Abstract
Background
Grade 3 1p/19q co-deleted oligodendroglioma is an uncommon primary CNS tumor with a high rate of progression and recurrence. This study examines the benefit of surgery after progression and identifies predictors of survival.
Methods
This is a single-institution retrospective cohort study of consecutive adult patients with anaplastic or grade 3 1p/19q co-deleted oligodendroglioma diagnosed between 2001 and 2020.
Results
Eighty patients with 1p/19q co-deleted grade 3 oligodendroglioma were included. The median age was 47 years (interquartile range 38–56) and 38.8% were women. All patients underwent surgery, including gross total resection (GTR) for 26.3% of patients, subtotal resection (STR) for 70.0% of patients, and biopsy for 3.8% of patients. Forty-three cases (53.8%) progressed at a median of 5.6 years, and the median overall survival (OS) was 14.1 years. Among 43 cases of progression or recurrence, 21 (48.8%) underwent another resection. Patients who underwent a second operation had improved OS (P = .041) and survival after progression/recurrence (P = .012), but similar time to subsequent progression as patients who did not have repeat surgery (P = .50). Predictors of mortality at initial diagnosis included a preoperative Karnofsky Performance Status (KPS) under 80 (hazard ratio [HR] 5.4; 95% CI 1.5–19.2), an STR or biopsy rather than GTR (HR 4.1; 95% CI 1.2–14.2), and a persistent postoperative neurologic deficit (HR 4.0; 95% CI 1.2–14.1).
Conclusions
Repeat surgery is associated with increased survival, but not time to subsequent progression for progressing or recurrent 1p/19q co-deleted grade 3 oligodendrogliomas recur. Mortality is associated with a preoperative KPS under 80, lack of GTR, and persistent postoperative neurologic deficits after the initial surgery.
Keywords: grade 3 oligodendroglioma, progression, recurrence, repeat surgery, survival prediction
Key Points.
Re-resection after progression of grade 3 1p/19q co-deleted oligodendroglioma is associated with increased survival.
Low preop Karnofsky Performance Status (KPS), lack of gross total resection (GTR), and postop neurologic deficits predict decreased survival for grade 3 1p/19q co-deleted oligodendroglioma.
Importance of the Study.
Grade 3 1p/19q co-deleted oligodendrogliomas (IDH-1/2 mutants and not otherwise specified [NOS]) are primary CNS tumors that nearly always progress or recur after treatment and have variable overall survival. There are limited data guiding efforts to identify patients at higher risk of mortality based on clinical and operative factors, and no data on the utility of further debulking when progression or recurrence inevitably occurs. In this study, we provide updated survival analysis for grade 3 1p/19q co-deleted oligodendrogliomas and identify low Karnofsky Performance Status (KPS), lack of gross total resection (GTR), and postoperative neurologic deficits as associated with poorer survival. We find that when progression or recurrence does occur, it is often asymptomatic and that further debulking can improve survival. Our findings will help neuro-oncology teams prognosticate the long-term survival of patients after their initial operations. They also provide evidence to support further debulking after progression or recurrence.
World Health Organization (WHO) grade 3 oligodendroglioma is an uncommon subtype of infiltrative glioma that comprises less than 1% of newly diagnosed intra-axial brain tumors.1 The diagnosis has evolved over time from histopathologic examination alone to the inclusion of cytogenetic (1p/19q co-deletion) and genetic (IDH1/2 mutation) data.2 Treatment involves surgery followed by radiation therapy with or without concurrent as well as adjuvant chemotherapy.3,4 Surgeons typically attempt gross total resection (GTR) when there is an acceptably low risk of neurologic morbidity given survival benefits observed with maximal cytoreduction.5–8
Long-term survival is variable and can be hard to predict.1,7,9,10 Despite surgery and adjuvant treatment, grade 3 oligodendroglioma usually progresses or recurs, and there is little data to guide the next steps of treatment once this occurs. This study examines the clinical course of grade 3 1p/19 co-deleted oligodendroglioma (IDH1/2 mutant and not otherwise specified [NOS] cases), including whether surgery after progression or recurrence improves prognosis.
Materials and Methods
Objectives and Study Design
The primary objectives of this study were to determine whether a repeat surgery after disease progression or recurrence improves overall survival (OS), survival after a progression or recurrence event, and subsequent progression-free survival (PFS) after a progression or recurrence event. Secondary objectives included (1) characterizing the importance of radiographic features and (2) identifying predictors of OS in a multivariate analysis.
Adult patients diagnosed with anaplastic or grade 3 oligodendroglioma that harbored 1p/19q co-deletion on cytogenetic testing at the Brigham and Women’s Hospital (BWH) and Dana Farber Cancer Institute (DFCI) between 2001 and 2020 were included. Patients who had operations outside of BWH were included if they received oncologic care or opinions at DFCI. All patients who had IDH1 R132H immunohistochemistry or IDH1/2 next-generation sequencing performed harbored mutations, but immunohistologic and genetic testing was not conducted for most patients prior to 2011. Patients without IDH1 or IDH1/2 testing were still included to better characterize long-term outcomes and improve external generalizability. Patients with fewer than 6 months of postoperative follow-up (except those who died within 6 months of surgery) and patients with unknown extent of resection at initial resection were excluded.
This study was approved by the Mass General Brigham Institutional Review Board (Protocol #2015P002352).
Data Collection and Categorization
Clinical charts and public obituary records were reviewed to complete data abstraction. Extent of resection was determined by review of radiographic imaging and radiology reports. A GTR was defined as no T1 contrast enhancement or infiltrative T2 disease seen on postoperative magnetic resonance imaging (MRI) scans. Radiographic progression was determined using the Response Assessment in Neuro-Oncology Criteria for 35 (81%) cases of progression, and for the remainder of cases where imaging was not available for review, an oncologist or radiologist report of progression was considered sufficient to determine progression.11 When biopsy was followed by resection within 3 months of the biopsy, the resection was classified as the index operation.
Data Handling and Statistical Analysis
Given a limited number of observations in each stratum, data were aggregated for the multivariate analysis of survival based on initial disease-related and surgical factors: Karnofsky Performance Status (KPS) was considered high (80 or higher) or low (70 or lower); tumor location was categorized as frontal or nonfrontal; and extent of resection was categorized as GTR or subtotal resection (STR) and biopsy. The latter was done because there were only 3 biopsy cases. A post hoc subset analysis was conducted to determine how clinical outcomes varied between GTR, near-total resection (NTR), and STR/biopsy for cases with imaging available for review. Cases were coded as NTR when there was only a thin rim (under <5 mm) of nonenhancing tumor left at the end of a resection. For 78 cases for which any imaging (including pre- and postoperative) was available for review, tumors were considered eloquent based on proximity to motor, language, and vision structures. Details for T1 contrast enhancement were available for 77 patients and for diffusion restriction were available for 72 patients. Missing data were managed with pairwise elimination.
Statistical analysis was conducted on R 4.2.1 (The R Project). Fisher’s exact test was used to compare proportions, and the Mann–Whitney U test was used to compare averages given small sample sizes and lack of normality. The Mantel–Cox log-rank test was used to identify statistical significance for univariate survival analysis. The Cox proportional-hazards test was used to identify predictors of survival in a multivariate model. Graphics were generated with GraphPad Prism.
Results
A total of 80 consecutive patients with 1p/19q co-deleted grade 3 oligodendroglioma were included. The median age was 47 years (interquartile range [IQR] 38–56) and 38.8% of patients were women (Table 1). The most common preoperative presenting KPS was 80 (IQR 80–90). Most tumors involved the frontal lobe (78.8%). T1 contrast enhancement and diffusion restriction were observed in 56.6% and 22.5% of tumors in which preoperative MRI imaging was available for review, respectively. Further, 47.4% of tumors were considered eloquent for this study given their location near radiographic structures associated with motion, language, and vision.
Table 1.
Clinical Characteristics
| Variable | Overall (n = 80) | IDH mutant (n = 59) | IDH NOS (n = 21) |
|---|---|---|---|
| Age, median (IQR) | 47.2 (37.6–56.3) | 49.5 (37.7–57.9) | 42.6 (37.6–49.1) |
| Female sex, n (%) | 31 (38.8%) | 21 (35.6%) | 10 (47.6%) |
| Location, n (%) | |||
| Frontal | 63 (78.9%) | 45 (76.3%) | 18 (85.7%) |
| Parietal | 12 (15.0%) | 8 (13.6%) | 4 (19.0%) |
| Temporal | 9 (11.3%) | 8 (13.6%) | 1 (4.8%) |
| Occipital | 3 (3.8%) | 3 (5.1%) | 0 (0.0%) |
| Other | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Radiographic features, n (%) | |||
| T1 contrast enhancement | 43 (53.8%) | 32 (54.2%) | 11 (52.4%) |
| Diffusion restriction | 16 (20.0%) | 11 (18.6%) | 5 (23.8%) |
| Extent of resection, n (%) | |||
| Biopsy | 3 (3.8%) | 3 (5.1%) | 0 (0.0%) |
| Subtotal resection | 56 (70.0%) | 41 (69.5%) | 15 (71.4%) |
| Gross total resection | 21 (26.3%) | 15 (25.4%) | 6 (28.6%) |
| Adjuvant chemotherapy, n (%) | 76 (95.0%) | 56 (94.9%) | 20 (95.2%) |
| Adjuvant radiation therapy, n (%) | 55 (68.8%) | 42 (71.2%) | 13 (61.9%) |
| Progression, n (%) | 43 (53.8%) | 29 (49.2%) | 14 (66.7%) |
IDH, isocitrate dehydrogenase; IQR, interquartile range; NOS, not otherwise specified.
All patients underwent surgery. GTR was achieved in 21 cases (26.3%) and STR was achieved in 56 (70.0%) cases; the remainder had biopsy alone (3.8%). In 43 cases with STR with available pre- and postoperative imaging for review, 14 had an NTR (32.6% of all STR cases). Five patients (6.3%) had postoperative surgical complications and 2 patients (2.5%) needed an urgent reoperation for (1 for hematoma and 1 for wound washout). Eight patients (10.0%) had postoperative neurologic deficits that were new or worse than their preoperative deficits and persisted up to at least 1 month after surgery.
The presence of IDH1 and IDH1/2 mutational testing was confirmed in 59 cases (73.8%) using immunohistochemistry for the IDH1 R132H variant (n = 42), next-generation sequencing revealing IDH1 mutations (n = 16), and next-generation sequencing revealing IDH2 mutations (n = 1). The remainder of cases (26.3%) were considered 1p/19q co-deleted grade 3 oligodendroglioma, NOS. Patient age, gender, and extent of resection did not differ between patients with IDH-mutant and IDH-NOS cases (all P > .05). An additional 28 patients had cytogenetic testing and 14 patients had oncologic genetic screening done as previously described.12 The most common chromosomal abnormalities aside from 1p/19q co-deletions included losses in chromosome 9 (35.7%), chromosome 4 (25.0%), and chromosome 13 (14.3%) and gain in chromosome 7 (21.4%) The most frequent genetic mutations observed were mutations in TERT promotor (78.6%), PIK3R1 (64.3%), CIC (50%), ATM (28.6%), and ARID (21.4%).
After surgery, 22 patients (27.5%) either had adjuvant chemotherapy alone, 1 patient (1.3%) had radiation therapy alone, 54 patients (67.5%) had both, and 3 patients (3.8%) had neither. Among patients who received chemotherapy, 72 patients (88.9%) received temozolomide and 4 patients (4.9%) received combinatorial procarbazine, lomustine, and vincristine (PCV).
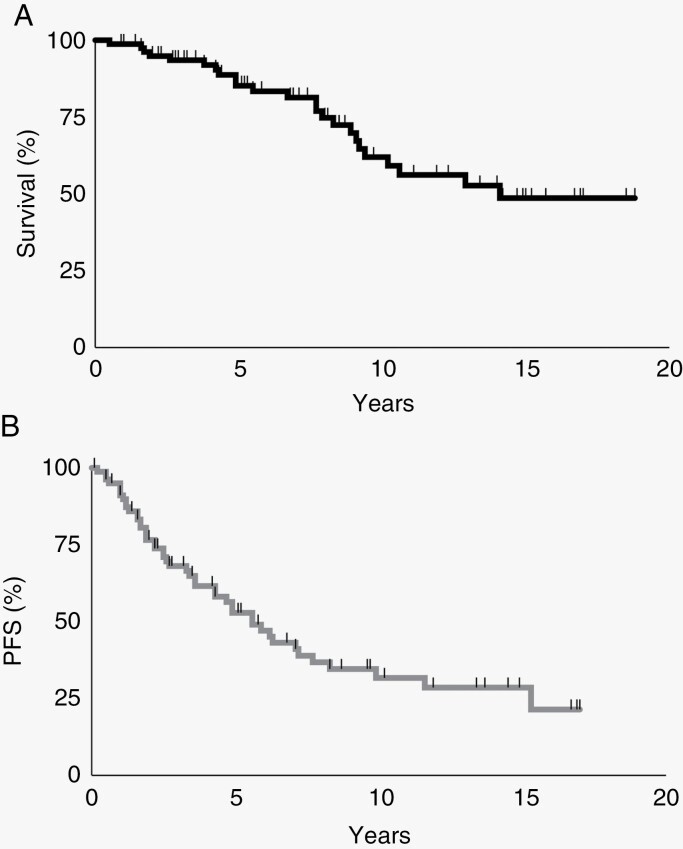
The median clinical follow-up was 6.9 years (IQR 3.5–10.3) and 43 (53.8%) cases progressed or recurred by follow-up, including 49.2% of IDH-mutant cases and 66.7% of IDH-NOS cases (P = .21). Progression or recurrence was noted on surveillance imaging in 39 cases (90.7%) and due to a new symptom prompting imaging in the other cases. Median PFS was 5.6 years and median OS was 14.1 years accounting for censored patients (Figure 1a and b). Twenty-one patients (48.8%) underwent another operation at a median time of 3 months after radiographic progression or recurrence; 18 of these reoperated patients (85.7%) had an STR, 2 had GTR (9.5%), and 1 had biopsy (4.8%).
Figure 1.
Overall survival (a) and progression-free survival (b) are demonstrated for 80 1p/19q co-deleted grade 3 1p/19q co-deleted oligodendrogliomas. Tick marks indicate censored patients.
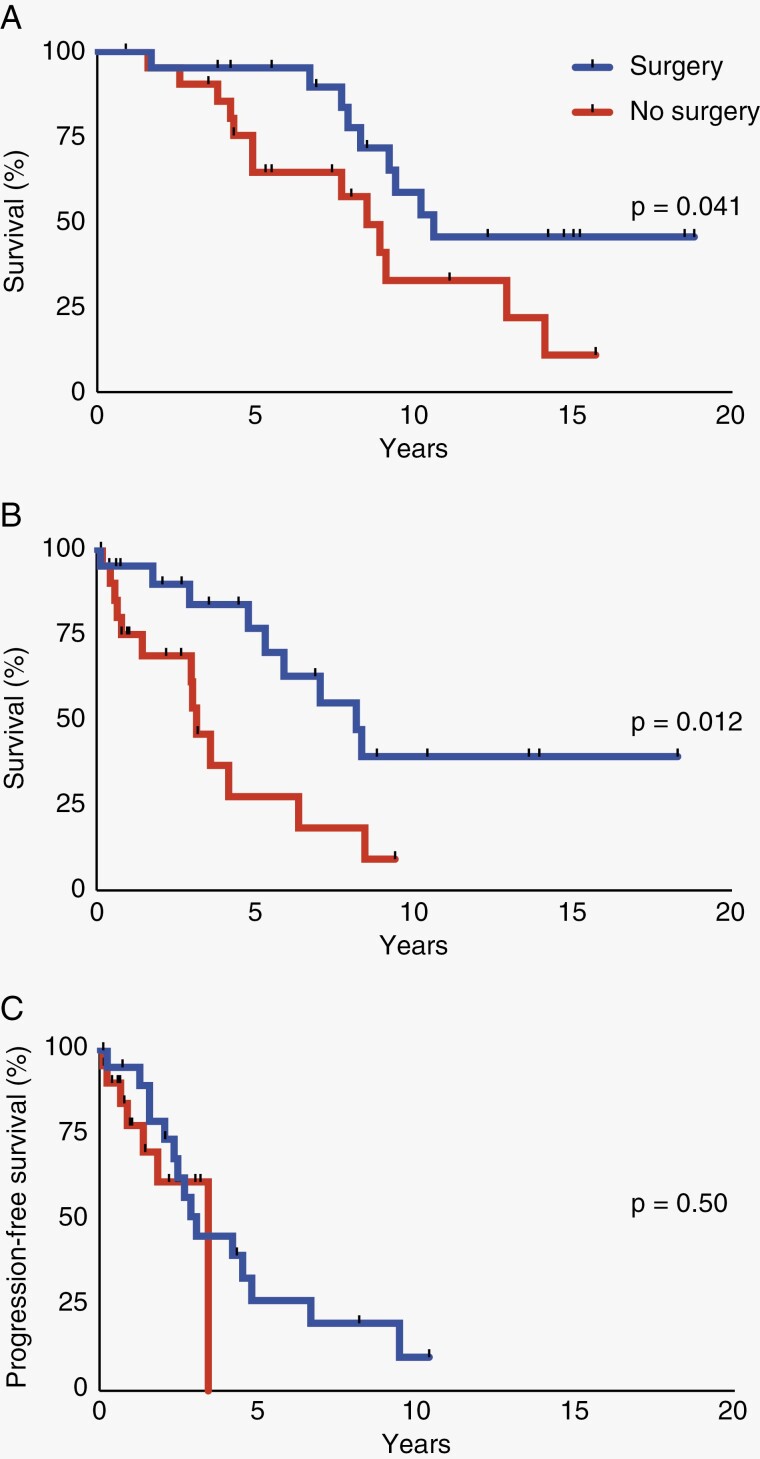
At progression, 31 patients (72.1%) and 19 patients (44.2%) received chemotherapy and radiation therapy, respectively. The age, sex, location, eloquence, and rates of postprogression chemotherapy and radiation therapy were similar between patients who had repeat surgery and those who did not (Table 2). Repeat surgery was associated with improved OS (P = .041) and survival after progression/recurrence (P = .012) (Figure 2a and b), but did not impact the time to the next disease progression compared to patients who did not have repeat surgery (Figure 2c). All patients had a WHO grade 3 1p/19q co-deleted oligodendroglioma on repeat pathology.
Table 2.
Comparison of Patients With Progressive or Recurrent Tumors Who Underwent Repeat Surgery and Patients Who Did Not
| Variable | Overall (n = 43) | Repeat Surgery (n = 21) | No Repeat Surgery (n = 22) | P-value |
|---|---|---|---|---|
| Age, median (IQR) | 44.5 (52.9–35.5) | 44.3 (32.1–51.2) | 46.7 (38.0–54.9) | .24 |
| Sex, n (%) | 26 (60.5%) | 16 (76.2%) | 10 (45.5%) | .06 |
| Frontal location, n (%) | 31 (72.0%) | 17 (18.0%) | 14 (63.6%) | .31 |
| Eloquence, n (%)** | 19 (46.3%) | 8 (40.0%) | 11 (52.4%) | .43 |
| Chemotherapy, n (%)* | 31 (72.1%) | 16 (51.6%) | 15 (48.4%) | .74 |
| Radiation therapy, n (%)* | 19 (44.2%) | 9 (47.4%) | 10 (52.6%) | .99 |
IQR, interquartile range.
*Refers to chemotherapy and radiation therapy received after progression/recurrence.
**2 cases do not have data available on eloquence.
Figure 2.
Patients who received surgery after progression/recurrence have improved overall survival (OS) (a) and survival after progression/recurrence (b), but not subsequent progression after the second surgery (c) compared to patients who did not receive a repeat surgery. Tick marks indicate censored patients. Patients who received surgery after progression/recurrence are represented in the upper curve in (a) and (b) and the upper curve at years 0-3 in (c).
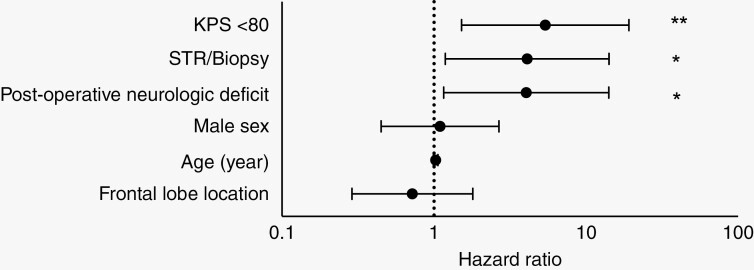
A multivariate Cox proportional-hazards model for mortality was constructed using age, sex, tumor location, initial KPS, extent of resection at initial operation, and new or worsened persistent postoperative neurological deficits after the initial operation. The strongest predictors of mortality were a preoperative KPS under 80 (hazard ratio [HR] 5.4; 95% CI 1.5–19.2), STR or biopsy rather than GTR (HR 4.1; 95% CI 1.2–14.2), and a persistent postoperative neurologic deficit (HR 4.0; 95% CI 1.2–14.1) (Figure 3). This model was repeated after separating biopsy, STR, and GTR into discrete categories. Extent of resection demonstrated a stepwise association with mortality, as STR (HR 4.0; 95% CI 1.2–14.0) and biopsy (HR 6.2; 95% CI 0.5–70.8) were associated with progressively increased risk of mortality compared to GTR, though this was not statistically significant. These predictive models were repeated in a subset of 66 patients with available pre- and postoperative imaging available to distinguish STR and NTR. Mortality was associated with STR or biopsy (odds ratio [OR] 5.8, 95% CI 1.5–23.2) and trended toward an association with NTR (OR 2.4; 95% CI 0.5–12.0) compared to GTR. When STR and biopsy were separated into discrete categories, there was a statistically nonsignificant stepwise association with decreased extent of resection and mortality, as NTR (OR 2.4; 95% CI 0.5–12.0), STR (OR 5.8; 95% CI 1.4–23.7), and biopsy (OR 6.1; 95% CI 0.5–77.5) compared to GTR.
Figure 3.
In a multivariate Cox proportional-hazards model, reduced overall survival (OS) is associated with a preoperative Karnofsky Performance Status (KPS) under 80, a postoperative neurologic deficit that persists at least 30 days postoperatively, and a subtotal resection (STR)/biopsy procedure rather than a gross total resection (GTR). Age, sex, and location (frontal lobe vs. nonfrontal) are not associated with OS in this model. *P < .05; **P < .01.
A separate univariate analysis was conducted to examine the impact of preoperative radiographic findings given deficiencies in availability of these data. Diffusion restriction on preoperative imaging was associated with decreased OS (P = .005; Supplementary Figure 1), but not PFS (P = .97). Preoperative T1 contrast enhancement was not associated with either PFS or OS (P > .05 for both).
Discussion
WHO grade 3 oligodendrogliomas indolently progress years after resection and adjuvant therapy, and the decision of whether to and when to operate again is difficult and highly personalized. This study identifies that surgery after progression or recurrence for WHO grade 3 1p/19q co-deleted oligodendrogliomas, IDH1/2 mutant and NOS, improves overall patient survival and survival after progression or recurrence. It also implicates several additional predictors of survival for this challenging disease, including initial extent of resection, preoperative KPS, and the presence of new postoperative neurologic deficits.
We noted that GTR, both at initial diagnosis and at recurrence, is associated with improved OS. Similar to our findings, Shin et al. identified GTR as associated with survival in a series of newly diagnosed “anaplastic” oligodendrogliomas.10 Conversely, Bush et al. noted that volumetric extent of resection was not associated with survival in a series of 40 newly diagnosed 1p/19q co-deleted, IDH1/2 mutant grade 3 oligodendroglioma.7 This variability in results may be due to differing methodology and statistical power. GTRs are associated with improved survival for other glial-lineage tumors such as grade 2 oligodendrogliomas and glioblastoma (GBM), consistent with our finding that a complete resection has benefits in grade 3 1p/19q co-deleted oligodendroglioma as well.5,13,14 Further studies should continue understanding the risk and benefit of NTRs, which may reduce neurologic complications but may also confer a higher risk of mortality.
A low preoperative KPS was strongly predictive of poor OS independent of tumor location and extent of resection. Poor baseline KPS should not be used as a sole determinant for surgical candidacy but may be beneficial to follow for prognostication. A prior report suggests that it may also help predict short-term outcome after surgery and may be helpful to identify patients needing closer follow-up or outpatient services.15
We also confirm that postoperative deficits reduce survival despite an extensive resection, as has been similarly reported with GBM.16 Cellular infiltration of adjacent brain structures can obscure the boundaries between tumor and normal brain, but there is an expanding armamentarium to aid surgeons, ranging from multimodality neuro-monitoring, fluorescence guidance, and increasingly sophisticated neuro-navigation.17–22 The goal of surgery should remain an aggressive of a resection as possible while minimizing risk of postoperative deficit.
When progression or recurrence occurs, repeat surgery may offer a survival benefit through cytoreduction and diagnosis. Over 95% of repeat operations were resections rather than biopsy alone, and all subsequent diagnoses were tumor rather than radiation necrosis. Debulking more immediately improves neurologic deficits related to mass effect, which prior studies identify as associated with survival.23 Most repeat surgeries were STRs, potentially because the invasive or scarred nature of progressive and recurrent disease limited safe GTR. Regardless, the effect of repeat surgery after surgery was clinically significant particularly if able to achieve a GTR or substantial STR.
There is growing knowledge on the genomic landscape of grade 3 oligodendrogliomas, but further study is needed to determine the prognostic value and therapeutic implications of various mutations.24TERT promotor mutations are commonly found in oligodendrogliomas and may be associated with a more favorable prognostic course.25,26 The CIC gene is located on chromosome 19q, and mutations in this gene are associated with oligodendrogliomas and may contribute to their pathogenesis.27PI3K mutations have also been identified in low-grade gliomas, but their prognostic value remains unknown in this pathology.28,29
This study has several limitations. Notably, both 1p/19q co-deleted, IDH1/2 mutant grade 3 oligodendroglioma as well as 1p/19q co-deleted grade 3 oligodendroglioma, NOS, were included in this study. This was done to improve statistical power for analyses given the rarity of the pathology and to improve generalizability, so clinicians may cautiously interpret these findings on patients who have not had IDH1 R132H immunohistochemistry or IDH1/2 mutational analyses. We anticipate that most IDH1/2 NOS patients in our series harbored tumors that were IDH1/2 mutant based on historical data demonstrating that essentially all 1p19q co-deleted grade 3 oligodendrogliomas harbor IDH1 mutation and the rare tumors that are IDH1 wild-type are in fact IDH2 mutant.30 In addition, radiographic interpretation can be limited by image capture techniques and interrater reliability that may have varied over the 20-year span of this patient series.31 Another limitation is the relatively short median follow-up, which is potentially relevant given the longer survival often noted for WHO grade 3 oligodendrogliomas.10 This limits our ability to discuss the prognostic factors for these tumors which are typically associated with a longer survival time, but our center continues to follow many of the patients included in the present study. Furthermore, there is a possibility that certain patients who did not undergo repeat surgery were deemed to be poor surgical candidates, which has the potential to skew results in favor of repeat resection. We attempted to mitigate this by collecting data relevant to performance status and functionality for patients at various time points throughout follow-up. The median survival in this series is higher than previous estimates. This may reflect improving survival overall for this disease, but it may also be a partial overestimate if a significant number of patients lost to follow-up were deceased. We attempted to mitigate this through review of public obituary records. Further studies conducted in a multicenter format are needed to validate these findings, and multicenter study designs may be necessary given the rarity of this pathology and new genomic subgroupings.
Conclusion
The median OS is 14.1 years for WHO grade 3, 1p/19q co-deleted oligodendroglioma. When these tumors progress or recur, repeat surgery is associated with increased survival, but not with time to subsequent progression. Decreased survival was associated with an initial preoperative KPS under 80, a lack of GTR at first resection, and persistent postoperative neurologic deficits after the first surgery.
Supplementary Material
Acknowledgments
We have no acknowledgments to disclose.
Contributor Information
Saksham Gupta, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Noah L Nawabi, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; College of Medicine, Medical University of South Carolina, Charleston, SC, USA.
Siva Emani, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Lila Medeiros, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Joshua D Bernstock, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Julia Duvall, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Patrick Ng, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Timothy R Smith, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Patrick Y Wen, Center for Neuro-Oncology, Dana-Farber Cancer Center, Brigham and Women’s Hospital, Boston, MA, USA.
David A Reardon, Center for Neuro-Oncology, Dana-Farber Cancer Center, Brigham and Women’s Hospital, Boston, MA, USA.
Omar Arnaout, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interest statement
J.D.B. reports an equity position in Treovir LLC and is a member of the POCKiT Diagnostics Board of Scientific Advisors. D.A.R. reports the following potentially competing interests: honoraria for consultation from Abbvie; Advantagene; Agenus; Agios; Amgen; AnHeart Therapeutics; Avita Biomedical, Inc.; Bayer; Boston Biomedical; Boehringer Ingelheim; Bristol-Myers Squibb; Celldex; Deciphera; Del Mar Pharma; DNAtrix; Ellipses Pharma; EMD Serono; Genenta; Genentech/Roche; Hoffman-LaRoche, Ltd; Imvax; Inovio; Janssen Research & Development, LLC; Johnson & Johnson; Pharmac; Kintara; Kiyatec; Medicenna Biopharma, Inc.; Merck; Merck KGaA; Monteris; Neuvogen; Novartis; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Sumitono Dainippon Pharma; Pyramid; Taiho Oncology, Inc.; Vivacitas Oncology, Inc., Y-mabs Therapeutics.
Authorship statement
Project ideation: S.G., N.L.N., S.E., T.R.S., O.A. Data collection: S.G., N.L.N., S.E., L.M., J.D.B., J.D., P.N. Data analysis: S.G., S.E., J.D.B., P.N. Data interpretation: S.G., S.E., J.D., P.Y.W., D.A.R., T.R.S., O.A. Manuscript writing: S.G., N.L.N., J.D., P.Y.W., D.A.R. Critical review of the manuscript: all authors.
References
- 1. Ostrom QT, Patil N, Cioffi G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung C, Laperriere N.. Radiation therapy and grade II/III oligodendroglial tumors. CNS Oncol. 2015;4(5):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van den Bent MJ, Chang SM.. Grade II and III oligodendroglioma and astrocytoma. Neurol Clin. 2018;36(3):467–484. [DOI] [PubMed] [Google Scholar]
- 5. Kinslow CJ, Garton ALA, Rae AI, et al. Extent of resection and survival for oligodendroglioma: a U.S. population-based study. J Neurooncol. 2019;144(3):591–601. [DOI] [PubMed] [Google Scholar]
- 6. Alattar AA, Brandel MG, Hirshman BR, et al. Oligodendroglioma resection: a Surveillance, Epidemiology, and End Results (SEER) analysis. J Neurosurg. 2018;128(4):1076–1083. [DOI] [PubMed] [Google Scholar]
- 7. Bush NAO, Young JS, Zhang Y, et al. A single institution retrospective analysis on survival based on treatment paradigms for patients with anaplastic oligodendroglioma. J Neurooncol. 2021;153(3):447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong JB, Roh TH, Kang SG, et al. Survival, prognostic factors, and volumetric analysis of extent of resection for anaplastic gliomas. Cancer Res Treat. 2020;52(4):1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garnier L, Vidal C, Chinot O, et al. Characteristics of anaplastic oligodendrogliomas short-term survivors: a POLA Network Study. Oncologist. 2022;27(5):414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shin DW, Lee S, Song SW, et al. Survival outcome and prognostic factors in anaplastic oligodendroglioma: a single-institution study of 95 cases. Sci Rep. 2020;10(1):20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 12. Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141(6):751–758. [DOI] [PubMed] [Google Scholar]
- 13. Jakola AS, Skjulsvik AJ, Myrmel KS, et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28(8):1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunawan PY, Islam AA, July J, et al. Karnofsky Performance Scale and neurological assessment of neuro-oncology scale as early predictor in glioma. Asian Pac J Cancer Prev. 2020;21(11):3387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahman M, Abbatematteo J, De Leo EK, et al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg. 2017;127(1):123–131. [DOI] [PubMed] [Google Scholar]
- 17. Unsgard G, Lindseth F.. 3D ultrasound-guided resection of low-grade gliomas: principles and clinical examples. Neurosurg Focus. 2019;47(6):E9. [DOI] [PubMed] [Google Scholar]
- 18. Schupper AJ, Yong RL, Hadjipanayis CG.. The neurosurgeon’s armamentarium for gliomas: an update on intraoperative technologies to improve extent of resection. J Clin Med. 2021;10(2):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. You H, Qiao H.. Intraoperative neuromonitoring during resection of gliomas involving eloquent areas. Front Neurol. 2021;12:658680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu K, Bi WL, Essayed W, et al. Integration of microanatomy, neuronavigation, dynamic neurophysiologic monitoring, and intraoperative multimodality imaging for the safe removal of an insular glioma: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 2021;21(1):E28–E29. [DOI] [PubMed] [Google Scholar]
- 21. Kiesel B, Freund J, Reichert D, et al. 5-ALA in suspected low-grade gliomas: current role, limitations, and new approaches. Front Oncol. 2021;11:699301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Widhalm G, Olson J, Weller J, et al. The value of visible 5-ALA fluorescence and quantitative protoporphyrin IX analysis for improved surgery of suspected low-grade gliomas. J Neurosurg. 2019;133(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zetterling M, Berhane L, Alafuzoff I, Jakola AS, Smits A.. Prognostic markers for survival in patients with oligodendroglial tumors; a single-institution review of 214 cases. PLoS One. 2017;12(11):e0188419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holdhoff M, Cairncross GJ, Kollmeyer TM, et al. Genetic landscape of extreme responders with anaplastic oligodendroglioma. Oncotarget. 2017;8(22):35523–35531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arita H, Matsushita Y, Machida R, et al. TERT promoter mutation confers favorable prognosis regardless of 1p/19q status in adult diffuse gliomas with IDH1/2 mutations. Acta Neuropathol Commun. 2020;8(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee Y, Koh J, Kim SI, et al. The frequency and prognostic effect of TERT promoter mutation in diffuse gliomas. Acta Neuropathol Commun. 2017;5(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brito C, Tomás A, Azevedo A, et al. PIK3CA mutations in diffuse gliomas: an update on molecular stratification, prognosis, recurrence, and aggressiveness. Clin Med Insights Oncol. 2022;16:11795549211068804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Broderick DK, Di C, Parrett TJ, et al. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004;64(15):5048–5050. [DOI] [PubMed] [Google Scholar]
- 30. Labussiere M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010;74(23):1886–1890. [DOI] [PubMed] [Google Scholar]
- 31. Aboud O, Shah R, Vera E, et al. Challenges of imaging interpretation to predict oligodendroglioma grade: a report from the Neuro-Oncology Branch. CNS Oncol. 2022;11(1):CNS83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.