Abstract
Introduction: The cardiovascular autonomic functions can be tested by a Battery of five tests developed by Ewing and Clark in 1981 in Edinburgh. Yogic practices are immensely useful for physical, mental and spiritual development required for better autonomic function. Aim and Objectives: To assess the ANS (Autonomic function system) function with help of Ewing’s Battery tests in yoga participants and healthy participants not practicing yoga. Materials and Methods: A cross-sectional study was conducted on 270 participants which were divided into two groups viz: 135 in healthy control (Group I) and 135 in yoga group (Group II). Subjects with informed consent between 40-50 years, were included in control (Group I) and those practicing yoga for past minimum 3 months were included in Group II. Anthropometric measurements were done and parasympathetic tests like Heart rate (HR) response to standing from the supine posture, to Valsalva maneuvers and to slow deep breathing were done. Also, sympathetic tests, Blood Pressure (BP) response to cold in cold pressor test (CPT), to sustained handgrip test and to standing from lying posture were carried out. Results: P value was found to be statically significant among yoga group as compared with healthy control group in all the sympathetic and parasympathetic tests except in CPT. As per the Ewing’s criteria, normal, early, diseased and severe CAN (Cardiac autonomic neuropathy) in healthy controls findings were 11.11%, 58.51%, 37.03%, 17.77% and in yoga participants findings were 37.7%, 34.8%, 6.66% and 8.88% respectively. According to Bellavere’s classification, maximum diseased CAN were found in healthy control as compared to yoga group. As per AIIMS (All India Institute of Medical Sciences) criteria, parasympathetic neuropathy was observed in 11.85% of the healthy controls and in 6.66% of the yoga group, and that maximum sympathetic neuropathy was observed in 11.11% of the healthy patients and only 3.7% of the yoga group. Conclusion: More emphasis should be given on implementation of yoga from early ages at the institutional levels, hospital levels. Yoga practices will suffice and lead to improvement of unhealthy ANS condition. Overall, Yoga showed better ANS function than healthy control group.
Keywords: Yoga, AFT, CAN, AIIMs criteria, Ewing criteria
Introduction
The autonomic nervous system regulates visceral functions such as respiratory rate, HR, digestion, and maintenance of internal homeostasis. It also plays a crucial role in attention, emotional stability, self-regulation, and social affiliation [1]. Various tests have been mentioned to describe the mechanism of autonomic function in various systems like the cardiovascular, gastrointestinal, pupillary, and neuroendocrine systems [2]. Autonomic cardiovascular functions can be tested by a battery of five tests developed by Ewing and Clark in 1981 in Edinburgh, and later validated by the American Diabetes Association [3,4]. These tests are used to determine autonomic function of a person by stimulating cardiovascular reflexes, and they are widely used for clinical assessment of CAN. The tests have good sensitivity, specificity, reproducibility, are easy to perform, and are well standardized [5]. Ewing’s parasympathetic function includes HR response to standing from the supine posture (30:15 ratio), to Valsalva maneuvers-Valsalva ratio (VR) and to slow deep breathing, while the tests for sympathetic function include the BP response to cold, to sustained HGT, and to actively standing from the supine posture. The results obtained from stimulation of cardiovascular reflexes can be compared with standard guidelines and classified as normal or abnormal autonomic function. Ewing’s criteria showed the normal values for various sympathetic and parasympathetic tests. The normal range for HR response to standing from the supine posture, Valsalva maneuver, and slow deep breathing include 1.01-1.03, 1.11-1.20, and 11-14, respectively, while the normal range for BP response CPT, sustained handgrip, and standing from a lying posture include 11-15 mmHg, 11-15 mmHg, and 11-20 mmHg respectively [6,7].
Yoga is believed to have originated in the Indian subcontinent, and yogic practices are immensely useful for physical, mental, and spiritual development [8]. The positive effect and other benefits of yoga were observed mainly on cardio-respiratory and autonomic functions [9]. Yoga also causes a reduction in systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial BP along with resting pulse rate.
Hence, we conducted this study and included two groups of healthy participants not practicing yoga, and a regular yoga-practicing group to assess ANS function.
Materials and methods
An analytical cross-sectional study with 270 participants was conducted in the Department of Physiology, Zydus Medical College and Hospital, Dahod, Gujarat, India after obtaining consent from the Institutional Ethical Committee, and the study lasted from December 2020 to March 2020.
Inclusion and exclusion criteria
The selection criteria included 135 participants in a healthy control group and 135 in a yoga group.
Group I - Healthy control
Subjects who gave informed consent and between 40 and 50 years of age were included. Participants regularly practicing yoga, who were not interested in the study, below 40 and above 45 years, with history of surgeries, cardiovascular or neurological illness, smoking, alcoholism, or any other addiction as well as female participants in the bleeding phase of their menstrual cycle were excluded from Group I.
Group II - Yoga group
Participants who gave informed consent, between 40 and 50 years as well as those undergoing yoga training by an expert for a minimum of 3 months with one-hour duration of daily practice were included. Subjects who were not interested, below 40 and above 45 years, diagnosed with diabetes, with a history of surgeries, cardiovascular or neurological illness, smoking, alcoholism, or any other addiction as well as females in the bleeding phase of their menstrual cycle were excluded from Group II.
All participants were informed about the analytical cross-sectional study and a written consent was obtained; case study forms were also maintained for all accordingly. Furthermore, a brief explanation of the procedure was given, and volunteers were familiarized with all the equipment used in the study and also instructed to discontinue the test if they faced any discomfort and also to report immediately. All the AFT were performed after a light breakfast in morning.
Anthropometric assessment was done as per the guidelines given in the anthropometry procedures manual published by the National Health and Nutrition Examination Survey (NHANES) [10]. Height, weight, and body mass index (BMI) were recorded for all participants and ANS function tests were then performed.
Parasympathetic tests
HR response to standing from the supine posture
The subject was asked to lie supine, fully relaxed on a couch for 10 minutes. We recorded ECG and calculated basal HR then the participant was made to stand up immediately. HR was again calculated as the ratio between the R-R interval at beats 30 and 15 of the ECG recorded immediately upon standing. This was termed as the 30:15 ratio [4,11].
HR response to Valsalva maneuver
The participant was asked to sit on a chair. The nostrils were closed with a nose clip mouthpiece put into the mouth and connected to a manometer. The recording was taken on the ECG machine, then the subject was asked to breathe forcefully into the mercury manometer and maintain the expiratory pressure at 40 mmHg for 10-15 seconds. The ECG was recorded during the maneuver and 45 seconds after the maneuver. The VR was calculated between the maximum (after release of strain) and minimum R-R interval (during strain) [4,11].
HR response to slow deep breathing (expiratory-inspiratory ratio)
The participants were asked to comfortably lie supine and we recorded ECG and respiratory events. Subjects were asked to breathe deeply at a rate of 6 breaths/min, followed by slow and deep expiration, and changes in HR between inspiration and expiration were averaged over six cycles.
Sympathetic function tests
BP response to CPT
The basal BP of the participants recorded and then they were asked to submerge one of their upper limbs in cold water (temp at 2-4°C) kept in a pot for 1 minute, and their BPs were recorded at 30 and 60 seconds of submersion of limb [4,11].
BP response to sustained hand grip
Before the exercise, the participants were allowed to rest in a quiet room for 10 minutes to reduce their anxiety, then their resting BPs were measured by the auscultatory method using a mercury sphygmomanometer. Instructions were given to the subjects to perform (dominant hand) hand grip in a dynamometer, giving as much pressure as they could apply for 3-4 seconds, which was the maximum voluntary contraction followed by sustained hand grip exercise, maintaining a pressure of 30% of maximum activity for 5 minutes with the dynamometer. We recorded BP at this time and again after 5 minutes after completion of the exercise. Difference between the diastolic BP before and after the exercise was considered the response [4,11].
BP response to active standing from a supine posture
The participants were kept in a fully relaxed patient supine position for 10-20 minutes and their basal BPs was measured. Then, the participants were instructed to stand up abruptly from the supine position with the BP cuff still in place, and within 15 seconds of standing, their BPs were again recorded. Difference in the BPs from standing to supine positions considered the measure of postural BP change [4,11].
Statistical analysis
The mean and S.D. were utilized to compare the values using Excel, whereas comparison between the groups was done using the independent student “t” test. The values were expressed as mean ± SD, and P < 0.05 was taken for statistical significance. All statistical calculations and analyses were performed using SPSS for windows (statistical package for social sciences) version 16.
Result
Total male and female population in Group I i.e. healthy control group was 56.30% and 43.70% respectively with mean age 43.76±12.80. Total male and female population in Group II i.e. yoga group was 40% and 60% respectively with mean age 47.45±13.39 (Table 1).
Table 1.
Baseline characteristics of healthy control and yoga group (I, II)
| Group I Healthy Control N = 135 (%) | Group II Yoga N = 135 (%) | |
|---|---|---|
| Male | 56.30 | 40.00 |
| Female | 43.70 | 60.00 |
| Mean ± SD | Mean ± SD | |
| Age | 43.76±12.80 | 47.45±13.39 |
As per Ewing’s criteria, in Group I, normal tests, early, diseased and severe CAN were observed in 11.11%, 58.51%, 37.03%, and 17.77% of the healthy participants, respectively, while in Group II, % were seen as 37.7%, 34.8%, 6.66%, and 8.88% of Yoga participants, respectively (Figure 1).
Figure 1.
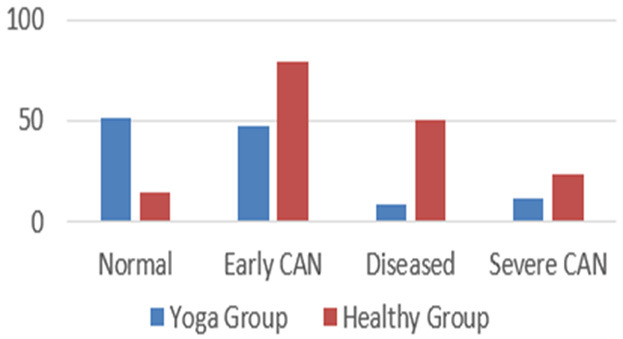
Findings of CAN based on Ewing’s criteria among healthy control & yoga group (I, II).
As per Bellavere’s criteria, Group I has 11.58% Diseased CAN participants while 11.1% diseased participants in Group II (Table 7).
Table 7.
Findings based on Bellavere’s criteria
| Category of CAN (%) | Healthy Control Group I | Yoga Group II |
|---|---|---|
| Normal CAN | 29.62 | 68.5 |
| Borderline CAN | 58.51 | 7.4 |
| Diseased CAN | 11.58 | 11.1 |
As per AIIMS criteria, parasympathetic neuropathy was observed in 11.85% in Group I and in 6.66% of Group II, and sympathetic neuropathy was observed in 11.11% of Group I and only 3.7% Group II (Figure 2).
Figure 2.
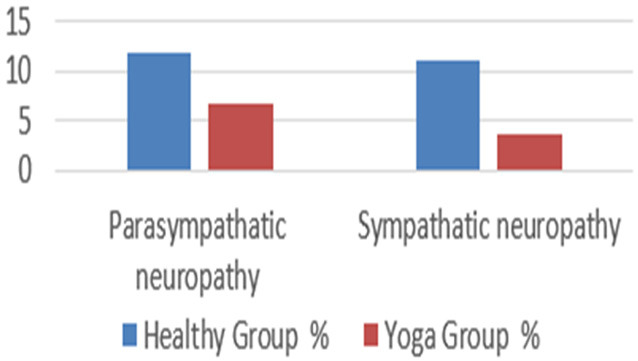
Findings of CAN (sympathetic tests) based on AIIMS’s AFT lab criteria among healthy control and yoga group (I, II).
Discussion
Baseline characteristics of the yoga group and the healthy control group
The mean SBP, DBP, HR, RBS, weight, and BMI parameters in the healthy control group were, respectively, 118.36 (6.73), 70.84 (7.16), 74.34 (9.40), 113.68 (11.17), 60.74 (8.25), and 23.48 (2.83) in an earlier study (Table 2). The mean age, weight, HR SBP, and DBP were 59.9 (9.9), 64.9 (9.3), 73.0 (9.1), 130.1 (10.3), and 83.7 (8.0), respectively, in the earlier study conducted by Pal et al. [8]. In a different study by Veerabhadrappa et al., the mean supine and standing blood pressure readings were, respectively, 112.08 8.99, 108.16 7.77, and 106.88 9.98, 104.56 7.93 before and after yoga training [13]. These values were lower than those in controls, demonstrating yoga’s positive impact on the body. Basal parameters were high in the healthy control Group I and low in the yoga practitioners Group II, indicating yoga is definitely beneficial for maintaining a healthy body and keeping BP, HR, and RBS levels within normal ranges. Yogic techniques have been proven to have considerable cardiovascular health advantages since they considerably lower HR, SBP, and DBP [8]. Hence, yoga needs to be given more attention.
Table 2.
Baseline parameters of healthy control and yoga group (I, II)
| Parameter | Healthy Group I | Yoga Group II | Yoga & healthy | |
|---|---|---|---|---|
|
|
|
|||
| Mean ± SD | Mean ± SD | T | P | |
| Height | 147.92±12.11 | 156.59±14.70 | 6.98 | 0.00* |
| Weight | 61.33±11.64 | 59.14±4.12 | -2.14 | 0.03* |
| BMI | 28.55±6.90 | 24.79±5.23 | -5.09 | 0.00* |
| Basal HR | 85.36±16.05 | 83.83±8.53 | -1.02 | 0.31 |
| RBS | 112.00±21.16 | 103.41±18.95 | -5.11 | 0.00* |
| SBP | 137.64±18.31 | 130.22±16.94 | -3.73 | 0.00* |
| DBP | 85.25±17.30 | 81.36±15.99 | -2.03 | 0.04* |
p-value ≤ 0.05 = statistically significant.
Parasympathetic and sympathetic tests
Test results for autonomic function were displayed in Table 3. The HR response to standing from a supine position (30:15 ratio), to the Valsalva manoeuvre, and to slow deep breathing were tests of the parasympathetic nervous system’s (PNS) functionality. CPT, BP response to sustained handgrip, and active standing from supine position were used as sympathetic nervous system (SNS) tests.
Table 3.
| Test No. | Normal (Score 0) | Borderline (Score 1) | Abnormal (Score 2) | |
|---|---|---|---|---|
| PARASYMPATHATIC TESTS | ||||
| 1. | HR response to standing from supine posture | ≥ 1.04 | 1.01-1.03 | ≤ 1.01 |
| 2. | HR rate response to Valsalva maneuver | ≥ 1.21 | 1.11-1.20 | ≤ 1.10 |
| 3. | HR response to slow deep breathing | > 15 | 11-14 | < 10 |
| SYMPATHATIC TESTS | ||||
| 4. | BP response Cold pressor test | 16 mmHg | 11-15 mmHg | < 10 mmHg |
| 5. | BP response to sustained handgrip | 16 mmHg | 11-15 mmHg | < 10 mmHg |
| 6. | BP response to standing from lying posture | ≤ 10 mmHg | 11-20 mmHg | ≥ 20 mmHg |
In test 1, or the HR reaction to standing from the supine position, our results demonstrated a lower increase in HR in the typical healthy group than in the yoga group. According to earlier research, the heart rate rose with standing until it reached its maximum at the 15th beat, following which it decreased to a stable level at around the 30th beat. This increase may be caused by the fact that when someone gets out of the supine position, gravity draws some blood to the hands, feet, and abdomen. This causes quick vasoconstriction, which causes a minor increase in heart rate. A gauge of parasympathetic activity is the ratio of the R-R interval between the 30th and 15th beats (30:15 ratio) [11]. Similar to this, Lin et al.’s investigation revealed that 5% of healthy subjects exhibited an aberrant HR response to standing from the supine position [6]. Our study’s findings are analogous to those of Madan Mohan, who found that after practising shavasana, HR and oxygen consumption significantly decreased [14]. After 6 weeks of training, a different study found that the shavasana group’s HR significantly decreased [15]. This finding suggests that regular yoga practise causes an autonomic equilibrium to gradually move towards a relative parasympathodominance, which lowers HR. Also, our study can be compared to another where a decline in the 30:15 ratio was noted [16].
In test 2, which measured the heart rate in reaction to the Valsalva manoeuvre, the healthy Group I recorded a lower heart rate than the yoga Group II. 52.6% of the patients in Anca et al.’s study had abnormal Valsalva manoeuvre scores [17]. However, a different study by Deepak D found that controls had an aberrant HR response to Valsalva manoeuvres [18], which may have been caused by weakened cardiovascular reflexes as well as reduced parasympathetic function as a result of low vagal tone. According to Pal et al., the Valsalva manoeuvre entails an abrupt, brief, and voluntary increase in intra-thoracic and intra-abdominal pressure that is brought on by straining [8]. The VR focuses more on the parasympathetic nervous system, which is influenced by factors like age, sex, patient position, expiratory pressure, duration of strain, and yoga technique [9]. In a study, Bharshankar JR et al. [19] investigated the impact of yoga on cardiovascular function in participants over the age of 40. They discovered that the VR was substantially higher in yoga practitioners than in controls, proving that the practise slows the decline in cardiovascular health that comes with ageing. Telles S and Khanam AA et al. observed no change in the Valsalva manoeuvre, which is in contrast to our findings [20,21].
According to the conventional reference values, the outcome of test 3, which measured the HR response to slow, deep breathing, revealed borderline changes in the yoga group (Group II) and as normal in the healthy control group (Group I). Heart rate variability, or HRV, is one of the numerous cardiovascular autonomic reflex tests (CARTs) that are included in the gold standard diagnostic criteria for CAN, according to Spallone V [22], and similar results were observed in the study by Tuppad et al., in which a decrease in this parameter was observed [23]. In the yoga and healthy control groups of Patil et al.’s study there was a decrease in sympathetic tone and an increase in parasympathetic tone, respectively [24]. When compared to the results from the first 7 days of yoga, deep breathing significantly improved in the study by Vinutha et al. [25]. This may be the case because the heart’s vagal innervations primarily mediate the fluctuation in HR with respiration. The gain of the afferent and efferent outputs at the nucleus tractus solitarius is influenced by the respiratory center’s neuronal output. Cardiovascular mechanoreceptors, pulmonary stretch receptors, and baroreceptors all control HR fluctuation. According to earlier research, cardiac mechanoreceptors, baroreceptors, and pulmonary stretch receptors all have a role in sinus arrhythmia [11]. After practising yoga poses and pranayama for three months, Phatale et al. found a statistically significant improvement in parasympathetic activity markers such orthostatic HR deep breathing [26].
Sympathetic function test
According to the results of test 4, or CPT, the yoga group (Group II CPT)’s was normal and the healthy control group’s (Group I) CPT was borderline [3]. In the aetiology of essential hypertension, the SNS is crucial. The increase in blood pressure is caused by sympathetic overactivity, which causes hypersensitivity to stress stimuli [27]. Moreover, studies have shown that dipping a hand into ice-cold water raises both SBP and DBP. Somatic fibres make up the reflex route’s afferent limb, whilst sympathetic fibres make up the efferent pathway [11]. Similar outcomes in the yoga group were reported by Bharshankar JR [19]. Also, the research by Rankhambe HB found that practising yoga can greatly reduce changes in cardiovascular parameters brought on by stress by redistributing the cardiovascular autonomic balance in favour of the parasympathetic side [28]. In around 12 weeks of yoga practise, Sardessai looked at how much a person’s capacity to handle stress improved as indicated by CPT [7]. The stress that the cold places on the circulatory system may be reduced by decreased sympathetic activity, increased vagal activity leading to enhanced parasympathetic tone, improved neuroendocrine balance, and other factors. Furthermore, the strengthening and stabilising of the autonomic and stress response systems, quieting of cortical areas involved in executive functions, progressive activation of the hypothalamic-pituitary-adrenal axis, midbrain and limbic systems, balancing of cortical areas by thalamic and medullary generators, as well as the eventual stimulation of the reward systems of the medial forebrain bundle help in parasympathetic dominance after yoga practise. Long-term meditation practise is also known to increase expression of genes relevant to telomere maintenance, insulin production, and energy metabolism while decreasing expression of genes linked to stress-related pathways [29].
Our findings for test 5, which measured the blood pressure response to a prolonged hand hold, indicated that the control group had higher mean values than the yoga group. According to Pal et al.’s study yoga practitioners had a higher mean BP response to continuous handgrip than did the controls [8]. Response to continuous handgrip improved following 7 days of yoga practise, according to Vinutha et al.’s study [25]. According to Phatale et al.’s study sympathetic activity caused DBP to drop from a mean value of 11.40 mmHg to 7.73 mmHg after continuous HGT [26]. Increases in DBP during isometric handgrip exercise are related with higher ambulatory blood pressure monitoring parameter values [30], and increased arterial stiffness in these patients appears to be a key vascular component predicting the BPR during handgrip exercise [31]. The yoga group may have had better results as a result of having reduced sympathetic drive, which is consistent with the idea that meditation practises allow for greater control of sympathetic drive. After the exercise was stopped, the SBP and DBP of the healthy persons reverted to normal within 5 minutes as a result of a reflex response that resulted in decreased sympathetic activity and increased parasympathetic activity [32].
Our findings for test 6, which measured the blood pressure response to rising from a supine position, indicated that the control group had higher mean values than the yoga group. According to published research, a sudden drop in standing blood pressure initiates the baroreceptor response, which causes the blood pressure to recover to normal within 15 seconds [11]. According to Phatale’s study on sympathetic activity, SBP decreased from 10.71 mmHg when laying down to 7.64 mmHg when standing up [26]. Our findings also indicated that 47.4% and 17.03% of the participants in the healthy group were borderline and abnormal, respectively. This may be because healthy people have increased resting parasympathetic activity as indicated by an increase in HRV, whereas low HRV is associated with poor health and increased sympathetic activity [33,34]. In the premeditation and postmeditation groups of the study by Ganguly et al., SBP decreased from lying to standing (7.23±1.28) (6.81±0.91) [35], while Papp et al. found that 8 weeks of hatha yoga significantly enhanced HRV, which suggests an increased vagal tone and decreased sympathetic activity [36].
According to Table 4, these autonomic tests were normal in 78.5%, 60.74%, 31.85%, and 85% of the healthy control group, and in 72.60%, 84.40%, and 80.70% of the yoga group, respectively. Ewing’s standards are described in Table 5 according to the accepted references [3,10,37]. Several researchers have found comparable results, however with somewhat varying percentages, which may be owing to the CAN adoption criteria, patient sample size, disease length, and ethnicity [12,38,39]. Figure 1 demonstrated that the yoga group had individuals with minimally diseased CAN. The parasympathetic component is represented by the Bellavere’s criteria [40], which are described in Table 6 according to a common reference [38,41]. Table 7 reveals that the yoga group included individuals who had only mild CAN illness. Despite the fact that they only comprise the parasympathetic tests, these percentages were higher than those in the Ewing’s criterion categorization. The standard values in Table 8 are determined by the AIIMS AFT lab guidelines [12], which evaluate sympathetic and parasympathetic testing separately. According to the test results, they are categorised as sympathetic neuropathy and parasympathetic neuropathy. Figure 2 demonstrates that maximum sympathetic neuropathy was seen in healthy patients and less in the yoga group, whereas parasympathetic neuropathy was seen more in healthy controls than in the yoga group. In order to better organise the patient’s care, AIIMS’s AFT Lab standards distinguish the values for sympathetic and parasympathetic neuropathy. Overall, the yoga group, which also included less impacted CAN participants, had less affected ANS function than the healthy controls according to Ewing’s criteria, Bellavere’s criteria, and the AIIMS AFT lab standards. This may be due to the fact that frequent yoga practise causes an incremental shift in autonomic homeostasis towards a relative parasympathodominance, which improves the outcomes of CAN [37].
Table 4.
Findings of autonomic function tests in healthy control & yoga group (I, II)
| Test | Healthy Control Group I | Yoga Group II | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| % | Normal | Borderline | Abnormal | Normal | Borderline | Abnormal |
| HR response to standing from supine posture | 78.5 | 8.14 | 13.33 | 72.60 | 9.62 | 17.70 |
| HR rate response to Valsalva maneuver | 60.74 | 22.20 | 17.03 | 84.40 | 9.60 | 6.00 |
| HR response to slow deep breathing | 31.85 | 23.70 | 44.44 | 80.70 | 11.80 | 7.40 |
| BP response Cold pressor test | 58.51 | 28.10 | 13.33 | 67.40 | 20.00 | 12.60 |
| BP response to sustained handgrip | 82.9 | 2.96 | 2.96 | 96.00 | 2.20 | 1.50 |
| BP response to standing from lying posture | 34.81 | 47.4 | 17.03 | 70.40 | 15.50 | 14.00 |
Table 5.
| CAN | Criteria |
|---|---|
| Normal | All tests normal or one test borderline |
| Early CAN | One of the three HR test abnormal and two borderlines |
| Definite CAN | Two HR test abnormal |
| Severe CAN | Two HR tests abnormal & one or both BP test abnormal |
Tests used - HR response to lying to standing, HR response to Valsalva maneuver, HR response to deep breathing test, BP response to sustained handgrip, BP response to standing from lying posture.
Table 6.
| Category of CAN | Score |
|---|---|
| No CAN | 0-1 |
| Early CAN | 2-3 |
| Definite CAN | 4-6 |
Tests used - (PARASYMPATHATIC TESTS) - HR response to lying to standing, HR response to Valsalva maneuver, HR response to deep breathing test.
Table 8.
Classification of CAN as per AIIMS AFT lab criteria [23]
| Category Of CAN | Criteria |
|---|---|
| Normal CAN | All tests normal or one test borderline |
| Early CAN | One test abnormal or two tests borderline |
| Definite CAN | Two tests abnormal |
Parasympathetic assessment - HR response to deep breathing test, HR response to Valsalva maneuver, HR response to Lying to standing (30:15 ratio); Sympathetic assessment - BP response to cold pressor test, BP response to sustained handgrip, BP response to standing from lying posture.
An idea that yoga practises lower allostatic load in stress response systems such that optimal homeostasis is restored is put out to explain the advantages of yoga practises in a variety of, frequently coexisting medical problems. It is theorised that stress causes an imbalance in the autonomic nervous system (ANS), leading to decreased PNS and increased SNS activity, underactivity of the principal inhibitory neurotransmitter system (GABA system), and increased allostatic burden. The vagus nerve, the primary peripheral conduit of the PNS, is stimulated by yoga-based activities, which are further theorised to lower allostatic load and rectify underactivity of the PNS and GABA systems [41]. As a result, we can draw the conclusion that yoga may be highly advantageous for obtaining good autonomic function. Yoga also produced superior results than the healthy control group, indicating that healthy people can enhance their autonomic function through consistent yoga practise. Yoga practitioners may have undergone changes as a result of the PNS dominating the SNS. Yoga practise lessens sympathetic hyperactivity brought on by stress and lowers anxiety levels [42-45]. These studies demonstrated that the regular yoga participant group had better results for the heart rate response to standing from a supine position, the heart rate response to the Valsalva manoeuvre, the heart rate response to slow, deep breathing, the CPT, the blood pressure response to sustained high heart rate, and the blood pressure response to standing from a lying position. We want to underline the value of yoga in preserving the body’s natural autonomic function. Daily exercise for 30 to 60 minutes should provide beneficial effects. As yoga practise results in a gradual shift of the autonomic equilibrium to parasympathetic dominance and a decrease in sympathetic activity, it should be incorporated into school curricula at least twice or three times each week. Yoga may be particularly helpful for people trying to improve their mental and physical health because it has therapies with solid scientific evidence that are virtually universally accessible [43-46]. This work amply supports other research by showing yoga’s positive effects on SNS and PNS. Yoga should therefore be regarded as a necessity for a better and healthier way of life.
In our study, it was established that those who practised yoga consistently had better outcomes than the healthy group in terms of their heart rate responses to standing from a supine position, heart rate responses to the Valsalva manoeuvre, slow deep breathing, CPT, and BP responses to continuous HGT. We want to emphasise the importance of yoga for maintaining the body’s natural autonomic function.
Limitations and future plan
One limitation of this study was the small sample size; therefore, similar research in the future should involve a larger sample size. Also, we hope to employ more parameters for assessing autonomic function and conduct more training sessions for the health to assess autonomic function.
Disclosure of conflict of interest
None.
References
- 1.Rukmani MR, Seshadri SP, Thennarasu K, Raju TR, Sathyaprabha TN. Heart rate variability in children with attention-deficit/hyperactivity disorder: a pilot study. Ann Neurosci. 2016;23:81–8. doi: 10.1159/000443574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryder RE, Hardisty CA. Which battery of cardiovascular autonomic function tests? Diabetologia. 1990;33:177–9. doi: 10.1007/BF00404047. discussion 180-1. [DOI] [PubMed] [Google Scholar]
- 3.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285:916–8. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8:491–8. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 5.Stranieri A, Abawajy J, Kelarev A, Huda S, Chowdhury M, Jelinek HF. An approach for Ewing test selection to support the clinical assessment of cardiac autonomic neuropathy. Artif Intell Med. 2013;58:185–93. doi: 10.1016/j.artmed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Lin K, Wei L, Huang Z, Zeng Q. Combination of Ewing test, heart rate variability, and heart rate turbulence analysis for early diagnosis of diabetic cardiac autonomic neuropathy. Medicine (Baltimore) 2017;96:e8296. doi: 10.1097/MD.0000000000008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sardessai SR, Pandarbale SS. Effect of cold pressor test on blood pressure in subjects with hypertensive first degree relatives. J Evol Med Dent Sci. 2017;6:3963–6. [Google Scholar]
- 8.Pal A, Srivastava N, Narain VS, Agrawal GG, Rani M. Effect of yogic intervention on the autonomic nervous system in the patients with coronary artery disease: a randomized controlled trial. East Mediterr Health J. 2013;19:452–8. [PubMed] [Google Scholar]
- 9.Sukhsohale ND, Phatak MS. Effect of short term and long term Brahmakumaris Raja Yoga meditation on physiological variables. Indian J Physiol Pharmacol. 2012;56:388–92. [PubMed] [Google Scholar]
- 10.National Health and Nutrition Examination Survey (NHANES). Anthropometry procedure manual [internet] Vol. C2007 Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf. [Google Scholar]
- 11.Pal GK. Textbook of practical physiology. 5th. India: Universities Press Pvt; 2020. pp. 248–58. [Google Scholar]
- 12.Pathak A, Gupta S, Kumar S, Agrawal S. Evaluation of cardiovascular autonomic nervous functions in diabetics: study in a rural teaching hospital. J Pract Cardiovasc Sci. 2017;3:150–7. [Google Scholar]
- 13.Veerabhadrappa SG, Baljoshi VS, Khanapure S, Herur A, Patil S, Ankad RB, Chinagudi S. Effect of yogic bellows on cardiovascular autonomic reactivity. J Cardiovasc Dis Res. 2011;2:223–7. doi: 10.4103/0975-3583.89806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madanmohan RUC, Balavittal V, Thombre DP, Gitananda S. Cardiorespiratory changes during Savitri pranayama and shavasan. The Yoga Rev. 1983;3:25–34. [Google Scholar]
- 15.Madanmohan , Bhavanani AB, Prakash ES, Kamath MG, Amudhan J. Effect of six weeks of shavasana training on spectral measures of short-term heart rate variability in young healthy volunteers. Indian J Physiol Pharmacol. 2004;48:370–3. [PubMed] [Google Scholar]
- 16.Pal GK, Velkumary S, Madanmohan Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J Med Res. 2004;120:115–21. [PubMed] [Google Scholar]
- 17.Anca M, Bajkó Z, Adina S, Rodica B. Cardiovascular autonomic neuropathy and sensorimotor polyneuropathy in type 2 diabetes mellitus. Acta Med Marisiensis. 2012:58. [Google Scholar]
- 18.Deepak D, Sinha AN, Gusain VS, Goel A. A study on effects of meditation on sympathetic nervous system functional status in meditators. J Clin Diagn Res. 2012:6. [Google Scholar]
- 19.Bharshankar JR, Mandape AD, Phatak MS, Bharshankar RN. Autonomic functions in Raja-yoga meditators. Indian J Physiol Pharmacol. 2015;59:396–401. [PubMed] [Google Scholar]
- 20.Telles S, Desiraju T. Autonomic changes in Brahma Kumaris Rajayoga meditation. Int J Psychophysiol. 1993;15:147–52. doi: 10.1016/0167-8760(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 21.Khanam AA, Sachdeva U, Guleria R, Deepak KK. Study of pulmonary and autonomic functions of asthma patients after yoga training. Indian J Physiol Pharmacol. 1996;40:318–24. [PubMed] [Google Scholar]
- 22.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–53. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 23.Tuppad SD. Family history, BMI and sympathetic hyperactivity in healthy offsprings of hypertensive parents. Int J Physiol. 2019;7:15–8. [Google Scholar]
- 24.Patil SG, Aithala MR, Naregal GV, Shanmukhe AG, Chopade SS. Effect of yoga on cardiac autonomic dysfunction and insulin resistance in non-diabetic offspring of type-2-diabetes parents: a randomized controlled study. Complement Ther Clin Pract. 2019;34:288–93. doi: 10.1016/j.ctcp.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Vinutha HT, Raghavendra BR, Manjunath NK. Effect of integrated approach of yoga therapy on autonomic functions in patients with type 2 diabetes. Indian J Endocrinol Metab. 2015;19:653–7. doi: 10.4103/2230-8210.163194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phatale SR, Shinde BV, Patil S, Shinde PU. A study of assessment of cardiac autonomic functions after yogasana and pranayama. ResearchGate. 2019 [Google Scholar]
- 27.Pramanik T, Regmi P, Adhikari P. Cold pressor test as predictor of hypertension. J The Univ Heart Ctr. 2009;4:177–80. [Google Scholar]
- 28.Rankhambe HB, Pande S. Effect of Sudarshan Kriya Yoga on cold pressor response in healthy young adults. Natl J Physiol Pharm Pharmacol. 2021;11:589–92. [Google Scholar]
- 29.Brown RP, Gerbarg PG, Muskin PR. DHUMALLtd. In: Tasman A, Kay J, Lieberman JC, editors. Alternative treatments in psychiatry. Psychiatry, 2nd ed. London, UK: John Wiley & Sons; 2003. pp. 2171–2. [Google Scholar]
- 30.Kempler M, Hajdú N, Putz Z, Istenes I, Vági O, Békeffy M, Schnabel K, Kempler P, Körei AE. Diabetic cardiovascular autonomic neuropathy, the handgrip test and ambulatory blood pressure monitoring parameters: are there any diagnostic implications? J Clin Med. 2020;9:3322. doi: 10.3390/jcm9103322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triantafyllou A, Dipla K, Koletsos N, Papadopoulos S, Zografou I, Zafeiridis AS, Mintziori G, Gkaliagkousi E, Zafeiridis A, Douma S. Blood pressure response to isometric handgrip exercise in diabetic patients without established CVD. J Hypertens. 2019;37:e141. [Google Scholar]
- 32.Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension: an update. Sports Med. 2000;30:193–206. doi: 10.2165/00007256-200030030-00004. [DOI] [PubMed] [Google Scholar]
- 33.Pahlm O, Sörnmo L. Special methods in electrocardioFigurey (specialmetoder inom elektrokardiografi; in Swedish) Lund: Studentlitteratur; 1998. [Google Scholar]
- 34.Sookan T, McKune AJ. Heart rate variability in physically active individuals: reliability and gender characteristics. Cardiovasc J Afr. 2012;23:67–72. doi: 10.5830/CVJA-2011-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganguly A, Hulke SM, Bharshanakar R, Parashar R, Wakode S. Effect of meditation on autonomic function in healthy individuals: a longitudinal study. J Fam Med Prim Care. 2020;9:3944–8. doi: 10.4103/jfmpc.jfmpc_460_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papp ME, Lindfors P, Storck N, Wändell PE. Increased heart rate variability but no effect on blood pressure from 8 weeks of Hatha yoga - a pilot study. BMC Res Notes. 2013;6:59. doi: 10.1186/1756-0500-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandelwal E, Jaryal AK, Deepak KK. Pattern and prevalence of cardiovascular autonomic neuropathy in diabetics visiting a tertiary care referral center in India. Indian J Physiol Pharmacol. 2011;55:119–27. [PubMed] [Google Scholar]
- 38.Yun JS, Kim JH, Song KH, Ahn YB, Yoon KH, Yoo KD, Park YM, Ko SH. Cardiovascular autonomic dysfunction predicts severe hypoglycemia in patients with type 2 diabetes: a 10-year follow-up study. Diabetes Care. 2014;37:235–41. doi: 10.2337/dc13-1164. [DOI] [PubMed] [Google Scholar]
- 39.Menon AS, Dixit A, Garg MK, Girish R. Cardiac autonomic neuropathy in patients with type 2 diabetes mellitus at high risk for foot ulcers. Indian J Endocrinol Metab. 2017;21:282–5. doi: 10.4103/ijem.IJEM_542_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellavere F, Bosello G, Fedele D, Cardone C, Ferri M. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1983;287:61. doi: 10.1136/bmj.287.6384.61-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Streeter CC, Gerbarg PL, Saper RB, Ciraulo DA, Brown RP. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571–9. doi: 10.1016/j.mehy.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Goleman D, Schwartz G. Meditation as an intervention in stress activity. J Consult Clin Psychol. 1876;44:456–66. doi: 10.1037//0022-006x.44.3.456. [DOI] [PubMed] [Google Scholar]
- 43.Kuppusamy M, Kamaldeen D, Pitani R, Amaldas J, Shanmugam P. Effects of Bhramari Pranayama on health-a systematic review. J Tradit Complement Med. 2017;8:11–16. doi: 10.1016/j.jtcme.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuppusamy M, Kamaldeen D, Pitani R, Amaldas J, Ramasamy P, Shanmugam P, Thirupathy VS. Effect of Bhramari pranayama practice on simple reaction time in healthy adolescents-a randomized control trial. Int J Adolesc Med Health. 2020;33:547–550. doi: 10.1515/ijamh-2019-0244. [DOI] [PubMed] [Google Scholar]
- 45.Bagya DA, Ganesan T, Maheshkumar K, Venkateswaran ST, Padmavathi R. Perception of stress among yoga trained individuals. Natl J Physiol Pharm Pharmacol. 2018;8:47–50. [Google Scholar]
- 46.Niva W, Lavanya Sekar J, Manikandan A, MaheshKumar K, Ganesan T, Vanishree S. Mahamantra chanting as an effective intervention for stress reduction among nursing professionals-a randomized controlled study. Adv Integr Med. 2021;8:27–32. [Google Scholar]


