Abstract
Objective:
The association between 25-hydroxyvitamin D (25(OH)D) and maternal depression (MD) is deemed to be inconclusive. The current analysis aimed to quantify the relationship between 25(OH)D serum concentrations, the main indicator of vitamin D nutritional status, and MD.
Design:
Dose–response meta-analysis.
Setting:
A systematic search in PubMed, Embase and Web of Science from inception to June 2019.
Participants:
Relevant observational studies reporting risk estimates and 95 % CI of random effects for 25(OH)D concentration on MD were identified.
Results:
Twelve observational studies with thirteen independent reports involving 10 317 pregnant women were included. Compared with the lowest category of 25(OH)D, the pooled OR for the highest category of MD was 0·49 (95 % CI 0·35, 0·63); a high heterogeneity was observed (P = 0·001, I 2 = 82·1 %). A non-linear association between 25(OH)D and MD was found (P for non-linearity = 0·001); the dose–response analysis indicated that the lowest pooled OR was at blood 25(OH)D concentrations of 90–110 nmol/l. Subgroup analyses suggested a stronger association between 25(OH)D and MD in summer time (OR 0·25, 95 % CI 0·08, 0·43) than in other seasons (OR 0·68, 95 % CI 0·52, 0·83) (P for interaction = 0·008). A visual inspection of funnel plot and Begg’s and Egger’s tests did not indicate any evidence of publication bias.
Conclusions:
Low circulating 25(OH)D is associated with MD, and our analysis suggests that they influence each other. Further randomised controlled trials would be needed to determine the direction of causation.
Keywords: Poor vitamin D status, Antepartum depression, Postpartum depression, Maternal depression, Meta-analysis
Depression is a common mental disorder and a leading cause of disability worldwide(1). It has been reported that 350 million people around the world are affected by depression, and as many as two-thirds of all persons who committed suicide may have had this condition(2). Women of child-bearing age are more vulnerable to suffer from depression due to strong biological, physical and social changes they experience(3). Approximately 15–30 % and 10–40 % of women experience depression during pregnancy and postpartum, respectively(4–7). Maternal depression (MD) (including antepartum depression and postpartum depression) is associated with adverse offspring development, such as preterm birth, low birth weight, intrauterine growth restriction, birth defects and low intelligence(8–10). During pregnancy, women need to provide nutrition for the growth and development of the fetus as well as for their own metabolic needs; hence, the demand for vitamins increases. However, due to reduced outdoor activities and sunlight exposure, pregnant women obtain less vitamin D from sun exposure, thereby making them susceptible to vitamin D deficiency. Over the past decades, the relationship between poor vitamin D status – as measured by serum concentration of the intermediary metabolite, 25-hydroxyvitamin D (25(OH)D) – and depression has been a research hotspot.
The aetiology of depression is not fully established; however, genetic, biological and environmental reasons, such as nutritional deficiency and inadequate sunlight exposure, are considered potential factors that could play a role in its pathophysiology(11). Depression is associated with dysregulated hypothalamic–pituitary–adrenal axis function, overactivity of the sympatho-adrenal system and increased level of inflammatory markers(12,13). Vitamin D, vitamin D receptors, vitamin D-activating enzyme 1α-hydroxylase and other vitamin D-related components play an important role in regulating neuronal function, neurotransmitter synthesis, inhibiting apoptosis and regulating neuron regeneration and differentiation(14–16), which have been associated with cognitive impairment and depressive symptoms.
The most widely accepted indicator of vitamin D status is blood 25(OH)D concentrations, the intermediary metabolite of vitamin that circulates after dietary or vitamin D synthesised in the skin after sun exposure is hydroxylated in the liver. Some epidemiological studies were conducted to evaluate the association between serum 25(OH)D and MD. However, the results have been inconclusive. Some studies have found that 25(OH)D has a protective effect on MD and recommended vitamin D supplement use for pregnant women(17,18), while other studies did not report a uniform relationship(19,20). In addition, the association of serum 25(OH)D concentrations with optimal mental health remains unknown. We, therefore, performed a dose–response meta-analysis of observational studies to describe the epidemiological evidence on the relationship between 25(OH)D and MD, and explored supplemental doses of vitamin D required to achieve the lowest risk of MD.
Methods
Search strategy
We comprehensively conducted a literature search of PubMed, Embase and Web of Science from inception to June 2019 for studies addressing the associations between 25(OH)D and MD based on the Meta-analysis Of Observational Studies in Epidemiology guidelines(21). We used the following search terms without restriction: ‘antenatal’ or ‘prepartum’ or ‘prenatal’ or ‘prepartal’ or ‘peripartum’ or ‘perinatal’ or ‘postpartum’ or ‘postnatal’ or ‘puerperal’ or ‘puerperium’ or ‘pregnant’ or ‘pregnancy’ or ‘gravida’ or ‘gestation’ or ‘gestational’ or ‘maternal’ (Mesh) and ‘vitamin D’ or ‘25-hydroxyvitamin D’ or ‘25(OH)D’ or ‘cholecalciferol’ or ‘ergocalciferol’ or ‘ergosterol’ or ‘7-dehydrocholesterol’ (Mesh) and ‘depression’ or ‘depressive symptoms’ or ‘mood disorders’ or ‘mood disturbance’ or ‘mood symptoms’ or ‘mental disorders’ or ‘mental health’ or’ psychological disorders’. Additionally, we reviewed reference lists of retrieved articles to identify additional relevant studies.
Study selection
Two investigators (D.C. and Q.T.) independently screened all articles. Studies that met the following criteria were included: (i) the study design was observational; (ii) 25(OH)D concentrations were estimated and MD was the outcome variable; (iii) the study reported OR or relative risk with 95 % CI for the association between 25(OH)D and MD, or provided sufficient information to allow for their calculation. The studies were limited to English language; non-human studies, clinical trials, reviews, letters and commentaries were excluded. If study populations were reported more than once, we included the result with the longest follow-up time; any result in one study stratified by the season of 25(OH)D measurement was treated as separate reports. Any discrepancy was solved through discussion with the third reviewer (S.L.).
Definition of depression and 25-hydroxyvitamin D measurement
Our primary outcome for all studies was depression diagnosed by one of the following: (i) a clinical diagnosis; (ii) a diagnosis using a validated rating scale with an established cut-off point, such as the twenty-one-item Depression, Anxiety and Stress Scales, or Center for Epidemiological Studies Depression Scale, or Beck Depression Scale, or Edinburgh Postnatal Depression Scale. The cut-off point of the twenty-one-item Depression, Anxiety and Stress Scale, Center for Epidemiological Studies Depression Scale and Beck Depression Scale was 14, 16 and 17, respectively, while that of Edinburgh Postnatal Depression Scale was diverse in different studies, ranging from 6 to 13.
In two studies, cord blood sample was collected to analyse the concentrations of 25(OH)D; many studies showed that maternal 25(OH)D levels are highly correlated with cord blood 25(OH)D concentrations; mean cord blood 25(OH)D concentrations approximated half of maternal 25(OH)D levels(22,23). So we converted 25(OH)D concentrations from cord blood to maternal blood with a conversion factor of 2(22,23). Meanwhile, we converted 25(OH)D concentrations from ng/ml to nmol/l with a conversion factor of 2·5.
Data extraction
Data extraction was conducted independently by two authors (D.C. and Q.T.). Information such as name of first author, year of publication, study location, number of participants, number of cases, study design and characteristics of study population at baseline (age and race) was extracted from the included studies. In addition, the duration of follow-up for cohort study, methods of exposure measurements and outcome measurements, risk estimates and corresponding 95 % CI, and covariates adjusted in the statistical analysis were extracted. The most adjusted risk estimates were extracted when available. Interobserver agreement was assessed using Cohen’s κ. Any disagreements were resolved by discussion with the third author (S.L.).
Quality assessment
Quality assessment was independently performed by two reviewers (D.C. and Q.T.). For cohort and case–control studies, the Newcastle–Ottawa Scale was used(24), it is a nine-point scale allocating points based on the selection process, comparability and identification of exposures and outcomes in the cohort. Scores of 0–3, 4–6 and 7–9 denoted low, moderate and high quality of studies, respectively. For cross-sectional studies, the quality assessment guideline recommended by the Agency for Healthcare Research and Quality was applied(25). The quality of articles was evaluated based on established questions, which were scored as follows: 1 point if the item was considered in the study; otherwise 0 point was assigned.
Data synthesis and statistical analysis
In this meta-analysis, OR and relative risk were considered as the effect level of association between 25(OH)D and MD. OR was deemed equivalent to relative risk. Multivariable adjusted risk was pooled where such estimates were available; otherwise we pooled the unadjusted estimate. Since exposure categories (dichotomous, multichotomous) might be different in different studies, the highest concentration of 25(OH)D was defined as the exposure group and the lowest was the reference group. We used the study-specific OR for the highest v. lowest category of 25(OH)D concentration for the current meta-analysis. Overall pooled OR was calculated using a random-effects model, which considers both within- and between-study variations.
Statistical heterogeneity among the studies was estimated by the Cochran Q test, reported as I 2 statistic, where values of <50, 50–75, >75 % were considered as low, moderate and high degrees of heterogeneity, respectively(26). If heterogeneity was significant, the Mantel–Haenszel random-effect model was used to pool the results, and when heterogeneity was negligible, a fixed-effect model was performed. We conducted sensitivity analysis by sequential and combinatorial algorithms to evaluate the change in between-study heterogeneity as one or more studies were excluded from the calculations. Potential publication bias was evaluated using a funnel plot and with Begg’s and Egger’s tests.
Subgroup analyses were conducted to explore potential sources of study heterogeneity and examine the robustness of the primary results. These included study design (cohort study, case–control study, cross-sectional study), MD type (postpartum depression, antepartum depression) and methods of MD measurements (depression scale and clinical diagnoses). Additionally, latitude (low, middle, high), race of participants (Caucasian, Asian, mixed), season of 25(OH)D measurement (summer, winter, all) and adjustment for different confounders (age, education, parity, history of depression, BMI and smoking) underwent subgroup analysis.
Dose–response analysis was performed on articles that reported more than two quantitative categories of 25(OH)D. For studies that reported concentrations at separate ranges of 25(OH)D levels, we estimated the midpoint in each category by calculating the average of lower and upper bounds. Where the lower boundary of the lowest category was unavailable, half of the upper boundary of that category was considered the assigned median; if the upper boundary of the highest category was not provided, the midpoint of this category was set at 1·5 times that of the lower level. To evaluate for a potential non-linear dose–response association between 25(OH)D and risk of MD, we used a restricted cubic spline regression model with four knots at percentiles 5, 35, 65 and 95 % of the distribution. If the P value for non-linearity was against the null hypothesis that 25(OH)D of the second and third spline transformation was equal to zero, the non-linear dose–response relationship between 25(OH)D and risk of MD was statistically significant(27). A trend curve of non-linear dose–response relationship was drawn. All analyses were performed with Stata statistical software (version 14.0). All tests were two-sided with a significance level of 0·05.
Result
Literature search and quality evaluation
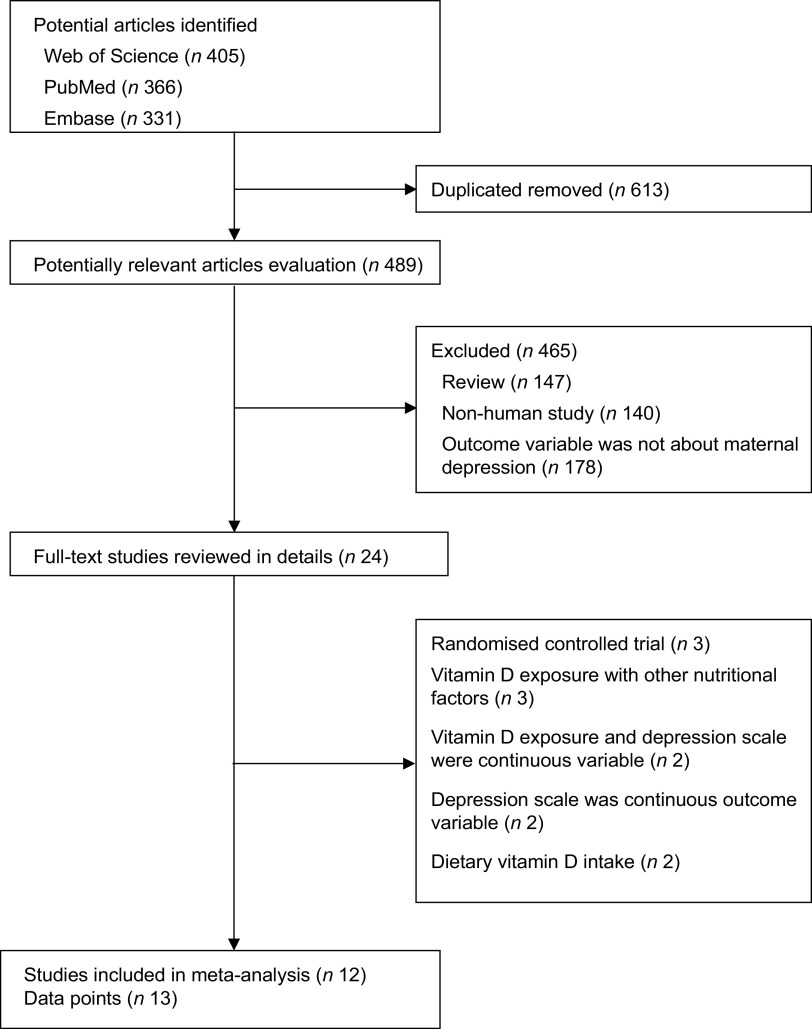
We initially retrieved 405 articles from the Web of Science, 366 from PubMed and 331 from Embase. After removing duplicates, 489 articles were identified as potentially relevant. After reviewing the titles and abstracts, 465 studies were excluded based on ineligibility according to the inclusion criteria. The full text of the remaining twenty-four articles was retrieved for review and detailed evaluation; twelve studies(19,20,28–37) with thirteen independent reports were finally included in the meta-analysis (Fig. 1). The average quality assessment score of all included cohort, case–control and cross-sectional studies was 7·0, 8·5 and 7·0, respectively, and all studies were of moderate or high quality (quality score ≥6) (Table 1). Interobserver agreement between reviewers for study inclusion was very high (κ = 0·97). In the dose–response analysis, two studies(33,35) were excluded because of no or having less than three categories of 25(OH)D concentrations. Finally, ten studies with eleven reports were included in the dose–response analysis of 25(OH)D concentrations with risk of MD.
Fig. 1.
Flowchart showing the relevant observational studies of vitamin D in relation to maternal depression
Table 1.
Characteristics of twelve studies and scores of quality assessment included in the meta-analysis
| Author (year) | Country | Age range/mean/median | Population | Study type | Follow-up | Categories of vitamin D | Cut-off point | Depression scale | Depression type | Methods of vitamin D measurements | Quality scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. (2017)(35) | China | 27 | 1491 | Cohort study | 3 months | <25, ≥25 nmol/l | ≥16 | CES-D | APD | Cord blood | 7 |
| Figueiredo et al. (2017)(34) | Brazil | 26·8 | 179 | Cohort study | – | <50, 50–70, >70 nmol/l | ≥13 | EPDS | APD | Venous blood | 8 |
| Huang et al. (2014)(31) | USA | ≥18 | 498 | Cross-sectional study | – | <72·25, 72·25–85, 85–98·5, ≥98·5 nmol/l | ≥14 | DASS-21 | APD | Venous blood | 7 |
| Brandenbarg et al. (2012)(28) | Netherlands | 31 | 4101 | Cohort study | 3 weeks | ≤29·9, 30–49·9, 50–79·9, ≥80 nmol/l | ≥16 | CES-D | APD | Venous blood | 7 |
| Cassidy-Bushrow et al. (2012)(13,29) | USA | 18–44 | 178 | Cross-sectional study | – | <32, 32–53, >53 nmol/l | ≥16 | CES-D | APD | Venous blood | 7 |
| Lin et al. (2019)(37) | China | – | 120 | Cross-sectional study | – | <50, 50–70, >70 nmol/l | ≥10 | EPDS | PPD | Venous blood | 7 |
| Abedi et al. (2018)(36) | Iran | 18–35 | 120 | Case–control study | – | <25, 25–50, 51–70, >70 nmol/l | ≥17 | Beck Depression Scale | PPD | Venous blood | 8 |
| Gould et al. (2015)(20) | Australian | 27 | 1037 | Cohort study | 6 months | <25, 25–50, >50 nmol/l | ≥13 | EPDS | PPD | Cord blood | 6 |
| Fu et al. (2015)(33) | China | 31 | 213 | Cohort study | 3 months | ≤25, >25 nmol/l | ≥12 | EPDS | PPD | Venous blood | 8 |
| Gur et al. (2014)(30) | Turkey | 28·5 | 194 | Cohort study | 6 months | ≤25, 25–50, ≥50 nmol/l | ≥12 | EPDS | PPD | Venous blood | 7 |
| Robinson et al. (2014)(32) | Australian | – | 706 | Cohort study | 5 months | <47, 47–58, 59–70, >70 nmol/l | ≥6 | EPDS | PPD | Venous blood | 6 |
| Nielsen et al. (2013)(19) | Denmark | ≥18 | 1480 | Nested case–control study | 72 months | <15, 15–24, 25–49, 50–79, 80–99, ≥100 nmol/l | – | Clinical diagnose | PPD | Venous blood | 9 |
CES-D, Center for Epidemiological Studies Depression Scale; APD, antepartum depression; EPDS, Edinburgh Postnatal Depression Scale; DASS-21, twenty-one-item Depression, Anxiety and Stress Scales; PPD, postpartum depression.
Study characteristics
This analysis included seven cohort studies(20,28,30,32,34,35,37), two case–control studies(19,36) and three cross-sectional studies(29,31,33). All studies used were published within the last 8 years from 2012 to 2019. Sample sizes of these studies ranged from 120 to 4101, with a total of 10 317 subjects, and the number of depression cases ranged from 23 to 651, with a total of 2553. Study locations were as follows: three in China(33,35,37), one in Brazil(34), two in Australia(20,32), one in Turkey(30), two in USA(29,31), one in Denmark(19), one in The Netherlands(28) and one in Iran(36). Methods of depression measurement used were one by clinical diagnosis(19), one by the twenty-one-item Depression, Anxiety and Stress Scale(31), one by Beck Depression Scale(36), three by Center for Epidemiological Studies Depression Scale(28,29,35) and six by Edinburgh Postnatal Depression Scale(20,30,32–34,37).
25-Hydroxyvitamin D and risk of maternal depression
Results of the random-effects meta-analysis combining the OR of MD in relation to 25(OH)D are shown in Fig. 2. Compared with the lowest category of 25(OH)D, the pooled OR of MD for the highest category was 0·49 (95 % CI 0·35, 0·63). A high heterogeneity was observed (P = 0·001, I 2 = 82·1 %). Compared with the lowest category of 25(OH)D, the pooled OR of antepartum depression and postpartum depression for the highest category was 0·51 (95 % CI 0·37, 0·65) and 0·48 (95 % CI 0·24, 0·72), respectively; a moderate heterogeneity was observed (P = 0·024, I 2 = 61·2 %) within the studies of antepartum depression, and a high heterogeneity was observed (P = 0·001, I 2 = 82·8 %) within the studies of postpartum depression.
Fig. 2.
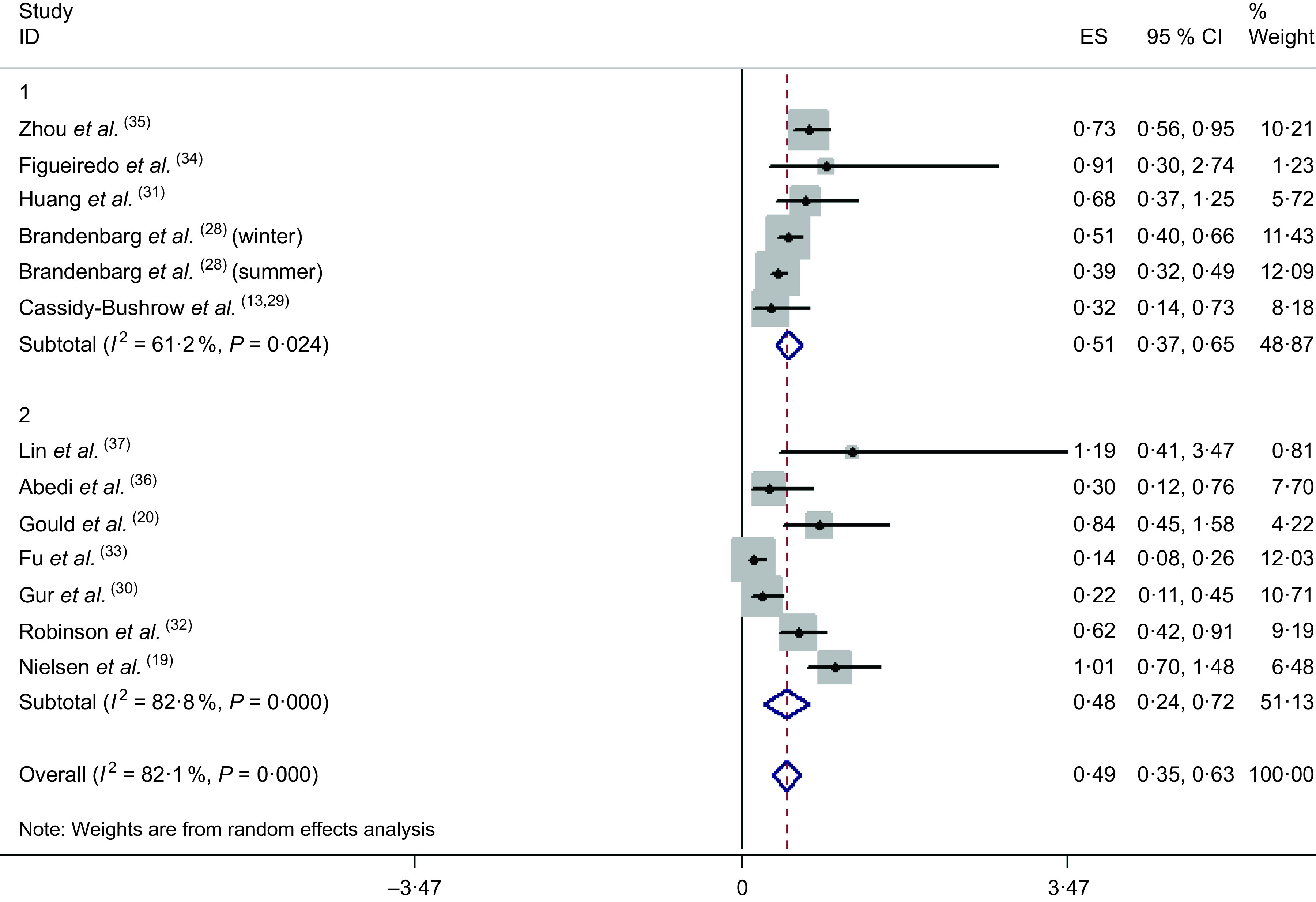
Association between 25(OH)D and risk of maternal depression (1, antepartum depression; 2, postpartum depression; ES, effect size)
Dose–response analysis of 25-hydroxyvitamin D with the risk of maternal depression
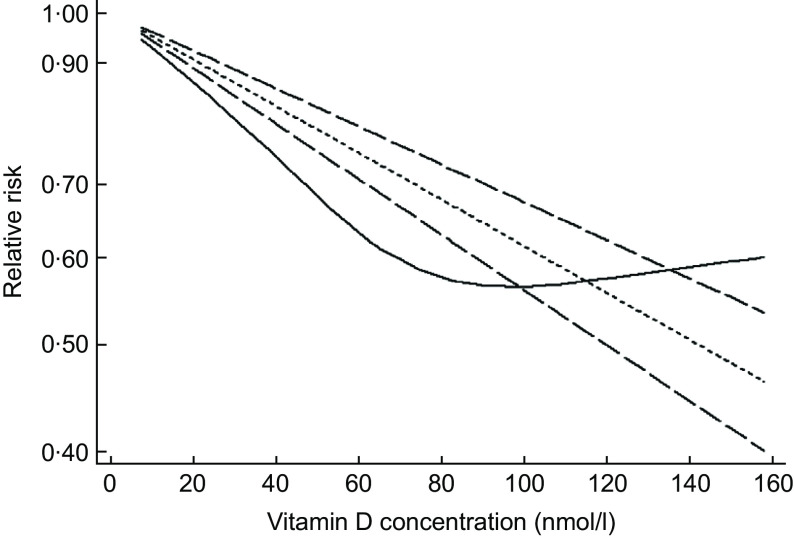
A total of ten studies with eleven reports were included in the dose–response analysis(19,20,28–32,34,36,37). In the cubic spline model (Fig. 3), we found evidence suggesting a non-linear association of 25(OH)D with the risk of MD (P for non-linearity = 0·001). As vitamin D concentrations increased, the risk of MD rapidly decreased to the lowest and then rose slowly. The lowest pooled OR was at 25(OH)D concentrations of 90–110 nmol/l.
Fig. 3.
Dose–response relation plots between 25(OH)D concentration and risk of maternal depression.  , lb with ref;
, lb with ref;  , ub with ref;
, ub with ref;  , rr with ref;
, rr with ref;  , rr_lin
, rr_lin
Subgroup analyses
In most of the subgroup analyses, an association between 25(OH)D and risk of MD was identified. A subgroup analysis of 25(OH)D measurement by season showed that 25(OH)D was negatively associated with MD, irrespective of the season it was measured. The risk was more pronounced for 25(OH)D measured in summer (OR 0·25, 95 % CI 0·08, 0·43) than in other seasons (OR 0·68, 95 % CI 0·52, 0·83). Additionally, depression type, study design, depression measurement, 25(OH)D measurement, latitude, race, adjustment for age, education, parity, history of depression, BMI and smoking did not influence the OR summary (Table 2).
Table 2.
Subgroup and meta-regression analysis of relative risk (RR) of maternal depression
| Subgroup | Number of studies | Pooled OR/RR | 95 % CI | P * | I 2 (%) | P † |
|---|---|---|---|---|---|---|
| Total | 13 | 0·49 | 0·35, 0·63 | 0·001 | 82·10 | |
| Depression type | ||||||
| APD | 6 | 0·51 | 0·37, 0·65 | 0·024 | 61·20 | 0·513 |
| PPD | 7 | 0·48 | 0·24, 0·72 | 0·001 | 82·80 | |
| Study design | ||||||
| Cohort study | 8 | 0·51 | 0·37, 0·65 | 0·002 | 69·30 | 0·711 |
| Case–control | 2 | 0·65 | –0·05, 1·34 | 0·037 | 86·90 | |
| Cross-sectional study | 3 | 0·34 | 0·04, 0·59 | 0·047 | 69·60 | |
| Depression measurement | ||||||
| CES-D | 4 | 0·49 | 0·34, 0·64 | 0·010 | 73·60 | 0·164 |
| EPDS | 6 | 0·41 | 0·17, 0·65 | 0·001 | 75·80 | |
| Others | 3 | 0·65 | 0·22, 1·08 | 0·021 | 74·20 | |
| 25(OH)D measurement | ||||||
| Venous blood | 11 | 0·43 | 0·29, 0·58 | 0·001 | 79·80 | 0·284 |
| Cord blood | 2 | 0·74 | 0·56, 0·93 | 0·718 | 0·00 | |
| Latitude | ||||||
| Low | 3 | 0·65 | 0·41, 0·88 | 0·702 | 0·00 | 0·462 |
| Middle | 10 | 0·46 | 0·31, 0·61 | 0·001 | 85·10 | |
| Race | ||||||
| Caucasian | 8 | 0·46 | 0·33, 0·59 | 0·003 | 67·10 | 0·347 |
| Asian | 3 | 0·51 | –0·04, 1·05 | 0·001 | 93·40 | |
| Mixed | 2 | 0·85 | 0·34, 1·37 | 0·919 | 0·00 | |
| Season of 25(OH)D measurement | ||||||
| Summer | 3 | 0·25 | 0·08, 0·43 | 0·201 | 28·50 | 0·008 |
| Winter | 2 | 0·46 | 0·28, 0·64 | 0·233 | 29·60 | |
| All | 8 | 0·68 | 0·52, 0·83 | 0·201 | 28·50 | |
| Age adjusted | ||||||
| Yes | 3 | 0·32 | 0·02, 0·63 | 0·040 | 69·00 | 0·477 |
| No | 10 | 0·53 | 0·39, 0·67 | 0·001 | 70·70 | |
| Education adjusted | ||||||
| Yes | 2 | 0·15 | 0·07, 0·24 | 0·345 | 0·00 | 0·159 |
| No | 11 | 0·54 | 0·41, 0·68 | 0·001 | 69·20 | |
| Parity adjusted | ||||||
| Yes | 3 | 0·24 | 0·04, 0·44 | 0·046 | 67·50 | 0·347 |
| No | 10 | 0·55 | 0·42, 0·68 | 0·005 | 61·90 | |
| History of depression adjusted | ||||||
| Yes | 1 | 0·84 | 0·28, 1·41 | – | – | 0·479 |
| No | 12 | 0·47 | 0·33, 0·62 | 0·001 | 82·80 | |
| BMI adjusted | ||||||
| Yes | 2 | 0·52 | 0·01, 1·04 | 0·103 | 62·40 | 0·809 |
| No | 11 | 0·49 | 0·33, 0·64 | 0·001 | 84·40 | |
| Smoking adjusted | ||||||
| Yes | 2 | 0·47 | –0·13, 1·07 | 0·039 | 76·40 | 0·909 |
| No | 11 | 0·51 | 0·35, 0·66 | 0·001 | 83·70 | |
APD, antepartum depression; PPD, postpartum depression; CES-D, Center for Epidemiological Studies Depression Scale; EPDS, Edinburgh Postnatal Depression Scale.
P for heterogeneity within each subgroup.
P for heterogeneity between subgroups in the meta-regression analysis.
Sensitivity analyses
Sensitivity analyses were performed to check for the robustness of results and to find the potential origins of heterogeneity. After dropping out four studies identified by combinatorial algorithms, which were primary origins of heterogeneity, there was still a statistically significant negative association (OR 0·67, 95 % CI, 0·56, 0·80), and no heterogeneity was observed (P = 0·29, I 2 = 17·2 %). By sequential algorithms, we excluded any single report in turn and pooled the results of remaining reports; a pooled OR of MD ranging from 0·49 (95 % CI, 0·40, 0·51; P = 0·001) to 0·59 (95 % CI 0·47, 0·71; P = 0·001) was observed, which indicated the result was robust.
Publication bias
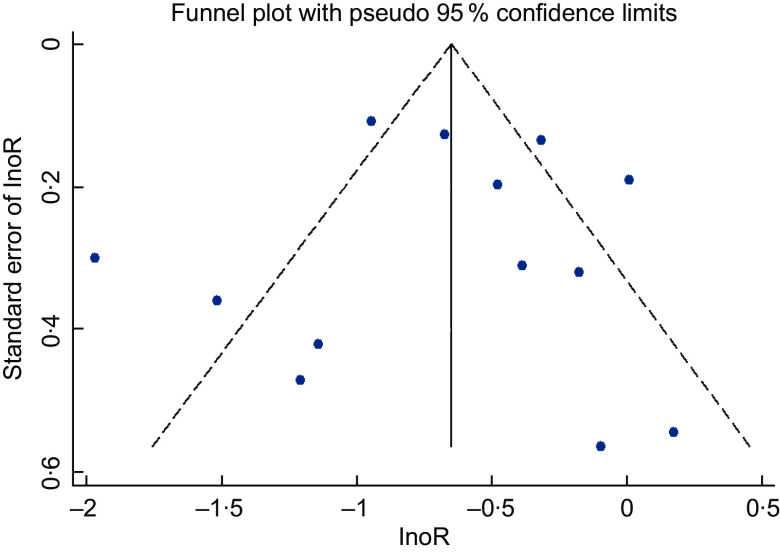
A visual inspection of the funnel plot did not suggest a substantial asymmetry (Fig. 4). Both the Begg’s rank correlation test (Z = 0·18, P = 0·855) and the Egger’s linear regression test (t = –0·09, P = 0·928) indicated no evidence of publication bias among the studies.
Fig. 4.
Funnel plot of 25(OH)D and risk of maternal depression
Discussion
To our knowledge, this is the first review to use a dose–response meta-analytic approach to quantitatively summarise the evidence and systematically estimate the effect of vitamin D status on MD risk. Based on our systematic analysis, we interpreted the range of serum 25(OH)D concentrations associated with optimal mental health. In thirteen reports, 25(OH)D is shown to be significantly related to a decreased risk of MD. Specifically, the dose–response meta-analysis indicated that a high blood 25(OH)D concentrations has a protective effect on MD. Notably, when blood 25(OH)D concentrations increased to 90–100 nmol/l, the risk of MD was the lowest.
Two valuable and important findings were obtained from the current analyses. Firstly, we inferred that 25(OH)D and MD were both a causality and a consequence. Some studies have demonstrated that vitamin D, a potential neurosteroid, may play an important role in certain mental processes, and its deficiency could lead to an improper functioning of hormones that control mood. Conversely, depression may also be a risk factor for developing vitamin D deficiency because of staying indoors, consumption of less-nutritious diets and inadequate exercises. In the subgroup analysis by season, an inverse relationship between 25(OH)D concentrations and MD symptoms was found, and the association became stronger in summer months. If depression was caused by vitamin D deficiency (mainly due to inadequate sunlight exposure), then the association between 25(OH)D concentrations and contemporaneous MD symptoms would be expected to be stronger in summer months when the levels are strongly influenced by sunlight exposure(38–40). This was clearly evident in the subgroup analysis by season. Regardless of the direction of causation, poor vitamin D status and depression are common among pregnant women, and although both appear to have substantial adverse health consequences, they are potentially reversible if enough attention can be paid to it.
Secondly, from our dose–response analysis, pregnant and lactating mothers may need to maintain blood 25(OH)D concentrations at about 90–100 nmol/l to reduce the risk of depression to the barest minimum. As Heaney has suggested(41), an equivalence value of 2·5 nmol/l may be used to reflect an input of 2·5 µg/d; herein, 90–100 nmol/l reflects an input of 90–100 µg/d of vitamin D. Studies estimating diet records have found that intakes of food sources would supply no more than 5·0 µg/d of vitamin D. Even at peak summer, maximal solar synthesis could account for less than 17·5 µg/d(41). In this regard, we inferred a need for about 67·5–77·5 µg/d of supplemental vitamin D from our dose–response analysis. The American Endocrine Society’s clinical practice guidelines suggest that pregnant and lactating women require at least 15·0 µg/d of supplemental vitamin D. It added that at least 37·5–50 µg/d of supplemental vitamin D may be needed to maintain a blood 25(OH)D level >75 nmol/l(42). The recommended dose in the current dose–response analysis is higher than that prescribed by the American Endocrine Society; however, it is below the tolerable upper intake level and well within the safe range delineated by Hathcock et al.(43).
Although poor vitamin D status may not be the only cause of depression, it is correlated with the aetiology and manifestation of depression. Therefore, vitamin D screening and intake of supplements could be an effective public health measure to reduce MD risk. To date, guidelines concerning vitamin D screening and supplements among pregnant women are conflicting. Clinical antenatal care guidelines in USA and Australia recommend screening for women only at an increased risk of vitamin D deficiency, including those with limited exposure to sunlight. In UK, pregnant women could obtain free vitamin D supplements from local health departments. However, similar policies are yet to be implemented in China and other developing countries(44). In conclusion, the evidence provided by the current meta-analysis shows that low 25(OH)D concentrations is implicated as not only a risk factor but also a consequence of MD, and supports the recommendation to scale-up vitamin D screening and use of supplements by pregnant women. Given the potential impact of season (sunlight exposure) on 25(OH)D concentrations, the dosage of supplement should be carefully estimated; in winter, pregnant women should moderately increase the dosage of vitamin D supplement.
The current study has several limitations. Firstly, twelve studies that measured 25(OH)D concentrations were selected independently of whether or not the assay used was standardised as currently recommended for accurate meta-analyses, and therefore the reported measurements might vary. Secondly, an association between 25(OH)D and MD derived from observational studies could just infer causality; further prospective studies and randomised controlled trials would be needed to confirm the causality. Another important limitation pertains to the observational study design; most employed a scale cut-off rather than a clinical depression diagnosis and used the unadjusted estimates as several important covariates, including life stress, social support and exercise, were missing. Lastly, 25(OH)D2 and 25(OH)D3 were not separately analysed in the dose–response analysis because 25(OH)D3 is the main part of 25(OH)D.
Acknowledgements
Acknowledgements: We express our appreciation and gratitude to all the authors of the original studies included in the current meta-analysis. Financial support: The present research was financially supported by ‘study on the effect of six sigma management on improving the quality of information on home page of medical records’ (2018YFYB010). Conflicts of interests: None. Authorship: D.C. performed study conception and design, literature search and data analysis. Q.T. performed literature search and drafting of manuscript. S.L. contributed to the literature search, data analysis and revised the article critically for important intellectual content. Ethics of human subject participation: None.
References
- 1. World Health Organization (2020) Depression. http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed September 2020).
- 2. Ledford H (2014) Medical research: if depression were cancer. Nature 515, 182–184. [DOI] [PubMed] [Google Scholar]
- 3. Marcus SM (2009) Depression during pregnancy: rates, risks and consequences–motherisk consequences – Motherisk Update 2008. Can J Clin Pharmacol 16, 15–22. [PubMed] [Google Scholar]
- 4. Marcus SM, Flynn HA, Blow FC et al. (2003) Depressive symptoms among pregnant women screened in obstetrics settings. J Women Health Gen-B 12, 373–380. [DOI] [PubMed] [Google Scholar]
- 5. Robertson E, Grace S, Wallington T et al. (2004) Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 26, 289–295. [DOI] [PubMed] [Google Scholar]
- 6. Field T (2010) Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev 33, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Savitz DA, Stein CR, Ye F et al. (2011) The epidemiology of hospitalized postpartum depression in New York State, 1995–2004. Ann Epidemiol 21, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grote NK, Bridge JA, Gavin AR et al. (2010) A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiat 67, 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aghajafari F, Nagulesapillai T, Ronksley PE et al. (2013) Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ 346, f1169. [DOI] [PubMed] [Google Scholar]
- 10. Schneuer FJ, Roberts CL, Guilbert C et al. (2014) Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am J Clin Nutr 99, 287–295. [DOI] [PubMed] [Google Scholar]
- 11. Saveanu RV & Nemeroff CB (2012) Etiology of depression: genetic and environmental factors. Psychiatr Clin North Am 35, 51–71. [DOI] [PubMed] [Google Scholar]
- 12. Amini S, Jafarirad S & Amani R (2019) Postpartum depression and vitamin D: a systematic review. Crit Rev Food Sci Nutr 59 1514–1520. [DOI] [PubMed] [Google Scholar]
- 13. Cassidy-Bushrow AE, Peters RM, Johnson DA et al. (2012) Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J Reprod Immunol 94, 202–209. [DOI] [PubMed] [Google Scholar]
- 14. Garcion E, Wion-Barbot N, Montero-Menei CN et al. (2002) New clues about vitamin D functions in the nervous system. Trends Endocrin Met 13, 100–105. [DOI] [PubMed] [Google Scholar]
- 15. Bertone-Johnson ER (2009) Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev 67, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandes de Abreu DA, Eyles D & Feron F (2009) Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 34, Suppl. 1, S265–S277. [DOI] [PubMed] [Google Scholar]
- 17. Accortt EE, Schetter CD, Peters RM et al. (2016) Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: preliminary evidence for moderation by inflammatory cytokines. Arch Womens Ment Health 19, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams JA, Romero VC, Clinton CM et al. (2016) Vitamin D levels and perinatal depressive symptoms in women at risk: a secondary analysis of the mothers, omega-3, and mental health study. BMC Pregnancy Childbirth 16, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen NO, Strom M, Boyd HA et al. (2013) Vitamin D status during pregnancy and the risk of subsequent postpartum depression: a case–control study. PLoS ONE 8, e80686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gould JF, Anderson AJ, Yelland LN et al. (2015) Association of cord blood vitamin D at delivery with postpartum depression in Australian women. Aust N Z J Obstet Gynaecol 55, 446–452. [DOI] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- 22. Bouillon R, Van Baelen H & De Moor P (1977) 25-Hydroxyvitamin D and its binding protein in maternal and cord serum. J Clin Endocrinol Metab 45, 679–684. [DOI] [PubMed] [Google Scholar]
- 23. Lamb AR, Lutenbacher M, Wallston KA et al. (2018) Vitamin D deficiency and depressive symptoms in the perinatal period. Arch Women Ment Health 21, 745–755. [DOI] [PubMed] [Google Scholar]
- 24. Wells GA, Shea B, O’Connell D et al. (2014) The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. Ottawa, ON: Ottawa Health Research Institute. [Google Scholar]
- 25. Rockville (2004) Evidence Reports. https://www.ncbi.nlm.nih.gov/books/NBK35156/ (accessed September 2020).
- 26. Higgins JP &Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- 27. Orsini N, Li RF, Wolk A et al. (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brandenbarg J, Vrijkotte TGM, Goedhart G et al. (2012) Maternal early-pregnancy vitamin D status is associated with maternal depressive symptoms in the Amsterdam born children and their development cohort. Psychosom Med 74, 751–757. [DOI] [PubMed] [Google Scholar]
- 29. Cassidy-Bushrow AE, Peters RM, Johnson DA et al. (2012) Vitamin D nutritional status and antenatal depressive symptoms in African American women. J Womens Health 21, 1189–1195. [DOI] [PubMed] [Google Scholar]
- 30. Gur EB, Gokduman A, Turan GA et al. (2014) Mid-pregnancy vitamin D levels and postpartum depression. Eur J Obstet Gynecol Reprod Biol 179, 110–116. [DOI] [PubMed] [Google Scholar]
- 31. Huang JY, Arnold D, Qiu CF et al. (2014) Association of serum vitamin D with symptoms of depression and anxiety in early pregnancy. J Womens Health 23, 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robinson M, Whitehouse AJO, Newnham JP et al. (2014) Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch Women’s Ment Health 17, 213–219. [DOI] [PubMed] [Google Scholar]
- 33. Fu CW, Liu JT, Tu WJ et al. (2015) Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG 122, 1688–1694. [DOI] [PubMed] [Google Scholar]
- 34. Figueiredo ACC, Trujillo J, Freitas-Vilela AA et al. (2017) Association between plasma concentrations of vitamin D metabolites and depressive symptoms throughout pregnancy in a prospective cohort of Brazilian women. J Psychiatr Res 95, 1–8. [DOI] [PubMed] [Google Scholar]
- 35. Zhou QF, Zhang MX, Tong SL et al. (2017) Maternal depression attenuates newborn vitamin D concentrations in winter-spring: a prospective population-based study. Sci Rep 7, 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abedi P, Bovayri M, Fakhri A et al. (2018) The relationship between vitamin D and postpartum depression in reproductive-aged Iranian women. J Med Life 11, 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin YH, Chen CM, Su HM et al. (2019) Association between postpartum nutritional status and postpartum depression symptoms. Nutrients 11, 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nanri A, Mizoue T, Matsushita Y et al. (2009) Association between serum 25-hydroxyvitamin D and depressive symptoms in Japanese: analysis by survey season. Eur J Clin Nutr 63, 1444–1447. [DOI] [PubMed] [Google Scholar]
- 39. Stewart R & Hirani V (2010) Relationship between vitamin D levels and depressive symptoms in older residents from a national survey population. Psychosom Med 72, 608–612. [DOI] [PubMed] [Google Scholar]
- 40. Rabenberg M, Harisch C, Rieckmann N et al. (2016) Association between vitamin D and depressive symptoms varies by season: results from the German Health Interview and Examination Survey for Adults (DEGS1). J Affect Disord 204, 92–98. [DOI] [PubMed] [Google Scholar]
- 41. Heaney RP & Armas LA (2015) Quantifying the vitamin D economy. Nutr Rev 73, 51–67. [DOI] [PubMed] [Google Scholar]
- 42. Holick MF, Binkley NC, Bischoff-Ferrari HA et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 96, 1911–1930. [DOI] [PubMed] [Google Scholar]
- 43. Hathcock JN, Shao A, Vieth R et al. (2007) Risk assessment for vitamin D. Am J Clin Nutr 85, 6–18. [DOI] [PubMed] [Google Scholar]
- 44. Yun C, Chen J, He Y et al. (2017) Vitamin D deficiency prevalence and risk factors among pregnant Chinese women. Public Health Nutr 20, 1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sempos CT, Heijboer AC, Bikle DD et al. (2018) Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br J Clin Pharmacol 84, 2194–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]