Abstract
Objective:
This review collates the published reports that focus on microbial and viral illnesses that can be transmitted by breast milk, donor milk and powdered infant formula (PIF). In this context, we attempt to define a risk framework encompassing those hazards, exposure scenarios, vulnerability and protective factors.
Design:
A literature search was performed for reported cases of morbidity and mortality associated with different infant feeding modes.
Setting:
Exclusive breast-feeding is the recommended for infant feeding under 6 months, or failing that, provision of donated human milk. However, the use of PIF remains high despite its intrinsic and extrinsic risk of microbial contamination, as well as the potential for adverse physiological effects, including infant gut dysbiosis.
Results:
Viable pathogen transmission via breast-feeding or donor milk (pasteurised and unpasteurised) is rare. However, transmission of HIV and human T-cell lymphotropic virus-1 is a concern for breast-feeding mothers, particularly for mothers undertaking a mixed feeding mode (PIF and breast-feeding). In PIF, intrinsic and extrinsic microbial contamination, such as Cronobacter and Salmonella, remain significant identifiable causes of infant morbidity and mortality.
Conclusions:
Disease transmission through breast-feeding or donor human milk is rare, most likely owing to its complex intrinsically protective composition of human milk and protection of the infant gut lining. Contamination of PIF and the morbidity associated with this is likely underappreciated in terms of community risk. A better system of safe donor milk sharing that also establishes security of supply for non-hospitalised healthy infants in need of breast milk would reduce the reliance on PIF.
Keywords: Breastfeeding, Infant formula, Human milk, Pathogen, Contamination, Infection risk
National and international health authorities recommend infants breastfeed to the exclusion of all other foods and fluids for the first 6 months of life and continue breast-feeding as part of a mixed diet well into early childhood(1,2). This remarkable unanimity recognises breast-feeding as necessary for physiological growth and development and immune protection during the amount of time that the infant is immune incompetent(3). Increasingly, it is recognised that the immunological resilience conferred through breast-feeding has lasting effects throughout life. Nevertheless, it is estimated that worldwide less than 40 % of infants under 6 months of age are exclusively breastfed(4). In Australia, although 96 % of mothers initiate breast-feeding, by 3 months only 39 % are still breast-feeding and only 15 % continue to 5 months(5). Powdered infant formula (PIF) is commonly used as a replacement for breast-feeding. The normalisation of its use has seen its change from a last resort intervention for infants who cannot be breastfed or fed with donor milk to a routine infant feeding practice(6,7). However, there is an unavoidable risk of intrinsic and extrinsic bacterial contamination with PIF use, in addition to the risks associated with the removal of the protective factors in breast milk that should be taken into consideration when deciding upon infant feeding mode.
Many structural and societal factors contribute to low breast-feeding rates. These include insufficient awareness of breast milk as a complex functional food; the lack of availability of donor milk, including pasteurised donor human milk (PDHM); little information about the comparative risks across all infant feeding modes; a poor appreciation of the intrinsic and extrinsic pathogen risks of PIF use and, no less important, gender discriminatory work and employment practices. Information on pathogen risk and morbidity associated with PIF use and recalls of PIF products due to microbial contamination are not always made available or easily accessible to the public. As a result, it is challenging for mothers to make a fully informed choice between the different infant feeding methods. Our systematic analysis of the scientific literature compares the reported pathogen risks of breast-feeding, PDHM, expressed breast milk (unpasteurised) and PIF use by collating available infant morbidity and mortality data. This review is intended as a comparative overview of the reported microbial risks involved across all the main feeding modes for infants, rather than an in-depth analysis of any particular risk. Human milk fortifiers (derived from sterilised human milk, where processing practices are not fully disclosed(8) and bovine milk) were not included in this review.
Factors affecting pathogen risk in infant feeding
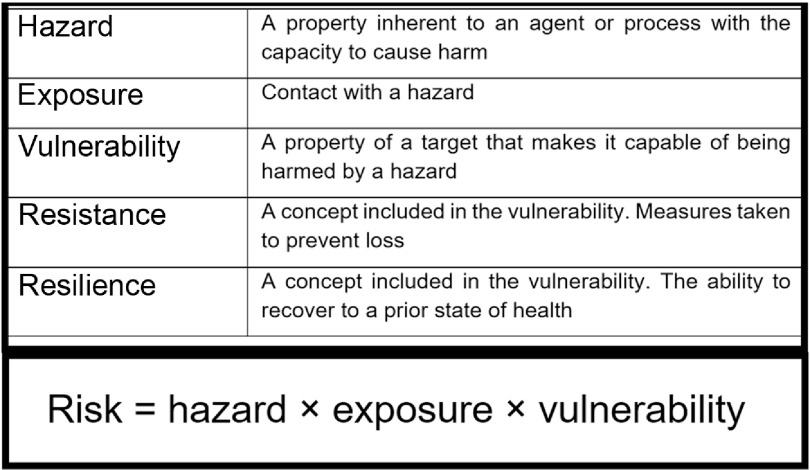
A hazard is any source or cause of potential damage. In regard to PIF use and breast milk feeding, the main hazard is the possible presence of pathogens and/or a reduced resilience against pathogens. Risk can be expressed as a product of hazard, exposure and vulnerability (Fig. 1). A hazard only becomes a risk to a target if it is exposed to that hazard and it is vulnerable. Risk may be mitigated by minimising exposure to the hazard or by reducing the target’s vulnerability. By including the latter, the assessment of risk also considers factors inherent to the milk, the processes required to produce and deliver it (Fig. 29–13) and factors inseparable from the feeding mode that mitigate hazards (e.g., antimicrobial properties of human milk) or reduce the infant’s vulnerability (e.g., immunological properties of human milk) (Table 1). Table 1 attempts to list the known hazards and mitigation factors. Non-biological hazards are mentioned for the sake of completeness (Table 1) but are beyond the scope of this review that focuses on infectious pathogens.
Fig. 1.
Risk equation and definitions of terms. Risk is an expression of the probability that exposure to a hazard will cause harm to a vulnerable organism or group. Resistance and resilience are both factors included in the broader definition of vulnerability
Fig. 2.
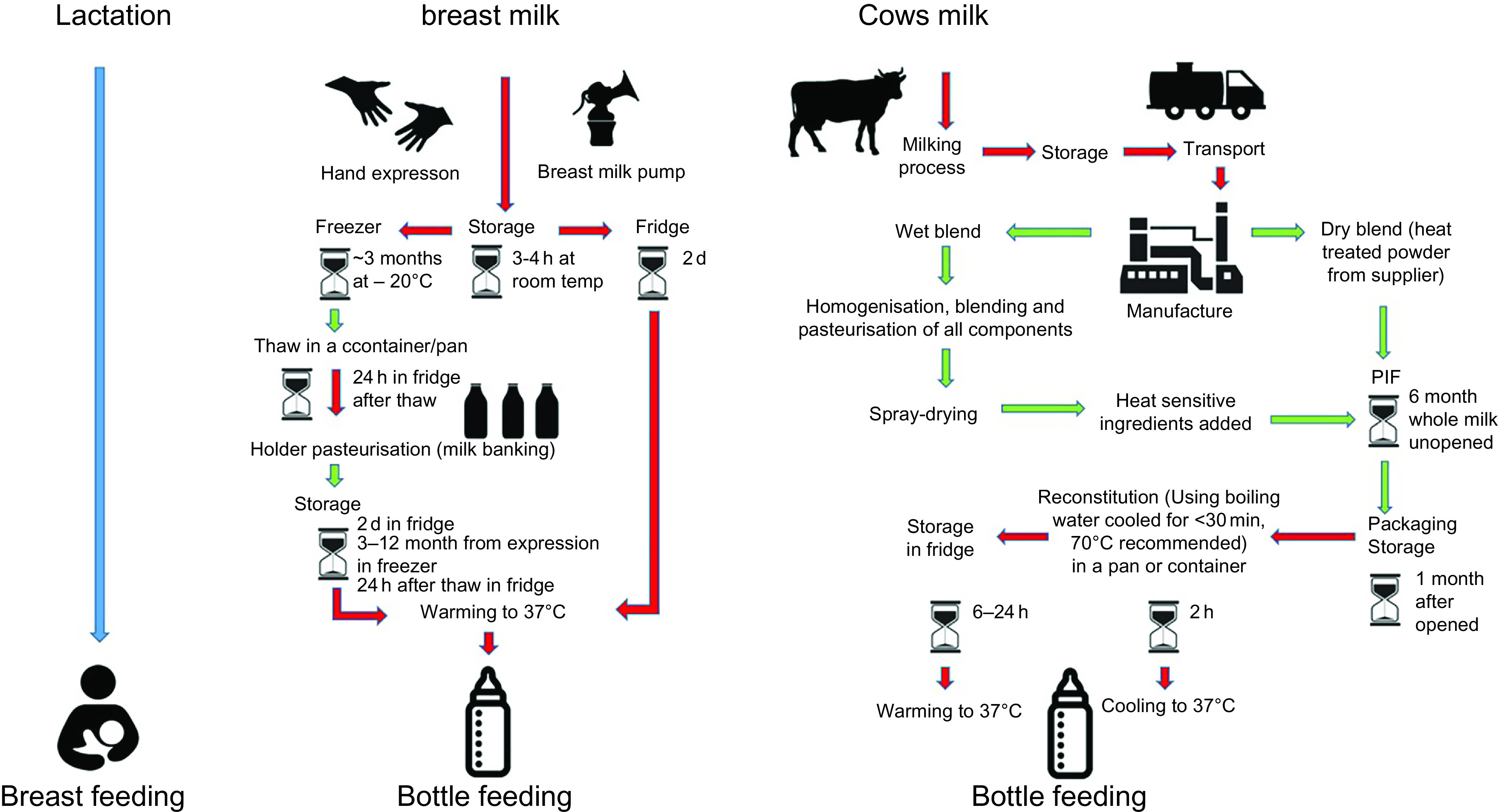
Flow diagram of infant feeding pathways. While in breast-feeding (blue) there is minimal external entry of pathogens other than from the maternal skin, all other modes of infant feeding have numerous entry points for pathogens from the environment(9–11). Red indicates those steps in which the food preparation and feeding processes are particularly prone to entry and/or growth of pathogens. Recommended safety steps for handling powdered infant formula (PIF) as set out by WHO(12) (not included in this diagram) should also be taken into consideration. The schematic is based on information taken from FAO 2004(13)
Table 1.
Hazards and mitigation factors related to breast-feeding, donated breast milk (PDHM) and PIF
| Infant feeding modes | Potential hazards | Exposure effects | Vulnerability effects |
|---|---|---|---|
|
Breast-feeding The process of feeding human milk to an infant, directly from the breast of a woman, usually the biological mother of the child. The milk is delivered directly from the mother’s mammary anatomy to the infant’s alimentary canal |
|
|
|
|
PDHM and expressed breast milk PDHM is expressed human milk that is refrigerated; transported; possibly frozen and defrosted; pooled (mixed together); pasteurised (Holder pasteurised – 62·5°C for 30 min); frozen; possibly transported; defrosted and warmed in preparation for feeding. The milk is either delivered to the infant’s alimentary canal from a container fitted with a rubber or silicone teat, or delivered through a tube if the infant is <32 weeks Expressed breast milk is the same as above but not usually pasteurised and is not handled by the milk bank |
|
|
|
|
PIF A modified animal (usually bovine or goat) milk-based partial or total replacement for human milk. The constituents of these products vary, even while their nutritional profiles are strictly prescribed. These are powdered milk products that must be reconstituted and are usually delivered to the infant’s alimentary canal from a container fitted with a rubber or silicone teat |
|
|
|
Literature review methodology
Search criteria
The databases Scopus and Web of Science were selected to retrieve peer-reviewed scientific publications. The search through the Web of Science databases comprised the Web of Science Core Collection, BIOSIS previews, CABI: CAB Abstracts®, Chinese Science Citation DatabaseSM, Current Contents Connect, KCI-Korean Journal Database, MEDLINE®, Russian Science Citation Index, SciELO Citation Index and Zoological Record. Initial search terms included (‘HIV’ or ‘Human immunodeficiency virus’) and (‘Human milk’ or ‘breastmilk’ or ‘breast milk’) and ‘Transmission’; (‘HTLV’ or ‘human T-lymphotropic virus’ or ‘human T-cell lymphotropic virus’ or ‘human T-cell leukemia-lymphoma virus’) and (‘Human milk’ or ‘breastmilk’ or ‘breast milk’) and ‘Transmission’; (‘Cronobacter sakazakii’ or ‘Enterobacter sakazakii’ or ‘salmonella’ or ‘salmonellosis’) and (‘PIF’ or ‘powdered infant formula’ or ‘infant formula’ or ‘milk substitute’ or ‘replacement formula’); and (‘donated milk’ and pathogen or morbidity).
For each topic, papers from all sources were integrated with duplicates removed and the final list curated by the lead author removing irrelevant publications based on title, abstract and finally full paper text in a three-step process (online Supplemental Fig. 1). After the initial search was completed, the reference list was expanded by including the term ‘donor milk’ as a more common term. Additional literature was extracted from the reference lists of publications retrieved through the search terms of the initial search.
Inclusion and exclusion criteria
Publications were only included in the review if they provided quantitative data of morbidity or mortality and specifically traced the source of the pathogen. Publications on pathogens known to be associated with PIF but without an examination or comment on the association with PIF in the reported specific case were excluded. Likewise, publications that did not specify the feeding mode were excluded as were publications on potential disease transmission through breast-feeding if the infant feeding mode was insufficiently specified, for example, breastfed exclusively, or mixed.
Pathogen risks associated with breast-feeding
Exclusive breast-feeding is not normally associated with any risk, even in the case of maternal infection (online Supplemental Table 1). The main hazards of breast-feeding are exposure to the maternal viral pathogens HIV and human T-cell lymphotropic virus-1 (HTLV-1). However, for both of these pathogens the actual transmission risk is not fully understood(14). Other viruses or pathogens may be present in breast milk but are rarely associated with subsequent infection in the infant (online Supplemental Table 1). This may be due to the concurrent presence of specific antibodies and antimicrobial factors in the breast milk(15). Breast-feeding has been described as a form of natural, broad-spectrum immunisation as it can, apart from passive immune transfer, elicit antigen-specific immune responses in the infant, boosting immunity to infectious diseases long-term(16). Consequently, the WHO recommends exclusive breast-feeding, except in the presence of a very limited number of pathogens as listed in online Supplemental Table 1.
As an example, human cytomegalovirus may also be transmitted via breast-feeding and infants can test positive. However, the vast majority of infants with cytomegalovirus remain asymptomatic. Symptomatic cytomegalovirus infection is usually only a concern for preterm infants (<32 weeks), and the approach to management remains controversial. One group has suggested that preterm infants receive pasteurised mothers milk(17), while others note that post-partum cytomegalovirus produces negligible severe outcomes and that the benefits of pasteurisation may not be justified by the risks or cost(18).
Hazard: HIV in breast milk
It is increasingly recognised that when infants are exclusively breastfed by an HIV positive mother, the infant usually remains HIV negative. However, an accurate transmission risk is difficult to obtain as studies designed to investigate this risk often either do not control for mixed feeding (combinations of breast-feeding and PIF and/or solid foods) or allow for variable amounts of mixed feeding even in the designated exclusive breast-feeding groups, which is a potential compounding hazard in its own right(19–22).
HIV transmission through breast-feeding is mainly seen in endemic areas, such as sub-Saharan Africa(23) with its high background prevalence of infant mortality and maternal under-nutrition.
In areas where HIV is endemic, the risk of HIV transmission through breast-feeding must be compared with the risks associated with PIF feeding, which include malnutrition, diarrhoea or pneumonia(24). This is illustrated well by an outbreak in Botswana in 2006. In an area where PIF use was high due to endemic HIV infection, heavy rains caused an outbreak of diarrhoea that disproportionally affected PIF-fed infants, regardless of HIV status. Of the 153 children hospitalised with diarrhoea, 88 % were not breastfed; of the thirty-three infants who died, twenty-seven were not breastfed. Non-breastfed infants were often undersupplied with PIF and therefore malnourished, an additional contextual determinant of the overall health outcome(25). Where PIF feeding cannot be done safely, breast-feeding is critical to infant survival, regardless of HIV status(25,26). Where HIV is endemic, lifelong maternal antiretroviral therapy is recommended for HIV positive mothers, and exclusive breast-feeding to 6 months and continued breast-feeding to at least 12 months is recommended as these measures are associated with a lower risk of infant morbidity and mortality then mixed or PIF feeding(24,27). Despite this recommendation, PIF use is common in heavily HIV-affected areas.
A meta-analysis was performed in all publications specifically reporting transmission rates for mother-to-child transmission rate of HIV. Across those sixteen studies, estimates of transmission were 6·2 % for exclusively breastfed infants, 15·3 % for mixed breastfed/PIF-fed infants and 12·7 % for exclusively PIF-fed infants (Table 2). In some reports, the definition of exclusive breast-feeding allowed for some solids or PIF to be used for short periods of time(21,28,34), which confounds interpretation. Additionally, while most reports had a study duration starting at 6 weeks of life, others started the study duration from birth, which may not clearly delineate between congenital and postnatal transmission (Table 2). The presence of HIV transmission in exclusively PIF-fed infants may indicate unsuccessful HIV testing for congenital transmission, or undisclosed occasional breast-feeding, which was noted in at least one of the studies, but is likely common(28).
Table 2.
Vertical postnatal HIV transmission data (2000–2018) separated by the infant feeding modes (breastfed, mixed-method and PIF-fed infants)*
| Year (country) | Exclusive breast-feeding definition in study | Exclusive breast-feeding | Mixed breast-feeding | PIF feeding | Antiretroviral therapy | Study duration | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence/total number of infants in study | % | Incidence/total number of infants in study | % | Incidence/total number of infants in study | % | Incidence/total number of infants in study | % | ||||
| 2000 (South Africa) | No liquids/solids introduced | 15/103 | 14·6 | 69/288 | 24·1 | 29/156 | 18·8 | No | 1 d–3 months | (19) | |
| 2005 (Uganda) | Only breast milk was received with no other concomitant fluid or feed. Infants who were switched to PIF before 4 months were still included in the EBF group | 19/119 | 16 | 10/49 | 20·4 | 4/108 | 3·7 | Short-term courses of zidovudine or nevirapine for mothers | Birth-6 months | (28) | |
| 2005 (Zimbabwe) | No liquids/solids or non-human milk except for vitamins or prescribed medicines for at least 3 months. One lapse in EBF was allowed as long as the non-breast milk item was not non-human milk | 8/156 | 5·1 | PBF 35/490 |
7·1 | Not reported | No | 6 weeks-18 months | (20) | ||
| MBF 156/1414 |
11 | ||||||||||
| 2007 (South Africa) | No liquids/solids except for vitamins or drugs (but up to 3 d of PIF feeding allowed in the EBF group) | 55/362 | 15 | MBF > 14 weeks 89/322, |
27 | 2/287 | 7 | Single-dose nevirapine provided for mothers and infants | 6–26 weeks | (21) | |
| MBF < 14 weeks 61/239 |
26 | ||||||||||
| 2010 (Nigeria) | Not defined | 5/27 | 18·8 | 32/47 | 68 | 11/228 | 4·8 | 231/304 mother and infant/only mother or only infant received ART | 76 | 6 weeks–17 months | (29) |
| 2014 (Tanzania) | Not defined | 43/497 | 8·7 | 6/22 | 27·3 | 4/40 | 10 | 469/561 received chemoprophylaxis | 86 | 4 weeks–18 months | (f) |
| 2014 (Nigeria) | Not defined | 3/94 | 3·2 | 10/63 | 15·9 | 6/426 | 1·4 | 431/583 received ART | 73·9 | 6 weeks-18 months | (23) |
| 2017 (Kenya) | Not defined | 10 364/167 166 | 6·2 | 3600/23 682 | 15·2 | 3672/27 856 | 9·7 | 68 % mother and child or mother only received some form of prophylaxis. 14 % prophylaxis status unknown | Birth-24 months | (31) | |
| 2018 (Cameroon) | No added supplements except vitamins. Feeding options described for the first 3 months only | 11/405 | 2·7 | 3/14 | 21·4 | 25/658 | 3·8 | All mothers received zidovudine | 6 weeks-24 months | (22) | |
| 2018 (Ethiopia) | Not defined | 15/691 | 3·3 | 3/10 | 37·5 | 4/63 | 7·5 | 407/764 mothers on prophylaxis | 53·3 | Birth–18 months | (32) |
| 353/764 mothers on HAART | 46·3 | ||||||||||
| 736/764 infants on prophylaxis | 97·9 | ||||||||||
| 2018 (Nigeria) | Not defined | 1/29 | 3·4 | 0/8 | 0 | 1/7 | 14·3 | 2/44 mother-infant pairs received ART | 95·2 | 1–18 months | (33) |
| Total | n/a | 10 539/169 549 | 6·2 | 4074/26 648 | 15·2 | 3758/29 829 | 12·6 | n/a | n/a | n/a | |
PIF, powdered infant formula; MBF, mixed breast-feeding; PBF, predominantly breast-feeding; EBF, exclusively breast-feeding; EFF, exclusive formula feeding; ART, antiretroviral therapy; HAART, highly active antiretroviral therapy.
This review records the incidence in each study of vertical HIV transmission, the total number of infants in the study and the percentage of the transmission rate. It should be noted that some of the included studies began their study from birth, which may not differentiate accurately between congenital and postnatal transmission.
Exposure effects
Exposure to antiretroviral therapy for both the infant and mother during pregnancy and breast-feeding has been shown to reduce the risk of transmission(35), as this reduces the viral load. Mixed feeding is also associated with higher rates of HIV transmission (Table 219–23,28–33).
Vulnerability effects
Breast milk contains antimicrobial substances that support host defence(36) and protects against infections through entero-mammary pathways(37). Secretory IgA, an anti-inflammatory antibody, is the first line of defence against pathogens gaining entry through the mucosal lining of the gut(3). During the first few months of life where the infant’s secretory immune system (production of antibodies) is deficient, and the mucosal gut barrier is not established adequately(38), the infant is reliant on the supply of antibodies from breast milk(39). Therefore, the absence of breast milk can disrupt the mucosal barrier. Breast milk colonises the gut with Bifidobacteria for which breast milk oligosaccharides provide the favourable growth conditions(40). In contrast, infants fed with reconstituted PIF or prematurely with solid foods show an increased gut pH and altered microbiota(41,42). This dysbiosis, along with increased disruption of the mucosal barrier(3) and increased gut permeability, may result in translocation of luminal contents(43) and make the infant more vulnerable to pathogens(44). Altered gut mucosal lining and permeability may be particularly important in HIV transmission(45), which could explain in part the increased HIV transmission under mixed feeding conditions.
Hazard: HTLV-1 in breast milk
HTLV-1 is a retrovirus that causes life-long infection. It is transmitted sexually or through an iatrogenic route in adults or through breast milk. While infants remain largely asymptomatic, about 10 % of adult HTLV-1 carriers develop adult T-cell leukaemia(46). Adult T-cell leukaemia has a high mortality rate and is linked to mother-to-child transmission of HTLV-1 (rather than adult infection of HTLV-1)(46). At least 5–10 million people are living with HTLV-1. Areas of high prevalence are found in parts of Africa, Peru, Iran, Brazil, Japan(46) and central Australia(47), although the true prevalence of HTLV-1 is unknown(48). The exact portion of infection globally that comes from mother-to-child transmission is not known and varies regionally(46). Our meta-analysis included all publications reporting morbidity and mortality thought to be due to mother-to-child transmission of HTLV-1. Across eleven retrieved studies, the overall transmission rates were 5·9, 14·1, 23·7 and 4·3 % for infants breastfed for <6, <12, >12 months and PIF-fed infants, respectively (Table 349–59). Two reports were excluded in these overall rates due to incidence rates being reported without sufficient information about the duration of breast-feeding(49,50).
Table 3.
Vertical postnatal HTLV-1 transmission data (1984–2018) separated by the infant feeding modes and duration of breast-feeding (breastfed for <6 months, >6 months, mixed-method and PIF-fed infants)
| Year (country) | Breast-feeding <6 months | Breast-feeding >6 months | Mixed feeding | PIF feeding | Study duration | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidence/total number of infants in study | % | Incidence/total number of infants in study | % | Incidence/total number of infants in study | % | Incidence/total number of infants in study | % | |||
| 1986 (Japan) | Not reported | 13/16 (BF duration not specified) | 25 | Not reported | Not reported | Birth-18 months | (49) | |||
| 1987 (Japan) | Not reported | BF (duration not specified) 11/24 | 46 | Not reported | 1/11 | Tested at 12 months | (50) | |||
| 1987 (Japan) | Not reported | 12 months 5/8 | 63 | 12 months 2/7 | 29 | Not reported | Tested at 12 months and 18 months | (51) | ||
| –18 months 5/18 |
28 | 18 months 3/29 | 10 | |||||||
| 1990 (Japan) | Not reported | 17/44 | 39 | 5/49 | 10 | 0/10 | 0 | Retrospective study of 1–13 year olds | (52) | |
| 1991 (Japan) | <6 months 4/90 | 4·4 | >7 months 20/139 | 14·4 | Not reported | 9/158 | 5·7 | Tested every 3 months for 1 year, then 6 months for 2 years | (53) | |
| 1991 (Japan) | <3 months 2/39 | 5·1 | <12 months 6/23 | 26·1 | Not reported | 2/12 | 16·7 | Tested every 6 months | (54) | |
| <6 months 5/22 | 22·7 | >12 months 5/13 | 38·5 | |||||||
| 1996 (Gabon) | <6 months not reported | >8 months 4/34 | 11·8 | Not reported | Not reported | Tested every 6 months until 2/4 years old | (55) | |||
| 1997 (Japan) | <6 months 2/51 | 3·9 | >6 months 13/64 | 20·3 | Not reported | 4/162 | 2·5 | Infant s > 30 months were tested | (56) | |
| 1997 (Jamaica) | <6 months not reported | <12 months 8/86 |
9 | Not reported | Not reported | Birth–24 months | (57) | |||
| >12 months 19/60 |
32 | |||||||||
| 1999 (French Guyana) | <3 months 1/35 | 2·9 | 7–9 months 1/13, | 7·7 | Not reported | 0/23 | 0 | Retrospective study of 0–12 year olds | (58) | |
| 4–6 months 1/17 | 5·9 | 10–12 months 7/68 | 10·3 | |||||||
| >12 months 9/70 | 12·9 | |||||||||
| 2018 (Brazil) | Not reported | <12 months 7/145 | 4·8 | Not reported | Not reported | Birth–until BF stopped | (59) | |||
| >12 months 34/143 | 23·8 | |||||||||
| Total | 15/254 | 5·9 | <12 months 88/624 | 14·1 | 10/85 | 11·8 | 16/376 | 4·3 | n/a | |
| >12 months 72/304 | 23·7 | |||||||||
This review records the incidence in each study of vertical HTLV-1 transmission, the total number of infants in the study and the percentage of the transmission rate (incidence/total number of infants in study (%)). It should be noted that no definitions were found for feeding modes; therefore, it is not known if breast-feeding was strictly exclusive or if other solids and/or liquids were allowed. Therefore, all HTLV-1 reports must be viewed as potentially having some PIF exposure.
Exposure effects
The mechanism of transmission during breast-feeding is not well understood(46). Mechanisms for both transmission through disrupted and intact epithelium have been reported(60). The transmission rate is lower when the duration of breast-feeding is shorter than 6–12 months(46,59). Since these studies do not specify whether ‘breast-feeding’ refers to exclusive or mixed modes, it is not known whether and how much a ‘mixed feeding mode’ might increase the risk of HTLV-1 transmission. In some regions of high prevalence, breast-feeding mothers lacking screening and education are unaware of their HTLV-1 status and may transmit the virus unknowingly(47). Increased pro-viral load(59,61) increases transmission, irrespective of breast-feeding duration(61). Additional independent variables for increased transmission also include more than one child with HTLV-1 and mothers who are >26 years old(59). No clinical trials have been undertaken to test the efficacy of antiretroviral therapy for HTLV-1 positive mothers, which may help to reduce viral loads and transmission(46).
Vulnerability effects
The major factor in the infants’ vulnerability to HTLV-1 seems to be the protective presence of the maternal HTLV-1 antibody in the milk for the first 6–12 months(46,53,54). Although there has been a strong focus on the effects of mixed feeding on HIV transmission, no such studies have been conducted for HTLV-1. The integrity of the infant gut may be a factor in transmission of HTLV-1. However, no reports were found where mixed feeding was included as a variable.
Pasteurised donor human milk and expressed breast milk
In this review, PDHM refers to donor milk that is handled by staff in milk banks following prescribed processes, that is, low temperature long time heat treated (Holder pasteurisation). Expressed milk refers to milk that is expressed for either the mother’s own child or unofficially shared with other mothers and handled in an unregulated manner and is not pasteurised(17). Holder pasteurisation is generally recognised as a necessary step for donor milk to ensure compliance with microbiological safety standards(62). The current pasteurisation standards are based on the destruction in cows’ milk of Coxiella burnetii, the cause of Q fever(63). The temperature and time established for the pathogen control C. burnetii, however, significantly reduces the protein, lipid and lactose content compared with unpasteurised breast milk(64), as well as reducing protein function, antibody content, antioxidants, vitamin C, B12, growth factors(65) and the natural microbiota present in milk(66). For this reason, milk banks in Norway rely on donor screening rather than pasteurisation, and the provision of unpasteurised human milk has not been associated with any increase in infant morbidity(67). Limited supply of donated milk in milk banks usually limits PDHM to premature infants in hospitals(9). Despite the prevalence of bacterial contamination present in expressed breast milk and PDHM before and after pasteurisation (up to 25 % being discarded as a result)(68,69) clinically significant infection events associated with either of these feeding modes are rare (online Supplemental Table 2).
Hazards: transmissible maternal viral pathogens and opportunistic pathogens
The main hazards include maternal pathogens present in the breast milk and contamination with a pathogen after expression. In PDHM, the main isolates before and after pasteurisation are Staphylococci and the spore-forming, heat resistant Bacillus cereus, respectively(68,70). The presence of Staphylococcus aureus pre-pasteurisation may produce heat resistant enterotoxins, and these may still be present after pasteurisation(71). The presence of heat stable enterotoxins or bacteria (Bacillus cereus) emphasises the importance of post-pasteurisation checks(70).
Exposure effects
For PDHM, donor screening is used to reduce exposure risk, although there are no global regulatory frameworks for milk banking in place(72). Therefore, screening and processing practices differ and are based on screening requirements for blood and tissue donation(9) and processing techniques used in the dairy industry as a basis(73). This results in a variable product and therefore has variable risks and benefits(74). The entry points for bacterial contamination for both PDHM and expressed milk include unhygienic expression (improperly washed hands or breast pump), unhygienic storage (incorrect temperature or storage duration), non-compliant pasteurisation, improperly sterilised feeding equipment (parenteral tube, enteral tube, bottle or cup) and through contamination during fortification of donated milk for premature infants(69,75).
The majority of cases of infant morbidity collected for this review were associated with unpasteurised milk (not sourced through milk banks) and expressed with both manual and electric breast pumps (online Supplemental Table 2). However, if expressed milk is handled properly, there is little evidence for any significant risk to infants, in contrast to PIF use, which is associated with a direct intrinsic and extrinsic pathogen risk as well as substantial intolerance reactions. Health authorities routinely advise against the use of expressed milk, notably if shared informally between mothers, due to the lack of regulatory control and lack of pasteurisation. However, with appropriate hygiene, there is no strong evidence that under share arrangements without monetary incentives, the quality and microbial contamination is systematically different between supervised, formally or informally shared milk(76). It thus appears that it is specifically the education in appropriate handling and storage of expressed milk, rather than necessarily pasteurising milk (with its potential lowering of quality), that yields the greatest health benefits and that sharing as such does not increase the risk above that of bottle feeding with own milk.
Vulnerability effects
Thermal pasteurisation, as generally practiced, significantly reduces the milk’s nutritional and bioactive components(77), as well as most beneficial bacteria naturally present(66). Despite a partial loss of function, PDHM is still associated with better clinical outcomes than reconstituted PIF in the protection against opportunistic pathogens. Studies have found that feeding preterm infants with PDHM (rather than reconstituted PIF) significantly reduces the incidence of necrotising enterocolitis(78–80), late onset sepsis(81) and bronchopulmonary dysplasia(82). However, not all studies have found a difference in necrotizing enterocolitis rates between PDHM and reconstituted PIF-fed infants(83). There are a number of reasons why PDHM might not show the expected beneficial effects. Over-treatment of PDHM may aggravate the loss of biological functions still present in expressed raw milk. Also, prolonged storage of expressed milk in a fridge (>72 h)(84) or freezer (>3 months)(85) reduces bactericidal activity. Increased vulnerability might also result from the absence, as with any form of bottle feeding, of the entero-mammary transfer of beneficial microbiota through breast-feeding(37) and the missing adaptive responses of the maternal immune system in the wake of breast-feeding(3).
Risks associated with powdered infant formula
Pathogen risk is inherent to PIF use as the manufacturing process itself introduces direct, albeit small, pathogen risks that cannot be completely eliminated(86). Contamination risks are associated with both dry and wet blending methods(10). Although dry blending tends to have a lower contamination risk due to the dry environment(10), batch testing of dried milk samples does not always detect contamination due to uneven distribution(87). Recall of PIF due to either later-detected contamination or increased infant morbidity is a regular occurrence (online Supplemental Table 4).
In addition to the risks associated with manufacturing, PIF reconstitution also yields sources of contamination. Potential extrinsic pathogen entry points include reconstitution water, utensils, bottles and teats, the preparers’ hygiene practices or the storage duration/conditions. Contamination of reconstituted PIF with faecal bacteria and inadequate sterilisation of the bottle and teat is common(11). Some feeding bottles designed with a silicone straw inside are particularly difficult to clean according to WHO recommendations, which recommend scrubbing all internal and external surfaces with hot soapy water(12) and are thus prone to biofilm formation.
PIF are mostly based on modified bovine milk with added vegetable oils, vitamins, minerals and processing agents. Due to the heat processing of bovine milk, PIF contains a range of pro-inflammatory advanced glycation end-products(88).
Use of reconstituted PIF is associated with an increased risk of gut inflammation, alteration of the gut microbiome and disruption of the epithelium barrier(42), all of which may increase the risk of pathogen entry into the bloodstream(89), thus increasing the infant’s vulnerability. Some infant formulas are supplemented with probiotics(90), and some with a synthetic human oligosaccharide(91). It should, however, be noted that there are over 200 interactive human oligosaccharides present in breast milk(92) and that the health benefits of probiotics in infant formula are reported in comparison with conventional infant formula and not benchmarked against donor milk. Thus, the reported effects are primarily a mitigation of the detrimental effects of un-supplemented infant formula.
There are a wide range of pathogens that have been linked to contamination of PIF (online Supplemental Table 3), including bacterial spores(93) and bacteria that resist desiccation(94). Salmonella and Cronobacter are the two most critical genera based on prevalence and health impact reported in the literature. While both pathogens are regularly detected in batches of PIF (online Supplemental Table 4), morbidity is mostly associated with Salmonella enterica. However, there may also be an underappreciated link between contamination of PIF with spores and infant morbidity(95).
Hazard: Salmonella enterica
Salmonella enterica are non-spore forming gram-negative Enterobacteriaceae, with a growth range of 5·2–46·2°C, are able to survive desiccation and, in certain circumstances, are able to survive freezing and some heat treatments(96). Although there is no dose-specific model for infants <9 months, existing data suggest a very low infective dose (possibly as low as <10 CFU)(97). Salmonella contamination of milk substitutes is associated with non-typhoidal S. enterica(10). It affects both term and premature infants and, based on reports in the literature, is generally associated with large outbreaks (although the true incidence rates are not known). The natural reservoirs of Salmonella are foods of animal origin including raw milk, human contact with animals, the faecal-oral route or exposure to other infants with diarrhoea(97). Non-typhoidal Salmonella infection can lead to gastroenteritis, diarrhoea, fever and vomiting, or more serious and potentially fatal conditions in premature infants including bacteremia, meningitis or severe levels of dehydration(98). This is particularly pertinent in resource-poor settings where medical attention and clean water are not readily available. While the detection of Salmonella is by far the most commonly identified reason for morbidity associated with contaminated PIF, no reports could be found for the overall morbidity or mortality rates for Salmonella associated with PIF contamination(99). While reports of Salmonella outbreaks associated with PIF exist for resource-rich settings, no such reports could be found for resource-poor settings, despite high levels of Salmonella infection in infants in these regions(100,101). It remains a common cause of neonatal sepsis in Africa and is thought to be associated with unhygienic conditions in the home environment(102). However, the association with PIF is unknown and calls for urgent research into the prevalence and incidence of Salmonella infections in PIF-fed infants. Our meta-analysis included all reports commenting on either morbidity or mortality traceable to a Salmonella contamination of PIF. Based on the reports found, there were 407 cases of Salmonella infant infection from twelve unrelated outbreaks that could be attributed to contaminated PIF in the past 50 years. From the compiled data, one was fatal (0·2 %) (Table 4103–114). These morbidity and mortality rates for Salmonella infection from contaminated PIF may well represent a substantial under-reporting. Non-typhoidal Salmonella is a common foodborne pathogen worldwide and is the most frequently isolated enteric bacterial pathogen in children under 5 in the USA(115). The source of the infection is often difficult to find and is frequently not even investigated.
Table 4.
Salmonella outbreaks attributed to contamination of PIF
| Year | Country | Symptomatic/deaths | Source of the contamination | Outbreak | Recall | References |
|---|---|---|---|---|---|---|
| 1968 | USA | 12/0 | Manufacturing environment (spray driers) | Yes | ? | (104) |
| 1987 | UK | 46/1 | Manufacturing environment (hole in inner liner of spray drier) | Yes | Yes | (105) |
| 1993 | Spain | 3/0 | Manufacturing environment (production equipment) | Yes | Yes | (106) |
| 1996 | Spain | 45/0 | Source not identified but attributed to the supplier of ingredients for the PIF brand | Yes | Yes | (107) |
| 1998 | UK and France | 17/0 | Manufacturing environment implicated through isolation in one brand of PIF | Yes | Yes | (108) |
| 2002 | USA | 11/0 | PIF mixed by the hospital | Yes | Yes | (109) |
| 2003 | Australia | 7/0 | Manufacturing environment (spray driers) | Yes | Yes | (110) |
| 2004 | Korea | 28/0 | Attributed to infected staff mixing PIF at NICU | Yes | Yes | (111) |
| 2008 | France | 136/0 | Traced back to one production line and two brands | Yes | Yes | (112) |
| 2008 | Spain | 31/0 | Manufacturing environment implicated through isolation in one brand of PIF | Yes | Yes | (113) |
| 2017 | France/Europe | 39/0 | Traced back to one production line and several brands | Yes | Yes | (114) |
| 2018 | France/Europe | 32/0 | Manufacturing environment implicated through isolation in one brand of PIF | Yes | Yes | (115) |
| n/a | n/a | 407/1 | n/a | n/a | n/a | Total |
NICU, neonatal intensive care unit.
Exposure effects
All outbreaks of Salmonella infections caused by contamination of PIF were either traced back to manufacturing equipment or the raw milk product(103,105,106,109,116,117) or isolated from unopened tins of PIF, implicating the manufacturing environment. The only exception was an outbreak in a neonatal intensive care unit in 2001, which was attributed to infected staff(108). Small amounts of Salmonella may grow to an infectious dose through unhygienic reconstitution, storage and feeding conditions(104).
Vulnerability effects
As previously noted, PIF feeding can increase vulnerability to bacterial pathogens due to gut microbiome dysbiosis(41,44) or lack of passive maternal immune protection(3).
Hazard: Cronobacter sakazakii
Cronobacter are an opportunistic gram-negative Enterobacteriaceae with a growth range from 5 to 47°C, are able to survive desiccated conditions for up to 2 years and, once ingested, can pass through the blood-brain barrier(118). Cronobacter sakazakii (or Enterobacter sakazakii prior to 2007)(118) is a ubiquitous pathogen that rarely causes disease except in vulnerable populations, including infants and the elderly(118). Cronobacter infection is almost exclusively associated with premature infants, or term infants within the first 2 months of life(119), and has not been associated with large outbreaks. Although the natural reservoir is not known, Cronobacter infection in infants is usually associated with PIF use(119). Morbidities associated with Cronobacter include meningitis, necrotising enterocolitis, bacteremia and sepsis, all of which can lead to fatality or permanent sequelae(118). Mortality rates are estimated to be between 33 and 80 %(120). The infectious dose for neonates is estimated to be between 1 × 103 CFU and 1 × 104 CFU in a single feed(97) which is most likely subject to the degree of vulnerability of the infant.
Estimates of invasive Cronobacter infection incidence in infants in the USA from 2003 to 2009 were 0·66 per 100 000(121). This is likely underestimated as Cronobacter is not notifiable in all states of the USA or in most countries, even in resource-rich settings(97). Data for the incidence of Cronobacter infection outside of resource-rich settings could not be found.
A meta-analysis of all the reports containing comments on Cronobacter-associated morbidity or mortality identified fourteen reports of Cronobacter outbreaks or cases in neonates where the source was investigated and attributed to PIF use. Of the sixty-two cases in these reports, thirty-eight cases were invasive (61·2 %) and eleven were fatal (17·7 %); eight of fourteen studies were considered to be outbreaks (Table 5122–135).
Table 5.
Cases of Cronobacter in neonates attributed to contamination of PIF. Note that there are records of other Cronobacter infections in neonates; however, all cases where the source of contamination was not investigated were excluded
| Year | Country | Exposure/symptomatic/death | Source of infection | Outbreak | Recall | References |
|---|---|---|---|---|---|---|
| 1989 | Iceland | 4/3/1 | Reconstituted PIF | Yes | No | (123) |
| 1989 | USA | 4/4/0 | Manufacturing environment | Yes | No | (124) |
| 1990 | USA | 1/1/0 | Blender | No | No | (125) |
| 2001 | Belgium | 6/6/2 | Reconstituted PIF+ unopened PIF | Yes | Yes | (126) |
| 2001 | Israel | 5/2/0 | PIF and blender | Yes | No | (127) |
| 2001 | USA | 10/3/1 | Reconstituted PIF + unopened powdered milk | Yes | Yes | (128) |
| 2002 | Belgium | 1/1/1 | PIF implicated | No | Yes | (129) |
| 2004 | New Zealand | 5/1/1 | Reconstituted PIF | Yes | No | (130) |
| 1994 | France | 17/8/3 | Reconstituted PIF + unopened PIF | Yes | No | (131) |
| 2008 | USA | 1/1/0 | Reconstituted PIF + unopened PIF | No | No | (132) |
| 2010 | Spain | 1/1/0 | Unopened PIF | No | No | (133) |
| 2010 | Mexico | 2/2/0 | Reconstituted PIF + unopened PIF | No | No | (134) |
| 2011 | USA | 4/4/2 | Reconstituted PIF + unopened PIF + nursery water | Yes | No | (135) |
| 2016 | USA | 1/1/0 | PIF | No | Yes | (136) |
| n/a | n/a | 62/38/11 | n/a | n/a | n/a |
Exposure effects
Cronobacter contamination has been traced back to unopened tins of PIF(125,130,136), powdered milk(127) and once back to the manufacturing environment(123). Several other studies on the incidence of bacteria in PIF found that it is common for there to be small amounts of bacterial contamination from the manufacturing environment.(137) Incidence of morbidity and mortality from Cronobacter infection is often associated with unhygienic practices during reconstitution and storage, including leaving reconstituted PIF for too long before consuming(122), improper cleaning of blenders(124,126) and reconstituting with water that is insufficiently heated(136). A recent study demonstrated that the bacterial load on the hands of the caregiver may also pose a risk through the hand-spoon-PIF route and should also be considered in any risk assessment(138). It is unclear whether the major source of the Cronobacter is the manufacturing environment or lies in the environment during reconstitution. Whatever the origin, if Cronobacter is present, unhygienic reconstitution conditions allow for growth to reach an infective dose(139).
Vulnerability effects
Cronobacter infection is highly specific to preterm and <2 months old infants, suggesting that the development of the immune system, presence of maternal immune components and a healthy microbiome in the infant gut (which may outcompete ingested Cronobacter)(118) are highly relevant to the degree of vulnerability of the infant. The absence of breast milk is associated with increased gut permeability and dysbiosis and therefore increased risk of pathogen translocation through the gut lining.
Outcomes and conclusions
This review collated cases of infant morbidity or mortality where the source of infection could be traced to breast-feeding, donor breast milk or PIF use, in an effort to better compare the risks associated with infant feeding modes. However, large gaps in the literature hampered this task. It is likely that issues such as PIF contamination remain significantly under-reported, making it challenging to gauge the risks associated with major hazards such as Salmonella. While the published peer-reviewed literature may give an incomplete picture of the different risks, important observations can still be made about each feeding mode.
The primary perceived risk associated with breast-feeding is maternal virus transmission, the greatest concern being HIV. However, when the mother is provided with antiretroviral therapy and exclusive breast-feeding is practiced, the transmission rate is very low. A mixed feeding mode appears to increase the transmission rate, which may be due to increased exposure of infants to the virus as a consequence of the damaging effects of PIF on the infant digestive system(140). Moreover, mortality resulting from withholding breast milk is higher than the infant mortality rate due to HIV infection(25,26). The design of future studies needs to pay greater attention to the definitions of exclusive (from birth) breast-feeding and the impact of early supplementation and later mixed feeding modes, which in many reports are frequently observed as confounding variables.
Proper management of breast-feeding for HTLV-1 positive mothers is complex. Although antenatal screening and strict withholding of breast-feeding for all HTLV-1 positive mothers have greatly lowered vertical transmission rates in Japan(141), this also carries its own risks and consequences associated with PIF use, which would be magnified in resource-poor settings. The risk of HTLV-1 transmission must be weighed against the risks associated with PIF use and the loss of the benefits associated with breast-feeding. Monitoring of the pro-viral load in the milk may be a feasible option to assess transmission risk. Additionally, clinical trials should be conducted in order to assess the efficacy of antiretroviral therapy in lowering transmission risk. Finally, further research is needed to evaluate the effect of mixed feeding on transmission risk. An alternative practicable approach might also be the systematic provision of PHDM or pasteurisation of the mothers’ own milk until the endemic presence of HTLV-1 in vulnerable communities has been removed.
Pathogen risk cannot be eliminated from the PIF manufacturing process. Salmonella is the pathogen of most concern as it has been repeatedly associated with large outbreaks traced back to PIF contamination. Recent assessments have concluded that final quality control measures may not be enough to ensure microbial safety(142). The reconstitution from powder additionally introduces extrinsic pathogen risks from contaminated water(143), contaminated bottles, teats or utensils and improper storage conditions which allows for bacterial growth. While all of these risks may be adequately mitigated by strict adherence to hygienic practices (using 70°C water with sterilised equipment), the formula-fed infant will still be left more vulnerable due to the lack of protective and developmental components in PIF.
A full assessment of the true risk associated with infant feeding modes also needs to encompass indirect hazard mechanisms. This includes any delay or disturbance in gut maturation through PIF-induced gut dysbiosis(42) or through lack of the protective and developmental factors in breast milk(3), the involvement of (possibly sub-clinical) inflammation(42,144) and other immune responses. Presently, there are insufficient data on the frequency of hospitalised infant morbidity linked to PIF use, and non-hospitalised infants with infections are not captured systematically. Therefore, the true incidence of PIF with primary or secondary contamination leading to illness may be greatly underestimated. Better documentation and quantification of the impact of diarrheal disease and pneumonia in infants fed with reconstituted PIF is needed, and clinical trials of PIF need to be benchmarked against health benefits of breast-feeding or donor milk.
Ultimately, a wider availability of PDHM could be a highly valuable public health measure if it decreases reliance on PIF. The available published data indicate that the risk of infant infection through PDHM or expressed milk is low. Indeed, most cases of bacterial contamination can be attributed to unhygienic handling of donor milk, including handling errors during the pasteurisation process. Proper decontamination of collection equipment and user hygiene are, therefore, key preventative strategies. While Holder pasteurisation can limit opportunistic pathogens, challenges remain around heat-stable spores(68) and the potential impact of thermal treatment on milk quality. To resolve these issues, one solution would be to advance non-thermal pasteurisation methods. Alternatively, a greater emphasis could be placed on testing the functional integrity of the processed batches of milk and rigorously comparing the advantages associated with unpasteurised donor milk on preterm infants with pathogen-related risks(145).
Acknowledgements
Acknowledgements: The contributions to the technical discussions by Kevin Condon and Lynne-McKensey Hall, Australian Breast Milk Bank and the editorial support by Suze Coppinger are highly appreciated by authors. Financial support: The authors acknowledge the financial support, for this study, from the University of Sydney for establishing Centre for Excellence in Advanced Food Enginomics. K.B. acknowledges support from the Joyce Beatrice Pirchan Research Scholarship. Conflict of interest: None (all authors). Authorship: R.B.B. conceptualised the review and defined the scope of the systematic review process. K.B. drafted the initial manuscript, which was extensively edited by A.S. and P.V. N.B. reworked the risk analysis section and provided a public health perspective. All authors including F.D. and N.K. provided commentary and approved the final manuscript. Ethics of human subject participation: N/A (review).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020000555.
click here to view supplementary material
References
- 1.Centers for Disease Control and Prevention (2019) Breastfeeding. https://www.cdc.gov/nutrition/infantandtoddlernutrition/breastfeeding/index.html (accessed February 2020).
- 2.The World Health Organization (2019) Appropriate complementary feeding. e-Library of Evidence for Nutrition Actions (eLENA). https://www.who.int/elena/titles/complementary_feeding/en/ (accessed February 2020).
- 3.Brandtzaeg P (2010) The mucosal immune system and its integration with the mammary glands. J Pediatr 156, S8–S15. [DOI] [PubMed] [Google Scholar]
- 4.Victora CG, Bahl R, Barros AJ et al. (2016) Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490. [DOI] [PubMed] [Google Scholar]
- 5.Australian Institute of Health and Welfare (2011) 2010 Australian National Infant Feeding Survey: Indicator Results. Canberra: AIHW. ISBN: 978-1-74249-269-8 Cat. no: PHE 156. [Google Scholar]
- 6.Berry NJ & Gribble KD (2008) Breast is no longer best: promoting normal infant feeding. Matern Child Nutr 4, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Victora CG, Morris SS, Barros FC et al. (1998) The NCHS reference and the growth of breast-and bottle-fed infants. J Nutr 128, 1134–1138. [DOI] [PubMed] [Google Scholar]
- 8.Czank C, Simmer K & Hartmann PE (2010) Design and characterization of a human milk product for the preterm infant. Breastfeed Med 5, 59–66. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann B, Pang W, Keil A et al. (2007) Best practice guidelines for the operation of a donor human milk bank in an Australian NICU. Early Hum Dev 83, 667–673. [DOI] [PubMed] [Google Scholar]
- 10.Kent RM, Fitzgerald GF, Hill C et al. (2015) Novel approaches to improve the intrinsic microbiological safety of powdered infant milk formula. Nutrients 7, 1217–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson S, Sahanggamu D, Fatmaningrum D et al. (2017) ‘Unfit for human consumption’: a study of the contamination of formula milk fed to young children in East Java, Indonesia. Trop Med Int Health 22, 1275–1282. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization (2007) How to Prepare Formula for Bottle-Feeding at Home. Geneva: World Health Organization. [Google Scholar]
- 13.World Health Organization (2004) Enterobacter sakazakii and Other Microorganisms in Powdered Infant Formula: Meeting Report. Italy: Food & Agriculture Org. [Google Scholar]
- 14.Gribble KD & Hausman BL (2012) Milk sharing and formula feeding: infant feeding risks in comparative perspective? Australas Med J 5, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Workowski KA & Bolan GA (2015) Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64, 1. [PubMed] [Google Scholar]
- 16.Verhasselt V (2015) Is infant immunization by breastfeeding possible? Philos Trans R Soc B Biol Sci 370, 20140139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picaud JC, Buffin R, Gremmo-Feger G et al. (2018) Review concludes that specific recommendations are needed to harmonise the provision of fresh mother’s milk to their preterm infants. Acta Paediatr 107, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jim W-T, Chiu N-C, Ho C-S et al. (2015) Outcome of preterm infants with postnatal cytomegalovirus infection via breast milk: a two-year prospective follow-up study. Medicine 94, e1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutsoudis A, Pillay K, Spooner E et al. (1999) Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. Lancet 354, 471–476. [DOI] [PubMed] [Google Scholar]
- 20.Iliff PJ, Piwoz EG, Tavengwa NV et al. (2005) Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 19, 699–708. [DOI] [PubMed] [Google Scholar]
- 21.Coovadia HM, Rollins NC, Bland RM et al. (2007) Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 369, 1107–1116. [DOI] [PubMed] [Google Scholar]
- 22.Nlend AEN, Motaze ACN, Sandie A et al. (2018) HIV-1 transmission and survival according to feeding options in infants born to HIV-infected women in Yaounde, Cameroon. BMC Pediatr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalu SO, Reynolds F, Petra GB et al. (2014) Infant feeding choices practiced among HIV positive mothers attending a Prevention of Mother to Child Transmission (PMTCT) of HIV program in Nnewi, Nigeria. J AIDS Clin Res 5, 1000300. [Google Scholar]
- 24.WHO (2019) Infant feeding for the prevention of mother-to-child transmission of HIV. e-Library of Evidence for Nutrition Actions (eLENA). https://www.who.int/elena/titles/hiv_infant_feeding/en/ (accessed November 2019).
- 25.Creek TL, Kim A, Lu L et al. (2010) Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr 53, 14–19. [DOI] [PubMed] [Google Scholar]
- 26.Rollins NC, Ndirangu J, Bland RM et al. (2013) Exclusive breastfeeding, diarrhoeal morbidity and all-cause mortality in infants of HIV-infected and HIV uninfected mothers: an intervention cohort study in KwaZulu Natal, South Africa. PLoS ONE 8, e81307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Alliance for Breastfeeding Action (2018) Understanding International Policy on HIV and Breastfeeding: A Comprehensive Resource, 2nd ed. Ireland: WABA. https://waba.org.my/understanding-international-policy-on-hiv-and-breastfeeding-a-comprehensive-resource/ (accessed February 2020). [Google Scholar]
- 28.Magoni M, Bassani L, Okong P et al. (2005) Mode of infant feeding and HIV infection in children in a program for prevention of mother-to-child transmission in Uganda. AIDS 19, 433–437. [DOI] [PubMed] [Google Scholar]
- 29.Ugochukwu E & Kalu S (2010) Early infant diagnosis of HIV infection in southeastern Nigeria: prevalence of HIV infection among HIV-exposed babies. West Afr J Med 29, 3–7. [DOI] [PubMed] [Google Scholar]
- 30.Mwendo EM, Mtuy TB, Renju J et al. (2014) Effectiveness of prevention of mother-to-child HIV transmission programmes in Kilimanjaro region, northern Tanzania. Trop Med Int Health 19, 267–274. [DOI] [PubMed] [Google Scholar]
- 31.Mwau M, Bwana P, Kithinji L et al. (2017) Mother-to-child transmission of HIV in Kenya: a cross-sectional analysis of the national database over nine years. PLoS ONE 12, e0183860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirkuzie AH (2018) Implementation and outcomes of guideline revisions for the prevention of mother-to-child HIV transmission in Mother Support Programme, Addis Ababa, Ethiopia. PLoS ONE 13, e0198438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afolabi AY, Bakarey AS, Kolawole OE et al. (2018) Investigation of mother-to-child transmission of HIV in pregnancy and among HIV-exposed infants accessing care at a PMTCT clinic in southwest Nigeria. J Immunoassay Immunochem 39, 403–415. [DOI] [PubMed] [Google Scholar]
- 34.Natchu UCM, Liu E, Duggan C et al. (2012) Exclusive breastfeeding reduces risk of mortality in infants up to 6 mo of age born to HIV-positive Tanzanian women. Am J Clin Nutr 96, 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flynn PM, Taha TE, Cababasay M et al. (2018) Prevention of HIV-1 transmission through breastfeeding: efficacy and safety of maternal antiretroviral therapy versus infant nevirapine prophylaxis for duration of breastfeeding in HIV-1-infected women with high CD4 cell count (IMPAACT PROMISE): a randomized, open label, clinical trial. J Acquir Immune Defic Syndr 77, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lönnerdal B (2013) Bioactive proteins in breast milk. J Paediatr Child Health 49, 1–7. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez JM (2014) The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr 5, 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandtzaeg P (2007) Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25, 5467–5484. [DOI] [PubMed] [Google Scholar]
- 39.Brandtzaeg P, Nilssen D, Rognum T et al. (1991) Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol Clin North Am 20, 397–439. [PubMed] [Google Scholar]
- 40.Cacho NT & Lawrence RM (2017) Innate immunity and breast milk. Front Immunol 8, 584. doi: 10.3389/fimmu.2017.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SA, Lim JY, Kim B-S et al. (2015) Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr Res Pract 9, 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker M (2014) Formula Supplementation of the breastfed infant: assault on the gut microbiome. Clin Lact 5, 128–132. [Google Scholar]
- 43.Mai V, Torrazza RM, Ukhanova M et al. (2013) Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS ONE 8, e52876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezirtzoglou E, Tsiotsias A & Welling GW (2011) Microbiota profile in feces of breast-and formula-fed newborns by using fluorescence insitu hybridization (FISH). Anaerobe 17, 478–482. [DOI] [PubMed] [Google Scholar]
- 45.Kutty PK (2012) HIV transmission through breastmilk: the science behind the understanding of current trends and future research. Med J Malaysia 67, 644–651. [PubMed] [Google Scholar]
- 46.Rosadas C & Taylor GP (2019) Mother-to-child HTLV-1 transmission: unmet research needs. Front Microbiol 10, 999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Einsiedel LJ, Pham H, Woodman RJ et al. (2016) The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med J Aust 205, 305–309. [DOI] [PubMed] [Google Scholar]
- 48.Zihlmann KF, Mazzaia MC & Alvarenga AT (2017) Meanings of breastfeeding interruption due to infection by human T cell lymphotrophic virus type 1 (HTLV-1). Acta Paul Enferm 30, 80–86. [Google Scholar]
- 49.Nakano S, Ando Y, Saito K et al. (1986) Primary infection of Japanese infants with adult T-cell leukaemia-associated retrovirus (ATLV): evidence for viral transmission from mothers to children. J Infect 12, 205–212. [DOI] [PubMed] [Google Scholar]
- 50.Ando Y, Nakano S, Saito K et al. (1987) Transmission of adult T-cell leukemia retrovirus (HTLAI) from mother to child: comparison of bottle- with breast-fed babies. Jpn J Cancer Res 78, 322–324. [PubMed] [Google Scholar]
- 51.Hino S, Sugiyama H, Doi H et al. (1987) Breaking the cycle of HTLV-I transmission via carrier mothers’ milk. Lancet 330, 158–159. [DOI] [PubMed] [Google Scholar]
- 52.Tsuji Y, Doi H, Yamabe T et al. (1990) Prevention of mother-to-child transmission of human T-lymphotropic virus type-I. Pediatrics 86, 11–17. [PubMed] [Google Scholar]
- 53.Takahashi K, Takezaki T, Oki T et al. (1991) Inhibitory effect of maternal antibody on mother-to-child transmission of human T-lymphotropic virus type I. Int J Cancer 49, 673–677. [DOI] [PubMed] [Google Scholar]
- 54.Hirata M, Hayashi J, Noguchi A et al. (1992) The effects of breast-feeding and presence of antibody to p40tax protein of human t-cell lymphotropic virus type-i on mother to child transmission. Int J Epidemiol 21, 989–994. [DOI] [PubMed] [Google Scholar]
- 55.Nyambi PN, Ville Y, Louwagie J et al. (1996) Mother-to-child transmission of human T-cell lymphotropic virus types I and II (HTLV-I/II) in Gabon: a prospective follow-up of 4 years. J Acquir Immune Defic Syndr Hum Retrovirol 12, 187–192. [DOI] [PubMed] [Google Scholar]
- 56.Takezaki T (1997) Short-term breast-feeding may reduce the risk of vertical transmission of HTLV-I. Leukemia 11, 60–62. [PubMed] [Google Scholar]
- 57.Wiktor SZ, Pate EJ, Rosenberg PS et al. (1997) Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breast-feeding. J Hum Virol 1, 37–44. [PubMed] [Google Scholar]
- 58.Ureta-Vidal A, Angelin-Duclos C, Tortevoye P et al. (1999) Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: implication of high antiviral antibody titer and high proviral load in carrier mothers. Int J Cancer 82, 832–836. [DOI] [PubMed] [Google Scholar]
- 59.Paiva AM, Assone T, Haziot ME et al. (2018) Risk factors associated with HTLV-1 vertical transmission in Brazil: longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. Sci Rep 8, 7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pique C & Jones KS (2012) Pathways of cell-cell transmission of HTLV-1. Front Microbiol 3, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li HC, Biggar RJ, Miley WJ et al. (2004) Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J Infect Dis 190, 1275–1278. [DOI] [PubMed] [Google Scholar]
- 62.Moro GE, Billeaud C, Rachel B et al. (2019) Processing of donor human milk: update and recommendations from the European Milk Bank Association (EMBA). Front Pediatr 7, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enright JB, Sadler WW & Thomas RC (1957) Pasteurization of milk containing the organism of Q fever. Am J Public Health Nations Health 47, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piemontese P, Mallardi D, Liotto N et al. (2019) Macronutrient content of pooled donor human milk before and after Holder pasteurization. BMC Pediatr 19, doi: 10.1186/s12887-019-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wesolowska A, Sinkiewicz-Darol E, Barbarska O et al. (2019) Innovative techniques of processing human milk to preserve key components. Nutrients 11, 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandez L, Ruiz L, Jara J et al. (2018) Strategies for the preservation, restoration and modulation of the human milk microbiota. Implications for human milk banks and neonatal intensive care units. Front Microbiol 9, 2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grøvslien AH & Grønn M (2009) Donor milk banking and breastfeeding in Norway. J Hum Lact 25, 206–210. [DOI] [PubMed] [Google Scholar]
- 68.Mullié C, Obin O, Outurquin G et al. (2018) Breastmilk donations: bacteriological assessment, analysis of causes of non-compliance and suggestions for improvement. Arch Pédiatr 25, 263–268. [DOI] [PubMed] [Google Scholar]
- 69.Boo NY, Nordiah AJ, Alfizah H et al. (2001) Contamination of breast milk obtained by manual expression and breast pumps in mothers of very low birthweight infants. J Hosp Infect 49, 274–281. [DOI] [PubMed] [Google Scholar]
- 70.Lewin A, Delage G, Bernier F et al. (2019) Banked human milk and quantitative risk assessment of Bacillus cereus infection in premature infants: a simulation study. Can J Infect Dis Med Microbiol 2019, 6348281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Almutawif Y, Hartmann B, Lloyd M et al. (2017) A retrospective audit of bacterial culture results of donated human milk in Perth, Western Australia. Early Hum Dev 105, 1–6. [DOI] [PubMed] [Google Scholar]
- 72.PATH (2019) Providing Lifesaving Breast Milk for Every Newborn. https://path.azureedge.net/media/documents/PATH_HMB_Toolkit_0._Global_Implementation_Framework.pdf (accessed February 2020).
- 73.Holsinger V, Rajkowski K & Stabel JR (1997) Milk pasteurisation and safety: a brief history and update. Rev Sci Tech 16, 441–466. [DOI] [PubMed] [Google Scholar]
- 74.Hartmann BT (2019) Benefit by design: determining the ‘value’of donor human milk and medical products derived from human milk in NICU. Semin Perinatol 43, 151157. [DOI] [PubMed] [Google Scholar]
- 75.Becker GE, Smith HA & Cooney F (2016) Methods of milk expression for lactating women. Cochrane Database Syst Rev issue 9, CD006170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perrin MT, Fogleman AD, Davis DD et al. (2018) A pilot study on nutrients, antimicrobial proteins, and bacteria in commerce-free models for exchanging expressed human milk in the USA. Matern Child Nutr 14, e12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peila C, Moro G, Bertino E et al. (2016) The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: a review. Nutrients 8, e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan S, Schanler RJ, Kim JH et al. (2010) An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 156, 562–567.e561. [DOI] [PubMed] [Google Scholar]
- 79.Chowning R, Radmacher P, Lewis S et al. (2016) A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth. J Perinatol 36, 221. [DOI] [PubMed] [Google Scholar]
- 80.Quigley M, Henderson G, Anthony M et al. (2007) Formula milk versus donor breast milk for feeding preterm or low birth weight infants (Review). Cochrane Database Syst Rev issue 4, CD002971. [DOI] [PubMed] [Google Scholar]
- 81.Hair AB, Peluso AM, Hawthorne KM et al. (2016) Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk–based diet. Breastfeed Med 11, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Villamor-Martínez E, Pierro M, Cavallaro G et al. (2018) Donor human milk protects against bronchopulmonary dysplasia: a systematic review and meta-analysis. Nutrients 10, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schanler RJ, Lau C, Hurst NM et al. (2005) Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 116, 400–406. [DOI] [PubMed] [Google Scholar]
- 84.Silvestre D, Löpez M, March L et al. (2006) Bactericidal activity of human milk: stability during storage. Br J Biomed Sci 63, 59–62. [DOI] [PubMed] [Google Scholar]
- 85.Takci S, Gulmez D, Yigit S et al. (2012) Effects of freezing on the bactericidal activity of human milk. J Pediatr Gastroenterol Nutr 55, 146–149. [DOI] [PubMed] [Google Scholar]
- 86.Farmer III JJ (2015) My 40-year history with Cronobacter/Enterobacter sakazakii–lessons learned, myths debunked, and recommendations. Front Pediatr 3, 84. doi: 10.3389/fped.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zink D (2003) Powdered infant formula: an overview of manufacturing processes. Acedido em Mar 4, 2008. [Google Scholar]
- 88.Birlouez-Aragon I, Pischetsrieder M, Leclère J et al. (2004) Assessment of protein glycation markers in infant formulas. Food Chem 87, 253–259. [Google Scholar]
- 89.Claud EC & Walker WA (2001) Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J 15, 1398–1403. [DOI] [PubMed] [Google Scholar]
- 90.Chassard C, de Wouters T & Lacroix C (2014) Probiotics tailored to the infant: a window of opportunity. Curr Opin Biotechnol 26, 141–147. [DOI] [PubMed] [Google Scholar]
- 91.Coulet M, Phothirath P, Allais L et al. (2014) Pre-clinical safety evaluation of the synthetic human milk, nature-identical, oligosaccharide 2′-O-fucosyllactose (2′ FL). Regul Toxicol Pharmacol 68, 59–69. [DOI] [PubMed] [Google Scholar]
- 92.German JB, Freeman SL, Lebrilla CB et al. (2008) Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutrition Workshop Series. Pediatric Program 62, 205–212. doi: 10.1159/000146322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haughton P, Garvey M & Rowan NJ (2010) Emergence of Bacillus cereus as a dominant organism in Irish retailed Powdered Infant Formulae (PIF) when reconstituted and stored under abuse conditions. J Food Safety 30, 814–831. [Google Scholar]
- 94.Juma NA, Manning G & Forsythe SJ (2016) Desiccation survival of Acinetobacter spp. in infant formula. Food Control 68, 162–166. [Google Scholar]
- 95.Barash JR, Hsia JK & Arnon SS (2010) Presence of soil-dwelling clostridia in commercial powdered infant formulas. J Pediatr 156, 402–408. [DOI] [PubMed] [Google Scholar]
- 96.Food Standards Australia New Zealand (2013) Salmonella (non-typhoidal). FSANZ Australia. 1–11. https://www.foodstandards.gov.au/publications/Documents/Salmonella%20(non-typhoidal).pdf (accessed June 2019). [Google Scholar]
- 97.World Health Organization (2006) Enterobacter sakazakii and Salmonella in powdered infant formula: meeting report. Microbiol Risk Assess Series 10, 7–10. [Google Scholar]
- 98.Jones TF, Ingram LA, Fullerton KE et al. (2006) A case-control study of the epidemiology of sporadic Salmonella infection in infants. Pediatrics 118, 2380–2387. [DOI] [PubMed] [Google Scholar]
- 99.Cummings PL, Sorvillo F & Kuo T (2010) Salmonellosis-related mortality in the United States, 1990–2006. Foodborne Pathog Dis 7, 1393–1399. [DOI] [PubMed] [Google Scholar]
- 100.Feasey NA, Archer BN, Heyderman RS et al. (2010) Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis 16, 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohan A, Munusamy C, Tan Y-C et al. (2019) Invasive Salmonella infections among children in Bintulu, Sarawak, Malaysian Borneo: a 6-year retrospective review. BMC Infect Dis 19, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gordon MA (2011) Invasive non-typhoidal Salmonella disease – epidemiology, pathogenesis and diagnosis. Curr Opin Infect Dis 24, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collins RN, Treger MD, Goldsby JB et al. (1968) Interstate outbreak of Salmonella newbrunswick infection traced to powdered milk. JAMA 203, 838–844. [PubMed] [Google Scholar]
- 104.Rowe B, Hutchinson DN, Gilbert RJ et al. (1987) Salmonella ealing infections associated with consumption of infant dried milk. Lancet 330, 900–903. [DOI] [PubMed] [Google Scholar]
- 105.Louie KK, Paccagnella AM, Osei WD et al. (1993) Salmonella serotype Tennessee in powdered milk products and infant formula – Canada and United States, 1993. J Am Med Assoc 270, 432. [Google Scholar]
- 106.Usera MA (1996) Interregional foodborne salmonellosis outbreak due to powdered infant formula contaminated with lactose-fermenting Salmonella virchow. Eur J Epidemiol 12, 377–381. [DOI] [PubMed] [Google Scholar]
- 107.Threlfall EJ, Ward LR, Hampton MD et al. (1998) Molecular fingerprinting defines a strain of Salmonella enterica serotype Anatum responsible for an international outbreak associated with formula-dried milk. Epidemiol Infect 121, 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bornemann R, Zerr DM, Heath J et al. (2002) An outbreak of salmonella serotype Saintpaul in a children’s hospital. Infect Control Hosp Epidemiol 23, 671–676. [DOI] [PubMed] [Google Scholar]
- 109.Forsyth JRL, Bennett NM, Hogben S et al. (2003) The year of the salmonella seekers – 1977. Aust N Z J Public Health 27, 385–389. [DOI] [PubMed] [Google Scholar]
- 110.Park JK, Seok WS, Choi BJ et al. (2004) Salmonella enterica serovar London infections associated with consumption of infant formula. Yonsei Med J 45, 43–48. [DOI] [PubMed] [Google Scholar]
- 111.Brouard C, Espié E, Weill FX et al. (2007) Two consecutive large outbreaks of Salmonella enterica serotype Agona infections in infants linked to the consumption of powdered infant formula. Pediatr Infect Dis J 26, 148–152. [DOI] [PubMed] [Google Scholar]
- 112.Rodriguez-Urrego J, Herrera-Leon S, Echeita-Sarriondia A et al. (2010) Nationwide outbreak of Salmonella serotype Kedougou associated with infant formula, Spain, 2008. Eurosurveillance 15, 19–23. [PubMed] [Google Scholar]
- 113.European Food Safety Authority, Prevention ECfD Control (2018) Multi-Country Outbreak of Salmonella Agona Infections Linked to Infant Formula. EFSA Supporting Publications 15, EN-1365.
- 114.European Centre for Disease Prevention and Control & European Food Safety Authority (2019) Multi-Country Outbreak of Salmonella Poona Infections Linked to Consumption of Infant Formula. EFSA Supporting Publication 16, EN-1594.
- 115.Scallan E, Mahon BE, Hoekstra RM et al. (2013) Estimates of illnesses, hospitalizations and deaths caused by major bacterial enteric pathogens in young children in the United States. Pediatr Infect Dis J 32, 217–221. [DOI] [PubMed] [Google Scholar]
- 116.Rowe SY, Rocourt JR, Shiferaw B et al. (2004) Breast-feeding decreases the risk of sporadic salmonellosis among infants in FoodNet sites. Clin Infect Dis 38, S262–S270. [DOI] [PubMed] [Google Scholar]
- 117.Jones G, de la Gandara MP, Herrera-Leon L et al. (2019) Outbreak of Salmonella enterica serotype Poona in infants linked to persistent Salmonella contamination in an infant formula manufacturing facility, France, August 2018 to February 2019. Eurosurveillance 24, 1900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holý O & Forsythe S (2014) Cronobacter spp. as emerging causes of healthcare-associated infection. J Hosp Infect 86, 169–177. [DOI] [PubMed] [Google Scholar]
- 119.Jason J (2012) Prevention of invasive Cronobacter infections in young infants fed powdered infant formulas. Pediatrics 130, e1076–e1084. [DOI] [PubMed] [Google Scholar]
- 120.Lai KK (2001) Enterobacter sakazakii infections among neonates, infants, children, and adults: case reports and a review of the literature. Medicine 80, 113–122. [DOI] [PubMed] [Google Scholar]
- 121.Patrick ME, Mahon BE, Greene SA et al. (2014) Incidence of cronobacter spp. infections, United States, 2003–2009. Emerg Infect Dis 20, 1520–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Biering G, Karlsson S, Clark NC et al. (1989) Three cases of neonatal meningitis caused by Enterobacter sakazakii in powdered milk. J Clin Microbiol 27, 2054–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simmons BP, Gelfand MS, Haas M et al. (1989) Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect Control Hosp Epidemiol 10, 398–401. [DOI] [PubMed] [Google Scholar]
- 124.Noriega FR, Kotloff KL, Martin MA et al. (1990) Nosocomial bacteremia caused by Enterobacter-sakazakii and Leuconostoc-mesenteroides resulting from extrinsic contamination of infant formula. Pediatr Infect Dis J 9, 447–449. [PubMed] [Google Scholar]
- 125.Van Acker J, De Smet F, Muyldermans G et al. (2001) Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J Clin Microbiol 39, 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bar-Oz B, Preminger A, Peleg O et al. (2001) Enterobacter sakazakii infection in the newborn. Acta Paediatr 90, 356–358. [PubMed] [Google Scholar]
- 127.Himelright I, Harris E, Lorch V et al. (2002) Enterobacter sakazakii infections associated with the use of powdered infant Formula – Tennessee, 2001. MMWR Morb Mortal Wkly Rep 51, 297–300. [PubMed] [Google Scholar]
- 128.International Baby Foods Action Network (2002) How safe are infant formulas? The death of a week old infant in Belgium: Baby Milk Action IBFAN UK. Press release 10 May 2002. http://www.babymilkaction.org/archives/10978 (accessed June 2019).
- 129.Jarvis C (2005) Fatal Enterobacter sakazakii infection associated with powdered infant formula in a neonatal intensive care unit in New Zealand. Am J Infect Control 33, e19. [Google Scholar]
- 130.Caubilla-Barron J, Hurrell E, Townsend S et al. (2007) Genotypic and phenotypic analysis of Enterobacter sakazakii strains from an outbreak resulting in fatalities in a neonatal intensive care unit in France. J Clin Microbiol 45, 3979–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Centers for Disease and Prevention (2009) Cronobacter species isolation in two infants – New Mexico, 2008. MMWR 58, 1179–1183. [PubMed] [Google Scholar]
- 132.Simón MD, Sabaté S, Cristina Osanz A et al. (2010) Investigation of a neonatal case of Enterobacter sakazakii infection associated with the use of powdered infant formula. Enferm Infecc Microbiol Clin 28, 713–715. [DOI] [PubMed] [Google Scholar]
- 133.Parra Flores J, Arvizu Medrano S, Silva Sanchez J et al. (2011) Two cases of hemorrhagic diarrhea caused by Cronobacter sakazakii in hospitalized nursing infants associated with the consumption of powdered infant formula. J Food Prot 74, 2177–2181. [DOI] [PubMed] [Google Scholar]
- 134.Centers for Disease Control and Prevention (2013) CDC Update: Investigation of Cronobacter Infections Among Infants in the United States. USA: CDC. [Google Scholar]
- 135.Tatev (2017) Enfamil Class Action Lawsuit. Consumer Law Magazine. http://consumerlawmagazine.com/enfamil-class-action-lawsuit/ (accessed June 2019).
- 136.Flores JP, Medrano SA, Sánchez JS et al. (2011) Two cases of hemorrhagic diarrhea caused by Cronobacter sakazakii in hospitalized nursing infants associated with the consumption of powdered infant formula. J Food Prot 74, 2177–2181. [DOI] [PubMed] [Google Scholar]
- 137.Márquez M, Hernández A, Echevarría JW et al. (2019) Microbiological contamination in children’s powdered formulas in two Honduran hospitals. Rev Chil Nutr 46, 571–578. [Google Scholar]
- 138.Cho TJ, Hwang JY, Kim HW et al. (2019) Underestimated risks of infantile infectious disease from the Caregiver’s typical handling practices of infant formula. Sci Rep 9, 9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu Y & Matthews K (2019) Prevalence and genetic diversity of Cronobacter species isolated from four infant formula production factories in China. Front Microbiol 10, 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith MM & Kuhn L (2000) Exclusive breast-feeding: does it have the potential to reduce breast-feeding transmission of HIV-1? Nutr Rev 58, 333–340. [DOI] [PubMed] [Google Scholar]
- 141.Kashiwagi K, Furusyo N, Nakashima H et al. (2004) A decrease in mother-to-child transmission of human T lymphotropic virus type I (HTLV-I) in Okinawa, Japan. Am J Trop Med Hyg 70, 158–163. [PubMed] [Google Scholar]
- 142.Avis de l’Anses,Saisine n° (2018) -SA-0264 Saisine liée n° 2005-SA-0313; French Agency for Food, Environmental and Occupational Health and Safety (ANSES Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail, Maisons-Alfort, France, 1–30. https://www.anses.fr/en/system/files/EAUX2012sa0114EN.pdf (accessed March 2020).
- 143.Marino DD (2007) Water and food safety in the developing world: global implications for health and nutrition of infants and young children. J Am Diet Assoc 107, 1930–1934. [DOI] [PubMed] [Google Scholar]
- 144.Moodley-Govender E, Mulol H, Stauber J et al. (2015) Increased exclusivity of breastfeeding associated with reduced gut inflammation in infants. Breastfeed Med 10, 488–492. [DOI] [PubMed] [Google Scholar]
- 145.Lindemann P, Foshaugen I & Lindemann R (2004) Characteristics of breast milk and serology of women donating breast milk to a milk bank. Arch Dis Child Fetal Neonatal Ed 89, F440–F441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020000555.
click here to view supplementary material