Abstract
Filamentous cells of Escherichia coli can be produced by treatment with the antibiotic cephalexin, which blocks cell division but allows cell growth. To explore the effect of cell size on chemotactic activity, we studied the motility and chemotaxis of filamentous cells. The filaments, up to 50 times the length of normal E. coli organisms, were motile and had flagella along their entire lengths. Despite their increased size, the motility and chemotaxis of filaments were very similar to those properties of normal-sized cells. Unstimulated filaments of chemotactically normal bacteria ran and stopped repeatedly (while normal-sized bacteria run and tumble repeatedly). Filaments responded to attractants by prolonged running (like normal-sized bacteria) and to repellents by prolonged stopping (unlike normal-sized bacteria, which tumble), until adaptation restored unstimulated behavior (as occurs with normal-sized cells). Chemotaxis mutants that always ran when they were normal sized always ran when they were filament sized, and those mutants that always tumbled when they were normal sized always stopped when they were filament sized. Chemoreceptors in filaments were localized to regions both at the poles and at intervals along the filament. We suggest that the location of the chemoreceptors enables the chemotactic responses observed in filaments. The implications of this work with regard to the cytoplasmic diffusion of chemotaxis components in normal-sized and filamentous E. coli are discussed.
Escherichia coli organisms have about six flagella, located randomly around their surfaces, that serve to propel the bacterium. When flagellar rotation is counterclockwise, the flagella form a bundle at one end to push the bacterium forward; this is known as a “run” and typically lasts about 1 to 2 s. When the rotation is clockwise, the flagella pull in opposing directions, causing the bacterium to “tumble” for less than a second. The bacteria alternately run and tumble in the absence of stimuli. When an attractant is encountered, running is promoted and tumbling is suppressed, which causes the bacteria to swim towards the attractant. When a repellent is encountered, the bacteria tumble, which prevents them from swimming into repellent. Adaptation to the attractant or repellent returns the bacteria to unstimulated behavior despite the continued presence of attractant or repellent.
Detection of attractants and repellents is mediated by a series of chemoreceptors in the cytoplasmic membrane, the methyl-accepting chemotaxis proteins (MCPs). Binding of repellents induces phosphorylation of the soluble cytoplasmic protein CheY, whereas binding of attractants results in CheY dephosphorylation. CheY interacts with the flagellar motor components and controls the direction of flagellar spin; phosphorylated CheY (CheY-P) stimulates clockwise rotation (tumbling), while unphosphorylated CheY results in counterclockwise rotation (running). For recent reviews of motility and chemotaxis see references 7 and 30.
The MCPs are located primarily at the poles of E. coli (22). During chemotactic responses, CheY must diffuse from the MCPs to the randomly located flagella (28). Diffusion of CheY is likely not a limiting factor in chemotactic responses (6, 15, 28), due to the small size of the normal E. coli cell (approximately 2 μm long and 0.5 μm wide). If cell division is blocked by a β-lactam antibiotic, such as cephalexin, growth continues and results in formation of a nonseptated cell, known as a filament or snake, that can be at least 50 times longer than a normal E. coli organism (12, 27). Despite their increased size, filaments are still motile (5).
Segall et al. reported that when attractants were introduced by micropipette to nonmotile E. coli filaments bearing polyhooks, only those polyhooks near the mouth of the pipette displayed altered spin bias (28). They concluded from these data that the chemotactic signal is inactivated as it diffuses through the cytoplasm, decreasing in strength by one-third every 2 μm (28). The mediator of this signal was subsequently shown to be CheY-P (13, 32), which undergoes dephosphorylation as it diffuses through the cytoplasm. In an elongated filamentous cell, it is unlikely that CheY-P from the cell poles reaches flagella all along the cell body. Further characterization of E. coli filaments may elucidate how they overcome the expected diffusion limitations of CheY-P necessary for motility and chemotaxis in cells of filamentous size.
Here we describe the motility and chemotaxis of filaments of chemotactically normal E. coli and also of filaments of E. coli chemotaxis mutants. We compare the motility and chemotaxis of filaments to those properties of normal-sized cells. Further, we show that the MCPs in filaments are located in clusters that are present not only at the poles but also all along the filament and suggest that these locations allow the filaments to rely on CheY-P diffusion for chemotactic responses.
MATERIALS AND METHODS
Bacteria.
The chemotactically wild-type E. coli strains were RP437 (25) and AW405 (3). The chemotaxis excitation mutants had deletions of cheA (RP1788) (19), cheW (RP1078) (19), cheY (RP5232) (J. S. Parkinson, unpublished data), and cheZ (RP1616) (J. S. Parkinson, unpublished data), and the chemotaxis adaptation mutants had deletions of cheB (RP4971) (J. S. Parkinson, unpublished data) and cheR (RP1254) (J. S. Parkinson, unpublished data). Except for AW405, all were kindly provided by John S. Parkinson.
Media.
Tryptone broth contained 1% tryptone and 0.5% NaCl. Minimal medium was made up according to the Vogel-Bonner recipe for its inorganic components (31); 50 mM dl-lactate was used as the carbon and energy source, and the required amino acids (l-histidine, l-leucine, and l-threonine for AW405 and l-histidine, l-leucine, l-methionine, and l-threonine for the other strains) were present at 1 mM.
Growth conditions.
After storage at −75°C, bacteria were grown by shaking them overnight at 35°C in 10 ml of tryptone broth in a 125-ml flask. The cells were then adapted to 10 ml of minimal medium by shaking them at 35°C in a 125-ml flask overnight.
These cells were diluted 100-fold into 10 ml of minimal medium and shaken at 35°C in a 125-ml flask for 2 h. Cephalexin (60 μg/ml) was then added, and the culture was grown with shaking at 35°C. For fluorescence microscopy, bacteria were grown in tryptone broth instead of minimal medium.
Motility and chemotaxis.
All experiments to determine motility and chemotaxis were done in minimal growth medium, not in the usual (1, 2) chemotaxis medium. We made observations of cells on slides at the liquid-glass interface with ×40 magnification at 28°C in samples taken every 30 minutes from the 35°C cultures. Finally, 3 to 3.5 h after addition of cephalexin (optical density at 590 nm of 0.4 to 0.6) was chosen as a standard time for observations. Chemotaxis was evaluated by a temporal assay (21); i.e., attractant or repellent was added to an aliquot, and microscopic observations were then made. For quantitation, the paths of 10 individual motile cells were monitored. At any given time, motile cells ranged from 25 to 95% of the total cell numbers; the remainder of the cells were presumably stuck to the glass surface.
Fluorescence microscopy.
Wild-type E. coli (AW405) or a ΔcheW strain (RP1078) from overnight tryptone broth cultures were diluted 1:100 in tryptone broth and grown to an optical density at 590 nm between 0.4 and 0.6 with or without 60 μg of cephalexin per ml. A 250-μl aliquot was removed, and the bacteria were washed twice in chemotaxis medium (10 mM potassium phosphate buffer [pH 7.0], 100 μM EDTA). Bacteria were then prepared for immunofluorescence microscopy according to the procedure of Maddock and Shapiro (22). The anti-Tsr serum used in these experiments recognizes Tsr and Tar (J. S. Parkinson, personal communication). Slides were stored at 4°C until they were viewed on a Zeiss Axioscope at a ×630 magnification under oil immersion with a Princeton Micromax camera and appropriate filter sets. Images were obtained by using IPLab Spectrum 3.2 and Adobe PhotoShop 5.0.
RESULTS
Growth of filaments.
Upon addition of cephalexin to an E. coli culture (see Materials and Methods), cell division is blocked but growth continues; consequently, single-celled filaments are produced (12, 27). In minimal medium, this development occurred up to 6 h after addition of cephalexin. Results with samples of 3- and 6-h treatments are shown in Fig. 1 and 2, respectively. Essentially identical rates of growth of filaments were obtained for the chemotactically wild-type strain and for the cheA, cheB, cheR, cheW, cheY, and cheZ chemotaxis mutants.
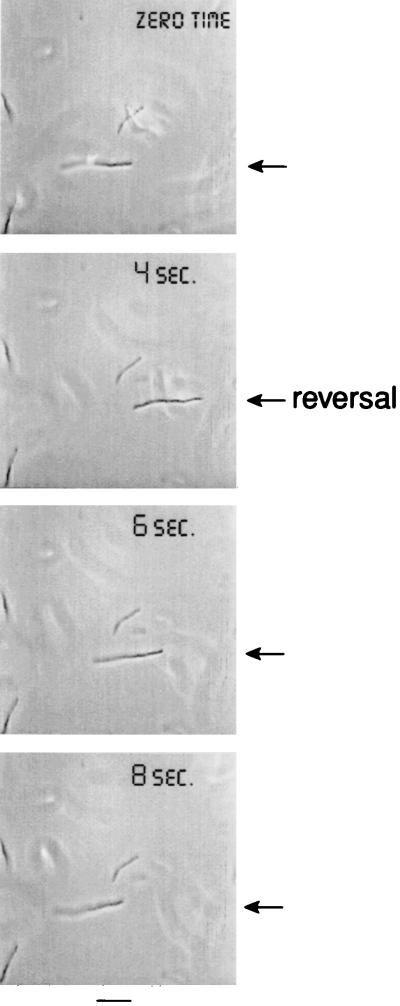
FIG. 1.
Reversal of filament movement at 3 h after addition of cephalexin. At zero time to 4 s the filament swims to the right. Then after a brief stop (about 0.5 s), the filament swims to the left. At other times (not shown), the filament continues to swim in its original direction after a stop. Arrows point to the filament. Bar, 5 μm.
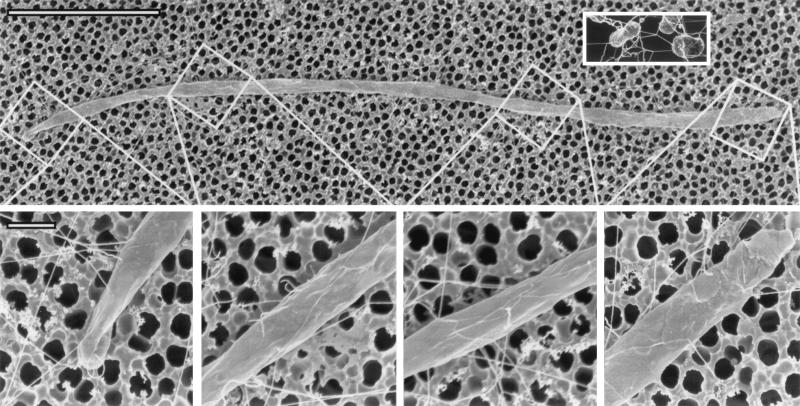
FIG. 2.
Electron micrograph of a filamentous cell of E. coli at 6 h after addition of cephalexin. The top panel shows a 25-μm-long filament, 10 to 15 times longer than a normal-sized E. coli organism. The inset shows untreated cells, which are about 2 μm long. The bottom panel shows four parts of the filament at a higher magnification, ×38,800; note that flagella are visible in the four parts (flagella are difficult to discern in the top panel because of the lower magnification). At this higher magnification, flagella can be seen equally distributed along the entire length of the filament. Flagella appear straight in such scanning electron micrographs, rather than curly as in transmission electron micrographs. The diameters of the flagella rule out the possibility that these are pili. Bar in top panel, 5 μm; bar in bottom panel, 0.5 μm.
Flagella in filaments.
Filamentous cells of E. coli were examined by scanning electron microscopy. Filaments had numerous flagella (Fig. 2), which shows that cell division is not required for the synthesis of flagella. Just as in normal-sized E. coli, the flagella of a filament are distributed randomly along the entire cell surface. This distribution resembles that previously known for filaments with polyhooks (14, 28).
Running and stopping in chemotactically normal filaments.
In order to characterize the motility of filaments, we observed the cells by light microscopy. The filaments were motile and could run (Fig. 1); however, filaments appeared to be too elongated to tumble and instead they stopped. All of the motile filaments continuously ran and stopped with a frequency of stops shown in Table 1. Running was either in the same direction as before the stop or in the opposite direction (Fig. 1). Filaments in minimal medium were motile for at least 6 h after addition of cephalexin.
TABLE 1.
Chemotactic responses of filamentous E. coli cells compared to those of normal-sized E. coli cells
| Cephalexin treatment | Mean length (μm) ± SD | Mean no. of tumbles or stops/10 s ± SD | With 5 × 10−5 M l-serine
|
With 1.5 × 10−2 M sodium benzoate
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| % of motile cells responding | No. of tumbles or stopsa | Running speed (μm/s)a | Adaptation time (s)b | % of motile cells responding | Nature of tumbling or stoppingc | Adaptation time (s)d | |||
| − | 1.5 ± 0.2 | 7 ± 2e | 100 | 0 | 19 ± 2 | 240 | 100 | Continuouse | 50 |
| + | 7.5 ± 1.2 | 6 ± 2f | 100 | 0 | 11 ± 2 | 90 | 100 | Continuousf | 30 |
During the first 60 seconds.
Appearance of first tumbles.
During the first 10 seconds.
Appearance of first runs.
Tumbling, no stopping.
Stopping, no tumbling.
Effect of stimuli on chemotactically normal filaments.
Addition of attractants to chemotactically normal E. coli organisms not treated with cephalexin causes continuous running and blocks tumbling, whereas addition of repellents induces continuous tumbling. In either case, this is followed by adaptation (a return to running and tumbling). In order to study the chemotactic responses of filaments, we added l-serine (an attractant) to a final concentration of 5 × 10−5 M or sodium benzoate (a repellent) to a final concentration of 1.5 × 10−2 M and monitored the responses by light microscopy. We also monitored the responses of normal-sized cells not treated with cephalexin under identical conditions. The results of these comparative experiments are shown in Table 1. All motile filaments responded to attractants by continuous running and to repellents by continuous stopping. All motile normal-sized cells under these conditions responded to attractants by continuous running and to repellents by continuous tumbling. In every case, these responses were followed by adaptation. In general, the chemotactic responses of filament-sized and normal-sized E. coli organisms were similar. However, the speed of the filament-sized cells after addition of attractant (11 μm/s) was lower than the speed of the normal-sized cells (19 μm/s) and shorter times were required for adaptation of the filament-sized cells to attractant (90 s compared to 240 s for the normal-sized cells) and repellent (30 s compared to 50 s for the normal-sized cells).
Filaments of chemotaxis mutants.
A variety of chemotaxis mutants was used to characterize the chemotactic response of filaments. Some normal-sized (not treated with cephalexin) E. coli chemotaxis mutants, the cheA, cheR, cheW, and cheY mutants, run but do not tumble (11, 19, 24, 26), but the cheR mutant tumbles with the addition of repellents (11, 26). Filaments of these mutants always ran without stopping. Addition of repellent, 1.5 × 10−2 M benzoate, to cheR mutant filaments failed to produce the stopping observed in the wild-type filaments.
The E. coli cheB and cheZ chemotaxis mutants continuously tumble, but they will run upon addition of attractants (24, 33). Filaments of these mutants were found to be in the stopped mode (gently thrashing about). Addition of l-serine induced running in the cheB mutant (10−3 M) and cheZ mutant (10−4 M) filaments.
Chemoreceptors are localized in filaments.
MCPs have been shown to be concentrated at the poles of normal-sized E. coli cells (22). It has been proposed that this localization is involved in the proper response of the bacteria to ligand during chemotaxis (4, 18, 20). Since cephalexin-induced filaments undergo chemotactic responses, we sought to determine the subcellular location of the chemoreceptors in such filaments.
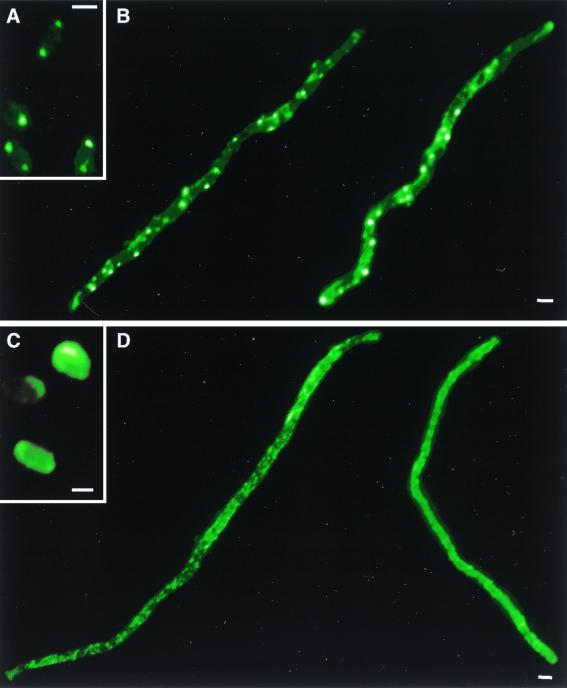
Filaments were labeled with anti-MCP serum and visualized by immunofluorescence microscopy to determine the location of the MCPs. Chemoreceptors were localized to the poles of normal-sized E. coli cells (Fig. 3A) as has been previously shown (22). In filaments, however, fluorescence was observed at intervals along the entire cell body and was not restricted to the poles (Fig. 3B). This result indicates that the MCPs are concentrated at both the filament poles and at regions along the filament length.
FIG. 3.
Chemoreceptor localization in filamentous cells of E. coli. Bacteria were immunolabeled with fluorescent anti-MCP serum to identify the subcellular locations of chemoreceptors. (A) Untreated wild-type E. coli (AW405); (B) cephalexin-treated AW405 filaments; (C) untreated cheW mutant; (D) cephalexin-treated cheW filaments. Images are representative (>90%) of those from three independent experiments. Bars, 1 μm.
The cytoplasmic protein CheW has been shown to be necessary for the localization of the chemoreceptors in normal E. coli, because deletion of CheW results in randomization of MCP location (22). As in cells not treated with cephalexin (Fig. 3C), cephalexin-treated cheW filaments displayed a diffuse fluorescence pattern (Fig. 3D). Thus, MCP localization in filaments requires CheW.
DISCUSSION
To explore chemotactic responses in large bacteria, we generated cephalexin-produced filaments of E. coli and characterized their motility and chemotaxis. Filamentous cells of chemotactically wild-type E. coli had flagella located along the entire cell (Fig. 2). The filaments were motile (Fig. 1), and they alternately ran and stopped. Addition of attractant to the chemotactically wild-type filaments caused prolonged running, while addition of repellent caused stopping. Following these responses, filaments adapted, returning to their unstimulated behavior. Thus, running of filaments is equivalent to running of normal-sized bacteria and stopping is equivalent to tumbling of normal-sized bacteria. The running and stopping responses of filaments were similar to the running and tumbling responses of normal-sized cells (Table 1).
Chemotaxis mutants have been very important in the analysis of chemotaxis in normal bacteria. To compare the chemotaxis of filament-sized cells to that of normal-sized cells, we generated filaments of these mutants and characterized their motility. Filaments of the running cheA, cheR, cheW, and cheY mutants ran continuously. Filaments of the tumbly cheB and cheZ mutants were in a stopping mode, gently thrashing about, until addition of attractant induced running. Thus, the chemotaxis of filamentous mutants is very similar to that of normal-sized mutant cells.
Fluorescence microscopy revealed that the MCPs in filaments are located in regions both at the poles and along the cell body (Fig. 3B). These results indicate also that the total amount of MCP located in clusters along the length of the filament is greater (by at least 10-fold) than the amount of MCP at the poles. This is in contrast to what occurs in normal-sized cells, which display a majority (80%) of fluorescence at their poles (22). It is not known at this time whether these MCP clusters along the filament length are anchored, perhaps to cell division sites, or whether they are able to migrate in the membrane. Our results demonstrate that CheW is required for the formation of both polar and nonpolar MCP clusters in filaments, as deletion of CheW abrogates any MCP localization (Fig. 3D).
Chemotaxis in normal-sized E. coli is dependent on the diffusion of CheY-P from MCPs, which are located primarily at the cellular poles (22), to the flagella, which are randomly placed along the cell body. The diffusion constant of CheY in the bacterial cytoplasm has been estimated to be 10 μm2/s (6), which suggests that diffusion of CheY-P is sufficient to account for response initiation times in normal-sized E. coli (about 0.1 s) (15, 29). If MCPs were localized strictly at the poles of filaments, the short half-life of CheY-P and the increased length of the cells might be expected to disrupt proper chemotactic responses due to limitations (16, 28) imposed by diffusion. However, the work presented here demonstrates that filaments of E. coli are motile and chemotactic and that their chemotactic responses are similar to those of normal-sized cells. Potential difficulties arising from the size of these cells appear to be offset by the presence of laterally localized MCP clusters along the cell body. We hypothesize that E. coli filaments sense chemoattractants and chemorepellents via MCPs concentrated to regions at the poles and all along the cell body and that this mechanism allows normal chemotactic responses. In this model, local pools of CheY, present at a concentration of 10 μM in the cytoplasm (8), are acted upon by polar and laterally located MCP clusters. CheY-P then diffuses from the MCP cluster to nearby flagella. A uniform concentration of stimulus may therefore be expected to induce responses in every flagellum along the filament, as flagella are never more than 1 to 2 μm from an MCP cluster (Fig. 2 and 3). Nonuniform concentrations of stimulus, in which the stimulus concentration differs significantly between the ends of the filament, are expected to generate responses only at the flagella near the stimulus. This hypothesis is supported by the observations of Segall et al. that only those polyhooks in the vicinity of locally applied attractant displayed altered bias (28).
Chemotactic signaling responses appear to be carried out by changes in membrane potentials in large bacteria (20 to 40 μm in length) such as Spirillum volutans (17, 23) and Spirochaeta aurantia (9, 10). Ishihara et al. (14) and Segall et al. (28) have considered the possibility of an action potential propagated along the length of an E. coli filament. They have suggested that this does not occur, and our results are consistent with this conclusion. Moreover, we suggest a sensing mechanism involving diffusion of CheY-P from polar and laterally localized MCPs as an alternative to a mechanism involving action potentials. Thus, motilities and chemotaxes are similar in normal-sized and filament-sized E. coli cells and similar molecular mechanisms may explain their chemotaxis.
ACKNOWLEDGMENTS
We thank Paul A. Sims and Ralph M. Albrecht for the electron microscopy. We are grateful to Sandy Parkinson for anti-Tsr serum. Sebastian Bednarek generously supplied fluorescence microscopy equipment.
The research reported here was supported by a grant to J.A. (GM52214) from the National Institutes of Health, a grant to J.A. (IBN-9807789) from the National Science Foundation, and a grant to L.L.K. (GM-55984) from the National Institutes of Health. L.L.K. acknowledges the Dreyfus Foundation for support. J.E.G. acknowledges the NIH Biotechnology Training Program (T32GM08349) for support.
REFERENCES
- 1.Adler J. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol. 1967;46:175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- 2.Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong J B, Adler J, Dahl M M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967;93:390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray D, Levin M D, Morton-Firth C J. Receptor clustering as a cellular mechanism to control sensitivity. Nature. 1998;393:85–88. doi: 10.1038/30018. [DOI] [PubMed] [Google Scholar]
- 5.Cui C, Smith D O, Adler J. Characterization of mechanosensitive channels in Escherichia coli cytoplasmic membrane by whole-cell patch clamp recording. J Membr Biol. 1995;144:31–42. doi: 10.1007/BF00238414. [DOI] [PubMed] [Google Scholar]
- 6.Elowitz M B, Surette M G, Wolf P-E, Stock J B, Leibler S. Protein mobility in the cytoplasm of Escherichia coli. J Bacteriol. 1999;181:197–203. doi: 10.1128/jb.181.1.197-203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falke J J, Bass R B, Butler S L, Chervitz S A, Danielson M A. The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol. 1997;13:457–512. doi: 10.1146/annurev.cellbio.13.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gegner J A, Graham D R, Roth A F, Dahlquist F W. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992;70:975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- 9.Goulbourne E A J, Greenberg E P. Chemotaxis of Spirochaeta aurantia: involvement of membrane potential in chemosensory signal transduction. J Bacteriol. 1981;148:837–844. doi: 10.1128/jb.148.3.837-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulbourne E A J, Greenberg E P. A voltage clamp inhibits chemotaxis of Spirochaeta aurantia. J Bacteriol. 1983;153:916–920. doi: 10.1128/jb.153.2.916-920.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goy M F, Springer M S, Adler J. Failure of sensory adaptation in bacterial mutants that are defective in a protein methylation reaction. Cell. 1978;15:1231–1240. doi: 10.1016/0092-8674(78)90049-1. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood D, O'Grady F. Comparison of the response of Escherichia coli and Proteus mirabilis to seven β-lactam antibiotics. J Infect Dis. 1973;128:211–222. doi: 10.1093/infdis/128.2.211. [DOI] [PubMed] [Google Scholar]
- 13.Hess J F, Oosawa K, Kaplan N, Simon M I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988;53:79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara A, Segall J E, Block S M, Berg H C. Coordination of flagella on filamentous cells of Escherichia coli. J Bacteriol. 1983;155:228–237. doi: 10.1128/jb.155.1.228-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasuja R, Keyoung J, Reid G P, Trentham D R, Khan S. Chemotactic responses of Escherichia coli to small jumps of photoreleased l-aspartate. Biophys J. 1999;76:1706–1719. doi: 10.1016/S0006-3495(99)77329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch A L. What size should a bacterium be? A question of scale. Annu Rev Microbiol. 1996;50:317–348. doi: 10.1146/annurev.micro.50.1.317. [DOI] [PubMed] [Google Scholar]
- 17.Krieg N R, Tomelty J P, Wells J S., Jr Inhibition of flagellar coordination in Spirillum volutans. J Bacteriol. 1967;94:1431–1436. doi: 10.1128/jb.94.5.1431-1436.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levit M N, Liu Y, Stock J B. Stimulus response coupling in bacterial chemotaxis: receptor dimers in signaling arrays. Mol Microbiol. 1998;30:459–466. doi: 10.1046/j.1365-2958.1998.01066.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Parkinson J S. Role of CheW protein in coupling membrane receptors to the intracellular signaling system of bacterial chemotaxis. Proc Natl Acad Sci USA. 1989;86:8703–8707. doi: 10.1073/pnas.86.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Levit M, Lurz R, Surette M G, Stock J B. Receptor-mediated protein kinase activation and the mechanism of transmembrane signaling in bacterial chemotaxis. EMBO J. 1997;16:7231–7240. doi: 10.1093/emboj/16.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macnab R M, Koshland D E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci USA. 1972;69:2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddock J R, Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993;259:1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- 23.Metzner P. Die Bewegung und Reizbeantwortung der bipolar gegeisselten Spirillen. Jahrb Wiss Bot. 1920;59:325–412. [Google Scholar]
- 24.Parkinson J S. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J Bacteriol. 1978;135:45–53. doi: 10.1128/jb.135.1.45-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkinson J S, Houts S E. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J Bacteriol. 1982;151:106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkinson J S, Revello P T. Sensory adaptation mutants of E. coli. Cell. 1978;15:1221–1230. doi: 10.1016/0092-8674(78)90048-x. [DOI] [PubMed] [Google Scholar]
- 27.Rolinson G N. Effect of β-lactam antibiotics on bacterial cell growth rate. J Gen Microbiol. 1980;120:317–323. doi: 10.1099/00221287-120-2-317. [DOI] [PubMed] [Google Scholar]
- 28.Segall J E, Ishihara A, Berg H C. Chemotactic signaling in filamentous cells of Escherichia coli. J Bacteriol. 1985;161:51–59. doi: 10.1128/jb.161.1.51-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segall J E, Manson M D, Berg H C. Signal processing times in bacterial chemotaxis. Nature. 1982;296:855–857. doi: 10.1038/296855a0. [DOI] [PubMed] [Google Scholar]
- 30.Silversmith R E, Bourret R B. Throwing the switch in bacterial chemotaxis. Trends Microbiol. 1999;7:16–22. doi: 10.1016/s0966-842x(98)01409-7. [DOI] [PubMed] [Google Scholar]
- 31.Vogel H J, Bonner D M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 32.Wylie D, Stock A, Wong C-Y, Stock J. Sensory transduction in bacterial chemotaxis involves phosphotransfer between Che proteins. Biochem Biophys Res Commun. 1988;151:891–896. doi: 10.1016/s0006-291x(88)80365-6. [DOI] [PubMed] [Google Scholar]
- 33.Yonekawa H, Hayashi H, Parkinson J S. Requirement of the cheB function for sensory adaptation in Escherichia coli. J Bacteriol. 1983;156:1228–1235. doi: 10.1128/jb.156.3.1228-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]