Abstract
Objective: Magnetic resonance imaging (MRI) of the brain or spine examines the findings as well as the time interval between the onset of symptoms and other adverse effects in coronavirus disease that first appeared in 2019 (COVID-19) patients. The goal of this study is to look at studies that use neuroimaging to look at neurological and neuroradiological symptoms in COVID-19 patients. Methods: We try to put together all of the research on how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes neurological symptoms and cognitive-behavioral changes and give a full picture. Results: We have categorized neuroimaging findings into subtitles such as: headache and dizziness; cerebrovascular complications after stroke; Intracerebral Hemorrhage (ICH); Cerebral Microbleeds (CMBs); encephalopathy; meningitis; encephalitis and myelitis; altered mental status (AMS) and delirium; seizure; neuropsychiatric symptoms; Guillain-Barre Syndrome (GBS) and its variants; smell and taste disorders; peripheral neuropathy; Mild Cognitive Impairment (MCI); and myopathy and myositis. Conclusion: In this review study, we talked about some MRI findings that show how COVID-19 affects the nervous system based on what we found.
Keywords: COVID-19, magnetic resonance imaging, neurological symptoms, neuropsychological symptoms, cognitive-behavioral disorders
Introduction
Magnetic resonance imaging (MRI) is one of the most important, powerful, and widely used types of imaging. This method can provide high spatial resolution without a penetration tissue depth limit, no ionizing radiation, multi-planar imaging, or affecting the function of soft tissues. MRI is a useful method that is used in many medical and biomedical fields, such as diagnosis, treatment, follow-up, evaluation of treatment, cancer imaging, inflammation detection, perfusion imaging, and targeted MRI-guided drug delivery [1-6].
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is what causes the coronavirus disease 2019 (COVID-19), which has since spread to 215 countries [7]. At first, it was thought that COVID-19 only affected the respiratory system. Now, we know that it affects more than just the respiratory system. Symptoms range from mild ones like fever, headache, myalgia, diarrhea, sputum production, sore throat, fatigue, cough, and shortness of breath to life-threatening ones like acute respiratory distress syndrome (ARDS), acute cardiac injury, and septic shock [8], as well as neurological symptoms. It is known that coronaviruses have a neuroinvasive propensity, and COVID-19 may present a wide range of neurological symptoms [7,9,10].
Viruses’ DNA and even proteins can often be found in samples of nervous system tissue, like cerebrospinal fluid or brain. This suggests that viruses can directly invade the nervous system and damage nerves through the blood circulation pathway and the neuronal pathway. Some of the other ways that COVID-19 infections damage the nervous system are hypoxic injury, immune injury, and angiotensin-converting enzyme 2 (ACE2) [9,11-13]. AMS, seizure, focal neurological impairments, neuropathy, including hypogeusia and hyposmia, and, in rare cases, ascending weakness like Guillain-Barré syndrome (GBS) [7,14,15].
Using neuroscience, MR imaging of the brain or spine evaluates the results and the interval between the onset of symptoms and other side effects in patients with COVID-19 [16,17]. MRI examinations for patients with COVID-19-related diseases of the central nervous system (CNS) and peripheral nervous system (PNS) are possible [8], and low CNS abnormalities are allowable [18]. However, CNS disorders are strongly linked to increased cerebrovascular wall contrast on MRI in patients with COVID-19 [10,19-22].
Cognitive impairment from a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been seen in people with meningitis, encephalitis, encephalopathy, and acute cerebrovascular disease, as well as in people with no obvious structural cause [23]. For patients with COVID-19 to have the least amount of illness and death, it is important to be aware of and catch neurological and neuroradiological symptoms as soon as possible [14].
The goal of this study is to look at studies that use MRI to review the neurological and neuroradiological signs of cognitive-behavioral disorders in COVID-19 patients.
Materials and methods
Study design
This is a review study that tries to gather all the information about how SARS-CoV-2 causes neurological symptoms and cognitive-behavioral changes and gives a comprehensive overview of this topic.
Literature search strategy
The article search began on November 12, 2021, and finished on February 19, 2023. For classifying and sorting articles, Mendeley Desktop software version 1.19.5 was utilized. The following template was used to put the keywords for the search into the PubMed database and the Google Scholar search engine: ((COVID-19)) AND (“Magnetic Resonance Imaging”) AND (((Neurological Manifestation))).
Eligibility criteria
Inclusion and exclusion criteria in this study are shown in Figure 1.
Figure 1.
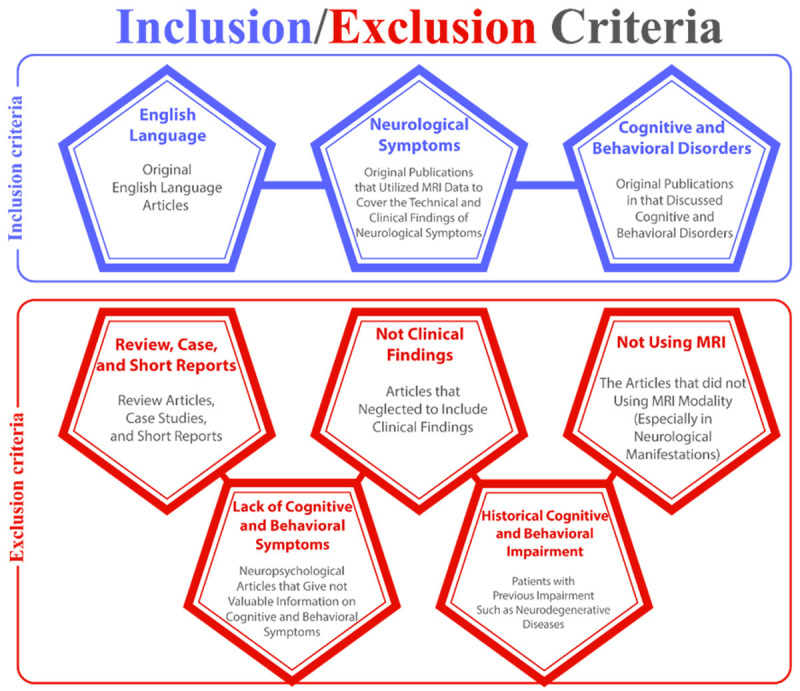
Inclusion and exclusion criteria of the study.
MR imaging
MRI can find cysts, tumors, bleeding, swelling, developmental and anatomical problems, infections, inflammatory diseases, and problems with blood vessels. It can determine whether a shunt is working and spot brain damage brought on by an accident or stroke.
One or more models of the devices listed have been used in different articles. Some of these devices were 64-channel head coils: Optima MR 450w GEM 1.5T (GE Healthcare, Mmilwaki, USA), Ingenia 1.5T, Philips Healthcare (Philips Medical, Best, The Netherlands), Magnetom Aera 1.5T (Siemens Healthineers, Germany), Prismafit 3.0T (Siemens Healthineers, Germany), Skyra 3.0T (Siemens Healthineers, Germany), Vida 3.0T (Siemens Healthineers, Germany), Signa Architect (GE Healthcare, Milwaki, USA), Discovery 750W (GE Healthcare, Milwaki, USA), 1.5T Philips Achieva system (Philips Medical, Best, The Netherlands), and postmortem brain MRI was acquired in a 7T Siemens Magnetom scanner (Siemens, Erlangen, USA) with a 32-channel coil (Nova Medical, Wilmington, USA).
People with COVID-19 who have neurological symptoms or cognitive and behavioral disorders are given an MRI and/or a functional MRI (fMRI). 3D T1 weighted spin echo pre- and/or post contrast or 3D T1 magnetization-prepared rapid acquisition with gradient-recalled echo (MPRAGE), diffusion weighted imaging (DWI), 2D or 3D fluid-attenuated inversion recovery (FLAIR), susceptibility weighted imaging (SWI), and gradient echo T2 weighted are some imaging methods. In addition to these protocols, in some patients, arterial spin labeling (ASL) or diffusion tensor imaging (DTI) is done.
T1 and T2 are technical terms that describe different ways that MRI images are made. More precisely, T1 and T2 relate to the time taken between the magnetic pulses and the image taken. Different structures or chemicals in the CNS can be found with these different methods [24]. FLAIR is an MRI sequence that produces high T2 weighting, removes the cerebrospinal fluid (CSF) signal, and minimizes the contrast between gray and white matter (GM and WM) [25]. DWI is a signal contrast method generated as a function of Brownian motion differences. DWI is a method for evaluating molecular function and microarchitecture in the human body [26]. ASL is an MRI technique for non-invasively measuring brain perfusion in tissues. Benefiting from magnetically marked incoming blood contrast, ASL prevents exogenous contrast [27]. DTI is a neuroimaging method that uses MRI to estimate where the brain’s WM is, how it is oriented, and how it is different from other parts of the brain [28].
Data collection
Data extraction
During the first search, about 272 publications, including original studies, review articles, case reports, and short reports, were found. However, review articles, case reports, and short reports were not included. However, the literature review’s references were studied. All of the publications in the reference list were evaluated, and data on cognitive-behavioral disorders as well as neurological manifestations were completely extracted. The following parameters were considered throughout the search: 1) first author, 2) date of publication, 3) MRI modality or psychosocial testing, and 4) neurological manifestations, including cognitive-behavioral disorders.
Data analysis
After doing database searches and gathering publications, all were divided into neurological, cognitive, and behavioral categories.
Neurological manifestations are often divided into three categories: 1) the CNS, 2) the PNS, and 3) neuromuscular disease.
Cognitive-behavioral articles also generally include: 1) common cognitive and behavioral disorders, and 2) other cognitive and behavioral disorders.
Results
CNS
Headache and dizziness
Anomia, myalgia, and headache have all been reported in patients with COVID-19 since the beginning of the pandemic (Table 1) [29]. Headaches are more common in some places and cultures than others. This is because people have different genes that make them more likely to get headaches and different ways of expressing pain [30]. COVID-19 headaches have been studied for their pathogenesis. COVID-19-related headaches may be caused by sex, obesity, lower socioeconomic status, or genetic predisposition [31,32]. For example, headache disorders are less common in Asia than in Latin America [33]. In the context of COVID-19, this means that the chance of getting headaches may depend on cultural, genetic, or geographical factors. In addition, novel SARS-CoV-2 strains that are more common in certain geographical locations may be more closely linked to specific symptoms, such as headaches, than other types [34].
Table 1.
Neuroimaging findings in COVID-19 disease and disorders
| Central Nervous System (CNS) | |||||
|---|---|---|---|---|---|
|
| |||||
| Diseases or disorders | First Author (Year) | Sample Size (percentage of men and women) | Mean Age (range) [years] | Relative Frequency Percent | MRI Findings |
| Headaches and dizziness | [111] Lu (2020) | 60 (56.7%, 43.3%) | 44.10 | Headache = 25% | Patients with COVID-19 had statistically significant higher GM volumes (GMV) on both sides of the brain in the olfactory cortex, hippocampi, insula, left Rolandic operculum, left Heschl’s gyrus, and right cingulate gyrus. |
| [5] Kremer (2020) | 64 (67%, 33%) | 66 (20-92) | Headache = 16% | The severe acute respiratory syndrome coronavirus was suspected in 56% of the abnormal brain MRIs. | |
| [140] Yoon (2020) | 150 (65.33%, 34.67%) | 63.6 (22-96) | Headache = 8% | Fourteen percent had an MRI, and twenty-six percent had both a CT and an MRA. Neuroimaging studies revealed anomalies in 17 percent of patients. | |
| [68] Lersy (2020) | 58 (66%, 34%) | 62 (55-70) | Headache = 5% | In patients with neurological symptoms related to COVID-19, abnormalities, especially leptomeningeal enhancement, and increased inflammatory markers in CSF are common, whereas SARS-CoV-2 detection in CSF remains sparse. | |
| [89] Karadaş (2020) | 239 (55.6%, 44.4%) | 46.46 (19-88) | Headache = 27.6% | In the frontal and posterior regions of the head, the symptoms of a headache were frequently observed. | |
| Dizziness = 6.7% | |||||
| [38] Jain (2020) | 3218 (60.7%, 39.3%) | 64 (2 weeks-105 years) | Headache = 3.8% | Patients with AMS or delirium, strokes, and mechanical falls or trauma were the most common reasons for neuroimaging studies. There were a few less common symptoms, including syncope (4%), headache (3.8%), dizziness (2.8%), seizures (2.1%), and ataxia (1.4%), among them. | |
| Dizziness = 2.8% | |||||
| [7] Scullen (2020) | 27 (52%, 48%) | 59.8 (35-91) | Headache = 7.4% | CT revealed 63% of neurologic findings, MRI showed 30%, and EEG showed 44%. Diffuse hypoattenuation, hemorrhages in the subcortical parenchyma, and hypodensities in deep structures are the most common findings. The corpus callosum, the basal ganglia, and deep WM were all found to be affected by MRI findings. There was only one patient with an irregular proximal focal stenosis of the supraclinoid internal carotid artery among patients with a large-territory stroke. | |
| [81] Liottaa (2020) | 509 (55.2%, 44.8%) | 58.5 | Headache = 37.7% | A brain MRI was performed on 3.14% of the patients. In 42.2% of patients, neurologic symptoms were present at the onset of COVID-19, in 62.7 percent of patients, and at any time during the disease course in 82.3 percent of patients. | |
| Dizziness = 29.7% | |||||
| [16] García-Azorín (2021) | 233 (57.9%, 42.1%) | 61.1 | Headache = 12.9% | A brain MRI was performed in 26.0% of cases, and abnormal findings were found in 35.7%, including 16 cases of stroke-related findings, two cases of encephalitis-related changes, and two cases of non-specific WM lesions. A total of 13 patients underwent spinal MRIs, with 4 displaying degenerative signs and one displaying meningeal and nerve root enhancements. | |
| [19] Altunisik (2021) | 32 (40.6%, 59.4%) | 39.94 (24-55) | Daily Headache = 53.12% | MRI results did not show an increase in intracranial pressure in patients with persistent headaches following COVID-19. The majority of the current data on elevated ICP findings come from small series and case reports of patients with a severe disease spectrum; thus, other factors other than elevated ICP may play a role in headache persistence. Patients with a wide range of severe illnesses have provided the majority of the current data on increased ICP. This suggests that increased ICP isn’t the only factor in the recurrence of headaches. | |
| Migraine = 21.87% | |||||
| Tension Headache = 25% | |||||
| Dizziness = 37.5% | |||||
| [128] Planchuelo-Gómez (2023) | 42 (26%, 74%) | 43.8 | 100% | Individuals with chronic headache after COVID-19 resolution exhibited a variety of alterations in GM and WM structure. The changes in GM were minor, affecting anterior regions such as the pars orbitalis, fusiform gyrus, and frontal pole. On the one hand, the observed WM alterations were widespread, including the majority of the WM tracts, and seemed to be connected to WM fiber bundle degradation. The WM alterations, on the other hand, created a scenario similar to but milder than migraine. | |
| Cerebrovascular Complications after Stroke | [5] Kremer (2020) | 64 (67%, 33%) | 66 (20-92) | 27% | The severe acute respiratory syndrome coronavirus was suspected in 56 percent of the abnormal brain MRIs. |
| [22] Chougar (2020) | 73 (65.8%, 34.2%) | 58.5 | 70.6% | The MRI scans of 30 patients (22 non-ICU and 8 ICU) revealed no significant abnormalities aside from the changes common in elderly patients and pathological findings in 58.9% of patients. | |
| [140] Yoon (2020) | 150 (65.33%, 34.67%) | 63.6 (22-96) | 8% | Fourteen percent had an MRI, and twenty-six percent had both a CT and an MRA. Neuroimaging tests revealed anomalies in the brains of 17% of people. | |
| [51] Lin (2020) | 2054 (57%, 43%) | 64 (50-75) | 11% | There were six cases of abnormalities in the brain’s cranial nerves and three patients with a microhemorrhage pattern consistent with critical illness-associated microbleeds-in the 51 patients who underwent MR imaging examinations. | |
| [52] Mahammedi (2020) | 108 (63.8%, 36.2%) | 69 (16-62) | 31% | Brain MRI was performed on 18% of patients, and 35% had acute abnormalities on brain MRIs. | |
| [7] Scullen (2020) | 27 (52%, 48%) | 59.8 (35-91) | 11.1% | In patients with AD, MRI results frequently showed diffuse involvement of the deep WM, corpus callosum, and basal ganglia. | |
| [48] Büttner (2021) | 34 (76.5, 23.5%) | 67.5 (7 months-82 years) | 5.9% | Some 38.2% had shown brain imaging abnormalities either on initial or follow-up neuroimaging. | |
| [16] García-Azorín (2021) | 233 (57.9%, 42.1%) | 61.1 | 27% | Brain MRI: 16 cases of stroke-related findings; 2 patients with encephalitis-related changes; and 2 cases of non-specific WM lesions. Degenerative signs were found in four of the cases, while meningeal and nerve root enhancement was found in one. | |
| [100] Ray (2021) | 52 (58%, 42%) | 9 (1-17) | 8% | An abnormality in neuroimaging was found in 74% of those who underwent the procedure. One ischaemic involving the anterior and middle right cerebral artery, one intraparenchymal hemorrhage in the right frontal lobe, and one (1%) had bilateral hyperintensities within the claustra as a result of ADEM were found. | |
| [77] Helms (2020) | 140 (71.4%, 28.6%) | 62 (52-70) | 6.25% | Some 28.57% had abnormalities in the WM of their brains that were found to be intraparenchymal. | |
| Intracerebral Hemorrhage (ICH) | [46] Katz (2020) | 86 (56%, 44%) | 67.4 (25-94) | Pure intracranial hemorrhage = 16.3% | An MR venogram revealed a hemorrhagic venous infarction in the left temporal lobe of a patient with a cerebral hemorrhage. |
| [88] Cleret de Langavant (2021) | 26 (73%, 27%) | 58.3 (16-86) | Subdural hemorrhage = 3.8% | All of the patients had normal brain MRIs. | |
| [22] Chougar (2020) | 73 (65.8%, 34.2%) | 58.5 | microhemorrhage = 11.3% | MRIs revealed bilateral edematous changes in the thalami, basal ganglia, and midbrain, as well as variable contrast enhancement. One MRI case involved the substantia nigra. These findings include WM lesions with angiocentric enhancement as well as abnormalities in the basal ganglia, including substantia nigra involvement, which may indicate vasculitis and/or inflammation. | |
| [52] Mahammedi (2020) | 108 (64%, 36%) | 71 (60.5-79) | Intracranial hemorrhage = 6% | Brain MRI was performed on 20 (18%) of the 108 patients. Seven of the MRIs (or 35% of the total) revealed acute abnormalities in the brain. Neuroimaging features in hospitalized COVID-19 patients were found to be variable, lacking specific patterns, but dominated by acute ischemic infarctions and intracranial hemorrhages, as shown in this research. | |
| [5] Kremer (2020) | 37 (81%, 19%) | 61 (8-78) | ICH lesion = 54% | FLAIR and diffusion-weighted sequences show nonconfluent multifocal WM hyperintense lesions with variable enhancement, with associated hemorrhagic lesions in 11 of 37 patients and extensive and isolated WM microhemorrhages in nine of 37 patients. Microhemorrhages in a 57-year-old man with abnormal wakefulness after sedation were found by axial SWI in the WM of the brain. cerebellar peduncles, subcortical WM, internal capsule, and corpus callosum. | |
| [48] Büttner (2021) | 34 (75.8%, 24.2%) | 67.5 (7 months, 82 years) | Hemorrhagic manifestation = 26.5% | It was discovered that a 57-year-old man had multiple microbleeds in the superficial and deep WM of his brain. | |
| [129] Lersy (2020) | 69 (67%, 33%) | 65 (21-86) | Extensive WM micro-hemorrhage = 9% | Six of the patients had extensive WM microhemorrhages with atypical involvement of the corpus callosum. Imaging revealed vessel wall thickening with homogeneous and concentric enhancement, suggesting vasculitis. | |
| Subarachnoid hemorrhage = 4% | |||||
| [45] Gorgulu (2021) | 42 (50%, 50%) | 73.5 (22-98) | Cerebral hemorrhage = 28.6% | Anatomical localization of ICH included 41.7% (n = 5) lobar, 16.7% (n = 2) basal ganglia, 16.7% (n = 2) cerebellum, and 25% (n = 3) other areas. | |
| [59] Sawlani (2021) | 3,403 (66%-33%) | 59.7 (32-91) | Microhaemorrhage = 12/20, 60% | MRI abnormalities were found in 20 patients, and CT abnormalities were found in 18 patients, in 23% of the patients. The microhemorrhage in the corpus callosum splenium was the most recurrent and consistent finding on the MRI scans. | |
| [40] Martin (2022) | 7 (43%, 57%) | 44 (11-74) | Punctate brain hemorrhage = 43% | One subject had a normal MRI and CT scan, while the other six had abnormal results. Five patients had abnormalities in the cerebrovascular system. Only one SAH, three microhemorrhages, and one stroke were reported. | |
| [118] Mahammedi (2021) | 135 (64%, 36%) | 68.2 (17-94) | Intracranial hemorrhage = 10% | After subarachnoid and parenchymal hemorrhages, microhemorrhage was the most common intracranial hemorrhage. T2/FLAIR hyperintense lesions with associated microbleeds (with and without restricted diffusion) and confluent symmetric T2/FLAIR hyperintense lesions involving the deep and subcortical WM without restricted diffusion were the most common MR imaging findings of WM disease. | |
| [140] Yoon (2020) | 150 (65%, 35%) | 63.6 (22-96) | Hemorrhage = 7.3% | A CT or MR scan found anomalies in 26 (17%) of the patients, and 11 (42%) of the patients had hemorrhages. There were microhemorrhages found in 7 of the 11 patients who had intracranial hemorrhage. | |
| Cerebral Microbleeds (CMBs) | [48] Büttner (2021) | 34 (76%, 24%) | 67.5 (7 months, 82 years) | Microbleed = 20.6% | The corpus callosum is the most severely affected area of the brain in this situation, with multiple microbleeds. Four patients in this group had a microbleed pattern that was consistent with critical illness encephalopathy. A similar neuroimaging pattern has been observed in other studies involving COVID-19 patients. |
| [56] Elizondo (2021) | 47 | - | 12.5% | Basal ganglia, cerebellum, cerebellum and/or juxtacortical cortex, cerebellum, and deep and periventricular WM were found to have microbleeds. | |
| Encephalopathy | [52] Mahammedi (2020) | 108 (63.8%, 36.2%) | 71 (16-62) | Acute encephalopathy = 25% | MRIs of the brain were performed on 20 of the 108 patients who were a part of the research. Of the 20 patients who had MRI scans, acute abnormalities in the brain were found in seven of them. Systemic toxemia, viremia, and/or hypoxic effects can all result in a non-specific cortical pattern of T2 FLAIR hyperintense signal with associated diffusion restriction. |
| PRES = 25% | |||||
| Nonspecific encephalopathy = 50% | |||||
| [7] Scullen (2020) | 76 (52%, 48%) | 59.8 (35-91) | Encephalopathy = 74% | The basal ganglia frequently showed FLAIR changes on MRI or CT, or discrete hypodensities, and SWI in several patients revealed petechial changes similar to necrotizing encephalopathy. In one patient, there were FLAIR changes in the cortical region of the bilateral precentral frontal gyri, as well as deep structural changes typical of anoxic ischemia. | |
| Acute necrotizing encephalopathy = 7% | An electrographic nonspecific encephalopathy with mild to moderate clinical AMS affected the majority of patients over the course of their illness. These patients frequently had non-specific imaging findings such as deep structure hypodensity or hypoattenuation. It was found that in severe cases, CT and MRI findings were consistent with idiopathic absence epilepsy (IAE) and acute necrotizing encephalopathy, two other viruses that can cause encephalitides. | ||||
| [37] Meppiel (2020) | 222 (61.3%, 38.7%) | 65 (53-72) | 30.2% | Only six patients (9%) had small acute cerebral infarctions unrelated to symptoms, and one patient had a typical reversible lesion of the corpus callosum spleen. | |
| [100] Ray (2021) | 52 (58%, 42%) | 9 (1-17) | 52% of COVID-19 neurology patients had isolated encephalopathy. | The abnormalities were found in 28 (58%) of the 46 MRI scans and 11 CT scans of the 52 patients. The corpus callosum splenium showed signs of mild encephalopathy in one case. Consistent symptoms in 22 of 25 cases have been found in children with the Pediatric Inflammatory Multisystem Syndrome (PIMS-TS). Patients with mild encephalopathy were found to have a reversible splenial lesion in the corpus callosum in more than 40% of cases. | |
| 48% | |||||
| PIMS-TS neurology group: encephalopathy | |||||
| [18] Lindan (2021) | 38 | 0-18 | 2.6% | Patients with fever and encephalopathy were observed. MRI revealed an unexpected pattern of cerebral microhemorrhages and infarct foci. | |
| [140] Yoon (2020) | 150 (65.3%, 34.7%) | 63.6 (22-96) | Leukoencephalopathy = 27% | CT scans were performed on 141 patients; MRI scans were performed on 21 others; and a combination of CT and MRI scans was performed on another 31 patients. Twenty-six (17%) of the patients had abnormal neuroimaging studies, including hemorrhage, infarction, and leukoencephalopathies. | |
| [41] Klironomos (2020) | 185 (74.5%, 25.5%) | 62 | Leukoencephalopathy = 44% | Both patients showed signs of dynamic processes, with leukoencephalopathy regressing and leptomeningeal enhancement growing in the same time period. One patient with diffuse symmetric leukoencephalopathy improved clinically and partially resolved during follow-up. There were symmetrical WM changes in both the occipital and frontal regions of one patient, as well as reduced diffusion in those regions. These findings could point to hypoxic-induced diffuse leukoencephalopathy. | |
| [126] Lang (2021) | 93 (67%, 33%) | 63 | Leukoencephalopathy = 6.4% | Neuroimaging findings such as intracranial hemorrhage (n = 24), infarction (n = 4), or a combination of these were found in 26% of the patients studied. | |
| [51] Lin (2020) | 2054 (57%, 43%) | 64 (50-75) | PRES = 1.1% | T2 FLAIR hyperintensity or CT hypoattenuation in confluence was used to determine PRES from prior literature. | |
| [71] Azab (2021) | 977 (45.4%, 54.6%) | 60.15 | Acute necrotizing encephalopathy = 13.7% | SARS-CoV-2 encephalitis was detected in the CSF, and brain MRI showed increased intensity in the right mesial temporal lobe. Another case of acute necrotizing encephalitis has been reported. It appeared on MRI scans as a “ring enhancement”. | |
| [70] Pons-Escoda (2020) | 103 (61%, 39%) | 74 (50.2-90) | Encephalopathy = 23.5% | The brains of 17 patients were imaged using MRI. One patient was left out of this study. There were no abnormalities found in the brain images of four patients who had suffered from encephalopathy due to prolonged sedation. | |
| [124] Uginet (2022) | 39 (89.7% 10.3%) | 66.5 | 85% | In 29 of the 34 patients with COVID-19 encephalopathy who had high-resolution vessel wall imaging, they found a circular enhancement and thickening of the basilar and vertebral arteries (85%), but no correlation with ischemia or microbleeds. | |
| [58] Uginet (2021) | 707 | 64.6 | 4.4% | COVID-19 encephalopathy severity was not related to pneumonia severity. 92% of patients (23/25) had abnormal MRI findings, and 85% (17/20) had intracranial vessel gadolinium enhancement, indicating that the blood-brain barrier had been disrupted, while 85.7 percent (6/7 patients) had increased CSF/serum quotient of albumin. The SARS-CoV2-induced endotheliitis is consistent with altered brain homeostasis and vascular dysfunction, even though other pathophysiological mechanisms may be at play. | |
| [141] Espindola (2021) | 58 (43.1%, 56.9%) | 51.6 | 41.4% | Only 12.1% of the 58 participants in the study were found to have CVD in their brain MRI, six of whom had intracranial hemorrhage, and one had a TIA. | |
| [65] Helms (2020) | 32 | 62 (52-70) | 18.7% | Of the 118 patients examined, 32 underwent brain MRI. A subarachnoid contrast enhancement on brain images in six of the 32 patients with encephalopathy suggests that the blood meningeal barrier has abnormal permeability. | |
| Meningitis, encephalitis and myelitis | [38] Jain (2020) | 3218 (60.7%, 39.3%) | 64 (2 weeks-105 years) | Encephalitis = 2.5% | Imaging findings consistent with encephalitis were seen in only one patient (2.5%). |
| [91] Kandemirli (2021) | 27 (44.4%, 55.6%) | 63 (34-87) | Leptomeningeal enhancement = 18.5% | Only post-contrast 3D FLAIR images showed leptomeningeal enhancement in one case; post-contrast T1WI or TurboFlash T1WI images did not show any evidence of this enhancement whatsoever. | |
| [68] Lersy (2021) | 58 (66%, 34%) | 62 (55-70) | Leptomeningeal enhancement = 38% | Brain MR images showed leptomeningeal enhancement in 20% of patients. | |
| 4% of demyelinating lesions have acute inflammation. | |||||
| [63] Sanchez (2020) | 841 (56.2%, 43.8%) | 66.42 | Encephalitis < 1% | To rule out inflammation, one patient had encephalitis, which manifested as an apparent stroke mimic in FLAIR sequences of brain MRI (14th day from onset, stage IIA) with bilateral temporal hyperintensity. | |
| [37] Meppiel (2020) | 222 (61.3%, 38.7%) | 65 (53-72) | Encephalitis = 9.5% | Neuroimaging revealed various acute nonvascular lesions in 14 of the 21 people with encephalitis who underwent the procedure. | |
| [62] Rifino (2021) | 137 (66%, 34%) | 64.9 (30-95) | Encephalitis = 3.6% | The vigilance and/or consciousness of 49 patients were disturbed. They all had a CT scan or an MRI of the brain. SARS-CoV-2 RT-PCR was performed in the CSF of twenty-one patients. Clinical features, CSF data, and neuroimaging led us to conclude that encephalitis was present in five of the patients, one of whom had been infected with HSV-1 and another had been diagnosed with necrotizing encephalitis. | |
| Necrotizing encephalitis < 1% | |||||
| [71] Azab (2021) | 977 (45%, 55%) | 60.15 | Viral encephalitis = 16.2% | Only 34 patients with encephalitis had MRIs done because of the limited availability. The MRI findings of COVID-19 encephalitis included WM lesions, demyelinating hyperintensities, leptomeningeal enhancement, and necrotic hemorrhage. MRI microvascular brain lesions were found in approximately 21 patients with encephalitis. There is a possibility that this is the result of a COVID-19-mediated vascular brain injury. | |
| Acute necrotizing encephalopathy = 13.7% | |||||
| [16] García-Azorín (2021) | 233 (57.9%, 42.1%) | 61.1 | Meningoencephalitis = 3.6% | Two patients were diagnosed with encephalitis after abnormal findings were found in 57 of 219 (26.0%) patients who underwent a brain MRI. | |
| Encephalitis < 1% | |||||
| [18] Lindan (2021) | 38 | 0-18 | Encephalomyelitis-like changes of the brain = 42% | Anti-N-methyl-D-aspartate receptor (anti-NMDAR) autoimmune encephalitis was diagnosed in a patient with brain changes similar to those seen in ADEM. When the term “ADEM-like” is used to describe an imaging phenotype that resembles an ADEM pattern, it means that the pattern is similar in some way. As a result, one patient developed a long-term, T2 hyperintense central cord myelitis. | |
| Myelitis = 21% | |||||
| [9] Khedr (2021) | 117 Patients with definite COVID-19, n = 55 (50%, 50%) | Definite | Encephalitis = 5% | An MRI can show brain edema and inflammation as evidence of encephalitis. | |
| Patients with probable COVID-19, n = 62 (56%, 44%) | COVID-19 = 51.5 | Meningoencephalitis < 1% | |||
| Probable = 60.3 | |||||
| Altered mental status and delirium | [5] Kremer (2020) | 37 (81%, 19%) | 61 (8-78) | Alteration of consciousness = 73% | There were three distinct patterns in the brain MRI of patients with severe COVID-19: signal abnormalities in the medial temporal lobe, non-confluent multifocal WM hyperintense lesions on FLAIR and diffusion with variable enhancement, and extensive and isolated WM microhemorrhages. |
| Confusion = 32% | |||||
| Agitation = 19% | |||||
| [65] Helms (2020) | 140 (71.4%, 28.6%) | 62 (52-70) | Delirium with a combination of acute attention, awareness, and cognition disturbances = 84.3% | Patients with WM microhemorrhages and one with a left frontal intraparenchymal hematoma were found in the 28 MRI scans; these were found in eight of the 28 patients. A total of four patients had FLAIR hyperintensities, with two patients showing small foci of contrast enhancement and two patients showing diffusion hyperintensities. During postcontrast T1 or FLAIR imaging, a hyperintensity and/or enhancement in the subarachnoid space was observed in 17 of these patients (60.7%). | |
| Unexpected state of agitation = 69.3% | |||||
| [100] Ray (2021) | 52 (58%, 42%) | 9 (1-17) | Acute psychosis = 3.84% | One had an abnormal T2 signal in the hippocampus and cortical diffusion restriction due to limbic encephalitis; one had an abnormal T2 signal in the periventricular; and one had signal changes in the intraorbital segment of the right optic nerve consistent with demyelination in a child with acute demyelinating syndrome. | |
| [91] Kandemirli (2021) | 27 patients with MRI (78%, 22%) | The median age of patients with MRI: 63 (34-87) | - | The right frontal cortical hyperintensity and symmetrical WM hyperintensity were clearly visible on axial FLAIR images taken at the midbrain and centrum semiovale. Linear hyperintensity was also visible on the frontal sulci. Axial b2000 DWI showed a frontal increase in signal with a correspondingly low ADC. Axial T1WI revealed effacement of the right frontal sulcal region. The pial-subarachnoid enhancement on post-contrast T1WI was mild. Radial and centro semiovale axial SWI revealed blooming artifacts in the frontal sulci. FLAIR showed bilateral leptomeningeal enhancement after contrast. | |
| [125] Gunbey (2021) | 354 (42%, 58%) | 65.2 | Syncope = 13% | CT and MR imaging detected abnormalities in 4.7% and 28% of cases, respectively. Neuroimaging results showed infarcts, parenchymal hemorrhages, and encephalitis, as well as cortical signal abnormalities, the PRES, and cranial nerve involvement. MRI results (n = 103 total) showed that the WML classification ratios in patients were as follows: none 31% (n = 32), mild 16.9% (n = 17), moderate 19.7% (n = 20), and severe 32.4% (n = 34). | |
| Altered mental status = 3% | |||||
| [131] Radmanesh (2020) | 242 (62%, 38%) | 68.7 | Altered mental status = 42.1% | 42 (41.2% of the patients) had WM microangiopathic changes, 29 (28.4% of the patients) had chronic infarcts, and 1 patient had a meningioma that was found by chance.The imaging of the brains of no patients with AMS revealed any cases of acute or subacute infarction or acute intracranial hemorrhage. | |
| Syncope/fall = 32.6% | |||||
| [5] Kremer (2020) | 64 (67%, 33%) | 66 (20-92) | Confusion = 53% | A total of 36 (56%) brain MRIs were deemed abnormal, possibly due to the SARS-CoV-2 virus. | |
| Impaired consciousness = 39% | |||||
| Agitation = 31% | |||||
| Seizure | [67] Khedr (2021) | 19 (36.8%, 63.2%) | 47 (35-65) | 100% | There was diffuse cerebral oedema, leptomeningeal enhancement with T2 and FLAIR hyperintensities in the frontal lobes and/or bilateral medial temporal and/or thalamic oedema. |
| [68] Lersy (2021) | 58 (66%, 34%) | 62 (55-70) | 10% | MR images of the brains of five of the six patients who had seizures yielded GM lesions in one (2%) and FLAIR hyperintensities in the mesial temporal lobes in one (2%) of the patients. | |
| Neuropsychiatric Symptoms | [79] Du (2022) | 19 (42%, 58%) | 50.5 | Dyspnea = 42% | The RecCOVID group had significantly higher amplitude of ALFF values in the left precentral gyrus (PreCG), middle frontal gyrus, inferior frontal gyrus of the operculum, inferior frontal gyrus of the triangle, insula, hippocampus, parahippocampal gyrus, fusiform gyrus, and postcentral. |
| Fatigue = 37% | |||||
| Myalgia = 21% | |||||
| [142] Voruz (2022) | 50 | - | Severe = 18% | Long-term memory and executive dysfunctions are caused by SARS-CoV-2 infection, which is linked to changes in large-scale functional brain connections. | |
| Moderate = 42% Mild = 40% | |||||
| Peripheral Nervous System (PNS) | |||||
| Guillain-Barre Syndrome (GBS) and its Variants | [68] Lersy (2021) | 58 (66%, 34%) | 62 (55-70) | 2% | Neuroimaging was performed on the patient with Guillain-Barré syndrome. Guillain-Barré syndrome-related multiple cranial nerve enhancement was seen in brain MRI images, but spinal MRIs were found to be normal. |
| [51] Lin (2020) | 2054 (57%, 43%) | 64 (50-75) | 1 patient with MFS | CT or MR imaging of the brain was performed on 278 (14%) patients. Neuronal enhancement after IV gadobutrol administration was clearly visible in an enlarged, T2-hyperintense left oculomotor nerve on MR imaging of the brain. | |
| [52] Mahammedi (2020) | 108 (63.8%, 36.2%) | 69 (16-62) | 2 cases of GBS, 1 case of MFS | Acute neuroimaging abnormalities were found in 51 out of 108 patients. Two GBs patients and one MFS patient had cranial nerve and cauda equina enhancement, respectively. | |
| Smell and Taste Disorders | [51] Lin (2020) | 2054 (57%, 43%) | 64 (50-75) | 33% | Each of the diagnostic olfactory bulb sequences was performed on all 12 patients. The olfactory bulb volume in none of the patients was altered. However, 4 of 12 patients had an abnormally increased olfactory bulb signal on post-contrast T2 FLAIR, which could indicate intrinsic T2 prolongation or, potentially, contrast enhancement. On post-contrast T2 FLAIR images of the olfactory bulb, they discovered evidence of olfactory neuritis in four patients with COVID-19. |
| [17] Strauss (2020) | 12 (50%, 50%) | 58.25 | 100% | The T2 FLAIR signal intensity in the normalized olfactory bulb was significantly different between patients with COVID-19 and controls with anosmia. Intraneural T2 signal hyperintensity was seen in four of the 12 COVID-19 patients compared to none of the 12 anosmia-free controls. The 3D FLAIR signal intensity in the olfactory bulb was higher in patients with COVID-19 and neurologic symptoms when compared to a control group of patients who had olfactory dysfunction of the same age. | |
| [134] Altundag (2021) | 24 with anosmia due to COVID-19 | 39.3 | 100% anosmia | There were CT measurements of the cleft width and volumes and MRI measurements of the signal intensity, the bulb volumes, and the olfactory depths of the nasal passages, as well as the T2-weighted signal intensity. Anosmic patients with SARS-CoV-2 (group 1) or non-SARS-CoV-2 viral infection (group 2) had significantly wider olfactory clefts (OCs) than healthy controls. Healthy controls had lower OC volumes, and the T2 signal in the OC area was higher in groups 1 and 2 compared to groups 2. Between groups 1, 2, and 3, there was no discernible difference in olfactory bulb volume or sulci depth as measured by MRI. | |
| [91] Kandemirl (2021) | 23 (39.10%, 60.9%) | 29 (22-41) | 100% | Olfactory bulb volumes, sulcus depth, morphology, signal intensity, and nerve filia architecture were all assessed quantitatively and qualitatively using MRI. An abnormality in signal intensity in 91.3% of cases was found to be diffusely increased or scattered hyperintense foci or microhemorrhages. 34.8% of cases had clumping of the olfactory filia, and 17.4% had thinning and scarcity of the filia. A primary abnormality in olfactory cortical signals was found in 21.7% of cases. Olfactory bulb volume was 62 millimeters in diameter on average. On the right, the median olfactory sulcus depth was 6.8 mm, while on the left, it was 6.3 mm. | |
| [136] Yildirim (2022) | Persistent COVID-19 related OD: 31 (32%, 68%) | COVID-19-related persistent OD: 32.5 | 100% | There was a significant difference in COVID-19-related olfactory dysfunction (OD) compared to post-infectious olfactory dysfunction. On the other hand, there was no significant difference in the proportion of COVID-19-related and post-infectious OD with deformed bulb morphology and elevated olfactory bulb signal intensity in the other OD groups. | |
| Post-Infectious OD: 97 (39%, 61%) | Post-Infectious: 45.9 | ||||
| [41] Klironomos (2020) | 185 (74%, 26%) | 62 | 19% | Slight contrast enhancement was seen in two patients, and seven of 37 (or 19%) had abnormally high T2-weighted FLAIR sequence signals in the olfactory bulb. | |
| [111] Lu (2020) | 60 (56.67%, 43.32%) | 44.10 | 3.33% loss of smell and 6.67% loss of taste | Significantly larger volumes were found in the bilateral olfactory, hippocampal, insula, left Heschl’s gyrus, left Rolandic operculum, and right cingulate gyrus. | |
| [133] Eliezer (2020) | 20 (50%, 50%) | 34.6 (21-53) | - | The severity of the olfactory score was found to be significantly correlated with the degree of OC obstruction. The olfactory bulb (OB) volume in COVID-19 patients and healthy subjects did not differ significantly. Between the first and second MRIs, there was no significant difference in the OB volume (OBV). Olfactory function loss is linked to reversible OC obstruction in SARS-CoV2-infected patients. | |
| [137] Guney (2021) | 41 (48.72%, 51.22%) | 40.27 | Approximately 100% had a history of smell disorder | Patients with COVID-19 had significantly smaller left, right, and mean olfactory bulb volumes and olfactory sulcus depths (OSDs) than control individuals. People with COVID-19 infection and a smell disorder who are in the chronic phase of their illness see a significant decrease in OBV. When compared to normal healthy cases, OSD values were found to be lower during the chronic period. | |
| [132] Burulday (2021) | 23 (56.5%, 43.5%) | 37.08 (19-73) | Smell = 100% | COVID-19 disease affects the Obs’ periphery but not the center smell regions of the insular gyrus and corpus amygdala. The significance of their research is to identify MRI abnormalities in individuals with COVID-19 who have olfactory issues. | |
| [136] Yildirim (2022) | 31 (67.7%, 32.3%) | 32.5 | Olfactory dysfunction = 100% | Whereas COVID-19-related anosmia has reduced OB volume and white matter tract integrity of olfactory areas, it is not as severe as other post-infectious OD. In COVID-19-related OD, trigeminosensory activation was stronger. These results may indicate that COVID-19 related OD has a better maintained central olfactory system than COVID-19 related OD. Persistent COVID-19-related anosmia may be caused by OB injury. | |
| [143] Esposito (2022) | 27 (37%, 63%) | - | Olfactory loss = 100% | More segregated processing within areas more functionally related to the anterior piriform cortex was associated with more residual olfactory impairment. While olfactory loss was a lasting COVID19 symptom, greater neural connection within the olfactory brain was connected with a recent SARSCoV2 infection. The functional connectivity of the anterior piriform cortex, the greatest cortical receiver of olfactory bulb afferent axons, explained the interindividual diversity in sensory impairment. | |
| [138] Campabadal (2022) | 23 (13%, 87%) | 51.96 | Olfactory dysfunction = 100% | Reduced GM volume and higher MD in olfactory-related regions explain post-acute COVID-19 patients’ chronic olfactory impairments. | |
| [139] Ammar (2022) | 11 (64%, 36%) | 41.5 | Olfactory dysfunction = 100% | Individuals with anosmia reported OB imaging abnormalities that may be quantitatively assessed using the T2 FLAIR-Signal intensity ratio (SIR) and OB volumes. After the patient regained smell, the T2 FLAIR-SIR and OB volumes substantially normalized. This lends credence to the underlying mechanism of transitory OB inflammation as a cause of Olfactory Dysfunction in COVID-19 patients. | |
| [135] Çetin (2022) | 15 (73.3%, 26.7%) | 25.1 | Ansomia = 100% | According to the conclusions of this research, there is a link between loss of taste and smell and MRI findings. | |
| Peripheral neuropathy | [62] Rifino (2021) | 1760 (66%, 34%) | 64.9 (30-95) | 22.6% | In one case, the brain MRI was normal, but the spine MRI showed diffuse degeneration. When the brain MRI was normal, the roots of the cauda appeared conglutinate and showed a slight hyperintense signal in T2 sequences in another case. |
| [144] Michaelson (2021) | 14 (100%) | 57 (33-82) | 100% | Plexopathies, peripheral neuropathies, and entrapment neuropathies are examples of peripheral neurological problems. | |
| Cognitive-behavioral Disorders | |||||
| Mild Cognitive Impairment | [96] Hosp (2021) | 29 (62%, 38%) | 65.2 | - | In order to examine the effects of atrophy on partial volume effects, MRI scans were performed. In addition, the raters were made aware of four cases of cerebral (micro) infarctions and given instructions to rate any abnormalities that went beyond the scope of structural defects and possibly expected diaschisis. |
| [5] Kremer (2020) | 37 (81%, 19%) | 61 (8-78) | Alteration of consciousnes = 73% | FLAIR and diffusion-weighted sequences with variable enhancement, associated hemorrhagic lesions, and extensive and isolated white-matter micro-hematomas in the medial temporal lobe were used to identify signal abnormalities in the temporal lobe. Patients with ICH lesions had more severe clinical presentations and higher admission rates to ICUs (20 of 20 patients [100%] vs. 12 of 17 patients without hemorrhage). | |
| Confusion = 32% | |||||
| [16] García-Azorín (2021) | 233 (54.9%, 45.1%) | 61.1 | Altered mental status = 23.6% | A brain MRI revealed abnormal findings, including stroke-related changes and WM lesions caused by encephalitis. A total of 13 patients underwent a spinal MRI, which revealed signs of degeneration, as well as enhancement of the meninges and nerve roots. | |
| [65] Helms (2020) | 140 (100% men) | 62 | Delirium (84.3%) with a combination of acute attention, awareness, and cognition disturbance | Associating FLAIR and diffusion hyperintensities with multiple infra and supratentorial white-matter microhemorrhages in the brain. FLAIR hyperintensities in WM that are confluent, with small contrast enhancement foci. | |
| Neuromuscular Disorders | |||||
| Myopathy and Myositis | [63] Sanchez (2020) | 841 (56.2%, 43.8%) | 66.4 | Myopathy = 3.1% | In addition to reviewing electronic medical records, laboratory parameters, radiologic examinations (head CT or brain MRI), and neurophysiologic tests, if necessary, such as EEG and EMG, they also conducted neuropsychological evaluations. In 26 patients (3.1%), we found evidence of myopathy in the form of hyperCKemia in three of the patients. |
| [9] Khedr (2021) | 117 Patients with definite COVID-19, n = 55 (50%, 50%) | Definite | Myositis = 4.8% | A C4-T4 cervicalodorsal myelopathy was found on an imaging study; it was most likely caused by secondary occlusion of the anterior spinal artery following acute COVID-19 pneumonia, according to results from an imaging study performed after the patient had been hospitalized. | |
| Patients with probable COVID-19, n = 62 (56%, 44%) | COVID-19 = 51.5 | ||||
| Probable = 60.3 | |||||
| [18] Lindan (2021) | 38 | 0-18 | Myositis = 10% | Multifocal T2 bright lesions in the brain WM, vasculitic patterns with ischaemic lesions, enhancing neuritis or polyradiculitis, venous thrombosis, splenial lesions of the corpus callosum, longitudinally extensive myelitis, and myositis were found in the brain, cranial nerves, and spinal cord, respectively. Myositis of the visible musculature of the neck or face was found in 36% of patients with the multisystem inflammatory syndrome in children (MIS-C). There have been reports of myositis in adults who have COVID-19. Neuroradiologists should be aware of the possibility of myositis as a possible cause of neck swelling in children with MIS-C. Finally, splenial lesions and myositis of the neck and face were the most common findings in children with MIS-C. | |
Patients with mild or moderate SARS-CoV-2 infections are more likely to experience headaches, according to one study [35]. Headaches can be caused by the activation of the trigeminovascular system, which can happen when the branches of the trigeminal nerve in the nasal cavity are stimulated [36]. The occurrence of post-viral headaches is another possible indicator of CNS involvement. The close relationship between migraine, tension-type headache (TTH), and new daily persistent headache (NDPH) shows that emotional factors may play a role in the cause of headaches caused by COVID-19. It’s possible that the causes of COVID-19 headaches are multiple. However, it is still unclear how much of this headache is due to an increase in intracranial pressure (ICP) [19].
Cerebrovascular complications after stroke
Patients with COVID-19 may have neurological symptoms like ischemic strokes, encephalitis, and other life-threatening problems [5].
Meppiel et al. found that in people with acute ischaemic cerebrovascular syndrome, neurological symptoms showed up between 7 and 18 days after the first COVID-19 symptoms. There were 22.8% of patients with acute ischaemic cerebrovascular syndrome who had multi-territory ischaemic strokes, with 28.1% having large vessel thrombosis. The median duration of follow-up was 24 (17-34) days, with a high rate of short-term mortality (28/222, 12.6%). Patients with a sudden neurologic deficit due to an acute vascular lesion on a cerebral MRI or CT scan, people with a temporary focused deficit and a normal MRI, and people with cerebral venous thrombosis were all tested for stroke [37].
For patients with large-territory ischemic stroke, all but one displayed irregular proximal focal stenosis of the supraclinoid internal carotid artery. Patients who had signs of an acute ischemic stroke in large-vessel areas on CT angiography (CTA) or MR angiography (MRA), with or without signs of large-vessel occlusion (LVO), were given an informal diagnosis of “COVID-19-associated vasculopathy” [7].
Recurring neurological symptoms were seen in 33% of patients, according to Garca-Azorin et al. Most patients had general symptoms and abnormal findings from general laboratory testing (97.7%), and 99.4% of patients had abnormal outcomes. SARS-CoV-2 positive results were identified in just one of the 51 instances whose CSF assay results were abnormal. Seventy-four combinations of symptoms were observed [16].
Ischemic stroke happens more often in older people, who are also more likely to have symptoms of damage to the corticospinal tract, but are less likely to need oxygen or have ARDS. ARDS is less frequently seen in stroke patients. A total of 11 patients (including six proximal artery occlusions and two internal carotid artery dissections or occlusions) were found to have major arterial infarctions in the Kremer et al. 2020 research. COVID-19-related respiratory Symptoms began in 15 of 17 individuals with an ischemic stroke prior to this acute episode, but stroke symptoms began 2 days earlier in 2 cases. However, it now seems well established that people with COVID-19 have an increased risk of thrombotic events. There were MRI abnormalities in the majority of patients, with substantial and diverse results beyond the severe respiratory illness (half of the patients had ARDS, and 11% died); cerebrovascular disease was seen in the majority of people (especially ischemic stroke; large artery infarctions occurred more frequently than watershed cerebral infarctions) [5].
The most common result of neuroimaging was an acute stroke, which was seen in 92.5% of patients with positive results and in 1.1% of COVID-19 hospitalized patients. When age, body mass index (BMI), and high blood pressure were taken into account, patients with a major ischemic or bleeding stroke had a much higher risk of dying than COVID-19 patients who didn’t have neuroimaging [38].
Intracerebral hemorrhage (ICH)
ICH is a potentially fatal type of stroke that happens when an artery inside the brain breaks, causing a persistent accumulation of minute blood products in various brain regions known as cerebral microbleeds [39].
Martin et al. (2022) looked at the brain MR (7 Tesla) and CT scans of seven COVID-19 patients who had died and had a minimally invasive autopsy (MIA). The scans showed a number of abnormalities in the splenium, basal ganglia, WM, hippocampi, and posterior cortico-subcortical regions. The most prevalent observation was punctate cerebral hemorrhage (three out of seven cases). Signal abnormalities were found in both corticospinal pathways, bilateral basal ganglia, hippocampi, cortical and subcortical parietal bilaterally, and in the right frontal lobe in one patient with bilateral frontal and parietal subarachnoid hemorrhage (SAH). Another patient was found to have increased T2 and FLAIR signals in the splenium of the corpus callosum, which were accompanied by punctate hemorrhagic foci. The third patient exhibited some nonspecific multifocal WM abnormalities with elevated T2 and FLAIR signals and some punctate hemorrhagic foci in the right frontal WM [40].
Neuroimaging was done on 185 people with COVID-19 at Karolinska University Hospital. The average age was 62, and 138 of the people were men. A total of 222 CT brain scans, 47 MRI brain scans, and 7 MRI spinal scans were carried out. Hemorrhage was seen in 38 of the 39 patients who underwent MRI, including one ICH (2.6%) and one intracerebellar hemorrhage (2.6%), as well as nine SAHs (23%). Intra-axial susceptibility anomalies were the most prevalent result (29 of 39) in brain MRI patients, often with an oval shape indicative of microvascular disease and a preference for the corpus callosum (23 of 39) and juxtacortical regions. Macrohemorrhagic symptoms were also detected, although vascular imaging revealed no obvious abnormalities [41]. Microhemorrhages were linked to an increased risk of death [42]. It is usual to see vascular and inflammatory alterations, as well as frequent nonspherical susceptibility abnormalities, in MRI images of patients with coronavirus illness who were hospitalized. Coronavirus disease 2019 sufferers exhibited an extensive range of central and PNS involvement, including both inflammation and vasoconstriction [41].
In a look back at 37 people, most of them had ICH lesions (20 of 37), which meant they had a more severe clinical presentation and were more likely to be admitted to critical care units (20 of 20 patients). In people with ICH lesions, it took longer (33 days on average) between the start of symptoms (mostly breathing problems) and a brain MRI than in people without lesions. People with hemorrhagic lesions were more likely to have leukocytosis, anemia, and renal failure than those who didn’t have lesions. There is an association between bleeding and worse respiratory, neurologic, and biological health [43].
A retrospective, multicenter observational study with a total of one hundred patients was done, and nine of those patients (9%) presented with an ICH. Lobar hemorrhages affected five people, or 55.6% of the total population (one patient had bilateral frontal hemorrhages; there was no sign of cerebral venous thrombosis (CVT) on the MR venogram). Patients with lobar hemorrhage did not have high blood pressure, diabetes, or chronic renal failure (CRF). There were no incidences of hypertension, diabetes, or chronic renal failure (CRF) among the lobar hemorrhage patients. In four distinct cases, basal ganglia hemorrhages happened (putamen being the most common). The majority of the six patients, six in all, had severe COVID-19. Increased D-dimer levels by the median day 17 of their hospitalization were indicative of ICH occurring more frequently in patients receiving therapeutic or prophylactic anticoagulation doses, according to a previous study. Only two patients in our study developed symptomatic ICH on days 7 or 13 of hospitalization; both individuals had severe COVID-19 at the time. When the remaining patients at our hospital were brought in on the day they were hospitalized, they showed ICH-related symptoms. The absence of identified risk factors for lobar hemorrhages and their occurrence early in the course of COVID-19 sickness lends some credence to the concept of a causal relationship between COVID-19 and ICH. Despite the fact that the pathways that lead to ICH with COVID-19 are still being investigated, two possibilities have been proposed. To begin, both direct and indirect endothelial dysfunction are possible (by way of inflammatory and thrombotic responses). Second, COVID-19 may disrupt the renin-angiotensin system, leading to a loss of cerebral blood flow autoregulation and ICH [44].
23 trials were looked at in a meta-analysis that looked at 148 COVID-19 patients with intracerebral hemorrhage (ICH). The incidence of ICH was found to be 0.7%. The bulk of the patients (65.8%) were older men with comorbidities, with systemic hypertension being the most common (54%), and intraparenchymal (lobar) hemorrhage being the most frequent kind of hemorrhage (62.6%). Fifty percent of the patients received anticoagulation therapy in some form [45].
In a study, J.M. Katz and his colleagues found that 20.8% of 72 patients with infarction had related bleeding in the brain. This includes 9 people who had both bleeding and infarction at the same time, as well as 6 people who had hemorrhagic transformation, one of whom had hemorrhagic venous infarction [46].
Cerebral microbleeds (CMBs)
Researchers found that cerebral microhemorrhages are most likely to happen in the corpus callosum, juxtacortical WM, and brainstem [47].
In a study that looked back at CT and MRI scans of the brains of 34 hospitalized COVID-19 patients, it was found that nine of them had signs of bleeding. Microbleeds were the most prevalent, occurring in 7 individuals. Four cases of focal sulcal convexity SAH, three cases of superficial convexity hemosiderosis, and one case of loco-typico hematoma followed this. Microbleeds have also been seen in severely ill patients receiving therapy in intensive care units (ICUs). This is especially true for ARDS patients who are receiving improved respiratory therapy. The typical neuroimaging pattern observed in this setting consists of many microbleeds in brain tissue, with the corpus callosum being the most severely injured. There were four individuals in this group who showed a pattern of microbleeds compatible with critical illness encephalopathy. This pattern has also been seen in prior studies that looked at the neuroimaging characteristics of COVID-19 patients. Furthermore, individuals exhibited many microbleeds that were not emphasized in the corpus callosum but rather damaged the WM in a superficial and deep way. This constellation might be the outcome of therapeutic anticoagulation, which could possibly be accompanied by an ARDS or severe illness component. The SARS-CoV-2 virus may have disrupted the coagulation system in severe instances of COVID-19, and possible viral endothelium disturbances mediated by the ACE2-receptor may also be implicated in the etiology of these microbleeds. Both of these elements may have a role in the etiology of COVID-19. Other hemorrhagic signs seen included convexity hemosiderosis, isolated cortical SAH, and frank cerebral hematomas. Iatrogenic anticoagulation and unstable blood pressure are two more probable causes of these events. It is worth noting that in certain individuals, both sulcal SAH and superficial siderosis were shown to be topographically related to the vascular boundary zones, indicating a failure in cerebral auto vasoregulation [48].
In their study, Aikaterini Fitsiori et al. reported the MR scans of nine people with a consistent pattern of abnormal results (2 women and 7 men). The SARS-CoV-2 virus was discovered in all cases. Each person had either a severe (5/9) or moderate (4/9) episode of ARDS, necessitating a lengthy stay in the ICU. The presence of microbleeds in an unusual distribution, with a predilection for the corpus callosum, was discovered consistently throughout their MRI exams. Microbleeds were also seen in the middle cerebellar peduncles (5/9) and the internal capsule (5/9). These are also uncommon locations. The majority of individuals exhibited subcortical problems as well [49].
Patients given CT, MRI, EEG, CSF analysis, and autopsies if they died were found in an evaluation of 32 COVID-19 patients who got sicker and sicker over time and were treated at a tertiary care center for severe CNS involvement at the same time. Eight of the 32 COVID-19 patients who were very sick, or 25%, had serious problems with their CNS. With the exception of one case, neuroimaging or autopsies indicated many brain microbleeds. Three of the patients had experienced additional SAH, and two had extra-minor ischemic lesions. An MRI was used on three distinct people to image the cerebral vascular wall. All patients had contrast enhancement of vessel walls in major cerebral arteries, indicating the presence of vascular wall disorders with an inflammatory component [50].
A total of 2054 patients with laboratory-confirmed cases of COVID-19 were included in a retrospective cohort study done at two hospitals in New York. The average age of these patients was 64, with women accounting for 43 percent. 278 patients had their brains scanned utilizing CT or MR technologies. 3.6 percent of the patients that were scanned had parenchymal hematomas, and the majority of these patients (n = 6) had anticoagulation-related hemorrhages. This study emphasizes the dangers of starting anticoagulant medication in response to prothrombotic characteristics in COVID-19 patients. 26 percent of the 51 individuals who received MR imaging exams displayed acute or subacute abnormalities. Three individuals showed a microhemorrhage pattern consistent with severe illness associated with microbleeds (5.9%), and one patient had a SAH (2.0%). Twenty-six of the patients who received MR imaging revealed foci of age-indeterminate microhemorrhage. This equates to a percentage of 51%. The majority of these people (n = 17) had one to three microhemorrhages, which prevented further characterization from being undertaken. Seven of the individuals had a higher burden of microhemorrhage (> 15 foci), accounting for 14% of the total. One of these patients had a cortical and subcortical distribution, sparing the deep GM structures in a pattern consistent with cerebral amyloid angiopathy; another had predominant involvement of the basal ganglia and cerebellum, with both the microhemorrhage distribution and clinical history consistent with a hypertensive etiology; and a third had foci consistent with previously treated widespread metastatic disease. Another patient appeared with a general pattern that may be linked to a number of chronic infarcts, but this person also had a history of hypertension. The three patients who were still alive and had a higher burden of microhemorrhage had a plethora of foci, the vast majority of which were found in the splenium of the corpus callosum. There were also many foci along the rest of the corpus callosum, internal capsules, and juxtacortical WM. There were no foci that encompassed the cerebellum, the brain stem, the deep GM, or the cortex. The distribution of microbleeds in all three people was not typical for either cerebral amyloid angiopathy or hypertensive microhemorrhage; rather, it was most consistent with critical illness-associated microhemorrhage. Although all three patients had intubation and mechanical breathing throughout their lengthy hospitalizations in the critical care unit, none of them got extracorporeal membrane oxygenation. Two of the three people had a history of hypertension, but none had a history of epileptic seizures or usage of antiepileptic medicines. Two additional patients had microhemorrhages confined to the splenium of the corpus callosum, increasing the total number of patients with fewer than 15 foci of microhemorrhage to 14. With the exception of one patient, all of the patients in our cohort with acute parenchymal hematomas were men, with a median age of 68 years. Six of the 10 hematomas were greater than five centimeters in diameter, and they all featured intraventricular extension, surrounding edema, a shift in the midline, and a herniation lower down. 26 of the 51 patients had microhemorrhages, with three having severe microhemorrhages mostly affecting the corpus callosum. These microhemorrhages were analogous to those seen in a recent case report of a patient with COVID-19 and posterior reversible (leuko) encephalopathy syndrome (PRES), and they were also compatible with the results of other recent observational research that identified four COVID-19 patients who had microhemorrhages in the corpus callosum. High-altitude cerebral edema and microhemorrhages are thought to be caused by increased cerebral venous pressure. Because of the increased intrathoracic pressure, positive pressure breathing may impair cerebral venous return. Instead of being caused by the virus, the callosal microhemorrhage detected in COVID-19 might have been induced by mechanical ventilation [51].
According to Chougar et al.’s study, 43 of the 73 patients who came with acute neurological symptoms had aberrant MRI results (58.9%), including 8 with multiple microhemorrhages and specific involvement of the corpus callosum. Microhemorrhages (5) were more common in ICU patients than in non-ICU patients (20.6% vs. 2.7%), and the corpus callosum was linked to the status of people. Patients who have had microhemorrhages in the past often have a longer partial thromboplastin time after taking anticoagulant drugs. Extracorporeal membrane oxygenation was used on 28.6% of people with multiple microhemorrhages in the critical care unit [22].
Encephalopathy
Pyramidal tract dysfunction (like muscle weakness, stiffness, hyperreflexia, and Babinski symptoms), seizures, and headaches are common with encephalopathy. Signs of encephalopathy (5% of the time) include confusion, loss of consciousness, pathological wakefulness when sedatives are stopped, agitation, and hallucinations. Patients admitted to the ICU had a greater rate of encephalopathy (present in 41 of 47 ICU patients [87%] vs. 6 of 11 non-ICU patients [55%]) [22]. In the absence of other criteria for encephalitis, encephalopathy was defined as AMS that lasted for 24 hours and was linked to seizure activity and/or focal neurologic symptoms. There was no evidence of encephalitis. According to the reporting physician, they determined that COVID-19 associated encephalopathy (CAE) was the cause of encephalopathy if there was no other explanation, such as toxic or metabolic factors [37].
In the study conducted by Mahammedi et al., 725 consecutive COVID-19 patients who were hospitalized were examined. 15% of them, or 108 in total, met the eligibility standards. Non-contrast brain CT was conducted on 107 (99%) of the 108 patients; head and neck CTA on 17 (16%) of the patients; and brain MRI on 20 (18%) of the patients. Ten of these patients, or 50%, had an MRI of the brain with and without intravenous (IV) contrast; ten of these patients, or 50%, had an MRA of the head and neck; and three of these patients had an extra MRI of the whole spine to evaluate lower extremity weakness. Among the 108 patients, 71 (or 66%) had no acute findings on brain CT; nevertheless, 7 (or 35%) had acute abnormalities on brain MRI. There was a statistically significant association between the prevalence of AMS and the patient’s age (72.11 vs. 64.18 years), particularly [52].
There have been reports of severe neurologic diseases in COVID-19, but they are rare, show up in different ways, and get worse quickly [53,54]. Patients of Scullen and his colleagues changed in ways that were hard to predict along one of three presentation spectrums. During their disease, most of the patients developed severe electrographic nonspecific encephalopathy, and their clinical AMS ranged from mild to severe. Imaging abnormalities of a general nature were often seen in these patients, such as low density or low attenuation of deep tissues. Because their symptoms are so bad, these people can be told that they have COVID-19-associated encephalopathy. One of our patients had status epilepticus without convulsions, which is similar to how COVID-19-associated epileptic encephalopathy is described in medical literature [7,55].
In the study of 481 brain MRIs, 9.7% of patients were hospitalized with COVID-19 pneumonia. A SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) test showed microbleeds, osmotic demyelination, arterial thrombosis, ischemic infarcts, venous thrombosis, metabolic cerebellar syndrome, and posterior relapse. Additionally, they discovered two cases of posterior reversible encephalopathy syndrome (0.41%), of which one was due to a COVID-19 infection [56].
In a retrospective study, a brain MRI was done on 25 people who had confirmed instances of COVID-19. This group consisted of 19 men, ranging in age from 38 to 85 years. The results show that COVID-19-related encephalopathy can be measured with systematic multimodal MRI. They suggest that any cause of brain hyperperfusion, such as arterial hypertension, inflammation, and sepsis, as well as any source of diminished brain oxygenation, such as microthrombosis or even mild anemia, should be limited. They also give evidence of a critical role for any treatment that has the capacity to reduce the damage produced by COVID-19 to endothelial cells [57].
Another study looked at a group of people who had been diagnosed with a coronavirus infection. This group consists of 31 people who have all been diagnosed with acute encephalopathy. Concurrent neurological disorders such as stroke and meningitis were checked out before including these individuals. Based on severity, a distinction was drawn between severe and moderate instances of COVID-19 encephalopathy. There was no link found between the severity of the pneumonia and the COVID-19 encephalopathy. There was no difference between the groups and 28 of the 31 patients, or 90%, experienced ARDS [58].
There were 3,403 people who tested positive for SARS-CoV-2 infection, and their medical records were reviewed retrospectively. Out of the 3,403 people who were diagnosed with COVID-19, 167 (4.9%) had neurological symptoms or signs that needed to be checked out with neuroimaging. The most common symptoms were delirium (44/167 cases, or 26 percent), focal neurology (37/167 cases, or 22 percent), and altered awareness (34/167 cases, or 20 percent). Neuroimaging found abnormalities in 23% of patients, with CT revealing 18 abnormalities and MRI revealing 20 abnormalities. The most common neuroradiological result was microhemorrhage, with a preference for the splenium of the corpus callosum (12/20, 60%). This was followed by an acute or subacute infarct (5/20, 25%), watershed WM hyperintensities (4/20, 20%), and susceptibility alterations in the superficial veins on SWI (3/20, 15%). Acute hemorrhage was another neuroradiological result. Several imaging patterns were identified on the MRI, including stroke, acute hemorrhagic necrotizing encephalopathy, hypoxic-ischaemic alterations, acute disseminated encephalomyelitis (ADEM)-like abnormalities, and WM hyperintensities. The occurrence of microhemorrhages was the most common finding [59].
Meningitis, encephalitis, and myelitis
The following are the most prevalent types of encephalitis: (a) limbic encephalitis; (b) cytotoxic lesion of the corpus callosum (CLOCC); (c) radiological ADEM; (d) radiological acute hemorrhagic necrotizing encephalopathy; and (e) miscellaneous encephalitis. Encephalitis was characterized as aberrant FLAIR hyperintensity in the brain parenchyma, including the GM, WM, and/or basal ganglia, with varying enhancement. In the instance of limbic encephalitis, this aberration was largely located in the medial temporal and inferior frontal lobes [5].
Varatharaj and colleagues discovered that the average age of their patients was 71 years old. Full clinical datasets were available for 125 of 153 patients, accounting for 82 percent of the total. 39 patients out of 125 presented with AMS, including nine with unexplained encephalopathy (23%) and seven with encephalitis (18%). This accounted for 31% of all cases. An AMS, which includes encephalopathy or encephalitis as well as serious psychiatric illnesses, was the second most common symptom. This symptom was more common in those under the age of 30 [60].
Altered mental status and delirium
When people with COVID-19 go to the hospital, they often show signs of AMS, which is a neurological complication. AMS has been linked to death and longer hospital stays in a number of studies. Recent research shows that patients in the ICU often show signs of encephalopathy, such as agitation, confusion, and damage to the corticospinal tract [5].
A total of 58 of 64 consecutively hospitalized patients in Strasbourg were found to have neurological symptoms. The majority of them (67%) occurred when no sedation was given, and they included agitation (69%), confusion (65%), and diffuse corticospinal tract signs (67%) [50].
In a study of people with multiple sclerosis [61], it was found that anti-CD20 antibodies were linked to more severe forms of COVID-19, such as severe symptoms like AMS.
Between February 24th and April 30th, a total of 1760 COVID-19 patients were admitted to ASST Papa Giovanni XXIII, and 1650 of them were either discharged or are still being treated at the hospital. 137 of them had neurologic symptoms following the onset of COVID-19 symptoms. 49 of the patients showed evidence of disrupted alertness and/or consciousness, classifying them as having an AMS. Everyone had a brain CT scan or MRI. CSF testing was performed on twenty-one patients, and SARS-CoV-2 RT-PCR was performed on all of them. After analyzing their clinical symptoms, CSF data, and neuroimaging, we determined that five people had encephalitis. One patient had HSV1-related encephalitis, another had necrotizing encephalitis, and two had SARSCoV-2 identified in their CSF via RT-PCR. There was a tendency toward increased mortality in patients with cardiovascular disease when compared to those with PNS involvement (38.5% vs. 11.8%), but this trend was not evident when patients with AMS were examined (38.5% vs. 28.1%). Individuals with PNS involvement exhibited a significantly greater prevalence of moderate to severe ARDS as compared to patients with cardiovascular illness (87.1% vs. 42%) and patients with profoundly disturbed mental states (87.1% vs. 55.6%) [62].
In March 2020, 57.4% of the Spanish people who were diagnosed with COVID-19 and admitted to the hospital said they had some kind of neurological symptom. This result was confirmed after a thorough examination of the instances. Consciousness disturbances were common, occurring more often in older people and those in more severe and advanced COVID-19 stages (relative to less severe stages) (38.9% vs. 7.2%). During COVID-19 stage III, two patients appeared with low degrees of consciousness and pyramidal symptoms. In both cases, the patient’s brain MRI came out negative. According to the findings, severe hypoxia (platelet-aggregation factor inhibitor level less than 300) was the primary cause of the majority of cases of altered consciousness and was closely associated with the disease’s advanced stage. Previous research suggests that this global brain dysfunction occurs in the context of multiorgan failure caused by a combination of factors such as hypoxia, blood-brain barrier (BBB) dysfunction, cerebrovascular disease, toxic metabolites (uremia, ammonium, and electrolyte dysregulation), and cytokine release syndrome, as seen in chimeric antigen receptor T-cell therapy-associated neurotoxicity [63].
Hospitalized people with SARS-Cov-2 from four hospitals in the Chinese province of Hubei were chosen for a retrospective cohort study between January 18 and March 10, 2020. There were 1268 individuals in all, with 162 (12.8%) experiencing CNS symptoms. There were 34 instances of somnolence, 71 cases of unconsciousness, 69 cases of coma, and 50 cases of dysphoria among these symptoms. When each symptom was assessed separately, somnolence was shown to be the earliest indication, arriving 12 days after the onset of symptoms, while coma was discovered to be the last symptom, coming 16 days after the onset of symptoms. The highest risk of death was associated with coma, then unconsciousness, somnolence, and dysphoria [64].
In France, a study was done in the two ICUs of the Strasbourg University Hospital as part of a two-center cohort study. The study enlisted the participation of all 140 patients. Twenty-two of the patients showed normal neurological examination findings (15.7%). There were 118 cases of delirium, a condition that causes severe problems with cognition, awareness, and attention. Despite receiving high infusion rates of sedative treatments and neuroleptics, 88 patients developed an unexpected state of agitation. Patients in the COVID-19 research who had delirium or neurological symptoms required more mechanical breathing than patients in the control group who did not have delirium or neurological symptoms. A systemic inflammatory response to SARS-CoV-2 might result in secondary consequences such as delirium or neurological symptoms. According to a recent study, the majority of patients, or 84 percent, who are admitted to critical care units for ARDS caused by COVID-19 may develop neurological symptoms, most notably delusional symptoms. ICU delirium is characterized by variable deficits in attention and cognition that occur over a short period of time and are not explained by pre-existing neurocognitive illnesses. These symptoms may appear at any point throughout the patient’s stay in the critical care unit. Delirium has been linked to poorer outcomes in critically ill patients, such as longer hospital stays, an increased risk of long-term neurocognitive and neuropsychiatric problems, and even mortality in critical illness survivors. 118 patients had delirium or abnormal neurological examinations at some point during their stay in the ICU. When these patients were admitted to the ICU, 22 (18.6%) showed indications of delirium and/or corticospinal tract dysfunction. Delirium was observed in 97 of 122 patients (or 79.5 percent) throughout their stay in the critical care unit based on a positive confusion assessment method for the ICU (CAM-ICU). The CAM-ICU test detected delirium in 97 of 122 patients; 84 (86.6 percent) of those patients were found to have hyperactive delirium, while the remaining patients were found to have hypoactive delirium. Despite having noninvasive ventilation and kinesitherapy, the 11/11 patients who experienced auto-extubation required quick reintubation owing to sudden respiratory failure. Each of these individuals was suffering from delirium on the day of their auto-extubation. The duration of invasive mechanical breathing was considerably longer in patients with delirium and/or abnormal neurological examination than in patients with a normal neurological examination and no delirium. Furthermore, patients with delirium had a longer stay in the critical care unit. In this study, we talk about how patients with COVID-19-induced ARDS were more likely to have delirium and/or neurological symptoms and had a worse prognosis than patients who did not have delirium and had normal neurological tests. Delirium and/or neurological symptoms necessitated the use of invasive mechanical breathing for an extended period of time, as well as abnormally high doses of sedation and neuroleptics. Patients who did not have delirium and whose neurological evaluation was normal, on the other hand, were able to be extubated and released from the critical care unit quicker. Delirium was also linked to severe hypoxemia after reintubation, which may have contributed to the lengthy process of weaning the patient off the ventilator. Another approach to delirium increased the risk of possibly deadly inadvertent extubation. The majority of COVID-19 delirium patients in the ICU were male. The most noticeable signs of delirium are acute issues with attention and consciousness, as well as cognitive functioning. As previously stated, delirium (positive CAM-ICU) or acute encephalopathy may be caused by systemic inflammatory reactions to SARS-CoV-2 rather than by SARS-CoV-2 itself. This is due to the fact that SARS-CoV-2 produces inflammation throughout the body. In a multicenter study of 497 patients, Salluh et al. discovered an incidence of delirium of 32.3% using the CAM-ICU score. Khan et al., on the other hand, recently found that delirium occurred in 16.5 percent of the 2742 ICU patient participants. We discovered that the prevalence of delirium in COVID-19 patients was relatively high, at 79.5% [65].
Seizure
It is likely that COVID-19 will cause some SARS-CoV-2 patients to have seizures because of low oxygen levels, metabolic problems, organ failure, or even brain damage. This is a reasonable assumption, since neurotropism is a trait that most coronaviruses share and has already been proven. In the case of encephalitis, SARS-CoV infection has been said to be able to directly cause seizures. On the other hand, seizures caused by hypoxia, metabolic derangement, medications, multiorgan failure, or even brain damage have been said to be able to cause seizures indirectly. Seizures are quite rare in COVID-19 patients [66].
Khedr (2021), Participants were monitored as part of a retrospective cohort research project in Upper Egypt between June 1 and August 10, 2020 (Assiut and Aswan). There were 439 COVID-19 cases, and 19 patients, or 4.3 percent, had acute symptomatic seizures. 0.68 percent (3 out of 19) of the 439 people studied experienced new-onset seizures without an underlying illness. The remaining 0.46 percent (2 out of 19) had been diagnosed with controlled epilepsy with breakthrough seizures earlier. The majority of cases (14 individuals, or 3.19%) had initial pathology that might explain the occurrence of seizures (post-COVID-19 stroke, COVID-related encephalitis, elderly ischemic stroke patients, patients with simultaneous pineal and brain tumors). The goal of their study was to evaluate the frequency of seizures and classify them as primary or secondary. The determination of the kinds of seizures associated with COVID-19, as well as the issues that may emerge from having many seizures or having status epilepticus, as well as the relationship with laboratory and imaging results Seizures are a typical side effect of a post-COVID-19 infection. They saw seizures that had recently begun as well as seizures that were a result of the initial brain injury (post-COVID-19 encephalitis or stroke as well as an old stroke). The accumulation of cytokines both centrally and peripherally, as well as fever and hypoxia, may all play a role in the development of seizures [67].
Lersy (2020), In France, clinical, laboratory (CSF analysis), and brain MR imaging data from 58 people with COVID-19 and neurological symptoms were collected and analyzed retrospectively. Patients with neurological symptoms caused by COVID-19 are more likely to show abnormalities on brain MR imaging, such as leptomeningeal enhancement, as well as higher inflammatory markers in their CSF, while SARS-CoV-2 detection in their CSF remained limited. The MRI of the brain in 17 people was found to be normal and unrelated to the current acute episode (32%). On their brain MR images, twenty patients, or 38%, displayed leptomeningeal enhancement. These individuals regularly had WM lesions that were broad, ill-defined, and confluent in supratentorial WM. The pathogenic brain MR pictures included 36 images, or 68 percent of the total. Gray matter lesions were seen in one patient (2%) with status epilepticus alterations and one patient (2%) with FLAIR hyperintensities affecting the mesial temporal lobe. FLAIR hyperintensities were found in 4 patients (8%), 2 (4%) had acute inflammatory demyelinating lesions, and 1 had FLAIR hyperintensities involving the middle cerebellar peduncles (2%). Brain MR scans were performed on five of the six people who were having seizures, and the findings were normal in two of them [68].
Pranusha Pinna (2020) examined the data records from 50 COVID-19 patients who underwent evaluations by American neurology services. There were 48 percent of these patients of African American heritage and 24 percent of Latino descent. The AMS was seen in 60% of the patients (n = 30), making it the most frequently observed symptom. Cerebrovascular incidents occurred in 40% (n = 20) of patients and were classified as follows: Ischemic stroke occurred in 20% (n = 10) of patients, ICH in 8% (n = 4) of patients, non-aneurysmal SAH in 8% (n = 4) of patients, and transient ischemic attack (TIA) in 4% (n = 2) of patients. Short-term memory impairment was the most prevalent cognitive deficit, with headache coming in second at 24% (n = 12), and new-onset seizures, also known as “breakthrough seizures”, occurring in 26% of patients (n = 13). The characteristics of the headache and the seizure pattern were not reported. 14 percent of patients (n = 7) had hypoxic ischemic brain injury, and five had abnormal extraocular movement. MRI scans were not performed as part of this inquiry [69].
Pons-Escoda (2020) performed research that comprised a cross-sectional investigation of 2249 people diagnosed with COVID-19, and 103 of those people (61% males, 39% women, a mean age of 74 (50.2-90)) underwent neuroimaging. They focused on those who arrived with neurologic symptoms that necessitated neuroimaging and analyzed one of the largest groups of COVID-19 patients. This study’s findings were made public. Despite the enormous number of COVID-19 patients, the researchers discovered a considerable percentage of symptomatic patients with negative neuroimaging data. As a result, drawing any inferences regarding the particular correlations that exist between neuroimaging and COVID-19 is difficult. It seems reasonable that virus-associated coagulopathy, if it exists, would increase the risk of cerebrovascular accidents (in their experience, possibly more hemorrhagic). A CT scan was performed on all three patients due to their convulsions. They showed no symptoms of being very ill [70].
Romero-Sánchez (2020), There were 56.2% of men and 43.8% of women among the 841 individuals hospitalized with COVID-19 in Spain’s population during March 2020. The mean age of these patients was 66.4. Myalgias, headaches, and dizziness were the nonspecific symptoms that manifested themselves the majority of the time throughout the early stages of the illness. Six individuals had seizures, accounting for 0.7% of the total population. However, only one of these patients had a history of epilepsy. Status epilepticus did not aggravate any of these cases in any way. Four instances were found in patients who were in the severe stage of the illness, and two of those cases happened after cerebral hemorrhages. In three of the individuals, the onset of seizures was localized. It is noteworthy that a history of cognitive impairment (CI) was shown to be related to seizures in the setting of COVID-19. There was no correlation between the severity of the illness and the kind of seizure, as well as any other medical evidence [63].
Azab (2021), a multicenter retrospective study, looked at 977 patients in Egypt who had SARS-CoV-2 and developed neurological problems after getting COVID-19. These patients were 45% male and 55 percent female, with a mean age of 60.15 years (18 and older). Based on the information they had, 277 people had seizures after being exposed to COVID-19. They can’t tell if COVID-19 is the cause of these seizures because they haven’t ruled out any other possible causes. They did not conduct an EEG or MRI on those people due to a lack of resources. Patients’ first seizures were looked at, and there was no sign that they were more likely to happen suddenly [71].
García-Azorín (2021), A 233-case multicenter study of patients with neurological signs of COVID-19 in Spanish society (57.9% men, 42.1% women, and a median age of 61.1 years). Stroke accounted for 27% of all occurrences, followed by AMS (23.6%), neuromuscular symptoms (23.6%), anosmia (17.6%), headache (12.9%), and seizures (11.6 percent). A brain MRI was done in 57 out of 219 patients (26.0%), with abnormal results in 20 of the 57 instances (35.7%). These aberrant results comprised 16 instances of stroke-related abnormalities, 2 cases of encephalitis-related alterations, and 2 cases of non-specific WM lesions. EEG was done on 36 (15.4 percent) of the cases; the findings were normal in 13/36 (36.1%) of the patients and exhibited diffuse slowing in 14/36 (38.9%). Moreover, it indicated symptoms of encephalopathy in 6/16.7% of the patients and focal epileptic activity in 13.9% [16].
Neuropsychiatric symptoms
Patients with COVID-19 often have respiratory symptoms, but neurological and neuropsychiatric problems are also being reported more and more [60,72]. Through histopathological tests on the brains of COVID-19 patients who had died, it has also been shown that SARS-CoV-2 can infect the CNS [73]. This includes milder symptoms like dizziness and anosmia [74], as well as more serious conditions like acute demyelinating encephalopathy [75], meningitis [53], and strokes in rare cases [76,77]. Recently, patients with COVID-19 from the United Kingdom who experienced neurologic and psychiatric complications during the acute phase of their disease were reported, and ICH and AMS were among the most common symptoms [60]. The same was found in a Wuhan study of 214 patients, in which 78 patients had unspecified neurological symptoms and 13 patients were diagnosed with a new cerebrovascular disease during an acute infection [29]. In a UK-wide surveillance study of acute COVID-19, it was found that cerebrovascular events and AMS were common neuropsychiatric symptoms [60]. 59% of the people with AMS had neuropsychiatric disorders, which shows the wide range of COVID-19-related symptoms [78].
There were 167 (19.9%) patients with neuropsychiatric symptoms in Romero, Carlos Manuel, et al.’s 2020 study, with insomnia being the most common symptom (13%), followed by anxiety (8.1%), depression (5.2%), and psychosis (1.3%). There was no correlation between any of these signs and the severity of the disease [63].
There were 23 patients (59%) in the Aravinthan Varatharaj et al. 2020 study who met the clinical case definitions for psychiatric diagnoses as classified by the notifying psychiatrist or neuropsychiatrist, and 21 (92%) of these were new diagnoses. In 23 patients with neuropsychiatric disorders, ten (43%) had new-onset psychosis, six (26%) had a neurocognitive (dementia-like) syndrome, and four (17%) had an affective disorder [60].
Ten mild cases and nine severe cases were recovered from COVID-19 (RecCOVID) in Du et al.’s study, according to World Health Organization (WHO) guidelines. Most people with RecCOVID went to the hospital because they had a fever (79%) and a cough (74%), as well as dyspnea (42%), loss of smell (42%), loss of taste (37%), and tiredness (37%). Fever (11%), cough (37%), olfactory loss (5%), and taste loss (5% each) significantly improved after one year of follow-up. However, at the 1-year follow-up, the RecCOVID group had more chest tightness and headaches (36% fewer) than the SARS-CoV-2-infected group. At the one-year follow-up, 32% of patients still had dyspnea, 21% had fatigue, and 36% had myalgia [79].
Researchers found that the RecCOVID group had significantly higher amplitudes of low-frequency fluctuation (ALFF) in the left precentral gyrus (PreCG), middle frontal gyrus, inferior frontal gyrus of the operculum, inferior frontal gyrus of the triangle, insula, hippocampus, parahippocampal gyrus, fusiform gyrus, postcentral gyrus, and angular gyrus. Among the clusters, the left hippocampus had the highest peak [79].
Cerebellar ataxia with myoclonus
The cerebellum, a region of the brain that controls muscle coordination, is frequently the site of ataxia injuries. Alcohol abuse, stroke, tumors, brain degeneration, multiple sclerosis, certain medications, and genetic disorders are just a few of the conditions that can result in ataxia [80].
There were 509 total patients, of whom 228 (44.8%) were female and 281 (55.2%) were male. 95.8% of COVID-19’s neurological symptoms included one or more of the following: myalgias, headache, encephalopathy, dizziness, dysgeusia, or anosmia. On the other hand, only 0.2 to 1.4% of patients had focal motor and sensory deficits, ataxia, ischemic and hemorrhagic stroke, movement disorders, or seizures. ADEM and GBS were not found in any patients. Patients reported having neurologic symptoms at a median rate of 2 [81].
Mohammed Azab et al. examined 1,500 people in total, but they only discovered that 190 of them (105 men and 85 women) had ataxia. Optic neuritis, seizures, and ataxia were the most common symptoms. Acute ischemic strokes and cerebral venous sinus thrombosis occurred in a small number of patients [71].
Miller fisher syndrome (MFS)
Ataxia is thought to be caused by the cerebellum, while areflexia is thought to be caused by the lower motor neurons. In 2019, the new coronavirus disease has been linked to new cases of MFS, but no imaging results have been found. COVID-19 patients usually have fever, shortness of breath, and cough, but headaches, ataxia, CI, anosmia, and stroke are also possible neurological symptoms. COVID-19-associated MFS has not previously been documented with imaging. High-resolution imaging of the orbits and retro-orbital region, including gadolinium-enhanced MRI of the left cranial nerve III, was notable for its striking enlargement, prominent enhancement with gadolinium, and T2 hyper-intense signal. Neither the signals nor the enhancement of any other cranial nerves were abnormal. There were no abnormalities in the brain’s MR imaging. A cerebellar abnormality that was visible on the MRI did not contribute to the patient’s ataxia. Neither meningitis, encephalitis, demyelination, nor an infarct were discovered during this investigation. MR imaging of the spine, which could have provided an imaging correlate for the patient’s areflexia, was not carried out. MFS, which is more prevalent in men than women and typically precedes an upper respiratory illness, is the cause of one to five percent of Guillain-Barré syndrome cases in western countries. Diplopia (78%), ataxia (48%), or both (34%), are common symptoms in MFS. The patient had diplopia in addition to COVID-19 symptoms, which the patient’s clinical examination revealed was the result of a cranial nerve III palsy. On MR imaging, T2 hyperintensity and enhancement of the affected cranial nerve III from the cavernous sinus to the orbit were seen. Preliminary imaging findings support the hypothesis that this is the first case of MFS linked to a COVID-19 infection to be reported [82].
One of the people in a study done by E. Lin in 2020 was found to have diplopia, ataxia, and areflexia, which are all forms of Guillain-Barré syndrome that affect the cranial nerves. By using orbital sequences for MR imaging of the brain, it was possible to see a T2-hyperintense left oculomotor nerve enlargement and significant nerve enhancement after IV gadobutrol administration [51].
Dementia
Along with chronic diseases like heart and lung diseases, high blood pressure, diabetes, obesity, and cancer, dementia has been found to be a risk factor for COVID-19. The reasons behind this association are still unknown, though. Individuals with dementia are more likely to be infected with COVID-19 because of the higher risk of infection associated with living in care or nursing homes, being dependent on outside caregivers, or otherwise not being able to maintain personal hygiene or preventative health measures on one’s own [83].
Among the 12,863 people who were tested in a Brazilian cohort study, 6228 were hospitalized and 6631 were not; 6232 were hospitalized. Age, male sex, BMI, and chronic conditions, such as high blood pressure, diabetes, cancer, and cardiovascular disease, were found to be associated with an increased risk of hospitalization. However, there was no correlation between all-cause dementia, Alzheimer’s disease (AD), or Parkinson’s disease (PD). According to a univariate analysis of 932 COVID-19-positive inpatients and 5300 COVID-19-negative inpatients, those with all-cause dementia, AD, and PD were more likely to be hospitalized for COVID-19-related reasons. These findings suggest that COVID-19-infected elderly patients are more likely to suffer from severe outcomes if they have dementia due to any cause [83].
In Spain’s retrospective study, COVID-19 was confirmed in 281 of 477 adult cases that died. Around 30 percent of participants in each group had some degree of CI. Of these, 21.1% had dementia (of which 4.3% had mild dementia, 10.0% had moderate dementia, and 6.8% had severe dementia), and 8.9% had some form of mild cognitive impairment (MCI). Following hypertension (69.4%), diabetes (33.8%), and cardiovascular disease (33.5%), CI was the fourth most common comorbidity. Patients with confirmed COVID-19 CI were diagnosed with AD (9.3%), mixed (7.2%), and vascular CI (4.8%), respectively. A significant number of patients in both groups (32.1% vs. 14.7%) had encephalopathy, which was more common in those with CI. In terms of medical care, only one patient with CI was admitted to the ICU, and fewer patients with CI received non-invasive mechanical ventilation (7.1%) than those without the condition (25.4%). Patients with CI received more palliative care than those without CI (79.2% vs. 66.3%) [84].
PNS
GBS and its variants
Zhao et al. described the first GBS patient from COVID-19 [85]. As part of the “long COVID-19 syndrome”, GBS has been documented. In spite of this, there are reports that GBS is a viral infection-related paralysis. In most cases, the acute onset of a viral infection occurs within a few days [86].
COVID-19 has also been linked to GBS variants that hurt the brain, like Miller-Fisher syndrome and cranial polyneuritis [87].
Patients with Guillain-Barré-related PNS disorders were found in one patient (2%), according to François Lersy and colleagues. In addition, a Guillain-Barré syndrome patient underwent spinal cord MR imaging. A brain MRI revealed multiple cranial nerve enhancements in one patient (2%) [68].
Lin et al. (2020) conducted a retrospective cohort study in New York City, enrolling 2054 patients with laboratory-confirmed COVID-19, of whom 278 (14%) underwent brain imaging with either CT or MRI. The first patient showed signs of MFS, a cranial nerve variant of Guillain-Barré syndrome, including diplopia, ataxia, and areflexia. After IV gadobutrol, MR imaging of the brain revealed an enlarged, T2-hyperintense left oculomotor nerve with marked nerve enhancement. After IV immunoglobulin treatment, this patient had negative serum antiganglioside testing, and the symptoms improved. MR imaging revealed enhanced optic nerve sheaths and posterior tendon capsules in a second patient with painless diplopia and a right abductor palsy [51].
In another study with 725 people, neuroimaging was done on people with COVID-19 who had sudden neurological symptoms. Cauda equina enhancement was found in two patients with Guillain-Barré syndrome who underwent MRI. A 62-year-old man with MFS had bilateral facial nerve palsy, ophthalmoplegia, areflexia, and polyradiculopathy, but his CSF tested negative for SARS-CoV-2 [52].
In another study, there were 2249 COVID-19 patients, 112 of whom had head neuroimaging, and only one of these patients had Guillain-Barré syndrome and normal neuroimaging findings (CT) [70].
According to Mohammed A. Azab et al., 102 patients in their case series had GBS. As there are no other possible causes, they are almost certainly connected to COVID. The neurological and CSF tests confirmed the presence of GBS. The COVID-19 nasopharyngeal swab PCR was positive in all patients with GBS. This study does not have any MRI data for these patients [71].
There were 439 patients with confirmed or probable COVID-19 in another study, and 222 of those patients had neurological symptoms. 42 patients had problems with their PNS. Most of them had anosmia and ageusia (31), while the others had Guillain-Barré syndrome (4), peripheral neuropathy (3), myasthenia gravis (MG, 2), or myositis (2). In this study, GBS was found to be a common neurological complication of COVID-19. It was found that four patients had the classic symptoms of acute symmetric flaccid weakness, with absent reflexes and numbness of four limbs, 2-3 weeks after COVID-19. Degenerative polyradiculoneuropathy was diagnosed through the use of nerve conduction, F waves, H reflexes, and electromyograms [9]. A wide range of neurological conditions were found in patients with SARS-CoV-2 infection, including encephalitis (N = 8), encephalopathy (N = 6), cerebrovascular events (ischemic strokes (N = 4), and vein thrombosis (N = 2)), other CNS disorders (N = 4), and GBS (N = 2). A total of 26 patients were diagnosed with neurological disorders. For the diagnosis of GBS, electrophysiology results associated with clinical and CSF testing were utilized [88].
At the end of a research project involving 841 patients hospitalized with COVID-19 (mean age of 66.4 years, 56.2% men), Romero-Sanchez et al. found that 57.4% of them experienced some form of neurological symptom. There has been one case of Guillain-Barré syndrome reported [63].
There was only one case of Guillain-Barré syndrome among the 239 patients studied by Karadaş et al. in 2020 (133 men and 106 women) [89].
Smell and taste disorders
Smell and taste disturbances (STDs) are common in patients with COVID-19. STDs appear early in the course of the disease, are more common in SARS-CoV-2 infections than in other upper respiratory tract infections, and may persist long after the onset of respiratory symptoms. Inflammation and the subsequent dysfunction of supporting non-neuronal cells in the mucosa are thought to be the primary causes of the loss of olfactory sensory neurons and taste buds that occurs in STDs. There have been other hypotheses about the possible causes of chemosensory dysfunction, and there have been conflicting reports about the direct infection of sensory neurons by SARS-CoV-2 [90].
Olfactory tests indicated that patients were anemic at the time of imaging. 73.9% of cases showed olfactory cleft opacification on CT, with a preference for the mid and posterior segments. Olfactory bulb volumes were less than normal in 43.5% of cases, and sulci were shallow in 60.9% of cases. 54.2% of all cases showed a deviation from the bulb’s normal inverted J shape. Of the cases, 91.3 percent had abnormalities in the olfactory bulb signal intensity manifested as scattered hyperintense foci or microhemorrhages that were diffusely increased. Clumping of olfactory filia was found in 34.8% of cases; thinning with a lack of filia was found in 17.4% of cases. There was an abnormality in the olfactory cortical signal in 21.7% of the cases [91].
Between March 4 and May 9, 14 percent, or 278, of the 2054 people in New York City with COVID-19 who went to two hospitals had CT or MR brain imaging done. It was found that 43% of these patients were female and that the median age was 64 (interquartile range, 50-75 years). There were 58 (21%) patients who had acute or subacute neuroimaging findings, the most common of which were cerebral infarctions (11%) and parenchymal hematomas (3.6%). Some 26 of the 51 MR imaging patients had acute or subacute findings, including six cases of cranial nerve abnormalities (four patients had olfactory bulb abnormalities) and three patients with a microhemorrhage pattern consistent with critical illness-associated microbleeds. All patients with COVID-19 whose clinical status allowed for additional scan time were given thin-section coronal T2 imaging through the olfactory bulbs in their brain MRIs by the department. For a total of 13 patients, both diagnostic olfactory bulb sequences and high-resolution 3D-T2 FLAIR images were used. Olfactory bulb volume was unchanged in any of the patients studied. On the other hand, four of the 12 patients had an abnormally high olfactory bulb signal on post-contrast T2 FLAIR, which could be due to an intrinsic T2 prolongation or even contrast enhancement. When it came to the four patients who had olfactory bulb signal abnormalities, one had documented anosmia, but the other three did not. Patients with COVID-19 showed hyperintensity of the olfactory bulb on post-contrast T2 FLAIR images, which could be a biomarker of disease and an imaging correlate of the common anosmia experienced by patients [51].
Peripheral neuropathy
It is common for hands and feet to become weak, numb, and painful when the peripheral nerves in the body are damaged as a result of brain or spinal cord damage. It can also have an impact on other bodily functions, such as digestion, urination, and circulation [92].
Retrospective research in Italy found that 7.8 percent of 1760 COVID-19 patients had symptoms or signs of CNS/PNS dysfunction. The patients ranged in age from 30 to 95 years old, with 34% of them being female. Their average age was 64.9. The three most common neurological symptoms were cardiovascular disease (CVD) (53 patients; 38.7%), PNS disease (31 patients; 22.6%), and AMS (49 patients; 35.8%). 17 GBS, 9 critical illness myopathy and neuropathy (CRIMYNE), 2 brachial plexopathies, and 3 peripheral polyneuropathy (PNP) patients were found to have PNS involvement. ARDS was significantly more common in patients with PNS involvement compared to patients with CVD (87.1% vs. 42%) and in patients with AMS (87.1% vs. 55.6%), and the mean length of stay was significantly longer in patients with PNS involvement compared with patients with CVD. Brain MRIs were performed in patients with AMS and clinical vignettes. Based on clinical characteristics, CSF data, and neuroimaging, encephalitis was diagnosed in 5 patients, and brain MRI was normal in 64 clinical vignette patients [62].
The D-dimer concentration in the blood was 2.737 mg/L on average. Patients with acute CNS disease had a significantly higher level (8.77) than those with acute PNS disease (0.79). There was a mean serum ferritin level of 544.7 ng/mL. However, it was not statistically different between patients with acute CNS illness and those with PNS illness (787.2 to 463.9). The average percentage of lymphocytes was 18.6 percent in this study. For those suffering from acute CNS disease, the score was 16, compared to 23.1 for those with PNS disease. In patients with encephalitis, an MRI was performed, and encephalitis was documented by the presence of brain edema and signs of inflammation in the MRI [9].
Cognitive-behavioral symptoms
Following a serious illness, cognitive deficits are frequent, long-lasting, and debilitating. They’re becoming more widely recognized as a common side effect of COVID-19. There are numerous potential causes of cognitive sequelae, including the illness itself and the care it receives. Hypoxia, ventilation, sedation, delirium, cerebrovascular events, and inflammation are some of the more common symptoms [93].
Amnestic MCI (aMCI) and non-amnestic MCI (naMCI) are two types of MCI. Most people who have aMCI experience memory loss [94]. A common symptom of naMCI is a decline in executive function and attentional abilities, two non-memory domains [95].
Mild cognitive impairment
Concentration
Researchers at the University Medical Center Hamburg-Eppendorf (UKE) conducted a cross-sectional study by randomly interviewing patients from the outpatient clinic and excluding those who had been admitted to the ICU. In total, 21 patients were approached, with 18 agreeing to take part in the study. Out of the 18 participants, 9 (50%) reported attention deficits, 8 (44.4%) concentration deficits, 8 (44.4%) short-term memory deficits, 5 (27.8%) difficulty finding words, 3 (16.7%) fatigue, 2 (11.1%) severe mood swings, and 1 (5.6%) sustained lack of energy, phonophobia, and incoherent thoughts. For the two worst-affected patients, they performed cranial MRI, lumbar puncture, and other diagnostic procedures to rule out structural pathologies and acute inflammation. A neuropsychological evaluation revealed deficiencies in memory, executive functions, and attention [78].
Sixteen different hospitals participated in a retrospective study from March 23, 2020, to April 27, 2020. With a mean age of 61 years, thirty men (81%) and seven women (19%) met the inclusion criteria. The most common neurologic sign was a change in consciousness (27 of 37, or 73%), followed by abnormal wakefulness when the sedation was stopped (15 of 37, or 41%), confusion (12 of 37, or 32%), and agitation (seven of 37, or 19%) [5].
Two French ICUs at Strasbourg University Hospital were studied in a bicentric cohort study. With an average age of 62, the 140 patients had a simplified acute physiology score II (SAPS II) of 49. Twenty-two patients (15.7%) had a neurological examination that was normal. Acute attention, awareness, and cognition disturbances were observed in 118 of the 153 patients (84.3%) who developed delirium [77].
Attention
An investigation into the neurological and cognitive effects of COVID-19 was carried out in one study. These findings are based on information gleaned from a single neuro-COVID-19 register, which includes prospectively examined patients with RT-PCR confirmation of their SARS-CoV2 infection and new neurological symptoms who were consecutively admitted for inpatient treatment at the Department of Internal Medicine at the University Hospital Freiburg between April 20th and May 12th, 2020. The MoCA test found 14 patients (54%) with mild to moderate impairment (Montreal cognitive assessment (MoCA) 18-25) and four (15%) with severe impairment (MoCA 10-17) who did not meet the cut-off. Specific deficits were found in executive functions as well as visual construction, memory, and attention, according to the MoCA domain scores [96].
Memory loss
Between March 27th and June 20th, 2020, 87 patients were admitted to the COVID-19 Rehabilitation Unit for the purpose of research by Alemanno et al. The Mini Mental State Evaluation (MMSE), MoCA, Hamilton Rating Scale for Depression, and the Functional Independence Measure (FIM) were used to evaluate patients. The data were divided into four groups based on the type of respiratory assistance provided during the acute phase: Group 1 (orotracheal intubation), Group 2 (non-invasive ventilation using Biphasic Positive Airway Pressure), Group 3 (Venturi Masks), and Group 4 (no oxygen therapy). a month after being discharged from a hospital. Study results showed that 74.2% of patients in Group 1, 94.4% in Group 2, 89.5% in Group 3, and 71.8% in Group 4 had deficits, as shown by the total score analysis of MoCA scores. A one-way ANOVA on the main factor “total score” revealed significant group differences. When comparing the two groups, Group 1 had higher scores than Group 3. Subdomains of short-term memory (attention, abstraction), long-term memory (long-term storage), and space-time orientation were significantly different between the two groups [97].
In a cross-sectional study, people who had gotten better from mild to moderate disease and went back to their outpatient clinic for follow-up care after taking COVID-19 were watched for an average of 85 days. The cognitive status screening test for MCI found that the modified telephone interview was much harder for 14 (78%) of the patients than for 10 healthy people the same age. COVID-19 had the most effect on short-term memory, attention, and concentration, but screening results were not linked to hospitalization, treatment, viremia, or acute inflammation. In the two patients with the most severe symptoms, MRI and lumbar puncture were used to rule out structural pathologies and acute inflammation, respectively. A thorough neuropsychological evaluation showed that the patient had trouble paying attention, making decisions, and remembering things [78].
COVID-19’s neurological and neuro-cognitive effects were examined in a separate study. These findings are based on information gleaned from a single neuro-COVID-19 register, which includes prospectively examined patients with RT-PCR confirmation of their SARS-CoV2 infection and new neurological symptoms who were consecutively admitted for inpatient treatment at the Department of Internal Medicine at the University Hospital Freiburg between April 20th and May 12th, 2020. Only four patients (or 15 percent) were classified as severely impaired on the MoCA scale, the other fourteen (54 percent) were classified as mildly to moderately impaired. Specific deficits were found in executive functions as well as visual construction, memory, and attention, according to the MoCA domain scores [96].
Behavioral symptoms
Stress and anxiety
Salomona et al. in 2021 compared MRI scans taken before and after the outbreak of COVID-19 to examine volumetric changes. This group’s images were compared to those of 50 people who had been scanned twice before the pandemic and served as a control group. Participants in the study who were exposed to COVID-19 showed volumetric increases in their bilateral amygdalae, putamen, and three anatomical regions in the ventral anterior temporal cortex next to each other: the medial part of the anterior temporal lobe, the fusiform cortex, and the parahippocampal cortex. Over time, the changes in the amygdala went away, which suggests that the pandemic caused temporary changes in the size of brain regions that are usually affected by stress and anxiety [98].
At the Neurological Outpatient Clinic in Zabrze, Poland, all MS patients were routinely checked for signs of COVID-19 infection and past contact with an infected person. Patients who had COVID-19 symptoms or who had confirmed contact with an infected person were told to take the COVID-19 test. The study included all 41 patients who tested positive for SARS-CoV-2 infection. There were additional examinations done following recovery in 26 subjects. After infection with COVID-19, the results of additional tests (brain MRI, electroneurography, EEG, color duplex Doppler, visual evoked potentials, brainstem auditory evoked potentials, and psychological assessment) in most MS patients were similar to those obtained prior to infection (before infection). The results of a psychological evaluation showed that anxiety was found in 42.31% of patients [99].
For a planned national cohort study in the United Kingdom, the CoroNerve study group has set up a secure online network of quick-response notification portals. Doctors of psychiatry and pediatric neurology were asked to warn any patients under 18 with neurological or mental health problems who might have SARS-CoV-2. Of the 52 patients, 48 (92%) had either MRI or CT scans of the brain or spine, with abnormal results in 28 (58%) of those scans. Because of limbic encephalitis, one patient had an abnormal T2 signal in the hippocampi and a restriction in cortical diffusion. Other patients had other neurological symptoms [100].
Fatigue
Versace et al. looked at 12 patients 9 to 13 weeks after the start of their illness. These patients had recovered from typical COVID-19 pneumonia with neurological complications, said they were very physically and mentally tired, and had been tested with the Fatigue Rating Scale (FRS) and the Frontal Assessment Battery (FAB). TMS protocols, such as short-interval intracortical inhibition (SICI), long-interval intracortical inhibition (LICI), and short-latency afferent inhibition (SAI), which all measure GABAA-mediated inhibition, were used to measure intracortical activity. There were ten healthy subjects (HS) in the TMS study who were matched for age, gender, and educational level. According to the FRS score of 8.1, post-COVID-19 patients reported significant fatigue and presented pathological scores at the FAB in accordance with Italian normative data of 12.2. TMS showed a marked decrease in SICI and disruption of LICI compared to HS. The SAI was also reduced slightly [101].
Affective disorders
Anxiety and rumination depression
Anxiety and depression are affective reactions that have important adaptive functions. However, the recurrence, persistence, and intensity of these responses can have a negative impact on both the individual’s mental and physical health. Depression can set in after a person has suffered a real or imagined loss. Anxiety is defined as an emotional state marked by subjective feelings of tension and apprehension as well as autonomic nervous system responses [102].
Brooks et al. 2020 conducted a review of the literature based on 26 studies. Of the 26 studies taken into consideration, there is only one that addresses the long-term psychological effects of quarantine. According to the study, 7% of participants experienced anxiety symptoms, and 17% reported feeling angry. However, 4-6 months after the quarantine ended, these symptoms had decreased to 3% and 6%, respectively [103]. In a longitudinal study (Wang et al., 2020), the COVID-19 pandemic did not lead to significant changes in anxiety and depression [104].
Researchers looked at the emotional effects of the COVID-19 pandemic quarantine on the participants using the State-Trait Anxiety Inventory (STAI), the Beck Depression Inventory-II (BDI-II), and the Positive and Negative Affect Scale (PNA-110). After two weeks of isolation, depression symptoms went up a little bit, but state anxiety went down in every category that was looked at. Most of the time, this drop was statistically significant, but the effect was small or nonexistent. These results support the idea that being alone doesn’t make anxiety worse, but rather makes it better [102].
A total of 26 patients were assessed psychologically as part of the research. Anxiety and fear affected 42.31 percent of those who took part in the research. Every patient had a normal range of cognitive abilities [99].
Anxiety and stress disorders were found to affect 23% and 27% of medical professionals in China, respectively. The COVID-19 pandemic appears to have a greater impact on patients’ neuropsychiatric health [113]. Even in the early stages of the disease, patients report symptoms of anxiety, depression, fatigue, and cognitive dysfunction. Prior to the outbreak of the COVID-19 pandemic, anxiety in MS patients was estimated to be 22.1%. Even so, the results differed greatly among the studies cited [105]. It appears that infection with the COVID-19 virus is associated with an increase in anxiety [99].
Psychological distress and stress
COVID-19’s outbreak led to an increase in stress and anxiety, which is not a surprise. Most of the time, these were more common in young adults who were healthy, which suggests that the high rate of psychological distress was caused by things other than actual health risk, like social isolation and financial insecurity caused by the health crisis [106,107].
In one study, researchers thought that the stress of the outbreak might have caused changes in the brains of healthy people who had not been infected with the virus. There were 50 participants who had MRI scans prior to and following the outbreak of COVID-19. This group’s images were compared to those of 50 people who had been scanned twice before the pandemic and served as a control group. After the COVID-19 outbreak and lockdown, the test group members’ bilateral amygdalae, putamen, and anterior temporal cortices grew in size in a way that had never been seen before. This could be explained by a gradual decrease in the volumetric change effect as a function of time after lockdown (TFL). If the volumetric change effect is not tied to a big event outside the body, it may be harder to notice [73].
Neuromuscular disorders
Patients with neuromuscular diseases may be particularly vulnerable to the COVID-19 pandemic. It has quickly led to changes in the delivery of clinical care and education for neuromuscular disorders that are likely to have long-lasting effects on the field [108].
COVID-19 has a wide range of neurological symptoms. More commonly, symptoms like nausea and dizziness are present earlier in the disease’s progression. Greater severity is linked to AMS, neuromuscular symptoms, and stroke [16].
Myopathy and myositis
Dermatomyositis (DM), Polymyositis (PM), Necrotizing Myopathy (NM), and Inclusion Body Myositis (IBM) are the four different types of idiopathic inflammatory myopathies, also known as myositis. The use of skeletal muscle MRI and the detection of myositis-specific autoantibodies have become important diagnostic tools [109].
Myopathies and disorders of the neuromuscular junction can happen together, even though they are usually different. This has been shown by a number of inherited or acquired conditions. Although the clinical phenotypes of those affected can vary, repetitive nerve stimulation usually shows a decline, and muscle biopsy often shows myopathic findings in those affected [110].
A summary of manifestations in COVID-19 disease is shown in Figure 2.
Figure 2.
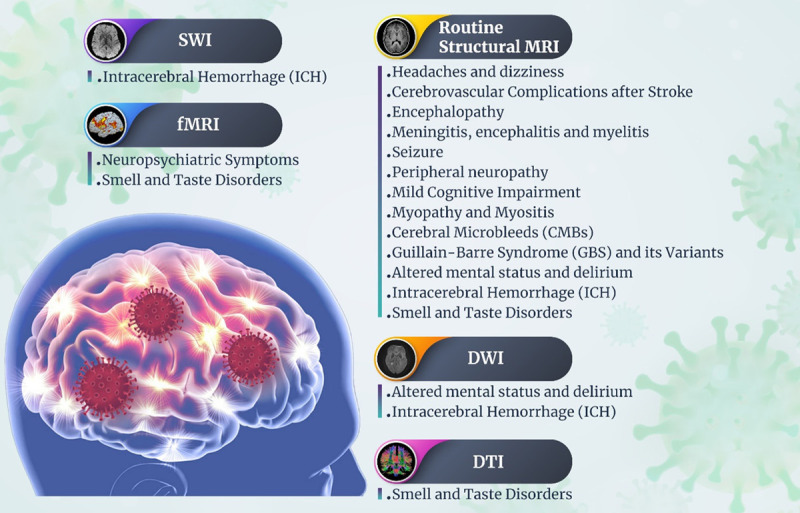
Neuroimaging techniques and common neurological manifestations in COVID-19.
Discussion
Since COVID-19 is so complicated, it may be difficult to understand all of the systems involved. In this regard, medical imaging, such as MRI, is beneficial. SARS-CoV-2-related neurological symptoms and cognitive-behavioral abnormalities were investigated in this research, which intended to collect and analyze imaging data from a variety of perspectives.
The results of this study show that COVID-19 can cause a wide range of neurological symptoms, such as headaches, dizziness, changes in consciousness, strokes, and encephalopathy. Most of the time, these symptoms show up in severe cases of COVID-19, but they can also show up in mild cases.
Headache and dizziness are common symptoms of COVID-19, although no definitive connections have been discovered between the symptoms and MRI findings. This has resulted in reports of headaches and dizziness accompanied by a wide range of MRI results. Grey matter bilateral volume increase in various brain regions, leptomeningeal enhancement, ischemic stroke, widespread hypoattenuation parenchymal subcortical hemorrhage, spinal degenerative symptoms, and focal hypodensities within deep structure are only a few of the possible signs and symptoms [7,16,43,111]. The causes of these headaches are not yet fully understood but may be linked to factors such as genetic predisposition or activation of the trigeminovascular system. It is also unclear how much of these headaches are caused by an increase in intracranial pressure.
During the first phase of a COVID-19 infection, a person may continue to have an increased risk of stroke [112]. Several hypotheses propose a causal relationship between infectious/inflammatory syndromes and an increased risk of stroke, with potential mechanisms including a prothrombotic state, modifications to lipid metabolism and platelet aggregation, changes in endothelial function, and plaque instability or rupture [113,114]. Patients with COVID-19 may develop a hypercoagulable condition because of SARS-CoV-2 binding to the angiotensin-converting enzyme 2 receptor, which is the currently accepted mechanism [115,116]. D-dimer levels and platelet counts are commonly high in critically ill patients with SARS-CoV-2, which may increase the risk of acute cerebrovascular disease [11]. Stroke risk may be increased by COVID-19 via a variety of methods that have been identified but not yet confirmed. Hypercoagulability, as seen by elevated D-dimer levels, enhanced systemic inflammation, sometimes known as a “cytokine storm”, and cardio-embolism are all symptoms of a more severe condition [9,44].
Vascular problems are common in people over 60 who have had a stroke [5,7,16,22,48,51,52,77,100]. Males are much more likely than females to be affected by these disorders. Neuroimaging techniques, such as MRI and CT scans, are often used to monitor and diagnose these diseases. MRI findings are often more thorough than CT data because of their greater contrast resolution and ability to identify vascular abnormalities. An MRI scan indicated a variety of abnormalities, including microhemorrhage patterns and lesions, as well as reduced oxygen flow to the arteries that provide oxygenated blood to the brain’s tissue [7,16,51,77,100].
This could be because there wasn’t enough follow-up after the infection or control of vascular risk factors during the pandemic. It could also be because of a proinflammatory state or endothelial dysfunction [16,40,63]. The gradient-echo T2*-weighted MRI is very sensitive to the detection of localized regions of signal loss that reflect the aftereffects of silent microbleeds. Hemosiderin deposition causes inhomogeneities in the local magnetic field, leading to the T2* effect. Microbleeds seen on T2*-weighted MRI are confirmed to be hemosiderin deposits via pathology [117].
COVID-19 patients sometimes have microhemorrhage, also known as microbleeds or ICH. We can promptly and accurately detect the condition by using modern MRI methods such as SWI, FLAIR, or DWI to visualize cerebral microbleeds [5,7,52,118]. Kremer found that vascular symptoms, such as microhemorrhage, negatively impacted the functioning of WM regions. An unusually low reaction to sedation was seen as a result of this impairment [5]. The basal ganglia, thalamus, brainstem, and cerebellum are the most common locations of microbleeds caused by arterial hypertension, whereas the parieto-occipital cortical regions are the most common in cerebral amyloid angiopathy [40,44,51].
Endothelial dysfunction may contribute to CMBs by letting red blood cells leak into the brain in a certain area. CMBs have many well-known causes, such as chronic high blood pressure, cerebral amyloid angiopathy, and damage to many axons. Microbleeds are increasingly being documented in critically ill individuals with respiratory failure [119]. Many ideas have been offered to explain the pathophysiological mechanisms at play in COVID-19, but consensus has yet to be reached. Leukoencephalopathy and CMBs have been linked to severe COVID-19 infection, with hypoxemia-induced brain alterations being proposed as a possible explanation [45,48,91]. Simultaneously, imaging studies have shown CMBs throughout the brain. The reported changes may be the result of the virus itself, the severity of the sickness, or a complication of the disease. It’s well accepted that SARS-CoV-2 may bind angiotensin-converting enzyme 2 (ACE2) and activate inflammatory and thrombotic pathways to gain entry to and harm endothelial cells in the lungs, heart, and kidneys [36,120,121].
How or why these types of haemorrhages arise inside the brain (pathophysiology) is still an unknown. There is substantial evidence that ICH is a risk factor for COVID-19. Primary ICH may develop with or without the presence of risk factors, including arterial hypertension (HTN) or anticoagulant medication (pre-treatment of an unrelated disease or COVID-19 thromboprophylaxis). Secondary causes of ICH include cerebral sinus thrombosis, acute ischemic stroke, and complications from revascularization therapies [122]. The risk of thromboembolic events in patients with COVID-19 is supported by increasing data. It has been postulated that variables such as platelet activity, endothelial dysfunction, and stagnation have a role. The significant inflammatory response seen in COVID-19 individuals in the latter stages may also contribute to thrombotic microangiopathy and/or disseminated intravascular coagulation (DIC). Patients with COVID-19 are at high risk for thromboembolic complications; one study found that 30% of individuals with the disease suffered from pulmonary emboli. Furthermore, previous research has shown imaging evidence of microthrombi [38,115].
Encephalopathy and other neurologic symptoms are possible side effects of hypoxemia in patients hospitalised with severe COVID-19 who have respiratory impairment and/or other organ failure. Acute brain damage may also result from metabolic disturbances or the side effects of medications in patients with severe illnesses. There are a number of neurological disorders in which encephalopathy may both be a presenting symptom and a clinical component. COVID-19 encephalopathy may be caused by a number of different neurological problems, including ischemic stroke, hemorrhagic encephalitis, and others [15,54,58,65,81,123,124].
When it comes to encephalopathy, several MRI methods may be used to differentiate between the various types [51,52,125]. COVID-19 encephalopathy has the potential to affect both children and adults [100]. There is an increased risk of leukoencephalopathy, infraction, and cerebral hemorrhage in males over the age of 60 and in individuals with COVID-19-induced encephalopathy [126]. Although there are numerous potential pathophysiological causes of acute encephalopathy, it appears that COVID-19-induced endotheliitis is the most frequently reported culprit for the associated homeostatic and cerebrovascular function problems [58].
There are numerous neuroimaging abnormalities present in patients with COV-19-induced encephalopathy. A cortical FLAIR signal abnormality, acute ischemic stroke, leptomeningeal enhancement (often mild), and other signs of encephalitis are the most often seen acute abnormalities in neuroimaging studies in patients with COVID-19-related encephalopathy. By utilizing arterial spin labeling MRI sequences, physicians at hospitals may check for signs of impaired perfusion in patients. Multifocal hyperintense lesions on T2-weighted and diffusion-weighted sequences in the white matter or medial temporal lobe were common MRI abnormalities. On MRI, adult patients with COVID-19-related encephalopathy and a small number of children with COVID-19 multisystem inflammatory syndrome have been found to have hyperintense lesions in the splenium of the corpus callosum. Several factors may contribute to the development of the encephalopathy seen in patients infected with COVID-19. Possible explanations include neurotropism and hypoxic/metabolic alterations brought on by the severe inflammatory response caused by a cytokine storm. Brain dysfunction on a global scale is the product of a cytokine storm’s hypoxia- and metabolism-inducing assaults. Common clinical manifestations include changes in level of consciousness, from moderate disorientation and delirium to coma. Symmetrical, multi-focal lesions with consistent thalamic involvement are the most often seen imaging finding on MRI MRI T2/FLAIR. The brainstem, white matter of the cerebrum, cortical and subcortical white matter, and the cerebellum are also often affected [25,117,123].
Patients presenting with encephalopathy and COVID-19 have been documented to have viral meningoencephalitis. A number of risk factors for developing encephalitis as a result of COVID-19 were identified. Complications from a COVID-19 infection, such as encephalitis, may be more likely in the elderly or in those who have preexisting medical conditions. Patients with severe COVID-19 disease are also at substantially higher risk for developing encephalitis as a consequence of their illness. Diffuse WM hyperintensities and hemorrhagic lesions on FLAIR and T2 sequences are frequent MRI brain findings. Cerebral edema and venous thrombosis are two other, less frequent MRI diagnoses. The pathophysiology of COVID-19-related encephalitis has been hypothesized to occur by three different mechanisms: direct invasion of the nervous system, systemic inflammation, and molecular mimicry. Evidence suggests that the primary mechanism of encephalitis in COVID-19 is not the direct invasion of SARS-CoV-2 virus into the central nervous system. Encephalitis may occur if the SARS-CoV-2 virus invades the brain parenchyma directly. Transsynaptic transmission or hematogenous invasion are both potential entry points for SARS-CoV-2. During transmission across synapses, SARS-CoV-2 reaches target cells via binding to the angiotensin II (ACE-II) receptor on the cell membrane of peripheral nerve cells. Then, it makes use of functional axonal transport to make its way back to the central nervous system [127]. It has been proposed that direct SARS-CoV-2 viral invasion into the brain is less likely to be the primary mechanism producing encephalitis in COVID-19 [66,111,120].
During COVID-19, patients may have neurological symptoms such as headache, dizziness, hyposmia, delirium, meningitis, encephalitis, and severe cerebrovascular accidents. These neurological symptoms may be the only noticeable symptoms at first when someone is diagnosed with COVID-19 [7,19,33,39,53,65,124,127,128]. Patients infected with COVID-19 have an increased risk for neurological symptoms and inflammation, which may lead to diseases including stroke, meningitis, encephalitis, and myelitis. Patients with pre-existing neurological conditions may be at a higher risk of developing severe COVID-19 and neurological complications. Meningoencephalitis seems to be an immunological or inflammatory process based on the presence of inflammatory symptoms on neuroimaging, even when the direct effects of the virus are disregarded as possible causes of the disease. This is true regardless of whether or not the individual is experiencing any of the virus’s symptoms [16,37,43,62,63,71,91,129].
People who have COVID-19 may have encephalopathy with sleepiness and a lower level of consciousness. Others may have severe delirium and agitation that needs to be calmed down. In older persons, delirium is a frequent, early, and fatal complication of COVID-19, often resulting in acute or chronic neurocognitive impairment significantly impacted by inflammatory and hypoxic-ischemic pathways. Seizures, a hallmark of toxic-metabolic encephalopathy, have been reported in individuals with COVID-19, and are often accompanied by symptoms of the corticospinal tract. Mechanical ventilation, vasopressor use, restraint use, benzodiazepine or continuous opioid infusions, and a lack of family visitation were all associated with an increased risk of delirium in COVID-19 ICU patients [65,67,78]. It is reasonable to anticipate that some COVID-19 patients may experience seizures as a result of hypoxia, metabolic derangements, organ failure, or possibly brain damage caused by COVID-19. Neurotropism is a feature shared by most coronaviruses and has previously been established, and SARS-CoV-2 infection has the potential to cause seizures in the context of encephalitis. However, seizures caused by hypoxia, metabolic derangement, medications, multiorgan failure, or brain damage can also indirectly cause seizures.
Any deviation from the patient’s original mental state is classified as AMS. A broad variety of diseases may lead to acute brain malfunction, which is usually the basis of AMS. AMS emerges as a shift in either the intensity or nature of one’s consciousness. In hospitalized patients, AMS is a frequent neurological symptom of the COVID-19 infection. As of yet, the main factors that lead to AMS are unknown. Diffuse brain dysfunction due to severe systemic illness (also known as encephalopathy or delirium) or more direct invasive effects on the brain may also be precipitated by viral infection, leading to AMS (i.e., encephalitis). AMS may be caused by a combination of factors such as hypoxia, blood-brain barrier (BBB) dysfunction, cerebrovascular disease, toxic metabolites, and cytokine release syndrome. Metabolic encephalopathy is the leading cause of AMS in COVID-19 patients and is linked to older age, dementia, and cerebrovascular diseases. Delirium is a common symptom of COVID-19 infection, just as it is in other acute infections, and it is more common in elderly people who are already weak [5,65,91,100,125,130,131].
The association between dementia and COVID-19 is complex and not yet fully understood. However, there are some possible reasons why individuals with dementia may be at higher risk of contracting the virus and experiencing severe outcomes. One reason is that individuals with dementia are more likely to reside in care or nursing homes where there is a higher risk of infection. Additionally, they may have difficulty maintaining personal hygiene or preventative health measures on their own, making them more vulnerable to infection.
Hyperintensity and signal abnormalities were often seen in MR images of COVID-19 patients with delirium and AMS symptoms [5,17,43,65,125].
Also, it’s clear that there isn’t enough research into COVID-19 diseases using fMRI and DTI. Extensive exposure to the COVID-19 illness has been linked to a wide range of neuropsychiatric symptoms, including weakness, aches and pains, mental confusion, and impaired judgment. These results support the need for more rs-fMRI research [77,79,91].
When severe respiratory symptoms emerge, neuropsychiatric involvement becomes obvious. COVID-19 individuals may have a variety of adverse effects, including delirium, depression, anxiety, insomnia, and psychosis. It is essential to emphasize that the pathophysiological mechanisms involved in acute and ‘long-COVID’ neuropsychiatric symptoms are diverse. In acute COVID-19 infection, SARS-CoV-2 reaches the brain by hematogenous transmission or retrograde transport down the axons of the olfactory and vagus nerves. SARS-CoV-2 cell entrance is primarily enhanced at the neuronal level via the ACE2 receptor, which is found on the surface of neuronal cells. Intracellular inflammatory responses in the context of acute SARS-CoV-2 infection cause lysosomal, mitochondrial, and endoplasmic reticulum dysfunction, whereas protein misfolding and intracellular protein aggregation buildup may cause long-term neurodegenerative pathways. Neuropsychiatric abnormalities in ‘long-COVID’ syndrome have been related to ‘ACE2-rich’ brain areas stretching from the somatosensory cortex to the rectal/orbital gyrus, temporal lobe, thalamus, and hypothalamus, and beyond, to the brainstem and cerebellar regions [5,65,91,100,125,131].
During COVID-19, people may not be able to smell because infection and the death of supporting cells, microvillar cells, and vascular pericytes have changed the way olfactory sensory neurons work. The idea that olfactory sensory neurons can be directly infected needs more research. However, other inflammation-related processes, such as localized mucosal edema and airflow blockage, are also possible. Many respiratory viruses, particularly coronaviruses, have been linked to cases of olfactory dysfunction. Inflammatory changes in the respiratory mucosa and mucous discharge are often seen after the onset of respiratory symptoms. Conversely, COVID-19 patients often describe a loss of taste or smell without any accompanying nasal congestion or discharge. These characteristics point to a possible role for a regional impairment in airflow conduction or sensorineural injury in the development of olfactory and gustatory dysfunction. Imaging investigations on people infected with SARS-CoV-2 have shown that the small passageways that enable inspired air to reach the olfactory epithelium expand and get blocked up. Many disorders affecting smell and taste have been connected to the COVID-19 gene. A major contributor to this result is the presence of significant differences in OB volume [74,90,91,132-136]. In the most severe form of COVID-19 illness, OBs decrease relative to their normal size. If performed early enough, an MRI may be able to detect these alterations in the early stages of the disease, which might lead to an earlier and more accurate diagnosis of severe COVID-19 consequences [17,41,111,132,134,136-138]. Although Lin et al. [51] and Eliezer et al. [133] didn’t find any significant changes in OB volume in COVID-19 patients, two investigations did discover a change in OB volume and signal intensity [136,139]. The need for more research into brain structure and function is evident.
Conclusion
COVID-19 has a wide range of clinical effects, from infections with no symptoms to severe pneumonia with breathing problems, damage to many organs, and death. COVID-19 can have a variety of effects on the CNS and PNS. As a result, we have divided the titles into separate groups of manifestations (vascular lesions, inflammatory lesions, neuropsychiatric disorders, and so on). Several imaging findings related to the neurological effects of COVID-19 were described in our review study. According to our findings, neuroimaging investigations, particularly in the CNS, are recommended to evaluate various aspects of COVID-19 disease.
Disclosure of conflict of interest
None.
Author’s contributions
S.Gh and M.M contributed to the conception and design of the study; M.O., M.Gh., H.H., Gh.Y., Z.Kh., A.K., Z.P., N.A., S.Sh.Sh.J., R.Kh., Sh.B., F.B., and M.Z. contributed to the data collection. S.Gh, M.M, and S.M contributed to drafting the text and preparing the figures.
Abbreviations
- AD
Alzheimer’s disease
- ADEM
Acute disseminated encephalomyelitis
- ALFF
Amplitudes of low-frequency fluctuation
- aMCI
Amnestic MCI
- AMS
Altered mental status
- anti-NMDAR
Anti-N-methyl-D-aspartate receptor
- ARDS
Acute respiratory distress syndrome
- ASL
Arterial spin labeling
- BBB
Blood brain barrier
- BDI-II
Beck depression inventory-II
- BMI
Body mass index
- CAE
COVID-19 associated encephalopathy
- CAM-ICU
Confusion assessment method for the ICU
- CI
Cognitive impairment
- CLOCC
Cytotoxic lesion of the corpus callosum
- CMBs
Cerebral microbleeds
- CNS
Central nervous system
- COVID-19
coronavirus disease of 2019
- CRG
Chronic renal failure
- CRIMYNE
Critical illness myopathy and neuropathy
- CSF
Cerebrospinal fluid
- CTA
Computed tomography angiography
- CVD
Cardiovascular disease
- CVT
Cerebral venous thrombosis
- DM
Dermatomyositis
- DTI
Diffusion tensor imaging
- DWI
Diffusion weighted imaging
- EEG
Electroencephalography
- FAB
Frontal assessment battery
- FIM
Functional independence measure
- FLAIR
Fluid-attenuated inversion recovery
- fMRI
Functional magnetic resonance imaging
- FRS
Fatigue rating scale
- GBS
Guillain-Barre syndrome
- GM
Gray matter
- GMV
Gray matter volume
- HS
Healthy subjects
- IAE
Idiopathic absence epilepsy
- IBM
Inclusion body myositis
- ICH
Intracerebral hemorrhage
- ICP
Intracranial pressure
- ICU
Intensive care units
- IV
Intravenous
- LICI
Long-interval intracortical inhibition
- LVO
Large-vessel occlusion
- MCI
Mild cognitive impairment
- MFS
Miller Fisher syndrome
- MIA
Minimally invasive autopsy
- MIS-C
Multisystem inflammatory syndrome in children
- MMSE
Mini mental state evaluation
- MoCA
Montreal cognitive assessment
- MPRAGE
Magnetization-prepared rapid acquisition with gradient-recalled echo
- MR
Magnetic resonance
- MRA
Magnetic resonance angiography
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- naMCI
non-amnestic MCI
- NDPH
New daily persistent headache
- NM
Necrotizing myopathy
- OB
Olfactory bulb
- OBV
Olfactory bulb volume
- OCs
olfactory clefts
- OD
Olfactory dysfunction
- OSDs
Olfactory sulcus depths
- PD
Parkinson’s disease
- PIMS-TS
Pediatric inflammatory multisystem syndrome
- PM
Polymyositis
- PNA-110
Positive and negative affect scale
- PNP
Peripheral polyneuropathy
- PNS
Peripheral nervous system
- PreCG
Precentral gyrus
- PRES
Posterior reversible (leuko) encephalopathy syndrome
- QA
Quantitative anisotropy
- RecCOVID
Recovered from COVID-19
- rs-fMRI
Resting-state functional magnetic resonance imaging
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SAH
Subarachnoid hemorrhage
- SAI
Short-latency afferent inhibition
- SAPS II
Simplified acute physiology score II
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SICI
Short-interval intracortical inhibition
- STAI
State-Trait anxiety inventory
- STDs
Smell and taste disturbances
- SWI
Susceptibility weighted imaging
- TFL
Time following lockdown
- TTH
Tension-type headache
- WM
White matter
References
- 1.Maghsoudinia F, Tavakoli MB, Samani RK, Hejazi SH, Sobhani T, Mehradnia F, Mehrgardi MA. Folic acid-functionalized gadolinium-loaded phase transition nanodroplets for dual-modal ultrasound/magnetic resonance imaging of hepatocellular carcinoma. Talanta. 2021;228:122245. doi: 10.1016/j.talanta.2021.122245. [DOI] [PubMed] [Google Scholar]
- 2.Ansari L, Sharifi I, Ghadrijan H, Azarpira N, Momeni F, Zamani H, Rasouli N, Mohammadi M, Mehdizadeh A, Abedi-Firouzjah R. Synthesis, characterization and MRI application of cobalt-zinc ferrite nanoparticles coated with DMSA: an in-vivo study. Appl Magn Reson. 2021;52:33–45. [Google Scholar]
- 3.Ghaderi S, Divband B, Gharehaghaji N. Magnetic resonance imaging property of doxorubicin-loaded gadolinium/13X zeolite/folic acid nanocomposite. J Biomed Phys Eng. 2020;10:103–110. doi: 10.31661/jbpe.v0i0.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghaderi S, Ghaderi K, Ghaznavi H. Using marker-controlled watershed transform to detect Baker’s cyst in magnetic resonance imaging images: a pilot study. J Med Signals Sens. 2022;12:84–89. doi: 10.4103/jmss.JMSS_49_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremer S, Lersy F, Anheim M, Merdji H, Schenck M, Oesterlé H, Bolognini F, Messie J, Khalil A, Gaudemer A, Carré S, Alleg M, Lecocq C, Schmitt E, Anxionnat R, Zhu F, Jager L, Nesser P, Mba YT, Hmeydia G, Benzakoun J, Oppenheim C, Ferré JC, Maamar A, Carsin-Nicol B, Comby PO, Ricolfi F, Thouant P, Boutet C, Fabre X, Forestier G, de Beaurepaire I, Bornet G, Desal H, Boulouis G, Berge J, Kazémi A, Pyatigorskaya N, Lecler A, Saleme S, Edjlali-Goujon M, Kerleroux B, Constans JM, Zorn PE, Mathieu M, Baloglu S, Ardellier FD, Willaume T, Brisset JC, Caillard S, Collange O, Mertes PM, Schneider F, Fafi-Kremer S, Ohana M, Meziani F, Meyer N, Helms J, Cotton F. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. 2020;95:e1868–e1882. doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 6.Maghsoudinia F, Tavakoli MB, Samani RK, Motaghi H, Hejazi SH, Mehrgardi MA. Bevacizumab and folic acid dual-targeted gadolinium-carbon dots for fluorescence/magnetic resonance imaging of hepatocellular carcinoma. J Drug Deliv Sci Technol. 2021;61:102288. [Google Scholar]
- 7.Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L. Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg. 2020;141:e437–e446. doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khedr EM, Abo-Elfetoh N, Deaf E, Hassan HM, Amin MT, Soliman RK, Attia AA, Zarzour AA, Zain M, Mohamed-Hussein A, Hashem MK, Hassany SM, Aly A, Shoyb A, Saber M. Surveillance study of acute neurological manifestations among 439 Egyptian patients with COVID-19 in Assiut and Aswan University Hospitals. Neuroepidemiology. 2021;55:109–118. doi: 10.1159/000513647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islamoglu Y, Celik B, Kiris M. Facial paralysis as the only symptom of COVID-19: a prospective study. Am J Otolaryngol. 2021;42:102956. doi: 10.1016/j.amjoto.2021.102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Ling Q, Manyande A, Wu D, Xiang B. Brain imaging changes in patients recovered from COVID-19: a narrative review. Front Neurosci. 2022;16:855868. doi: 10.3389/fnins.2022.855868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27. e11. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehmani R, Segan S, Maddika SR, Lei YW, Broka A. Spectrum of neurologic & neuroimaging manifestation in COVID-19. Brain Behav Immun Health. 2021;13:100238. doi: 10.1016/j.bbih.2021.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12:e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Azorín D, Abildúa MJA, Aguirre MEE, Fernández SF, Moncó JCG, Guijarro-Castro C, Platas MG, Delgado FR, Andrés JML, Ezpeleta D. Neurological presentations of COVID-19: findings from the Spanish Society of Neurology neuroCOVID-19 registry. J Neurol Sci. 2021;423:117283. doi: 10.1016/j.jns.2020.117283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauss SB, Lantos JE, Heier LA, Shatzkes DR, Phillips CD. Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. AJNR Am J Neuroradiol. 2020;41:1882–1887. doi: 10.3174/ajnr.A6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, Stivaros SM, Palasis S. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5:167–177. doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altunisik E, Sut SK, Sahin S, Baykan AH. Is increased intracranial pressure a factor in persistent headache after coronavirus disease 2019? J Nerv Ment Dis. 2021;209:640–644. doi: 10.1097/NMD.0000000000001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan M, Qin Y, Xue X, Zhu S. Death from Covid-19 of 23 Health Care Workers in China. N Engl J Med. 2020;382:2267–2268. doi: 10.1056/NEJMc2005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller E, Brandi G, Winklhofer S, Imbach L, Kirschenbaum D, Frontzek KJ, Steiger P, Dietler SA, Haeberlin MI, Willms JF, Porta F, Waeckerlin A, Abela IA, Lutterotti A, Stippich C, Jelcic I. Central nervous system disorders in severe SARS-CoV-2 infection: detailed clinical work-up of eight cases. Stroke. 2020 doi: 10.1161/STROKEAHA.120.031224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chougar L, Shor N, Weiss N, Galanaud D, Leclercq D, Mathon B, Belkacem S, Ströer S, Burrel S, Boutolleau D, Demoule A, Rosso C, Delorme C, Seilhean D, Dormont D, Morawiec E, Raux M, Demeret S, Gerber S, Trunet S, Similowski T, Degos V, Rufat P, Corvol JC, Lehéricy S, Pyatigorskaya N. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology. 2020;297:E313–E323. doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toniolo S, Scarioni M, Di Lorenzo F, Hort J, Georges J, Tomic S, Nobili F, Frederiksen KS. Dementia and COVID-19, a bidirectional liaison: risk factors, biomarkers, and optimal health care. J Alzheimers Dis. 2021;82:883–898. doi: 10.3233/JAD-210335. [DOI] [PubMed] [Google Scholar]
- 24.Grover VP, Tognarelli JM, Crossey MM, Cox IJ, Taylor-Robinson SD, McPhail MJ. Magnetic resonance imaging: principles and techniques: lessons for clinicians. J Clin Exp Hepatol. 2015;5:246–255. doi: 10.1016/j.jceh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kates R, Atkinson D, Brant-Zawadzki M. Fluid-attenuated inversion recovery (FLAIR): clinical prospectus of current and future applications. Top Magn Reson Imaging. 1996;8:389–396. [PubMed] [Google Scholar]
- 26.Baliyan V, Das CJ, Sharma R, Gupta AK. Diffusion weighted imaging: technique and applications. World J Radiol. 2016;8:785–798. doi: 10.4329/wjr.v8.i9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology. 2016;281:337–356. doi: 10.1148/radiol.2016150789. [DOI] [PubMed] [Google Scholar]
- 28.Lemaire JJ, Cosnard G, Sakka L, Nuti C, Gradkowski W, Mori S, Hermoye L. White matter anatomy of the human deep brain revisited with high resolution DTI fibre tracking. Neurochirurgie. 2011;57:52–67. doi: 10.1016/j.neuchi.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma S, Ferreira-Valente A, de CWAC, Abbott JH, Pais-Ribeiro J, Jensen MP. Group differences between countries and between languages in pain-related beliefs, coping, and catastrophizing in chronic pain: a systematic review. Pain Med. 2020;21:1847–1862. doi: 10.1093/pm/pnz373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu T, Mack JA, Salvatore M, Prabhu Sankar S, Valley TS, Singh K, Nallamothu BK, Kheterpal S, Lisabeth L, Fritsche LG, Mukherjee B. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open. 2020;3:e2025197. doi: 10.1001/jamanetworkopen.2020.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carcamo Garcia MH, Garcia Choza DD, Salazar Linares BJ, Diaz MM. Neurological manifestations of patients with mild-to-moderate COVID-19 attending a public hospital in Lima, Peru. eNeurologicalSci. 2021;23:100338. doi: 10.1016/j.ensci.2021.100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stovner LJ, Nichols E, Steiner TJ, Abd-Allah F, Abdelalim A, Al-Raddadi RM, Ansha MG, Barac A, Bensenor IM, Doan LP, Edessa D, Endres M, Foreman KJ, Gankpe FG, Gopalkrishna G, Goulart AC, Gupta R, Hankey GJ, Hay SI, Hegazy MI, Hilawe EH, Kasaeian A, Kassa DH, Khalil I, Khang Y-H, Khubchandan J, Kim YJ, Kokubo Y, Mohammed MA, Mokdad AH, Moradi-Lakeh M, Nguyen HLT, Nirayo YL, Qorbani M, Ranta A, Roba KT, Safiri S, Santos IS, Satpathy M, Sawhney M, Shiferaw MS, Shiue I, Smith M, Szoeke CEI, Truong NT, Venketasubramanian N, weldegwergs Kg, Westerman R, Wijeratne T, Tran BX, Yonemoto N, Feigin VL, Vos T, Murray CJL. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. doi: 10.1016/S1474-4422(18)30322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Z, Meng K, Meng G. Genomic recombination events may reveal the evolution of coronavirus and the origin of SARS-CoV-2. Sci Rep. 2020;10:21617. doi: 10.1038/s41598-020-78703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 36.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meppiel E, Peiffer-Smadja N, Maury A, Bekri I, Delorme C, Desestret V, Gorza L, Hautecloque-Raysz G, Landre S, Lannuzel A, Moulin S, Perrin P, Petitgas P, Sella IF, Wang A, Tattevin P, de Broucker T. Neurologic manifestations associated with COVID-19: a multicentre registry. Clin Microbiol Infect. 2021;27:458–466. doi: 10.1016/j.cmi.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, Bilaloglu S, Hochman K, Raz E, Galetta S, Horwtiz L. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korres G, Kitsos DK, Kaski D, Tsogka A, Giannopoulos S, Giannopapas V, Sideris G, Tyrellis G, Voumvourakis K. The prevalence of dizziness and vertigo in COVID-19 patients: a systematic review. Brain Sci. 2022;12:948. doi: 10.3390/brainsci12070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin MDGM, Paes VR, Cardoso EF, Neto CEBP, Kanamura CT, Leite CDC, Otaduy MCG, Monteiro RAA, Mauad T, da Silva LFF, Castro LHM, Saldiva PHN, Dolhnikoff M, Duarte-Neto AN. Postmortem brain 7T MRI with minimally invasive pathological correlation in deceased COVID-19 subjects. Insights Imaging. 2022;13:7. doi: 10.1186/s13244-021-01144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klironomos S, Tzortzakakis A, Kits A, Öhberg C, Kollia E, Ahoromazdae A, Almqvist H, Aspelin Å, Martin H, Ouellette R, Al-Saadi J, Hasselberg M, Haghgou M, Pedersen M, Petersson S, Finnsson J, Lundberg J, Falk Delgado A, Granberg T. Nervous system involvement in coronavirus disease 2019: results from a retrospective consecutive neuroimaging cohort. Radiology. 2020;297:E324–E334. doi: 10.1148/radiol.2020202791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jegatheeswaran V, Chan MWK, Chakrabarti S, Fawcett A, Chen YA. Neuroimaging findings of hospitalized COVID-19 patients: a Canadian retrospective observational study. Can Assoc Radiol J. 2022;73:179–186. doi: 10.1177/08465371211002815. [DOI] [PubMed] [Google Scholar]
- 43.Kremer S, Lersy F, de Sèze J, Ferré JC, Maamar A, Carsin-Nicol B, Collange O, Bonneville F, Adam G, Martin-Blondel G, Rafiq M, Geeraerts T, Delamarre L, Grand S, Krainik A, Caillard S, Constans JM, Metanbou S, Heintz A, Helms J, Schenck M, Lefèbvre N, Boutet C, Fabre X, Forestier G, de Beaurepaire I, Bornet G, Lacalm A, Oesterlé H, Bolognini F, Messié J, Hmeydia G, Benzakoun J, Oppenheim C, Bapst B, Megdiche I, Henry Feugeas MC, Khalil A, Gaudemer A, Jager L, Nesser P, Talla Mba Y, Hemmert C, Feuerstein P, Sebag N, Carré S, Alleg M, Lecocq C, Schmitt E, Anxionnat R, Zhu F, Comby PO, Ricolfi F, Thouant P, Desal H, Boulouis G, Berge J, Kazémi A, Pyatigorskaya N, Lecler A, Saleme S, Edjlali-Goujon M, Kerleroux B, Zorn PE, Matthieu M, Baloglu S, Ardellier FD, Willaume T, Brisset JC, Boulay C, Mutschler V, Hansmann Y, Mertes PM, Schneider F, Fafi-Kremer S, Ohana M, Meziani F, David JS, Meyer N, Anheim M, Cotton F. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297:E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benny R, Singh RK, Venkitachalam A, Lalla RS, Pandit RA, Panchal KC, Pardasani V, Chanchalani G, Basle M, Bolegave V, Manoj H, Shetty AN, Shah AM, Pai P, Banthia NM, Patil SG, Chafale V, Pujara B, Shah S, Mehta N, Thakkar VV, Patel V, Shetty KV. Characteristics and outcomes of 100 consecutive patients with acute stroke and COVID-19. J Neurol Sci. 2021;423:117348. doi: 10.1016/j.jns.2021.117348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorgulu U, Bayındır H, Bektas H, Kayipmaz AE, San I. Coexistence of neurological diseases with COVID-19 pneumonia during the pandemic period. J Clin Neurosci. 2021;91:237–242. doi: 10.1016/j.jocn.2021.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz JM, Libman RB, Wang JJ, Filippi CG, Sanelli P, Zlochower A, Gribko M, Pacia SV, Kuzniecky RI, Najjar S, Azhar S. COVID-19 severity and stroke: correlation of imaging and laboratory markers. AJNR Am J Neuroradiol. 2021;42:257–261. doi: 10.3174/ajnr.A6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dixon L, McNamara C, Gaur P, Mallon D, Coughlan C, Tona F, Jan W, Wilson M, Jones B. Cerebral microhaemorrhage in COVID-19: a critical illness related phenomenon? Stroke Vasc Neurol. 2020;5:315–322. doi: 10.1136/svn-2020-000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Büttner L, Bauknecht HC, Fleckenstein FN, Kahn J, Tietze A, Bohner G, Siebert E. Neuroimaging findings in conjunction with severe COVID-19. Rofo. 2021;193:822–829. doi: 10.1055/a-1345-9784. [DOI] [PubMed] [Google Scholar]
- 49.Fitsiori A, Pugin D, Thieffry C, Lalive P, Vargas MI. COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients. J Neuroimaging. 2020;30:593–597. doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keller E, Brandi G, Winklhofer S, Imbach LL, Kirschenbaum D, Frontzek K, Steiger P, Dietler S, Haeberlin M, Willms J, Porta F, Waeckerlin A, Huber M, Abela IA, Lutterotti A, Stippich C, Globas C, Varga Z, Jelcic I. Large and small cerebral vessel involvement in severe COVID-19: detailed clinical workup of a case series. Stroke. 2020;51:3719–3722. doi: 10.1161/STROKEAHA.120.031224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin E, Lantos JE, Strauss SB, Phillips CD, Campion TR Jr, Navi BB, Parikh NS, Merkler AE, Mir S, Zhang C, Kamel H, Cusick M, Goyal P, Gupta A. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. AJNR Am J Neuroradiol. 2020;41:2001–2008. doi: 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahammedi A, Saba L, Vagal A, Leali M, Rossi A, Gaskill M, Sengupta S, Zhang B, Carriero A, Bachir S, Crivelli P, Paschè A, Premi E, Padovani A, Gasparotti R. Imaging of neurologic disease in hospitalized patients with COVID-19: an Italian multicenter retrospective observational study. Radiology. 2020;297:E270–E273. doi: 10.1148/radiol.2020201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boucher A, Desforges M, Duquette P, Talbot PJ. Long-term human coronavirus-myelin cross-reactive T-cell clones derived from multiple sclerosis patients. Clin Immunol. 2007;123:258–267. doi: 10.1016/j.clim.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendez Elizondo EF, Valdez Ramírez JA, Barraza Aguirre G, Dautt Medina PM, Berlanga Estens J. Central nervous system injury in patients with severe acute respiratory syndrome coronavirus 2: MRI findings. Cureus. 2021;13:e18052. doi: 10.7759/cureus.18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henry-Feugeas MC, Gaudemer A, Lescure X, Dossier A, Sonneville R, Ehmer C, Choquet C, Israel T, Simon AR, Borie R, Amarenco P, Khalil A. Covid-19 and dysregulated cerebral perfusion: observations with multimodal MRI. medRxiv. 2020 2020.2007.2012.20100941. [Google Scholar]
- 58.Uginet M, Breville G, Assal F, Lövblad KO, Vargas MI, Pugin J, Serratrice J, Herrmann FR, Lalive PH, Allali G. COVID-19 encephalopathy: clinical and neurobiological features. J Med Virol. 2021;93:4374–4381. doi: 10.1002/jmv.26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawlani V, Scotton S, Nader K, Jen JP, Patel M, Gokani K, Denno P, Thaller M, Englezou C, Janjua U, Bowen M, Hoskote C, Veenith T, Hassan-Smith G, Jacob S. COVID-19-related intracranial imaging findings: a large single-centre experience. Clin Radiol. 2021;76:108–116. doi: 10.1016/j.crad.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M, Coles JP, Manji H, Al-Shahi Salman R, Menon DK, Nicholson TR, Benjamin LA, Carson A, Smith C, Turner MR, Solomon T, Kneen R, Pett SL, Galea I, Thomas RH, Michael BD CoroNerve Study Group. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghadiri F, Sahraian MA, Shaygannejad V, Ashtari F, Ghalyanchi Langroodi H, Baghbanian SM, Mozhdehipanah H, Majdi-Nasab N, Hosseini S, Poursadeghfard M, Beladimoghadam N, Razazian N, Ayoubi S, Rezaeimanesh N, Eskandarieh S, Naser Moghadasi A. Characteristics of COVID-19 in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;57:103437. doi: 10.1016/j.msard.2021.103437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rifino N, Censori B, Agazzi E, Alimonti D, Bonito V, Camera G, Conti MZ, Foresti C, Frigeni B, Gerevini S, Grimoldi M, La Gioia S, Partziguian T, Quadri S, Riva R, Servalli MC, Sgarzi M, Storti B, Vedovello M, Venturelli E, Viganò M, Callegaro A, Arosio M, Sessa M. Neurologic manifestations in 1760 COVID-19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. 2021;268:2331–2338. doi: 10.1007/s00415-020-10251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, González E, Redondo-Peñas I, Perona-Moratalla AB, Del Valle-Pérez JA, Gracia-Gil J, Rojas-Bartolomé L, Feria-Vilar I, Monteagudo M, Palao M, Palazón-García E, Alcahut-Rodríguez C, Sopelana-Garay D, Moreno Y, Ahmad J, Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao TS, Zeng HL, Zhang X, Chen X, Jiang WL, Du J, Liu HY, Zhao J, Yuan Y, Peng XF, Li JC, Yang T, Liu BC, Li HJ, Zhang XA, Fang LQ, Lu QB, Cui F, Liu W. Neurological manifestations in COVID-19 patients and their application in predicting fatal disease: a retrospective cohort study. J Microbiol Immunol Infect. 2022;55:445–453. doi: 10.1016/j.jmii.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, Studer A, Radosavljevic M, Kummerlen C, Monnier A, Boulay C, Fafi-Kremer S, Castelain V, Ohana M, Anheim M, Schneider F, Meziani F. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haidar MA, Shakkour Z, Reslan MA, Al-Haj N, Chamoun P, Habashy K, Kaafarani H, Shahjouei S, Farran SH, Shaito A, Saba ES, Badran B, Sabra M, Kobeissy F, Bizri M. SARS-CoV-2 involvement in central nervous system tissue damage. Neural Regen Res. 2022;17:1228–1239. doi: 10.4103/1673-5374.327323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khedr EM, Shoyb A, Mohammaden M, Saber M. Acute symptomatic seizures and COVID-19: hospital-based study. Epilepsy Res. 2021;174:106650. doi: 10.1016/j.eplepsyres.2021.106650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lersy F, Benotmane I, Helms J, Collange O, Schenck M, Brisset JC, Chammas A, Willaume T, Lefebvre N, Solis M, Hansmann Y, Fabacher T, Caillard S, Mertes PM, Pottecher J, Schneider F, Meziani F, Fafi-Kremer S, Kremer S. Cerebrospinal fluid features in patients with coronavirus disease 2019 and neurological manifestations: correlation with brain magnetic resonance imaging findings in 58 patients. J Infect Dis. 2021;223:600–609. doi: 10.1093/infdis/jiaa745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pinna P, Grewal P, Hall JP, Tavarez T, Dafer RM, Garg R, Osteraas ND, Pellack DR, Asthana A, Fegan K, Patel V, Conners JJ, John S, Silva ID. Neurological manifestations and COVID-19: experiences from a tertiary care center at the frontline. J Neurol Sci. 2020;415:116969. doi: 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pons-Escoda A, Naval-Baudín P, Majós C, Camins A, Cardona P, Cos M, Calvo N. Neurologic involvement in COVID-19: cause or coincidence? A neuroimaging perspective. AJNR Am J Neuroradiol. 2020;41:1365–1369. doi: 10.3174/ajnr.A6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azab MA, Azzam AY, Salem AE, Reda A, Hassanein SF, Sabra M, Gadelmoula IS. Neurological problems in the context of COVID-19 infection in Egypt. A multicenter retrospective analysis. Interdiscip Neurosurg. 2021;26:101345. doi: 10.1016/j.inat.2021.101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, Ambrosy AP, Sidney S, Go AS. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 74.Hornuss D, Lange B, Schröter N, Rieg S, Kern WV, Wagner D. Anosmia in COVID-19 patients. Clin Microbiol Infect. 2020;26:1426–1427. doi: 10.1016/j.cmi.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woo MS, Malsy J, Pöttgen J, Seddiq Zai S, Ufer F, Hadjilaou A, Schmiedel S, Addo MM, Gerloff C, Heesen C, Schulze Zur Wiesch J, Friese MA. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2:fcaa205. doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du YY, Zhao W, Zhou XL, Zeng M, Yang DH, Xie XZ, Huang SH, Jiang YJ, Yang WH, Guo H, Sun H, Liu JY, Liu P, Zhou ZG, Luo H, Liu J. Survivors of COVID-19 exhibit altered amplitudes of low frequency fluctuation in the brain: a resting-state functional magnetic resonance imaging study at 1-year follow-up. Neural Regen Res. 2022;17:1576–1581. doi: 10.4103/1673-5374.327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ilg W, Bastian AJ, Boesch S, Burciu RG, Celnik P, Claaßen J, Feil K, Kalla R, Miyai I, Nachbauer W, Schöls L, Strupp M, Synofzik M, Teufel J, Timmann D. Consensus paper: management of degenerative cerebellar disorders. Cerebellum. 2014;13:248–268. doi: 10.1007/s12311-013-0531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, Koralnik IJ. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7:2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lantos JE, Strauss SB, Lin E. COVID-19-associated miller fisher syndrome: MRI findings. AJNR Am J Neuroradiol. 2020;41:1184–1186. doi: 10.3174/ajnr.A6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tahira AC, Verjovski-Almeida S, Ferreira ST. Dementia is an age-independent risk factor for severity and death in COVID-19 inpatients. Alzheimers Dement. 2021;17:1818–1831. doi: 10.1002/alz.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martín-Jiménez P, Muñoz-García MI, Seoane D, Roca-Rodríguez L, García-Reyne A, Lalueza A, Maestro G, Folgueira D, Blanco-Palmero VA, Herrero-San Martín A, Llamas-Velasco S, Pérez-Martínez DA, González-Sánchez M, Villarejo-Galende A. Cognitive impairment is a common comorbidity in deceased COVID-19 patients: a hospital-based retrospective cohort study. J Alzheimers Dis. 2020;78:1367–1372. doi: 10.3233/JAD-200937. [DOI] [PubMed] [Google Scholar]
- 85.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shoraka S, Ferreira MLB, Mohebbi SR, Ghaemi A. SARS-CoV-2 infection and Guillain-Barré syndrome: a review on potential pathogenic mechanisms. Front Immunol. 2021;12:674922. doi: 10.3389/fimmu.2021.674922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raciti L, Calabrò RS. Neurological complications of COVID-19: from pathophysiology to rehabilitation. An overview. Acta Biomed. 2021;92:e2021317. doi: 10.23750/abm.v92i4.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cleret de Langavant L, Petit A, Nguyen QTR, Gendre T, Abdelhedi J, Djellaoui A, Seddik L, Lim L, Faugeras F, Salhi H, Wahab A, Fechtenbaum L, Dormeuil A, Hosseini H, Youssov K, Fénelon G, Bapst B, Brugières P, Tuilier T, Kalsoum E, Matignon MB, Oniszczuk J, Gallien S, Vindrios W, Melica G, Scain AL, Esser R, Rostain L, Guillaud C, Dubos-Lascu G, Saada N, Guillet H, Khellaf M, Bardel B, Ayache SS, Lefaucheur JP, Pawlotsky JM, Fourati S, Bachoud-Lévi AC. Clinical description of the broad range of neurological presentations of COVID-19: a retrospective case series. Rev Neurol (Paris) 2021;177:275–282. doi: 10.1016/j.neurol.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karadaş Ö, Öztürk B, Sonkaya AR. A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci. 2020;41:1991–1995. doi: 10.1007/s10072-020-04547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mastrangelo A, Bonato M, Cinque P. Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci Lett. 2021;748:135694. doi: 10.1016/j.neulet.2021.135694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kandemirli SG, Altundag A, Yildirim D, Tekcan Sanli DE, Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol. 2021;28:28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung T, Prasad K, Lloyd TE. Peripheral neuropathy: clinical and electrophysiological considerations. Neuroimaging Clin N Am. 2014;24:49–65. doi: 10.1016/j.nic.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaywant A, Vanderlind WM, Alexopoulos GS, Fridman CB, Perlis RH, Gunning FM. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46:2235–2240. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 95.Csukly G, Sirály E, Fodor Z, Horváth A, Salacz P, Hidasi Z, Csibri É, Rudas G, Szabó Á. The differentiation of amnestic type MCI from the non-amnestic types by structural MRI. Front Aging Neurosci. 2016;8:52. doi: 10.3389/fnagi.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hosp JA, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M, Thurow J, Wagner D, Waller C, Niesen WD, Frings L, Urbach H, Prinz M, Weiller C, Schroeter N, Meyer PT. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144:1263–1276. doi: 10.1093/brain/awab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, Angelone S, Brugliera L, Tettamanti A, Beretta L, Iannaccone S. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One. 2021;16:e0246590. doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salomon T, Cohen A, Barazany D, Ben-Zvi G, Botvinik-Nezer R, Gera R, Oren S, Roll D, Rozic G, Saliy A, Tik N, Tsarfati G, Tavor I, Schonberg T, Assaf Y. Brain volumetric changes in the general population following the COVID-19 outbreak and lockdown. Neuroimage. 2021;239:118311. doi: 10.1016/j.neuroimage.2021.118311. [DOI] [PubMed] [Google Scholar]
- 99.Nowak-Kiczmer M, Kubicka-Bączyk K, Niedziela N, Adamczyk B, Wierzbicki K, Bartman W, Adamczyk-Sowa M. The course of COVID-19 infection in patients with multiple sclerosis-The experience of one center based on the population of Upper Silesia. Mult Scler Relat Disord. 2021;52:102984. doi: 10.1016/j.msard.2021.102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ray STJ, Abdel-Mannan O, Sa M, Fuller C, Wood GK, Pysden K, Yoong M, McCullagh H, Scott D, McMahon M, Thomas N, Taylor M, Illingworth M, McCrea N, Davies V, Whitehouse W, Zuberi S, Guthrie K, Wassmer E, Shah N, Baker MR, Tiwary S, Tan HJ, Varma U, Ram D, Avula S, Enright N, Hassell J, Ross Russell AL, Kumar R, Mulholland RE, Pett S, Galea I, Thomas RH, Lim M, Hacohen Y, Solomon T, Griffiths MJ, Michael BD, Kneen R CoroNerve study group. Neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents in the UK: a prospective national cohort study. Lancet Child Adolesc Health. 2021;5:631–641. doi: 10.1016/S2352-4642(21)00193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Versace V, Sebastianelli L, Ferrazzoli D, Romanello R, Ortelli P, Saltuari L, D’Acunto A, Porrazzini F, Ajello V, Oliviero A, Kofler M, Koch G. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin Neurophysiol. 2021;132:1138–1143. doi: 10.1016/j.clinph.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Canet-Juric L, Andrés ML, Del Valle M, López-Morales H, Poó F, Galli JI, Yerro M, Urquijo S. A longitudinal study on the emotional impact cause by the COVID-19 pandemic quarantine on general population. Front Psychol. 2020;11:565688. doi: 10.3389/fpsyg.2020.565688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang C, Pan R, Wan X, Tan Y, Xu L, McIntyre RS, Choo FN, Tran B, Ho R, Sharma VK, Ho C. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. 2020;87:40–48. doi: 10.1016/j.bbi.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boeschoten RE, Braamse AMJ, Beekman ATF, Cuijpers P, van Oppen P, Dekker J, Uitdehaag BMJ. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331–341. doi: 10.1016/j.jns.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 106.Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020;288:112954. doi: 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor S, Landry CA, Paluszek MM, Fergus TA, McKay D, Asmundson GJG. COVID stress syndrome: concept, structure, and correlates. Depress Anxiety. 2020;37:706–714. doi: 10.1002/da.23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology. 2020;94:959–969. doi: 10.1212/WNL.0000000000009566. [DOI] [PubMed] [Google Scholar]
- 109.Carstens PO, Schmidt J. Diagnosis, pathogenesis and treatment of myositis: recent advances. Clin Exp Immunol. 2014;175:349–358. doi: 10.1111/cei.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nicolau S, Kao JC, Liewluck T. Trouble at the junction: when myopathy and myasthenia overlap. Muscle Nerve. 2019;60:648–657. doi: 10.1002/mus.26676. [DOI] [PubMed] [Google Scholar]
- 111.Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, Jia T, Zhao Y, Wang D, Xiao A, Yin B. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ameriso SF, Wong VL, Quismorio FP Jr, Fisher M. Immunohematologic characteristics of infection-associated cerebral infarction. Stroke. 1991;22:1004–1009. doi: 10.1161/01.str.22.8.1004. [DOI] [PubMed] [Google Scholar]
- 114.Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation. 2011;124:346–354. doi: 10.1161/CIRCULATIONAHA.110.968776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33:1536–1540. doi: 10.1161/01.str.0000018012.65108.86. [DOI] [PubMed] [Google Scholar]
- 118.Mahammedi A, Ramos A, Bargalló N, Gaskill M, Kapur S, Saba L, Carrete H Jr, Sengupta S, Salvador E, Hilario A, Revilla Y, Sanchez M, Perez-Nuñez M, Bachir S, Zhang B, Oleaga L, Sergio J, Koren L, Martin-Medina P, Wang L, Benegas M, Ostos F, Gonzalez-Ortega G, Calleja P, Udstuen G, Williamson B, Khandwala V, Chadalavada S, Woo D, Vagal A. Brain and lung imaging correlation in patients with COVID-19: could the severity of lung disease reflect the prevalence of acute abnormalities on neuroimaging? A global multicenter observational study. AJNR Am J Neuroradiol. 2021;42:1008–1016. doi: 10.3174/ajnr.A7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fanou EM, Coutinho JM, Shannon P, Kiehl TR, Levi MM, Wilcox ME, Aviv RI, Mandell DM. Critical illness-associated cerebral microbleeds. Stroke. 2017;48:1085–1087. doi: 10.1161/STROKEAHA.116.016289. [DOI] [PubMed] [Google Scholar]
- 120.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 121.Go RS, Winters JL, Leung N, Murray DL, Willrich MA, Abraham RS, Amer H, Hogan WJ, Marshall AL, Sethi S, Tran CL, Chen D, Pruthi RK, Ashrani AA, Fervenza FC, Cramer CH 2nd, Rodriguez V, Wolanskyj AP, Thomé SD, Hook CC. Thrombotic microangiopathy care pathway: a consensus statement for the mayo clinic complement alternative pathway-thrombotic microangiopathy (CAP-TMA) Disease-Oriented Group. Mayo Clin Proc. 2016;91:1189–1211. doi: 10.1016/j.mayocp.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 122.Margos NP, Meintanopoulos AS, Filioglou D, Ellul J. Intracerebral hemorrhage in COVID-19: a narrative review. J Clin Neurosci. 2021;89:271–278. doi: 10.1016/j.jocn.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shah P, Patel J, Soror NN, Kartan R. Encephalopathy in COVID-19 patients. Cureus. 2021;13:e16620. doi: 10.7759/cureus.16620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uginet M, Breville G, Hofmeister J, Machi P, Lalive PH, Rosi A, Fitsiori A, Vargas MI, Assal F, Allali G, Lovblad KO. Cerebrovascular complications and vessel wall imaging in COVID-19 encephalopathy-a pilot study. Clin Neuroradiol. 2022;32:287–293. doi: 10.1007/s00062-021-01008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Günbey HP, Rona G, Adıgüzel Karaoysal Ö, Batırel A, Özen Barut B. Correlation of neuroimaging and thorax CT findings in patients with COVID-19: a large single-center experience. Turk J Med Sci. 2021;51:2850–2860. doi: 10.3906/sag-2105-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lang M, Li MD, Jiang KZ, Yoon BC, Mendoza DP, Flores EJ, Rincon SP, Mehan WA Jr, Conklin J, Huang SY, Lang AL, Giao DM, Leslie-Mazwi TM, Kalpathy-Cramer J, Little BP, Buch K. Severity of chest imaging is correlated with risk of acute neuroimaging findings among patients with COVID-19. AJNR Am J Neuroradiol. 2021;42:831–837. doi: 10.3174/ajnr.A7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A. Encephalitis as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes, and predictors. Eur J Neurol. 2021;28:3491–3502. doi: 10.1111/ene.14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Planchuelo-Gómez Á, García-Azorín D, Guerrero Á L, Rodríguez M, Aja-Fernández S, de Luis-García R. Structural brain changes in patients with persistent headache after COVID-19 resolution. J Neurol. 2023;270:13–31. doi: 10.1007/s00415-022-11398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lersy F, Anheim M, Willaume T, Chammas A, Brisset JC, Cotton F, Kremer S. Cerebral vasculitis of medium-sized vessels as a possible mechanism of brain damage in COVID-19 patients. J Neuroradiol. 2021;48:141–146. doi: 10.1016/j.neurad.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Antoniello D, Milstein MJ, Dardick J, Fernandez-Torres J, Lu J, Patel N, Esenwa C. Altered mental status in COVID-19. J Neurol. 2022;269:12–18. doi: 10.1007/s00415-021-10623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Radmanesh A, Raz E, Zan E, Derman A, Kaminetzky M. Brain imaging use and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. AJNR Am J Neuroradiol. 2020;41:1179–1183. doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burulday V, Sayar MS, Bayar Muluk N. Peripheral and central smell regions in COVID-19 positive patients: an MRI evaluation. Acta Radiol. 2022;63:1233–1242. doi: 10.1177/02841851211034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eliezer M, Hamel AL, Houdart E, Herman P, Housset J, Jourdaine C, Eloit C, Verillaud B, Hautefort C. Loss of smell in patients with COVID-19: MRI data reveal a transient edema of the olfactory clefts. Neurology. 2020;95:e3145–e3152. doi: 10.1212/WNL.0000000000010806. [DOI] [PubMed] [Google Scholar]
- 134.Altundag A, Yıldırım D, Tekcan Sanli DE, Cayonu M, Kandemirli SG, Sanli AN, Arici Duz O, Saatci O. Olfactory cleft measurements and COVID-19-related anosmia. Otolaryngol Head Neck Surg. 2021;164:1337–1344. doi: 10.1177/0194599820965920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Çetin H, Ateş A, Taydaş O, Elmas B, Güçlü E. Magnetic resonance imaging findings in COVID-19-related anosmia. Turk J Med Sci. 2022;52:1506–1512. doi: 10.55730/1300-0144.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yildirim D, Kandemirli SG, Tekcan Sanli DE, Akinci O, Altundag A. A comparative olfactory MRI, DTI and fMRI study of COVID-19 related anosmia and post viral olfactory dysfunction. Acad Radiol. 2022;29:31–41. doi: 10.1016/j.acra.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Güney B, Bacaksızlar Sarı F, Özdemir MY, Çullu N, Doğan E, Togan T. Changes in olfactory bulbus volume and olfactory sulcus depth in the chronic period after COVID-19 infection. Acta Otolaryngol. 2021;141:786–790. doi: 10.1080/00016489.2021.1946138. [DOI] [PubMed] [Google Scholar]
- 138.Campabadal A, Oltra J, Junqué C, Guillen N, Botí M, Sala-Llonch R, Monté-Rubio GC, Lledó G, Bargalló N, Rami L, Sánchez-Valle R, Segura B. Structural brain changes in post-acute COVID-19 patients with persistent olfactory dysfunction. Ann Clin Transl Neurol. 2023;10:195–203. doi: 10.1002/acn3.51710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ammar A, Distinguin L, Chetrit A, Safa D, Hans S, Carlier R, Lechien JR, Edjlali M. Transient modifications of the olfactory bulb on MR follow-up of COVID-19 patients with related olfactory dysfunction. J Neuroradiol. 2022;49:329–332. doi: 10.1016/j.neurad.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoon BC, Buch K, Lang M, Applewhite BP, Li MD, Mehan WA Jr, Leslie-Mazwi TM, Rincon SP. Clinical and neuroimaging correlation in patients with COVID-19. AJNR Am J Neuroradiol. 2020;41:1791–1796. doi: 10.3174/ajnr.A6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Espíndola OM, Brandão CO, Gomes YCP, Siqueira M, Soares CN, Lima M, Leite A, Torezani G, Araujo AQC, Silva MTT. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int J Infect Dis. 2021;102:155–162. doi: 10.1016/j.ijid.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Voruz P, Cionca A, Jacot de Alcântara I, Nuber-Champier A, Allali G, Benzakour L, Lalive PH, Lövblad KO, Braillard O, Nehme M, Coen M, Serratrice J, Reny JL, Pugin J, Guessous I, Ptak R, Landis BN, Adler D, Griffa A, Van De Ville D, Assal F, Péron JA. Brain functional connectivity alterations associated with neuropsychological performance 6-9 months following SARS-CoV-2 infection. Hum Brain Mapp. 2023;44:1629–1646. doi: 10.1002/hbm.26163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Esposito F, Cirillo M, De Micco R, Caiazzo G, Siciliano M, Russo AG, Monari C, Coppola N, Tedeschi G, Tessitore A. Olfactory loss and brain connectivity after COVID-19. Hum Brain Mapp. 2022;43:1548–1560. doi: 10.1002/hbm.25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Michaelson NM, Malhotra A, Wang Z, Heier L, Tanji K, Wolfe S, Gupta A, MacGowan D. Peripheral neurological complications during COVID-19: a single center experience. J Neurol Sci. 2022;434:120118. doi: 10.1016/j.jns.2021.120118. [DOI] [PMC free article] [PubMed] [Google Scholar]


