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tipih T & Burt FJ Crimean-congo hemorrhagic fever virus: Advances in vaccine development. BioResearch Open Access 9, 137–150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan MSA et al. Computational formulation and immune dynamics of a multi-peptide vaccine candidate against Crimean-Congo hemorrhagic fever virus. Mol. Cell. Probes 55, 101693 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Tahir Ul Qamar M et al. Development of a Novel Multi-Epitope Vaccine Against Crimean-Congo Hemorrhagic Fever Virus: An Integrated Reverse Vaccinology, Vaccine Informatics and Biophysics Approach. Front. Immunol 12, 2313 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao C et al. Immunogenicity of a multi-epitope DNA vaccine against hantavirus. Hum. Vaccin. Immunother 8, 208–215 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Dowall SD et al. A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum. Vaccines Immunother 12, 519–527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramírez de Arellano E et al. Phylogenetic Characterization of Crimean-Congo Hemorrhagic Fever Virus, Spain. Emerg. Infect. Dis 23, 2078 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spengler JR et al. Heterologous protection against Crimean-Congo hemorrhagic fever in mice after a single dose of replicon particle vaccine. Antiviral Res. 170, 104573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawman DW et al. Crimean-Congo Hemorrhagic Fever Mouse Model Recapitulating Human Convalescence. J. Virol 93, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golden JW et al. GP38-targeting monoclonal antibodies protect adult mice against lethal Crimean-Congo hemorrhagic fever virus infection. Sci. Adv (2019). doi: 10.1126/sciadv.aaw9535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez SE et al. Vesicular Stomatitis Virus-Based Vaccine Protects Mice against Crimean-Congo Hemorrhagic Fever. Sci. Rep (2019). doi: 10.1038/s41598-019-44210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spengler JR et al. Viral replicon particles protect IFNAR−/− mice against lethal Crimean-Congo hemorrhagic fever virus challenge three days after vaccination. Antiviral Res. 191, 105090 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zivcec M, Spiropoulou CF & Spengler JR The use of mice lacking type I or both type I and type II interferon responses in research on hemorrhagic fever viruses. Part 2: Vaccine efficacy studies. Antiviral Res. 174, 104702 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinkula J et al. Immunization with DNA Plasmids Coding for Crimean-Congo Hemorrhagic Fever Virus Capsid and Envelope Proteins and/or Virus-Like Particles Induces Protection and Survival in Challenged Mice. J. Virol 91, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spik K et al. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine 24, 4657–4666 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Jameson LJ, Morgan PJ, Medlock JM, Watola G & Vaux AGC Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick. Borne. Dis 3, 95–99 (2012). [DOI] [PubMed] [Google Scholar]


