Abstract
Insects act as vectors to carry a wide range of bacteria and viruses that can cause multiple vector-borne diseases in humans. Diseases such as dengue fever, epidemic encephalitis B, and epidemic typhus, which pose serious risks to humans, can be transmitted by insects. Due to the absence of effective vaccines for most arbovirus, insect control was the main strategy for vector-borne diseases control. However, the rise of drug resistance in the vectors brings a great challenge to the prevention and control of vector-borne diseases. Therefore, finding an eco-friendly method for vector control is essential to combat vector-borne diseases. Nanomaterials with the ability to resist insects and deliver drugs offer new opportunities to increase agent efficacy compared with traditional agents, and the application of nanoagents has expanded the field of vector-borne disease control. Up to now, the reviews of nanomaterials mainly focus on biomedicines, and the control of insect-borne diseases has always been a neglected field. In this study, we analyzed 425 works of the literature about different nanoparticles applied on vectors in PubMed around keywords, such as“nanoparticles against insect,” “NPs against insect,” and “metal nanoparticles against insect.” Through these articles, we focus on the application and development of nanoparticles (NPs) for vector control, discussing the lethal mechanism of NPs to vectors, which can explore the prospect of applying nanotechnology in the prevention and control of vectors.
1. Introduction
Vector-borne diseases (VBDs), such as malaria, dengue, ZIKA, Chikungunya, and Japanese Encephalitis, impose an important global burden on public health in the past and present. These diseases are not communicable directly among humans, but when favorable conditions are formed for the interaction of pathogens, hosts, and the environment [1]. In recent years, the reproduction and spreading of insect-borne diseases become more widespread with the change in social and other environmental factors such as international trade and global climatic alterations, and the threat to human health from insect-borne diseases is increasing [2]. According to WHO's latest World Malaria Report, there were an estimated 241 million malaria cases and 627 000 malaria deaths worldwide in 2020, which represents about 14 million more cases in 2020 compared to 2019 and 69 000 more deaths. Besides, from September 2019 to November 2021, Dengue was endemic in Pakistan with a total of 102,404 cases including 278 deaths (case fatality ratio (CFR): 0.27%) have been reported. Hence, we need to face the emerging and re-emerging major VBDs and the challenges of their control.
Worryingly, there are currently no effective vaccines or drugs for most VBDs due to the amazing rate of mutation of the virus. Therefore, vector control is the most important means to control VBDs. In 2017, WHO published the global vector control response: an integrated approach for the control of VBDs that mentioned that an estimated 663 million malaria cases were averted in Africa, more than half of which was due to the widespread deployment and measures of vector control. Hence, effective vector control interventions also can dramatically reduce disease treatment costs.
The larvae of vectors are usually targeted with pesticides and other insect growth regulators, which is an available method for controlling many VBDs. Different strategies such as indoor residual spraying and insecticide-impregnated bed nets are also applied. However, the “3R” problems (resistance, residue, and rampant) caused by the applications of these chemical treatments cannot be ignored. Insecticide resistance plays an important role in difficulties in vector control; a variety of vectors such as mosquitoes [3], ticks [4], and lice [5] were found to be resistant to chemical pesticides in different countries and regions. With the development of resistance, the dosage of chemical pesticides continues to increase, and pesticide residues become more and more serious. Researchers found that there were 113 pesticide residues in drinking water samples from 31 countries worldwide [6]. In addition, a study observed a positive exposure-response relationship for 31 pesticides, which concluded an increased risk of cancer among humans [7]. Therefore, alternative interventions with different active ingredients, capable of controlling insecticide-resistant vectors which transmit outdoors, are urgently required to ensure the sustainability of insect control. Nowadays, NPs provide a new option for controlling vectors with the development of nanotechnology. According to Benelli's review, in addition to mosquitoes that transmit encephalitis and Zika, Au NPs can be used against insect vectors that transmit leishmaniasis and trypanosomiasis, such as ticks, flies, and sandflies [8]. Au NPs are not the only kind of nanoparticles effective against insect vectors, but it was found that different kinds of nanoparticles NPs displayed insecticidal activity against vectors, including TiO2, Ag, CuO, and Pd, [9–12]. However, how do NPs work on insects? We have limited knowledge about the rationales for their enhanced toxicity at low concentrations. The latest review mentioned that the toxicity of nanoparticles is not followed according to their physical-chemical properties, such as shape, size, surface area, surface charge, solubility, and others. They provided a hypothesis that the number of atoms/ions/molecules per NPs is the source of toxicity [13]. Indeed, this hypothesis needs to be verified. Not only be used as an effective pesticide for insects but NPs can also be added as a new composition for pesticides [14–16]. Cao reported mesoporous silica NPs with a double-shelled hollow structure to deliver pyraclostrobin against the fungus Phomopsis asparagi [17]. However, with the deepening of the research, simply loaded drugs can no longer be satisfied the demand of people, and the controlled release nanosized pesticide systems based on NPs came into generation. For example, Shan synthesized a biodegradable and light-responsive amphiphilic polymer, which is stable without UV, and the release rate of pesticide from the NPs gradually increased after treatment with UV [18]. Except for controlled-release drugs, studies showed that NPs can reduce pesticide consumption by protecting active ingredients against hydrolysis and photolysis, which is beneficial to the environment [19–21]. On the other hand, the greener synthesis methods of NPs are more respectful to the environment compared to chemical pesticides. NPs synthesized using biological resources such as plants and plant extracts, bacteria, fungi, yeast, and algae as precursors show low toxicity and high biocompatibility [22–24]. Although previous studies show the potential application of NPs on insects related to public health, the safety of NPs and their influence on enemies remain unknown. Zielinska summarized the nanoecotoxicology of polymeric NPs, showing no risk to the environment or human health in their potential applications [25]. However, the other review revealed the two opposite conclusions. NPs can promote the growth of plants in lower concentrations by promoting photosynthesis and increasing the size of the xylem and phloem. Higher concentrations of NPs can also inhibit the growth of plants by destroying the cellular structure of plants [26]. However, in how to define the high and low concentrations of different nanoparticles, there is no clear standard. In this review, we focus on the application and development of nanoparticles (NPs) for vector control, discussing the lethal mechanism of NPs to vectors and the safety of nanoparticles in the environment, which can explore the prospect of applying nanotechnology in the prevention and control of vectors.
2. Classification of Nanoparticles against Insects
We analyzed 425 journal articles which adopted to search the following keywords initially: “nanoparticles against insect,” “NPs against insect,” and “metal nanoparticles against insect,” in PubMed. The thrree major types of nanoparticles (NPs) were identified in this review (Figure 1). Type 1 are metal-based NPs (for example, Ag, Cu, and Ti) which is the most widely used to resist insect, Type 2 are nonmetal-based NPs (for example, Si and Ca), and Type 3 include some complex polymers (for example, chitosan and plant extract). For Type 1 reagents, the element with the largest proportion is Ag, due to its significant impact on insect antioxidant and detoxifying enzymes, leading to ROS-mediated apoptosis, DNA damage, and autophagy [27]. In Type 1, most metals work in this way. However, the main insecticidal principle is different in Type 2; for example, the toxicity of SiO2 NPs is due to desiccation, body wall abrasion, and spiracle blockage [28], followed by physical sorption of waxes and lipids, leading to insect dehydration. In addition, nanomaterials used as insecticides can be classified according to different functions. Type 1 mainly includes NPs for directly using as insecticides, and metal-based NPs are the most widely applied in this type. In Type 2, NPs serve as carriers to encapsulate active ingredients to control insects. The first nanoabamectin pesticide recently approved in China adopts the three-dimensional reticulated nanomaterials as the carrier and uses the reticulated nanoscale drug-loading space inherent in the structure of nanomaterials to achieve encapsulation of raw materials without agglomeration (Pesticide Registration Certificate No. PD20210374).
Figure 1.
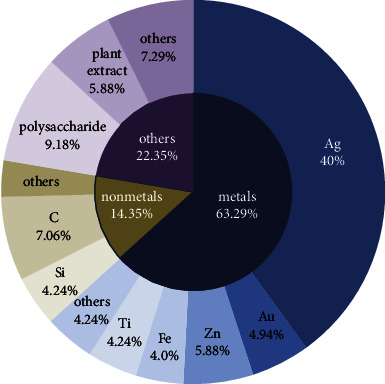
Classification of NPs against insects. NPs used to resist insects can be roughly divided into the three categories according to different properties. Among them, metal-based NPs account for the highest proportion, reaching 63.29%, and nonmetal-based NPs, such as Si- and C-based, account for only 14.35%.
3. Different Methods of NPs Synthesis
According to the discipline classification of synthesizing NPs, it can be divided into physical, chemical, and biological methods (Figure 2). The physical method refers to slicing bulk material to get nanosized particles, which mainly includes vacuum condensation, the evaporation-condensation method, and the mechanical ball grinding method [29]. Conversely, the chemical method creates nanostructures by controlling the deposition and growth of atoms and molecules, including solvothermal [30], sol-gel [31], and microemulsion methods [32]. As the most widely used chemical method, the superior advantage of the solvothermal method is the use of no catalysts, but the synthesis mostly prefers toxic solvents to fabricate required materials [33]. However, with the development of synthetic technology, many simpler methods without the use of harsh chemicals to prepare NPs were presented. Li reported a novel solvothermal approach to synthesizing carbon nanoparticles (CNPs), in contrast with previous methods, this synthesis process uses glucose and ammonium oxalate as the carbon source and glycol as the solvent, and neither strong acid treatment nor further surface modification was necessary [34]. The current advancements in solvothermal synthesis methods for NPs indicate that there are many chemical synthesis research opportunities.
Figure 2.
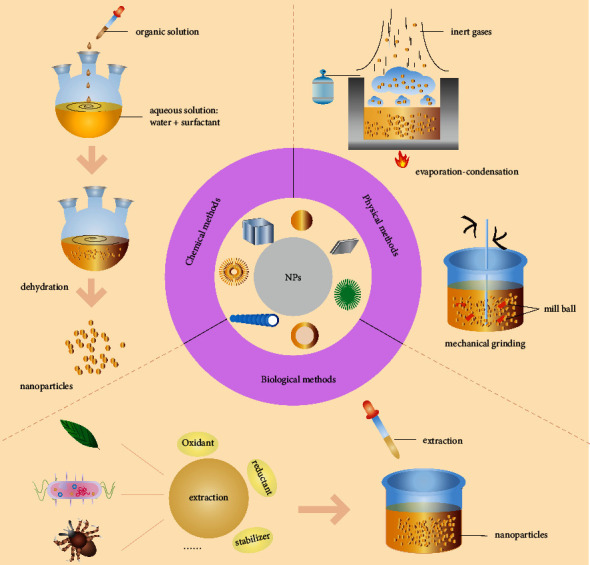
The three different synthesis methods of nanoparticles: the chemical method: different substances react chemically to form nanoparticles, the physical method: the particle size of the material is made to the nanometer scale by physical means, and the biological method: synthesis of nanoparticles from biological extracts.
Nevertheless, the latest research proposal is the biological method. Nowadays, 1917 articles have been published on PubMed in 2021 (keywords: bio nanoparticle). As an emerging green-synthesis strategy in nanotechnology, the biological method uses living templates that include plants and microorganisms such as plant extracts, viruses, bacteria, and other biomolecules for the synthesis of NPs. Recently, products approach to synthesizing NPs has focused on the use of plant components. These plant-derived nanopesticides can also be subdivided into different types based on their usage, such as plant-derived NPs and nanoemulsions prepared with essential oils extracted from plant parts. For example, iron oxide (Fe3O4) NPs are synthesized by a Rhus Coriaria extract and possess better and enhanced properties than the chemical method [35], and CopaPlu nanoemulsion also showed great antibacterial activity against Paracoccidioides. In addition, plants such as Melia azedarach leaf, Azadirachta indica leaf, and Nasturtium officinale are also explored for the synthesis of Cu-ZnO, CuO, and MoO3 NPs [36–38]. In these studies, the presence of phytochemicals in their extract functions as natural stabilizing and reducing agents for NPs production. Furthermore, enzymes and other biomolecules produced by microorganisms can also reduce metal ions to metal NPs. Different microbial such as Saccaropolyspora hirsute and B. licheniformis are also used in the synthesis of silver, ZnO, and NPs [39, 40]. By the way, the employment of different biological as reducing and stabilizing agents leads to biosynthetic NPs with optical properties, including different sizes, shapes, and differential functional properties on vectors [41, 42]. In a study, different ZnO NP shapes were obtained from Fusarium keratoplasticum and Aspergillus niger, where A. niger synthesized nanorod-shaped NPs showed enhanced antibacterial properties against pathogenic bacteria and UV-protection than F. keratoplasticum synthesized hexagonal NPs [43]. Generally speaking, bioderived NPs are regarded as safe, biocompatible, and environmentally friendly particles that cause less harmful effects to human health but are also time-consuming biological screening and expensive. There are pros and cons to different synthesis methods, and the appropriate synthesis method should be selected according to the requirements (Table 1).
Table 1.
Advantages and disadvantages of different synthesis methods.
| Advantages | Disadvantages | |
|---|---|---|
| Physical methods | Simple methodology [44] | The lower yield of NPs |
| Higher energy-consuming | ||
| Higher input cost [45] | ||
|
| ||
| Chemical methods | Homogenous NPs with high accuracy | The usage of toxic chemicals [46, 47] |
| The consumption of less energy [48] | ||
|
| ||
| Biological methods | Do not involve toxic chemicals in the preparation protocols | Highly demanding |
| Time-consuming | ||
| Requiring technology and practical microbiological experience to ensure cell culture and nanoparticle purification under aseptic conditions [49, 50] | ||
4. Application of NPs in Vector-Insects
4.1. Toxicity of NPs to Insects
The application of NPs obtained through various synthetic pathways as new pesticides has attracted more attention recently. In the last 5 years, more than 600 studies outlining the toxicity of NPs towards various vector-insects have been published. These NPs including gold, silver, titanium oxide, semiconductors, and silica-based nanomaterials have been tested against a wide range of vectors, covering mosquito, lice, and fly [16, 51]. Among all, the large majority of the researchers focused on mosquito vectors due to their huge ecological and physiological plasticity [52]. According to research, most of NPs have obvious acute toxicity against mosquito (Table 2). The published literature was carried out to examine the effectiveness of Nelumbo nucifera synthesized Ag NPs against larvae of An. subpictus (LC50 = 0.69 mg/L) and Cx. quinquefasciatus (LC50 = 1.10 mg/L) [63]. Palanisamy describes the toxicity of Ag NPs biosynthesized using cheap leaf extract of Berberis tinctoria against larval instars (I–IV) of Ae. Albopictus with LC50 of 4.97 ppm (I instar), 5.97 ppm (II), 7.60 ppm (III), and 9.65 ppm (IV) [64]. Notably, the NPs obtained as described above showed different toxicity against mosquitoes, even if they are the same NPs. Barik tested the three types of silica NPs, including lipophilic, hydrophilic, and hydrophobic, to assess the larvicidal properties of different mosquito species. In Ae. aegypti, the LC50 was 5-fold less than hydrophilic when treated with hydrophobic silica NPs [65].
Table 2.
Various NPs with their applicative measures against the different mosquito species.
| Nanoparticles | Source | Target's species | Lethal indices (LC50) |
|---|---|---|---|
| Ag | Bacillus marisflavi [53] | Ae. aegypti | 13.96 ppm |
| Ag | Cx. quinquefasciatus | 24.54 ppm | |
| Ag | An. stephensi | 29.14 ppm | |
|
| |||
| Ag | Ipomoea batatas [54] | Ae. aegypti | 17.578 μg/mL |
| Ag | Cx. quinquefasciatus | 10.069 μg/mL | |
| Ag | An. stephensi | 12.568 μg/mL | |
|
| |||
| Ag | Cassia roxburghii [55] | Ae. aegypti | 26.35 μg/mL |
| Ag | Cx. quinquefasciatus | 28.67 μg/mL | |
| Ag | An. stephensi | 31.27 μg/mL | |
|
| |||
| Au | Parmelia sulcata [56] | Ae. aegypti | 70.16 ppm |
|
| |||
| ZnO | Cucurbita [57] | Cx. tritaeniorhynchus | 39.007 ppm |
|
| |||
| ZnO | Chemical method [58] | Cx. quinquefasciatus | 291.0 mg/L |
|
| |||
| ZnO | Pseudomonas aeruginosa [59] | Culex pipiens | 75 ppm |
|
| |||
| ZnO | Cucurbita [59] | Cx. tritaeniorhynchus | 44.68 ppm |
|
| |||
| MgO | Penicillium chrysogenum [60] | An. stephensi | 12.5–15.5 ppm |
|
| |||
| MgO | Chemical method [58] | Cx. quinquefasciatus | 83.4 mg/L |
|
| |||
| CuO | Chemical method [58] | Cx. quinquefasciatus | 100.8 mg/L |
|
| |||
| CuO | Tridax procumbens [61] | Ae. aegypti | 4.209 mg/L |
|
| |||
| SiO2 | Chemical method [58] | Cx. quinquefasciatus | 27.81 mg/L |
|
| |||
| Se | Nilgirianthus ciliatus [62] | Ae. aegypti | 0.92 mg/L |
Besides, a recent review by Benelli pointed out that NPs could not only be used as mosquito larvicides, ovicides, and adulticides [66, 67] but also reduce mosquito longevity and fecundity [68]. In Thelma's work, the synthesized Ag NPs exhibited significant ovicidal activity. The LC50 values for the ovicidal activity were 13.96 ppm, 63.31 ppm, and 24.54 ppm for Ae. aegypti, Cx. quinquefasciatus, and An. stephensi, respectively, which revealed that Ae. aegypti was more susceptible to Ag NPs followed by Cx. quinquefasciatus and An. stephensi [53]. In other studies, growth inhibition was also found in An. stephensi and Cx. quinquefasciatus at all the concentrations tested in silica NPs [65].
4.2. NPs Act as Transport Agents against Vector Insert
Pesticide microencapsulation technology is to encapsulate liquid or solid pesticides in capsule materials to prepare tiny capsule preparations with a particle size of 0.1–10.0 μm, to protect the active ingredients of pesticides from light, air, and other external factors. Decomposition, especially for environmentally sensitive pesticides, making them into microcapsule formulations can greatly improve their effective utilization and achieve high-efficiency mosquito killing effects [69]. In addition, microencapsulation technology not only has the advantages of prolonging drug release time, reducing solvent harm, improving drug efficacy, and improving stability [70] but also can play a huge potential in improving the performance of drug preparations. The technology of microencapsulation enables nanotechnology to work. Compared with traditional drugs, the use of nanomaterials as carriers for drug delivery can protect drugs, delay drug release, improve drug utilization, and reduce drug side effects, so it has more obvious advantages in the biological field (Figure 3).
Figure 3.
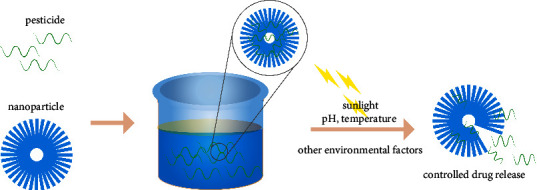
Nanoparticles act as carriers to loading pesticides. Nanoparticles encapsulate pesticides to protect them from external factors. Meanwhile, under the stimulation of these external factors, active components will be released to achieve the controllable effect.
Some selected nanomaterials, such as ZIF-8, have been tested to stabilize and boost the efficacy of dinotefuran against vector [71]. Zhang adopted a nanocarrier (star polycation, SPc) based on electrostatic interaction with the fluorine group in flufenoxuron to form a flufenoxuron/SPc complex, which reduced the particle size of flufenoxuron from 933 nm to 70 nm and significantly improved larval mortality [72]. Among them, the nanohollow microsphere has an internal cavity structure that can be used to load other small molecule substances, which can efficiently load drugs and proteins to achieve the purpose of targeted delivery. For example, SiO2 is classic mesoporous material with outstanding properties such as biocompatibility, pore volume, and specific surface area. Studies confirm that SiO2 NPs are relatively stable in water and phosphate-buffered saline, but in cell media, the release of drug molecules can be regulated by controlled corrosion of silica [73]. In addition, NPs have high UV reflectivity and strong stability, which can be used for the protection of light-sensitive drugs. After the Bacillus thuringiensis, chitinase was loaded with silica NPs, the pH tolerance, thermal stability, and UV resistance were improved, and the insecticidal activity against Caenorhabditis elegans was enhanced [74]. Mesoporous silica also could protect protein Cry 5B from nematode pepsin digestion, while relatively rapidly transporting protein into the intestinal lumen of nematodes [75]. Besides protecting the drug from consumption, the use of NPs could enhance the solubility of insecticides. Water-soluble insecticides can be prepared by modifying chitosan and porous silica-based NPs [76–78]. The use of solid lipid mixtures of NPs can also solve the evaporation or volatilization problems associated with the application of insecticides. All these studies highlight the potential advantages of NPs.
4.3. Mode of Action of NPs against Vector Insects
Studies have shown that the insecticidal effect of NPs on vector insects occurs in the following ways:
Due to the scale effect of NPs, the adhesion of pesticides in the environment can be improved, increasing the possibility of insect exposure to NPs in the environment. After that, NPs could dehydrate cells through stratum corneum adsorption, leading to several morphological and histological abnormalities in insects. Among different NPs, silica and aluminum are represented to bind with the cuticle layer of ticks, leading to physical uptake of lipids and waxes, cell dehydration, and eventual cell death [79] (Figure 4(a)). Experiments by Sultana found that the carbon-dotted silver NPs had high toxicity to Anopheles stutzeri and Culex quinquefasciatus. SEM analysis in this experiment showed that the above carbon-dotted silver NPs caused deformation of larvae, while X-ray analysis proved nanohybrids in the treated. The presence of Ag in mosquito tissues suggests that their death may be due to the toxicity of nano-Ag at the cellular level. In addition, HR-TEM also showed stratum corneum and cellular tissue damage [80]. Third-instar Aedes aegypti larvae exposed to ZnO NPs (1.57 mg/mL, 24 hours) prepared from Lobelia were found to have abdominal contractions, altered thorax shape, midgut lesions, and loss of lateral hairs, anal gills, and brushes, while the accumulation of ZnO NPs was observed in the chest and abdomen [81].
NPs enter the insect body, causing oxidative stress in the insect, destroying the protein in the insect body, and disturbing the normal physiological function of the insect. In vivo studies, DNA damage and oxidative stress have been found when cellular uptake NPs [82]. Previous research studies have shown that NPs have insecticidal activity due to its cytotoxicity. Some NPs, such as TiO2, can absorb UV in the environment, the electrons are excited to become highly active after absorbing energy, and holes are generated in the valence band. The oxygen in the air combines with the e− to produce O2−. H2O undergoes oxidation reaction with produced holes to generate reactive oxygen species (ROS), such as H2O2, O2−, and H2O2 reacting with glycosides, unsaturated fatty acids, proteins, and other substances in the cell, resulting in the death of cells (Figure 4(b)). Besides, the metal ions contained in the material enter the cell during the process of outward release and bind to the amino acids S and P in proteins, reducing cell membrane permeability [83]. Once inside cells, NPs may cause damage to DNA and inhibit the activity of intracellular enzymes [84]. Very recently, Chimkhan outlined that Ag NPs ingested by Bacillus thuringiensis led to mortality and detrimental effects on Aedes aegypti larvae. The results showed that six protein expressions in A. aegypti larvae were involved in DNA and protein damage, inhibition of cell proliferation, and cell apoptosis, indicating that NPs can affect basic physiological regulation in insects [85].
The expression of target genes in insects is affected after NPs enter the body of the insect. The effect of NPs on gene expression in insects was published in 2011, and Nair and Choi evaluated the expression of GST genes, which are linked with the occurrence of oxidative stress after Chironomus riparius had been exposed to the different concentrations of Cd and Ag NPs for various time [86]. The result shows that all GST genes have up-down regulation to varying levels; among them Delta3, Sigma4, and Epsilonl GST classes have the highest expression levels. However, the expression of genes is not static. Studies have shown that after short-term (24 h) exposure to nanoinsecticides, the expression of detoxification-related genes decreases, but after long-term (48 h) exposure, the expression of detoxification-related genes (P-gp and hr96) increases significantly. Expression of reproductive system-related genes (cyp314, vtg, and dmrt93) varies with concentration and time [87]. Recently, Zhang analyzed the effect of lufenuron/SPc nanocomplexes on insect gene expression by RNA-seq technology and found that it not only significantly downregulated insect cuticle-related genes (cut) and inhibited the formation of insect cuticles but also upregulated endocytosis-related genes, promoting drug intake [72]. This suggests that NPs can improve insect toxicity by affecting insect gene expression (Figure 4(c)).
Figure 4.
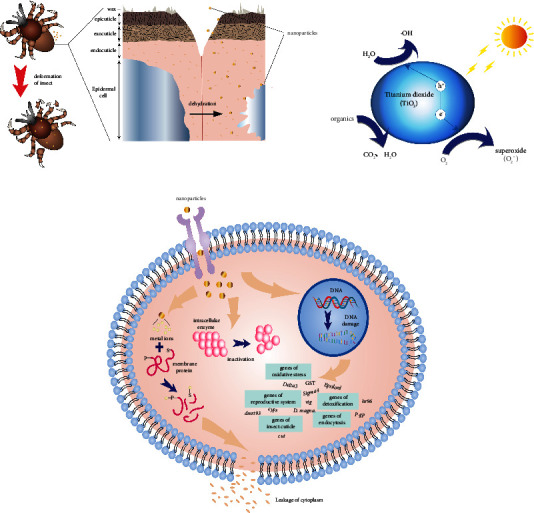
Mode of action of NPs against vector insects. (a) The NPs bind to the body surface of vectors, leading to the physical absorption of lipids and waxes, causing cell dehydration and deformation of the larvae. (b) Photocatalytic mechanism of titanium dioxide, generating peroxides such as reactive oxygen species causing oxidative stress in vectors. (c) Several ways in which NPs can cause the death of vectors by affecting cells: (1) metal NPs release metal ions that reduce the permeability of cell membranes and cause cytoplasmic leakage; (2) NPs reduce enzyme activity; and (3) NPs cause DNA damage and also affect the related gene expression.
4.4. The Safety of the NPs
The small size, large surface, and more active area account for the extensive use of NPs in various applications, such as in drug delivery [88], diagnostics [89], food science [90], electronics [91], and several other biological and nonbiological areas. With the increased interest in nanotechnology in the last decade, the safety of NPs in the environment has also been considered. Therefore, in addition to the toxicity of NPs to vector insects, the impact of NPs on nontarget organisms in the environment has become the focus of attention. In a study, Chakraborty et al. used zebrafish as a model organism to investigate the toxic effects of silver NPs, gold NPs, and several metal oxide NPs (TiO2, Al2O3, CuO, NiO, and ZnO) on it [92]. The correlation between successful hatching efficiency and embryotoxicity is an important parameter for evaluating nanotoxicity. The researchers learned about embryotoxicity by assessing the relationship between hatching success and hours after exposure to TiO2 NPs. The study found that titanium dioxide NPs can cause premature hatching of zebrafish embryos in a dose-dependent manner. Through gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses, Lu et al. found that silver NPs caused the accumulation of reactive oxygen species (ROS) and malondialdehyde (MDA) in zebrafish embryos, inhibiting the superoxide dismutase (SOD), catalase (CAT), and mitochondrial complex I-V activity, and downregulated the expression of SOD, CAT, and mitochondrial complex I-IV chain-related genes, ultimately leading to zebrafish embryotoxicity [93]. However, some studies have also shown that TiO2 NPs can reduce the toxicity of pesticides to zebrafish. To study the bioconcentration and cardiotoxicity mechanism of azoxystrobin (AZ) under the action of TiO2 NPs, zebrafish embryos were exposed for 72 h after fertilization. The results showed that the presence of TiO2 significantly reduced AZ accumulation in larvae compared with AZ alone and thus notably decreased AZ-induced cardiotoxicity, including changes in gene expression of heart rate, pericardium edema, venous thrombosis, sinus venosus, and bulbus arteriosus distance [94]. This suggests that insecticides mixed with NPs help to slow the release of active molecules, thereby helping to reduce the toxic effects of insecticides [95, 96]. The effects of Ag NPs synthesized by T. asiatica aqueous extract on the predation rate of Culex quinquefasciatus by zebrafish were also evaluated in indoor and field experiments. It was found that the predation of mosquito larvae was slightly higher than in normal laboratory conditions in the silver-exposed environment, indicating that Ag NPs can be used at low doses to reduce the number of mosquito vectors without adversely affecting the predation of natural enemies [97]. In addition to acute toxicity, whether NPs have long-term toxicity to organisms has also been investigated. Al2O3 NPs and ZnO NPs are fed uninterrupted daily to rats for 75 days, and the result found that NPs caused hepato-renal toxicity at all parameter levels and inhibited the expression of mitochondrial transcription factor A gene and peroxisome proliferator-activated receptor gamma coactivator [98]. Chronic exposure of the Northern Pike (Esox lucius) and their primary prey species Yellow Perch (Perca flavescens) to Ag NPs caused the length- and weight-at-age (indices of growth rate) of the Northern Pike decline and the per capita availability of Yellow Perch declined by over 30% [99]. Despite considerable efforts to understand the toxicity and safety of these NPs, many of these questions are not yet fully answered. The differences in the toxicity of these NPs may depend on their biophysical properties, including size, surface area, surface charge, and aggregation state, which have been shown to influence the distribution and deposition of NPs in different organ systems and alter their molecular interactions with various proteins and other macromolecules [100].
4.5. Challenges and Opportunities
Nanotechnology is an ecological branch of vector control, and previous studies indicated its great potential for a practical application already, but several questions remain to be solved as follows:
How to improve the economic efficiency of NPs biosynthesis.
The potential connection between loading efficiency and the relevant property of NPs, such as size, shape, and crystal structure.
The mechanism and correlation between the dose of different kinds of NPs and acute or long-term toxicity of organisms.
Effects of NPs residue on generations of vectors and nontarget organisms in the environment.
The above challenges cannot be solved by short-term experiments, and people should work for long-term experimental results to confirm conclusions.
5. Conclusions
Historically and currently, vector control is a very effective means of preventing and controlling infectious diseases. However, environmental changes, pesticide resistance, and population growth have created a new set of challenges for current vector control. There is an urgent need to develop new vector control tools using all our existing interventions, including insecticide and nonpesticide-based control methods. As a rapidly developing new discipline, the application of nanotechnology has been involved in many fields such as medicine, agriculture, catalysis, and biosensing. Compared with traditional chemical and physical methods to synthesize nanomaterials, green synthesis methods have better sustainability and environmental safety. In addition to possibly having some anti-insect effects on their own, NPs can also be effective in carrying pesticides [76–78]. In this review, we found that almost every type of NPs have been used for insect resistance, including metal, nonmetal, and other substances with particle sizes on the nanometer scale. The novel method for obtaining these NPs is the biological synthesis approach, which has better sustainability and environmental safety compared with traditional chemical methods. In addition to possibly possessing insecticidal efficiency on their own, NPs that are synthesized by different methods can also be effective in carrying pesticides, solving the evaporation or volatilization issues associated with pesticide application, and helping to slow the release of the active ingredient, thereby reducing the pesticide's toxic effects. Overall, NPs have provided new ideas for the prevention and control of infectious disease vector insects by elaborating on the insecticide mechanism and safety. Due to the diversity of species, nanoparticles have an immeasurable potential for vector control. Nevertheless, despite efforts to elucidate the mechanisms of nanoparticle toxicity in the three different aspects, our knowledge in the related field is still lacking, the information on the effects of NPs on different types of vector insects is unclear, and the impact of the size, shape, and charge of NPs on various potential mechanisms of action is also to be verified. Because of the uncertainty of the toxicity mechanism of NPs, their sublethal effects on nontarget organisms also require further monitoring, especially genotoxicity and fine physiological and behavioral modifications. In addition, exploring the stability of NPs in the environment, finding suitable standard production methods, and developing commercial products based on nano pesticides are also the keys to the next step to applying NPs in practice.
Acknowledgments
This work was supported by the Jiangsu Science and Technology Plan Projects (BK20221196, BE2017620, and BE2022682).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Chala B., Hamde F. Emerging and Re-emerging vector-borne infectious diseases and the challenges for control: a review. Frontiers in Public Health . 2021;9 doi: 10.3389/fpubh.2021.715759.715759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warpeha K. M., Munster V., Mullié C., Chen S. H. Editorial: emerging infectious and vector-borne diseases: a global challenge. Frontiers in Public Health . 2020;8:p. 214. doi: 10.3389/fpubh.2020.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanyalew T., Natea G., Amenu D., Yewhalaw D., Simma E. A.-O. Composition of mosquito fauna and insecticide resistance status of Anopheles gambiae sensu lato in Itang special district, Gambella, Southwestern Ethiopia. Malaria Journal . 2022;21(1):p. 125. doi: 10.1186/s12936-022-04150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yawa M., Nyangiwe N., Jaja I. F., Marufu M. C., Kadzere C. T. Acaricide resistance of Rhipicephalus decoloratus ticks collected from communal grazing cattle in South Africa. Journal of Advanced Veterinary and Animal Research . 2022;9(1):33–41. doi: 10.5455/javar.2022.i566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadi J., Azizi K., Alipour H. A.-O., et al. Frequency of pyrethroid resistance in human head louse treatment: systematic review and meta-analysis. Parasite . 2021;28:p. 86. doi: 10.1051/parasite/2021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Nahhal I., El-Nahhal Y. Pesticide residues in drinking water, their potential risk to human health and removal options. Journal of Environmental Management . 2021;299 doi: 10.1016/j.jenvman.2021.113611.113611 [DOI] [PubMed] [Google Scholar]
- 7.Varghese J. V., Sebastian E. M., Iqbal T., Tom A. A. Pesticide applicators and cancer: a systematic review. Reviews on Environmental Health . 2020;36(4):467–476. doi: 10.1515/reveh-2020-0121. [DOI] [PubMed] [Google Scholar]
- 8.Benelli G. Gold nanoparticles - against parasites and insect vectors. Acta Tropica . 2018;178:73–80. doi: 10.1016/j.actatropica.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Jayaseelan C., Abdulhaq A., Ragavendran C., Mohan S. Phytoconstituents assisted biofabrication of copper oxide nanoparticles and their antiplasmodial, and antilarval efficacy: a novel approach for the control of parasites. Molecules . 2022;27(23):p. 8269. doi: 10.3390/molecules27238269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathivanan D., Kamaraj C., Suseem S. R., Gandhi P. R., Malafaia G. Seaweed Sargassum wightii mediated preparation of TiO(2) nanoparticles, larvicidal activity against malaria and filariasis vectors, and its effect on non-target organisms. Environmental Research . 2023;225 doi: 10.1016/j.envres.2023.115569.115569 [DOI] [PubMed] [Google Scholar]
- 11.Kamaraj C., Vimal S., Ragavendran C., Priyadharsan A., Marimuthu K., Malafaia G. Traditionally used medicinal plants mediate the biosynthesis of silver nanoparticles: methodological, larvicidal, and ecotoxicological approach. Science of the Total Environment . 2023;873 doi: 10.1016/j.scitotenv.2023.162402.162402 [DOI] [PubMed] [Google Scholar]
- 12.Minal S. P., Prakash S. Laboratory analysis of Au-Pd bimetallic nanoparticles synthesized with Citrus limon leaf extract and its efficacy on mosquito larvae and non-target organisms. Scientific Reports . 2020;10(1) doi: 10.1038/s41598-020-78662-y.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali M. What function of nanoparticles is the primary factor for their hyper-toxicity? Advances in Colloid and Interface Science . 2023;314 doi: 10.1016/j.cis.2023.102881.102881 [DOI] [PubMed] [Google Scholar]
- 14.P Ferreira T., Haddi K., F T Corrêa R., et al. Prolonged mosquitocidal activity of Siparuna guianensis essential oil encapsulated in chitosan nanoparticles. PLoS Neglected Tropical Diseases . 2019;13(8):p. 7624. doi: 10.1371/journal.pntd.0007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos T. S., de Souza Varize C., Sanchez-Lopez E., et al. Entomopathogenic fungi-mediated AgNPs: synthesis and insecticidal effect against plutella xylostella (Lepidoptera: plutellidae) Materials . 2022;15(21):p. 7596. doi: 10.3390/ma15217596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thabet A. F., Boraei H. A., Galal O. A., et al. Silica nanoparticles as pesticide against insects of different feeding types and their non-target attraction of predators. Scientific Reports . 2021;11(1) doi: 10.1038/s41598-021-93518-9.14484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao L., Zhang H., Zhou Z., et al. Fluorophore-free luminescent double-shelled hollow mesoporous silica nanoparticles as pesticide delivery vehicles. Nanoscale . 2018;10(43):20354–20365. doi: 10.1039/c8nr04626c. [DOI] [PubMed] [Google Scholar]
- 18.Shan P., Lu Y., Lu W., et al. Biodegradable and light-responsive polymeric nanoparticles for environmentally safe herbicide delivery. ACS Applied Materials & Interfaces . 2022;14(38):43759–43770. doi: 10.1021/acsami.2c12106. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y., Wang S. J., Jia H. J., et al. Pectin functionalized metal-organic frameworks as dual-stimuli-responsive carriers to improve the pesticide targeting and reduce environmental risks. Colloids and Surfaces B: Biointerfaces . 2022;219 doi: 10.1016/j.colsurfb.2022.112796.112796 [DOI] [PubMed] [Google Scholar]
- 20.Feng J. G., Yang J. H., Shen Y. M., et al. Mesoporous silica nanoparticles prepared via a one-pot method for controlled release of abamectin: properties and applications. Microporous and Mesoporous Materials . 2021;311 doi: 10.1016/j.micromeso.2020.110688.110688 [DOI] [Google Scholar]
- 21.Taran M., Etemadi S., Safaei M. Microbial levan biopolymer production and its use for the synthesis of an antibacterial iron(II,III) oxide-levan nanocomposite. Journal of Applied Polymer Science . 2017;134(12) doi: 10.1002/app.44613.44613 [DOI] [Google Scholar]
- 22.Kurhade P., Kodape S., Choudhury R. Overview on green synthesis of metallic nanoparticles. Chemical Papers . 2021;75(10):5187–5222. doi: 10.1007/s11696-021-01693-w. [DOI] [Google Scholar]
- 23.Soltys L., Olkhovyy O., Tatarchuk T., Naushad M. Green synthesis of metal and metal oxide nanoparticles: principles of green chemistry and raw materials. Magnetochemistry . 2021;7(11):p. 145. doi: 10.3390/magnetochemistry7110145. [DOI] [Google Scholar]
- 24.Jadoun S., Arif R., Jangid N. K., Meena R. K. Green synthesis of nanoparticles using plant extracts: a review. Environmental Chemistry Letters . 2021;19(1):355–374. doi: 10.1007/s10311-020-01074-x. [DOI] [Google Scholar]
- 25.Zielińska A., Carreiró F., Oliveira A. M., et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules . 2020;25(16):p. 3731. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M., Chang J., Wang Z., Zhang H., Wang T. Advances in transport and toxicity of nanoparticles in plants. Journal of Nanobiotechnology . 2023;21(1):p. 75. doi: 10.1186/s12951-023-01830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benelli G. A.-O. Mode of action of nanoparticles against insects. Environmental Science and Pollution Research . 2018;25(13):12329–12341. doi: 10.1007/s11356-018-1850-4. [DOI] [PubMed] [Google Scholar]
- 28.Shoaib A., Elabasy A., Waqas M., et al. Entomotoxic effect of silicon dioxide nanoparticles on Plutella xylostella (L.) (Lepidoptera: plutellidae) under laboratory conditions. Toxicological and Environmental Chemistry . 2018;100(1):80–91. doi: 10.1080/02772248.2017.1387786. [DOI] [Google Scholar]
- 29.Teo B. K., Sun X. H. From top-down to bottom-up to hybrid nanotechnologies: road to nanodevices. Journal of Cluster Science . 2006;17(4):529–540. doi: 10.1007/s10876-006-0086-5. [DOI] [Google Scholar]
- 30.Marinescu L., Ficai D., Ficai A. A.-O., et al. Comparative antimicrobial activity of silver nanoparticles obtained by wet chemical reduction and solvothermal methods. International Journal of Molecular Sciences . 2022;23(11):p. 5982. doi: 10.3390/ijms23115982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirghani M., Osman A. Modified sol-gel process for synthesis of molybdenum oxide-doped titanium dioxide. MethodsX . 2022;9 doi: 10.1016/j.mex.2022.101742.101742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J., Ma Y., Chen Q., et al. Effect of water-oil ratio on the photocatalytic performance of visible light-active BiVO4 nanoparticles prepared by inverse microemulsion-calcination method. Chemosphere . 2022;299 doi: 10.1016/j.chemosphere.2022.134454.134454 [DOI] [PubMed] [Google Scholar]
- 33.Ndlwana L., Raleie N., Dimpe K. M., et al. Sustainable hydrothermal and solvothermal synthesis of advanced carbon materials in multidimensional applications: a review. Materials . 2021;14(17):p. 5094. doi: 10.3390/ma14175094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hongren L., Feng L., Aimin D. A solvothermal method to synthesize fluorescent carbon nanoparticles and application to photocatalysis and electrocatalysis. Luminescence . 2015;30(6):740–744. doi: 10.1002/bio.2813. [DOI] [PubMed] [Google Scholar]
- 35.Piro N. S., Hamad S. M., Mohammed A. S., Barzinjy A. A. Green synthesis magnetite (Fe₃O₄) nanoparticles from Rhus coriaria extract: a characteristic comparison with a conventional chemical method. IEEE Transactions on NanoBioscience . 2023;22(2):308–317. doi: 10.1109/tnb.2022.3187344. [DOI] [PubMed] [Google Scholar]
- 36.Shaheen I., Ahmad K. S. Chromatographic identification of “green capping agents” extracted from Nasturtium officinale (Brassicaceae) leaves for the synthesis of MoO(3) nanoparticles. Journal of Separation Science . 2020;43(3):598–605. doi: 10.1002/jssc.201900840. [DOI] [PubMed] [Google Scholar]
- 37.Anwaar S., Maqbool Q., Jabeen N., et al. The effect of green synthesized CuO nanoparticles on callogenesis and regeneration of oryza sativa L. Frontiers of Plant Science . 2016;7:p. 1330. doi: 10.3389/fpls.2016.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathania D., Sharma A., Kumar S., Srivastava A. K., Kumar A., Singh L. Bio-synthesized Cu-ZnO hetro-nanostructure for catalytic degradation of organophosphate chlorpyrifos under solar illumination. Chemosphere . 2021;277 doi: 10.1016/j.chemosphere.2021.130315.130315 [DOI] [PubMed] [Google Scholar]
- 39.Sholkamy E. N., Ahamd M. S., Yasser M. M., Eslam N. Anti-microbiological activities of bio-synthesized silver Nano-stars by Saccharopolyspora hirsuta. Saudi Journal of Biological Sciences . 2019;26(1):195–200. doi: 10.1016/j.sjbs.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raliya R., Tarafdar J. C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in clusterbean (cyamopsis tetragonoloba L.) Agricultural Research . 2013;2(1):48–57. doi: 10.1007/s40003-012-0049-z. [DOI] [Google Scholar]
- 41.Govindarajan M., Benelli G. J. J. E. One-pot fabrication of silver nanocrystals using Ormocarpum cochinchinense: biophysical characterization of a potent mosquitocidal and toxicity on non-target mosquito predators. Journal of Asia-Pacific Entomology . 2016;19(2):377–385. doi: 10.1016/j.aspen.2016.04.003. [DOI] [Google Scholar]
- 42.Govindarajan M., Benelli G. Facile biosynthesis of silver nanoparticles using Barleria cristata: mosquitocidal potential and biotoxicity on three non-target aquatic organisms. Parasitology Research . 2016;115(3):925–935. doi: 10.1007/s00436-015-4817-0. [DOI] [PubMed] [Google Scholar]
- 43.Mohd Yusof H. A.-O., Abdul Rahman N., Mohamad R. A.-O., Hasanah Zaidan U., Samsudin A. A.-O. Antibacterial potential of biosynthesized zinc oxide nanoparticles against poultry-associated foodborne pathogens: an in vitro study. Animals . 2021;11(7):p. 2093. doi: 10.3390/ani11072093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu C., De S., Balu A. M., Ojeda M., Luque R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chemical Communications . 2015;51(31):6698–6713. doi: 10.1039/c4cc09876e. [DOI] [PubMed] [Google Scholar]
- 45.Shedbalkar U., Singh R., Wadhwani S., Gaidhani S., Chopade B. A. Microbial synthesis of gold nanoparticles: current status and future prospects. Advances in Colloid and Interface Science . 2014;209:40–48. doi: 10.1016/j.cis.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Hao C., Li J., Zhang Z., et al. Enhancement of photocatalytic properties of TiO(2) nanoparticles doped with CeO(2) and supported on SiO(2) for phenol degradation. Applied Surface Science . 2015;331:17–26. doi: 10.1016/j.apsusc.2015.01.069. [DOI] [Google Scholar]
- 47.Kharisov B. I., Kharissova O. V., Ortiz Méndez U., De La Fuente I. G. Decoration of carbon nanotubes with metal nanoparticles: recent t. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry . 2016;46(1):55–76. doi: 10.1080/15533174.2014.900635. [DOI] [Google Scholar]
- 48.Albanese A., Tang P. S., Chan W. C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annual Review of Biomedical Engineering . 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 49.Iravani S. Bacteria in nanoparticle synthesis: current status and future prospects. International Scholarly Research Notices . 2014;2014:1–18. doi: 10.1155/2014/359316.359316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah M., Fawcett D., Sharma S., Tripathy S., Poinern G. Green synthesis of metallic nanoparticles via biological entities. Materials . 2015;8(11):7278–7308. doi: 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali M. M., Ramadan M. A., Ghazawy N. A., Afify A., Mousa S. A. Photochemical effect of silver nanoparticles on flesh fly larval biological system. Acta Histochemica . 2022;124(3) doi: 10.1016/j.acthis.2022.151871.151871 [DOI] [PubMed] [Google Scholar]
- 52.Bonizzoni M., Gasperi G., Chen X., James A. A. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends in Parasitology . 2013;29(9):460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thelma J., Balasubramanian C. Ovicidal, larvicidal and pupicidal efficacy of silver nanoparticles synthesized by Bacillus marisflavi against the chosen mosquito species. PLoS One . 2021;16(12) doi: 10.1371/journal.pone.0260253.260253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavithra Bharathi V., Ragavendran C., Murugan N., Natarajan D. Ipomoea batatas (Convolvulaceae)-mediated synthesis of silver nanoparticles for controlling mosquito vectors of Aedes albopictus, Anopheles stephensi, and Culex quinquefasciatus (Diptera:Culicidae) Artificial Cells, Nanomedicine, and Biotechnology . 2017;45(8):1568–1580. doi: 10.1080/21691401.2016.1261873. [DOI] [PubMed] [Google Scholar]
- 55.Muthukumaran U., Govindarajan M., Rajeswary M. Green synthesis of silver nanoparticles from Cassia roxburghii—a most potent power for mosquito control. Parasitology Research . 2015;114(12):4385–4395. doi: 10.1007/s00436-015-4677-7. [DOI] [PubMed] [Google Scholar]
- 56.Gandhi A. D., Murugan K., Umamahesh K., Babujanarthanam R., Kavitha P., Selvi A. Lichen Parmelia sulcata mediated synthesis of gold nanoparticles: an eco-friendly tool against Anopheles stephensi and Aedes aegypti. Environmental Science and Pollution Research . 2019;26(23):23886–23898. doi: 10.1007/s11356-019-05726-6. [DOI] [PubMed] [Google Scholar]
- 57.Velsankar K., Sudhahar S., Maheshwaran G., Krishna Kumar M. Effect of biosynthesis of ZnO nanoparticles via Cucurbita seed extract on Culex tritaeniorhynchus mosquito larvae with its biological applications. Journal of Photochemistry and Photobiology B: Biology . 2019;200 doi: 10.1016/j.jphotobiol.2019.111650.111650 [DOI] [PubMed] [Google Scholar]
- 58.Chakrabarti A., Patra P. Relative larvicidal property of common oxide nanostructures against Culex quinquefasciatus. IET Nanobiotechnology . 2020;14(5):389–395. doi: 10.1049/iet-nbt.2020.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdo A. M., Fouda A. A.-O., Eid A. M., et al. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and their activity against pathogenic microbes and common house mosquito, Culex pipiens. Materials . 2021;14(22):p. 6983. doi: 10.3390/ma14226983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fouda A., Awad M. A., Eid A. M., et al. An eco-friendly approach to the control of pathogenic microbes and Anopheles stephensi malarial vector using magnesium oxide nanoparticles (Mg-NPs) fabricated by Penicillium chrysogenum. International Journal of Molecular Sciences . 2021;22(10):p. 5096. doi: 10.3390/ijms22105096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muthamil Selvan S., Vijai Anand K., Govindaraju K., et al. Green synthesis of copper oxide nanoparticles and mosquito larvicidal activity against dengue, zika and chikungunya causing vector Aedes aegypti. IET Nanobiotechnology . 2018;12(8):1042–1046. doi: 10.1049/iet-nbt.2018.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meenambigai K., Kokila R., Chandhirasekar K., et al. Green synthesis of selenium nanoparticles mediated by nilgirianthus ciliates leaf extracts for antimicrobial activity on foodborne pathogenic microbes and pesticidal activity against Aedes aegypti with molecular docking. Biological Trace Element Research . 2022;200(6):2948–2962. doi: 10.1007/s12011-021-02868-y. [DOI] [PubMed] [Google Scholar]
- 63.Benelli G., Maggi F., Romano D., et al. Nanoparticles as effective acaricides against ticks-A review. Ticks and Tick-Borne Diseases . 2017;8(6):821–826. doi: 10.1016/j.ttbdis.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 64.Kumar P. M., Murugan K., Madhiyazhagan P., et al. Biosynthesis, characterization, and acute toxicity of Berberis tinctoria-fabricated silver nanoparticles against the Asian tiger mosquito, Aedes albopictus, and the mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoides. Parasitology Research . 2016;115(2):751–759. doi: 10.1007/s00436-015-4799-y. [DOI] [PubMed] [Google Scholar]
- 65.Barik T. K., Kamaraju R., Gowswami A. Silica nanoparticle: a potential new insecticide for mosquito vector control. Parasitology Research . 2012;111(3):1075–1083. doi: 10.1007/s00436-012-2934-6. [DOI] [PubMed] [Google Scholar]
- 66.Rajaganesh R., Murugan K., Panneerselvam C., et al. Fern-synthesized silver nanocrystals: towards a new class of mosquito oviposition deterrents? Research in Veterinary Science . 2016;109:40–51. doi: 10.1016/j.rvsc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Sujitha V., Murugan K., Paulpandi M., et al. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitology Research . 2015;114(9):3315–3325. doi: 10.1007/s00436-015-4556-2. [DOI] [PubMed] [Google Scholar]
- 68.Benelli G., Maggi F., Pavela R., et al. Mosquito control with green nanopesticides: towards the One Health approach? A review of non-target effects. Environmental Science and Pollution Research . 2018;25(11):10184–10206. doi: 10.1007/s11356-017-9752-4. [DOI] [PubMed] [Google Scholar]
- 69.Wang C., Zhu H., Li N., et al. Dinotefuran nano-pesticide with enhanced valid duration and controlled release properties based on a layered double hydroxide nano-carrier. Environmental Sciences: Nano . 2021;8(11):3202–3210. doi: 10.1039/d1en00661d. [DOI] [Google Scholar]
- 70.Cui J., Sun C., Wang A., et al. Dual-Functionalized pesticide nanocapsule delivery system with improved spreading behavior and enhanced bioactivity. Nanomaterials . 2020;10(2):p. 220. doi: 10.3390/nano10020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma S., Ji Y., Dong Y., Chen S., Wang Y., Lü S. An environmental-friendly pesticide-fertilizer combination fabricated by in-situ synthesis of ZIF-8. Science of the Total Environment . 2021;789:147845–114755. doi: 10.1016/j.scitotenv.2021.147845. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L., Yan S., Li M., et al. Nanodelivery system alters an insect growth regulator’s action mode: from oral feeding to topical application. ACS Applied Materials & Interfaces . 2022;14(30):35105–35113. doi: 10.1021/acsami.2c08239. [DOI] [PubMed] [Google Scholar]
- 73.Ahmadi F., Sodagar-Taleghani A., Ebrahimnejad P., et al. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. International Journal of Pharmaceutics . 2022;625 doi: 10.1016/j.ijpharm.2022.122099.122099 [DOI] [PubMed] [Google Scholar]
- 74.Qin X., Xiang X., Sun X., Ni H., Li L. Preparation of nanoscale Bacillus thuringiensis chitinases using silica nanoparticles for nematicide delivery. International Journal of Biological Macromolecules . 2016;82:13–21. doi: 10.1016/j.ijbiomac.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 75.Wu C. C., Hu Y., Miller M., Aroian R. V., Sailor M. J. Protection and delivery of anthelmintic protein Cry5B to nematodes using mesoporous silicon particles. ACS Nano . 2015;9(6):6158–6167. doi: 10.1021/acsnano.5b01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Etesami H. Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agriculture, Ecosystems & Environment . 2018;253:98–112. doi: 10.1016/j.agee.2017.11.007. [DOI] [Google Scholar]
- 77.Huang B., Chen F., Shen Y., et al. Advances in targeted pesticides with environmentally responsive controlled release by nanotechnology. Nanomaterials . 2018;8(2):p. 102. doi: 10.3390/nano8020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaud M. A.-O., Souto E. B., Zielinska A., et al. Nanopesticides in agriculture: benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics . 2021;9(6) doi: 10.3390/toxics9060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zaheer T., Ali M. M., Abbas R. Z., et al. Insights into nanopesticides for ticks: the superbugs of livestock. Oxidative Medicine and Cellular Longevity . 2022;2022:18. doi: 10.1155/2022/7411481.7411481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sultana N., Raul P. K., Goswami D., Das B., Gogoi H. K., Raju P. S. Nanoweapon: control of mosquito breeding using carbon-dot-silver nanohybrid as a biolarvicide. Environmental Chemistry Letters . 2018;16(3):1017–1023. doi: 10.1007/s10311-018-0712-0. [DOI] [Google Scholar]
- 81.Banumathi B., Vaseeharan B., Ishwarya R., et al. Toxicity of herbal extracts used in ethno-veterinary medicine and green-encapsulated ZnO nanoparticles against Aedes aegypti and microbial pathogens. Parasitology Research . 2017;116(6):1637–1651. doi: 10.1007/s00436-017-5438-6. [DOI] [PubMed] [Google Scholar]
- 82.Demir E. An in vivo study of nanorod, nanosphere, and nanowire forms of titanium dioxide using Drosophila melanogaster: toxicity, cellular uptake, oxidative stress, and DNA damage. Journal of Toxicology and Environmental Health, Part A . 2020;83(11-12):456–469. doi: 10.1080/15287394.2020.1777236. [DOI] [PubMed] [Google Scholar]
- 83.Foldbjerg R., Jiang X., Miclăuş T., Chen C., Autrup H., Beer C. Silver nanoparticles – wolves in sheep’s clothing? Toxicology Research . 2015;4(3):563–575. doi: 10.1039/c4tx00110a. [DOI] [Google Scholar]
- 84.Chen Z., Shi J., Zhang Y., Han S., Zhang J., Jia G. DNA oxidative damage as a sensitive genetic endpoint to detect the genotoxicity induced by titanium dioxide nanoparticles. Nanomaterials . 2022;12(15):p. 2616. doi: 10.3390/nano12152616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chimkhan N., Thammasittirong S. N., Roytrakul S., Krobthong S., Thammasittirong A. Proteomic response of Aedes aegypti larvae to silver/silver chloride nanoparticles synthesized using Bacillus thuringiensis subsp. israelensis metabolites. Insects . 2022;13(7):p. 641. doi: 10.3390/insects13070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nair P. M., Choi J. Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquatic Toxicology . 2011;101(3-4):550–560. doi: 10.1016/j.aquatox.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Aksakal F. I., Arslan H. Detoxification and reproductive system-related gene expression following exposure to Cu(OH)2 nanopesticide in water flea (Daphnia magna Straus 1820) Environmental Science and Pollution Research . 2020;27(6):6103–6111. doi: 10.1007/s11356-019-07414-x. [DOI] [PubMed] [Google Scholar]
- 88.Mitchell M. J., Billingsley M. M., Haley R. M., Wechsler M. E., Peppas N. A., Langer R. A.-O. Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery . 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gonzalez-Avila G., Sommer B., García-Hernandez A. A., Ramos C., Flores-Soto E. Nanotechnology and matrix metalloproteinases in cancer diagnosis and treatment. Frontiers in Molecular Biosciences . 2022;9 doi: 10.3389/fmolb.2022.918789.918789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dos Santos C. A., Ingle A. P., Rai M. The emerging role of metallic nanoparticles in food. Applied Microbiology and Biotechnology . 2020;104(6):2373–2383. doi: 10.1007/s00253-020-10372-x. [DOI] [PubMed] [Google Scholar]
- 91.Wang B., Li Z., Sebesta C., et al. Multichannel power electronics and magnetic nanoparticles for selective thermal magnetogenetics. Journal of Neural Engineering . 2022;19:26015–26022. doi: 10.1088/1741-2552/ac5b94. [DOI] [PubMed] [Google Scholar]
- 92.Chakraborty C., Sharma A. R., Sharma G., Lee S. S. Zebrafish: a complete animal model to enumerate the nanoparticle toxicity. Journal of Nanobiotechnology . 2016;14(1):p. 65. doi: 10.1186/s12951-016-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu C., Lv Y., Kou G., et al. Silver nanoparticles induce developmental toxicity via oxidative stress and mitochondrial dysfunction in zebrafish (Danio rerio) Ecotoxicology and Environmental Safety . 2022;243 doi: 10.1016/j.ecoenv.2022.113993.113993 [DOI] [PubMed] [Google Scholar]
- 94.Nie H., Pan M., Chen J., et al. Titanium dioxide nanoparticles decreases bioconcentration of azoxystrobin in zebrafish larvae leading to the alleviation of cardiotoxicity. Chemosphere . 2022;307 doi: 10.1016/j.chemosphere.2022.135977.135977 [DOI] [PubMed] [Google Scholar]
- 95.Okey-Onyesolu C. F., Hassanisaadi M., Bilal M., et al. Nanomaterials as nanofertilizers and nanopesticides: an overview. ChemistrySelect . 2021;6(33):8645–8663. doi: 10.1002/slct.202102379. [DOI] [Google Scholar]
- 96.Oliveira J. 1-Nano-biopesticides: present concepts and future perspectives in integrated pest management. Advances in Nano-Fertilizers and NanoPesticides in Agriculture . 2021:1–27. [Google Scholar]
- 97.Murugan K., Venus J. S. E., Panneerselvam C., et al. Biosynthesis, mosquitocidal and antibacterial properties of Toddalia asiatica-synthesized silver nanoparticles: do they impact predation of guppy Poecilia reticulata against the filariasis mosquito Culex quinquefasciatus? Environmental Science and Pollution Research . 2015;22(21):17053–17064. doi: 10.1007/s11356-015-4920-x. [DOI] [PubMed] [Google Scholar]
- 98.Yousef M. I., Mutar T. F., Kamel M. A. E. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicology Reports . 2019;6:336–346. doi: 10.1016/j.toxrep.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Slongo B. D., Hayhurst L. D., Drombolis P. C. T., Metcalfe C. D., Rennie M. D. Whole-lake nanosilver additions reduce northern pike (Esox lucius) growth. Science of the Total Environment . 2022;838(2) doi: 10.1016/j.scitotenv.2022.156219.156219 [DOI] [PubMed] [Google Scholar]
- 100.Kreyling W. G., Semmler-Behnke M., Seitz J., et al. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhalation Toxicology . 2009;21(1):55–60. doi: 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]


