Abstract
Objectives:
Colorectal cancer (CRC) is the third and second most prevalent cancer in men and women, respectively. Various epidemiological studies indicated that dietary factors are implicated in the aetiology of CRC and its precursor, colorectal adenomas (CRA). Recently, much attention has been given to the role of acid–base balance in the development of chronic diseases including cancers. Therefore, the aim of the current study is to examine the association of diet-dependent acid load and the risk of CRC and CRA.
Design:
In this case–control study, potential renal acid load (PRAL) was computed based on dietary intake of participants assessed via a validated FFQ. Negative PRAL values indicated a base-forming potential, while positive values of PRAL implied acid-forming potential of diet. Logistic regression was used to derive OR and 95 % CI after adjusting for confounders.
Setting:
Tehran, Iran.
Participants:
A total of 499 participants aged 30–70 years were included in the study (240 hospital controls, 129 newly diagnosed CRC and 130 newly diagnosed CRA). The current study was conducted between December 2016 and September 2018.
Results:
After adjusting for potential confounders, a higher PRAL was associated with increased odds of CRC and CRA. The highest v. the lowest tertile of PRAL for CRC and CRA was OR 4·82 (95 % CI 2·51–9·25) and OR 2·47 (95 % CI 1·38–4·42), respectively.
Conclusions:
The findings of the current study suggested that higher diet-dependent acid load is associated with higher risk of CRC and CRA.
Keywords: Colorectal cancer, Potential renal acid load, Colorectal adenomas
Colorectal cancer (CRC) is the third and second most prevalent cancer in men and women, respectively. Worldwide, countries in Asia have witnessed the sharpest increase in CRC rate. CRC is the fourth common cancer among Iranian people and its incidence has been increasing in both men and women in recent years(1,2).
A variety of genetic and environmental factors play a role in the development of CRC(3). CRC usually originates from lesions named colorectal adenomas (CRA). Prevalence of adenomas varies between 30 and 50 %, and its environmental risk factors are almost similar to CRC(4). Various epidemiological studies indicated that dietary factors can contribute to or prevent from CRC development(5,6). Many of these studies investigated the relationship between nutrients, foods, food groups, dietary patterns and diet quality indices with CRC or CRA(7–11). The findings of these studies showed that adopting a diet high in plant-based foods and low in animal and processed food products was associated with lower risk of CRC and CRA(12–14).
Recently, much attention has been given to the role of dietary acid–base balance in the development of chronic diseases including CVD, type 2 diabetes, cancers and overall mortality(15–17). Short-term imbalanced acid–base is quickly corrected without significant clinical effects. However, long-term diet-dependent acid load, as a consequence of high protein consumption, may raise the level of insulin-like growth factor-1 that is associated with increased risk of CRC(17,18). Furthermore, some studies showed that extracellular acidity stimulated the invasive potential of cancer cells(19,20). However, the evidence for the relationship between diet-dependent acid load and the risk of cancers is limited(17,21).
According to our knowledge, until now, no study has examined the association between diet-dependent acid load and the risk of CRC and CRA. Therefore, the aim of the current study is to examine the association of diet-dependent acid load and the risk of CRC and CRA (as an important precursor of CRC) in a sample of Iranian adults.
Methods
Study design and population
This was a hospital-based case–control study, conducted in three major referral hospitals for CRC (Taleghani, Shohadae Tajrish and Emam Hosein) in Tehran, Iran between December 2016 and September 2018. The study participants had already been recruited for another study(22). To be included as CRC cases in original study, patients had to be diagnosed with CRC, no longer than 3 months before the interview date, confirmed by colonoscopy and pathology reports, with no history of cancers, polyps and inflammatory bowel diseases. CRA cases were either patients with rectal bleeding or asymptomatic individual with positive faecal occult blood tests who were referred to colonoscopy procedures. Patients with histologically confirmed adenomatous polyps were assigned to the CRA group. All cases were aged between 30 and 70 years old. Over the same period of time, age (±10 year) and sex-matched controls were recruited from patients admitted to same hospitals as cases for conditions unrelated to cancer, colorectal polyps, inflammatory bowel diseases and dietary restriction due to chronic diseases such as orthopedic disorders, traumas, acute surgical conditions, nose, and ears and skin problems. Overall, 536 participants (268 controls, 134 CRC and 134 CRA) were recruited in the current study. Subjects with incomplete FFQ and implausible energy intake estimates (outside the range of ±3 sd from the mean) were excluded (28 controls, five CRC and four CRA). The final sample consisted of 499 participants (240 controls, 129 CRC and 130 CRA).
Covariates’ assessment
Data regarding socio-demographic characteristics, family history of CRC and other cancers, tobacco use, medical history of diseases, medications and vitamin/mineral supplements and cooking techniques were obtained by a multicomponent questionnaire. Weight was measured with precision of 0·1 kg and height with the precision of 0·1 cm and then BMI was calculated. Alcohol intake consumption question was not included in the questionnaire due to prohibition of alcohol intake in Iran as an Islamic country. Furthermore, physical activity was assessed by a validated questionnaire in which participants were asked to specify the time of their leisure and occupational activity which were weighted based on intensity level(23,24). Metabolic equivalent task per 24 h was then calculated according to these specifications. Moreover, the reason for not considering possible dietary confounders such as whole grains, fibres, red and processed meat as confounders is that these are used in calculating amounts of nutrients which are part of potential renal acid load (PRAL) such as Mg and K.
Dietary assessment
Usual dietary intake of participants was assessed using a valid and reliable semi-quantitative FFQ, consisting of 148 foods and beverages(25). Dietary intake assessment referred to 1 year before diagnosis for cases and 1 year before interview for controls. Trained interviewers asked all participants to specify their intake frequency of given serving for each item on a daily, weekly and monthly basis. Collected data were then converted to daily frequencies. Daily gram intake of each item was calculated using the corresponding daily frequency and the portion size. Energy and nutrients for every participant were estimated using United States Department of Agriculture (USDA) food composition data. For traditional Iranian food items, such as traditional Iranian bread, which were not included in the USDA database, the Iranian food composition table was used(26).
Diet-dependent acid load calculation
While various methodologies were proposed to estimate the diet-dependent acid load, PRAL, used in previous studies, was selected for the purpose of the current study(27,28). PRAL is an estimation of endogenous acid production which exceeds the amount of alkali produced from foods consumed daily. Also, animal protein to potassium ratio (A:K) and net endogenous acid production (NEAP) were calculated to see their linear trend across PRAL as simpler indicators of acid load. Calculations are detailed below:
PRAL (mEq/d) = [Protein (g/d) × 0·49] + [P (mg/d) × 0·037] – [K (mg/d) × 0·201] – [Ca (mg/d) × 0·013] – [Mg (mg/d)] × 0·026]
Negative PRAL values indicate a base-forming potential, while positive values of PRAL imply acid-forming potential of diet(29).
A:K ratio = animal protein (g/d)/potassium (g/d)(30)
Net endogenous acid production (mEq/d) = [54·5 × protein (g/d)]/[0·0366 × potassium (mEq/d)] – 1·02(28).
Statistical analysis
Data analysis was performed by Statistical Package Software for Social Science, version 24 (SPSS Inc.). Kolmogorov–Smirnov test was used to check the normality of data. To compare general characteristics of controls with CRA and CRC cases, the Mann–Whitney U test was used for continuous variables and χ 2 used for categorical variables. PRAL scores were categorised into tertiles based on 33·3th and 66·66th percentile values of PRAL. Dietary consumption across tertile of PRAL scores was assessed using median value for each tertile of PRAL and modelling this value as a continuous variable in general linear regression adjustment for age and energy intake. Unconditional logistic regression was used to estimate OR with 95 % CI of CRC and CRA according to tertiles of PRAL after adjustment for potential confounders in one model, adjusted for age, comorbidity, family history of cancer, common ways of cooking, level of salt intake, physical activity and Ca supplement use. A P-value < 0·05 was considered as statistically significant.
To designing figures, participants were categorised into four groups (group 1 = < –1 sd, group 2 = –1 sd – mean, group 3 = mean – +1 sd and group 4 = > +1 sd) based on mean and ±1 sd of PRAL. To estimate OR and 95 % CI, group 1 values were used as reference in regression models and other groups compared with group 1 and OR and CI calculated based on it and then logarithm (log) of OR were used for Y-axis and sd for X-axis.
Results
Characteristics of study population
Table 1 provides the main characteristics of study participants. Given that the selection of participants followed a frequency matched design, age and gender did not differ in controls and CRC and CRA cases. There was no statistically significant difference among participants with regard to BMI, smoking status, family history of CRC in first-degree relatives, vitamin D supplement, multivitamin use and energy intake. However, CRC cases were more likely to have family history of cancer in first-degree relatives than controls (51·2 % v. 32·9 %; P-value < 0·01). Compared with controls, adenoma cases were more likely to have at least one comorbidity (36·9 % v. 15·8 %; P-value < 0·01) and the use of Ca supplement was higher in adenoma cases than controls (24·6 % v. 14·6; P-value = 0·01). Moreover, the median value for physical activity was significantly higher in controls than adenomas (OR 39·02 (95 % CI 36·51–41·33) v. OR 37·75 (95 % CI 34·67–40·40); P-value < 0·01). Level of the salt intake was lower in controls compared with cancer and adenoma cases (P-value = 0·002). Common ways of cooking among controls differed significantly from cancer and adenomas patients (P-value < 0·05). More specifically, cancer cases consumed more fried and grilled foods compared with controls and consumption of steamed food was lower in adenomas than controls.
Table 1.
Baseline characteristics of the study participants
| Controls (n 240) | Cancers (n 129) | Adenomas (n 130) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | P * | P † |
| Age (years) | ‡ | ‡ | ||||||
| Median | 56 | 59 | 58 | |||||
| Q1–Q3 | 50–61·7 | 49·2–64 | 51–64 | |||||
| Gender (male) | 133 | 55·4 | 66 | 51·2 | 59 | 45·4 | ‡ | ‡ |
| BMI (kg/m2) | 0·23 | 0·95 | ||||||
| Median | 26·5 | 25·7 | 26·7 | |||||
| Q1–Q3 | 24·1–29·4 | 23·0–29·7 | 23·8–29·4 | |||||
| Smoking (yes) | 42 | 17·5 | 26 | 20·2 | 27 | 20·8 | 0·53 | 0·11 |
| Comorbidities (yes)§ | 38 | 15·8 | 17 | 13·2 | 48 | 36·9 | 0·84 | <0·01¶ |
| Diabetes (yes) | 20 | 8·3 | 12 | 9·3 | 12 | 9·2 | 0·75 | 0·77 |
| Hypertension (yes) | 13 | 5·4 | 4 | 3·1 | 23 | 17·7 | 0·34 | <0·01¶ |
| CHD (yes) | 5 | 2·1 | 1 | 0·8 | 13 | 10·0 | 0·31 | <0·01¶ |
| Family history of cancer in first degree (yes) | 89 | 32·9 | 66 | 51·2 | 48 | 36·9 | <0·01¶ | 0·43 |
| Colorectal cancer family history in first-degree relatives (yes) | 18 | 7·5 | 10 | 7·8 | 17 | 13·1 | 0·15 | 0·08 |
| Common ways of cooking food | ||||||||
| Fried | 55 | 22·9 | 40 | 31 | 18 | 13·8 | <0·01¶ | 0·06 |
| Boiled | 81 | 33·8 | 41 | 31·8 | 34 | 26·2 | 0·75 | 0·82 |
| Grilled | 5 | 2·1 | 0 | 0 | 4 | 3·1 | 0·02¶ | 0·52 |
| Steam cook | 3 | 1·3 | 2 | 1·6 | 0 | 0 | 0·32 | <0·01¶ |
| Combined | 96 | 40 | 46 | 35·7 | 74 | 56·9 | <0·01¶ | 0·06 |
| Level of salt intake | ||||||||
| Low | 127 | 52·9 | 44 | 34·1 | 44 | 34·1 | <0·01¶ | <0·01¶ |
| Normal | 79 | 32·9 | 64 | 49·6 | 64 | 49·6 | 0·08 | 0·08 |
| High | 34 | 14·2 | 21 | 16·3 | 21 | 16·3 | 0·07 | 0·07 |
| Physical activity (MET/h/d) | 0·85 | <0·01‖ | ||||||
| Median | 39·02 | 39·30 | 37·75 | |||||
| Q1–Q3 | 36·51–41·33 | 37·17–40·90 | 34·67–40·40 | |||||
| Monthly intake of 1250 mcg vitamin D supplement (yes) | 56 | 23·3 | 28 | 21·7 | 40 | 30·8 | 0·72 | 0·11 |
| Daily intake of 500 mg Ca supplement (yes) | 35 | 14·6 | 28 | 21·7 | 32 | 24·6 | 0·08 | 0·01¶ |
| Multivitamin use every day (yes) | 18 | 7·5 | 16 | 12·4 | 9 | 7 | 0·12 | 0·84 |
| Energy intake (kJ/d) | 0·35 | 0·35 | ||||||
| Median | 9549·0 | 9154·9 | 9221·6 | |||||
| Q1–Q3 | 8122·1–11 140·9 | 7714·9–11 087·0 | 7648·0–11 608·6 | |||||
MET, metabolic equivalent.
P-value between cancers and controls.
P-value between adenomas and controls.
Matched variables of the study.
Comorbidities are defined as diabetes, hypertension and CHD.
Significant difference (χ 2, P-value < 0·05).
Significant difference (Mann–Whitney U, P-value <0·05).
Dietary intakes across tertiles
Table 2 shows dietary intake of energy, food groups and nutrients, according to tertiles of PRAL. Dietary intake of red meat (β = 0·23), poultry (β = 0·24), egg (β = 0·11), protein (β = 0·14), animal protein (β = 0·25) and net endogenous acid production (β = 0·82) and protein to potassium ratio (β = 0·65) increased significantly across increasing tertiles of PRAL (P for trend < 0·001), while dietary intake of dairy products (β = –0·11), legumes (β = –0·12), fruits (β = –0·48), vegetables (β = –0·35), carbohydrates (β = –0·67), Mg (β = –0·12) and K (β = –0·39) decreased significantly across the increasing tertiles of PRAL (P for trend <0·01). Intake of Ca (861·16 (sd 309·37) v. 873·92 (sd 297·56) mg/d) in third tertile was significantly lower compared with first tertile of PRAL (P for trend < 0·001). Compared with first tertile of PRAL, energy intake (10 327·58 (sd 3022·98) v. 9641·65 (sd 2628·30) kJ/d) was significantly higher in third tertile of PRAL (P for trend < 0·01).
Table 2.
Dietary intake of study participants (controls, colorectal cancer cases and colorectal adenoma cases) across tertiles (T) of potential renal acid load score (PRAL)
| Characteristics | Potential renal acid load score tertiles (mEq/d) | Standardised regression coefficient (β) | |||||
|---|---|---|---|---|---|---|---|
| T1 (<−6·0) (n 166) | T2 (−6·0 to 3·9) (n 166) | T3 (>3·9) (n 166) | |||||
| Mean | sd | Mean | sd | Mean | sd | ||
| Foods* | |||||||
| Red meat (g/d) | 20·3 | 16·8 | 25·0 | 18·2 | 32·4† | 21·5 | 0·23 |
| Processed meat (g/d) | 1·7 | 5·3 | 4·2 | 12·7 | 2·8 | 7·0 | 0·04 |
| Poultry (g/d) | 33·5 | 24·1 | 36·5 | 22·9 | 52·8† | 36·7 | 0·24 |
| Sea foods (g/d) | 15·0 | 16·9 | 13·3 | 17·1 | 19·4 | 26·9 | 0·06 |
| Egg (g/d) | 21·55 | 14·60 | 23·0 | 20·6 | 28·3† | 24·7 | 0·11 |
| Dairy products (g/d) | 276·9 | 166·7 | 233·3 | 136·9 | 242·2† | 175·4 | −0·11 |
| Whole grain (g/d) | 79·6 | 76·6 | 83·3 | 72·6 | 119·7† | 124·9 | 0·13 |
| Nuts and seeds (g/d) | 21·3 | 23·0 | 18·3 | 26·5 | 22·0 | 32·0 | −0·03 |
| Legumes (g/d) | 54·7 | 43·8 | 44·0 | 30·9 | 46·7† | 37·5 | −0·12 |
| Fruits (g/d) | 410·9 | 183·2 | 279·3 | 144·4 | 221·9† | 130·5 | −0·48 |
| Vegetables (g/d) | 238·0 | 133·5 | 165·8 | 81·9 | 149·2† | 110·0 | −0·35 |
| Added sugar (g/d) | 42·3 | 34·2 | 44·7 | 27·3 | 49·5 | 27·8 | 0·04 |
| Nutrients* | |||||||
| Protein (g/d) | 72·4 | 23·6 | 69·7 | 17·9 | 85·4† | 25·7 | 0·14 |
| Animal protein (g/d) | 37·5 | 13·5 | 37·9 | 11·8 | 48·5† | 16·6 | 0·25 |
| Carbohydrate (g/d) | 287·0 | 90·7 | 264·0 | 84·4 | 293·2† | 105·1 | −0·67 |
| Fat (g/d) | 105·1 | 33·3 | 103·4 | 31·8 | 112·3 | 37·0 | 0·01 |
| Ca (mg/d) | 873·9 | 297·5 | 768·5 | 249·8 | 861·1† | 309·3 | −0·08 |
| Mg (mg/d) | 378·8 | 134·7 | 328·5 | 103·6 | 368·9† | 147·7 | −0·12 |
| K (mg/d) | 3758·8 | 1130·4 | 2972·4 | 783·2 | 298·4† | 997·2 | −0·39 |
| P (mg/d) | 1331·7 | 436·80 | 1228·7 | 340·8 | 1463·0 | 476·7 | 0·04 |
| NEAP (mEq/d)* | 30·7 | 4·8 | 39·9 | 3·4 | 51·9† | 8·8 | 0·82 |
| A:K* | 0·3 | 0·1 | 0·5 | 0·1 | 0·6† | 0·1 | 0·65 |
| Energy intake (kJ/d)‡ | 9641·6 | 2628·3 | 9229·6 | 2342·6 | 10 327·5† | 3022·9 | 0·10 |
NEAP, net endogenous acid production; A:K ratio, the ratio of animal protein to potassium.
Adjusted for age (linear) and energy intake (linear).
P < 0·01, using general linear regression to compare the dietary intake of participant across tertiles of PRAL.
Adjusted for age (linear).
Association of potential renal acid load with colorectal cancer and adenomas risk
Tables 3 and 4 present the OR and 95 % CI of CRC and CRA across tertiles of PRAL, respectively. After adjusting for potential confounding variables including age, comorbidities, family history of cancers, common ways of cooking, level of the salt intake, physical activity and Ca supplement consumption, the odds (95 % CI) of having CRC and CRA were 4·82 (2·51–9·25) and 2·47 (1·38–4·42) times higher at the highest compared with the lowest tertile of PRAL.
Table 3.
OR and corresponding 95 % CI for the risk of colorectal cancer across tertiles (T) of potential renal acid load score (PRAL)
| Model | T1 (<−6·0 mEq/d) | T2 (–6·0 to 3·9 mEq/d) | T3 (>3·9 mEq/d) | P for trend | ||
|---|---|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | |||
| Number of controls/cases | 109/21 | 69/52 | 62/56 | |||
| Crude | Ref | 3·9 | 2·1, 3·7 | 4·6 | 2·6, 8·4 | <0·01 |
| Model 1* | Ref | 4·4 | 2·3, 8·5 | 4·8 | 2·5, 9·2 | <0·01 |
OR adjusted for age (linear), comorbidity (in categories: diabetes, hypertension and CHD), cancer family history (in categories: yes, no), common ways of cooking (in categories: fried, boiled, grilled, steam cooked and combined), level of salt intake (in categories: low, normal and high), physical activity (linear) and Ca supplement use (in categories: yes, no).
Table 4.
OR and 95 % CI for the risk of colorectal adenomas based on tertiles (T) of potential renal acid load score (PRAL)
| Model | T1 (<−6·0 mEq/d) | T2 (−6·0–3·9 mEq/d) | T3 (>3·9 mEq/d) | P for trend | ||
|---|---|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | |||
| Number of controls/adenomas | 109/37 | 69/45 | 62/48 | |||
| Crude | Ref | 1·9 | 1·13, 3·2 | 2·2 | 1·3, 3·8 | <0·01 |
| Model 1* | Ref | 2·0 | 1·16, 3·6 | 2·4 | 1·3, 4·4 | <0·01 |
OR adjusted for age (linear), comorbidity (in categories: diabetes, hypertension and CHD), cancer family history (in categories: yes, no), common ways of cooking (in categories: fried, boiled, grilled, steam cooked and combined), level of salt intake (in categories: low, normal and high), physical activity (linear) and Ca supplement use (in categories: yes, no).
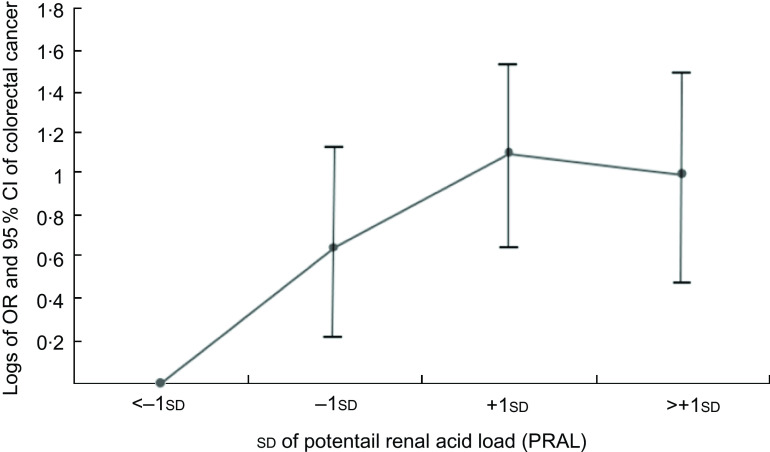
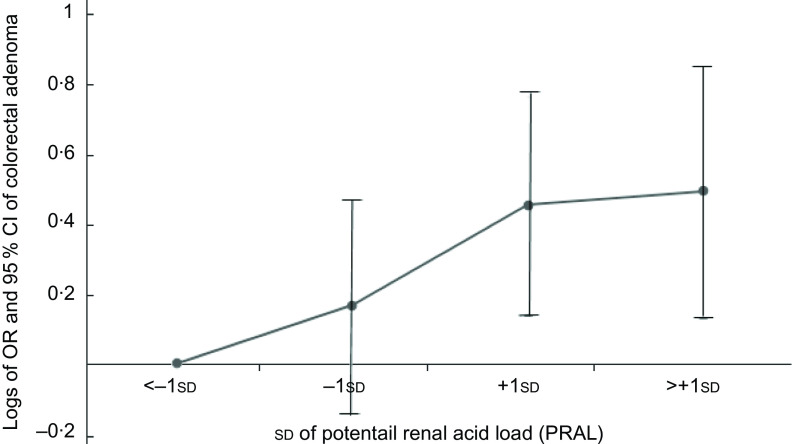
Figures 1 and 2 show the logs of the odds (95 % CI) of CRC and CRA according to sd of PRAL, respectively. After adjusting for potential confounding variables, risk of having CRC and CRA increased through increasing PRAL.
Fig. 1.
Participants categorised into four groups based on mean and ±1 sd of PRAL: <−1 sd (≤−14·62), from −1 sd to mean (from −14·61 to −1·42), mean to +1 sd (from −1·41 to 11·78) and >+1 sd: ≥11·79. To estimate logs of odds and 95 % CI, −1 sd values were used as reference in regression models and then logarithm (log) of odds was used for Y-axis and sd for X-axis
Fig. 2.
Participants categorised into four groups based on mean and ±1 sd of PRAL: <−1 sd (≤−14·62), from −1 sd to mean (from −14·61 to −1·42), mean to +1 sd (from −1·41 to 11·78) and >+1 sd: ≥11·79. To estimate logs of odds and 95 % CI, −1 sd values were used as reference in regression models and then logarithm (log) of odds was used for Y-axis and sd for X-axis
Discussion
In this case–control study, we found that higher diet-dependent acid load scores were significantly and positively associated with increase in risk of CRC and CRA, among Iranian adults. In addition, the intake of animal products (red meat, poultry, egg, protein and animal protein) was positively associated with diet-dependent acid load while intakes of plant-based foods (legumes, fruits and vegetables) were negatively related to diet-dependent acid load.
A few epidemiological studies have evaluated the association between dietary acid–base disequilibrium and risk of cancers(21). A prospective cohort study examined the relationship between urine pH (represented as net acid excretion) and bladder cancer based on a hypothesis that low urine PH is an important potential risk factor for bladder cancer. The current study indicated that there was no significant association between net acid excretion and bladder cancer, but in long-term smokers a non-significant increased risk was seen(31). Another prospective cohort study showed that higher diet-dependent acid load (represented as PRAL) was significantly related to increased risk of breast cancer especially in estrogen reseptor (ER) negative and triple negative breast cancer(17). The results of our study together with those of previous studies suggested a role of acid–base balance of diet in the development of cancer. Moreover, our findings showed a relationship between diet-dependent acid load and the risk of CRA as a main precursor of CRC.
The results of the present study showed that, among all food groups which have an impact on diet-dependent acid load, animal protein, total protein, poultry and red meat were positively associated with acidity, while fruits and vegetables had the highest impact on the base potential of diet. These findings are in line with the proposition that food groups can affect the acid–base balance. Meats, dairy products and eggs, which are high in sulphur containing amino acids, increase diet-dependent acid load by increasing hydrogen ion [H+] concentration, whereas fruits, vegetables and legumes have base-producing potential because they are rich in K and Mg which consume hydrogen ions when metabolised(32,33).
Although there is no consensus on how diet-dependent acid-load affects the risk of CRC, several mechanisms have been proposed. Existing evidence indicated that short-term diet induced acid–base imbalance as a result of high protein meals is transient and does not have much clinical consequences(34); however, on the long term, there are some potential hormonal and non-hormonal mechanisms to explain the effect of diet-dependent acidosis on CRC. Acidogenic diet contributes to mild metabolic acidosis which induces cortisol secretion excess(35). Studies have reported that cortisol signalling may have a role in carcinogenesis. Chronic cortisol production leads to insulin resistance and activation of insulin-like growth factor-1, which may result in colorectal carcinogenesis and growth and malignant transformation of adenomatous polyps through mitogen-activated protein kinase (MAPK) pathway and phosphoinositide 3-kinase (PI3K) pathway(32,36,37). On the other hand, diet-dependent acid load could decrease circulating adiponectin(38) and prevent its anti-proliferative and apoptotic activities(39). Evidence on the impact of protein intake, as a net acid producer, on leptin level is limited and the results are inconsistent(40,41). Leptin has proinflammatory, proliferative, anti-apoptotic and mitogenic activities, leading to CRC(42).
Furthermore, long-term diet high in red meat, processed meats, dairy and eggs results in sulphate and phosphate production, which are ultimately net-acid producers (H+). If the release of acids into the circulation exceeds the amount of bicarbonate (HCO3-) which is the final metabolite of K and Mg from vegetables and fruits, low grade diet-dependent acidosis occurs(32,34). Several studies have shown an inverse relationship between vegetables and fruits intake and CRC and CRA, while many studies have suggested that red meat and processed meats increase the risk of CRC and CRA(8,43). Considering that higher PRAL was associated with a higher risk of CRC and CRA in the present study and higher PRAL scores were associated with higher intake of red meat and lower intake of vegetables and fruits, our study confirms the protective role of high consumption of fruits and vegetables and low consumption of red meat for CRC cancer and CRA. According to mentioned associations between fruits, vegetables and red meats with CRC and CRA, it should be considered that the associations between PRAL and the outcomes could possibly be due to these risk factors and protective factors and PRAL may be one of the several mechanisms linking these foods with CRC. Several mechanisms have been proposed linking these foods to CRC. For example, for plant foods reducing the plasma concentration of inflammatory markers by antioxidant compounds, inhibiting CRC cell growth by holding down the activation of NF-κB pathway by phytochemical compounds(44,45) and promoting cell cycle arrest and apoptosis, inhibition of cell migration and invasion by fibre have been noted(46). Also, for red meats, high fat and polycyclic aromatic hydrocarbons and heterocyclic amines produced from cooking over high heat have been suggested(47).
Our study has some strengths. First, to our knowledge, the current study is the first to explore the association between diet-dependent acid load and the risk of CRC and CRA. Second, using a validated FFQ allowed for an accurate estimation of the main exposure of interest, which is dietary intake and gave us the opportunity to control for several important confounders(24,48). On the other hand, there are some limitations in the present study. Since dietary intake was evaluated through a self-administered FFQ, measurement errors were inevitable. Selection and recall bias are inevitable in case–control studies. However, we tried to reduce these biases by including incident cases along with hospital controls and employing validated FFQ performed by trained interviewers. We did not have information about participants’ kidney function as an important determinant of acid–base equilibrium. However, we did not include patients with chronic kidney, liver and lung diseases in the study and the results were adjusted for comorbidities such as blood pressure and type 2 diabetes.
Conclusion
In conclusion, findings from this case–control study suggested that higher diet-dependent acid load is associated with higher risk of CRC and CRA. Further intervention studies are needed to determine whether diets with lower acid load could reduce the risk of CRC and CRA as a precursor of colorectal malignancies. Also, studies to confirm present findings in other populations are warranted.
Acknowledgements
Acknowledgements: The authors thank all study participants without whom the current study was impossible. Financial support: This investigation received no financial support. Conflict of interest: The authors declare no conflicts of interest. Authorship: In the current study, the author contributions are as follows: Conceptualisation and methodology: E.H. and A.S.; analysis: S.K.N., N.R. and B.R.; investigation: S.J.N.P.R. and A.B.; resources: A.S. and E.H.; writing: S.J.N., N.R., E.H. and A.S.; review and editing: G.S., B.R. and F.N.; visualisation: A.S. and E.H.; supervision: A.S. and E.H.; project administration: A.S. and E.H. Ethics of human subject participants: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (approval number: 0450/1118). Written informed consent was obtained from all subjects/patients.
References
- 1. Khosravi Shadmani F, Ayubi E, Khazaei S et al. (2017) Geographic distribution of the incidence of colorectal cancer in Iran: a population-based study. Epidemiol Health 39, e2017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rafiemanesh H, Pakzad R, Abedi M et al. (2016) Colorectal cancer in Iran: epidemiology and morphology trends. EXCLI J 15, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuipers EJ, Grady WM, Lieberman D et al. (2015) Colorectal cancer. Nat Rev Dis Primers 1, 15065–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Øines M, Helsingen LM, Bretthauer M et al. (2017) Epidemiology and risk factors of colorectal polyps. Best Pract Res Clin Gastroenterol 31, 419–424. [DOI] [PubMed] [Google Scholar]
- 5. Aune D, Chan DS, Lau R et al. (2011) Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 343, d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baena R & Salinas P (2015) Diet and colorectal cancer. Maturitas 80, 258–264. [DOI] [PubMed] [Google Scholar]
- 7. Randi G, Edefonti V, Ferraroni M et al. (2010) Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev 68, 389–408. [DOI] [PubMed] [Google Scholar]
- 8. Kunzmann AT, Coleman HG, Huang WY et al. (2016) Fruit and vegetable intakes and risk of colorectal cancer and incident and recurrent adenomas in the PLCO cancer screening trial. Int J Cancer 138, 1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kunzmann AT, Coleman HG, Huang WY et al. (2015) Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr 102, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farinetti A, Zurlo V, Manenti A et al. (2017) Mediterranean diet and colorectal cancer: a systematic review. Nutrition 43–44, 83–88. [DOI] [PubMed] [Google Scholar]
- 11. Schwingshackl L & Hoffmann G (2015) Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 115, 780–800. [DOI] [PubMed]
- 12. Tayyem RF, Bawadi HA, Shehadah I et al. (2017) Dietary patterns and colorectal cancer. Clin Nutr 36, 848–852. [DOI] [PubMed] [Google Scholar]
- 13. Schwingshackl L, Schwedhelm C, Galbete C et al. (2017) Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients 9, 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azeem S, Gillani SW, Siddiqui A et al. (2015) Diet and colorectal cancer risk in Asia: a systematic review. Asian Pac J Cancer Prev 16, 5389–5396. [DOI] [PubMed] [Google Scholar]
- 15. Kucharska AM, Szostak-Wegierek DE, Waskiewicz A et al. (2018) Dietary acid load and cardiometabolic risk in the Polish adult population. Adv Clin Exp Med 27, 1347–1354. [DOI] [PubMed] [Google Scholar]
- 16. Kiefte-de Jong JC, Li Y, Chen M et al. (2017) Diet-dependent acid load and type 2 diabetes: pooled results from three prospective cohort studies. Diabetologia 60, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park YM, Steck SE, Fung TT et al. (2018) Higher diet-dependent acid load is associated with risk of breast cancer: findings from the sister study. Int J Cancer 144, 1834–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furstenberger G & Senn HJ (2002) Insulin-like growth factors and cancer. Lancet Oncol 3, 298–302. [DOI] [PubMed] [Google Scholar]
- 19. Kato Y, Ozawa S, Miyamoto C et al. (2013) Acidic extracellular microenvironment and cancer. Cancer Cell Int 13, 89–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez-Zaguilan R, Seftor EA, Seftor RE et al. (1996) Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis 14, 176–186. [DOI] [PubMed] [Google Scholar]
- 21. Fenton TR & Huang T (2016) Systematic review of the association between dietary acid load, alkaline water and cancer. BMJ Open 6, e010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bahrami A, Houshyari M, Jafari S et al. (2019) Dietary patterns and the risk of colorectal cancer and adenoma: a case control study in Iran. Gastroenterol Hepatol Bed Bench 12, 217–225. [PMC free article] [PubMed] [Google Scholar]
- 23. Kriska AM, Knowler WC, LaPorte RE et al. (1990) Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 13, 401–411. [DOI] [PubMed] [Google Scholar]
- 24. Aadahl M & Jorgensen T (2003) Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc 35, 1196–1202. [DOI] [PubMed] [Google Scholar]
- 25. Esfahani FH, Asghari G, Mirmiran P et al. (2010) Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. J Epidemiol 20, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azar M & Sarkisian E (1980) Food Composition Table of Iran: National Nutrition and Food Research Institute. Tehran: Shaheed Beheshti University. [Google Scholar]
- 27. Remer T, Dimitriou T & Manz F (2003) Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr 77, 1255–1260. [DOI] [PubMed] [Google Scholar]
- 28. Frassetto LA, Todd KM, Morris RC Jr et al. (1998) Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68, 576–583. [DOI] [PubMed] [Google Scholar]
- 29. Remer T & Manz F (1994) Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr 59, 1356–1361. [DOI] [PubMed] [Google Scholar]
- 30. Zwart SR, Hargens AR & Smith SM (2004) The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. Am J Clin Nutr 80, 1058–1065. [DOI] [PubMed] [Google Scholar]
- 31. Wright ME, Michaud DS, Pietinen P et al. (2005) Estimated urine pH and bladder cancer risk in a cohort of male smokers (Finland). Cancer Causes Control 16, 1117–1123. [DOI] [PubMed] [Google Scholar]
- 32. Robey IF (2012) Examining the relationship between diet-induced acidosis and cancer. Nutr Metab (Lond) 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Remer T (2000) Acid-base in renal failure: influence of diet on acid-base balance. Semin Dial 13, 221–226. [DOI] [PubMed] [Google Scholar]
- 34. Pizzorno J, Frassetto LA & Katzinger J (2010) Diet-induced acidosis: is it real and clinically relevant? Br J Nutr 103, 1185–1194. [DOI] [PubMed] [Google Scholar]
- 35. Espino L, Suarez ML, Santamarina G et al. (2005) Effects of dietary cation-anion difference on blood cortisol and ACTH levels in reproducing ewes. J Vet Med A Physiol Pathol Clin Med 52, 8–12. [DOI] [PubMed] [Google Scholar]
- 36. Zhang R, Xu GL, Li Y et al. (2013) The role of insulin-like growth factor 1 and its receptor in the formation and development of colorectal carcinoma. J Int Med Res 41, 1228–1235. [DOI] [PubMed] [Google Scholar]
- 37. Nosho K, Yamamoto H, Taniguchi H et al. (2004) Interplay of insulin-like growth factor-II, insulin-like growth factor-I, insulin-like growth factor-I receptor, COX-2, and matrix metalloproteine-7, play key roles in the early stage of colorectal carcinogenesis. Clin Cancer Res 10, 7950–7957. [DOI] [PubMed] [Google Scholar]
- 38. Disthabanchong S, Niticharoenpong K, Radinahamed P et al. (2011) Metabolic acidosis lowers circulating adiponectin through inhibition of adiponectin gene transcription. Nephrol Dial Transplant 26, 592–598. [DOI] [PubMed] [Google Scholar]
- 39. Gonullu G, Kahraman H, Bedir A et al. (2010) Association between adiponectin, resistin, insulin resistance, and colorectal tumors. Int J Colorectal Dis 25, 205–212. [DOI] [PubMed] [Google Scholar]
- 40. Izadi V, Saraf-Bank S & Azadbakht L (2014) Dietary intakes and leptin concentrations. ARYA Atheroscler 10, 266–272. [PMC free article] [PubMed] [Google Scholar]
- 41. Weigle DS, Breen PA, Matthys CC et al. (2005) A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 82, 41–48. [DOI] [PubMed] [Google Scholar]
- 42. Rodríguez AJ, Mastronardi C & Paz-Filho G (2013) Leptin as a risk factor for the development of colorectal cancer. Transl Gastrointest Cancer 2, 211–222. [Google Scholar]
- 43. Abid Z, Cross AJ & Sinha R (2014) Meat, dairy, and cancer. Am J Clin Nutr 100, Suppl. 1, 386s–393s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li YH, Niu YB, Sun Y et al. (2015) Role of phytochemicals in colorectal cancer prevention. World J Gastroenterol 21, 9262–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jaganathan SK, Vellayappan MV, Narasimhan G et al. (2014) Chemopreventive effect of apple and berry fruits against colon cancer. World J Gastroenterol 20, 17029–17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng H, Lazarova DL & Bordonaro M (2014) Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol 6, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Demeyer D, Mertens B, De Smet S et al. (2016) Mechanisms linking colorectal cancer to the consumption of (processed) red meat: a review. Crit Rev Food Sci Nutr 56, 2747–2766. [DOI] [PubMed] [Google Scholar]
- 48. Asghari G, Rezazadeh A, Hosseini-Esfahani F et al. (2012) Reliability, comparative validity and stability of dietary patterns derived from an FFQ in the Tehran lipid and glucose study. Br J Nutr 108, 1109–1117. [DOI] [PubMed] [Google Scholar]