Abstract
Objective:
The objective of this scoping review was to examine the research question: In the adults with or without cardiometabolic risk, what is the availability of literature examining interventions to improve or maintain nutrition and physical activity-related outcomes? Sub-topics included: (1) behaviour counseling or coaching from a dietitian/nutritionist or exercise practitioner, (2) mobile applications to improve nutrition and physical activity and (3) nutritional ergogenic aids.
Design:
The current study is a scoping review. A literature search of the Medline Complete, CINAHL Complete, Cochrane Database of Systematic Reviews and other databases was conducted to identify articles published in the English language from January 2005 until May 2020. Data were synthesised using bubble charts and heat maps.
Setting:
Out-patient, community and workplace.
Participants:
Adults with or without cardiometabolic risk factors living in economically developed countries.
Results:
Searches resulted in 19 474 unique articles and 170 articles were included in this scoping review, including one guideline, thirty systematic reviews (SR), 134 randomised controlled trials and five non-randomised trials. Mobile applications (n 37) as well as ergogenic aids (n 87) have been addressed in several recent studies, including SR. While primary research has examined the effect of individual-level nutrition and physical activity counseling or coaching from a dietitian/nutritionist and/or exercise practitioner (n 48), interventions provided by these practitioners have not been recently synthesised in SR.
Conclusion:
SR of behaviour counseling or coaching provided by a dietitian/nutritionist and/or exercise practitioner are needed and can inform practice for practitioners working with individuals who are healthy or have cardiometabolic risk.
Keywords: Diet, Exercise, Scoping review, Dietitian, Nutritionist, Counseling, Mobile applications
For individuals living in economically developed environments, rates of non-communicable diseases associated with overnutrition, such as type 2 diabetes mellitus and many forms of heart disease, are serious concerns(1). In addition to the decreasing quality of life(2) and potential lifespan(3), these diseases collectively contribute to extreme economic burdens to the individual and society as a whole(3). Nutrition and physical activity are each independent risk factors for the development of cardiometabolic diseases and associated mortality(4). Despite knowledge of the benefits of improved dietary intake and physical activity, three quarters of Americans follow an eating pattern low in fruits and vegetables(5) and only half of adults meet the minimum aerobic physical activity recommendations(6).
Population-level improvement of nutrition and physical activity behaviours may decrease development and progression of cardiometabolic disease. This may, in turn, result in improved quality of life and a decreased burden of personal and national health care costs. To improve health behaviours on a population level, evidence-based guidance is needed to inform nutrition and physical activity practitioners working with clients in the community, workplace or out-patient settings.
The aim of a scoping review is to map the availability of research, both systematic reviews (SR) and guidelines as well as controlled trials, in areas of interest to determine where resources are available to guide practice, and where evidence is still needed(7). Additionally, a scoping review can identify which current topics still require SR and evidence-based practice guidelines to inform practitioners working with individuals who are healthy or who have cardiometabolic risk factors. This scoping review was conducted to determine if current evidence was available on relevant nutrition and physical activity interventions for the general population. Specific areas of interest that require clarification or are important to policy or practice were identified by practitioners currently working with clients in the field and are addressed in the individual research questions.
The objective of this scoping review is to address the overarching research question: In adults in the ‘general population’, including non-athletes or recreational athletes with or without cardiometabolic risk factors, what is the extent, range and nature of literature examining interventions to improve or maintain nutrition and physical activity and related outcomes? Specific research questions examined availability of research describing:
Question 1 (Q1). Individual-level nutrition and physical activity counseling or coaching provided by a dietitian/nutritionist and/or exercise practitioner;
Question 2 (Q2). Mobile applications (apps) and/or wearable technology;
Question 3 (Q3). Nutritional ergogenic aids of interest.
Methods
This scoping review was conducted with the framework introduced by Arksey and O’Malley(8) and developed by Levac et al.(9) and the Joanna Briggs Institute(7). This scoping review was registered on Open Science Framework (osf.io/pc6sy)(10) and adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for scoping reviews(11).
Eligibility criteria
A full description of eligibility criteria can be found in Table 1. The target population for this scoping review was adults in the ‘general’ population living in economically developed countries, such as the USA(12). The authors recognised that currently, a ‘general’ population does not imply a ‘healthy’ population, since cardiometabolic risk factors may exist in a majority of adults. Thus, this scoping review included individuals with no risk, risk for and diagnosed with cardiometabolic disease.
Table 1.
Eligibility criteria for studies including in scoping review examining effect of nutrition and physical activity interventions in the general population
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Peer-review status | Peer-reviewed and published in a juried publication in a peer-reviewed section within the publication | Non-peer-reviewed articles, such as government reports, grey literature |
| Population | • Humans • Adults (≥18 years old) • Non- or recreational athletes (<10 h training/week) |
• Animal studies • <18 years old • Elite athletes (>10 h training/week) • Highly trained • Well-trained • Wrestling, soccer or other team sports |
| Setting | Out-patient, community | In-patient |
| Health status | • No diagnosed cardiometabolic diseases or mental health disorder • At risk for or diagnosed with non-acute cardiometabolic disease • Non-severe anxiety or depression • Non-alcoholic fatty liver disease • Osteoarthritis • Sarcopenia |
Persons with medical conditions that limit their generalisability to the general population, such as • Acute, terminal or critical illnesses • Dialysed or post-organ transplant • Post-surgical patients • Chronic diseases such as COPD or HIV/AIDS • Spinal cord injury • Cancer or studies targeting cancer survivors • Heart failure, stroke • Pregnancy, lactation, postpartum • Morbidly obese (BMI >/= 40) or who have PCOS; bariatric surgery • Active military • IBD • Severe and persistent mental illness • Institutionalised (nursing home, hospitalised, prison) |
| Interventions/exposures | • Q1: Nutrition AND exercise counseling or coaching • Q1: Must include some individual-level counseling or coaching • Q1: Counseling or coaching must be provided by at least one of the following: Dietitian, Diet Tech, Nutritionist (if in country where this is dietitian-equivalent), Health Coach, Personal Trainer, Exercise Practitioner Otherwise Specified • Q1: Nutrition counseling topics could include ○ Increased consumption of fruits, vegetables, whole grains, fat-free or low-fat dairy, and lean proteins ○ Limited consumption of Na, saturated fat, trans-fat, and sugar-sweetened food and beverages ○ Balanced diet plans such as Mediterranean, DASH, MyPlate • Q1: Physical activity coaching could include ○ Aerobic activities that involve repeated use of large muscles, such as walking, cycling and swimming ○ Resistance training designed to improve physical strength ○ Reduction of sedentary behaviours ○ Optional or access to guided physical activity or exercise classes allowed • Q2: Wearable technology and/or mobile application (app) to assess and intervene in nutrition AND physical activity • Q3: Nutritional ergogenic aids including carbohydrate replacement, caffeine, branch-chained amino acids, creatine, collagen, multivitamins, n-3 fatty acids and exogenous ketones |
• Q1: Counseling or coaching for nutrition OR physical activity only • Q1: Group level counseling or coaching only • Q1: Counseling or coaching provided by: Physician, Nurse, Psychologist, Community Health Worker, Paraprofessional/Peer, any other provider not specified for included • Q1: Nutrition counseling topics could not include special/controlled diets (e.g., low-carbohydrate diet) • Q1: PA interventions excluded ○ Physical activity counseling solely focused on balance, flexibility or gait ○ Stress management interventions (e.g., meditation or yoga or tai chi-based interventions that have minimal aerobic or strength-building activities) ○ Counseling interventions aimed at fall prevention, cognitive functioning • Q1 and Q2: Interventions or exposures that do not consider the combination of nutrition AND physical activity • Q3: Dietary supplements other than those indicated. BCAA metabolites |
| Comparators | Must have a comparison group that is a true control not receiving the intervention. Includes usual care, minimal intervention, attention control no intervention Q3: Placebo controlled |
No comparison group. Comparison group is an alternative intervention, with no true control Q1: Comparison group includes counseling or coaching for nutrition only or physical activity only. Comparison group receives only group counseling for nutrition and physical activity Q3: Not placebo controlled |
| Study design preferences | Systematic reviews and evidence-based practice guidelines Controlled clinical trials (RCT, non-RCT) Q3: RCT only |
Narrative reviews, commentary/letters to the editor, case studies Observational studies, including cross-sectional studies, cohort studies Q3: Non-RCT |
| Minimum study duration | No limits | No limits |
| Size of study groups | ≥10 participants/group | <10 participants/group |
| Study drop-out rate | No limits | No limits |
| Outcomes | Q1–Q2: Diet and physical activity (behaviour), intermediate and health outcomes Q3: Physical activity, anthropometric and body composition Intermediate outcomes: Dietary intake, physical activity, body composition (FM, FFM, BMD), anthropometrics, glucose homoeostasis measures/pre-diabetes, BP, lipid profile, intermediate CVD measures (e.g., intima media thickening), CRP Intermediate outcomes must be measured before and after the trial Health outcomes: mortality, quality of life, CVD/events, type 2 diabetes, metabolic syndrome, malnutrition (overweight/obesity/underweight), anxiety disorders, depression, osteoarthritis, osteoporosis/osteopenia, joint pain |
Outcomes other than those specified. Studies examining kinetics only Q3: Outcomes other than physical activity, body composition or anthropometrics |
| Year range | Primary studies: 2005–4 May 2020 Systematic reviews/meta-analyses: 2015 – May 4, 2020 |
Primary studies: Prior to 2005 Systematic reviews: Prior to 2015 |
| Language | Published in English language | Not published in English language |
| Location | Countries with developed economies | Countries that are not economically developed(12) |
BCAA, branched chain amino acid; BMD, bone mineral density; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; FFM, fat-free mass; FM, fat mass; IBD, irritable bowel disease; PCOS, polycystic ovarian syndrome; Q1, Question 1; Q2, Question 2; Q3, Question 3; RCT, randomised controlled trial.
Three areas of nutrition and physical activity interventions were explored in this scoping review: (1) counseling or coaching, (2) mobile applications and (3) nutritional ergogenic aids. Q1 examined the efficacy of nutrition and physical activity counseling or coaching provided by a dietitian/nutritionist and/or exercise practitioner (see Table 1 for specific criteria). For Q1 inclusion, study participants must have received at least some individual-level counseling in nutrition and/or physical activity. Q2 explored the efficacy of mobile apps and other wearable technology in nutrition and physical activity interventions. For these two questions, studies were required to be controlled trials, either randomised controlled trial (RCT) or non-RCT. Q3 examined efficacy nutritional ergogenic aids deemed as commonly used in the ‘general’ population (Table 1). For Q3 only (nutritional ergogenic aids), studies were required to be placebo-controlled RCT. Additionally, for Q3, studies were limited to those reporting anthropometric, body composition and performance outcomes. For all questions, primary studies were included if they were published in 2005 or later to balance a wide breadth of evidence with relevancy of interventions to the current population. SR answering at least one of the research questions were included if published in 2015 or later, since SR published earlier than 2015 may require updated information. Included studies were limited to those published in the English language due to resource constraints.
Search plan
Search strategies were written by an Information Specialist for the following databases via the Ebsco interface: Medline Complete, CINAHL Complete, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials and Food Science Source. Searches were run on 4 and 5 May 2020. Two methodological filters were used, one for SR and meta-analyses, and another observational and other study designs. Results were limited to English language and publication year 2005 forward. Results were managed and deduplicated in Endnote Software. A sample search strategy can be found in online supplementary material, Supplemental 1.
Study selection and data extraction
Article screening was conducted in two phases. In the first phase, each title/abstract was reviewed by at least one reviewer (M.R.) and 22·4 % of title/abstracts were reviewed by a second reviewer (A.Y.) using Rayyan screening software(13). Any discrepancies between authors were discussed until consensus was reached. Communication between reviewers throughout the screening process solidified eligibility criteria. Included title/abstracts moved to the second phase of the full-text review. Prior to the full-text review, authors collaborated to create a template allowing for standardised data extraction and coding, including but not limited to: study design, sample size, population age group, activity level, health status, research question addressed including details specific to the research question (e.g., practitioner delivering the intervention for Q1) and outcomes reported. One of two reviewers (M.R. or A.Y.) reviewed the full text, determined eligibility and extracted data for included articles. The second reviewer confirmed reason for exclusion or checked accuracy of extracted data. Relevant SR were searched for eligible articles that may have been missed by the databases search.
Synthesis of results
The study selection process was documented using a PRISMA flow chart(14). Data were analysed according to the specific research question addressed and types of studies included. A bubble chart was created to demonstrate publication trends according to the sub-question addressed. For each of the three questions, a heat map was created to demonstrate density of interventions according to the population, type of intervention and/or outcomes reported. As is customary for scoping reviews, critical appraisal of study quality and meta-analyses were not conducted.
Results
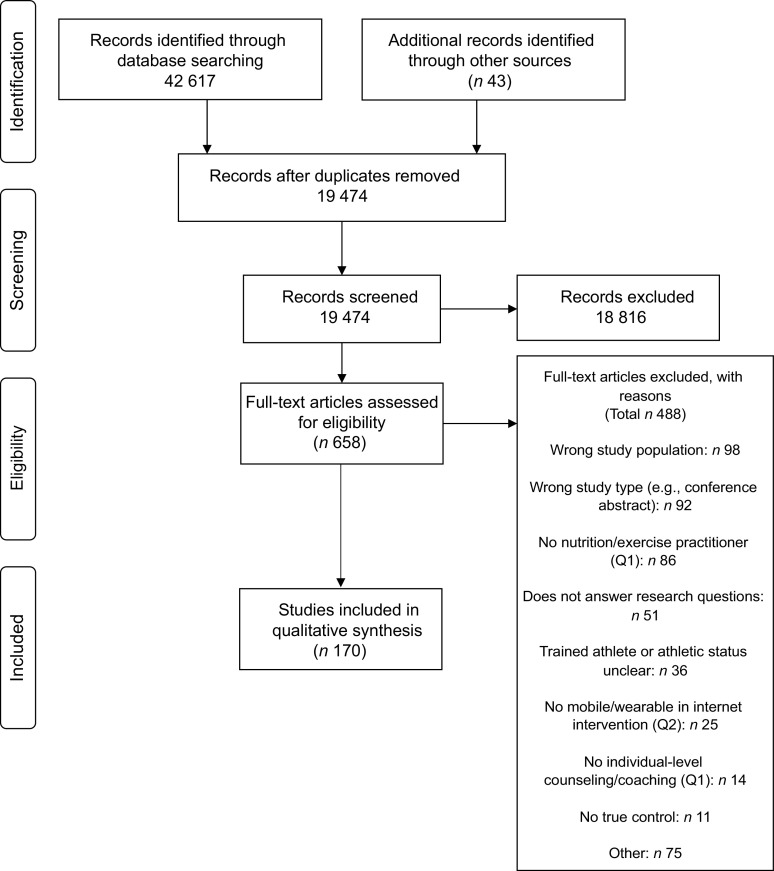
The databases and hand searches identified 19 474 unique articles. Following title/abstract screening, 657 full texts were reviewed, and 170 articles answering at least one of the research questions were included in this scoping review (Fig. 1). Eighty-three of the eighty-nine articles not meeting population criteria were specifically excluded for including participants with BMI ≥ 40 kg/m2. Of the articles included, one was an evidence-based practice guideline(15), thirty were SR(16–45), 134 were RCT(46–179) and five were non-RCT(180–184). Forty-eight of the articles were included for Q1 (counseling and coaching), thirty-seven articles were included for Q2 (mobile apps and wearables) and eighty-seven articles were included for Q3 (nutritional ergogenic aids of interest). While the rate of publication was relatively constant over the study period for articles examining nutrition and physical activity counseling and coaching (Q1), the number of primary research articles (not including SR) examining the effects of nutrition and physical activity mobile apps and/or wearables as well as nutritional ergogenic aids of interest grew considerably from approximately 2015–2020 (Fig. 2).
Fig. 1.
PRISMA flow chart(14) describing the study inclusion process for a scoping review examining the availability of studies with interventions including both nutrition and physical activity in the general population
Fig. 2.
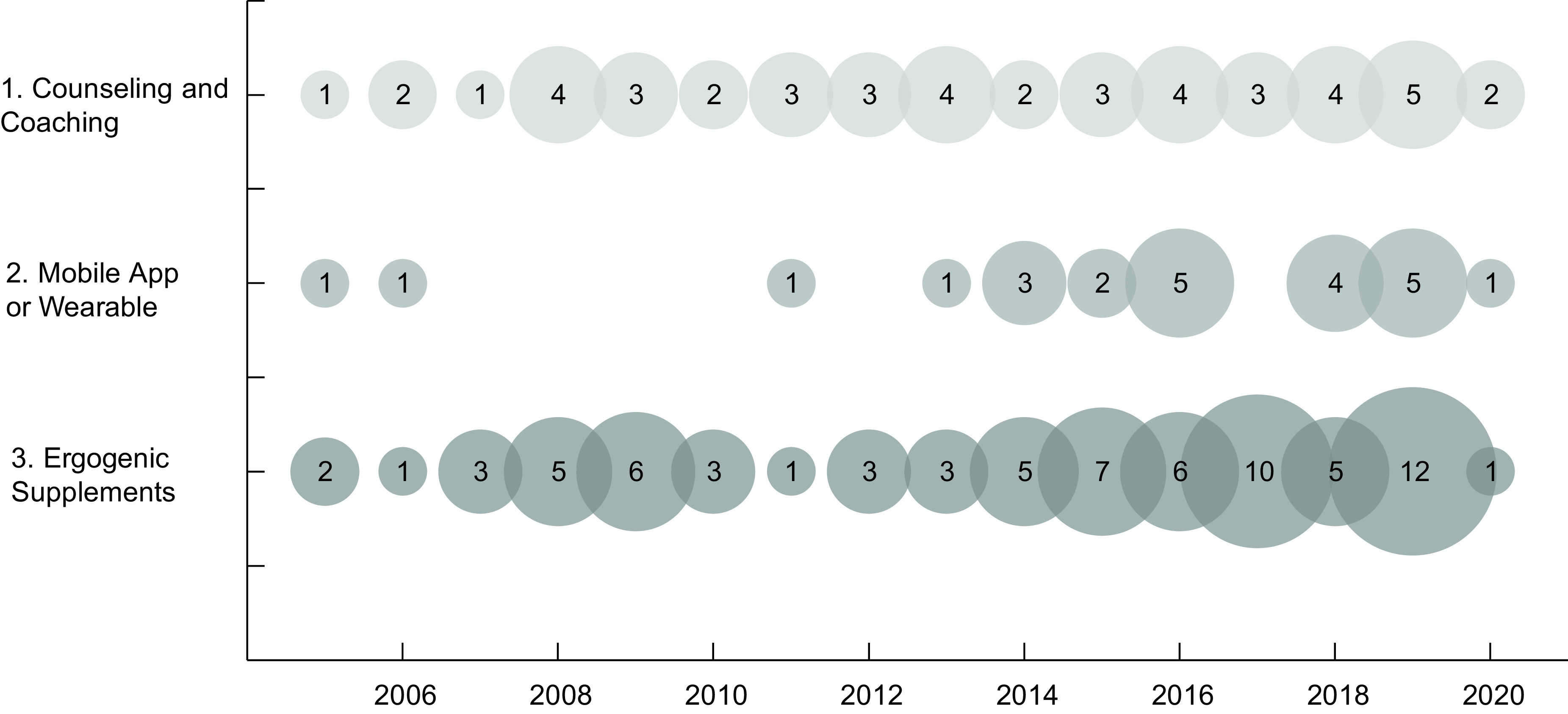
Bubble chart of publication trends in primary research articles published from 2005 to 2020 according to the research question addressed
Question 1: Individual-level nutrition and physical activity counseling or coaching provided by a dietitian/nutritionist or exercise practitioner
Forty-eight articles(15,32,46,47,49,51,52,56,69,74,76,78,83,89–91,101–104,108,109,111,120,121,128,130,133,138,139,142,147–151,154,157,160–162,169,171,172,177,180,182,184) representing thirty-eight studies met inclusion criteria and examined the effect of nutrition and physical activity counseling or coaching from a dietitian/nutritionist or exercise practitioner, including one evidence-based practice guideline, one SR, thirty-three RCT and three NRCT. The populations, intervention providers and reported outcomes are shown in Table 2. Of the thirty-three primary studies, twenty-eight targeted participants with cardiometabolic risk factors, primarily individuals with overweight or obesity. Five studies met eligibility criteria that targeted participants with cardiometabolic disease (type 2 diabetes mellitus and CVD)(46,83,104,147,161), and another five studies included participants with another morbidity, sarcopenia(69,121,149,172) and non-severe anxiety and depression(90) in four and one study, respectively. Two trials (entitled the TXT2Bfit and 40 something trials) included participants who were both at cardiometabolic risk and who did not have cardiometabolic risk factors but were at risk of weight gain(49,138,139) or were perimenopausal women(108,177). Sample sizes ranged from 28 to 11 827 participants and study durations ranged from 4 weeks to 8 years. Nutrition and physical activity counseling or coaching was provided by a dietitian/nutritionist only in fifteen studies(15,32,46,47,52,83,89,101,128,139,147,151,160,162,182), an exercise practitioner only in two studies(56,184) and both a dietitian/nutritionist and exercise practitioner in twenty-one studies(51,69,78,90,91,102,103,108,111,120,121,130,142,149,161,169,171,172,180). The greatest density of studies examined participants with cardiometabolic risk factors and interventions delivered by a dietitian/nutritionist and exercise practitioner or a dietitian/nutritionist only, and reporting anthropometric, glucose homoeostasis, blood pressure, lipid profile, dietary intake and physical activity outcomes. The one included SR reported the outcome of weight change(32). Exercise practitioners providing interventions were heterogeneous and included physiotherapists (n 4), exercise physiologists (n 7), physical trainer (n 1), physical activity ‘specialist’ or ‘coach’ (n 3), exercise or physical activity instructors (n 3) and health coaches (n 2) among others.
Table 2.
Primary studies examining the effect of nutrition and physical activity counseling/coaching according to the provider of intervention and outcomes reported (n 36 studies)
| Outcomes reported | Nutrition and exercise practitioner (n 21) | Nutrition practitioner only (n 14) | Exercise practitioner only (n 2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cardiometabolic disease risk (n 15)* | Cardiometabolic disease (n 2)† | Other morbidity (n 4) | Cardiometabolic disease risk (n 11)* | Cardiometabolic disease (n 3) | Other morbidity (n 0) | Cardiometabolic Disease Risk (n 2)* | Cardiometabolic disease (n 0) | Other morbidity (n 0) | |
| Mortality | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quality of life | 1(111) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CVD | 0 | 0 | 0 | 1(52) | 0 | 0 | 0 | 0 | 0 |
| Type 2 diabetes mellitus | 1(78) | 0 | 0 | 2(148,162) | 0 | 0 | 0 | 0 | 0 |
| Metabolic syndrome | 0 | 0 | 0 | 1(151) | 0 | 0 | 0 | 0 | 0 |
| Nutritional status | 1(103) | 0 | 0 | 0 | 0 | 0 | 1(184) | 0 | 0 |
| Anxiety/depression | 1(111) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bone health‡ | 0 | 0 | 2(69,90) | 0 | 0 | 0 | 0 | 0 | 0 |
| Anthropometrics | 13(51,78,91,102,103,109,111,120,130,169,171,177,180) | 1(104) | 3(90,121,172) | 9(47,49,89,101,139,151,160,162,182) | 3(4,147,683) | 0 | 2(56,184) | 0 | 0 |
| Body composition | 5(51,91,169,177,180) | 0 | 3(69,121,172) | 3(148,158) | 0 | 0 | 0 | 0 | 0 |
| Glucose homoeostasis | 7(51,78,109,120,130,177,180) | 0 | 1(69) | 4(47,89,151,162) | 1(46) | 0 | 1(184) | 0 | 0 |
| Blood pressure | 9(51,78,102,103,109,120,130,177,180) | 0 | 0 | 5(15,47,89,101,151) | 1(46) | 0 | 2(56,184) | 0 | 0 |
| Lipid profile | 9(51,78,102,103,109,120,130,177,180) | 0 | 0 | 6(47,89,101,151,160,182) | 2(46,83) | 0 | 1(184) | 0 | 0 |
| Inflammatory marker | 0 | 0 | 1(69) | 2(89,150) | 0 | 0 | 0 | 0 | 0 |
| Dietary intake | 11(51,78,102,103,108,109,130,142,169,171,180) | 0 | 3(90,121,172) | 6(89,101,128,139,151,160) | 1(46) | 0 | 1(184) | 0 | 0 |
| Physical activity | 12(51,78,102,103,108,109,111,120,142,169,171,180) | 0 | 2(69,172) | 7(49,89,101,128,139,151,160) | 1(46) | 0 | 1(184) | 0 | 0 |
Red colour = >5 studies, light orange colour = 1–5 studies, light yellow colour = no available studies.
Includes cardiovascular risk, type 2 diabetes mellitus risk, overweight and obesity and metabolic syndrome.
Simpson et al.(191) reported frailty index, which is not reported in the table.
Includes osteopenia, osteoporosis, osteoarthritic and bone mineral density/content.
Question 2: Nutrition and physical activity mobile apps and/or wearable technology
A total of thirty-six articles(18,19,21,22,30,34,35,37,40,42–44,48,59,80,87,94,95,97,100,106,109,113,119,123,126,129,140,141,143–145,147,176,181,183) representing thirty studies were included for Q2, which examined the effects of nutrition and physical activity mobile apps and/or wearables. Studies included were SR (n 12), RCT (n 16) and non-RCT (n 2). The populations, study designs and reported outcomes are shown in Table 3. Ten studies included participants who were healthy(22,34,37,40,80,87,94,97,123,181) and twenty-one studies included participants with cardiometabolic risk factors(18,19,21,22,34,35,40,48,59,94,95,97,100,106,109,113,126,129,140,141,183). Only six studies meeting eligibility criteria included participants with cardiometabolic diseases (type 2 diabetes mellitus and CVD)(30,42–44,147,176). Five studies included both participants who were both healthy and those who had cardiometabolic risk factors(22,34,40,94,97). Sample sizes ranged from 34 to 1007 participants and study durations ranged from 8 to 32 weeks. There were no patient-centred health outcomes reported for studies with participants who were healthy or at cardiometabolic risk. The greatest density of primary studies and SR examined individuals with cardiometabolic risk factors and reported anthropometric, dietary intake and physical activity outcomes. Six SR addressed the efficacy of mobile apps and wearables for nutrition and physical activity and were published from 2019 until the search date of 4 May 2020(18,21,34,35,40,44).
Table 3.
Heat map of controlled trials examining the effect of mobile apps and/or wearable devices for nutrition and physical activity according to the target populations and reported outcomes (n 30 studies)
| Outcome reported | Healthy (n 10) | Cardiometabolic risk (n 20)* | Cardiometabolic disease (n 6) | |||
|---|---|---|---|---|---|---|
| Controlled trials (n 7) | Systematic reviews (n 3) | Controlled trials (n 14) | Systematic reviews (n 7) | Controlled trials (n 2) | Systematic reviews (n 4) | |
| Mortality | 0 | 0 | 0 | 0 | 0 | 1(43) |
| Quality of life | 0 | 0 | 0 | 0 | 1(176) | 2(30,43) |
| CVD/events | 0 | 0 | 0 | 0 | 0 | 0 |
| Type 2 diabetes mellitus | 0 | 0 | 0 | 0 | 0 | 0 |
| Metabolic syndrome | 0 | 0 | 0 | 0 | 0 | 0 |
| Nutritional status | 0 | 0 | 0 | 0 | 0 | 0 |
| Anxiety/depression | 1(181) | 0 | 1(113) | 0 | 1(176) | 0 |
| Bone health | 0 | 0 | 0 | 0 | 0 | 0 |
| Anthropometrics | 4(80,87,95,123) | 2(37,40) | 12(48,59,95,100,106,109,113,126,129,140,141,183) | 6(18,19,21,22,35,40) | 2(147,176) | 2(43,44) |
| Body composition | 2(95,123) | 0 | 4(48,59,113,126) | 2(19,35) | 0 | 0 |
| Glucose homoeostasis | 1(97) | 1(37) | 4(48,97,109,126) | 1(35) | 1(176) | 3(42–44) |
| Blood pressure | 1(97) | 1(37) | 5(48,97,109,126,129) | 2(19,35) | 0 | 1(43) |
| Lipid profile | 1(97) | 1(37) | 4(48,97,100,109) | 1(35) | 0 | 1(42) |
| Inflammatory markers | 1(97) | 0 | 1(97) | 0 | 0 | 0 |
| Dietary intake | 4(87,94,123,181) | 2(34,37) | 8(48,94,106,109,113,140,141,183) | 4(19,21,34,35) | 0 | 1(30) |
| Physical activity | 4(87,94,123,181) | 3(34,37,40) | 9(59,94,100,106,109,113,126,140,141) | 7(18,19,21,22,34,35,40) | 0 | 2(30,43) |
| Cost effectiveness | 0 | 0 | 0 | 0 | 0 | 0 |
Red colour = >5 studies, light red colour = 1–5 studies, blue colour = no available studies.
Includes CVD risk, type 2 diabetes mellitus risk, overweight and obesity and metabolic syndrome.
Question 3: Nutritional ergogenic aids
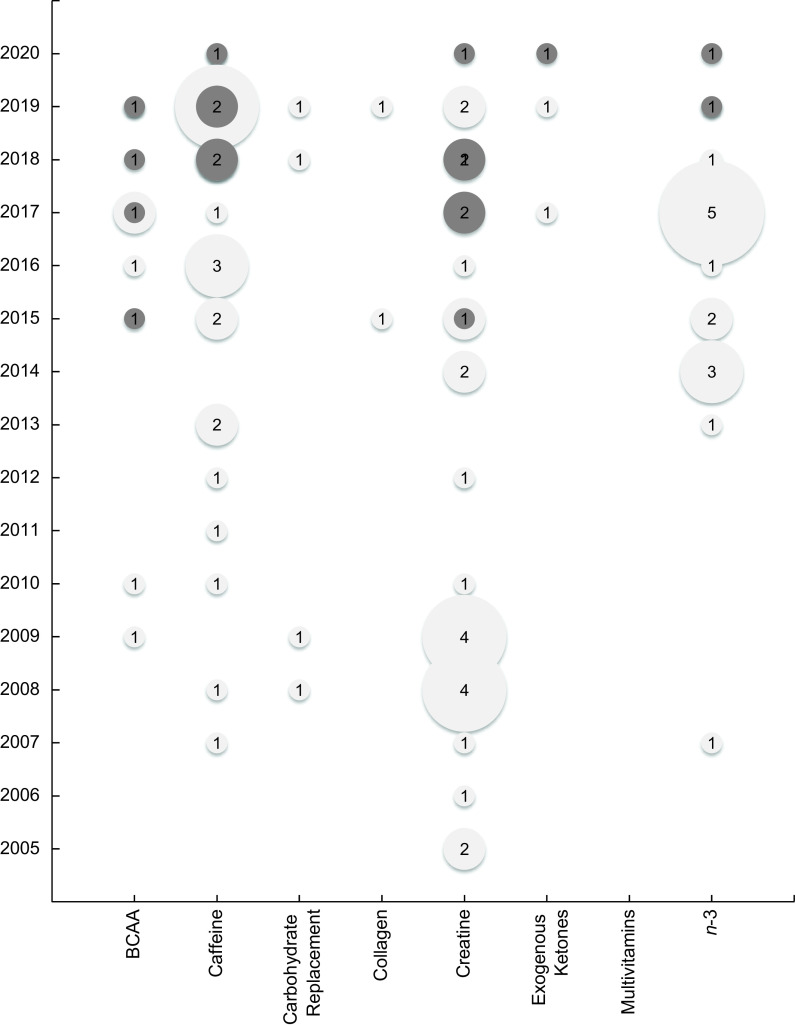
A total of eighty-seven articles, including seventeen SR(16,17,20,23–29,31,33,36,38,39,41,45) and seventy placebo-controlled RCT(50,53–55,57,58,60–68,70–73,75,77,79,81,82,84–86,88,92,93,96,98,99,105,107,110,112,114–118,122,124,125,127,131,132,134–137,146,152,153,155–157,159,163–168,170,173,175,178,179), examined the effect of nutritional ergogenic aids on physical activity, anthropometric and body composition outcomes. Sample sizes ranged from 10 to 118 participants and study durations ranged from 1 d to 1 year. Nearly all included articles focused on one dietary supplement of interest (branched chain amino acids, caffeine, carbohydrate replacement, collagen, creatine, exogenous ketones, multivitamins and n-3 fatty acids), with the exception of one RCT that assessed both creatine and carbohydrate supplementation(118) and one SR that assessed both creatine and the branched chain amino acid leucine(17).The most frequently examined ergogenic aid was creatine (n 6 SR(17,20,23,28,29,39) and 22 RCT(55,57,60–67,72,75,79,86,88,98,105,117,118,164,165,179)), followed by caffeine (n 5 SR(25–27,36,38) and 20 RCT(54,58,68,70,81,84,96,122,124,131,134,136,152,153,155,157,166–168,175)). There were no SR available for carbohydrate replacement (n 4 RCT(50,53,85,118)) or collagen (n 2 RCT(116,178)) in non- or recreational athletes. There were four SR(17,24,33,45) and six RCT(92,93,132,146,159,173) that focused on branched chain amino acids (primarily leucine); one SR(41) and two RCT(114,137) examined the effect of exogenous ketones; and two SR(16,31) and fifteen RCT(71,73,77,82,99,107,110,112,115,125,127,135,156,163,170) examined the effect of n-3 fatty acid supplementation. There were no placebo-controlled RCT or SR identified that evaluated the effect of multivitamins in the population of interest. Table 4 displays a heat map of the distribution of outcomes assessed in RCT and SR for each ergogenic aid of interest. Of the seventy included RCT, only two did not assess exercise/performance outcomes; one examined creatine(86) and that the other on n-3 fatty acids(107). None of the included RCT measured physical activity outcomes using metabolic equivalents of task and only two of the SRs assessed metabolic equivalents of task as an outcome measure of interest(31,33). For the nutritional ergogenic aids caffeine, creatine and n-3 fatty acid supplements, SR published in 2019 and 2020 were available (Fig. 3).
Table 4.
Heat map of placebo-controlled randomised controlled trials and systematic reviews examining the effect of ergogenic aids according to the supplement and reported outcomes (n 87 studies)
| Nutritional ergogenic supplement | Study design | Exercise/performance outcome | Anthropometric outcome | Body composition outcome |
|---|---|---|---|---|
| Branched chain amino acids (n 10) | RCT (n 6) | 6(92,93,132,146,159,173) | 2(93,132) | 3(93,132,173) |
| Systematic reviews (n 4) | 3(17,24,33) | 0 | 3(17,33,45) | |
| Caffeine (n 25) | RCT (n 20) | 20(54,58,68,70,81,84,96,122,124,131,134,136,152,153,155,157,166–168,175) | 0 | 0 |
| Systematic reviews (n 5) | 5(25–27,36,38) | 0 | 0 | |
| Carbohydrate replacement (n 4) | RCT (n 4) | 4(50,53,85,118) | 1(118) | 0 |
| Systematic reviews (n 0) | 0 | 0 | 0 | |
| Collagen (n 2) | RCT (n 2) | 2(116,178) | 2(116,178) | 2(116,178) |
| Systematic reviews (n 0) | 0 | 0 | 0 | |
| Creatine (n 28) | RCT (n 22) | 21(55,57,60–67,72,75,79,88,98,105,117,118,164,165,179) | 10(60,61,72,86,88,98,105,118,164,179) | 13(55,60–63,67,72,75,86,88,117,164,165) |
| Systematic reviews (n 6) | 4(17,28,29,39) | 0 | 4(17,20,23,39) | |
| Exogenous ketones (n 3) | RCT (n 2) | 2(114,137) | 0 | 0 |
| Systematic reviews (n 1) | 1(41) | 0 | 0 | |
| Multivitamins (n 0) | RCT (n 0) | 0 | 0 | 0 |
| Systematic reviews (n 0) | 0 | 0 | 0 | |
| n-3 (n 17) | RCT (n 15) | 14(71,73,77,82,99,110,112,115,125,127,135,156,163,170) | 5(82,107,115,127,163) | 5(73,77,127,163,192) |
| Systematic reviews (n 2) | 2(16,31) | 1(31) | 1(16) |
Red colour = >5 studies identified; orange colour = 1–5 studies identified; light yellow colour = no studies identified.
Fig. 3.
Bubble chart of placebo-controlled randomised controlled trials and systematic reviews published by year and by ergogenic aid. The bubble size is proportional to the number of studies published in the year for each ergogenic aid.  , RCT;
, RCT;  , SR
, SR
Discussion
This scoping review included 170 primary and secondary research articles that examined the effect of nutrition and physical activity interventions in individuals who were non-athletes or recreational athletes and who were healthy or had cardiometabolic risk. While primary research has been consistently available on the effect of individual-level nutrition and physical activity counseling or coaching from a dietitian or exercise practitioner, there has been little synthesis of these data in the 5 years of SR (2015–2020) examined. SR published prior to 2015 may be valuable for practice(185), but practitioners should be mindful that new evidence may shift conclusions. Additionally, newer SR may be more relevant to current circumstances (e.g., need for remote coaching/counseling during the COVID-19 pandemic). Mobile applications designed to improve nutrition and physical activity had been addressed in several primary studies over the past 5 years; these studies have been well-represented in SR. Regarding nutritional ergogenic aids of interest, recent SR were available for the supplements with relatively high publication activity (caffeine, creatine and n-3 fatty acids), particularly for the outcome of exercise performance. However, other commonly used ergogenic aids have relatively few SR available to guide practice.
Question 1: Individual-level nutrition and physical activity counseling or coaching provided by a dietitian/nutritionist or exercise practitioner
Prior education, experience, methodologies and assessment techniques can differ significantly among practitioners delivering nutrition and physical activity interventions. Studies in this scoping review included a range of practitioners providing nutrition and exercise counseling or coaching, particularly among exercise practitioners. In addition, state and federal regulations for scope of practice vary, potentially allowing less-than-qualified practitioners to provide nutrition and/or physical activity guidance. While decreasing standards may increase accessibility, there is also risk of lower quality care and, therefore, lower intervention efficacy when care is provided by non-qualified practitioners. Examining how provider qualifications impact outcomes may inform scope of practice for both dietitian/nutritionists and exercise practitioners working with different sub-groups of the ‘general’ population. For example, those with cardiometabolic disease or risk factors for cardiometabolic disease may require medical nutrition therapy provided by a Registered Dietitian, while direct coaching from an exercise practitioner may be required for individuals who are sedentary and/or have little exercise history. There were no studies included that investigated the effect on an intervention in individuals that had no cardiometabolic risk factors or disease. Most available primary studies investigated individuals with cardiometabolic risk, such as those with overweight or obesity, and investigated intermediate outcomes such as anthropometric measures, blood pressure, lab values and behavioural outcomes, which would indicate the prevention of progression towards cardiometabolic disease. A SR on the effects of nutrition and physical activity interventions in individuals with no risk factors may yield few results. However, signs and symptoms of cardiometabolic risk, such as incidence of overweight and pre-diabetic levels of fasting blood glucose, may overlap. Thus, in SR, it may be beneficial to group individuals with cardiometabolic risk factors, but without diagnosed disease.
The United States Preventative Task Force recently conducted a SR on the effect of behaviour counseling for nutrition and physical activity for individuals with cardiovascular risk on CVD outcomes(186). The current working version describes a beneficial effect on cardiovascular events, adiposity-related outcomes and many other health outcomes(187). The current scoping review focused on interventions delivered by nutrition and/or exercise practitioners specifically and included a broader range of participants. SR examining differences in outcomes according to the practitioner delivering the intervention can inform health care providers of the most effective methods to improve dietary intake and physical activity behaviours.
Question 2: Nutrition and physical activity mobile apps and wearable technology
Most studies examining the effectiveness of mobile apps in improving cardiometabolic risk factors have reported outcomes relating to energy intake, storage and output (dietary intake, anthropometrics and physical activity, respectively, Table 3). Fewer studies have assessed the influence of apps on treating those with cardiometabolic conditions, such as type 2 diabetes mellitus and CVD. This discrepancy may be intentional to curtail liability from self-diagnosis or self-treatment based on data or guidance from the app itself and in the absence of a qualified nutrition or exercise practitioner. However, several SR targeting individuals who are healthy or who have cardiometabolic risk factors are available to guide practitioners on the efficacy of utilising mobile apps with clients(18,19,21,22,30,34,35,37,40,42–44). Studies investigating individuals without cardiometabolic disease may offer valuable insights in broad-scale interventions implemented prior to individuals experiencing adverse symptoms of cardiometabolic risk and disease. Like the question investigating nutrition and physical activity counseling or coaching (Question 1), the highest density of evidence available examined individuals with cardiometabolic risk factors. These interventions most frequently reported outcomes that would indicate improved behaviours and intermediate outcomes that may indicate the prevention of cardiometabolic disease.
Use of and technology related to smartphone applications and forms of telehealth will likely continue to advance(188,189), particularly in light of the need for remote interventions due to the COVID-19 pandemic. Thus, the number of available studies in this domain may require further synthesis including examination of effective app components, differences between apps that simply track behaviour or biomarker data compared with those which provide recommendations and differences in apps developed directly by medical providers (hospitals, insurance providers) v. third-party companies.
Question 3: Nutritional ergogenic aids
Participants were healthy individuals without cardiometabolic risk factors in all except two studies investigating the effect of ergogenic aids(107,178). The greatest availability of research on nutritional ergogenic aids was for creatine, caffeine and n-3 fatty acids. Individuals typically use creatine to increase strength and power and may be of particular relevance to older individuals seeking to maintain or build strength, function and potentially cognition. While primary research on creatine as an ergogenic aid has waned in recent years, several SR have been published from 2017 to 2020, including in the ageing population(17,20,23,28,29,39), and these can be used as resources to guide practitioner advice on creatine supplementation. There is more availability of recent studies examining caffeine(26,27,36,38,190) and n-3 fatty acids(16,31) as ergogenic aids, but these have also been investigated in recent SR as recently as the year this search was conducted. When interpreting this evidence, practitioners should consider if the outcomes of interest align with the performance goals of the client including increased time spent exercising, enhanced endurance, strength or decreased pain. While little of the included research targeted individuals with cardiometabolic risk factors, the use of nutritional ergogenic aids may be common in these individuals to improve exercise endurance and capacity. Thus, when working with individuals with cardiometabolic risk factors, practitioners should consider how to appropriately interpret and modify conclusions and recommendations for clients.
Strengths and limitations
This scoping review had rigorous methods and comprehensively described interventions including both nutrition and physical activity. Another strength of this scoping review was inclusion of populations with a range of cardiometabolic risk that may be representative of the population in economically developed countries, such as the USA. This included individuals who were healthy, overweight or obese, or with cardiometabolic disease. However, the authors did set the parameter that studies would be excluded if they included participants with a BMI of ≥40 kg/m2, with the intention that this relatively arbitrary line may be a proxy for the point at which medical interventions may be necessary beyond ‘standard’ diet and exercise. This is evident in the few studies included that focused on individuals with cardiometabolic disease; most of which included some participants with BMI ≥ 40 kg/m2 and were thus excluded. Future studies may elucidate more relevant measures to stratify individuals who have therapeutic v. ‘general’ needs. Due to the wide breadth of nutrition and physical activity interventions, it was necessary to categorise populations, interventions and outcomes very broadly, thus masking heterogeneity between these studies. Future SR should consider how efficacy of interventions vary according to an individual’s cardiometabolic risk factors, diet and physical activity history and ability, and methods of data collection for dietary intake and physical activity outcomes. Improving understanding of how early interventions may prevent onset or progression of cardiometabolic risk factors prior to disease onset would allow for a development of a framework describing how interventions can be effectively individualised to specific clients but implemented on a broad scale. Increased attention to and rigor of data collection methods, including for dietary and physical activity behaviours, will improve quality of and certainty in evidence to inform practice.
Additional limitations of this scoping review were inclusion of evidence published in the English language only, which may have resulted in missing relevant studies published in other languages, and not all titles/abstracts were screened by two reviewers due to resource constraints and the wide breadth of evidence identified on the topic of interest. These limitations may have resulted in missing relevant articles published on the topics of interest. Also, while this scoping review aimed to identify primary studies published in the 15 years prior to the search and SR published in the 5 years prior to the search, as mentioned, earlier evidence may still be relevant and helpful to practitioners.
Conclusion
Interventions to improve or maintain both nutrition and physical activity can provide clients with the knowledge, skills and tools needed to prevent and treat cardiometabolic risk factors and disease. Several recent SR on the efficacy of nutrition and physical activity mobile apps and nutritional ergogenic aids can serve as evidence-based resources for health practitioners. Though consistent literature has been published examining the effect of providing nutrition and exercise counseling by practitioners in these fields, this evidence has not been synthesised. SR of these targeted interventions may inform scope of practice for dietitians and exercise practitioners working with individuals who are healthy or who have cardiometabolic risk factors. More research is needed examining the long-term effects of nutrition and physical activity interventions on patient-centred health outcomes.
Acknowledgements
Acknowledgements: The authors would like to acknowledge Janet Peterson, Dr PH, RDN, RCEP, WEMT, FACSM for her content expertise and contribution to developing the research questions and eligibility criteria. Financial support: This scoping review was supported by the Academy of Nutrition and Dietetics and the American Council on Exercise (no grant numbers). Conflicts of interest: M.R. is employed by the Academy of Nutrition and Dietetics. J.R. has provided contracting services with the American Council on Exercise. K.J. consults for US Highbush Blueberry Council, The Wonderful Company, Clif Bar & Co, Honey Stinger and NOW Foods. The authors have no other conflicts of interest to disclose. Authorship: All authors contributed to the development of the research question and sub-questions as well as eligibility criteria. M.R. and A.Y. screened article title/abstracts and full texts, extracted data and synthesised evidence. M.R., J.R. and K.J. wrote the first draft of the manuscript and all authors thoroughly reviewed and edited the manuscript and approve of the version submitted. Ethics of human subject participation: not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021002184.
click here to view supplementary material
References
- 1. World Health Organization (2014) Noncommunicable Disease Country Profiles 2014. Geneva, Switzerland: World Health Organization; available at https://apps.who.int/iris/bitstream/handle/10665/128038/9789241507509_eng.pdf;jsessionid=90C284D1B7F4B79C81A3CD363C6F02E3?sequence=1 (accessed May 2021).
- 2. Saboya PP, Bodanese LC, Zimmermann PR et al. (2016) Metabolic syndrome and quality of life: a systematic review. Rev Lat Am Enfermagem 24, e2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Muntner P, Alonso A et al. (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139, e56–e528. [DOI] [PubMed] [Google Scholar]
- 4. Carnethon MR (2009) Physical activity and cardiovascular disease: how much is enough? Am J Lifestyle Med 3, 44s–49s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. United States Department of Agriculture (2015) Dietary Guidelines for Americans. Chapter 2: Shifts Needed To Align With Healthy Eating Patterns. https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/chapter-2/current-eating-patterns-in-the-united-states/ (accessed May 2021).
- 6. Centers for Disease Control and Prevention (2019) QuickStats: percentage of adults who met federal guidelines for aerobic physical activity through leisure-time activity, by race/ethnicity – national health interview survey, 2008–2017. MMWR Morb Mortal Wkly Rep 68, 292. doi: 10.15585/mmwr.mm6812a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peters MDJ, Godfrey C, McInerney P et al. (2020) Chapter 11: scoping Reviews (2020 version). In JBI Manual for Evidence Synthesis [Aromataris E & Munn Z, editors]. Adelaide, Australia: JBI. [Google Scholar]
- 8. Arksey H & O’Malley L (2005) Scoping studies: towards a methodological framework. Int J Soc Res Methodol 8, 19–32. [Google Scholar]
- 9. Levac D, Colquhoun HO’ & Brien KK (2010) Scoping studies: advancing the methodology. Implement Sci 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rozga M (2020) Nutrition and Physical Activity Interventions for the General Population: an Academy of Nutrition and Dietetics and ACE Scoping Review. osf.io/pc6sy (accessed July 2020).
- 11. Tricco AC, Lillie E, Zarin W et al. (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169, 467–473. [DOI] [PubMed] [Google Scholar]
- 12. United Nations (2014) Country classification. https://www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf (accessed July 2020).
- 13. Ouzzani M, Hammady H, Fedorowicz Z et al. (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lennon SL, DellaValle DM, Rodder SG et al. (2017) 2015 Evidence analysis library evidence-based nutrition practice guideline for the management of hypertension in adults. J Acad Nutr Diet 117, 1445.e1417–1458.e1417. [DOI] [PubMed] [Google Scholar]
- 16. Abdelhamid A, Hooper L, Sivakaran R et al. (2019) The relationship between n-3, n-6 and total polyunsaturated fat and musculoskeletal health and functional status in adults: a systematic review and meta-analysis of RCTs. Calc Tissue Int 105, 353–372. [DOI] [PubMed] [Google Scholar]
- 17. Beaudart C, Rabenda V, Simmons M et al. (2018) Effects of protein, essential amino acids, B-hydroxy B-methylbutyrate, creatine, dehydroepiandrosterone and fatty acid supplementation on muscle mass, muscle strength and physical performance in older people aged 60 years and over. A systematic review of the literature. J Nutr Health Aging 22, 117–130. [DOI] [PubMed] [Google Scholar]
- 18. Dounavi K & Tsoumani O (2019) Mobile health applications in weight management: a systematic literature review. Am J Prev Med 56, 894–903. [DOI] [PubMed] [Google Scholar]
- 19. Cheatham SW, Stull KR, Fantigrassi M et al. (2018) The efficacy of wearable activity tracking technology as part of a weight loss program: a systematic review. J Sports Med Phys Fitness 58, 534–548. [DOI] [PubMed] [Google Scholar]
- 20. Chilibeck PD, Kaviani M, Candow DG et al. (2017) Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med 8, 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim H-N & Seo K (2020) Smartphone-based health program for improving physical activity and tackling obesity for young adults: a systematic review and meta-analysis. Int J Environ Res Public Health 17, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flores Mateo G, Granado-Font E, Ferré-Grau C et al. (2015) Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta-analysis. J Med Internet Res 17, e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forbes SC, Chilibeck PD & Candow DG (2018) Creatine supplementation during resistance training does not lead to greater bone mineral density in older humans: a brief meta-analysis. Front Nutr 5, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fouré A & Bendahan D (2017) Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients 9, 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grgic J (2018) Caffeine ingestion enhances Wingate performance: a meta-analysis. Eur J Sport Sci 18, 219–225. [DOI] [PubMed] [Google Scholar]
- 26. Grgic J & Pickering C (2019) The effects of caffeine ingestion on isokinetic muscular strength: a meta-analysis. J Sci Med Sport 22, 353–360. [DOI] [PubMed] [Google Scholar]
- 27. Grgic J, Grgic I, Pickering C et al. (2019) Wake up and smell the coffee: caffeine supplementation and exercise performance-an umbrella review of 21 published meta-analyses. Br J Sports Med 54, 681–688. [DOI] [PubMed] [Google Scholar]
- 28. Lanhers C, Pereira B, Naughton G et al. (2015) Creatine supplementation and lower limb strength performance: a systematic review and meta-analyses. Sports Med 45, 1285–1294. [DOI] [PubMed] [Google Scholar]
- 29. Lanhers C, Pereira B, Naughton G et al. (2017) Creatine supplementation and upper limb strength performance: a systematic review and meta-analysis. Sport Med 47, 163–173. [DOI] [PubMed] [Google Scholar]
- 30. Lunde P, Nilsson BB, Bergland A et al. (2018) The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: systematic review and meta-analyses. J Med Internet Res 20, e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng-Tao L, Zhang JM, Zhu WT (2020) n-3 Polyunsaturated fatty acid supplementation for reducing muscle soreness after eccentric exercise: a systematic review and meta-analysis of randomized controlled trials. BioMed Res Int 2020, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maciejewski ML, Shepherd-Banigan M, Raffa SD et al. (2018) Systematic review of behavioral weight management program move! for veterans. AmJ Prev Med 54, 704–714. [DOI] [PubMed] [Google Scholar]
- 33. Martínez-Arnau FM, Fonfría-Vivas R & Cauli O (2019) Beneficial effects of leucine supplementation on criteria for sarcopenia: a systematic review. Nutrients 11, 2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Milne-Ives M, Lam C, De Cock C et al. (2020) Mobile apps for health behavior change in physical activity, diet, drug and alcohol use, and mental health: systematic review. JMIR mHealth uHealth 8, e17046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puigdomenech Puig E, Robles N, Saigí-Rubió F et al. (2019) Assessment of the efficacy, safety, and effectiveness of weight control and obesity management mobile health interventions: systematic review. JMIR mHealth uHealth 7, e12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raya-González J, Rendo-Urteaga T, Domínguez R et al. (2020) Acute effects of caffeine supplementation on movement velocity in resistance exercise: a systematic review and meta-analysis. Sports Med 50, 717–729. [DOI] [PubMed] [Google Scholar]
- 37. Schoeppe S, Alley S, Van Lippevelde W et al. (2016) Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act 13, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Southward K, Rutherfurd-Markwick KJ & Ali A (2018) Correction to: the effect of acute caffeine ingestion on endurance performance: a systematic review and meta-analysis. Sports Med 48, 2425–2441. [DOI] [PubMed] [Google Scholar]
- 39. Stares A & Bains M (2020) The additive effects of creatine supplementation and exercise training in an aging population: a systematic review of randomized controlled trials. J Geriatr Phys Ther 43, 99–112. [DOI] [PubMed] [Google Scholar]
- 40. Sypes EE, Newton G & Lewis ZH (2019) Investigating the use of an electronic activity monitor system as a component of physical activity and weight-loss interventions in nonclinical populations: a systematic review. J Phys Act Health 16, 294–302. [DOI] [PubMed] [Google Scholar]
- 41. Valenzuela PL, Morales JS, Castillo-García A et al. (2020) Acute ketone supplementation and exercise performance: a systematic review and meta-analysis of randomized controlled trials. Int J Sports Physiol Perform, 1–11. doi: 10.1123/ijspp.2019-0918. [DOI] [PubMed] [Google Scholar]
- 42. Veazie S, Winchell K, Gilbert J et al. (2018) Rapid evidence review of mobile applications for self-management of diabetes. J Gen Intern Med 33, 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Veazie S, Winchell K, Gilbert J et al. (2018) Mobile applications for self-management of diabetes. J Med Syst 40, 210. [PubMed] [Google Scholar]
- 44. Wu X, Guo X & Zhang Z (2019) The efficacy of mobile phone apps for lifestyle modification in diabetes: systematic review and meta-analysis. JMIR mHealth uHealth 7, e12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu Z-R, Tan Z-J, Zhang Q et al. (2015) The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: a systematic review and meta-analysis. Br J Nutr 113, 25–34. [DOI] [PubMed] [Google Scholar]
- 46. Adachi M, Yamaoka K, Watanabe M et al. (2013) Effects of lifestyle education program for type 2 diabetes patients in clinics: a cluster randomized controlled trial. BMC Public Health 13, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Admiraal WM, Vlaar EM, Nierkens V et al. (2013) Intensive lifestyle intervention in general practice to prevent type 2 diabetes among 18 to 60-year-old South Asians: 1-year effects on the weight status and metabolic profile of participants in a randomized controlled trial. PLoS One 8, e68605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aktas MF, Mähler A, Hamm M et al. (2019) Lifestyle interventions in Muslim patients with metabolic syndrome-a feasibility study. Eur J Clin Nutr 73, 805–808. [DOI] [PubMed] [Google Scholar]
- 49. Allman-Farinelli M, Partridge SR, McGeechan K et al. (2016) A mobile health lifestyle program for prevention of weight gain in young adults (TXT2BFiT): 9-month outcomes of a randomized controlled trial. JMIR mHealth uHealth 4, e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andersson-Hall U, Pettersson S, Edin F et al. (2018) metabolism and whole-body fat oxidation following postexercise carbohydrate or protein intake. Int J Sport Nutr Exerc Metabol 28, 37–45. [DOI] [PubMed] [Google Scholar]
- 51. Arciero RJ, Gentile CL, Martin-Pressman R et al. (2006) Increased dietary protein and combined high intensity aerobic and resistance exercise improves body fat distribution and cardiovascular risk factors. Int J Sport Nutr Exerc Metab 16, 373–392. [DOI] [PubMed] [Google Scholar]
- 52. Aro A, Kauppinen A, Kivinen N et al. (2019) Life style intervention improves retinopathy status—the Finnish diabetes prevention study. Nutrients 11, 1691–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ballard TP, Melby CL, Camus H et al. (2009) Effect of resistance exercise, with or without carbohydrate supplementation, on plasma ghrelin concentrations and postexercise hunger and food intake. Metabolism 58, 1191–1199. [DOI] [PubMed] [Google Scholar]
- 54. Bazzucchi I, Felici F, Montini M et al. (2011) Caffeine improves neuromuscular function during maximal dynamic exercise. Muscle Nerve 43, 839–844. [DOI] [PubMed] [Google Scholar]
- 55. Bemben MG, Witten MS, Carter JM et al. (2010) The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging 14, 155–159. [DOI] [PubMed] [Google Scholar]
- 56. Bennett GG, Herring SJ, Puleo E et al. (2010) Web-based weight loss in primary care: a randomized controlled trial. Obesity 18, 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bernat P, Candow DG, Gryzb K et al. (2019) Effects of high-velocity resistance training and creatine supplementation in untrained healthy aging males. Appl Physiol Nutr Metab 44, 1246–1253. [DOI] [PubMed] [Google Scholar]
- 58. Black CD, Waddell DE & Gonglach AR (2015) Caffeine’s ergogenic effects on cycling: neuromuscular and perceptual factors. Med Sci Sports Exerc 47, 1145–1158. [DOI] [PubMed] [Google Scholar]
- 59. Byrne NM, Meerkin JD, Laukkanen R et al. (2006) Weight loss strategies for obese adults: personalized weight management program v. standard care. Obesity 14, 1777–1788. [DOI] [PubMed] [Google Scholar]
- 60. Burke DG, Candow DG, Chilibeck PD et al. (2008) Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int J Sport Nutr Exerc Metab 18, 389–398. [DOI] [PubMed] [Google Scholar]
- 61. Camic CL, Housh TJ, Zuniga JM et al. (2014) The effects of polyethylene glycosylated creatine supplementation on anaerobic performance measures and body composition. J Strength Cond Res 28, 825–833. [DOI] [PubMed] [Google Scholar]
- 62. Candow DG, Little JP, Chilibeck PD et al. (2008) Low-dose creatine combined with protein during resistance training in older men. Med Sci Sports Exerc 40, 1645–1652. [DOI] [PubMed] [Google Scholar]
- 63. Candow DG, Vogt E, Johannsmeyer S et al. (2015) Strategic creatine supplementation and resistance training in healthy older adults. Appl Physiol Nutr Metab 40, 689–694. [DOI] [PubMed] [Google Scholar]
- 64. Carter JM, Bemben DA, Knehans AW et al. (2005) Does nutritional supplementation influence adaptability of muscle to resistance training in men aged 48 to 72 years. J Geriatr Phys Ther 28, 40–47. [DOI] [PubMed] [Google Scholar]
- 65. Chami J & Candow DG (2019) Effect of creatine supplementation dosing strategies on aging muscle performance. J Geriatr Phys Ther 23, 281–285. [DOI] [PubMed] [Google Scholar]
- 66. Chilibeck PD, Chrusch MJ, Chad KE et al. (2005) Creatine monohydrate and resistance training increase bone mineral content and density in older men. J Nutr Health Aging 9, 352–353. [PubMed] [Google Scholar]
- 67. Chilibeck PD, Candow DG, Landeryou T et al. (2015) Effects of creatine and resistance training on bone health in postmenopausal women. Med Sci Sports Exerc 47, 1587–1595. [DOI] [PubMed] [Google Scholar]
- 68. Church DD, Hoffman JR, LaMonica MB et al. (2015) The effect of an acute ingestion of Turkish coffee on reaction time and time trial performance. J Int Soc Sports Nutr 12, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Colleluori G, Napoli N, Phadnis U et al. (2017) Effect of weight loss, exercise, or both on undercarboxylated osteocalcin and insulin secretion in frail, obese older adults. Oxid Med Cell Longev 2017, 4807046–4807046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Collier NB, Hardy MA, Millard-Stafford ML et al. (2016) Small beneficial effect of caffeinated energy drink ingestion on strength. J Strength Condi Res 30, 1862–1870. [DOI] [PubMed] [Google Scholar]
- 71. Corder KE, Newsham KR, McDaniel JL et al. (2016) Effects of short-term docosahexaenoic acid supplementation on markers of inflammation after eccentric strength exercise in women. J Sports Sci Med 15, 176–183. [PMC free article] [PubMed] [Google Scholar]
- 72. Cornish SM, Candow DG, Jantz NT et al. (2009) Conjugated linoleic acid combined with creatine monohydrate and whey protein supplementation during strength training. Int J Sport Nutr Exerc Metab 19, 79–96. [DOI] [PubMed] [Google Scholar]
- 73. Cornish SM, Myrie SB, Bugera EM et al. (2018) n-3 Supplementation with resistance training does not improve body composition or lower biomarkers of inflammation more so than resistance training alone in older men. Nutr Res 60, 87–95. [DOI] [PubMed] [Google Scholar]
- 74. Corpeleijn E, Feskens EJM, Jansen EHJM et al. (2006) Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia 49, 2392–2401. [DOI] [PubMed] [Google Scholar]
- 75. Cooke MB, Brabham B, Buford TW et al. (2014) Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur J Appl Physiol 114, 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Corpeleijn E, Feskens EJM, Jansen EHJM et al. (2007) Lifestyle intervention and adipokine levels in subjects at high risk for type 2 diabetes: the study on lifestyle intervention and impaired glucose tolerance Maastricht (SLIM). Diabetes Care 30, 3125–3127. [DOI] [PubMed] [Google Scholar]
- 77. Da Boit M, Sibson R, Sivasubramaniam S et al. (2017) Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am J Clin Nutr 105, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dale KS, Mann JI, McAuley KA et al. (2009) Sustainability of lifestyle changes following an intensive lifestyle intervention in insulin resistant adults: follow-up at 2-years. Asia Pac J Clin Nutr 18, 114–120. [PubMed] [Google Scholar]
- 79. Dalton RL, Sowinski RJ, Grubic TJ et al. (2017) Hematological and hemodynamic responses to acute and short-term creatine nitrate supplementation. Nutrients 9, 1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Day RS, Jahnke SA, Haddock CK et al. (2019) Occupationally tailored, web-based, nutrition and physical activity program for firefighters: cluster randomized trial and weight outcome. J Occup Environ Med 61, 841–848. [DOI] [PubMed] [Google Scholar]
- 81. Demura S, Yamada T & Terasawa N (2007) Effect of coffee ingestion on physiological responses and ratings of perceived exertion during submaximal endurance exercise. Percept Mot Skills 105, 1109–1116. [DOI] [PubMed] [Google Scholar]
- 82. DiLorenzo FM, Drager CJ & Rankin JW (2014) Docosahexaenoic acid affects markers of inflammation and muscle damage after eccentric exercise. J Strength Cond Res 28, 2768–2774. [DOI] [PubMed] [Google Scholar]
- 83. Droste DW, Iliescu C, Vaillant M et al. (2013) A daily glass of red wine associated with lifestyle changes independently improves blood lipids in patients with carotid arteriosclerosis: results from a randomized controlled trial. Nutr J 12, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Del Coso J, Salinero JJ, González-Millán C et al. (2012) Dose response effects of a caffeine-containing energy drink on muscle performance: a repeated measures design. J Int Soc Sports Nutr 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dupuy O & Tremblay J (2019) Impact of carbohydrate ingestion on cognitive flexibility and cerebral oxygenation during high-intensity intermittent exercise: a comparison between maple products and usual carbohydrate solutions. Nutrients 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Eliot KA, Knehans AW, Bemben DA et al. (2008) The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J Nutr Health Aging 12, 208–212. [DOI] [PubMed] [Google Scholar]
- 87. Epton T, Norman P, Dadzie A-S et al. (2014) A theory-based online health behaviour intervention for new university students (U@Uni): results from a randomised controlled trial. BMC Public Health 14, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ferguson TB & Syrotuik DG (2006) Effects of creatine monohydrate supplementation on body composition and strength indices in experienced resistance trained women. J Strength Cond Res 20, 939–946. [DOI] [PubMed] [Google Scholar]
- 89. Fernández-García JC, Martínez-Sánchez MA, Bernal-López MR et al. (2020) Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on the serum polyamine metabolome in individuals at high cardiovascular disease risk: a randomized clinical trial. Am J Clin Nutr, nqaa064. doi: 10.1093/ajcn/nqaa064. [DOI] [PubMed] [Google Scholar]
- 90. Forsyth A, Deane FP & Williams P (2015) A lifestyle intervention for primary care patients with depression and anxiety: a randomised controlled trial. Psychiatry Res 230, 537–544. [DOI] [PubMed] [Google Scholar]
- 91. Foster-Schubert KE, Alfano CM, Duggan CR et al. (2012) Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity 20, 1628–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fouré A, Nosaka K, Gastaldi M et al. (2016) Effects of branched-chain amino acids supplementation on both plasma amino acids concentration and muscle energetics changes resulting from muscle damage: a randomized placebo controlled trial. Clin Nutr 35, 83–94. [DOI] [PubMed] [Google Scholar]
- 93. Funderburk LK, Beretich KN, Chen MD et al. (2019) Efficacy of L-leucine supplementation coupled with resistance training in untrained midlife women. J Am Coll Nutr 39, 316–324. [DOI] [PubMed] [Google Scholar]
- 94. Garcia-Ortiz L, Recio-Rodriguez JI, Agudo-Conde C et al. (2018) Long-term effectiveness of a smartphone app for improving healthy lifestyles in general population in primary care: randomized controlled trial (evident II study). JMIR mHealth uHealth 6, e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gomez-Marcos MA, Patino-Alonso MC, Recio-Rodriguez JI et al. (2018) Short- and long-term effectiveness of a smartphone application for improving measures of adiposity: a randomised clinical trial – EVIDENT II study. Eur J Cardiovasc Nurs 17, 552–562. [DOI] [PubMed] [Google Scholar]
- 96. Gonglach AR, Ade CJ, Bemben MG et al. (2016) Muscle pain as a regulator of cycling intensity: effect of caffeine ingestion. Med Sci Sports Exerc 48, 287–296. [DOI] [PubMed] [Google Scholar]
- 97. Gonzalez-Sanchez J, Recio-Rodriguez JI, Fernandez-delRio A et al. (2019) Using a smartphone app in changing cardiovascular risk factors: a randomized controlled trial (evident II study). Int J Med Inform 125, 13–21. [DOI] [PubMed] [Google Scholar]
- 98. Graef JL, Smith AE, Kendall KL et al. (2009) The effects of 4 weeks of creatine supplementation and high-intensity interval training on cardiorespiratory fitness: a randomized controlled trial. J Int Soc Sports Nutr 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gray P, Chappell A, Jenkinson AM et al. (2014) Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. Int J Sport Nutr Exerc Metab 24, 206–214. [DOI] [PubMed] [Google Scholar]
- 100. Greene J, Sacks R, Piniewski B et al. (2013) The impact of an online social network with wireless monitoring devices on physical activity and weight loss. J Prim Care Community Health 4, 189–194. [DOI] [PubMed] [Google Scholar]
- 101. Hageman PA, Pullen CH, Hertzog M et al. (2014) Effectiveness of tailored lifestyle interventions, using web-based and print-mail, for reducing blood pressure among rural women with prehypertension: main results of the Wellness for Women: DASHing towards Health clinical trial. Int J Behav Nutr Phys Act 11, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hardcastle S, Taylor A, Bailey M et al. (2008) A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns 70, 31–39. [DOI] [PubMed] [Google Scholar]
- 103. Hardcastle SJ, Taylor AH, Bailey MP et al. (2013) Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: a randomised controlled trial with a 12-month post-intervention follow-up. Int J Behav Nutr Phys Act 10, 40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Haste A, Adamson AJ, McColl E et al. (2017) Web-based weight loss intervention for men with type 2 diabetes: pilot randomized controlled trial. JMIR Diabetes 2, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Herda TJ, Beck TW, Ryan ED et al. (2009) Effects of creatine monohydrate and polyethylene glycosylated creatine supplementation on muscular strength, endurance, and power output. J Strength Cond Res 23, 818–826. [DOI] [PubMed] [Google Scholar]
- 106. Hebden L, Cook A, van der Ploeg HP et al. (2014) A mobile health intervention for weight management among young adults: a pilot randomised controlled trial. J Hum Nutr Diet 27, 322–332. [DOI] [PubMed] [Google Scholar]
- 107. Hill AM, Buckley JD, Murphy KJ et al. (2007) Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr 85, 1267–1274. [DOI] [PubMed] [Google Scholar]
- 108. Hollis JL, Williams LT, Morgan PJ et al. (2015) The 40-Something Randomised Controlled Trial improved fruit intake and nutrient density of the diet in mid-age women. Nutr Diet 72, 316–326. [Google Scholar]
- 109. Hurkmans E, Matthys C, Bogaerts A et al. (2018) Face-to-face v. mobile versus blended weight loss program: randomized clinical trial. JMIR mHealth uHealth 6, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hutchins-Wiese H, Kleppinger A, Annis K et al. (2013) The impact of supplemental N-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging 17, 76–80. [DOI] [PubMed] [Google Scholar]
- 111. Imayama I, Alfano CM, Kong A et al. (2011) Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act 8, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jakeman JR, Lambrick DM, Wooley B et al. (2017) Effect of an acute dose of n-3 fish oil following exercise-induced muscle damage. Eur J Appl Physiol 117, 575–582. [DOI] [PubMed] [Google Scholar]
- 113. Jakicic JM, Davis KK, Rogers RJ et al. (2016) Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. JAMA 316, 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. James S & Kjerulf Greer B (2019) Influence of exogenous β-hydroxybutyrate on walking economy and rating of perceived exertion. J J Dietary Suppl 16, 463–469. [DOI] [PubMed] [Google Scholar]
- 115. Jannas-Vela S, Roke K, Boville S et al. (2017) Lack of effects of fish oil supplementation for 12 weeks on resting metabolic rate and substrate oxidation in healthy young men: a randomized controlled trial. PLoS One 12, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jendricke P, Centner C, Zdzieblik D et al. (2019) Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: a randomized controlled trial. Nutrients 11, 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Johannsmeyer S, Candow DG, Brahms CM et al. (2016) Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Exp Gerontol 83, 112–119. [DOI] [PubMed] [Google Scholar]
- 118. Koenig CA, Benardot D, Cody M et al. (2008) Comparison of creatine monohydrate and carbohydrate supplementation on repeated jump height performance. J Strength Cond Res 22, 1081–1086. [DOI] [PubMed] [Google Scholar]
- 119. Kruger J, Brennan A, Strong M et al. (2014) The cost-effectiveness of a theory-based online health behaviour intervention for new university students: an economic evaluation. BMC Public Health 14, 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kuller LH, Pettee Gabriel KK, Kinzel LS et al. (2012) The women on the move through activity and nutrition (WOMAN) study: final 48-month results. Obesity 20, 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lammes E, Rydwik E & Akner G (2012) Effects of nutritional intervention and physical training on energy intake, resting metabolic rate and body composition in frail elderly. A randomised, controlled pilot study. J Nutr Health Aging 16, 162–167. [DOI] [PubMed] [Google Scholar]
- 122. Lane MT & Byrd MT (2019) Effects of pre-workout supplements on power maintenance in lower body and upper body tasks. J Funct Morphol Kinesiol 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lara J, O’Brien N, Godfrey A et al. (2016) Pilot randomised controlled trial of a web-based intervention to promote healthy eating, physical activity and meaningful social connections compared with usual care control in people of retirement age recruited from workplaces. PLoS One 11, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lara B, Ruiz-Moreno C, Salinero JJ et al. (2019) Time course of tolerance to the performance benefits of caffeine. PLoS One 14, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lembke P, Capodice J, Hebert K et al. (2014) Influence of n-3 (n3) index on performance and wellbeing in young adults after heavy eccentric exercise. J Sports Sci Med 13, 151–156. [PMC free article] [PubMed] [Google Scholar]
- 126. Lisón JF, Palomar G, Mensorio MS et al. (2020) Impact of a web-based exercise and nutritional education intervention in patients who are obese with hypertension: randomized wait-list controlled trial. J Med Internet Res 22, e14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Logan SL & Spriet LL (2015) n-3 Fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community-dwelling older females. PLoS One 10, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Magriplis E, Sialvera TE, Papadopoulou A et al. (2019) Effectiveness and easiness of adherence to behavioural guidelines for diet and lifestyle changes for cholesterol-lowering: the increasing adherence of consumers to diet & lifestyle changes to lower (LDL) cholesterol (ACT) randomised controlled trial. J Hum Nutr Diet 32, 607–618. [DOI] [PubMed] [Google Scholar]
- 129. Martin CK, Miller AC, Thomas DM et al. (2015) Efficacy of SmartLoss, a smartphone-based weight loss intervention: results from a randomized controlled trial. Obesity 23, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Maruyama C, Kimura M, Okumura H et al. (2010) Effect of a worksite-based intervention program on metabolic parameters in middle-aged male white-collar workers: a randomized controlled trial. Prev Med 51, 11–17. [DOI] [PubMed] [Google Scholar]
- 131. Lane MT, Byrd MT, Bell Z et al. (2019) Effects of supplementation of a pre-workout on power maintenance in lower body and upper body tasks in women. J Funct Morphol Kinesiol 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mobley CB, Haun CT, Roberson PA et al. (2017) Effects of whey, soy or leucine supplementation with 12 weeks of resistance training on strength, body composition, and skeletal muscle and adipose tissue histological attributes in college-aged males. Nutrients 9, 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nakade M, Aiba N, Suda N et al. (2012) Behavioral change during weight loss program and 1-year follow-up: Saku control obesity program (SCOP) in Japan. Asia Pac J Clin Nutr 21, 22–34. [PubMed] [Google Scholar]
- 134. Nicks CR & Martin EH (2020) Effects of caffeine on inspiratory muscle function. Eur J Sport Sci 20, 813–818. [DOI] [PubMed] [Google Scholar]
- 135. Ochi E, Tsuchiya Y & Yanagimoto K (2017) Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contractions. J Int Soc Sports Nutr 14, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Olcina GJ, Timón R, Muñoz D et al. (2008) Caffeine ingestion effects on oxidative stress in a steady-state test at 75 % VO2 max. Sci Sports 23, 87–90. [Google Scholar]
- 137. O’Malley T, Myette-Cote E, Durrer C et al. (2017) Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab 42, 1031–1035. [DOI] [PubMed] [Google Scholar]
- 138. Partridge SR, McGeechan K, Hebden L et al. (2015) Effectiveness of a mHealth lifestyle program with telephone support (TXT2BFiT) to prevent unhealthy weight gain in young adults: randomized controlled trial. JMIR mHealth uHealth 3, e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Partridge SR, McGeechan K, Bauman A et al. (2016) Improved eating behaviours mediate weight gain prevention of young adults: moderation and mediation results of a randomised controlled trial of TXT2BFiT, mHealth program. Int J Behav Nutr Phys Act 13, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pellegrini CA, Verba SD, Otto AD et al. (2012) The comparison of a technology-based system and an in-person behavioral weight loss intervention. Obesity 20, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Polzien KM, Jakicic JM, Tate DF et al. (2007) The efficacy of a technology-based system in a short-term behavioral weight loss intervention. Obesity 15, 825–830. [DOI] [PubMed] [Google Scholar]
- 142. Puhkala J, Kukkonen-Harjula K, Aittasalo M et al. (2016) Lifestyle counseling in overweight truck and bus drivers – effects on dietary patterns and physical activity. Prev Med Rep 4, 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Recio-Rodriguez JI, Agudo-Conde C, Martin-Cantera C et al. (2016) Short-term effectiveness of a mobile phone app for increasing physical activity and adherence to the Mediterranean diet in primary care: a randomized controlled trial (evident II study). J Med Internet Res 18, 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Recio-Rodriguez JI, Agudo Conde C, Calvo-Aponte MJ et al. (2018) The effectiveness of a smartphone application on modifying the intakes of macro and micronutrients in primary care: a randomized controlled trial. The EVIDENT II study. Nutrients 10, 1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Recio-Rodríguez JI, Rodriguez-Sanchez E, Martin-Cantera C et al. (2019) Combined use of a healthy lifestyle smartphone application and usual primary care counseling to improve arterial stiffness, blood pressure and wave reflections: a randomized controlled trial (EVIDENT II Study). Hypertens Res 42, 852–862. [DOI] [PubMed] [Google Scholar]
- 146. Reule CA, Scholz C, Schoen C et al. (2017) Reduced muscular fatigue after a 12-week leucine-rich amino acid supplementation combined with moderate training in elderly: a randomised, placebo-controlled, double-blind trial. BMJ Open Sport Exerc Med 2, e000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ross KM & Wing RR (2016) Impact of newer self-monitoring technology and brief phone-based intervention on weight loss: a randomized pilot study. Obesity 24, 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Roumen C, Corpeleijn E, Feskens EJM et al. (2008) Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabetes Med 25, 597–605. [DOI] [PubMed] [Google Scholar]
- 149. Rydwik E, Lammes E, Frändin K et al. (2008) Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomized controlled pilot treatment trial. Aging Clin Exp Res 20, 159–170. [DOI] [PubMed] [Google Scholar]
- 150. Roumen C, Feskens EJM, Jansen EHJM et al. (2008) Changes in transferrin are related to changes in insulin resistance: the SLIM study. Diabetes Med 25, 1478–1482. [DOI] [PubMed] [Google Scholar]
- 151. Roumen C, Feskens EJM, Corpeleijn E et al. (2011) Predictors of lifestyle intervention outcome and dropout: the SLIM study. Eur J Clin Nutr 65, 1141–1147. [DOI] [PubMed] [Google Scholar]
- 152. Ruíz-Moreno C, Lara B, Brito de Souza D et al. (2020) Acute caffeine intake increases muscle oxygen saturation during a maximal incremental exercise test. Br J Clin Pharmacol 86, 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sabol F, Grgic J & Mikulic P (2019) The effects of 3 different doses of caffeine on jumping and throwing performance: a randomized, double-blind, crossover study. Int J Sports Physiol Perform 14, 1170–1177. [DOI] [PubMed] [Google Scholar]
- 154. Salas-Salvadó J, Díaz-López A, Ruiz-Canela M et al. (2019) Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: 1-year results of the PREDIMED-plus trial. Diabetes Care 42, 777–788. [DOI] [PubMed] [Google Scholar]
- 155. Salinero JJ, Lara B, Ruiz-Vicente D et al. (2017) CYP1A2 genotype variations do not modify the benefits and drawbacks of caffeine during exercise: a pilot study. Nutrients 9, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Schattin A, Baier C, Mai D et al. (2019) Effects of exergame training combined with n-3 fatty acids on the elderly brain: a randomized double-blind placebo-controlled trial. BMC Geriatr 19, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Schrader P, Panek LM & Temple JL (2013) Acute and chronic caffeine administration increases physical activity in sedentary adults. Nutr Res 33, 457–463. [DOI] [PubMed] [Google Scholar]
- 158. Schröder H, Cárdenas-Fuentes G, Martínez-González MA et al. (2018) Effectiveness of the physical activity intervention program in the PREDIMED-Plus study: a randomized controlled trial. Int J Behav Nutr Phys Act 15, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Shimomura Y, Inaguma A, Watanabe S et al. (2010) Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr Exerc Metab 20, 236–244. [DOI] [PubMed] [Google Scholar]
- 160. Sialvera TE, Papadopoulou A, Efstathiou SP et al. (2018) Structured advice provided by a dietitian increases adherence of consumers to diet and lifestyle changes and lowers blood low-density lipoprotein (LDL)-cholesterol: the increasing adherence of consumers to diet & lifestyle changes to lower (LDL) cholesterol (ACT) randomised controlled trial. J Hum Nutr Diet 31, 197–208. [DOI] [PubMed] [Google Scholar]
- 161. Simpson FR, Pajewski NM, Nicklas B et al. (2019) Impact of multidomain lifestyle intervention on frailty through the lens of deficit accumulation in adults with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 75, 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Smith GD, Bracha Y, Svendsen KH et al. (2005) Incidence of type 2 diabetes in the randomized multiple risk factor intervention trial. Ann Intern Med 142, 313–317. [DOI] [PubMed] [Google Scholar]
- 163. Smith GI, Julliand S, Reeds DN et al. (2015) Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr 102, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Spillane M, Schoch R, Cooke M et al. (2009) The effects of creatine ethyl ester supplementation combined with heavy resistance training on body composition, muscle performance, and serum and muscle creatine levels. J Int Soc Sports Nutr 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Stout JR, Sue Graves B, Cramer JT et al. (2007) effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years). J Nutr Health Aging 11, 459–464. [PubMed] [Google Scholar]
- 166. Tallis J, Duncan MJ, Wright SL et al. (2013) Assessment of the ergogenic effect of caffeine supplementation on mood, anticipation timing, and muscular strength in older adults. Physiol Rep 1, e00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Tallis J, Muhammad B, Islam M et al. (2016) Placebo effects of caffeine on maximal voluntary concentric force of the knee flexors and extensors. Muscle Nerve 54, 479–486. [DOI] [PubMed] [Google Scholar]
- 168. Tallis J & Yavuz HCM (2018) The effects of low and moderate doses of caffeine supplementation on upper and lower body maximal voluntary concentric and eccentric muscle force. Appl Physiol Nutr Metab 43, 274–281. [DOI] [PubMed] [Google Scholar]
- 169. Tanaka NI, Murakami H, Aiba N et al. (2019) Effects of 1-year weight loss intervention on abdominal skeletal muscle mass in Japanese overweight men and women. Asia Pac J Clin Nutr 28, 72–78. [DOI] [PubMed] [Google Scholar]
- 170. Tinsley GM, Gann JJ, Huber SR et al. (2017) Effects of fish oil supplementation on postresistance exercise muscle soreness. J Dietary Suppl 14, 89–100. [DOI] [PubMed] [Google Scholar]
- 171. van Wier MF, Ariëns GA, Dekkers JC et al. (2009) Phone and e-mail counselling are effective for weight management in an overweight working population: a randomized controlled trial. BMC Public Health 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. van Dongen EJI, Haveman-Nies A, Doets EL et al. (2020) Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: promuscle in practice. J Am Med Dir Assoc 21, 1065–1072. [DOI] [PubMed] [Google Scholar]
- 173. Verhoeven S, Vanschoonbeek K, Verdijk LB et al. (2009) Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr 89, 1468–1475. [DOI] [PubMed] [Google Scholar]
- 174. Waki K, Fujita H, Uchimura Y et al. (2014) DialBetics: a novel smartphone-based self-management support system for type 2 diabetes patients. J Diabetes Sci Technol 8, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wallman KE, Goh JW & Guelfi KJ (2010) Effects of caffeine on exercise performance in sedentary females. J Sports Sci Med 9, 183–189. [PMC free article] [PubMed] [Google Scholar]
- 176. Wayne N, Perez DF, Kaplan DM et al. (2015) health coaching reduces hba1c in type 2 diabetic patients from a lower-socioeconomic status community: a randomized controlled trial. J Med Internet Res 17, e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Williams LT, Hollis JL, Collins CE et al. (2014) Can a relatively low-intensity intervention by health professionals prevent weight gain in mid-age women? 12-Month outcomes of the 40-Something randomised controlled trial. Nutr Diabetes 4, e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zdzieblik D, Oesser S, Baumstark MW et al. (2015) Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutr 114, 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Zuniga JM, Housh TJ, Camic CL et al. (2012) The effects of creatine monohydrate loading on anaerobic performance and one-repetition maximum strength. J Strength Cond Res 26, 1651–1656. [DOI] [PubMed] [Google Scholar]
- 180. Haruyama Y, Muto T, Nakade M et al. (2009) Fifteen-month lifestyle intervention program to improve cardiovascular risk factors in a community population in Japan. Tohoku J Exp Med 217, 259–269. [DOI] [PubMed] [Google Scholar]
- 181. Mailey EL, Irwin BC, Joyce JM et al. (2019) Independent but not alone: a web-based intervention to promote physical and mental health among military spouses. Appl Psychol Health Well-Being 11, 562–583. [DOI] [PubMed] [Google Scholar]
- 182. Miller KE, Martz DC, Stoner C et al. (2018) Efficacy of a telephone-based medical nutrition program on blood lipid and lipoprotein metabolism: results of Our Healthy Heart. Nutr Diet 75, 73–78. [DOI] [PubMed] [Google Scholar]
- 183. West DS, Monroe CM, Turner-McGrievy G et al. (2016) A technology-mediated behavioral weight gain prevention intervention for college students: controlled, quasi-experimental study. J Med Internet Res 18, e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wilson MG, Castro Sweet CM, Edge MD et al. (2017) evaluation of a digital behavioral counseling program for reducing risk factors for chronic disease in a workforce. J Occup Environ Med 59, e150–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Johns DJ, Hartmann-Boyce J, Jebb SA et al. (2014) Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet 114, 1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. O’Connor EA, Evans CV, Rushkin MC et al. (2020) U.S. Preventive Services Task Force Evidence Syntheses, Formerly Systematic Evidence Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US). [Google Scholar]
- 187. U.S. Preventative Services Task Force (2020) Healthy Diet and Physical Activity to Prevent Cardiovascular Disease in Adults with Risk Factors: Behavioral Counseling Interventions. Rockville, MD: U.S. Preventative Services Task Force. [Google Scholar]
- 188. Rozga M, Handu D, Kelley K et al. (2021) Telehealth during the COVID-19 pandemic: a cross-sectional survey of registered dietitian nutritionists. J Acad Nutr Diet S2212-2672, 00036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Pew Research Center (2019) Mobile Fact Sheet. https://www.pewresearch.org/internet/fact-sheet/mobile/ (accessed March 2021).
- 190. Grgic J, Trexler ET, Lazinica B et al. (2018) Effects of caffeine intake on muscle strength and power: a systematic review and meta-analysis. J Int Soc Sports Nutr 15, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Simpson SA, McNamara R, Shaw C et al. (2015) A feasibility randomised controlled trial of a motivational interviewing-based intervention for weight loss maintenance in adults. Health Technol Assess 19, 1–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Hill AM, Worthley C, Murphy KJ et al. (2007) n-3 Fatty acid supplementation and regular moderate exercise: differential effects of a combined intervention on neutrophil function. Br J Nutr 98, 300–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021002184.
click here to view supplementary material