Abstract
Objective:
To validate the European Prospective Investigation into Cancer (EPIC) FFQ in Lebanon.
Design:
Validation of the EPIC FFQ was done against three 24-h recalls (24-HR). Unadjusted and energy-adjusted correlations, Bland–Altman plots and weighed kappa statistics were used to assess the agreement between the two methods.
Setting:
Lebanon.
Participants:
119 adults (staff and students) at a Lebanese University.
Results:
Good unadjusted and energy-adjusted correlation coefficients were found between data from the two methods which ranged from –0·002 (vitamin A) to 0·337 (carbohydrates) and were all statistically significant except for vitamin D, vitamin E, vitamin A, Se and niacin. Slight/fair agreement was reported through weighed kappa estimates for unadjusted data ranging from –0·05 (vitamin C) to 0·248 (Mg) and for energy-adjusted data ranging from –0·034 (vitamin A) to 0·203 (P). Individuals were categorised into exact and adjacent quartiles with an average of 78 % for unadjusted data and 70 % for energy-adjusted data, indicating a very good agreement between the EPIC FFQ and the average of the 24-HR data. The visual inspection of the Bland–Altman plots revealed an overestimation of energy, carbohydrates, protein and fat intakes by the FFQ method.
Conclusion:
Overall, when all tests were taken into consideration, the current study demonstrated an acceptable agreement of the EPIC FFQ with the 24-h dietary recall method and significantly good correlations between dietary intakes. Therefore, the EPIC FFQ can be considered a valid tool for assessing diet in epidemiological studies among Lebanese adults.
Keywords: Diet, Validation, FFQ, 24-h recalls, Adults, Lebanon
The prevalence of obesity in the Middle East, especially the Arab Gulf States, is growing rapidly; 75 % of adults are considered obese(1). Lebanon is a middle-income Middle Eastern country having food ingredients that are representative of the Mediterranean diet(2). Traditionally, Lebanese cuisine has included cereals and legumes, fresh vegetables, along with seafood, meat or chicken, filled or mixed with olive oil and herbs, ending up with common dishes known as ‘mezze’ and ‘stews’. The traditional Mediterranean diet consisted of fruits, vegetables, seeds, whole grains, non-refined cereals, olive oil and vegetable protein has shifted to a westernised dietary pattern based on animal proteins, low fibre, refined grains and high in sugar and saturated fats(3). However, Lebanon has experienced a dietary transition with the traditional Mediterranean diet being substituted by a more westernised diet in the past few years(2). This change in eating pattern has contributed to the increase in obesity and consequently, the prevalence of nutrition-related diseases (e.g., metabolic syndrome, diabetes, cancer and heart diseases) has grown among the Lebanese population over the last decade(4,5).
There is a need to study the link between food/nutrition and health outcomes through standardised and validated dietary tools(6). For such studies, rigorous methods to estimate short-term and long-term dietary intake are needed. However, thorough dietary methods are often expensive, time-consuming and demand a high commitment from participants(7). There are several dietary assessment methods including: diet records that ask individuals to report everything they consumed over several days/weeks, 24-h recalls (24-HR) that involve reporting food consumed in the past 24 h (including the Automated Self-Administered 24-h dietary recall and Intake-24 which are newer methods that reduce the burden on participants), FFQ, nutrition biomarkers, for example, urinary N or blood–lipid profile that confirm results of food intake(6,8–10). FFQ has numerous advantages compared with other dietary tools as they allow the assessment of food intake over a long-time interval and can estimate the past intake of large populations(11). Further, although FFQ are not the easiest dietary assessment tools to use, they are still deemed to be inexpensive, exert a low burden on participants and easy to administer(11,12).
Self-reported FFQ collect from individuals their frequency of consumption and portion size of several foods. In large surveys that primarily demonstrate an overview of the health status within a particular population, the methods employed for dietary evaluations (e.g., dietary patterns) should be feasible before assessments(7). FFQ assess the usual intake across a medium or long duration that is very crucial to be able to monitor individuals’ behaviours. Medical surveys often use FFQ to compare groups or people based on their intake of various food groups, and thereby FFQ is a suitable method of choice for such surveys(12). Yet, to minimse the burden on participants, ultimately an FFQ should be comprised of a limited number of food types. Additionally, it is necessary to adapt the food list according to the population’s food consumption habits(11). Similar to all other dietary tools, FFQ can exhibit measurement errors and it is strongly advised that they get validated among the studied population(7,11). In other words, FFQ ought to be culture and population specific(13). This means that it is unacceptable for them to be used cross-culturally (in different countries) except if they were validated in those countries(11,13).
The European Prospective Investigation into Cancer FFQ (EPIC FFQ) has been widely used for dietary assessment(14). It represents a gold standard assessment tool of the diet in nutrition epidemiological studies. The EPIC FFQ has been validated for use in adolescents and adults in the United Kingdom(15–17), in patient groups (celiac disease patients)(18) and in other European countries such as Italy(19) providing a reasonable assessment of habitual diet; however, no validation study of the EPIC FFQ has been done in the Middle East and North Africa region. Although FFQ are commonly used in the USA and European countries, nutrition epidemiology in the Middle East and North Africa region and Lebanon is considered poor due to the scarcity of rigour and representative dietary questionnaires, specifically FFQ(20). To date, there have been no studies on dietary patterns across different continents using a common FFQ. The aim of the current study was to validate an existing tool, the EPIC FFQ, in a new country context, Lebanon.
Materials and methods
The validation was done by comparing data collected from the EPIC FFQ with that collected from three 24-HR.
Participants
The sample consisted of adults aged 18 years and older who were staff and students at Lebanese American University in Lebanon. A total of 119 participants were eligible for the study. This number was also recommended by professionals in this field who confirm that more than 105 individuals are required to assess the agreement between tools used to evaluate dietary intakes(7,11). Exclusion criteria included adults who were suffering from a chronic disease such as cancer, Crohn’s disease, diabetes, heart disease, HIV/AIDS/multiple sclerosis, asthma, chronic obstructive pulmonary disease, cystic fibrosis or mental health disorder, having food intolerance or allergy, pregnant/breast-feeding, on any medication known to affect appetite or have undergone bariatric surgery.
Methodological procedure
Participants were approached by a licensed dietician through classroom and office visits during term where they were asked to fill out three 24-HR in paper form: two on weekdays and one on a weekend day providing qualitative (e.g., type of food) and quantitative (e.g., portion) details about what they consumed in the last 24 h. Participants were given guidance on how to use the 24-HR and were filled out on different days. One week after completing the 24-HR, participants were asked to fill in the adapted version of the EPIC FFQ. Additionally, their demographic characteristics were collected. Data were then entered electronically to an online survey in order to facilitate its analysis.
Measures
Socio-economic and physical characteristics
Self-reported age, body weight, height, education, income, race and marital status were collected to describe the socio-demographic characteristics of participants.
24-h recalls
The three 24-HR collected dietary data about foods and drinks consumed over the past 24 h. Participants were asked to fill out the second 24-HR on a weekday 2 d after completing the first one, while the third 24-HR to be completed in the weekend of the same week so that the data collected are representative of the individual’s overall dietary intake.
European Prospective Investigation into Cancer FFQ
The EPIC FFQ consists of 130 food items and one additional question for milk (131 items). The tool was adapted to reflect the Lebanese diet (online supplementary material, Supplemental Appendix 1). To adapt the EPIC FFQ to the Lebanese diet, the researcher (Lebanese) substituted some foods from the original EPIC FFQ with foods that are commonly consumed in Lebanon. In order to retain its international comparability, most food items from the original EPIC FFQ remained the same in each of the sections. Since students and staff at Lebanese American University were from different religions (Christians and Muslims), food items like pork and alcohol intake were kept unchanged, unlike other validation studies that took place in other Arab countries where pork and alcohol sections were excluded because participants were solely Muslims. The frequency of dietary intake of the adapted FFQ remained the same as the original version: never or less than once per month, 1–3 times per month, once a week, 2–4 times per week, 5–6 times per week, once a day, 2–3 times per day, 4–5 times per day and more than 6 times per day.
To ensure that adaptation was correct and improve content and face validity, the adapted version of the EPIC FFQ was cross-checked by nutrition academic staff at Lebanese American University.
Additionally, before the main validation study, the adapted version of EPIC FFQ was completed by ten adults in Lebanon as a pilot study. This step was essential to confirm the time required to complete the questionnaire and that the questions were easy to understand, and instructions were easy to follow. Also, any feedback from participants was taken into consideration and modifications were made such as changing unclear food items into more familiar ones.
Data analysis
The FFQ data were analysed through FETA software that is designed to derive dietary data (energy, macro- and micronutrients, etc.) specifically from EPIC FFQ(21). Data from the 24-HR were entered into the NUTRITICS software, which is a dietary analysis tool containing more than 750 000 food items(22). The mean (± sd) and median (with interquartile range) for energy and nutrients were derived from the adapted EPIC FFQ and three 24-HR. The adapted EPIC FFQ was compared with the average of three 24-HR. Pearson’s correlation (or Spearman’s rank correlation coefficient for non-normally distributed data) was used to measure the correlations of unadjusted, energy-adjusted, and age, gender and BMI-adjusted data between the energy and macro- and micronutrient intakes of the two methods(23,24). The residual method (from regression model) was used to obtain energy-adjusted data for nutrients correlations and age, gender, and BMI-adjusted data for energy and nutrients correlations(25). Moreover, the unadjusted and energy-adjusted data of energy and all nutrients were categorised into quartiles and weighed kappa statistics was used to determine the agreement between the FFQ method and the 24-HR method. The proportion of individuals categorised in same quartile by the FFQ and average 24-HR and in contiguous quartiles as well as opposite (and/or 1 quartile apart) was calculated. We interpreted weighed kappa results based on Cohen suggestion as follows: value < 0 indicates no agreement, 0–0·20 slight agreement, 0·21–0·40 fair, 0·41–0·60 moderate, 0·61–0·80 substantial and 0·81–1·00 nearly perfect agreement(26). The Bland–Altman plot was performed to estimate agreement between the two methods(27,28). The intake values, difference between FFQ and average of 24-HR, were plotted against the average intake values of these methods (intakes from FFQ + intakes from average of 24-HR divided by 2). Limits of agreements (95 %) were formed to illustrate the range of agreement between the two measures (mean ± 1·96 sd). Linear regression was performed to derive the slope coefficient for each nutrient where the average intake of the two measures was the independent variable and the intake difference was the dependent variable. Therefore, the slope coefficient was used to determine the degree of overestimation or underestimation of intakes from FFQ compared with the average of the three 24-HR. Data were analysed using IBM SPSS statistics version 25.
Results
We recruited 120 participants of those one was excluded due to completing only one 24-HR out of three, leaving a final sample of 119 participants. The median age of the validation study participants was 20 (3) years, and the median BMI was 22·7 (4·51) kg/m2 (Table 1). Almost all participants were single (99·2 %), and most of them were females (71·4 %) and non-smokers (75·6 %). More than 60 % of participants’ parents were university graduates and most of them were employees. The main source of income of participants was through the support of their families, and most participants reported a good/comfortable financial status with a family monthly income of > $3000. Participants had a family size of four to six persons, and more than 60% reported that two persons sleep in each room of the house.
Table 1.
Socio-demographic characteristics of study participants
| Socio-demographic characteristics | n | % | Median | Interquartile range |
|---|---|---|---|---|
| Gender | ||||
| Male | 34 | 28·6 | – | |
| Female | 85 | 71·4 | ||
| Age (years) | 20 | 3 | ||
| BMI (kg/m2) | 22·75 | 4·51 | ||
| Marital status | ||||
| Single | 118 | 99·2 | ||
| Married | 1 | 0·8 | ||
| Father’s educational level | ||||
| No education | 8 | 6·7 | ||
| Grade 9 (Brevet) | 12 | 10·1 | ||
| Grade 12 (Baccalaureate) | 25 | 21·0 | ||
| University graduate | 74 | 62·2 | ||
| Mother’s educational level | ||||
| No education | 2 | 1·7 | ||
| Grade 9 (Brevet) | 11 | 9·2 | ||
| Grade 12 (Baccalaureate) | 3 | 2·5 | ||
| University graduate | 73 | 61·3 | ||
| Father’s employment status | ||||
| Unemployed | 4 | 3·4 | ||
| Unable to work due to health problems | 4 | 3·4 | ||
| Employee | 99 | 83·2 | ||
| Full-time homeworker, parent or caregiver | 3 | 2·5 | ||
| Retired | 8 | 6·7 | ||
| Mother’s employment status | ||||
| Unemployed | 34 | 28·6 | ||
| Unable to work due to health problems | 1 | 0·8 | ||
| Employee | 45 | 37·8 | ||
| Full-time homeworker, parent or caregiver | 37 | 31·1 | ||
| Retired | 2 | 1·7 | ||
| Main source of income | ||||
| Family support | 98 | 82·4 | ||
| Self-support | 10 | 8·4 | ||
| Scholarship or stipend | 11 | 9·2 | ||
| Family monthly income | ||||
| < $500 | 3 | 2·5 | ||
| $500–$1499 | 23 | 19·3 | ||
| $1500–$2999 | 33 | 27·7 | ||
| > $3000 | 59 | 49·6 | ||
| Financial status | ||||
| Do not have enough to make ends meet | 5 | 4·2 | ||
| Have enough to make ends meet | 54 | 45·4 | ||
| Have more than enough to make ends meet | 59 | 49·6 | ||
| Family size | ||||
| Four or below | 48 | 40·3 | ||
| Five or above | 69 | 57·9 | ||
| Persons in each room of the house | ||||
| One | 41 | 34·5 | ||
| Two | 72 | 60·5 | ||
| Three | 3 | 2·5 | ||
| Four | 2 | 1·7 | ||
| Five | 1 | 0·8 | ||
| Smoking status | ||||
| Non-smoker | 90 | 75·6 | ||
| Ex-smoker | 2 | 1·7 | ||
| Smoker | 27 | 22·7 |
Table 2 presents the median (IQR) intake for energy, macronutrients and micronutrients calculated from the FFQ, the three 24-HR and their average. All data of energy and nutrients derived from FFQ were higher than those derived from the three 24-HR and their average. It can be seen that the intakes of energy and macronutrients are approximately 1·3 times high in FFQ than the average of three 24-HR. The difference of estimates of micronutrients ranged from 0·87 (niacin) to 2·56 (vitamin E) times higher through the FFQ method compared with 24-HR method.
Table 2.
Median IQR of energy and nutrients in the FFQ, average 24-h recalls, first 24-h recall, second 24-h recall and third 24-h recall
| FFQ | Average 24-HR | 24-HR 1 | 24-HR 2 | 24-HR 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Energy (kcal) | 2721·33 | 2048·3 | 2245·1 | 1124·33 | 2326·0 | 1534·0 | 2116·0 | 1368·0 | 1900·0 | 1243·0 |
| Carbohydrates (g) | 309·29 | 234·4 | 231·66 | 126·0 | 248·0 | 173·0 | 229·0 | 165·0 | 217·0 | 134·0 |
| Protein (g) | 117·34 | 81·76 | 87·0 | 34·63 | 100·0 | 61·0 | 76·0 | 56·0 | 79·0 | 56·0 |
| Fat (g) | 124·54 | 95·8 | 95·6 | 56·67 | 103·0 | 91·0 | 83·0 | 67·0 | 79·0 | 66·0 |
| Ca (mg) | 1243·86 | 826·58 | 669·0 | 475·33 | 684·0 | 696·0 | 653·0 | 557·0 | 612·0 | 599·0 |
| Vitamin D (μg) | 3·35 | 4·35 | 1·51 | 2·36 | 1·1 | 2·67 | 1·1 | 2·54 | 1·2 | 2·47 |
| Folate (μg) | 312·13 | 275·36 | 226·0 | 155·67 | 215·0 | 196·0 | 195·0 | 220·0 | 198·0 | 168·0 |
| Fe (mg) | 13·64 | 12·72 | 10·76 | 5·9 | 10·4 | 7·8 | 9·2 | 9·8 | 9·3 | 7·6 |
| Zn (mg) | 13·51 | 10·81 | 8·26 | 5·63 | 7·6 | 7·5 | 6·9 | 6·4 | 7·1 | 5·7 |
| Mg (mg) | 384·1 | 299·0 | 260·0 | 165·0 | 247·0 | 226·0 | 208·0 | 236·0 | 229·0 | 213·0 |
| Vitamin E (mg) | 22·1 | 19·47 | 8·6 | 8·37 | 8·4 | 9·4 | 7·6 | 10·0 | 7·6 | 9·10 |
| Vitamin C (mg) | 126·8 | 143·33 | 63·76 | 58·97 | 51·0 | 82·3 | 54·0 | 112·0 | 49·0 | 118·3 |
| Vitamin B12 (μg) | 8·26 | 10·24 | 3·16 | 3·01 | 2·5 | 3·8 | 2·4 | 4·16 | 2·9 | 4·0 |
| Vitamin A (μg) | 1417·48 | 1988·38 | 640·33 | 643·0 | 506·0 | 1161·0 | 336·0 | 911·0 | 454·0 | 1017·0 |
| Thiamine (mg) | 1·82 | 1·50 | 1·23 | 0·78 | 1·30 | 1·0 | 1·20 | 1·17 | 1·10 | 0·95 |
| Na (mg) | 3562·40 | 2760·85 | 2158·00 | 1497·33 | 2307·0 | 1881·0 | 2056·0 | 2002·0 | 1868·0 | 1694·0 |
| Se (μg) | 82·81 | 56·53 | 38·20 | 30·07 | 39·7 | 53·0 | 29·7 | 30·30 | 33·6 | 33·5 |
| Riboflavin (mg) | 2·71 | 1·77 | 1·17 | 0·81 | 1·2 | 1·24 | 0·95 | 1·09 | 1·1 | 0·91 |
| Pyridoxine (mg) | 2·7 | 2·33 | 1·46 | 0·84 | 1·6 | 1·12 | 1·2 | 1·42 | 1·3 | 1·32 |
| K (mg) | 4428·67 | 3372·37 | 2236·66 | 1121·33 | 2296·0 | 1531·0 | 2011·0 | 1679·0 | 2034·0 | 1742·0 |
| P (mg) | 1945·77 | 1209·23 | 1089·0 | 624·33 | 1132·0 | 898·0 | 963·0 | 806·0 | 965·0 | 663·0 |
| Niacin (mg) | 29·28 | 24·89 | 33·43 | 21·73 | 31·8 | 32·2 | 25·1 | 27·2 | 25·6 | 24·9 |
Table 3 lists the unadjusted and energy-adjusted correlation coefficients between the FFQ and the average of the three 24-HR of participants. Energy and nutrients in the unadjusted correlations were all statistically significant except for Se, K, niacin, vitamin D, vitamin E and vitamin A. Unadjusted and energy-adjusted correlation coefficients ranged from −0·002 (vitamin A) to 0·34 (carbohydrates). Energy-adjusted correlation coefficients were all statistically significant except for vitamin D, vitamin E, vitamin A, Se and niacin. Compared with unadjusted correlation coefficients, energy-adjusted correlation coefficients increased for protein, fat, folate, Fe, Mg, thiamine, Na, Se and K, decreased for Zn, vitamin E, riboflavin, pyridoxin and P and remained the same for carbohydrates, Ca, vitamin D, vitamin C, vitamin B12, vitamin A and niacin. The correlation coefficient of K intake became statistically significant after energy adjustment. For folate and P intakes, the significance level increased from < 0·05 to < 0·001 and decreased from < 0·001 to < 0·05, respectively. Adjusting for age, gender and BMI did not show any change in the correlation coefficient than through energy adjustment. Overall, a significant moderate correlation was observed between FFQ and average of the three 24-HR.
Table 3.
Correlation between energy and nutrients intake from FFQ and average of three 24-h recalls
| Unadjusted* | Energy adjusted*,† | Age, gender and BMI adjusted*,† | |
|---|---|---|---|
| Energy (kcal) | 0·33** | – | 0·33** |
| Carbohydrates (g) | 0·34** | 0·34** | 0·34** |
| Protein (g) | 0·18*** | 0·21*** | 0·21*** |
| Fat (g) | 0·27** | 0·29** | 0·29** |
| Ca (mg) | 0·26** | 0·26** | 0·26** |
| Vitamin D (μg) | 0·15 | 0·15 | 0·15 |
| Folate (μg) | 0·23*** | 0·24** | 0·24** |
| Fe (mg) | 0·30** | 0·31** | 0·31** |
| Zn (mg) | 0·19*** | 0·18*** | 0·18*** |
| Mg (mg) | 0·31** | 0·33** | 0·33*** |
| Vitamin E (mg) | 0·18 | 0·18 | 0·18 |
| Vitamin C (mg) | 0·20*** | 0·2*** | 0·2*** |
| Vitamin B12 (μg) | 0·21*** | 0·21*** | 0·21*** |
| Vitamin A (μg) | −0·002 | −0·002 | −0·002 |
| Thiamine (mg) | 0·32** | 0·34** | 0·34** |
| Na (mg) | 0·22*** | 0·22*** | 0·22*** |
| Se (μg) | 0·05 | 0·06 | 0·06 |
| Riboflavin (mg) | 0·26** | 0·26** | 0·26** |
| Pyridoxine (mg) | 0·25** | 0·25** | 0·25** |
| K (mg) | 0·18 | 0·18*** | 0·18*** |
| P (mg) | 0·26** | 0·23*** | 0·23*** |
| Niacin (mg) | 0·15 | 0·15 | 0·15 |
Spearman’s correlation.
Pearson’s correlation.
Correlation is significant at P < 0·01.
Correlation is significant at P < 0·05.
Table 4 shows the kappa statistics for unadjusted and energy-adjusted data. The weighed kappa estimates for unadjusted data ranged from –0·05 (vitamin C) to 0·248 (Mg). Weighed kappa values were statistically significant for energy, carbohydrates, protein, fat, Ca, Fe, Zn, Mg, vitamin E, thiamine, riboflavin and niacin. Weighed kappa values were not statistically significant for vitamin D, folate, vitamin C, vitamin B12, vitamin A, Na, Se, pyridoxin, K and P. After energy adjustment, weighed kappa values were reduced for energy and all nutrients but increased for vitamin C, vitamin B12, pyridoxin and P and remained unchanged for folate. Weighed kappa for energy-adjusted data ranged from –0·034 (vitamin A) to 0·203 (P). Overall, the weighed kappa statistics showed a slight-to-fair agreement between the FFQ and the average of the three 24-HR. The classification of subjects into the same quartile for unadjusted data ranged from 18 % (vitamin D) to 50 % (total energy). Exact plus adjacent agreement ranged from 58 % (vitamin D) to 92 % (carbohydrates) while the disagreement ranged from 4·5 % (total energy) to 38 % (vitamin D). For energy-adjusted data, the exact agreement ranged from 21 % (Ca) to 49 % (Na), whereas the exact plus adjacent agreement ranged from 58 % (Ca) to 94 % (vitamin E) and the disagreement ranged from 15 % (carbohydrates) to 38 % (folate).
Table 4.
Agreement (weighed kappa) and cross-classification of quartiles of energy and nutrients intake*
| Nutrients | Unadjusted data | Energy-adjusted data | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kw | 95 % CI | Exact agreement (%) | Exact agreement + adjacent (%) | Disagreement (%) | Kw | 95 % CI | Exact agreement (%) | Exact agreement + adjacent (%) | Disagreement (%) | |
| Energy (kcal) | 0·168 | 0·047, 0·289 | 50·7 | 88·5 | 4·5 | – | – | – | – | – |
| Carbohydrates (g) | 0·148 | 0·03, 0·265 | 47·3 | 92·5 | 5·3 | 0·06 | 0·004, 0·116 | 34·8 | 82·7 | 15·4 |
| Protein (g) | 0·14 | 0·015, 0·265 | 42·5 | 86·8 | 12·2 | 0·087 | −0·081, 0·265 | 33·2 | 74·1 | 27·9 |
| Fat (g) | 0·134 | 0·013, 0·265 | 29·0 | 74·5 | 23·5 | 0·052 | −0·022, 0·127 | 40·3 | 89·8 | 7·8 |
| Ca (mg) | 0·179 | 0·077, 0·281 | 35·2 | 71·4 | 25·8 | 0·042 | 0·012, 0·072 | 21·6 | 58·6 | 31·8 |
| Vitamin D (μg) | 0·062 | −0·043, 0·168 | 18·6 | 58·4 | 38·1 | 0·004 | −0·007, 0·014 | 22·9 | 65·7 | 33·1 |
| Folate (μg) | 0·084 | −0·035, 0·204 | 37·2 | 77·8 | 21·4 | 0·084 | −0·035, 0·204 | 30·4 | 63 | 38·8 |
| Fe (mg) | 0·215 | 0·068, 0·362 | 41·9 | 78·2 | 23·8 | 0·179 | −0·011, 0·369 | 35·2 | 67·2 | 26·8 |
| Zn (mg) | 0·115 | 0·023, 0·207 | 30·6 | 67·7 | 35·6 | 0·086 | 0·011, 0·161 | 25·8 | 67·2 | 32 |
| Mg (mg) | 0·248 | 0·095, 0·402 | 49·8 | 89·1 | 9·4 | 0·185 | 0·077, 0·294 | 25·8 | 64·3 | 28·4 |
| Vitamin E (mg) | 0·167 | −0·017, 0·352 | 33·9 | 78·2 | 21·4 | 0·042 | −0·046, 0·131 | 38·6 | 94·6 | 15·4 |
| Vitamin C (mg) | −0·05 | −0·106, 0·006 | 32·6 | 62·5 | 24·1 | 0·03 | −0·025, 0·084 | 26·6 | 62·2 | 33·2 |
| Vitamin B12 (μg) | 0·007 | −0·02, 0·035 | 34·4 | 71·5 | 25·9 | 0·15 | 0·018, 0·293 | 39·9 | 70·8 | 28·3 |
| Vitamin A (μg) | −0·003 | −0·111, 0·104 | 31·6 | 78 | 21·2 | −0·034 | −0·127, 0·059 | 32·3 | 66·3 | 29·1 |
| Thiamine (mg) | 0·211 | 0·011, 0·411 | 39·9 | 83·3 | 12·4 | 0·111 | −0·064, 0·286 | 35·9 | 81·7 | 19 |
| Na (mg) | 0·085 | −0·041, 0·21 | 49·5 | 84·6 | 15·3 | 0·046 | −0·025, 0·118 | 49·5 | 79·5 | 16·1 |
| Se (μg) | 0·075 | −0·021, 0·171 | 41·2 | 85·8 | 31·1 | −0·008 | −0·17, 0·154 | 47·9 | 85 | 17·5 |
| Riboflavin (mg) | 0·064 | −0·049, 0·176 | 37·9 | 80·4 | 16·9 | 0·058 | −0·003, 0·119 | 33·4 | 64·8 | 25·9 |
| Pyridoxine (mg) | 0·044 | −0·075, 0·162 | 49·8 | 83·8 | 6·9 | 0·056 | −0·009, 0·12 | 32·2 | 67·5 | 30·1 |
| K (mg) | 0·006 | −0·079, 0·09 | 41·2 | 85·2 | 10·2 | 0·018 | −0·028, 0·065 | 34·3 | 72·7 | 21·1 |
| P (mg) | 0·075 | −0·01, 0·16 | 44·5 | 84·7 | 12·8 | 0·203 | 0·05, 0·356 | 27·4 | 61·4 | 35·3 |
| Niacin (mg) | 0·181 | 0·001, 0·36 | 40·1 | 81·9 | 16·9 | 0·023 | −0·143, 0·189 | 34·6 | 71·8 | 25·4 |
Kw, weighed kappa.
Weighed K was performed between the FFQ and average of 24-HR.
Table 5 demonstrates the agreement between FFQ and average of the three 24-HR. It shows the mean difference with the 95 % limits of agreement (lower and upper) and the linear regression coefficients for energy, macronutrients and micronutrients where data of the average of three 24-HR were entered as predictor of FFQ data. The mean difference for energy (±sd) was 1212·7 ± 2630·3 with wide limits of agreement (−3942·7; 6368·1). For energy and macronutrients, a positive slope coefficient with P-value < 0·05 was found showing that the FFQ has overestimated higher energy and macronutrients intake levels. A positive slope was also found for all micronutrients except vitamin D (–0·45), vitamin C (–0·35), vitamin B12 (–0·28) and Se (0·17). Further, the visual inspection of the Bland–Altman plots (Fig. 1) also shows a pattern of overestimation of energy, carbohydrates, protein and fat intakes by the FFQ method. A greater number of data points is observed to be below the mean difference line v. above the mean difference line for energy, protein and fat intakes, and as the mean intake of energy and macronutrients increases, the difference increases indicating a slight proportional bias. This has also been evidenced through the linear regression that found a statistically significant t score (P-value < 0·05) for energy and macronutrients indicating that the null hypothesis that there is no proportional bias is rejected. Linear regression of all micronutrients data indicated a slight proportional bias except for Zn (P = 0·36), Mg (P = 0·54), vitamin E (P = 0·557), vitamin B12 (P = 0·065), vitamin A (P = 0·686), Se (P = 0·345), riboflavin (P = 0·244), pyridoxin (P = 0·954) and niacin (P = 0·27). β-coefficients were all close to 0 indicating that there is no huge proportional bias. Overall, the FFQ was shown to slightly overestimate nutrient intakes compared with the 24-HR.
Table 5.
Limits of agreement (LOA) and β-coefficients between FFQ and average of three 24-HR*
| Energy and nutrients | Mean difference (FFQ and average 24-HR) | sd | 95 % LOA lower, upper | β | P |
|---|---|---|---|---|---|
| Energy (kcal) | 1212·7 | 2630·3 | −3942·7, 6368·1 | 0·63 | < 0·001 |
| Carbohydrates (g) | 151·2 | 343·7 | −522·4, 824·9 | 0·71 | < 0·001 |
| Protein (g) | 49·8 | 83·1 | −113·1, 212·7 | 0·59 | < 0·001 |
| Fat (g) | 56·8 | 124·6 | −187·5, 301·1 | 0·47 | 0·001 |
| Ca (mg) | 664·5 | 769·8 | −844·3, 2173·4 | 0·42 | 0·003 |
| Vitamin D (μg) | 2·9 | 5·7 | −8·2, 14·0 | −0·45 | 0·007 |
| Folate (μg) | 169·1 | 252·9 | −326·7, 664·9 | 0·36 | 0·014 |
| Fe (mg) | 6·8 | 11·4 | −15·6, 29·2 | 0·43 | 0·001 |
| Zn (mg) | 8·3 | 10·5 | −12·4, 28·9 | 0·14 | 0·36 |
| Mg (mg) | 167·0 | 255·8 | −334·3, 668·4 | 0·08 | 0·54 |
| Vitamin E (mg) | 14·1 | 19·5 | −24·1, 52·4 | 0·09 | 0·557 |
| Vitamin C (mg) | 85·5 | 153·9 | −216·2, 387·2 | −0·35 | 0·026 |
| Vitamin B12 (μg) | 8·3 | 11·0 | −13·4, 30·0 | −0·28 | 0·065 |
| Vitamin A (μg) | 1520·8 | 2433·7 | −3249·2, 6290·8 | 0·08 | 0·686 |
| Thiamine (mg) | 0·8 | 1·3 | −1·7, 3·3 | 0·34 | 0·008 |
| Na (mg) | 2037·6 | 3031·7 | −3904·6, 7979·8 | 0·32 | 0·029 |
| Se (μg) | 56·1 | 66·9 | −75·1, 187·2 | −0·17 | 0·345 |
| Riboflavin (mg) | 1·8 | 1·6 | −1·2, 4·9 | 0·17 | 0·244 |
| Pyridoxine (mg) | 1·7 | 1·8 | −1·9, 5·2 | 0·01 | 0·954 |
| K (mg) | 2833·5 | 3146·5 | −3333·6, 9000·6 | 0·33 | 0·033 |
| P (mg) | 1141·9 | 1299·8 | −1405·7, 3689·5 | 0·42 | 0·004 |
| Niacin (mg) | 1·9 | 23·2 | −43·7, 47·5 | 0·17 | 0·27 |
Mean difference and LOA were derived through a one-sample t test. β-coefficients and P-values were derived through a linear regression of log-transformed data.
Fig. 1.
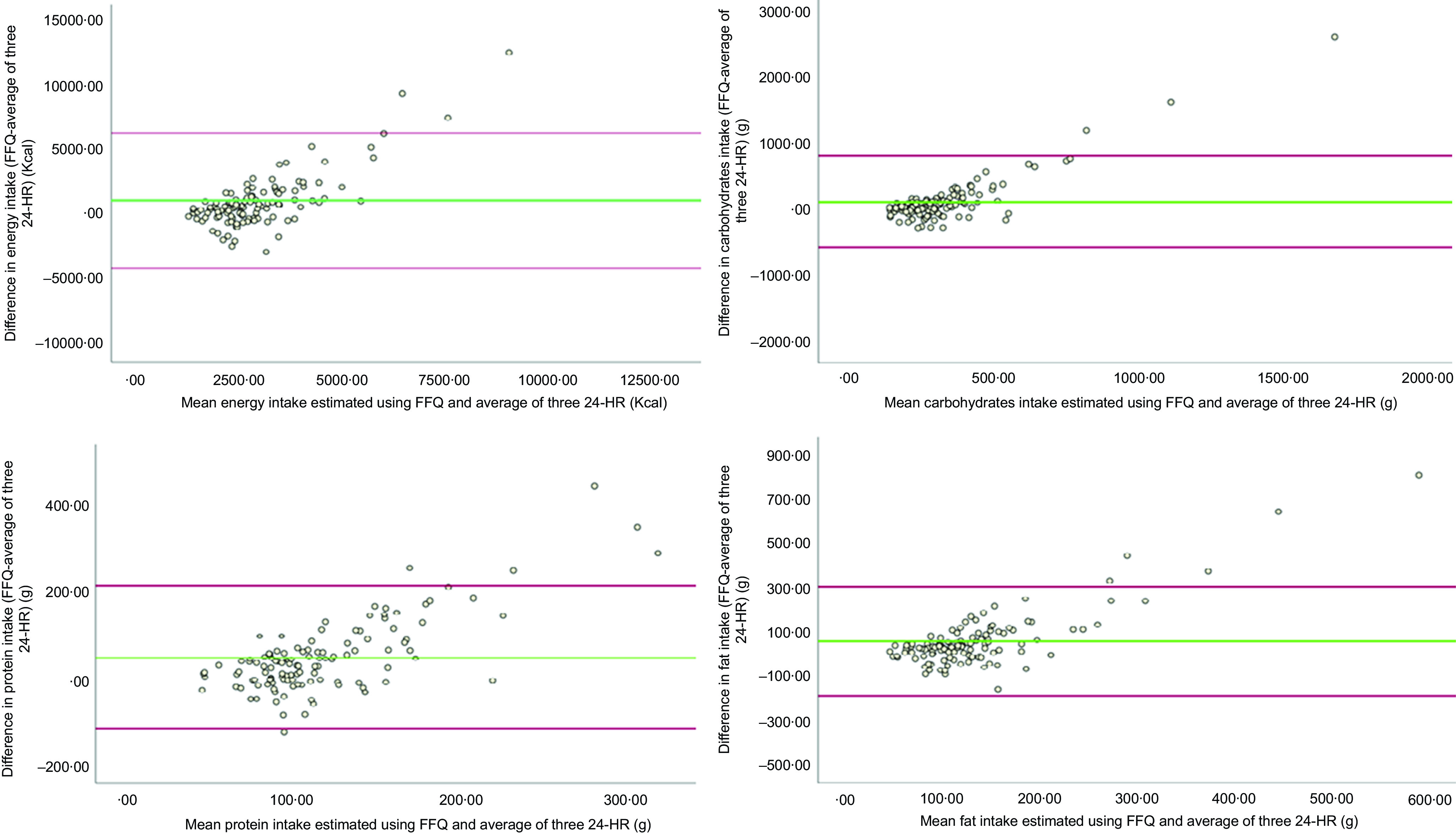
Bland–Altman plots for energy, carbohydrates, protein and fat intakes
Discussion
The EPIC FFQ is an easy-to-use gold standard tool that is widely used to assess the dietary intake of large populations. Nutrition epidemiology in Lebanon is deemed poor due to the scarcity of rigour and representativeness of dietary questionnaires, specifically FFQ(20). To the best of our knowledge, this is the first validation study of the EPIC FFQ for assessing dietary intake among adults in the Middle East and North Africa region, and especially in Lebanon. Although the FFQ showed overestimation of intake of energy and some nutrients in comparison with 24-HR, this validation study demonstrated an overall acceptable agreement compared with the 24-HR method and significantly good correlation between intakes.
In our study, the moderate correlation coefficients reported between the FFQ and the average of three 24-HR were statistically significant for all but six nutrients, and this has been similarly reported in validation studies from Bangladesh(11,29,30). The correlation coefficient for Zn intake in the study by Mumu et al.(11) between FFQ and three 24-HR was 0·161 which is very similar to that reported in the present study (0·192). Additionally, comparable validation studies of different FFQ done in Lebanon have found similar correlation coefficients with multiple 24-HR. For example, in a recent validation study by Harmouch-Karaki et al.(31) done among Lebanese adults, the correlation coefficient for Mg was 0·38 (P < 0·001) and for thiamine 0·33 (P < 0·001) compared with 0·31 (P < 0·001) and 0·32 (P < 0·001) in the present study, respectively. On the other hand, another recent study by Aoun et al. (32) conducted with Lebanese adults found higher correlation coefficients than the present study; however, they were not statistically significant for energy and several nutrients. For example, the correlation coefficient for energy was 0·998 (P = 0·098), 0·996 (P = 0·877) for fat, 0·967 (P = 0·073) for Fe, 0·987 (P = 0·348) for vitamin C and 0·973 (P = 0·289) for vitamin B12. After energy adjustment, the correlation coefficients in the present study were improved for protein, fat, folate, Fe Mg, thiamine, Na, Se and K intakes; however, for the majority of nutrients they showed no change or a decrease in correlation coefficients. The correlation of fat intake, which is a major predictor of CVD, slightly increased after adjusting for energy (0·27–0·29). It is argued that if the correlation coefficient of a specific nutrient increased after energy adjustment, the variability of this nutrient’s intake is linked to energy intake(13). In contrast, if the correlation coefficient decreased after energy adjustment, it means that the variability depends on systematic error of under and overestimation of that nutrient’s intake(13). Willet et al.(7) recommend that the demographic confounder should be controlled for in nutrition epidemiological research, and accordingly we adjusted for age, gender and BMI for unadjusted correlations in the current study. This is recommended because these confounders affect the between-person variation in food intake and usually manipulate the correlation between the dietary tools(7).
From the analysis of the data, it can be concluded that FFQ resulted in an overall overestimation of total energy, macronutrients and micronutrients intakes compared with the 24-HR. Similar findings have been found in previous research(13,33,34). It is widely accepted that an accurate estimation of energy intakes using self-report tools is hard to achieve; however, energy adjustment improves the estimation of other macro- and micronutrients(35). It is argued that when participants are asked to recall the frequency of different foods, they usually overestimate the overall intake(13). However, others suggest that FFQ generally contain a large list of foods that covers usual and local foods of the population under study, which explains the need for energy adjustment(36). The larger the food list is, the more inflated the estimates of total dietary intake will be when summing the foods(33), and in the present study we used a 130-food item FFQ which is considered a quite large food list. Moreover, participants tend to over-report the frequency of consumption of foods in an FFQ because of recall and social-desirability biases, and this leads to overestimation of dietary intake(11). Interestingly in our study, data collection was done during the COVID-19 pandemic, which might have manipulated the reporting of dietary intake of participants(37). Nevertheless, the current study indicates that there exists an agreement (slight/fair) between the FFQ and the average of three 24-HR for most of the nutrients, which is in line with what other validation studies, which validated different FFQ, have reported(38,39). A study by Sauvageot et al.(40) aimed to validate an FFQ against 3-d food record and found a slight/fair agreement between the two methods. For example, the study reported kappa values of 0·02 for energy, 0·12 for lipids, 0·22 for protein, 0·02 for Fe and 0·17 for K. Similar to the present study, these authors considered this agreement acceptable and the FFQ was validated for use among their specific population. Regarding the cross-classification of subjects into quartiles, the FFQ showed quite good results. Individuals were correctly categorised into the exact and adjacent quartiles with an average of 78 % for unadjusted data and 70 % for energy-adjusted data, which is similar to other studies(13,38,41).
One strength of the present study is that the food list of the EPIC FFQ was adapted to accurately reflect the Lebanese diet and hence it represents this population. Another strength is the statistical methodologies conducted in this paper. Although applying one to three statistical approaches is considered enough in such studies(42), the present study used several statistical methods to assess the validity of the EPIC FFQ(11).
A great challenge of validation studies is considered choosing a suitable reference method to validate the target dietary tool since there is not one gold standard tool for dietary intake measurement(7,13). Although other dietary tools (e.g., weighed food records) have been utilised in validation studies, they were not practical because of the increased cost involved. One limitation of the current study is that both dietary tools that we used rely on memory. However, the 24-HR have several advantages such as being inexpensive, quick to administer and able to collect detailed information on food consumed during the day. Moreover, the 24-HR require only short-term memory and are eligible to be used among all populations(12,33,43). A study by(43) mentions that 24-HR might sometimes have a higher objectivity than FFQ and that their use as a dietary tool does not alter the habitual diet of participants as the prospective food record dietary tool. In the current study, we collected 24-HR for 3 d and on both a weekend day and two weekdays to minimise the day-to-day variability. Our sample was selected from a university campus and contained a high proportion of young females who are educated and from a high socio-economic status and at a higher educational level; thus, caution should be taken regarding the generalisation to all Lebanese adults. This is the first Lebanese validation study of the FFQ, and future research should ensure a broader sample is selected. Another limitation of the present study is the use of Nutritics software which is based on UK guidelines which is different than the Lebanese nutrition guidelines. In the same context, there is no existing Lebanese software to analyse the dietary intake of Lebanese population. Estimating the dietary composition in Lebanon is challenging, and nutritionists should aim to continuously implement accurate food databases(44). In the present study, the 24-HR were collected 1 week after collecting the FFQ, data due to time restraint, which might be less representative than if they were collected throughout several months.
Conclusion
The current study showed that the EPIC FFQ is a valid tool to assess diet in epidemiological studies among Lebanese adults. Caution is needed as the EPIC FFQ may overestimate individuals’ dietary intake; however, this is not yet clear. Future studies should further assess the validity of the EPIC FFQ among Lebanese adults using nutritional biomarkers.
Acknowledgements
Acknowledgements: The authors acknowledge individuals or organisations that provided advice and/or support (non-financial). Financial support: This research received no external funding. Conflict of interest: There are no conflicts of interest. Authorship: Conceptualisation and study design: K.K., F.T. and V.H.; methodology: K.K. and F.T.; ethical approval applications preparation: K.K., F.T., V.H., M.B.1 and M.B.2; data collection: K.K., M.B.1 and M.B.2; formal data analysis: K.K.; data interpretation: K.K., F.T. and V.H.; writing – original first draft preparation: K.K.; writing – review and editing: K.K., F.T. and V.H.; supervision: F.T. and V.H. All authors have read and agreed to the published version of the manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Lebanese American University Institutional Review Board IRB#: LAU.SAS.MB3·2/Dec/2019. Written informed consent was obtained from all subjects/patients.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021002123.
click here to view supplementary material
References
- 1. Ng S, Zaghloul S, Ali H et al. (2010) The prevalence and trends of overweight, obesity and nutrition-related non-communicable diseases in the Arabian Gulf States. Obes Rev 12, 1–13. [DOI] [PubMed] [Google Scholar]
- 2. El-Kassas G & Ziade F (2016) Exploration of the dietary and lifestyle behaviors and weight status and their self-perceptions among health sciences university students in North Lebanon. Biomed Res Int 2016, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonaccio M, Bonanni A, Di Castelnuovo A et al. (2012) Low income is associated with poor adherence to a Mediterranean diet and a higher prevalence of obesity: cross-sectional results from the Moli-Sani study. BMJ Open 2, e001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mallat S, Geagea A, Jurjus R et al. (2016) Obesity in Lebanon: a National Problem. World J Cardiovasc Dis 6, 166–174. [Google Scholar]
- 5. Nasreddine L, Naja F, Chamieh M et al. (2012) Trends in overweight, obesity in Lebanon: evidence from two national cross-sectional surveys (1997, 2009). BMC Public Health 12, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satija A, Yu E, Willett W et al. (2015) Understanding nutritional epidemiology and its role in policy. Adv Nutr 6, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willett W (2013) Nutritional Epidemiology. New York: Oxford University Press. [Google Scholar]
- 8. Foster E, Lee C, Imamura F et al. (2019) Validity and reliability of an online self-report 24-hour dietary recall method (Intake24): A doubly-labelled water study and repeated measures analysis — CORRIGENDUM. J Nutr Sci 8. [DOI] [PMC free article] [PubMed]
- 9. Subar A, Kirkpatrick S, Mittl B et al. (2012) The automated self-administered 24-hour Dietary Recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Dietetics 112, 1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cade J (2016) Measuring diet in the 21st century: use of new technologies. Proc Nutr Soc 76, 276–282. [DOI] [PubMed] [Google Scholar]
- 11. Mumu S, Merom D, Ali L et al. (2020) Validation of a food frequency questionnaire as a tool for assessing dietary intake in cardiovascular disease research and surveillance in Bangladesh. Nutr J 19, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Subar A (2004) Developing dietary assessment tools. J Am Dietetic Assoc 104, 769–770. [DOI] [PubMed] [Google Scholar]
- 13. Liu L, Wang P, Roebothan B et al. (2013) Assessing the validity of a self-administered food-frequency questionnaire (FFQ) in the adult population of Newfoundland and Labrador, Canada. Nutr J 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Welch A, Luben R, Khaw K et al. (2005) The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J Hum Nutr Diet 18, 99–116. [DOI] [PubMed] [Google Scholar]
- 15. Bingham S (1997) Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol 26, 137S–151S. [DOI] [PubMed] [Google Scholar]
- 16. McKeown N, Day N, Welch A et al. (2001) Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am J Clin Nutr 74, 188–196. [DOI] [PubMed] [Google Scholar]
- 17. Lietz G, Barton K, Longbottom P et al. (2002) Can the EPIC food-frequency questionnaire be used in adolescent populations? Public Health Nutr 5, 783–789. [DOI] [PubMed] [Google Scholar]
- 18. Mazzeo T, Roncoroni L, Lombardo V et al. (2016) Evaluation of a modified Italian European prospective investigation into cancer and nutrition food frequency questionnaire for individuals with celiac disease. J Acad Nutr Dietetics 116, 1810–1816. [DOI] [PubMed] [Google Scholar]
- 19. Bonaccio M, Di Castelnuovo A, Pounis G et al. (2017) High adherence to the Mediterranean diet is associated with cardiovascular protection in higher but not in lower socioeconomic groups: prospective findings from the Moli-sani study. Int J Epidemiol 46, 1478–1487. [DOI] [PubMed] [Google Scholar]
- 20. Papazian T, Hout H, Sibai D et al. (2016) Development, reproducibility and validity of a food frequency questionnaire among pregnant women adherent to the Mediterranean dietary pattern. Clin Nutr 35, 1550–1556. [DOI] [PubMed] [Google Scholar]
- 21. Mulligan A, Luben R, Bhaniani A et al. (2014) A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 4, e004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nutritics (2020) Nutrition Analysis Software For Professionals [Internet]. Nutritics.com. [cited 12 December 2020]. https://www.nutritics.com/p/home (accessed December 2020).
- 23. Brunst K, Kannan S, Ni Y et al. (2015) Validation of a food frequency questionnaire for estimating micronutrient intakes in an urban US sample of multi-ethnic pregnant women. Matern Child Health J 20, 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El Kinany K, Garcia-Larsen V, Khalis M et al. (2018) Adaptation and validation of a food frequency questionnaire (FFQ) to assess dietary intake in Moroccan adults. Nutr J 17, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willett W, Sampson L, Stampfer M et al. (1985) Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122, 51–65. [DOI] [PubMed] [Google Scholar]
- 26. McHugh M (2012) Interrater reliability: the kappa statistic. Biochemia Medica 22, 276–282. [PMC free article] [PubMed] [Google Scholar]
- 27. Bartlett J & Frost C (2008) Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstetrics Gynecol 31, 466–475. [DOI] [PubMed] [Google Scholar]
- 28. Bland J & Altman D (1999) Measuring agreement in method comparison studies. Stat Meth Med Res 8, 135–160. [DOI] [PubMed] [Google Scholar]
- 29. Habibul CYA, Parvez F & Howe GR (2004) Validity of a food-frequency questionnaire for a large prospective cohort study in Bangladesh. Br J Nutr 92, 851–859. [DOI] [PubMed] [Google Scholar]
- 30. Lin P, Bromage S, Mostofa M et al. (2017) Validation of a dish-based semiquantitative food questionnaire in rural Bangladesh. Nutrients 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harmouche-Karaki M, Mahfouz M, Obeyd J et al. (2020) Development and validation of a quantitative food frequency questionnaire to assess dietary intake among Lebanese adults. Nutr J 19, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aoun C, Bou Daher R, El Osta N et al. (2019) Reproducibility and relative validity of a food frequency questionnaire to assess dietary intake of adults living in a Mediterranean country. PLoS One 14, e0218541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bowen L, Bharathi A, Kinra S et al. (2020) Development and evaluation of a semi-quantitative food frequency questionnaire for use in urban and rural India. Asia Pac J Nutr 21, 355–360. [PubMed] [Google Scholar]
- 34. Rodríguez M, Méndez H, Torún B et al. (2002) Validation of a semi-quantitative food-frequency questionnaire for use among adults in Guatemala. Public Health Nutr 5, 691–698. [DOI] [PubMed] [Google Scholar]
- 35. Subar A, Freedman L, Tooze J et al. (2015) Addressing current criticism regarding the value of self-report dietary data. J Nutr 145, 2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jaceldo-Siegl K, Knutsen S, Sabaté J et al. (2011) Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2) – Corrigendum. Public Health Nutr 14, 2079–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eftimov T, Popovski G, Petković M et al. (2020) COVID-19 pandemic changes the food consumption patterns. Trends Food Sci Tech 104, 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Araujo M, Yokoo E & Pereira R (2010) Validation and calibration of a semiquantitative food frequency questionnaire designed for adolescents. J Am Dietetic Assoc 110, 1170–1177. [DOI] [PubMed] [Google Scholar]
- 39. Bentzen S, Knudsen V, Christiensen T et al. (2016) Relative validity of a web-based food frequency questionnaire for patients with type 1 and type 2 diabetes in Denmark. Nutr Diabetes 6, e232–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sauvageot N (2013) Validation of the food frequency questionnaire used to assess the association between dietary habits and cardiovascular risk factors in the NESCAV study. J Nutr Food Sci 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernández-Ballart J, Piñol J, Zazpe I et al. (2010) Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 103, 1808–1816. [DOI] [PubMed] [Google Scholar]
- 42. Lombard M, Steyn N, Charlton K et al. (2015) Application and interpretation of multiple statistical tests to evaluate validity of dietary intake assessment methods. Nutr J 14, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee R & Nieman D (2013) Nutritional Assessment. New York: McGraw-Hill. [Google Scholar]
- 44. Hébert J, Hurley T, Steck S et al. (2014) Considering the value of dietary assessment data in informing nutrition-related health policy. Adv Nutr 5, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021002123.
click here to view supplementary material


