Abstract
Background: Thermal injury has a significant impact on disability and morbidity in pediatric patients. Challenges in caring for pediatric burn patients include limited donor sites for large total body surface area (TBSA) burn as well as optimization of wound management for long term growth and cosmesis. ReCell® technology produces autologous skin cell suspensions from minimal donor split-thickness skin samples, allowing for expanded coverage using minimal donor skin. Most literature on outcomes reports on adult patients. Objective: We present the largest to-date retrospective review of ReCell® technology use in pediatric patients at a single pediatric burn center. Method: Patients were treated at a quaternary care, free-standing, American Burn Association verified Pediatric Burn Center. A retrospective chart review was performed from September 2019 to March 2022, during which time twenty-one pediatric burn patients had been treated with ReCell® technology. Patient information was collected, including demographics, hospital course, burn wound characteristics, number of ReCell® applications, adjunct procedures, complications, healing time, Vancouver scar scale measurements, and follow-up. A descriptive analysis was performed, and medians were reported. Results: Median TBSA burn on initial presentation was 31% (ranging 4%-86%). The majority of patients (95.2%) had placement of a dermal substrate prior to ReCell® application. Four patients did not receive split thickness skin grafting with their ReCell® treatment. The median time between date of burn injury and first ReCell® application was 18 days (ranging 5-43 days). The number of ReCell® applications ranged from 1-4 per patient. Median time until wound was classified as healed was 81 days (ranging 39-573 days). The median maximum Vancouver scar scale measurement per patient at time healed was 8, ranging from 3-14. Five patients who received skin grafts had graft loss and three of these patients had graft loss from areas with ReCell®. Conclusion: ReCell® technology provides an additional method for wound coverage, either on its own or in conjunction with split thickness skin grafting, and is safe and effective in pediatric patients.
Keywords: ReCell® , autologous skin cell suspension, burns, pediatric
Introduction
Novel technology is constantly being developed to facilitate burn wound healing. Improving pediatric burn care is of significant interest as thermal injuries were the fourth leading cause of death in children between the ages of 1-4 years old in 2020 (https://wisqars.cdc.gov/fatal-leading). Not only are burn injuries associated with mortality and significant morbidity, but this care is costly on the healthcare system. The mean total cost of caring for a pediatric burn inpatient is estimated to be $83,535 per child [1].
The goal of pediatric burn care is to achieve wound healing in an expedient manner and minimize long-term scarring to improve functional outcomes. Deep partial thickness (second degree) and full thickness (third degree) burns typically require autologous split thickness skin grafts (STSG) to achieve definitive closure and minimize long term scarring. Despite being the standard of care, skin grafting is associated with several challenges, including post-procedural pain, potential graft failure, and donor site complications. In pediatric patients, using skin grafts for wound coverage can be limited by donor site availability for larger total body surface area (TBSA) burns.
The ReCell® Autologous Skin Cell Harvesting Device was developed in response to these challenges, with the goal of producing autologous skin cell suspensions (ASCS) using minimal donor split-thickness skin samples and without requiring cell culture [2]. ASCS are generated by incubating a donor split-thickness skin sample in proprietary enzyme and buffer solutions. Epidermal cells are then sterilely and mechanically dissociated, filtered, and sprayed onto the wound bed. The ReCell® device extracts approximately 1.7×106 viable cells per square centimeter (cm2) from the dermal-epidermal junction, and predominantly isolates keratinocytes, followed by fibroblasts and melanocytes [3]. The viability of the cells in the ReCell® suspension has been shown to be minimally affected by the spray application, with 76% of cells viable in the pre-spray samples compared to 70% viability from the post-spray samples [3]. This technology allows for an expansion ratio of up to 80:1 [2]. ASCS can be used for the treatment of burn wounds [4,5], STSG donor sites [6], chronic wounds [7-9], hypopigmented scars [10], acne scars [11], vitiligo [12-14], and large congenital melanocytic nevus [2,15]. Holmes et al. (2018) prospectively compared meshed STSG and ReCell® for treatment of deep partial-thickness burns on the same patient in a multi-center, randomized clinical trial [2]. They found comparable healing in this adult patient population, and the ReCell® donor site was smaller in size and less painful than that of the STSG donor site [2].
There is little published on the use of ReCell® in pediatric burn patients. In a prospective randomized clinical trial comparing treatment of pediatric patients who sustained partial thickness scald injuries with (1) standard dressing changes, (2) Biobrane®, a semipermeable silicone membrane, and (3) Biobrane® with ASCS using ReCell®, Wood et al. (2012) observed a higher percentage of wound healing in the children who received Biobrane® with ReCell® [16]. This study was not powered to detect statistical differences between ReCell® and other techniques, as it included a total of only thirteen patients with only five patients within the ReCell® group.
In this work, we present the largest retrospective review to-date of a single institution’s experience with utilizing ReCell® technology for the treatment of pediatric burns.
Materials and methods
Patient population
We conducted a retrospective chart review of all patients admitted to our American Burn Association (ABA)-verified pediatric burn center between September 2019 to March 2022. Using our institutional burn registry and electronic medical record system, we identified twenty-one pediatric patients who underwent treatment with ReCell®. The study was approved by the Institutional Review Board of Nationwide Children’s Hospital (Columbus, Ohio) and general consent was obtained (IRB #00001980).
Data collection
We performed retrospective chart review using our electronic medical record system. We collected patient demographic data, including age, sex, and race, and burn wound characteristics, such as mechanism of burn injury, burn TBSA, body regions with burn injury, and depth of the burn. We documented need for skin grafting, whether graft was meshed, need for dermal substrate placement prior to ReCell® application, number of surgical procedures and sedated dressing changes, length of hospital and intensive care unit (ICU) stay, and duration of follow-up. Duration of follow-up was defined from date of injury to last documented clinic visit in our hospital system. In terms of ReCell® therapy, we reported number of ReCell® applications, days between burn injury and first ReCell® application, time until wound was healed, and maximum Vancouver scar scale at healing. Vancouver scar scale is an objective and validated method for describing burn scars, that incorporates a summation of scar characteristics including pigmentation (0-2), vascularity (0-3), pliability (0-5), and height (0-3) [17]. Normal skin is given a score of 0 for each category. Our Vancouver scar scale measurements are conducted in an outpatient setting by certified occupational therapists. Healing was defined as the number of days post-burn until the wound was deemed closed and patient no longer required dressing changes.
We identified complications, including skin graft loss, loss of graft from areas with ReCell®, and infection within the treated burn area. We also documented whether patients required laser and/or surgical scar release, and number of treatments for each.
Analysis
We performed a descriptive analysis of our sample population and reported medians and ranges, as well as trends. We used Microsoft Excel software to generate tables and a graph.
Results
Demographics and burn wound characterization
Median age of the twenty-one pediatric burn patients was 5 years of age and ranged from 1 to 17 years old (Table 1). Females encompassed 33.3% of the study population. African American, Hispanic, and multi-racial patients made up 28.6%, 4.8%, and 4.8% of this population, respectively, with the remainder being Caucasian. The burn injuries occurred between September 2019 to March 2022, and mechanisms of injury included flame (67%), scald (29%), and grease (5%) burns, with flame being the most common. The median TBSA burn on initial presentation was 31%, ranging 4%-86%. All patients had partial thickness burns, with a median partial thickness TBSA of 10% (ranging 2.5-50%). Twelve patients had a full thickness burn, with a median full thickness TBSA of 28.3% (ranging 11-80%).
Table 1.
Patient demographics and burn wound characterization sorted by total body surface area (TBSA)
| TBSA (%) | Case no. | Age (years old) | Race | Sex | Mechanism of burn | Full thickness (%) | Partial thickness (%) |
|---|---|---|---|---|---|---|---|
| 4 | 6 | 5 | Caucasian | M | Flame | 0 | 4 |
| 12.5 | 2 | 3 | African American | M | Grease | 0 | 13 |
| 14 | 8 | 1 | Caucasian | F | Scald | 0 | 14 |
| 15.5 | 20 | 7 | African American | M | Scald | 0 | 15.5 |
| 16.5 | 21 | 12 | Caucasian | M | Flame | 11 | 5.5 |
| 19 | 9 | 4 | Multi-racial | F | Scald | 0 | 19 |
| 20.5 | 17 | 1 | Caucasian | F | Scald | 18 | 2.5 |
| 20.5 | 19 | 12 | Caucasian | M | Flame | 11.5 | 9 |
| 25 | 18 | 2 | African American | M | Flame | 15 | 10 |
| 26 | 14 | 11 | African American | M | Flame | 0 | 26 |
| 31 | 4 | 5 | Caucasian | F | Flame | 15 | 16 |
| 35 | 1 | 8 | Caucasian | F | Flame | 29.5 | 5.5 |
| 35 | 11 | 17 | Caucasian | M | Flame | 27 | 8 |
| 39 | 12 | 4 | African American | M | Flame | 33 | 6 |
| 41 | 3 | 1 | Caucasian | M | Scald | 0 | 41 |
| 46 | 7 | 15 | Caucasian | F | Flame | 42 | 4 |
| 46.5 | 13 | 4 | Hispanic | M | Scald | 0 | 46.5 |
| 50 | 5 | 2 | Caucasian | M | Flame | 0 | 50 |
| 50 | 16 | 2 | African American | M | Flame | 47 | 3 |
| 79.5 | 10 | 16 | Caucasian | M | Flame | 67 | 12.5 |
| 86 | 15 | 5 | Caucasian | F | Flame | 80 | 6 |
Burn wound management and clinical course
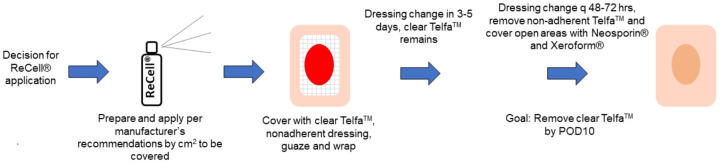
Our pathway for ReCell® application is outlined in Figure 1. From our cohort, four patients (19%) did not undergo any split thickness skin grafting with ReCell® application (Table 2). Of the patients who did undergo STSG, five patients (23.8%) had graft loss. Twenty patients (95.2%) had placement of skin substitute prior to ReCell® application, such as Primatrix® and biodegradable temporizing matrix (BTM). The median number of surgical procedures per patient was 4, ranging from 1-22, which included escharotomies, burn excisions, and skin grafting. The median number of sedated dressing changes was 7 per patient, ranging 3-41. The median length of stay (LOS) in the hospital after the initial burn injury was 41 days, ranging 9-181 days, and the median LOS in the ICU was 14 days, ranging 2-126 days, with only four patients not admitted to the ICU. The median number of total days hospitalized was 46 days, ranging 9-202 days, within recorded follow-up which included additional procedures requiring inpatient admission. The median length of follow-up was 190 days, ranging 24 to 693 days. In total five patients (23.8%) were discharged to rehab, whereas most of the cohort was able to return home.
Figure 1.
ReCell® pathway. A decision is made to apply ReCell® to the burn injury. ReCell® is prepared and applied per the manufacturer’s recommendations by square centimeters (cm2) for the area to be covered. ReCell® is covered with clear TelfaTM followed by non-adherent dressing, gauze, and a wrap. The dressings are changed in 3-5 days, while keeping the TelfaTM in place. The burn wound is assessed for hematoma, seroma, or infection. A dressing change is performed every 48-72 hours to remove non-adherent TelfaTM. Open areas are covered with Neosporin® and Xeroform®. The goal is to remove all clear TelfaTM by postoperative day 10.
Table 2.
Characterization of burn wound management and clinical course in pediatric ReCell® patients
| TBSA (%) | Case no. | Skin graft (Y/N) | Meshed ratio of skin graft | Graft loss (Y/N) | Dermal substrate prior to ReCell® | Number of surgical procedures | Number of sedated dressing changes | Length of initial hospital stay (days) | Length of stay in ICU (days) | Length of follow-up (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | N | None | 1 | 3 | 9 | 0 | 151 | ||
| 12.5 | 2 | N | Primatrix® | 3 | 5 | 25 | 0 | 193 | ||
| 14 | 8 | N | Primatrix® | 2 | 4 | 17 | 0 | 154 | ||
| 15.5 | 20 | Y | 3:1 | N | Primatrix®, Suprathel® | 3 | 3 | 29 | 2 | 51 |
| 16.5 | 21 | Y | 3:1, 2:1 | Y | BTM, Suprathel® | 4 | 5 | 36 | 0 | 59 |
| 19 | 9 | Y | Sheet, 2:1 | N | Primatrix® | 2 | 7 | 30 | 3 | 190 |
| 20.5 | 17 | Y | 3:1 | N | BTM, Primatrix® | 3 | 7 | 33 | 5 | 101 |
| 20.5 | 19 | Y | Sheet, 3:1 | N | BTM, Primatrix® | 5 | 6 | 41 | 8 | 160 |
| 25 | 18 | Y | Sheet, 3:1, 2:1 | N | BTM, Primatrix® | 10 | 8 | 74 | 30 | 211 |
| 26 | 14 | Y | Sheet, 4:1, 3:1 | Y | BTM | 5 | 6 | 48 | 33 | 693 |
| 31 | 4 | Y | 3:1 | N | Primatrix® | 3 | 4 | 33 | 19 | 469 |
| 35 | 1 | Y | Unmeshed, 4:1 | N | Primatrix®, BTM | 5 | 7 | 59 | 43 | 423 |
| 35 | 11 | Y | 3:1 | Y | Primatrix® | 6 | 10 | 46 | 5 | 172 |
| 39 | 12 | Y | Sheet, 4:1, 3:1 | N | BTM | 18 | 41 | 155 | 64 | 174 |
| 41 | 3 | Y | Unmeshed | Y | Primatrix®, BTM | 4 | 16 | 51 | 10 | 263 |
| 46 | 7 | Y | Sheet, 3:1, 2:1 | N | Primatrix®, BTM | 10 | 11 | 84 | 14 | 568 |
| 46.5 | 13 | Y | 4:1, 3:1 | N | Primatrix® | 4 | 11 | 35 | 11 | 174 |
| 50 | 5 | N | Primatrix®, Suprathel® | 4 | 10 | 41 | 23 | 24 | ||
| 50 | 16 | Y | Sheet, 3:1 | N | BTM, Primatrix® | 9 | 13 | 61 | 17 | 197 |
| 79.5 | 10 | Y | 3:1, 4:1 | N | BTM | 13 | 13 | 80 | 13 | 311 |
| 86 | 15 | Y | Sheet, 6:1, 4:1, 3:1, 2:1, 1.5:1 | Y | BTM, Primatrix® | 22 | 18 | 181 | 126 | 587 |
BTM: biodegradable temporizing matrix.
ReCell® therapy application and healing
In Table 3, the median time between date of burn injury and first ReCell® application was 18 days, ranging from 5-43 days. The number of ReCell® applications ranged from 1-4 per patient with 57.1% of patients requiring a single ReCell® application. Median time until a wound was classified as healed was 81 days, varying between 39-573 days from the initial injury. The median maximum Vancouver scar scale measurement per patient at time healed was 8, ranging from 3-14. Six patients did not have documented Vancouver scar scale at time healed. In Figure 2, the days between first ReCell® application and time healed normalized by TBSA is shown for each patient, with a median of 2.65 days per % TBSA. Six patients had documented burn wound infections within the areas treated with ReCell® along with STSG.
Table 3.
ReCell® therapy and healing in pediatric burn patients
| TBSA (%) | Case no. | Interval between injury and first ReCell® application (days) | Number of ReCell® applications | Time until wound healed (days) | Maximum Vancouver scar scale at healing |
|---|---|---|---|---|---|
| 4 | 6 | 5 | 1 | 39 | 7 |
| 12.5 | 2 | 16 | 1 | 50 | 4 |
| 14 | 8 | 13 | 1 | 49 | 6 |
| 15.5 | 20 | 15 | 1 | - | - |
| 16.5 | 21 | 22 | 1 | 59 | 3 |
| 19 | 9 | 18 | 1 | 43 | 3 |
| 20.5 | 17 | 19 | 1 | 68 | 14 |
| 20.5 | 19 | 15 | 1 | - | - |
| 25 | 18 | 31 | 2 | - | - |
| 26 | 14 | 13 | 1 | 98 | 9 |
| 31 | 4 | 19 | 1 | 54 | 8 |
| 35 | 1 | 16 | 2 | 79 | 10 |
| 35 | 11 | 13 | 2 | 109 | 13 |
| 39 | 12 | 30 | 3 | 168 | 9 |
| 41 | 3 | 43 | 1 | 209 | 7 |
| 46 | 7 | 23 | 3 | 113 | 11 |
| 46.5 | 13 | 12 | 2 | 83 | 8 |
| 50 | 5 | 13 | 1 | - | - |
| 50 | 16 | 22 | 4 | 197 | 11 |
| 79.5 | 10 | 39 | 4 | - | - |
| 86 | 15 | 19 | 3 | 573 | - |
Figure 2.
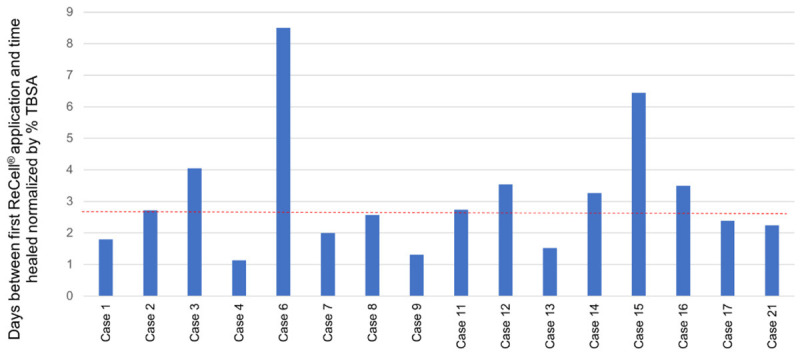
Healing time from ReCell® application. This shows the days between first ReCell® application and time healed normalized by total body surface area (TBSA). The red line represents the median at 2.65 days per TBSA.
Scar therapy and reconstructive procedures
Five patients did not require laser therapy for scar management, displayed in Table 4. Of those patients that did undergo laser treatment, the median number of days from injury to first laser treatment with fractional carbon dioxide (CO2) with/or without pulse dye laser was 136.5 days, ranging from 70-218 days. A range of 1-5 laser treatments were performed per patient. Seven patients (33.3%) underwent contracture release surgery ranging from 1-4 treatments per patient. Median number of days between injury and first contracture release was 147 days, ranging between 76-343 days.
Table 4.
Scar therapy and reconstructive procedures
| TBSA (%) | Case no. | Maximum Vancouver scar scale at healing | Laser treatment (Y/N) | Number of laser treatments | Surgical scar release treatment (Y/N) | Number of surgical scar release treatments |
|---|---|---|---|---|---|---|
| 4 | 6 | 7 | N | 0 | N | 0 |
| 12.5 | 2 | 4 | Y | 1 | N | 0 |
| 14 | 8 | 6 | N | 0 | N | 0 |
| 15.5 | 20 | N | 0 | N | 0 | |
| 16.5 | 21 | 3 | N | 0 | N | 0 |
| 19 | 9 | 3 | Y | 1 | N | 0 |
| 20.5 | 17 | 14 | Y | 3 | N | 0 |
| 20.5 | 19 | Y | 2 | Y | 1 | |
| 25 | 18 | Y | 1 | N | 0 | |
| 26 | 14 | 9 | Y | 3 | Y | 1 |
| 31 | 4 | 8 | Y | 4 | Y | 1 |
| 35 | 1 | 10 | N | 0 | N | 0 |
| 35 | 11 | 13 | Y | 2 | N | 0 |
| 39 | 12 | 9 | Y | 2 | N | 0 |
| 41 | 3 | 7 | Y | 3 | N | 0 |
| 46 | 7 | 11 | Y | 5 | Y | 3 |
| 46.5 | 13 | 8 | Y | 1 | N | 0 |
| 50 | 5 | Y | 1 | N | 0 | |
| 50 | 16 | 11 | Y | 3 | Y | 3 |
| 79.5 | 10 | Y | 3 | Y | 2 | |
| 86 | 15 | Y | 5 | Y | 4 |
Discussion
This series with twenty-one patients represents the largest case review series to date detailing the use of ReCell® in pediatric burn injuries. Most of the published literature on ReCell® evaluates its use in the adult population [4,7]. However, ReCell® is a particularly relevant technique for treating the pediatric patient population, given the smaller size of patients with limited donor sites and the ability of this technology to decrease the amount harvested for autografting [5]. Furthermore, data suggest that ASCS can be cost-saving for hospitals and decrease length of stay compared to STSG [18]. In terms of long-term outcomes, ReCell® has been shown to have similar aesthetics result compared to skin grafting in adults, but this remains an area for further study in children [5].
In contrast to Wood et al. (2012) which only evaluated pediatric patients with scald burns, our cohort represents a variety of burn mechanisms, including grease, scald, and flame injuries [16]. Although scald burns constitute majority of pediatric burn injuries, there was an overrepresentation of patients with flame burn injuries (67%) who underwent treatment with ReCell® in our study [19]. This is in line with flame injuries being more likely to cause severe burns that are deeper, thereby necessitating burn excision and coverage [19]. In our study population, median TBSA burn on initial presentation was 31%. At our institution, we often use ReCell® when we are treating burns with large TBSA requiring meshed grafts for coverage.
Within this study population, we identified four patients (19%) who did not undergo autografting with ReCell® application. We choose to use ReCell® alone if the wound bed is expected to heal without a skin graft but would still benefit from accelerated healing. The median burn TBSA on initial presentation for this ReCell® only group was 13.3%, ranging between 4-50%. One patient had a grease burn injury, another suffered a scald burn, and the remaining two had flame burn injuries. None of these patients had a full thickness burn injury, and only Case 6 with a burn TBSA of 4% did not undergo prior skin substitute placement. In addition, this group underwent ASCS application a median of 13 days after initial burn injury, ranging between 5-16 days. They all underwent only a single application of ReCell®. These results support the safe use of ASCS alone in select pediatric burn patients with relatively small burn TBSA and without full thickness burns, but the findings should be interpreted with caution given the small sample size.
Aside from the patient who underwent ReCell® only treatment without prior skin substitute, all the other twenty patients had placement of a skin substitute in our study population. This is consistent with our practice of applying skin substitutes to larger TBSA burns in order to limit grafting needs and prepare the wound bed for grafting. In addition, we reported a median number of sedated dressing changes of 7 per patient. At our institution, we tend to perform sedated burn dressing changes in larger TBSA burns to minimize pain and anxiety experienced by the child. Majority of patients who received ReCell® (81%) required admission to the ICU, which is in line with our use of this technology in larger and more severe burn injuries.
There was variability in terms of time to first ReCell® application and healing time, defined as wound being closed without requiring anymore dressing changes. At our center, ReCell® is most commonly used at the time of STSG, so the timing of ReCell® application depends on when the wound bed is appropriate for grafting or when it is decided that STSG will not be needed but ReCell® application might have some benefit. Timing of ReCell® application therefore varies with changes in the patient’s clinical status, whether prior application of dermal substrate is indicated, and with any concern for infection at the burn wound. For instance, Case 3 received ReCell® 43 days after their burn injury because their hospital course was complicated by sepsis, disseminated intravascular coagulation, acute kidney injury, and burn tissue infection. In fact, this patient did not receive STSG until 22 days after their injury, but they did undergo placement of dermal substitute initially. There was also a large range for healing time (39-573 days) reported within this cohort. Cases 3 and 15 required the longest amount of time to heal (209 and 573, respectively). Per chart review, both patients had been lost to follow-up for months.
Cosmetic results for patients treated with ReCell® are demonstrated in Figures 3, 4, 5 and 6. We reported a median maxium Vancouver scar scale measurement of 8 at time healed in this study. At our institution, patients with any kind of skin graft have a median Vancouver scar scale of 7. Therefore, cosmetic results were on par with STSG despite using less donor site. This was not a comparative study, but overall we saw good cosmetic results with ReCell® therapy and utilized relatively small donor sites. We did identify six patients with infections within areas treated with ReCell®, and of three of them experienced skin graft loss. The remainder were successfully treated with antibiotics.
Figure 3.
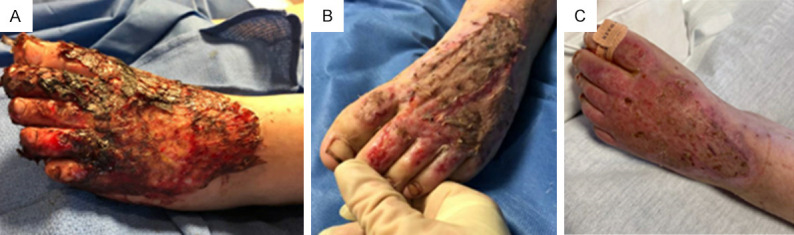
Case 1 with flame injury. A. Patient underwent sharp excision and placement of Primatrix® for left foot full thickness burn (1.5% total body surface area) on post-burn day 7. This picture was taken on post-burn day 10. B. Patient underwent placement of split thickness autograft and ReCell® on post-burn day 18 to the left foot. This is a photo of the left foot burn wound on post-burn day 35, two weeks after burn dressing change. C. This photograph of left foot burn wound was obtained on post-burn day 48.
Figure 4.
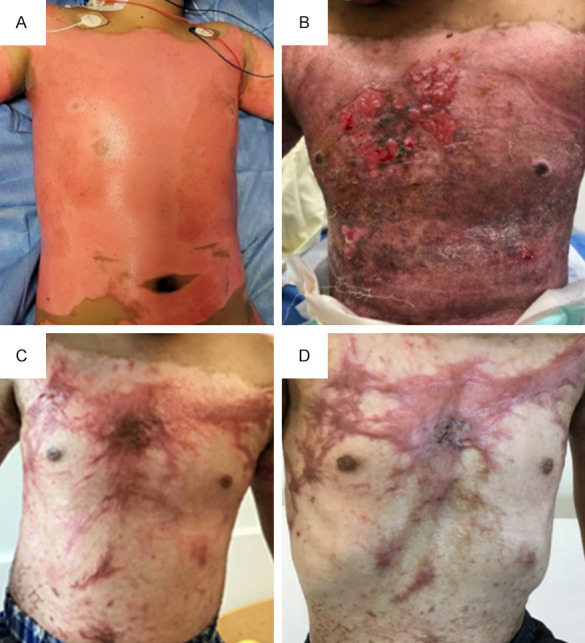
Case 13 with scald burn injury. A. 13% total body surface area (TBSA) full-thickness burn to anterior torso on post-burn day 1. B. ReCell® was applied on post-burn day 13 to the anterior torso (810 square centimeters) along with split thickness skin graft (STSG) meshed 4:1 (4% TBSA). Patient underwent repeat excision of burn wound to chest using VersaJetTM followed by placement of STSG (<1% TBSA) and re-application of ReCell® on post-burn day 22. This picture was taken on post-burn day 33. C. Anterior torso photographed on post-burn day 84 in clinic. D. Post-fractional carbon dioxide (CO2) ablative laser fenestration of the chest was performed on post-burn day 125. This picture of the chest was obtained at follow-up clinic visit on post-burn day 175.
Figure 5.
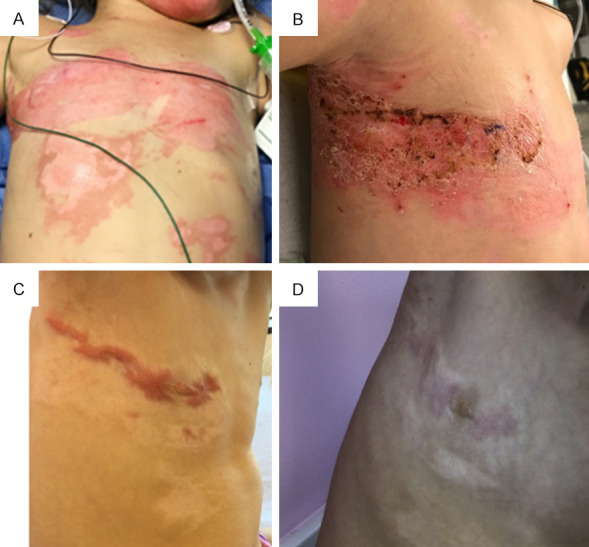
Case 4 with flame burn injury. A. There was 2% total body surface area (TBSA) burn to the right chest. This photo was taken on post-burn day 1. B. Patient underwent VersaJetTM excision to the right chest, followed by application of ReCell® to the chest (75 square centimeters) on post-burn day 20. There was no application of autologous skin graft to this area. This photo was taken on post-burn day 25 during a dressing change. C. Right chest was photographed in clinic on post-burn day 174. D. Patient underwent five rounds of fractional carbon dioxide (CO2) ablative laser fenestration of the right chest, with last treatment on post-burn day 453. Photo was taken on post-burn day 503 during a follow-up clinic visit.
Figure 6.
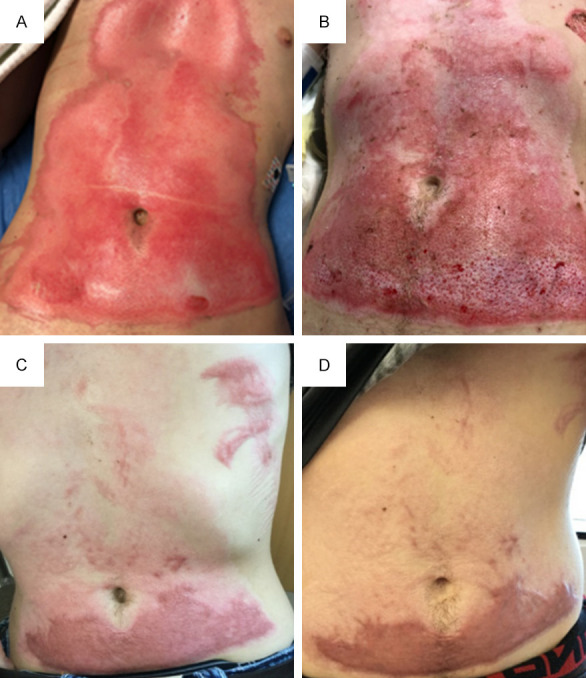
Case 11 with flame burn injury. A. Burn injury to abdomen, measuring 6% total body surface area (TBSA). B. Patient underwent VersaJetTM excision and ReCell® application to abdomen (200 square centimeters) on post-burn day 14. Photograph of abdomen was taken on post-burn day 27. C. Photograph of abdomen was obtained at follow-up clinic visit on post-burn day 110. D. Patient had one round of fractional carbon dioxide (CO2) ablative laser fenestration on post-burn day 144. Photograph of abdominal wound was taken on post-burn day 173 in clinic.
Contractures and scarring can be observed after a burn wound has healed and consequently may require further treatment. We found that majority (76.2%) of pediatric patients treated with ReCell® required laser treatment with fractional CO2 with/or without pulse dye laser, whereas a smaller number (33.3%) underwent contracture release surgery. It is unsurprising that majority of pediatric burn patients treated with ASCS went on to require laser therapy because we typically use this technology in conjunction with split thickness skin grafting on larger TBSA burns, a patient population already at high risk for contractures, scarring, pain, and pruritus. From the ReCell® only treated group, 50% required laser treatment and none had contracture release surgery.
This study describes our center’s experience with ReCell® therapy, showing that it is safe, effective, and well-tolerated in the pediatric population. After adopting this technology into our regular practice, we have been able to use thinner and more widely meshed split thickness autografts for burn patients. Use of ASCS consequently allows for smaller donor sites that heal more quickly, without sacrificing cosmesis. Our group’s use of this technology has continued to grow over time because, in our experience, there is shorter healing time and better cosmesis with ReCell® use. We have also noted improved skin pigmentation of burn wounds in patients with darker complexion using this technology.
Despite its benefits, one of the criticisms of ReCell® use is the increase in operative time to prepare the cell suspension during surgery [5]. At our center, it is typical for multiple burn surgeons to participate in each operation to reduce operative time. We can often dedicate one of these individuals to generating the ASCS, and therefore using this therapy does not increase the time that the patient is under anesthesia. In addition, at times we have incorporated trainees and advanced practice providers into the processing of ASCS intraoperatively.
Limitations of this study include, but are not limited to, the use of data from a single center institution and retrospective evaluation. In addition, there is some data lacking in our set, particularly for time healed and maximum Vancouver scar scale measurement, due to variability in follow-up and documentation. However, Nationwide Children’s Hospital Burn Center serves a large catchment area and is the only pediatric burn center in the region to provide follow-up care to these patients. Moreover, this study is not a comparative one, and conclusions about the impact of ReCell® on healing, functional outcomes, and cosmesis in comparison to skin grafting alone is yet to be determined in pediatric patients. Future research to compare these outcomes will require a larger sample size and prospective randomized controlled trials.
ReCell® therapy offers unique advantages in burn care, including the convenience of simultaneous preparation during the patient’s grafting operation, utilization of the patient’s own donor split-thickness skin sample for cell harvesting, and amplification of the amount of donor tissue available for coverage. This case review series highlights ongoing use of ReCell®, its safety and efficacy, and its potential to improve burn care in pediatric patients.
Disclosure of conflict of interest
None.
References
- 1.Carey K, Kazis LE, Lee AF, Liang MH, Li NC, Hinson MI, Lydon MK, Bauk H, Shapiro GD, Tompkins RG Multi-Center Benchmarking Study Working Group. Measuring the cost of care for children with acute burn injury. J Trauma Acute Care Surg. 2012;73(Suppl 2):S229–233. doi: 10.1097/TA.0b013e318265c88a. [DOI] [PubMed] [Google Scholar]
- 2.Holmes Iv JH, Molnar JA, Carter JE, Hwang J, Cairns BA, King BT, Smith DJ, Cruse CW, Foster KN, Peck MD, Sood R, Feldman MJ, Jordan MH, Mozingo DW, Greenhalgh DG, Palmieri TL, Griswold JA, Dissanaike S, Hickerson WL. A comparative study of the ReCell(R) device and autologous spit-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018;39:694–702. doi: 10.1093/jbcr/iry029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood FM, Giles N, Stevenson A, Rea S, Fear M. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell(R) kit. Burns. 2012;38:44–51. doi: 10.1016/j.burns.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Holmes JH 4th, Molnar JA, Shupp JW, Hickerson WL, King BT, Foster KN, Cairns BA, Carter JE. Demonstration of the safety and effectiveness of the RECELL(R) system combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries. Burns. 2019;45:772–782. doi: 10.1016/j.burns.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Gravante G, Di Fede MC, Araco A, Grimaldi M, De Angelis B, Arpino A, Cervelli V, Montone A. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33:966–972. doi: 10.1016/j.burns.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z, Guo D, Liu P, Cao X, Li S, Zhu J, Tang B. Randomized clinical trial of autologous skin cell suspension for accelerating re-epithelialization of split-thickness donor sites. Br J Surg. 2017;104:836–842. doi: 10.1002/bjs.10508. [DOI] [PubMed] [Google Scholar]
- 7.Chant H, Woodrow T, Manley J. Autologous skin cells: a new technique for skin regeneration in diabetic and vascular ulcers. J Wound Care. 2013;22:S11–15. doi: 10.12968/jowc.2013.22.Sup1.S10. [DOI] [PubMed] [Google Scholar]
- 8.Hu ZC, Chen D, Guo D, Liang YY, Zhang J, Zhu JY, Tang B. Randomized clinical trial of autologous skin cell suspension combined with skin grafting for chronic wounds. Br J Surg. 2015;102:e117–123. doi: 10.1002/bjs.9688. [DOI] [PubMed] [Google Scholar]
- 9.Hayes PD, Harding KG, Johnson SM, McCollum C, Teot L, Mercer K, Russell D. A pilot multi-centre prospective randomised controlled trial of RECELL for the treatment of venous leg ulcers. Int Wound J. 2020;17:742–752. doi: 10.1111/iwj.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busch KH, Bender R, Walezko N, Aziz H, Altintas MA, Aust MC. Combination of medical needling and non-cultured autologous skin cell transplantation (ReNovaCell) for repigmentation of hypopigmented burn scars. Burns. 2016;42:1556–1566. doi: 10.1016/j.burns.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Yu N, Liu Z, Zhang W, Long F, Zeng A, Zhu L, Wang X. The clinical efficacy of ReCell(R) autologous cell regeneration techniques combined with dermabrasion treatment in acne scars. Aesthetic Plast Surg. 2020;44:535–542. doi: 10.1007/s00266-019-01481-8. [DOI] [PubMed] [Google Scholar]
- 12.Cervelli V, De Angelis B, Balzani A, Colicchia G, Spallone D, Grimaldi M. Treatment of stable vitiligo by ReCell system. Acta Dermatovenerol Croat. 2009;17:273–278. [PubMed] [Google Scholar]
- 13.Yu N, Liu R, Yu P, Dong R, Chen C, Zeng A, Long F, Xia Z, Ma P, Tao Y, Liu Z. Repigmentation of nipple-areola complex after ReCell(R) treatment on breast vitiligo. J Cosmet Dermatol. 2022;21:2530–2534. doi: 10.1111/jocd.14399. [DOI] [PubMed] [Google Scholar]
- 14.Mulekar SV, Ghwish B, Al Issa A, Al Eisa A. Treatment of vitiligo lesions by ReCell vs. conventional melanocyte-keratinocyte transplantation: a pilot study. Br J Dermatol. 2008;158:45–49. doi: 10.1111/j.1365-2133.2007.08216.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill TB, Rawlins J, Rea S, Wood F. Treatment of a large congenital melanocytic nevus with dermabrasion and autologous cell suspension (ReCELL(R)): a case report. J Plast Reconstr Aesthet Surg. 2011;64:1672–1676. doi: 10.1016/j.bjps.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Wood F, Martin L, Lewis D, Rawlins J, McWilliams T, Burrows S, Rea S. A prospective randomised clinical pilot study to compare the effectiveness of Biobrane(R) synthetic wound dressing, with or without autologous cell suspension, to the local standard treatment regimen in paediatric scald injuries. Burns. 2012;38:830–839. doi: 10.1016/j.burns.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan T, Smith J, Kermode J, McIver E, Courtemanche DJ. Rating the burn scar. J Burn Care Rehabil. 1990;11:256–260. doi: 10.1097/00004630-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Kowal S, Kruger E, Bilir P, Holmes JH, Hickerson W, Foster K, Nystrom S, Sparks J, Iyer N, Bush K, Quick A. Cost-effectiveness of the use of autologous cell harvesting device compared to standard of care for treatment of severe burns in the United States. Adv Ther. 2019;36:1715–1729. doi: 10.1007/s12325-019-00961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moehrlen T, Szucs T, Landolt MA, Meuli M, Schiestl C, Moehrlen U. Trauma mechanisms and injury patterns in pediatric burn patients. Burns. 2018;44:326–334. doi: 10.1016/j.burns.2017.07.012. [DOI] [PubMed] [Google Scholar]