Abstract
Ultraviolet (UV) radiation is a major cause of multiple major skin diseases including skin cancer. It is crucial to discover new agents that can produce profound protective effects on UV-produced skin damage. Using a mouse model, in this study we determined the effects of NAD+ on UVC-induced skin damage and investigated the mechanisms underlying the effects, obtaining the following discoveries: First, UVC-induced skin’s green autofluorescence (AF) was highly correlated with the extent of UVC-indued skin’s damage; second, NAD+ administration profoundly decreased UVC-induced skin damage; third, NAD+ administration significantly attenuated UVC-induced decreases in the levels of mitochondrial superoxide dismutase and catalase; fourth, NAD+ administration significantly attenuated UVC-induced increase in the level of cyclooxygenase (COX) 2 - a marker of inflammation; fifth, NAD+ administration profoundly attenuated UVC-induced increase in double-strand DNA (dsDNA) damage; and sixth, NAD+ administration profoundly attenuated UVC-induced decreases in the ratios of Bcl-2/Bax - an index of apoptosis. Collectively, our study has found that NAD+ administration can profoundly decrease UVC-induced skin damage by attenuating oxidative stress, inflammation, DNA damage, and apoptosis, suggesting great potential of NAD+ as a protective agent for UVC-induced skin damage. Moreover, our study has further indicated that the skin’s green AF is a biomarker for predicting UVC-induced skin damage.
Keywords: NAD+, UV, skin damage, apoptosis, green autofluorescence
Introduction
NAD+ plays crucial roles in a number of major biological processes, including energy metabolism, mitochondrial function, cell death, DNA repair, and immunological functions [1,2]. Cumulating evidence has indicated that NAD+ can produce significant beneficial effects in multiple animal models of diseases, including ischemic brain injury and ischemic myocardial injury [3-5]. It is warranted to further determine the effects of NAD+ in various disease models and to further investigate the mechanisms underlying the effects of NAD+ on the tissue and organ injury [3].
UV irradiation is a critical cause for multiple skin diseases including skin cancer [6-8]. UV irradiation can produce multiple pathological changes of the skin, including photoaging and skin cancer [8,9], which results from its capacity in producing skin’s alterations including oxidative stress, inflammation, DNA damage, and apoptosis [10,11]. While multiple studies have found skin-protecting agents [12,13], it is highly significant to discover new agents which can provide profound protection against UV-induced skin damage.
There are three major types of UV irradiation, including UVA (320-400 nm), UVB (280-320 nm) and UVC (100-280 nm) [8]. Due to gradual depletion of the atmospheric ozone layer, there has been increased UVC radiation on the surface of the earth, which is increasingly becoming an important environmental concern [14]. Since it is ionizing irradiation, UVC is a strong mutagen that can cause cancer and immune-mediated diseases [14]. Most studies on protective agents against UV irradiation have focused at the agents that protect against UVB- or UVA-induced skin damage [12,13]. Therefore, it is highly valuable to search for the agents that can profoundly decrease UVC-induced skin damage.
It is of significance to discover biomarkers for non-invasive prediction of UV-induced skin damage, which can significantly increase our capacity of early diagnosis and intervention on UV-induced skin damage [15]. Our recent study has indicated that skin’s green AF intensity is a biomarker for predicting UVB/UVC-induced skin damage [15]. However, it is warranted to obtain further evidence to support and solidify this finding, so as to establish this novel approach in early diagnosis and intervention of UV-induced skin damage.
In our current study, we focused our study on determining the effects of NAD+ on UVC-induced skin damage of mice and investigated the mechanisms underlying the effects. We also further test the validity of the green AF-based prediction of UVC-induced skin damage. Our study has indicated that administration of NAD+ can profoundly decrease UVC-induced skin damage by attenuating oxidative stress, inflammation, DNA damage and apoptosis. Our study has also obtained further evidence indicating the validity of the green AF-based method in predicting UVC-induced skin damage.
Materials and methods
Materials
Formalin, hematoxylin, eosin, and NAD+ were purchased from Sigma-Aldrich (St. Louis, MO, USA). The antibodies of SOD2 and Actin were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody of γ-H2AX was purchased from Abcam (Cambridge, UK). The antibodies of Bcl-2, Bax, Catalase, and COX2 were procured from Cell Signaling Technology (Boston, MA, USA). All other reagents were of the highest grade commercially available.
Preparation of NAD+ solutions
The vehicle was made by mixture of equal volume of PBS and Glycerol. A stock solution of NAD+ at the final concentration of 10 mg/ml was made by addition of NAD+ into the vehicle. The NAD+ at the dosage of 1 mg/cm2 was administered to each ear.
Animal experiments
C57BL/6 male mice ages 6-8 weeks at the weight of 18-22 g (Lingchang Biotechnology Co., LTD., Shanghai) were kept in a SPF animal facility with controlled temperature and humidity. All animal experiments were conducted according to the animal protocol approved by the Bioethics Committee of the School of Biomedical Engineering, Shanghai Jiao Tong University. After a week of observation, the mice were irradiated with UVC at the irradiation dosages of 0, 0.3, 0.6, 0.9 J/cm2. 18-24 hr after the irradiation, the green AF of the ears were determined under a two-photon microscope (Nikon, Shanghai, China). Three days after the irradiation, the ears were collected and stored at -80°C.
Imaging of skin’s green AF
The imaging of the green AF of the skin was conducted as described previously [15]. The skin’s AF of mouse’s ears was imaged under a fluorescence microscope (A1 plus, Nikon Instech Co., Ltd., Tokyo, Japan) using its single-photon mode, with the excitation wavelength of 488 nm and the emission wavelength of 500-530 nm. The AF was quantified according to the following approach: Sixteen spots with the size of 100 μm × 100 μm on the scanned images were selected randomly. The average AF intensity of each layer was calculated, and subsequently the sum of the average AF intensity of each layer of each spot was calculated, which is defined as the AF intensity of each spot.
Histological assays
Histological assays were conducted as described previously [15]. Skin biopsies from the ears of the mice were obtained, which were placed immediately in 4% (w/v) paraformaldehyde buffer. After 12 to 24 h, paraffin embedding procedures were conducted on the samples. Hematoxylin & Eosin (H&E) staining was conducted according to the manufacturer’s protocol (C0105, Beyotime, Haimen, China).
Immunofluorescence staining
Paraffin sections were dragged, followed by incubation with 0.2% Triton X-100 in PBS for 10 min. After washes with PBS, the sections were blocked with 10% goat serum for 1 hr at room temperature and then incubated with anti-γ-H2AX antibody (ab81299, Cell Signaling Technology, MA, USA) (1:250 dilution) in PBS containing 1% goat serum overnight at 4°C. After three washes with PBS, the sections were incubated with Alexa Fluor® 647 goat anti-rabbit IgG (H+L) Secondary antibody (1:500 dilution, Molecular Probes, Eugene, Oregon, United States) in PBS containing 1% goat serum for 1 hr at room temperature. After three washes with PBS, the sections were counterstained with DAPI (1:1000 dilution, Beyotime Institute of Biotechnology) in PBS for 5 min at room temperature. The fluorescence images were photographed under a Leica confocal microscope (Leica Microsystems, Wetzlar, Germany).
Western blotting
The ear’s skin of mice was harvested and lysed in RIPA buffer (Millipore, Temecula, California, United States) containing 1% protease inhibitor cocktail, 1% phosphatase inhibitor cocktail (CWBio, Beijing, China) and 1 mM phenylmethanesulfonyl fluoride. The lysates were centrifuged at 12000 rpm for 20 min at 4°C. After quantifications of the protein samples using the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, Illinois, United States), 30 μg of total protein was electrophoresed through a 10% sodium dodecyl sulfate-polyacrylamide gel and then transferred to 0.45-μm nitrocellulose membranes. The blots were incubated overnight at 4°C with primary antibodies with the following antibody dilutions: anti-COX2 antibody (Cat#12282, Cell Signaling Technology, Danvers, MA) (1:1000 dilution), anti-SOD2 antibody (sc-133134, Santa Cruz, California, United States) (1:2000 dilution), anti-catalase antibody (Cat#14097, Cell Signaling Technology, Danvers, MA) (1:2000 dilution), anti-Bcl-2 antibody (Cat#3498, Cell Signaling Technology, Danvers, MA) (1:2000 dilution), anti-Bax antibody (Cat#5023, Cell Signaling Technology, Danvers, MA) (1:2000 dilution), anti-γ-H2AX antibody (ab81299, Abcam, Cambridge, UK) (1:4000 dilution) and anti-Actin antibody (sc-58673 Santa Cruz, California, United States) (1:1000 dilution). Subsequently, the blots were incubated with HRP conjugated Goat Anti-Rabbit IgG (H+L) (1:4000 dilution) (Jackson ImmunoResearch, PA, USA) or HRP conjugated Goat Anti-mouse IgG (1:4000 dilution, HA1006, HuaBio, Zhejiang Province, China). The protein signals were detected by an ECL detection system (Thermo Scientific, Pierce, IL, USA). The intensities of the bands were quantified by densitometry using Image J.
Statistical analysis
Results were presented as means ± standard error of the mean (SEM). Statistical analysis was conducted using a two-way analysis of variance followed by Bonferroni post hoc test. P values less than 0.05 were considered statistically significant.
Results
NAD+ administration profoundly decreased both UVC-induced skin damage and UVC-induced increases in the green AF of the mice’s skin
We determined the effects of irradiation with 0, 0.3, 0.6 and 0.9 J/cm2 UVC on the skin damage of mice: Irradiation with 0, 0.3, 0.6, and 0.9 J/cm2 UVC of the mice produced significant damage of the mice’s skin (Figure 1A). Quantifications of the skin damage indicated that UVC produced significant and dose-dependent damage of the skin (Figure 1B). Moreover, the dosages of the irradiation were highly correlated with the levels of the skin damage (Figure 1C).
Figure 1.
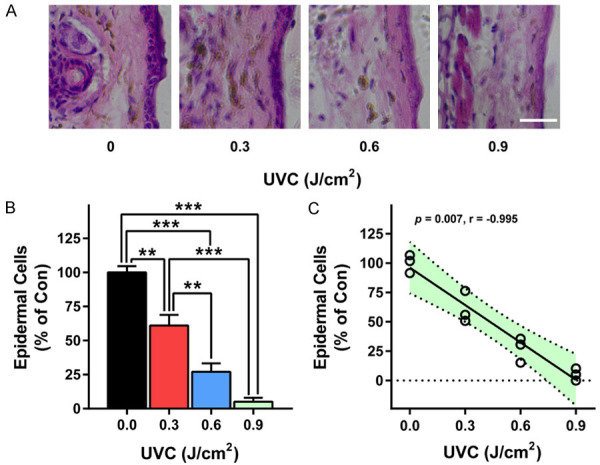
UVC induced dose-dependent damage of mice’s skin. A, B. Irradiation with 0, 0.3, 0.6 and 0.9 J/cm2 UVC produced dose-dependent damage of mice’s skin. C. The dosages of the irradiation were highly correlated with the levels of the skin damage. The mice were exposed to UVC irradiation at the dosages of 0, 0.3, 0.6 and 0.9 J/cm2. Three days after the irradiation, the skin’s damage was determined by H&E staining. N = 3. *, P < 0.05; **, P < 0.01. Scale bar = 100 μm.
We also determined effects of irradiation with 0, 0.3, 0.6, and 0.9 J/cm2 UVC on the green AF intensity of mice: Irradiation with 0, 0.3, 0.6, and 0.9 J/cm2 UVC produced obvious increases in the green AF of the mice’s skin (Figure 2A). Quantifications of the green AF intensity indicated that UVC produced significant and dose-dependent increases in the green AF intensity of the skin (Figure 2B). The dosages of the irradiation were highly correlated with the levels of the green AF intensity (Figure 2C). Moreover, there was a high correlation between the levels of the green AF intensity and the levels of the skin damage (Figure 2D).
Figure 2.
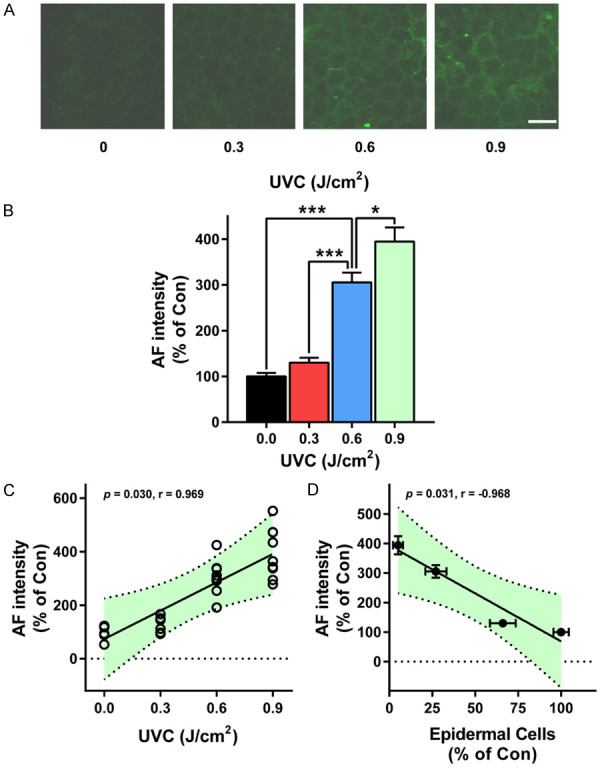
UVC induced dose-dependent increases in the green AF intensity of mice’s skin. A, B. Irradiation with 0, 0.3, 0.6 and 0.9 J/cm2 UVC produced significant and dose-dependent increases in the green AF of the mice’s skin. C. The dosages of the irradiation were highly correlated with the levels of the green AF intensity. D. There was a high correlation between the levels of the green AF intensity and the levels of the skin damage. The mice were exposed to UVC irradiation at the dosages of 0, 0.3, 0.6 and 0.9 J/cm2. Eighteen - 24 h after the irradiation, the skin’s green AF was determined under a confocal microscope. N = 9. *, P < 0.05; ***, P < 0.001. Scale bar = 20 μm.
We further determined the effects of NAD+ administration on the UVC-induced skin damage: NAD+ administration markedly decreased UVC-induced skin damage (Figure 3A). Quantifications of the data indicated that NAD+ administration significantly decreased UVC-induced skin damage (Figure 3B). NAD+ administration also obviously attenuated UVC-induced increases in the green AF intensity of the mice’s skin (Figure 3C). Quantifications of the data indicated that NAD+ administration significantly decreased UVC-induced increases in the green AF intensity of the mice’s skin (Figure 3D).
Figure 3.
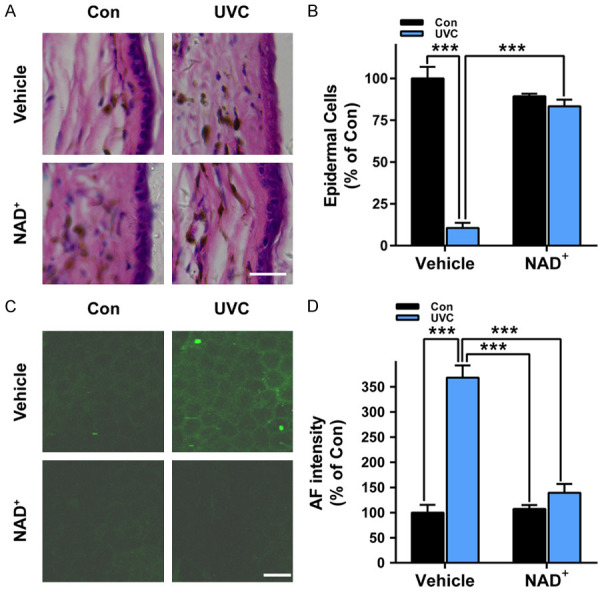
NAD+ administration profoundly attenuated both UVC-induced skin damage and UVC-induced increases in the green AF of the skin of mice. A, B. NAD+ administration significantly decreased UVC-induced skin damage. N = 3. ***, P < 0.001. Scale bar = 100 μm. C, D. NAD+ administration significantly attenuated UVC-induced increases in the green AF intensity of the mice’s skin. After the mice were administered with 1 mg/kg NAD+, the mice were exposed to UVC irradiation at the dosages of 0.6 J/cm2. Three days after the irradiation, the skin’s damage was determined by H&E staining. The skin’s green AF was determined under a confocal microscope. N = 10. ***, P < 0.001. Scale bar = 20 μm.
NAD+ administration significantly attenuated UVC-induced decreases in the levels of mitochondrial superoxide dismutase (SOD2) and catalase as well as UVC-induced increases in the COX2 levels of the mice’s skin
We found that UVC induced significant decreases in the SOD2 activity of the mice’s skin, which were significantly attenuated by NAD+ administration (Figure 4A and 4B). UVC also induced significant decreases in the levels of catalase of the mice’s skin, which were significantly attenuated by NAD+ administration (Figure 4C, 4D).
Figure 4.
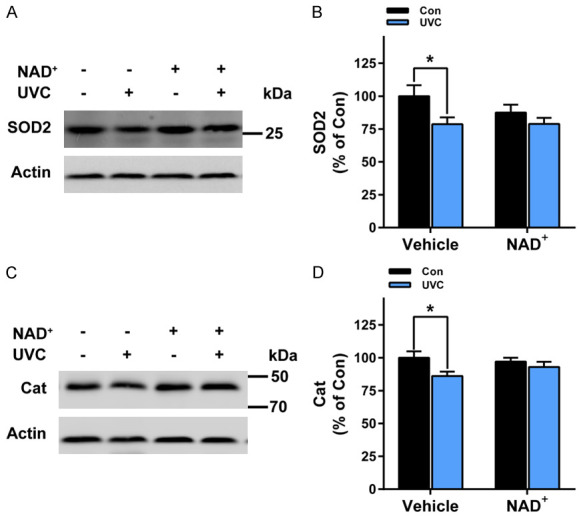
NAD+ administration significantly attenuated UVC-induced decreases in the levels of SOD2 and catalase in the mice’s skin. A, B. UVC induced significant decreases in SOD2 levels in the mice’s skin, which was significantly attenuated by the NAD+ administration. N = 16; *, P < 0.05. C, D. UVC induced significant decreases in catalase levels in the mice’s skin, which was significantly attenuated by the NAD+ administration. After the mice were administered with 1 mg/kg NAD+, the mice were exposed to UVC irradiation at the dosages of 0.6 J/cm2. One hr after the irradiation, the SOD2 levels and the catalase levels of the skin was determined by Western blot assays. N = 6. *, P < 0.05.
We further found that UVC induced significant increases in COX2 levels - a marker of inflammation [16] - of the mice’s skin (Figure 5A, 5B). Administration with NAD+ significantly attenuated the UVC-induced increases in the COX2 levels (Figure 5A, 5B).
Figure 5.
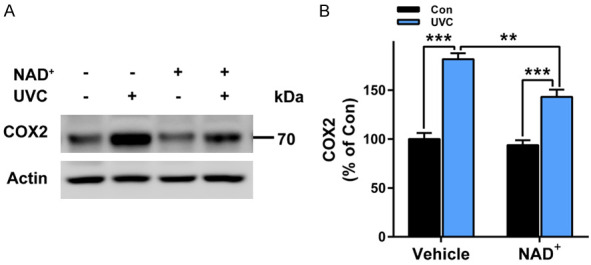
NAD+ administration significantly attenuated UVC-induced increases in COX2 levels of the mice’s skin. A, B. UVC induced significant increases in COX2 levels of the mice’s skin, which was significantly attenuated by the NAD+ administration. After the mice were administered with 1 mg/kg NAD+, the mice were exposed to UVC irradiation at the dosages of 0.6 J/cm2. One hr after the irradiation, the COX2 levels of the skin was determined by Western blot assays. N = 6; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
NAD+ administration significantly attenuated UVC-induced increases in dsDNA damage of the mice’s skin
Our Western blot assays showed that UVC induced significant increases in the phosphorylated protein levels of γ-H2AX - an index of dsDNA damage [17] (Figure 6A, 6B). NAD+ administration prevented the UVC-induced increases in γ-H2AX (Figure 6A, 6B). Our immunostaining assays also showed that UVC induced obvious increases in the protein levels of γ-H2AX, which were prevented by NAD+ administration (Figure 6C).
Figure 6.
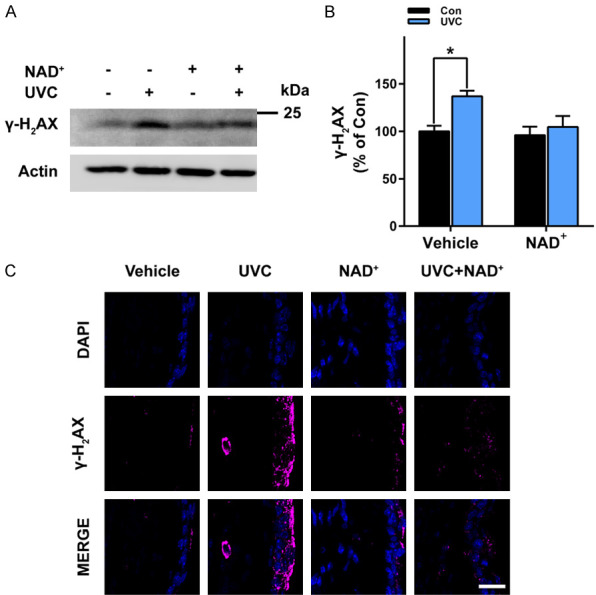
NAD+ administration significantly attenuated UVC-induced increases in γ-H2AX levels of the mice’s skin. A, B. UVC induced significant increases in the protein levels of γ-H2AX of the mice’s skin, which was significantly attenuated by the NAD+ administration. C. Immunostaining assays showed that UVC induced increases in the protein levels of γ-H2AX of the mice’s skin, which was attenuated by the NAD+ administration. After the mice were administered with 1 mg/kg NAD+, the mice were exposed to UVC irradiation at the dosages of 0.6 J/cm2. One hr after the irradiation, the γ-H2AX levels of the skin was determined by Western blot assays or immunostaining assays. N = 7. *, P < 0.05. Scale bar = 20 μm.
NAD+ administration significantly attenuated UVC-induced increases in the Bcl-2/Bax ratios of the mice’s skin
We further determined the effects of NAD+ administration on UVC-induced apoptotic signals: UVC induced significant decreases in the levels of Bcl-2 - a critical anti-apoptotic protein [18], which were prevented by NAD+ administration (Figure 7A, 7B). In contrast, UVC did not significantly affect the levels of Bax - an important pro-apoptotic protein [18], which were not affected by NAD+ administration (Figure 7A, 7C). Collectively, UVC induced significant decreases in the Bcl-2/Bax ratios, which were significantly attenuated by NAD+ administration (Figure 7D).
Figure 7.
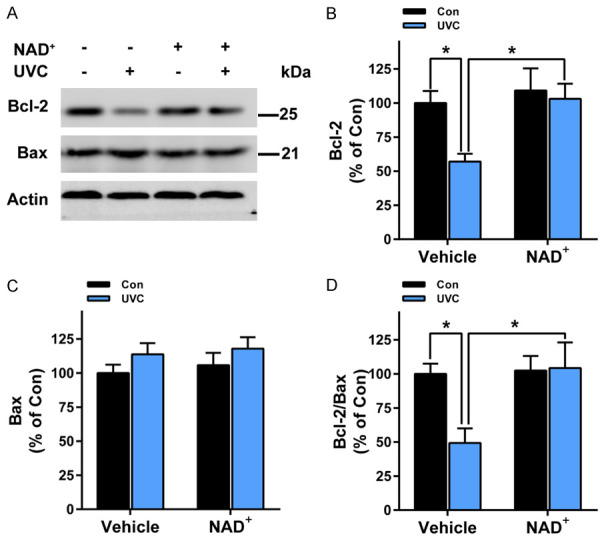
NAD+ administration significantly attenuated UVC-induced increases in the Bcl-2/Bax ratios of the skin of mice. A, B. UVC induced significant decreases in the levels of Bcl-2, which were prevented by NAD+ pre-treatment. A, C. UVC did not significantly affect the levels of Bax, which were not affected by NAD+ pre-administration. D. UVC induced significant decreases in the Bcl-2/Bax ratios, which were prevented by NAD+ administration. After the mice were administered with 1 mg/kg NAD+, the mice were exposed to UVC irradiation at the dosages of 0.6 J/cm2. One hr after the irradiation, the levels of Bcl-2 and Bax of the skin were determined by Western blot assays. N = 6; *, P < 0.05.
Discussion
The major findings of our study include: First, UVC-induced skin’s green AF was highly correlated with the extent of UVC-indued skin’s damage; second, NAD+ administration profoundly decreased UVC-induced skin damage; third, NAD+ administration significantly attenuated UVC-induced decreases in the levels of mitochondrial superoxide dismutase and catalase of the skin; fourth, NAD+ administration significantly attenuated UVC-induced increase in the level of COX2 of the skin; fifth, NAD+ administration profoundly attenuated UVC-induced increase in sDNA damage of the skin; and sixth, NAD+ administration profoundly attenuated UVC-induced decreases in the ratios of Bcl-2/Bax of the skin.
UV radiation is a major risk factor for a number of skin diseases including skin cancer [6-8]. While multiple studies have found skin-protecting agents, it is highly significant to discover new agents which can provide protection against UV-induced skin damage. Due to gradual depletion of the atmospheric ozone layer, there has been increased UVC radiation on the surface of the earth, which is increasingly becoming an important environmental concern [14]. Since it is ionizing irradiation, UVC is a strong mutagen that can cause cancer and immune-mediated diseases [14]. Therefore, it is highly valuable to search for the agents that can profoundly decrease UVC-induced skin damage. Our current study has provided strong evidence indicating that NAD+ is a potent agent that can decrease UVC-induced skin damage. Moreover, our study has indicated the mechanisms underlying the protective effects of NAD+ on UVC-induced skin damage: NAD+ can decrease UVC-induced skin damage by attenuating UVC-induced oxidative stress, inflammation, DNA damage and apoptosis.
Our study has indicated mechanisms underlying the NAD+-produced protection on UVC-induced skin damage: We found that NAD+ administration can profoundly attenuate UVC-induced decreases in the ratio of Bcl-2/Bax - an indicator of apoptotic signals, suggesting that NAD+ administration decreases UVC-induced skin damage at least partially by reducing UVC-induced apoptosis of the skin. Since dsDNA damage is an important inducer of apoptosis [19], our finding that NAD+ administration can attenuate UVC-induced increases in dsDNA damage has suggested that NAD+ administration can decrease the UVC-induced apoptosis at least partially by attenuating the UVC-induced dsDNA damage.
Since UVC is an ionizing radiation, UVC can induced increases oxidative stress in the skin [6-8], which is an important pathological factor in UVC-induced skin damage. Therefore, it is expected that UVC was shown in our study to induce decreased catalase activity and SOD2 activity. Out study showed that NAD+ administration can significantly attenuate the UVC-induced decreases in SOD2 activity and catalase activity, indicating that NAD+ decreases UVC-induced skin damage at least partially by attenuating oxidative stress. Since oxidative stress is an inducer of dsDNA damage [20] and apoptosis [21], our finding has suggested that NAD+ administration may attenuate the UVC-induced increases in dsDNA damage and apoptosis at least partially by decreasing oxidative stress. Inflammation is a critical pathological factor in UV-induced skin damage [22]. Our study has found that NAD+ administration can significantly attenuate UVC-induced increase in COX2 levels, suggesting that UVC-produced decreases in inflammation may be another important mechanism underlying the beneficial effects of NAD+ on UVC-induced skin damage.
Our studies have indicated that the skin’s green AF is a novel biomarker for diagnosis of multiple major diseases, including acute ischemic stroke and lung cancer [23]. Our recent study has also indicated that skin’s green AF intensity is a biomarker for predicting UVC-induced skin damage [15]. Our current study has provided further evidence supporting this finding: UVC-induced skin’s green AF was highly correlated with UVC-induced skin damage. Therefore, this novel method holds good potential for future applications in predicting UVC-induced skin damage.
In conclusion, our current study has not only indicated NAD+ as a new agent for decreasing UVC-induced skin damage, but also provided further evidence supporting the validity of our technology for predicting UVC-induced skin damage. It is warranted to further investigate the mechanisms underlying the validity of the technology.
Acknowledgements
The authors would like to acknowledge the funding supported by Shanghai Municipal Science and Technology Major Project (Grant No. 2017SHZDZX01) (to W.Y.) and a SJTUSTAR Medical-Engineering Interdisciplinary Youth Fund funded by Shanghai Jiao Tong University (YG2019QNA36) to Y.Z.
Disclosure of conflict of interest
None.
References
- 1.Ying W. NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006;11:3129–3148. doi: 10.2741/2038. [DOI] [PubMed] [Google Scholar]
- 2.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Ying W. NAD(+) deficiency is a common central pathological factor of a number of diseases and aging: mechanisms and therapeutic implications. Antioxid Redox Signal. 2019;30:890–905. doi: 10.1089/ars.2017.7445. [DOI] [PubMed] [Google Scholar]
- 4.Ying W, Wei G, Wang D, Wang Q, Tang X, Shi J, Zhang P, Lu H. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang B, Fu X, Guan S, Han W, Zhang J, Gan Q, Fang W, Ying W, Qu X. Exogenous NAD(+) administration significantly protects against myocardial ischemia/reperfusion injury in rat model. Am J Transl Res. 2016;8:3342–3350. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Weng QY, Fisher DE. UV signaling pathways within the skin. J Invest Dermatol. 2014;134:2080–2085. doi: 10.1038/jid.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Burke KE. Mechanisms of aging and development-A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech Ageing Dev. 2018;172:123–130. doi: 10.1016/j.mad.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 10.de Jager TL, Cockrell AE, Du Plessis SS. Ultraviolet light induced generation of reactive oxygen species. Adv Exp Med Biol. 2017;996:15–23. doi: 10.1007/978-3-319-56017-5_2. [DOI] [PubMed] [Google Scholar]
- 11.Mullenders LHF. Solar UV damage to cellular DNA: from mechanisms to biological effects. Photochem Photobiol Sci. 2018;17:1842–1852. doi: 10.1039/c8pp00182k. [DOI] [PubMed] [Google Scholar]
- 12.Guan LL, Lim HW, Mohammad TF. Sunscreens and photoaging: a review of current literature. Am J Clin Dermatol. 2021;22:819–828. doi: 10.1007/s40257-021-00632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancuso JB, Maruthi R, Wang SQ, Lim HW. Sunscreens: an update. Am J Clin Dermatol. 2017;18:643–650. doi: 10.1007/s40257-017-0290-0. [DOI] [PubMed] [Google Scholar]
- 14.Mohania D, Chandel S, Kumar P, Verma V, Digvijay K, Tripathi D, Choudhury K, Mitten SK, Shah D. Ultraviolet radiations: skin defense-damage mechanism. Adv Exp Med Biol. 2017;996:71–87. doi: 10.1007/978-3-319-56017-5_7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Ying W. UV-induced skin’s green autofluorescence is a biomarker for both non-invasive evaluations of the dosages of UV exposures of the skin and non-invasive prediction of UV-induced skin damage. Photochem Photobiol Sci. 2023;22:159–168. doi: 10.1007/s43630-022-00306-z. [DOI] [PubMed] [Google Scholar]
- 16.Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 17.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploumaki I, Triantafyllou E, Koumprentziotis IA, Karampinos K, Drougkas K, Karavolias I, Trontzas I, Kotteas EA. Bcl-2 pathway inhibition in solid tumors: a review of clinical trials. Clin Transl Oncol. 2023 doi: 10.1007/s12094-022-03070-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 21.Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 22.Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 23.Ying W. Phenomic studies on diseases: potential and challenges. Phenomics. 2023:1–15. doi: 10.1007/s43657-022-00089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


