Abstract
Background
Meningiomas occur in 80% of persons with neurofibromatosis 2 (NF2) and cause significant mortality and morbidity, yet there are no effective medical treatments. NF2-deficient tumors have constitutive activation of mammalian/mechanistic target of rapamycin (mTOR), and treatment with mTORC1 inhibitors results in growth arrest in a minority of tumors, with paradoxical activation of the mTORC2/AKT pathway. We studied the effect of vistusertib, a dual mTORC1/mTORC2 inhibitor, in NF2 patients with progressive or symptomatic meningiomas.
Methods
Vistusertib was administered orally at 125 mg twice daily for 2 consecutive days each week. The primary endpoint was the imaging response in the target meningioma, defined as a volume decrease of 20% compared with the baseline. Secondary endpoints included toxicity, imaging response of nontarget tumors, quality of life, and genetic biomarkers.
Results
Eighteen participants (13 female), median age of 41 (range, 18–61) years, were enrolled. In target meningiomas, the best response was partial response (PR) in 1/18 tumors (6%) and stable disease (SD) in 17/18 tumors (94%). For all measured intracranial meningiomas and vestibular schwannomas, the best imaging response was PR in 6/59 tumors (10%) and SD in 53 (90%). Treatment-related grade 3/4 adverse events occurred in 14 (78%) participants, and 9 participants discontinued treatment due to side effects.
Conclusions
Although the study did not meet the primary endpoint, vistusertib treatment was associated with high rates of SD in progressive NF2-related tumors. However, this dosing regimen for vistusertib was poorly tolerated. Future studies of dual mTORC inhibitors for NF2 should focus on optimizing tolerability and evaluating the relevance of tumor stability in participants.
Keywords: meningioma, mTOR, mTORC1, mTORC2, neurofibromatosis 2, NF2, vistusertib
Key Points.
Vistusertib treatment was associated with a high rate of meningioma and vestibular schwannoma stabilization in neurofibromatosis 2 (NF2) patients.
Vistusertib treatment at a dose of 125 mg twice daily on 2 consecutive days each week was not well tolerated by NF2 patients and alternative regimens should be considered in future trials.
Importance of this Study.
Meningiomas occur in 80% of patients with neurofibromatosis type 2 (NF2) and, to date, there are no effective medical therapies for these tumors. NF2-associated meningiomas demonstrate constitutive activation of mTORC1 and mTORC2 signaling, though previous clinical trials of everolimus (mTORC1 inhibitor) have shown cytostatic effects for vestibular schwannomas. Here, we report the results of a phase II single-arm study of the dual mTORC1/mTORC2 inhibitor vistusertib for progressive or symptomatic meningiomas in participants with NF2. While our prespecified imaging response rate was not met, vistusertib was associated with high rates of stable disease for NF2-associated tumors including progressive meningiomas. At the studied dosing, vistusertib was poorly tolerated and most participants discontinued the study prior to completing the clinical trial. These data further support the concept of mTOR inhibition as a therapeutic strategy for NF2-associated tumors but underscore the importance of drug tolerability in this population.
Neurofibromatosis 2 (NF2) is a neurogenetic tumor suppressor syndrome with a birth prevalence of 1 in 27,956.1 Persons with NF2 have an increased risk of multiple tumor types, including schwannomas, meningiomas, and ependymomas.2 While schwannomas (primarily bilateral vestibular schwannomas, VS) are the hallmark of NF2, meningiomas are the second most common tumor with a cumulative prevalence of 80% by age 70.3 In a long-term study of 119 persons with NF2, 74 had an evidence of meningioma (median, 3 per person). One-third of tumors demonstrated progressive linear growth exceeding 1 mm/year, and 7.3% of tumors had volumetric growth exceeding 20% per year.4 Meningiomas are a major contributor to the morbidity and mortality of NF2.
The current standard of care for the treatment of progressive or symptomatic meningiomas in NF2 is maximal safe surgical resection, with radiation reserved for the treatment of recurrent tumors or those with aggressive features (eg, WHO grades II or III), or for otherwise symptomatic but surgically inaccessible tumors. Given the young age of presentation for persons with NF2-associated meningiomas, many clinicians avoid or delay radiation therapy due to the risk of secondary malignancies. Many systemic therapies have been studied to treat recurrent or progressive meningiomas, though none have demonstrated clear clinical benefit for either sporadic or NF2-related meningioma.
Mammalian/mechanistic target of rapamycin (mTOR), a serine/threonine kinase, forms 2 distinct functional complexes called mTORC1 and mTORC2 that regulate cell growth, proliferation, and survival.5,6 We previously identified constitutive activation of mTORC1 in NF2-mutant meningioma cells, and in vitro treatment with rapamycin, a potent inhibitor of mTORC1 signaling, revealed a reduction in cell size.7 Based upon this work, a clinical trial of everolimus (a rapalog that selectively targets mTORC1) was performed in VS, demonstrating cytostatic effects.8–10 We subsequently identified that mTORC2 is constitutively activated in NF2-mutant meningioma cells by virtue of downstream activation of serum and glucocorticoid-regulated kinase 1 and hypothesized that this may explain why everolimus was only cytostatic in humans.11,12 There is also evidence that inhibition of mTORC1 activates multiple negative feedback mechanisms that lead to increased Akt signaling.13 Taken together, these observations led us to test the dual mTORC1/mTORC2 inhibitor, vistusertib (formerly AZD2014), in primary human meningioma cells in vitro, which convincingly demonstrated in vitro inactivation of both mTORC1 and mTORC2 signaling pathways and decreased cell proliferation with greater efficacy than rapamycin.11 Based on this information, we sought to test vistusertib in persons with NF2 and progressive meningiomas.
Materials and Methods
Study Design and Patients
This single-institution, open-label phase II trial enrolled persons with NF2 who had symptomatic or progressive meningiomas. The primary endpoint was the proportion of participants who achieved an imaging partial response (PR) to therapy, defined as ≥20% volume reduction in the target meningioma compared with baseline. Secondary endpoints included progression-free survival, progression-free survival at 6 months, proportion of subjects with an imaging PR in a nontarget meningioma or VS, and frequency of adverse events (AEs) related to vistusertib. Outcomes were correlated with germline NF2 pathogenic variants and immunohistochemistry (IHC) markers of mTORC1 and mTORC2 activation (pS6, pAKT, pNDRG) from archival tumor tissue.
The trial (NCT02831257) was approved by the Dana-Farber/Harvard Cancer Center institutional review board and vistusertib was supplied by AstraZeneca. All participants or their legal guardians provided informed consent.
Patients aged 18 years or older meeting National Institutes of Health14 or Manchester criteria15 for NF2 with a symptomatic or progressive meningioma were eligible for enrollment, with progression defined as an increase in target meningioma volume ≥20% OR ≥3 mm during the prior 2 years. Target meningiomas had to be measurable volumetrically and must not have been amenable to surgery or patients had to refuse surgery. Prior surgery or radiation was not a requirement for enrollment, though patients had to have received less than 3 prior chemotherapy regimens. Archival tumor tissue was required for immunohistochemistry, though not necessarily from the target tumor. Previous treatment with another mTORC1/mTORC2 dual inhibitor was prohibited. Finally, due to the risk of cardiac and metabolic toxicities with vistusertib, patients were excluded if they had a cardiac procedure within the last 12 months, had a left ventricular ejection fraction of <55%, uncontrolled type II diabetes, a prolonged QT interval, or required other medications that prolong QT interval.
Vistusertib was administered orally at a dose of 125 mg twice daily on 2 consecutive days each week, with 28 consecutive days defined as 1 treatment cycle. Sequential dose reductions to 100 mg—and then 75 mg—on the same schedule were applied for Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) grade 3 or 4 toxicities, provided recovery to grade 2 or less following drug hold. Participants were assessed by MRI with volumetric tumor analysis at baseline and every 3 cycles thereafter. The target meningioma was determined by the treating clinician, and central MRI review was performed by a central imaging core. Longitudinal tumor volume was additionally determined for up to 4 nontarget meningiomas and for any VS at each time point. Imaging response for tumors was defined according to REiNS criteria for NF-related tumors16: complete response (CR), complete disappearance of enhancing tumor; PR, decrease in tumor volume of 20% or more; progressive disease (PD), increase in tumor volume of 20% or more; and stable disease (SD) for all others. The best imaging response was defined as the smallest volume compared to baseline at any time point during treatment.
AEs were graded and attributed to vistusertib according to the CTCAE before each cycle; physical examination and laboratory monitoring were conducted every 4 weeks. Due to the relatively mild profile of adverse effects observed after the study began, the investigators expanded to allow alternating visits to be done through telehealth with required laboratory evaluations and/or electrocardiogram being done near home.
Quality of Life
NF2-specific quality of life (QoL) was evaluated using the NF2 Impact on Quality of Life (NFTI-QOL) questionnaire.17 This validated questionnaire assesses the 8 domains of hearing, dizziness and balance, facial palsy, sight, mobility and walking, role and outlook on life, pain, and anxiety and depression. Summary scores range from 0 to 24 with higher scores indicating worse NF2-specific QoL. Vestibular schwannoma-specific QoL was evaluated using the Penn Acoustic Neuroma Quality of Life (PAN-QOL) questionnaire, as previously described.18 This validated questionnaire assesses the 7 domains of hearing, balance, facial weakness, anxiety, energy, pain, and general function. Summary scores range from 0 to 100 with higher scores indicating worse VS-specific QoL.
Immunohistochemical Analysis of Tumor Samples
Tissue biomarkers were evaluated in archival formalin-fixed, paraffin-embedded tumor specimens. Antibodies used for IHC staining included phosphorylated ribosomal S6 S235/S236, phosphorylated Akt S473, and phosphorylated NDRG1 T346 (Cell Signaling Technology [CST], Danvers, MA). All histochemical staining was carried out by the Dana-Farber Cancer Institute/Harvard Cancer Center (DF/HCC) Pathology Core. The Core performed sectioning and immunostaining of paraffin-embedded formalin-fixed blocks for pNDRG1 T346 (CST 5482; 1:400), pS6 S235/6 (CST 2211; 1:400) and pAKT S473 (CST 4060; 1:50). Antigen retrieval was performed with EDTA for 15 min for pNDRG1 T346, or in sodium citrate for 45 min for pS6 S235/6 and pAKT S473 with Ventana automated stainer (Discovery Ultra, Ventana, Oro Valley, AZ). Slides were developed with the Ventana OmniMap anti-mouse HRP system (Ventana). All samples were run in parallel with positive and negative controls and were scored by a board-certified pathologist (ASR). Scoring of IHC staining was based on previously published criteria and incorporated staining intensity and extent of staining.19 The staining index score (0–7) was calculated by taking the sum of the staining extent score (0–4) and staining intensity (0–3). A staining index score of 0 was used to define tumors with negative expression and 1–7 indicated positive expression.
Genetic Analysis of the NF2 Gene
In order to identify germline pathogenic variants of participants, genomic DNA (gDNA) was extracted from blood samples using standard phenol/chloroform extraction methods. Following gDNA extraction, all NF2 coding exons were PCR amplified using flanking intronic primers as previously described.20 Sanger sequencing was performed by Eton Biosciences, Inc. (https://www.etonbio.com/).
Statistical Analysis
The primary endpoint was the proportion of participants achieving an imaging response in the target meningioma. We used natural history data for benchmarking.4 With the enrollment of 18 participants, we had 90% power and <5% significance using 1-sided binomial testing to determine the difference between an imaging response rate of 20% versus a historical control response rate of 1%. Based on this, we determined that if at least 2 participants achieved an imaging response in their target meningioma, vistusertib treatment at 125 mg BID twice weekly would be declared worthy of further investigation.
Baseline participant and disease characteristics are presented with standard descriptive summaries. Analysis of imaging response was performed on a per-patient and per-tumor basis to account for target and nontarget tumors. Proportion of PR was calculated and presented along with the exact binomial 95% CIs. Exact binomial test was used for testing proportions. Pearson correlation coefficient was used to estimate the correlation between continuous variables. For QoL studies, the minimal clinically important difference (MCID) was defined as half of the standard deviation of the summary score for the participants at baseline and rounded up to the nearest integer. Improvement and decline in QoL were defined as decreases and increases, respectively, greater than the MCID compared with the baseline. All p-values are reported as 2 sided. All analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC).
Results
Participants and Study Treatment
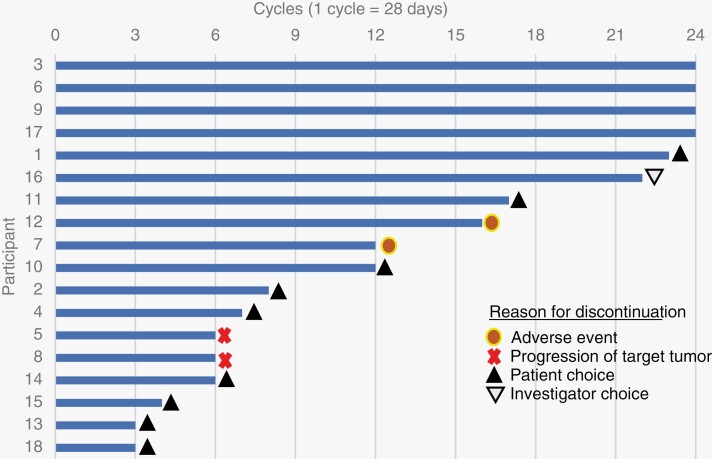
Eighteen participants (13 female), with a median age of 41 years (range, 18–61 years), were screened and enrolled between November 2016 and September 2017 (Table 1). According to the NF2 Genetic Severity Scoring System,21 7 participants (39%) were classified as severe (severity score = 3), 3 as moderate (17%, severity score = 2), and 8 as tissue mosaics (44%, severity score = 1). At baseline, the median volume for target meningiomas was 14 cc (range, 3–68 cc) with a median growth rate of 37% per year (range, −5% to 225% per year). The median volume for 20 nontarget meningiomas was 2.8 cc with a median growth rate of 14% per year, and the median volume for 21 VS was 2.7 cc with a median growth rate of 13% per year. All participants were evaluable for response and toxicity. The median duration of treatment was 12 months (range, 3–24 months) (Fig. 1A).
Table 1.
Participant Demographics (n = 18) and Disease Characteristics
| Participant demographics | N = 18 |
|---|---|
| Age, median (range) | 41 years (18–61 years) |
| Female, n (%) | 13 (72%) |
| Race, n (%) | |
| Caucasian | 17 (94%) |
| Other (not specified) | 1 (6%) |
| Ethnicity, n (%) | |
| Non-Hispanic | 16 (89%) |
| Not known | 2 (11%) |
| KPS at screening, median (range) | 85 (60–100) |
| Genetic severity score | |
| 1. Tissue mosaic, n (%) | 7 (39%) |
| 2. Classic, n (%) | 3 (17%) |
| 3. Severe, n (%) | 8 (44%) |
| Target meningioma | N = 18 |
| Volume, median (range) | 14.5 cc (3.3–68.2 cc) |
| Maximum linear dimension, median (range) | 32.8 mm (20.3–62.9 mm) |
| Annualized growth rate at enrollment (%), mean/median | 51%/33% |
| Nontarget meningiomas | N = 20 |
| Number of participants | 14 |
| Volume, median (range) | 2.8 cc (0.9–19.9 cc) |
| Annualized growth rate at enrollment (%), mean/median | 38%/14% |
| Vestibular schwannomas | N = 21 |
| Number of participants | 16 |
| Volume, median (range) | 2.70 cc (0.3–4.2 cc) |
| Maximum linear measurement (median, range) | 22.9 mm (10–37.2 mm) |
| Annualized growth rate at enrollment (%), mean/median | 20%/13% |
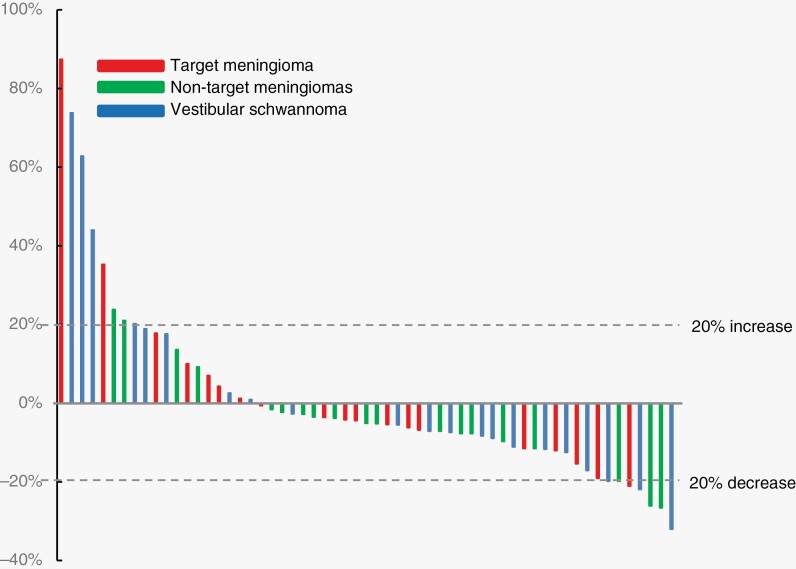
Figure 1.
(A) Duration of study treatment with reasons for treatment discontinuation and (B) best percentage change from baseline in meningiomas and schwannomas (n = 59).
Efficacy
The best imaging response to the treatment for target meningiomas was PR in 1 (6%) and SD in 17 (94%). For nontarget meningiomas (n = 20), the best imaging response was PR in 2 (10%) and SD in the remainder (90%). Among 20 evaluable VS, the best imaging response was PR in 3 (15%) and SD in 17 (85%). For all 59 intracranial tumors, the best imaging response was PR in 6 (10%) and SD in 53 (90%). The best response to therapy for all tumors is shown in Fig. 1B. PD was noted in 17/59 tumors (29%) during treatment, including 4 target meningiomas, 7 nontarget meningiomas, and 6 VS. Freedom from progression for 18 target meningiomas at 6, 12, and 24 months was 88%, 80%, and 64%; median progression-free survival was not reached (95% CI, 96 months, not available). Freedom from progression for 20 nontarget meningiomas at 6, 12, and 24 months was 100%, 90%, and 85%; for the 21 VS was 100%, 95%, and 78%; and for all 59 tumors was 100%, 95%, and 84%. Median progression-free survival was not reached (95% CI, 96 months, not available). For the 13 target meningiomas with progression at enrollment (ie, an annualized growth rate of >20%), 3 progressed on treatment (23%), whereas 9 had SD until study discontinuation (69%), and 1 had imaging response (8%).
NF2- and VS-Related Quality of Life During Vistusertib Therapy
Seventeen, 14, and 14 participants completed evaluation for NFTI-QOL at baseline, 4 months, and off study, respectively. The baseline average summary score was 9.2 (SD 3.8, MCID 2), with hearing problems identified as the greatest contributor to reduced NF2-related QoL. The average summary score decreased to 8.4 (SD 5.3) at 4 months and was 10.0 (SD 5.4) off study. Between baseline and 4 months, NF2-related QoL improved in 6 of 13 participants (46%), declined in 2 (15%), and was stable in 5 (38%). Between 4 months and off study, 8/11 (73%) participants reported a decline in NF2-related QoL participants, 1e (9%) reported improvement, and 2 (18%) reported stability.
Fifteen, 12, and 12 participants completed evaluation for Pan-QOL at baseline, 4 months, and off study, respectively. The baseline average summary score was 61.0 (SD 14.1, MCID 7), with facial function identified as the greatest contributor to reduced QoL related to VS. The average summary score at 4 months was 62.0 (SD 20.4) and was 57.4 (SD 18.4) off study. Nine participants had sufficient data at baseline and month 4 to determine change in QoL. Overall, significant improvement in VS-related QoL was reported in 3 participants (33%), decline in 3 (33%), and stable in 3 (33%). Eight participants had sufficient data at month 4 and off study to determine change in QoL. Significant improvement in VS-related QoL was reported in 5 participants (63%), decline in 1 (13%), and stable in 2 (25%).
Adverse Events
Treatment-related AEs of any grade and grade 3/4 occurred in 18 (100%) and 14 (78%) participants, respectively. AEs reported in ≥10% of participants (Table 2) included nausea (89%); diarrhea (84%); hypophosphatemia (72%); fatigue (67%); abdominal pain (56%); anorexia, mucositis, and dry mouth (39% each); dysgeusia, vomiting, and rash each (33% each); aspartate aminotransferase increased (28%); and constipation (22%). Twelve (67%) participants discontinued the study prior to completing 24 months, of these 9 (50%) discontinued due to participant choice, 1 (6%) discontinued due to investigator decision, and 2 (11%) discontinued due to AEs. No participants experienced a clinically significant decline in ejection fraction on echocardiography during treatment.
Table 2.
Treatment-Related Adverse Events in ≥10% of Patients
| Any grade, N = 18 | Grade 3 or 4, N = 18 | |
|---|---|---|
| Event | No. of patients (%) | |
| Any treatment-related adverse event | 18 (100%) | 14 (78%) |
| Nausea | 16 (89%) | |
| Diarrhea | 15 (84%) | |
| Hypophosphatemia | 13 (72%) | 10 (56%) |
| Fatigue | 12 (67%) | |
| Abdominal pain | 10 (56%) | 1 (6%) |
| Weight loss | 9 (50%) | 1 (6%) |
| Anorexia | 7 (39%) | 1 (6%) |
| Mucositis | 7 (39%) | |
| Dry mouth | 7 (39%) | |
| Dysgeusia | 6 (33%) | |
| Vomiting | 6 (33%) | |
| Skin disorders | 6 (33%) | |
| Aspartate aminotransferase increased | 5 (28%) | 1 (6%) |
| Constipation | 4 (22%) | |
| High cholesterol | 3 (17%) | |
| Hypertriglyceridemia | 3 (17%) | |
| Infection | 3 (17%) | |
| Pruritis | 3 (17%) | 1 (6%) |
| Alanine aminotransferase increased | 2 (11%) | |
| Anemia | 2 (11%) | |
| Headache | 2 (11%) | |
| Seizure | 2 (11%) | |
| Dyspareunia | 2 (11%) | |
Correlative Studies
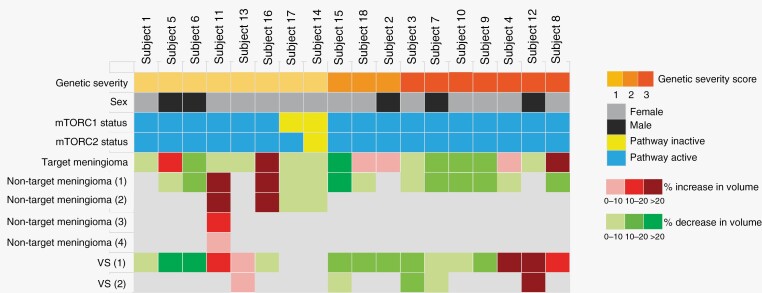
Archival tumor tissue collected during the study included 14 meningiomas, 3 schwannomas, and 1 collision tumor of schwannoma/meningioma. Activation of the mTORC1 pathway (defined as a staining index score of 1–7 for pS6) was noted in 13/14 (93%) meningiomas, in 2/3 (67%) schwannomas, and in the collision meningioma/schwannoma (100%); activation of mTORC2 pathway (defined as a staining index score of 1–7 for pNDRG1 or pAKT) was noted in 14/14 (100%) meningiomas, in 2/3 (67%) schwannomas, and in the collision meningioma/schwannoma (100%) (Table S1). There was no correlation between mTORC1 activation, mTORC2 activation, or genetic severity score with imaging response to vistusertib (Fig. 2).
Figure 2.
Genetic severity, activity of mTORC1 and mTORC2 pathways in archival tumors, and best clinical response in participants treated with vistusertib. Genetic severity was classified as severe (severity score = 3), moderate (severity score = 2), or tissue mosaics (severity score = 1) according to the NF2 Genetic Severity Scoring System.21
Discussion
In this trial involving NF2 patients with progressive or symptomatic meningiomas, we documented a partial imaging response in 6% of target meningiomas and 10% of all intracranial meningiomas and schwannomas. This response rate did not achieve the prespecified requirement to declare the dose of 125 mg BID twice weekly worthy of continued development for NF2 patients. Despite the low imaging response rate, this dosing regimen for vistusertib was associated with high rates of SD for progressive or symptomatic meningiomas. In review of all target and nontarget intracranial tumors, only 17/59 (29%) progressed during treatment, with a median time to progression not reached at 24 months.
One possible explanation for the low imaging response rate was the enrollment of participants with severe disease. Our cohort included a higher proportion with “severe” genetic severity (44%) compared with the Oxford NF2 population (13%), as determined by the analysis of germline NF2 pathogenic variants. We did not identify any relationship between genetic severity and response to vistusertib, and recent retrospective studies of NF2 meningioma have also failed to document a relationship between genetic severity and individual meningioma growth rate.22 Future studies are needed to determine whether germline NF2 variants (as opposed to somatic NF2 variants in tumor tissue) correlate with response to medical therapy.
The correlative studies of archival tumor specimens confirmed activation of the TORC1 and TORC2 pathways in virtually all tumors in our participants, but this activation status did not correlate with imaging response in target meningiomas. The inability to predict therapeutic response by pathway activation status may either reflect the use of nontarget tumors for many of our subjects, or that yet another signaling pathway alteration leads to mTORC1/2 inhibitor resistance. Future studies may consider collecting archival tissue from multiple tumors, as a further study of molecular signatures and signaling alterations is needed to improve our understanding of mTOR dependency, intertumoral variability, and to identify additional therapeutic targets.
In comparison to everolimus, vistusertib at 125 mg BID twice weekly was poorly tolerated by NF2 patients.8–10 In this trial, 50% of participants chose to discontinue treatment due to persistent grade 1/2 AEs. Despite the high rate of AEs, almost half of patients reported improvement in NF2-related QoL during the first 4 months of treatment. About three-fourths of participants reported a decline in NF2-related QoL at the time of treatment discontinuation. Because most tumors did not progress during treatment, a possible explanation for the decline in QoL is exacerbation of neurological symptoms due to medication effects. These findings emphasize the importance of identifying agents that are well tolerated by NF2 patients since they require extended treatment (ie, years) for their tumors.
Our trial has both notable strengths and limitations. First, this single-center trial successfully completed accrual for participants with NF2-related meningioma, confirming the willingness of these patients to enroll in clinical trials. Second, we incorporated measures of genetic severity into the study which confirmed that this cohort was more severely affected than the general NF2 population. We anticipate that the use of this prognostic factor will improve the design of NF2 clinical trials in the future. Third, the trial enrolled a relatively small number of patients given the rarity of NF2. The study was appropriately powered to identify a clinically significant imaging response rate but was not powered to identify clinical, genetic, or tumor characteristics that correlate with imaging outcomes. Thus, it is possible that a larger study would identify germline NF2 variants that influence response to mTOR inhibitors. Fourth, the trial did not include an internal comparator arm (either placebo or active control) given the limitations of enrolling sufficient participants in a single-center study. The lack of a comparator limits our ability to draw definitive conclusions about the relevance of SD for these tumors. Future studies of mTOR inhibitors should consider incorporating design features that determine the relevance of SD. Fifth, half of participants elected to stop treatment during the trial, thereby limiting information about tumor response to vistusertib and durability of any benefit in providing tumor stability. Finally, we used REiNS criteria in this study for determination of imaging response.16 Alternative criteria have been proposed for clinical trials of meningioma, and it is not certain which imaging criteria are most predictive of clinical benefit.
In conclusion, this prospective study of vistusertib in symptomatic or progressive NF2-associated meningiomas did not meet its prespecified target for advancing this dose in future clinical trials. However, we did show that dual mTORC1/mTORC2 inhibition with vistusertib stabilizes the majority of growing NF2-associated meningiomas and schwannomas, though the toxicity of the evaluated dose precludes further development in the NF2 population. Development of vistusertib has been discontinued by AstraZeneca, but alternative dual mTORC1/mTORC2 inhibitors, including potentially less toxic third-generation bi-steric mTOR inhibitors, should be considered for further study in NF2-related meningioma.
Acknowledgments
The authors would like to thank Dr. Miriam Smith for helpful comments regarding molecular analysis of participants. Our thanks to AstraZeneca for providing vistusertib for this trial and to Lisa Moore and Dr. Jan Cosaert for their support of the trial.
Contributor Information
Justin T Jordan, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Christina C Orr, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Raquel D Thalheimer, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Josephine V Cambillo, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Roberta L Beauchamp, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA.
Ghalib Shaikh, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA.
Alona Muzikansky, Biostatistics Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Anat Stemmer-Rachamimov, Department of Pathology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Marco Giovannini, Department of Head and Neck Surgery, David Geffen School of Medicine at UCLA and Jonsson Comprehensive Cancer Center (JCCC), University of California Los Angeles, Los Angeles, CA, USA.
Michel Kalamarides, Department of Neurosurgery, Hopital Pitie-Salpetriere, Sorbonne Université, Paris, France.
Fred G Barker, II, Neurosurgical Service, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Vijaya Ramesh, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA.
Scott R Plotkin, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Funding
Supported by Award Number W81XWH-16-1-0103 from the Department of Defense Neurofibromatosis Research Program.
Conflict of Interest statement
J.T.J.—Paid consulting for NF Network, Recursion Pharmaceuticals, CereXis, Health2047, and Navio Theragnostics. Royalties from Elsevier Publishing. C.C.O.—Paid consulting for AstraZeneca. R.D.T.—No disclosures. J.V.C.—No disclosures. R.L.B.—No disclosures. G.S.—No disclosures. A.M.—No disclosures. A.S.-R.—No disclosures. M.G.—Consults for NF2 Therapeutics and Puma Biotechnology. M.K.—Paid consulting for Recursion Pharmaceuticals. F.G.B.— No disclosures. V.R.—No disclosures. S.R.P.—Dr. Plotkin is co-founder of NFlection Therapeutics and NF2 Therapeutics and consults for AstraZeneca, SonalaSense, and Akouos.
Authorship
Study concept and design: J.T.J., S.R.P., V.R. Data collection: J.T.J., R.D.T., C.C.O., J.V.C., S.R.P., R.L.B., G.S. Analysis and interpretation of data: J.T.J., S.R.P., R.L.B., A.M., V.R., A.S.-R., M.G., M.K., F.G.B. Drafting of the manuscript: J.T.J., S.R.P., F.G.B. Revising of the manuscript: J.T.J., S.R.P., R.L.B., V.R., F.G.B.
References
- 1. Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005; 26(1):93–97. [DOI] [PubMed] [Google Scholar]
- 2. Mautner VF, Lindenau M, Baser ME, et al. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 1996; 38(5):880–885; discussion 885–6. [DOI] [PubMed] [Google Scholar]
- 3. Smith MJ, Higgs JE, Bowers NL, et al. Cranial meningiomas in 411 neurofibromatosis type 2 (NF2) patients with proven gene mutations: clear positional effect of mutations, but absence of female severity effect on age at onset. J Med Genet. 2011; 48(4):261–265. [DOI] [PubMed] [Google Scholar]
- 4. Goutagny S, Bah AB, Henin D, et al. Long-term follow-up of 287 meningiomas in neurofibromatosis type 2 patients: clinical, radiological, and molecular features. Neuro-Oncology. 2012; 14(8):1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Betz C, Hall MN.. Where is mTOR and what is it doing there? J Cell Biol. 2013; 203(4):563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guertin DA, Sabatini DM.. Defining the role of mTOR in cancer. Cancer Cell. 2007; 12(1):9–22. [DOI] [PubMed] [Google Scholar]
- 7. James MF, Han S, Polizzano C, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009/8; 29(15):4250–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giovannini M, Bonne NX, Vitte J, et al. mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma. Neuro-Oncology. 2014; 16(4):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goutagny S, Giovannini M, Kalamarides M.. A 4-year phase II study of everolimus in NF2 patients with growing vestibular schwannomas. J Neurooncol. 2017; 133(2):443–445. [DOI] [PubMed] [Google Scholar]
- 10. Goutagny S, Raymond E, Esposito-Farese M, et al. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J Neurooncol. 2015; 122(2):313–320. [DOI] [PubMed] [Google Scholar]
- 11. Beauchamp RL, James MF, DeSouza PA, et al. A high-throughput kinome screen reveals serum/glucocorticoid-regulated kinase 1 as a therapeutic target for NF2-deficient meningiomas. Oncotarget 2015; 6(19):16981–16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James MF, Stivison E, Beauchamp R, et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012; 10(5):649–659. [DOI] [PubMed] [Google Scholar]
- 13. Rozengurt E, Soares HP, Sinnet-Smith J.. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol Cancer Ther. 2014; 13(11):2477–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mulvihill JJ, Parry DM, Sherman JL, et al. NIH Conference. Neurofibromatosis 1 (Recklinghausen disease) and neurofibromatosis 2 (bilateral acoustic neurofibromatosis): an update. Ann Intern Med. 1990; 113(1):39–52. [DOI] [PubMed] [Google Scholar]
- 15. Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992; 84(304):603–618. [PubMed] [Google Scholar]
- 16. Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013; 81(21 Suppl 1):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hornigold RE, Golding JF, Leschziner G, et al. The NFTI-QOL: a disease-specific quality of life questionnaire for neurofibromatosis 2. J Neurol Surg B Skull Base. 2012; 73(2):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaffer BT, Cohen MS, Bigelow DC, Ruckenstein MJ.. Validation of a disease-specific quality-of-life instrument for acoustic neuroma: the Penn Acoustic Neuroma Quality-of-Life Scale. Laryngoscope. 2010/8; 120(8):1646–1654. [DOI] [PubMed] [Google Scholar]
- 19. Wen Q, Wang W, Luo J, et al. CGP57380 enhances efficacy of RAD001 in non-small cell lung cancer through abrogating mTOR inhibition-induced phosphorylation of eIF4E and activating mitochondrial apoptotic pathway. Oncotarget. 2016; 7(19):27787–27801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacoby LB, MacCollin M, Louis DN, et al. Exon scanning for mutation of the NF2 gene in schwannomas. Hum Mol Genet. 1994; 3(3):413–419. [DOI] [PubMed] [Google Scholar]
- 21. Halliday D, Emmanouil B, Pretorius P, et al. Genetic Severity Score predicts clinical phenotype in NF2. J Med Genet. 2017; 54(10):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abi Jaoude S, Peyre M, Degos V, et al. Validation of a scoring system to evaluate the risk of rapid growth of intracranial meningiomas in neurofibromatosis type 2 patients. J Neurosurg. 2020; 134(5):1–9. [DOI] [PubMed] [Google Scholar]